A multicenter investigation of 2,773 cases of bloodstream infections based on China antimicrobial surveillance network (CHINET)
- 1Institute of Antibiotics, Huashan Hospital, Fudan University, Shanghai, China
- 2Key Laboratory of Clinical Pharmacology of Antibiotics, National Health Commission, Shanghai, China
- 3Department of Hospital Infection Management, Huashan Hospital, Fudan University, Shanghai, China
- 4Clinical Microbiology Laboratory, The First Affiliated Hospital of Anhui Medical University, Hefei, China
- 5Clinical Microbiology Laboratory, Beijing Hospital, Beijing, China
- 6Department of Infectious Disease, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 7Clinical Microbiology Laboratory, The First Affiliated Hospital of Guangzhou Medical University, Guangzhou, China
- 8Clinical Microbiology Laboratory, The First Affiliated Hospital of Kunming Medical University, Kunming, China
- 9Department of Infectious Disease, Sir Run Run Shaw Hospital, Affiliated to Zhejiang University School of Medicine, Hangzhou, China
- 10Clinical Microbiology Laboratory, Sir Run Run Shaw Hospital, Affiliated to Zhejiang University School of Medicine, Hangzhou, China
- 11Clinical Microbiology Laboratory, Tongji Hospital Affiliated to Tongji Medical College of Huazhong University of Science and Technology, Wuhan, China
- 12Clinical Microbiology Laboratory, Peking Union Medical College Hospital, Beijing, China
- 13Clinical Microbiology Laboratory, Children’s Hospital of Fudan University, Shanghai, China
- 14Clinical Microbiology Laboratory, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
- 15Department of Hospital Infection Management, Ruijin Hospital Affiliated to Shanghai Jiaotong University School of Medicine, Shanghai, China
Background: Bloodstream infections (BSIs), especially hospital-acquired BSIs, are a major cause of morbidity and mortality. However, the details about the pathogens and antimicrobial resistance profile of BSIs across China are still lacking.
Methods: An investigation was conducted in 10 large teaching hospitals from seven geographic regions across China in 2016 based on China Antimicrobial Surveillance Network (CHINET) to profile the clinical and etiological features of BSIs.
Results: A total of 2,773 cases of BSIs were identified, a majority (97.3%) of which were monomicrobial. Overall, 38.4% (1,065/2,773) were community-acquired BSIs (CABSIs), and 61.6% (1,708/2,773) were hospital-acquired BSIs (HABSIs). Of the 2,861 pathogenic BSI isolates, 67.5% were Gram-negative bacteria, 29.6% were Gram-positive bacteria, and 2.9% were fungi. The top BSI pathogens were Escherichia coli, Klebsiella pneumoniae, coagulase-negative Staphylococci (CNS), Staphylococcus aureus, Enterococci, and Acinetobacter baumannii. Escherichia coli and K. pneumoniae isolates showed low susceptibility to penicillins, cephalosporins (except ceftazidime and cefepime), and ampicillin-sulbactam (13.1%–43.4% susceptible); moderate susceptibility (about 60% susceptible) to ceftazidime, cefepime, and aztreonam; and high susceptibility (>90%) to β-lactam/β-lactamase inhibitor combinations other than ampicillin-sulbactam, except K. pneumoniae strains to piperacillin-tazobactam (59.2% susceptible). HABSIs were associated with significantly higher prevalence of carbapenem-resistant and extended-spectrum β-lactamases-producing K. pneumoniae, methicillin-resistant S. aureus, methicillin-resistant CNS, and ampicillin-resistant Enterococci than CABSIs. Overall, 42.0% of the BSI due to S. aureus strains were resistant to methicillin.
Conclusions: The findings about BSIs in teaching hospitals across China add more scientific evidence to inform the appropriate management of the disease.
Bloodstream infection (BSI) is one of the well-recognized critical infectious diseases. The use of invasive procedures (e.g., intravenous catheters, mechanical ventilation, and dialysis), medications (e.g., immunosuppressants and biological products), and the growing population of immunocompromised or immunodeficient individuals have contributed to the high incidence of infections, including BSIs. The extensive and intensive use of broad-spectrum antibiotics has made the situation of antimicrobial resistance worse, and so the prevalence of drug-resistant bacteria is also increasing in BSIs (Yang et al., 2019). The antibiotic-resistant bacterial infections are difficult to manage, are associated with high mortality, and pose a great threat to health and life, which have caused heavy social and economic burden (Rodríguez-Créixems et al., 2008; Cassini et al., 2019). The widespread of carbapenem-resistant Gram-negative bacilli and other multidrug-resistant or extensively drug-resistant strains in recent years has brought great challenges to the antimicrobial treatment and narrowed the empirical treatment options for various infectious diseases, including BSIs (Hu et al., 2018).
Timely and accurate etiological diagnosis and early and appropriate antimicrobial treatment are crucial to optimal outcomes of BSIs (Rodríguez-Baño et al., 2010). The appropriateness of initial empirical treatment is dependent on the knowledge of local BSI pathogens and their resistance patterns. However, the data of large series of BSIs are still lacking in China even though such data are available internationally, which inform the changing BSI pathogens and their resistance profiles over time. The ongoing antimicrobial resistance surveillance in China only reported the BSI data in individual hospitals. Some additional reports on BSI cases are also available but of small sample size (Li et al., 2013; Chen et al., 2016; Zhou Y, et al., 2021; Zhou J, et al., 2021). Therefore, it is urgently needed to fully understand the distribution and resistance of BSI pathogens in China for better management of the disease. This investigation was based on the data from China Antimicrobial Surveillance Network (CHINET) (www.chinets.com) to conduct a prospective multicenter survey on BSIs for the first time in China. The findings will be conducive to appropriate empirical and targeted antimicrobial treatment of BSIs and inform the strategies for etiological diagnosis and antimicrobial therapy of BSIs in China.
Methods
Study design
Ten large teaching hospitals, including a children’s hospital, were selected from the members of CHINET program to participate in this investigation, covering seven provinces or municipalities across China. The participating hospitals ranged in size from 689 to 6,000 beds (2,576 on average). The patients with positive blood culture during treatment in any of the participating hospitals, outpatient clinic, or emergency department from 1 January to 31 December 2016 were enrolled in this study. BSI diagnosis is described according to the diagnostic criteria. Clinical data were retrieved from the hospital information system of each study center, including demographic data, clinical service at the onset of BSI, predisposing factors, disease status, anti-infective treatment, and outcomes. The pathogenic bacteria collected from each center were sent to the central laboratory, i.e., Clinical Microbiology Laboratory of Institute of Antibiotics, Huashan Hospital Affiliated to Fudan University, for species re-identification and re-testing of antimicrobial susceptibility. The distribution and resistance profile of pathogenic bacteria were analyzed.
The study protocol was reviewed and approved by the Institutional Review Board (IRB) of Huashan Hospital, Fudan University. The remaining nine participating centers (hospitals) agreed with the conclusion of the IRB of Huashan Hospital. Informed consent was waived for all the patients.
Study population
The patients with confirmed BSI were considered as the study population. The eligible patients were enrolled at each center if they met the diagnostic criteria for BSI (Ministry of Health of People’s Republic of China, 2001; Horan et al., 2008). The patients were excluded if the positive blood culture was due to skin contaminant or colonizing bacteria or medical history data were incomplete or missing. The first isolates from individual patients were collected and analyzed. Duplicate organisms from one patient were not collected and analyzed. Different organisms from various periods were collected to evaluate as independent episode or co-infection.
BSI diagnosis
BSI diagnosis was confirmed if the patient had one or more blood cultures positive for a pathogenic bacterium; and any of the symptoms or signs such as fever ≥38°C or <36°C, chills, or hypotension (systolic blood pressure ≤90 mmHg); and any of the following laboratory abnormalities: peripheral blood leukocytosis, or neutrophilia, or neutrophil left shift, or increased C-reactive protein (CRP) or procalcitonin (PCT). For the skin contaminant or colonizing bacteria (e.g., coagulase-negative Staphylococci), BSI was not considered unless the same bacterial strain was cultured from the blood samples taken from two different sites, or the bacterial strain isolated from blood was the same as that isolated from other body sites or intravascular catheter specimen. The BSI episodes were excluded if they were considered relapse rather than a separate infection.
BSIs were further differentiated as hospital acquired (HABSI) or community acquired (CABSI) according to where the BSI was acquired (Horan et al., 2008). HABSI was considered in a patient who had a positive blood culture and acquired BSI at least 48 h after admission or <48 h after discharge and he/she had no infection in the incubation period of infection at time of hospital admission. CABSI was considered in a patient who had a positive blood culture and acquired BSI in community or within 48 h after admission.
Microbiological methods
Blood cultures were sampled, according to predefined indications, in BacT/ALERT FA Plus and PF Plus (bioMérieux, Marcy-l’Etoile, France) blood culture bottles. Isolates retrieved from the blood culture bottles were processed locally and later shipped to Huashan Hospital for reference identification [matrix-assisted laser desorption/ionization time-of-flight spectrometry (MALDI-TOF), bioMérieux)] and antimicrobial susceptibility testing (broth microdilution method). All microbiological methods were consistent with current Clinical and Laboratory Standards Institute (CLSI) recommendations. The isolates were stored at −70°C for further tests. The results were interpreted according to the breakpoints recommended by CLSI in 2018 (CLSI, 2018b; CLSI, 2018b).
Statistical analysis
The antimicrobial susceptibility data were processed and analyzed using WHONET 5.6 software. The measurement data of clinical variables were compared statistically by two-sided t-test via SPSS 22.0 software. The enumeration data were compared by chi-square test. p<0.05 was considered statistically significant in univariate analysis.
Results
Patient and disease characteristics
A total of 6,235 blood-culture isolates were isolated from 5,613 patients from 10 participating cites during CHINET 2016. A total of 1,966 episodes were excluded as contaminants, and the majority were isolated with single or dual coagulase-negative Staphylococci. There were 874 episodes that were excluded as inadequate information. A total of 2,773 patients were identified with BSI diagnosis during the study period. Overall, 2,861 pathogenic strains were isolated, including monomicrobial BSI in 2,697 (97.3%) cases and polymicrobial BSI in 76 (2.7%) cases. Overall, 61.6% (1,709/2,773) of the BSIs were hospital acquired, and 38.4% (1,064/2,773) were community acquired. About 8.9% (248/2,773) of the BSIs occurred in outpatients or in patients visiting the emergency department and 91.1% (2,525/2,773) in hospitalized patients, of which 19.3% (488/2,525) stayed in the intensive care unit (ICU) and 80.7% (2,037/2,525) were treated in non-intensive care wards.
Most of the patients (86.2%, 2,390/2,773) were at least 18 years old. The primary site of infection was identified in 34.4% (955/2,773) of the cases. It was similar between HABSIs and CABSIs; besides, more CABSI patients also had urinary tract infection and biliary tract infection (Table 1).
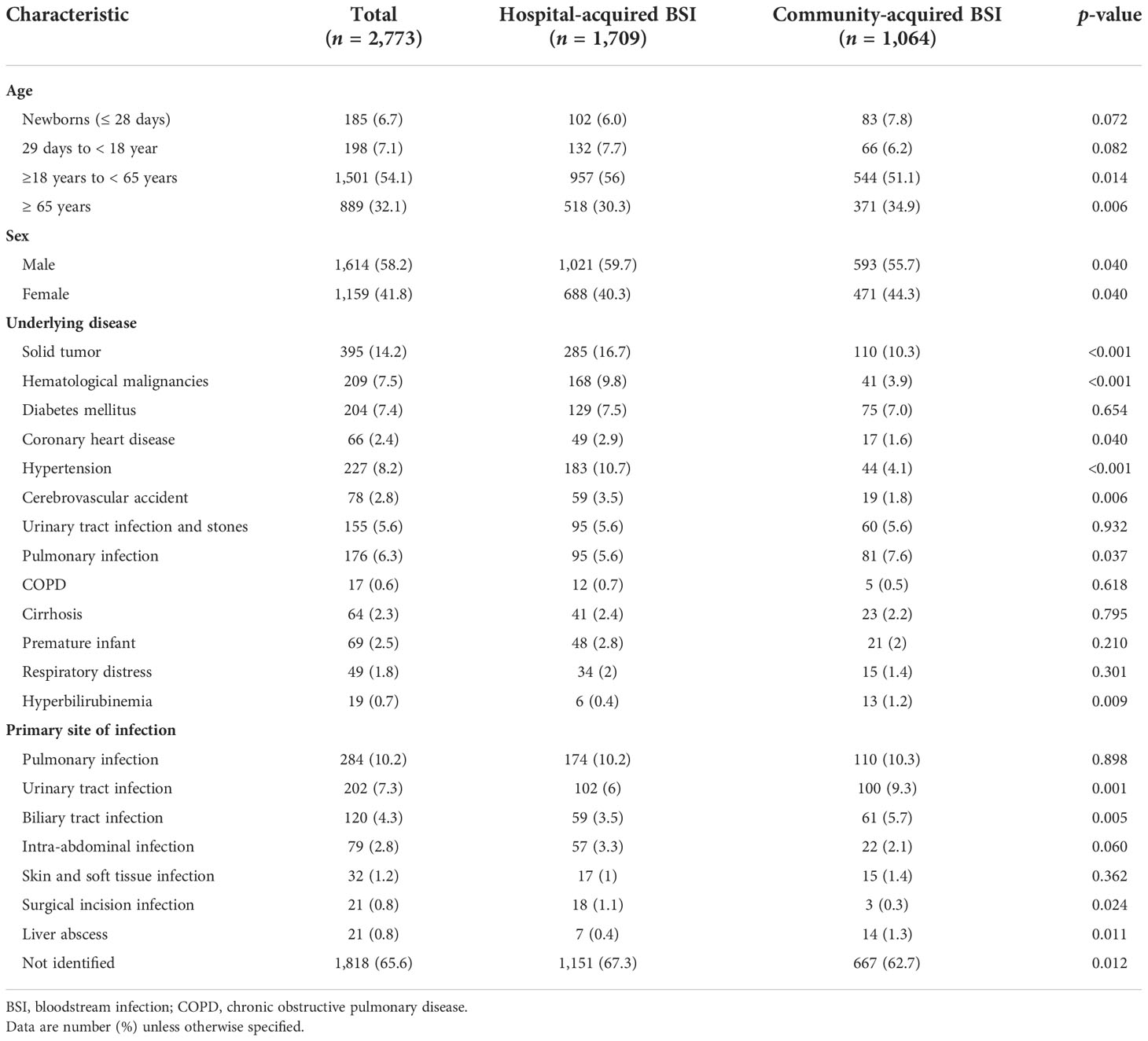
Table 1 Demographic and clinical data compared between hospital- and community-acquired bloodstream infections.
Fever was the prominent symptom of BSIs, reported in 88.0% (1,504/1,709) of the HABSI cases and 84.2% (896/1,064) of the CABSI cases. Intravascular catheters and other invasive procedures were the most prevalent predisposing factor for HABSIs, significantly higher than the prevalence in CABSIs (Table 2).
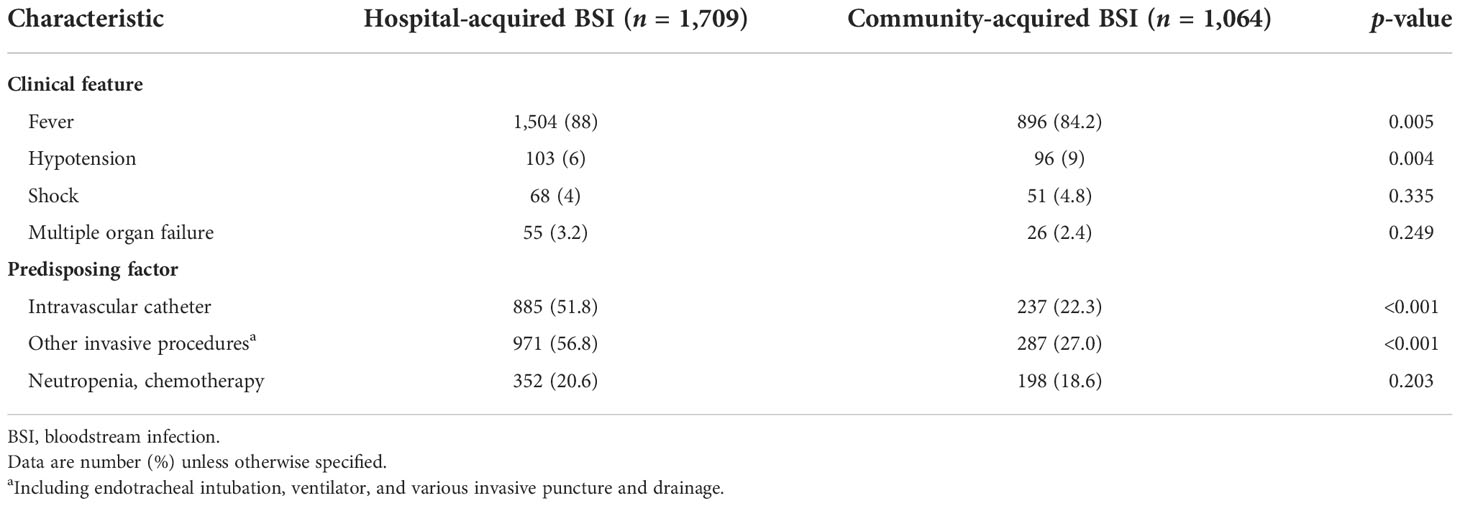
Table 2 Clinical features and predisposing factors compared between hospital- and community-acquired bloodstream infections.
BSI pathogens
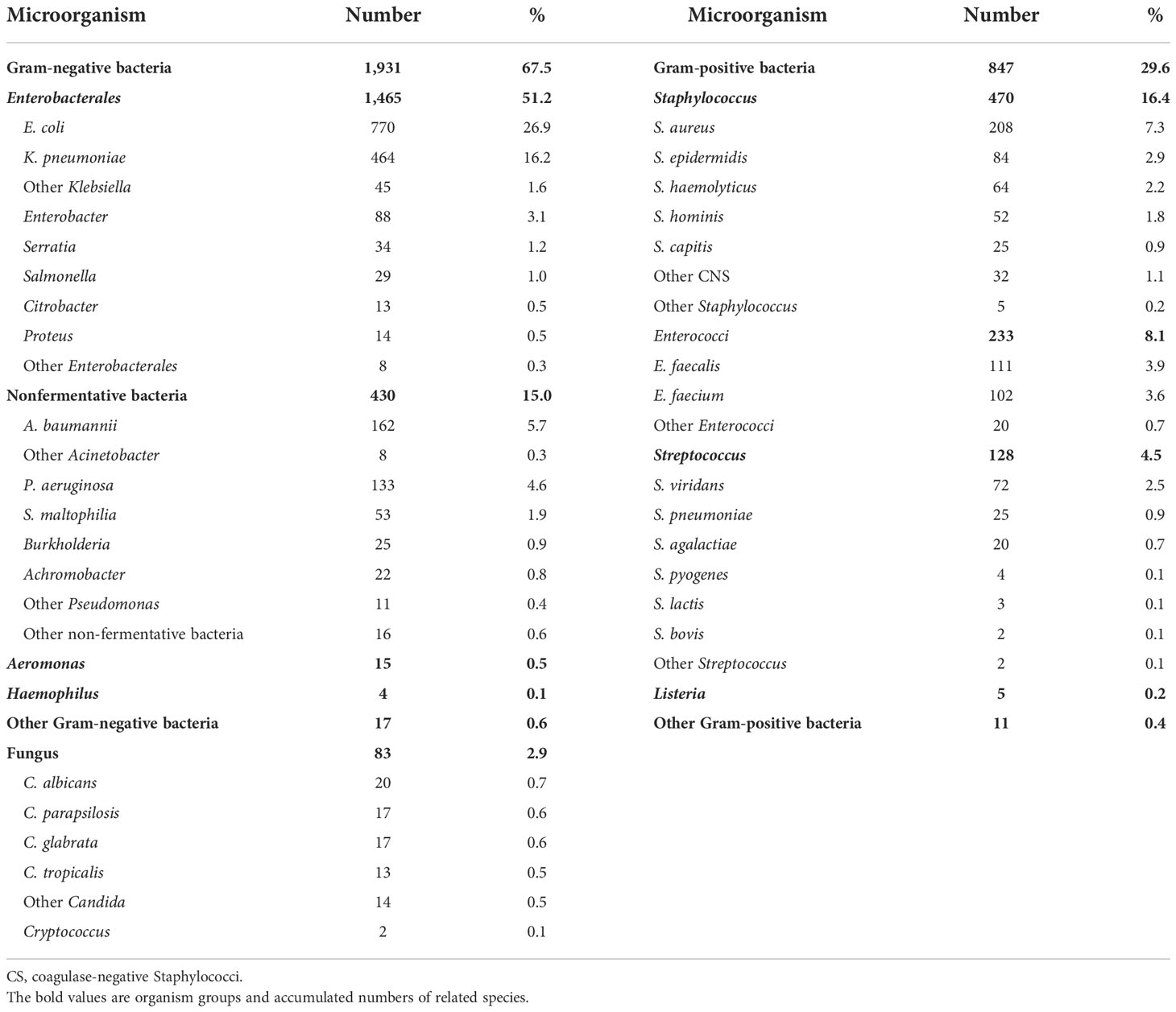
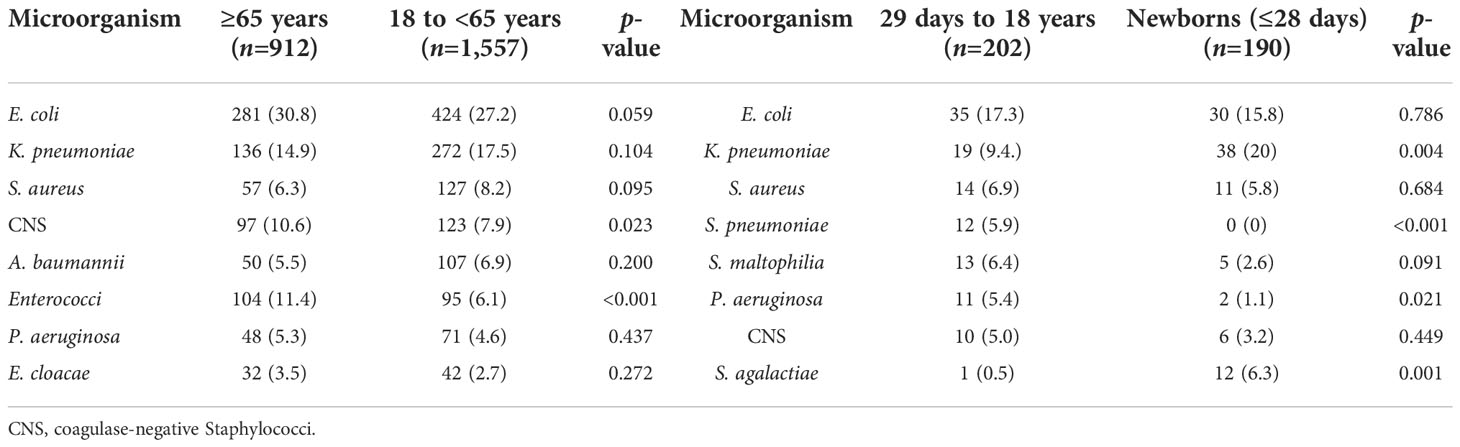
Of the 2,861 strains, the top 10 pathogens were E. coli, K. pneumoniae, coagulase-negative Staphylococci, S. aureus, Enterococci, A. baumannii, P. aeruginosa, Streptococcus, Enterobacter, and Candida species (Table 3). The prevalence of E. coli and S. aureus was significantly higher in CABSIs than in HABSIs, while HABSIs were associated with a significantly higher proportion of K. pneumoniae and A. baumannii than CABSIs. A significantly higher proportion of A. baumannii, coagulase-negative Staphylococci, and Enterococci were identified in the BSI pathogens isolated from ICU patients compared with that from non-ICU patients, while E. coli was more frequently isolated from non-ICU patients (Table 4). Enterococci and coagulase-negative Staphylococci were significantly associated with old patients (≥65 years) than younger adults (18–65 years) (Table 5).
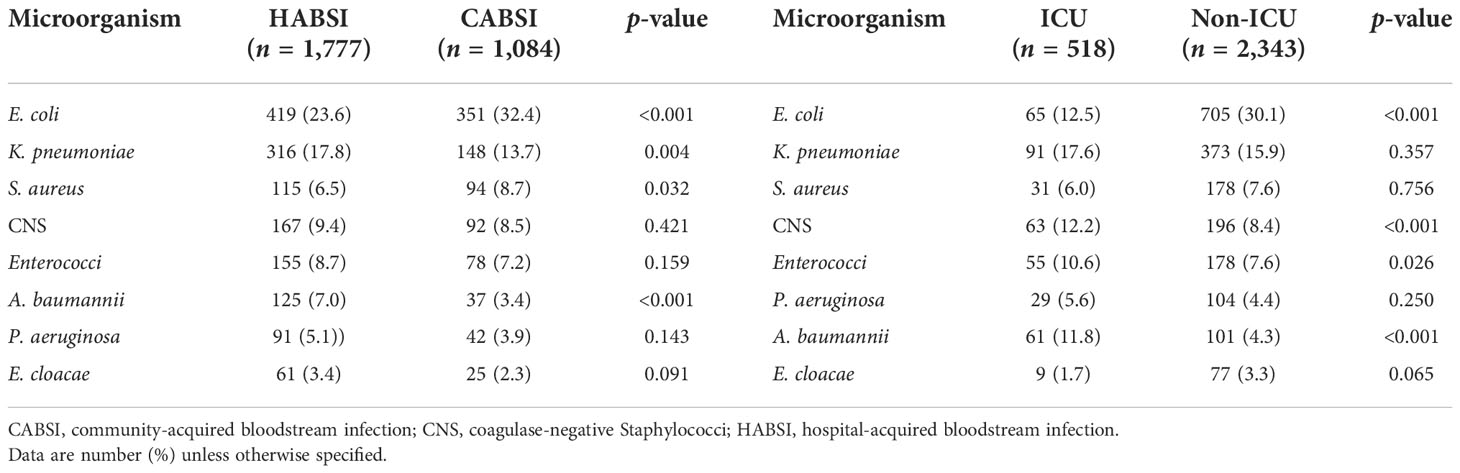
Table 4 Distribution of pathogens isolated from hospital-acquired versus community-acquired bloodstream infections and ICU versus non-ICU patients.
Antimicrobial susceptibility of BSI pathogens
About 60% of the E. coli and K. pneumoniae isolates were susceptible to ceftazidime, cefepime, and aztreonam, more than 90% susceptible to β-lactam/β-lactamase inhibitor combinations (excluding ampicillin-sulbactam), but only 13.1%–43.4% susceptible to penicillins, most cephalosporins, and ampicillin-sulbactam. However, K. pneumoniae strains showed a relatively lower resistance rate to piperacillin-tazobactam (59.2%) than penicillins, most cephalosporins, and ampicillin-sulbactam. About 41.3%–53.3% of the E. coli and K. pneumoniae isolates were susceptible to aminoglycosides (excluding amikacin), fluoroquinolones, and trimethoprim-sulfamethoxazole. Tigecycline and polymyxin E were highly active against E. coli and K. pneumoniae (>95% susceptible). More than half of the E. coli and K. pneumoniae isolates (60.7% and 57.9%) were resistant to cefotaxime. The E. coli isolates showed apparently lower resistance rate to imipenem than K. pneumoniae (0.25% vs. 33.2%). Approximately 8.6%–10.5% of the P. aeruginosa strains were resistant to piperacillin, piperacillin-tazobactam, carbapenems, and fluoroquinolones; 3.4%–8.6% resistant to ceftazidime, cefepime, ceftazidime-avibactam, aminoglycosides, and polymyxin E; and 15.5% resistant to aztreonam. More than 70% of the A. baumannii isolates were resistant to most of the antimicrobial agents tested, except polymyxin E (only 2.9% resistant), aminoglycosides, levofloxacin, ceftazidime, and trimethoprim-sulfamethoxazole (55.9%–66.2% resistant) (Table 6).
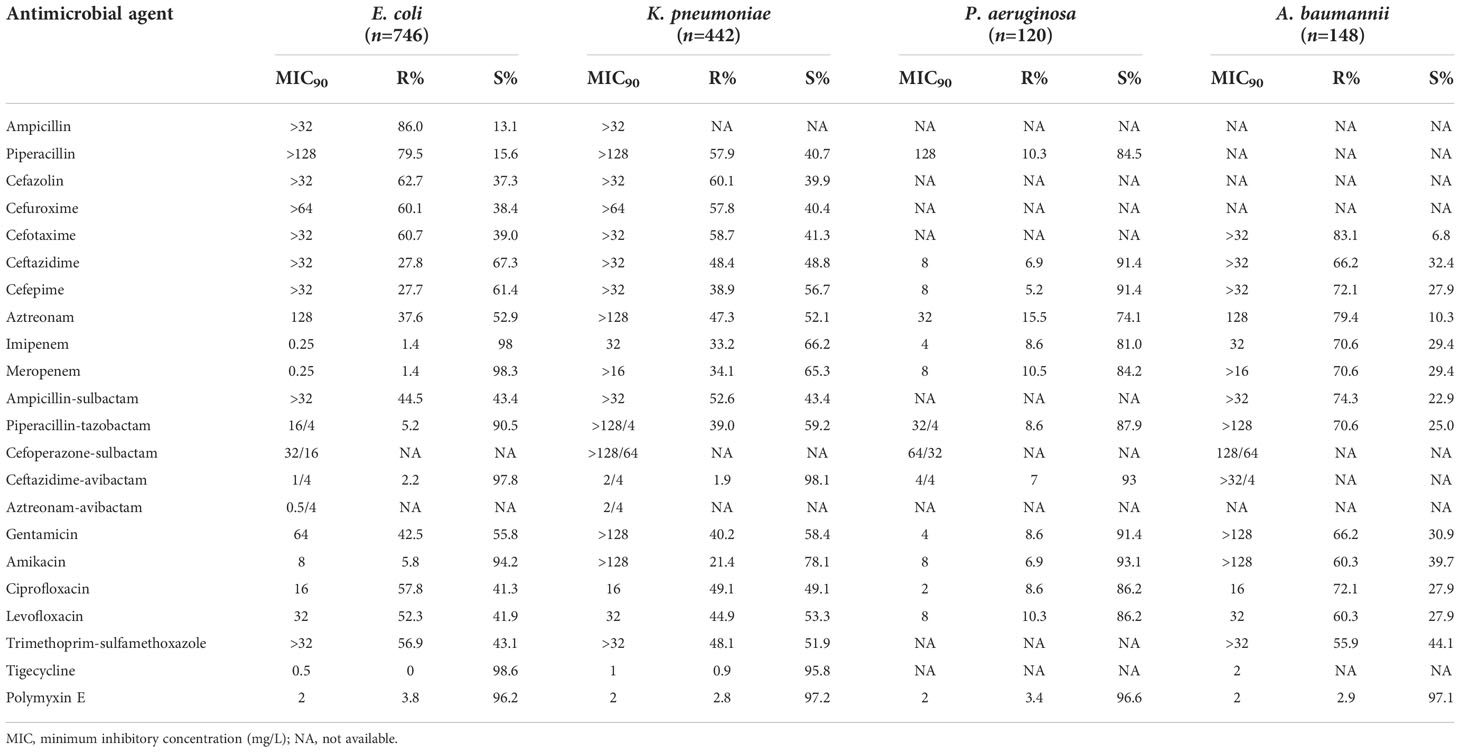
Table 6 Susceptibility of Gram-negative bacilli isolated from bloodstream infections to antimicrobial agents.
The prevalence of methicillin-resistant S. aureus (MRSA) was 42.0% in the S. aureus isolates. The prevalence of methicillin-resistant strains (MRCNS) was 93.7% in the CNS strains. None of the staphylococcal strains were resistant to vancomycin, but 1.5% of the MRCNS strains were resistant to linezolid (Table 7). Seven (7/102, 6.9%) of the E. faecalis strains were intermediate to linezolid but all susceptible to vancomycin. Three (3/111, 2.7%) of the E. faecium strains were resistant to vancomycin.
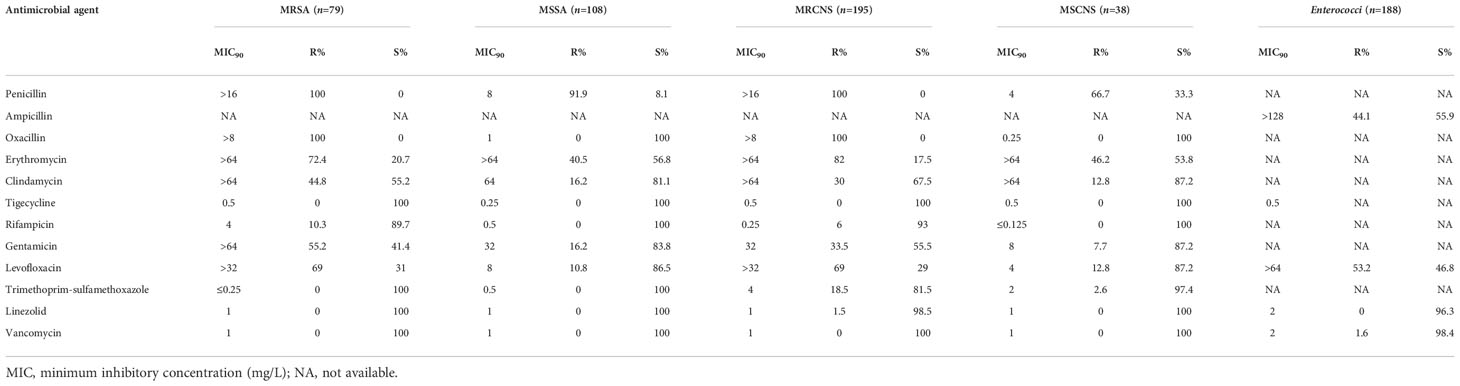
Table 7 Susceptibility of Gram-positive bacterial strains isolated from bloodstream infections to antimicrobial agents.
Prevalence of important resistant pathogens in different patient populations
Compared with CABSIs, HABSIs were associated with a higher proportion of resistant K. pneumoniae strains, especially carbapenem-resistant K. pneumoniae and ESBLs-producing K. pneumoniae. The prevalence of MRSA, MRCNS, and ampicillin-resistant Enterococci was significantly higher in HABSIs than in CABSIs. Compared with the strains isolated from non-ICU patients, the K. pneumoniae, P. aeruginosa, and A. baumannii isolated from ICU patients showed higher resistance rates, and the prevalence of carbapenem-resistant E. coli and MRSA was apparently higher. However, the prevalence of ESBL-producing E. coli was higher in the non-ICU patients compared with ICU patients. Most of the BSI isolates except P. aeruginosa and A. baumannii showed similar resistance level between old patients and young adults (Table 8).
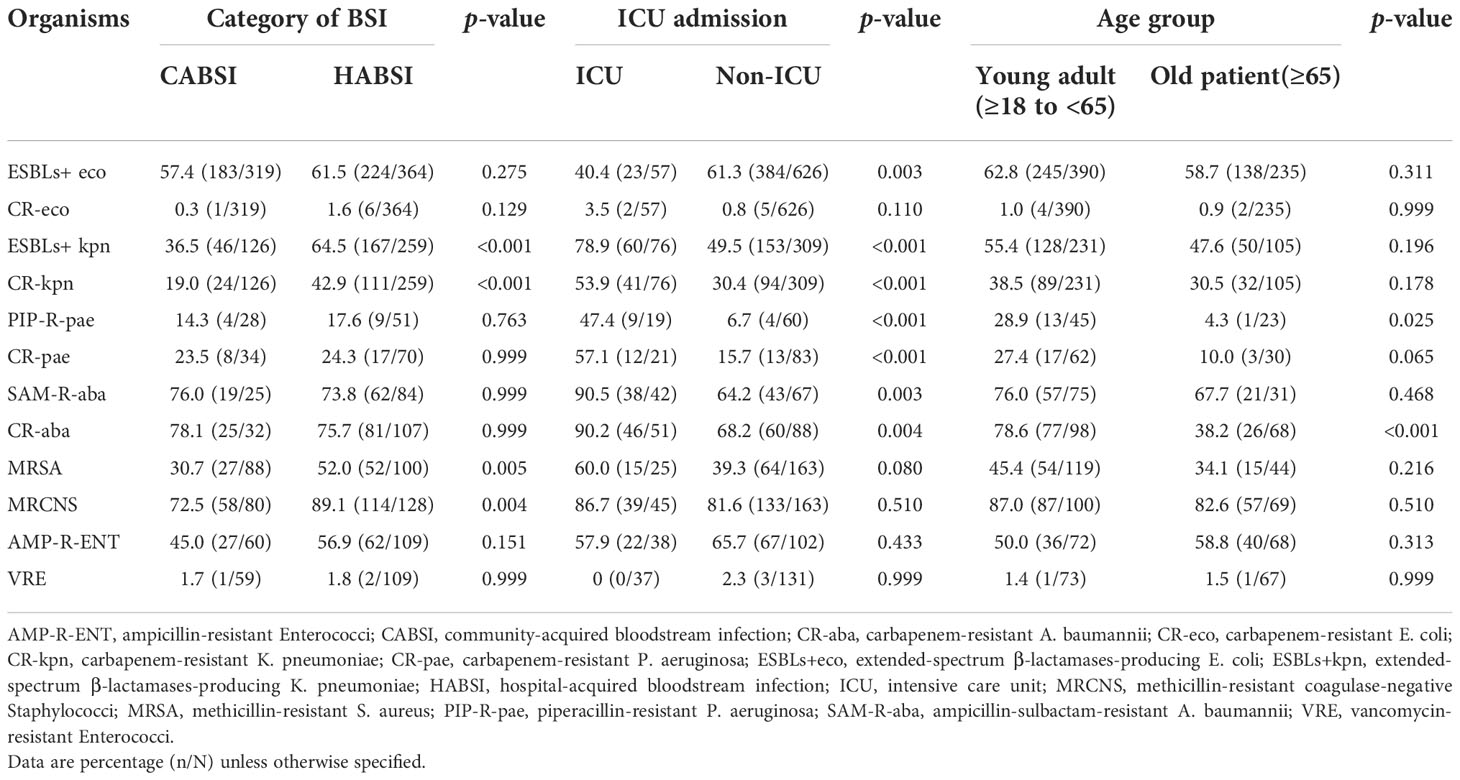
Table 8 Prevalence of important resistant pathogens isolated from bloodstream infections in different patient populations.
Antimicrobial therapy and outcomes
Most (79.8%, 2,212/2,773) of the patients received appropriate antimicrobial therapies for BSIs. Namely, at least one of the antimicrobial agents used to treat the BSIs was active against the baseline pathogenic isolate in antimicrobial susceptibility testing. Significantly higher percentage of the patients receiving appropriate antimicrobial therapies (63.4%, 1,757/2,773) than the patients receiving inappropriate antimicrobial therapies (42.8%, 240/561) responded well to the treatment. Inappropriate antimicrobial therapies were associated with significantly higher in-hospital mortality rate (18.5%, 104/561) of BSIs than appropriate antimicrobial therapies (7.0%, 155/2,212) (p <.01).
Discussion
It is important to understand the profile and patterns of antimicrobial resistance in BSIs for clinicians to prescribe more proper and appropriate antimicrobial therapies, especially in the context of growing antimicrobial resistance (Musicha et al., 2017). Therefore, this clinical investigation was conducted in the patients confirmed with BSI diagnosis, which is different from the data of isolates from positive blood culture collected from conventional antimicrobial resistance surveillance program. Generally, conventional surveillance cannot exclude all colonizers and contaminants and so cannot identify each of the real pathogens appropriately. In this series, 2,773 cases of BSIs were mostly monomicrobial (97.3%). About two-thirds (67.5%) of the 2,861 strains of pathogens isolated from BSIs were Gram-negative bacteria such as E. coli and Klebsiella. One-third of the pathogens were Gram-positive bacteria (29.6%) and fungus (2.9%). In contrast, SENTRY Antimicrobial Surveillance Program reported that S. aureus and CNS were among the top 3 pathogens of BSIs in various international regions during 1997–2002, and Gram-positive bacteria accounted for 45%–50% of all BSI pathogens. Since 2005, Gram-positive bacteria become less prevalent, and accordingly, the proportion of Gram-negative bacteria goes up slightly among BSI pathogens. Among the top 10 BSI pathogens, the proportion of Gram-negative bacteria increased from 33.5% before 2005 to 43.4% during 2013–2016 associated with higher resistance level (Biedenbach et al., 2004; Musicha et al., 2017; Pfaller et al., 2020). In the present report, the higher proportion of Gram-negative bacteria may be due to the following factors: the primary site of infection was mostly urinary tract, biliary tract, and abdominal infection; CNS isolates were considered as colonizer or contaminants and were thus excluded from analysis; only 37.5% of the BSIs were community acquired so that the Gram-positive pathogens such as S. pneumoniae were under-represented (4.5%) in our data.
This investigation revealed that the pathogens of BSIs varied with patient population and clinical setting. Escherichia coli and S. aureus were more prevalent in CABSIs than in HABSIs, which may be partly due to the fact that there is a higher proportion of urinary tract infections and skin and soft tissue infections in the primary source of CABSIs. Acinetobacter baumannii was more prevalent in HABSIs than in CABSIs. Additionally, ICU patients were associated with a higher proportion of A. baumannii and CNS isolates identified from BSIs than from non-ICU patients, which suggests such bacteria mostly originated from hospital services, such as invasive procedures. Non-ICU patients were associated with a higher proportion of E. coli than ICU patients, which is attributed to the higher percentage of CABSIs in non-ICU patients compared to that in ICU patients. The pathogens of BSIs also varied with age. Specifically, Enterococci were more prevalent in old patients, while S. agalactiae was more prevalent in newborns.
The BSI pathogens isolated from different patient populations showed different susceptibility profiles. HABSIs were associated with a higher prevalence of carbapenem-resistant K. pneumoniae, ESBL-producing K. pneumoniae, MRSA, MRCNS, and ampicillin-resistant Enterococci than CABSIs. ICU patients were associated with significantly higher resistance rates of K. pneumoniae, P. aeruginosa, and A. baumannii, and a higher prevalence of BSI isolates of carbapenem-resistant E. coli and MRSA than non-ICU patients. These findings suggest that the hospital-acquired BSI pathogens, especially in ICU patients, are generally more resistant than CABSI isolates.
The antimicrobial susceptibility of BSI pathogens indicated that about 50% or higher percentage of the E. coli and K. pneumoniae isolates were resistant to the commonly used broad-spectrum penicillins (including ampicillin-sulbactam), cephalosporins, gentamicin, and fluoroquinolones. This finding suggests that these antibiotics are not appropriate as empirical therapy to treat the BSIs caused by E. coli or K. pneumoniae, while β-lactam/β-lactamase inhibitor combinations (excluding ampicillin-sulbactam), amikacin, and carbapenems are appropriate treatment options. It is important to note that compared to E. coli, a higher percentage of K. pneumoniae strains were resistant to piperacillin-tazobactam and carbapenems. The antimicrobial therapy for the BSIs caused by K. pneumoniae should be based on susceptibility testing results. Polymyxins and tigecycline inhibited 96%–97% of the E. coli and K. pneumoniae isolates in vitro, but they are not appropriate as empirical therapy to treat BSIs because insufficient clinical evidence is available to support such empirical use (Katz et al., 2016; Zhou C, et al., 2021). Polymyxins and tigecycline should only be prescribed for the targeted treatment of specific pathogens based on susceptibility testing results. In this series of BSI cases, 33.2% and 34.1% of the K. pneumoniae isolates were resistant to imipenem and meropenem, respectively, much higher than the CHINET surveillance data in 2016 (15.4% resistant to imipenem and 17.9% resistant to meropenem) (Hu et al., 2017) and in 2020 (21.5% resistant to imipenem and 22.4% resistant to meropenem) (Hu et al., 2021). This may be related to the following conditions. More HABSIs than CABSIs (61.6% vs. 38.4%) cases were included in this analysis. HABSI isolates were more resistant than CABSI isolates, especially a higher percentage of carbapenem-resistant K. pneumoniae (CRKP) in HABSI isolates. The latest guidelines recommend taking ceftazidime-avibactam, meropenem-vaborbactam, and imipenem-relebactam as first-line treatment and cefiderocol as alternate therapy to treat the BSIs caused by CRKP (Tamma et al., 2021). The high proportion of CRKP in this series of BSI cases also reflects the increasing prevalence of CRE in the last 16 years in China. According to the CHINET report (Hu et al., 2021), the prevalence of CRKP increased from 2.9% to 3.0% in 2005 to the highest 25% to 26% in 2018, and 23% to 24% in 2019–2020. This is generally consistent with the global data (Diekema et al., 2019). The latest guidelines recommend the assay of carbapenemases for precision treatment of CRKP infections (Han et al., 2020; Yu et al., 2020), which is vital for the early diagnosis of CRKP BSIs. More than 80% of the P. aeruginosa isolates in this series of BSIs were susceptible to ceftazidime, piperacillin-tazobactam, aminoglycosides, and fluoroquinolones, which can be considered as empirical therapies. The prevalence of imipenem-resistant A. baumannii increased from 32.9% in 2005 to 72.9% in 2020 according to the CHINET report. The A. baumannii isolates were mostly resistant to carbapenems, β-lactam/β-lactamase inhibitor combinations, aminoglycosides, and fluoroquinolones (generally <40% susceptible), and more resistant to imipenem and meropenem compared with the data of foreign countries (70.6% vs. 55.3%) (Tamma et al., 2021). However, the A. baumannii isolates were highly susceptible to polymyxins and tigecycline.
The prevalence of MRSA was 42.0% in this series of BSI cases, comparable to the data in other regions (25%–50%) (You et al., 2017). The MRSA isolates were susceptible to vancomycin and linezolid, which can be used to treat MRSA BSIs, alone or in combination with rifampicin if necessary. Oxacillin and clindamycin are still appropriate therapies for the BSIs caused by methicillin-susceptible S. aureus (MSSA). The prevalence of vancomycin-resistant Enterococci (VRE) was much lower than that in the US (2.7% vs. >50%) (Mendes et al., 2016). Nearly half (44.1%) of the enterococcal isolates were resistant to ampicillin, which should not be considered in empirical therapy. Vancomycin and linezolid are still the good choice for treating the BSIs caused by ampicillin-resistant Enterococci due to their potent activity.
This multicenter clinical investigation reveals that Gram-negative bacteria especially E. coli and K. pneumoniae are more prevalent than Gram-positive bacteria such as Staphylococcus and fungus such as Candida species in the BSI pathogens. The distribution and antimicrobial resistance of BSI pathogens varied with patient population. Specifically, HABSIs and ICU patients were associated with a higher percentage of antibiotic-resistant pathogens than CABSIs and non-ICU patients. The BSI pathogens were highly resistant to the commonly used broad-spectrum penicillins and cephalosporins but relatively susceptible to carbapenems, β-lactam/β-lactamase inhibitor combinations, and vancomycin. The increasing prevalence of CRKP poses a serious challenge for BSI treatment. In this study, among 6,235 blood-culture isolates collected from 5,613 patients from 10 participating cites during CHINET 2016, 1966 episodes were excluded as contaminants, and the majority were isolated with single or dual coagulase-negative Staphylococci. One of the most likely reasons for such a high contamination rate is the contamination of the skin surface with colonized bacteria due to irregular skin disinfection during the collection of blood specimens. The findings in this study add more evidence to inform the empirical and targeted precision antimicrobial treatment for BSIs.
Data availability statement
The original contributions presented in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Author contributions
YinZ, DZ, and DL designed the study. HF, LL, and YaY, performed the experiments and wrote the manuscript. YuX, YiH, ZC, ZS, YuH, XA, YiX, XZ, YN, JS, YuY, JL, CZ, DS, CW, LH, SB, and YD performed the experiments. All authors contributed to the article and approved the submitted version.
Funding
This work was supported by the National Key Research and Development Program of China (2021YFC2701800 and 2021YFC2701803), National Natural Science Foundation of China (81861138052), the grants from China Antimicrobial Surveillance Network (Independent Medical Grants from Pfizer, 2020QD049), and Shanghai Antimicrobial Surveillance Network (grant no. 3030231003). The funders had no role in the study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling editor YWT declared a past co-authorship with the authors FH and YY.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Biedenbach, D. J., Moet, G. J., Jones, R. N. (2004). Occurrence and antimicrobial resistance pattern comparisons among bloodstream infection isolates from the SENTRY antimicrobial surveillance program (1997-2002). Diagn. Microbiol. Infect. Dis. 50, 59–69. doi: 10.1016/j.diagmicrobio.2004.05.003
Cassini, A., Högberg, L. D., Plachouras, D., Quattrocchi, A., Hoxha, A., Simonsen, G. S., et al. (2019). Burden of AMR collaborative group. attributable deaths and disability-adjusted life-years caused by infections with antibiotic-resistant bacteria in the EU and the European economic area in 2015: a population-level modelling analysis. Lancet Infect. Dis. 19, 56–66. doi: 10.1016/S1473-3099(18)30605-4
Chen, R., Rui, Q., Guo, T., Wang, T., Li, J., Yang, Z. (2016). Clinical and microbiological features of nosocomial blood stream infections in intensive care units. Chin. J. Infect. Chemother. 16 (6), 673–679. doi: 10.16718/j.1009-7708.2016.06.001
CLSI (2018a). “Performance standards for antimicrobial susceptibility testing. 28th ed,” in CLSI supplement M100 (Wayne, PA: Clinical and Laboratory Standards Institute).
CLSI (2018b). “Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically. 11th ed,” in CLSI standard M07 (Wayne, PA: Clinical and Laboratory Standards Institute).
Diekema, D. J., Hsueh, P. R., Mendes, R. E., Pfaller, M. A., Rolston, K. V., Sader, H. S., et al. (2019). The microbiology of bloodstream infection: 20-year trends from the SENTRY antimicrobial surveillance program. Antimicrob. Agents Chemother. 63, e00355–e00319. doi: 10.1128/AAC.00355-19
Han, R., Shi, Q., Wu, S., Yin, D., Peng, M., Dong, D., et al. (2020). China Antimicrobial surveillance network (CHINET) study group. dissemination of carbapenemases (KPC, NDM, OXA-48, IMP, and VIM) among carbapenem-resistant enterobacteriaceae isolated from adult and children patients in China. Front. Cell Infect. Microbiol. 10, 314. doi: 10.3389/fcimb.2020.00314
Horan, T. C., Andrus, M., Dudeck, M. A. (2008). CDC/NHSN surveillance definition of health care-associated infection and criteria for specific types of infections in the acute care setting. Am. J. Infect. Control 36, 309–332. doi: 10.1016/j.ajic.2008.03.002
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X., Xu, Y., et al. (2017). CHINET surveillance of bacterial resistance across China: report of the results in 2016. Chin. J. Infect. Chemother. 17, 481–491. doi: 10.16718/j.1009-7708.2017.05.001
Hu, F., Guo, Y., Zhu, D., Wang, F., Jiang, X., Xu, Y., et al. (2021). CHINET surveillance of bacterial resistance: results of 2020. Chin. J. Infect. Chemother. 21, 377–387. doi: 10.16718/j.1009-7708.2021.04.001
Hu, F., Zhu, D., Wang, F., Wang, M. (2018). Current status and trends of antibacterial resistance in China. Clin. Infect. Dis. 67 (suppl_2), S128–S134. doi: 10.1093/cid/ciy657
Katz, D. E., Marchaim, D., Assous, M. V., Yinnon, A., Wiener-Well, Y., Ben-Chetrit, E., et al. (2016). Ten years with colistin: a retrospective case series. Int. J. Clin. Pract. 70, 706–711. doi: 10.1111/ijcp.12830
Li, G., Zhu, D., Wang, F., Ni, Y., Sun, J., Xu, Y., et al. (2013). Frequency of isolation and antimicrobial susceptibility patterns of bacteria isolated from bloodstream infections in CHINET program in China during 2011. Chin. J. Infect. Chemother. 13, 241–247. doi: 10.16718/j.1009-7708.2013.04.002
Mendes, R. E., Castanheira, M., Farrell, D. J., Flamm, R. K., Sader, H.S., Jones, R. N. (2016). Longitudinal (2001-14) analysis of enterococci and VRE causing invasive infections in European and US hospitals, including a contemporary (2010-13) analysis of oritavancin in vitro potency. J. Antimicrob. Chemother. 71, 3453–3458. doi: 10.1093/jac/dkw319
Ministry of Health of People’s Republic of China (2001). Diagnostic criteria for hospital infections (Trial). Chin. Med. J. 81, 314–320.
Musicha, P., Cornick, J. E., Bar-Zeev, N., French, N., Masesa, C., Denis, B., et al. (2017). Trends in antimicrobial resistance in bloodstream infection isolates at a large urban hospital in Malawi (1998-2016): a surveillance study. Lancet Infect. Dis. 17, 1042–1052. doi: 10.1016/S1473-3099(17)30394-8
Pfaller, M. A., Carvalhaes, C. G., Smith, C. J., Diekema, D. J., Castanheira, M. (2020). Bacterial and fungal pathogens isolated from patients with bloodstream infection: frequency of occurrence and antimicrobial susceptibility patterns from the SENTRY antimicrobial surveillance program (2012-2017). Diagn. Microbiol. Infect. Dis. 97, 115016. doi: 10.1016/j.diagmicrobio.2020.115016
Rodríguez-Baño, J., de Cueto, M., Retamar, P., Gálvez-Acebal, J. (2010). Current management of bloodstream infections. Expert Rev. Anti Infect. Ther. 8, 815–829. doi: 10.1586/eri.10.49
Rodríguez-Créixems, M., Alcalá, L., Muñoz, P., Cercenado, E., Vicente, T., Bouza, E., et al. (2008). Bloodstream infections: evolution and trends in the microbiology workload, incidence, and etiology, 1985-2006. Med. (Baltimore) 87, 234–249. doi: 10.1097/MD.0b013e318182119b
Tamma, P. D., Aitken, S. L., Bonomo, R. A., Mathers, A. J., van Duin, D., Clancy, C. J., et al. (2021). Infectious diseases society of America guidance on the treatment of extended-spectrum β-lactamase producing enterobacterales (ESBL-e), carbapenem-resistant enterobacterales (CRE), and pseudomonas aeruginosa with difficult-to-Treat resistance (DTR-p. aeruginosa). Clin. Infect. Dis. 72, 1109–1116. doi: 10.1093/cid/ciab295
Yang, S., Xu, H., Sun, J., Sun, S. (2019). Shifting trends and age distribution of ESKAPEEc resistance in bloodstream infection, southwest China, 2012-2017. Antimicrob. Resist. Infect. Control 8, 61. doi: 10.1186/s13756-019-0499-1
You, J. H. S., Choi, K. W., Wong, T. Y., Ip, M., Wong, R.Y.-k., Tse, H.-t., et al. (2017). Disease burden, characteristics, and outcomes of methicillin-resistant staphylococcus aureus bloodstream infection in Hong Kong. Asia Pac J. Public Health 29, 451–461. doi: 10.1177/1010539517717365
Yu, H., Xu, X., Li, M., Yang, Q., Yang, Q., Zhang, R., et al. (2020). Consensus statement on laboratory detection and clinical report of carbapenemases among enterobacterales. Chin. J. Infect. Chemother. 20, 671–680. doi: 10.16718/j.1009-7708.2020.06.015
Zhou, J., Huang, X., Cao, T., Xu, F. (2021). Clinical features and antibiotic resistance profile of klebsiella pneumoniae bloodstream infection in children: report of 53 cases. Chin. J. Infect. Chemother. 21, 27–31. doi: 10.16718/j.1009-7708.2021.01.004
Zhou, C., Jin, L., Wang, Q., Wang, X., Chen, F., Gao, Y., et al. (2021). Bloodstream infections caused by carbapenem-resistant enterobacterales: Risk factors for mortality, antimicrobial therapy and treatment outcomes from a prospective multicenter study. Infect. Drug Resist. 14, 731–742. doi: 10.2147/IDR.S294282
Keywords: bloodstream infection, community-acquired, hospital-acquired, multicenter investigation, antimicrobial susceptibility testing
Citation: Hu F, Yuan L, Yang Y, Xu Y, Huang Y, Hu Y, Ai X, Zhuo C, Su D, Shan B, Du Y, Yu Y, Lin J, Sun Z, Chen Z, Xu Y, Zhang X, Wang C, He L, Ni Y, Zhang Y, Lin D, Zhu D and Zhang Y (2022) A multicenter investigation of 2,773 cases of bloodstream infections based on China antimicrobial surveillance network (CHINET). Front. Cell. Infect. Microbiol. 12:1075185. doi: 10.3389/fcimb.2022.1075185
Received: 20 October 2022; Accepted: 16 November 2022;
Published: 15 December 2022.
Edited by:
Wei Tang, Cepheid, United StatesReviewed by:
Congran Li, Chinese Academy of Medical Sciences, ChinaJihong Hu, National Center for Clinical Laboratories (NCCL), China
Copyright © 2022 Hu, Yuan, Yang, Xu, Huang, Hu, Ai, Zhuo, Su, Shan, Du, Yu, Lin, Sun, Chen, Xu, Zhang, Wang, He, Ni, Zhang, Lin, Zhu and Zhang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Dongfang Lin, lindongfang@fudan.edu.cn; Demei Zhu, zhu_dm@fudan.edu.cn
†These authors have contributed equally to this work
 Fupin Hu
Fupin Hu Lili Yuan3†
Lili Yuan3†  Yang Yang
Yang Yang Yuanhong Xu
Yuanhong Xu Chao Zhuo
Chao Zhuo Bin Shan
Bin Shan Yunsong Yu
Yunsong Yu Ziyong Sun
Ziyong Sun Yingchun Xu
Yingchun Xu Yibo Zhang
Yibo Zhang Dongfang Lin
Dongfang Lin