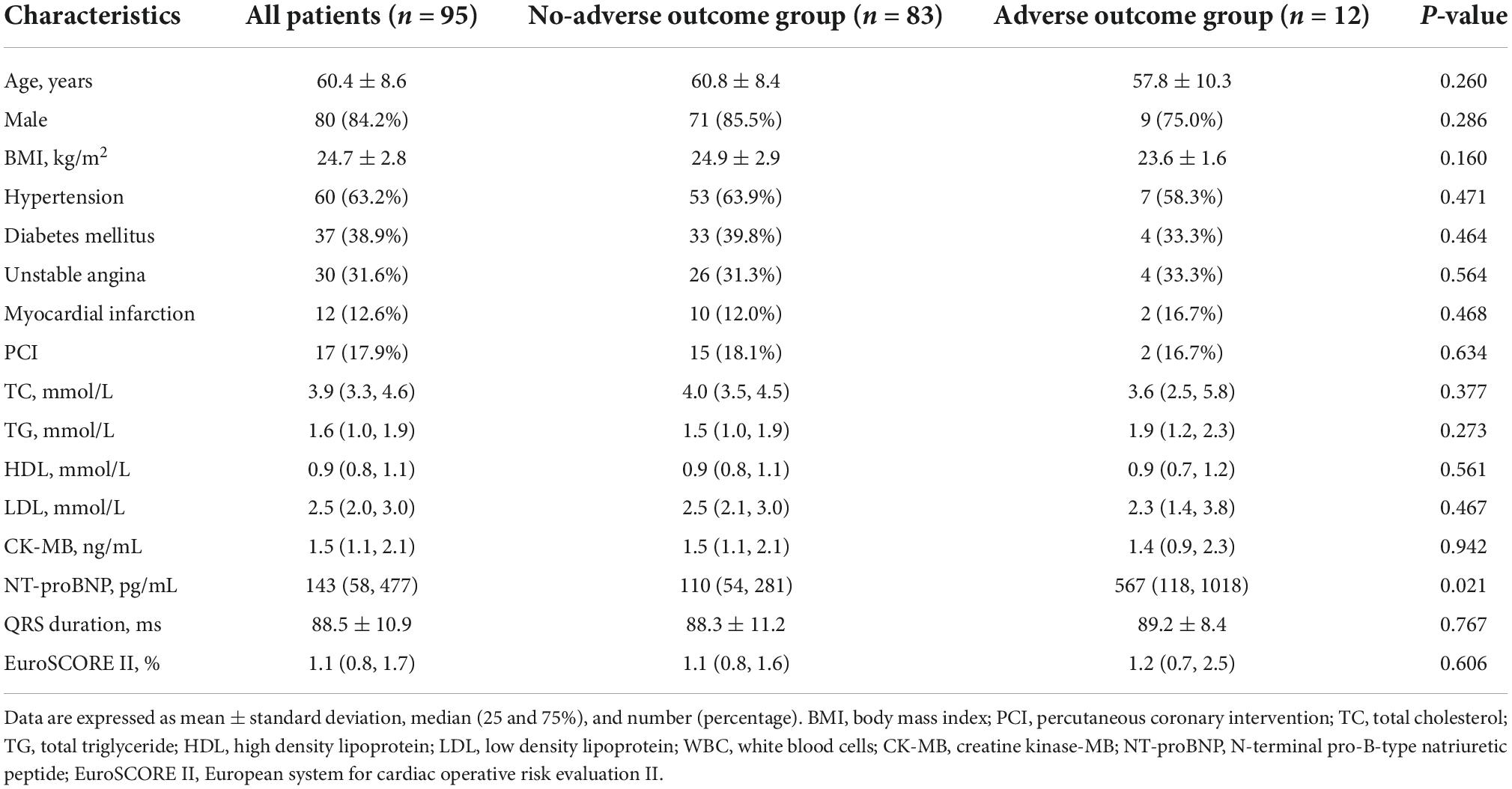
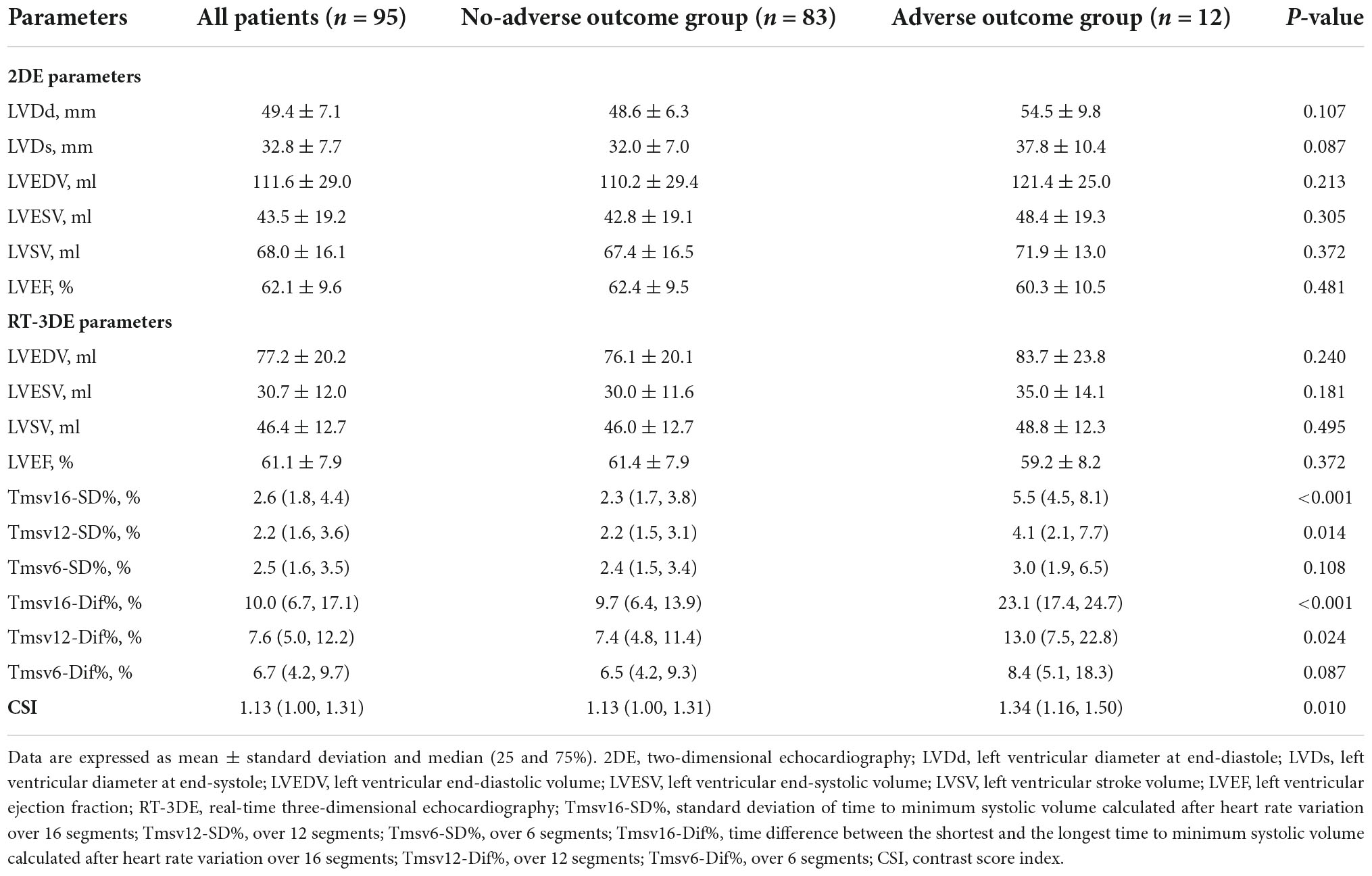
Predictive value of left ventricular dyssynchrony for short-term outcomes in three-vessel disease patients undergoing coronary artery bypass grafting with preserved or mildly reduced left ventricular ejection fraction
- Department of Medical Ultrasonics, The First Affiliated Hospital of Sun Yat-sen University, Guangzhou, China
Background and objective: Coronary artery bypass grafting (CABG) is the reference standard intervention in coronary artery disease (CAD) patients with three-vessel disease (3VD). We aimed to evaluate the predictive value of left ventricular (LV) dyssynchrony for short-term adverse outcomes in patients with 3VD undergoing CABG with preserved or mildly reduced LV ejection fraction (LVEF).
Materials and methods: This study involved ninety-five 3VD patients with preserved or mildly reduced LVEF undergoing scheduled on-pump CABG. The pre-operative diameters and volumes of LV and LVEF were obtained by two-dimensional echocardiography. LV dyssynchrony parameters were acquired by real-time three-dimensional echocardiography (RT-3DE) and analyzed by HeartModel quantification software. And the perfusion index of LV was obtained by contrast echocardiography. The clinical endpoints of short-term adverse outcomes comprised 30-day mortality and/or composite outcomes of postoperative complications. Univariate and multivariate logistic regression analyses were used to identify risk factors for the occurrence of post-CABG short-term adverse outcomes.
Results: Short-term adverse outcomes occurred in 12 (12.6%) patients. These patients had higher LV dyssynchrony parameters obtained through RT-3DE. The standard deviation (SD) of the time to minimum systolic volume (Tmsv) corrected by heart rate over 16 segments (Tmsv16-SD%) [odds ratio (OR), 1.362; 95% confidence interval (CI) (1.090–1.702); P = 0.006], one of the LV dyssynchrony parameters, was independently associated with short-term adverse outcomes. Patients with poor synchronization tended to spend more time in the intensive care unit (ICU) and hospital after surgery.
Conclusion: Pre-operative LV dyssynchrony parameter Tmsv16-SD% obtained through RT-3DE could be a useful additional predictor of postoperative short-term adverse outcomes in 3VD patients with preserved or mildly reduced LVEF undergoing CABG.
Introduction
Coronary atherosclerotic disease (CAD) is the most common heart disease and it remains a leading cause of death worldwide (1). CAD patients with three-vessel disease (3VD) have almost a twofold higher risk of mortality than patients with single-vessel disease (2, 3). As a safe and established intervention, coronary artery bypass grafting (CABG) is the standard care for 3VD patients (4, 5). Nevertheless, patients undergoing CABG remain at a high risk of adverse cardiovascular outcomes. In particularly, the highest risk exists during and shortly after surgery (6). Shroyer et al. (7) reported relatively high 30-day mortality and composite morbidity rates for CABG procedures (3.05 and 13.40%, respectively). Therefore, it is essential to identify risk factors to avoid short-term adverse postoperative outcomes after CABG.
The European System for Cardiac Operative Risk Evaluation (EuroSCORE) and its updated version EuroSCORE II are widely accepted risk models for cardiac surgery (8–10). In both models, left ventricular (LV) dysfunction is a validated risk factor, and its classification relies on the level of decrease in LV ejection fraction (LVEF). However, Jakobsen et al. (11) opined that depending on the measurement of LVEF obtained from echocardiography, in EuroSCORE, the model might have an unacceptable influence on risk classification. Some studies have identified that the EuroSCORE II model performs poorly in the low-risk patients and isolated cardiac surgery; it performs optimally in non-CABG surgery and in highest-risk patients (12, 13). In addition, most studies have focused on patients with severe LV dysfunction; however, patients with preserved or mildly reduced LVEF have usually been ignored. Therefore, additional indexes representing or associated with LV dysfunction could be potential and useful for post-CABG risk identification in 3VD patients with preserved or mildly reduced LVEF.
Left ventricular systolic dyssynchrony, which reflects the mechanical effects of asynchronous LV contraction, has been shown to be a prognostic indicator for some cardiovascular diseases and it also occurs in CAD patients (14–16). LV systolic dyssynchrony indexes can be acquired through real-time three-dimensional echocardiography (RT-3DE). This imaging technology can track myocardial trajectory in three-dimensional space and provide a quantitative and accurate evaluation of the synchronization (17).
Therefore, the main purpose of this study was to evaluate the predictive value of LV dyssynchrony parameters obtained from RT-3DE for short-term adverse outcomes after CABG in 3VD patients with preserved or mildly reduced LVEF. By documenting the advantages of this technology over conventional echocardiography, we hope that this study will find additional indexes besides LVEF and help to improve the standard of echocardiography care for 3VD patients before CABG in the future.
Patients and methods
Study population
Patients meeting the inclusion and exclusion criteria were enrolled from April 2018 to December 2021. The inclusion criteria were as follows: (1) CAD patients with 3VD confirmed by coronary angiography, waiting elective on-pump CABG; (2) LVEF >45% (Simpson’s method) (6, 18); and (3) sinus rhythm. The exclusion criteria were as follows: (1) the presence of chronic or acute pulmonary disease; (2) morbid obesity (body mass index >40 kg/m2); (3) moderate to severe valvular heart disease including stenosis, prolapse, and regurgitation; (4) cardiomyopathy; (5) previous pacemaker implantation; (6) prosthetic valve; (7) the presence of chronic or acute kidney disease; and (8) poor echocardiographic image quality. This study was registered at clinicaltrials.gov (NCT03235323) and was performed in accordance with the ethical standards of the Institutional Ethics Committee. Written informed consent was obtained from each patient. The enrolled patients underwent assessment consisting of demographic features, medical history, laboratory parameters, QRS duration, and echocardiographic imaging workup. Calculation of EuroSCORE II was done with the interactive calculator at www.euroscore.org.
Two-dimensional echocardiography imaging
Echocardiography imaging was recorded in DICOM format using EPIQ7 Philips Ultrasound systems (Philips Medical Systems, Andover, MA, USA) and X5-1 transducer and 3.5-mHz probe were used. The standard examination was completed per the guidelines of the American Society of Echocardiography (19). LVEF was measured using modified biplane Simpson’s method from apical 4- and 2-chamber views. Parameters including LV end-diastolic diameter (LVDd), LV end-systolic diameter (LVDs), LV end-diastolic volume (LVEDV), LV end-systolic volume (LVESV), LV stroke volume (LVSV), and LVEF were recorded.
Real-time three-dimensional echocardiography imaging and analysis
The RT-3DE full-volume datasets were acquired from the apical window of the entire LV. HeartModel acquisition (HMQ) mode, as a specific one-beat acquisition, was used to acquire 3DE datasets. At least three full-volume datasets throughout one cardiac cycle were obtained using this acquisition model. Gain and compression controls and time-gain compensation settings were optimized to ensure image quality. Data sets were acquired using a wide-angle acquisition (93 × 80 degrees) mode. Four wedge-shaped subvolumes (93 × 20 degrees each) were obtained from five consecutive cardiac cycles. Then, the digital echocardiographic videos were analyzed using fully automated quantification software (HeartModel, QLAB, version 2.0, Philips Medical Systems, Cleveland, OH, USA). The performed analytical procedure was described in detail in our previous study (20). Briefly, this software performed 3D endocardial border tracking throughout the cardiac cycle. For sequence analysis, the 16-segment model (apex excluded) was utilized based on the recommendations of the American Society of Echocardiography (19). The time to minimum systolic volume (Tmsv) was measured for each segment and displayed as a curved line throughout the cardiac cycle time. The standard deviation (SD) of the Tmsv was calculated after heart rate variation over 16 segments (Tmsv16-SD%), over 12 segments (Tmsv12-SD%), and over six segments (Tmsv6-SD%). The time difference (Dif) between the shortest and the longest time to the minimum systolic volume was calculated after heart rate variation over 16 segments (Tmsv16-Dif%), over 12 segments (Tmsv12-Dif%), and over six segments (Tmsv6-Dif%).
Myocardial contrast echocardiography
The standard procedure was described in our previous study (20). Briefly, ultrasound contrast agent Sonovue (Bracco, Milan, Italy) was administered intravenously. Imaging was performed using a low mechanical index under the myocardial perfusion pattern. No side effects were observed in any of the patients. A single perfusion score based on a 16-segment model of LV was assigned to each myocardial segment according to the degree of opacification at the peak contrast effect. Scores were graded as 1 = normal, 2 = reduced, or 3 = absent opacification. The contrast score index (CSI) was calculated by the sum of scores divided by the total number of segments.
Endpoints
The clinical endpoints comprised 30-day mortality and/or postoperative complications, defined as death within 30 days and/or the occurrence of at least one of the following complications within 7 days after surgery: (a) cardiac (reanimation, cardiogenic shock, pacemaker rhythm/implantation, new supraventricular arrhythmia, new ventricular arrhythmia, hypertensive crisis, intra-aortic balloon pump [IABP], and extracorporeal membrane oxygenation); (b) respiratory (duration of mechanical ventilation ≥24 h, reintubation, tracheotomy, and new pneumonia); (c) neurological (stroke, seizure, sopor, and coma); (d) surgical (reoperation for any reason); (e) infectious (sepsis and deep wound infection); and (f) acute renal failure requiring dialysis (21–23).
Postoperative follow-up
After the surgical procedure, all the patients were transferred to a cardiac surgical intensive care unit (ICU). The duration in ICU and length of postoperative hospital stays were recorded.
Statistical analysis
Continuous variables were expressed as means ± SD, or as median and interquartile range (IQR). Categorical variables were expressed as numbers and percentages. For comparing the mean values between the groups, the Student’s t-test was used for variables with a normal distribution, and the Mann–Whitney’s U-test was used for variables not satisfying the normal distribution. The chi-square test was used to compare categorical variables. The correlation of the Tmsv16-SD% and QRS duration and the relationship among dyssynchrony parameters were assessed using Spearman’ correlation analysis. Univariate logistic regression analysis was used to assess risk factors for post-CABG short-term adverse outcomes. Variables with P < 0.10 were included in the multivariate logistic regression model. The odds ratio (OR) from the regression were reported with 95% confidence interval (CI). The area under the receiver-operator characteristic (ROC) curve was calculated. The value closest to the upper left corner of the ROC curve determined the optimal sensitivity and specificity. The best cutoff value for predicting adverse outcome occurrence was determined using the Youden index according to the optimal sensitivity and specificity. The value was carried out to evaluate the prognostic performance of Tmsv16-SD% regarding the short-term clinical outcome. For all tests, P-value < 0.05 was significant. All analyses were conducted using SPSS 22.0 software (version 22.0, SPSS Inc., Chicago, IL, USA).
Results
Patients’ characteristics
A total of ninety-five 3VD patients were enrolled. Twelve patients (12.6%) showed adverse postoperative short-term outcomes. Some patients had more than one complication. The details about postoperative adverse outcomes were shown in Supplementary Table 1. Baseline characteristics are shown in Table 1. Patients suffering postoperative adverse outcomes had higher level of pre-operative N-terminal pro-brain natriuretic peptide (NT-proBNP) (P = 0.021). However, there were no significant differences in the other characteristics.
Comparison of echocardiographic characteristics
Two-dimensional echocardiography echocardiographic parameters are summarized in Table 2. There were no significant differences in LVDd, LVDs, LVEDV, LVESV, LVSV, or LVEF between the two groups.
A mathematical full-volume model of the LV was obtained through RT-3DE (Figure 1). Patients with good postoperative outcomes mostly showed consistent and overlapping curves (Figure 2). However, patients with adverse postoperative outcomes mostly presented with lagged time-volume curves (Figure 3). Patients with adverse postoperative outcomes had significantly higher LV dyssynchrony parameters obtained through RT-3DE, including Tmsv16-SD% (P < 0.001), Tmsv12-SD% (P = 0.014), Tmsv16-Dif% (P < 0.001), and Tmsv12-Dif% (P = 0.024).
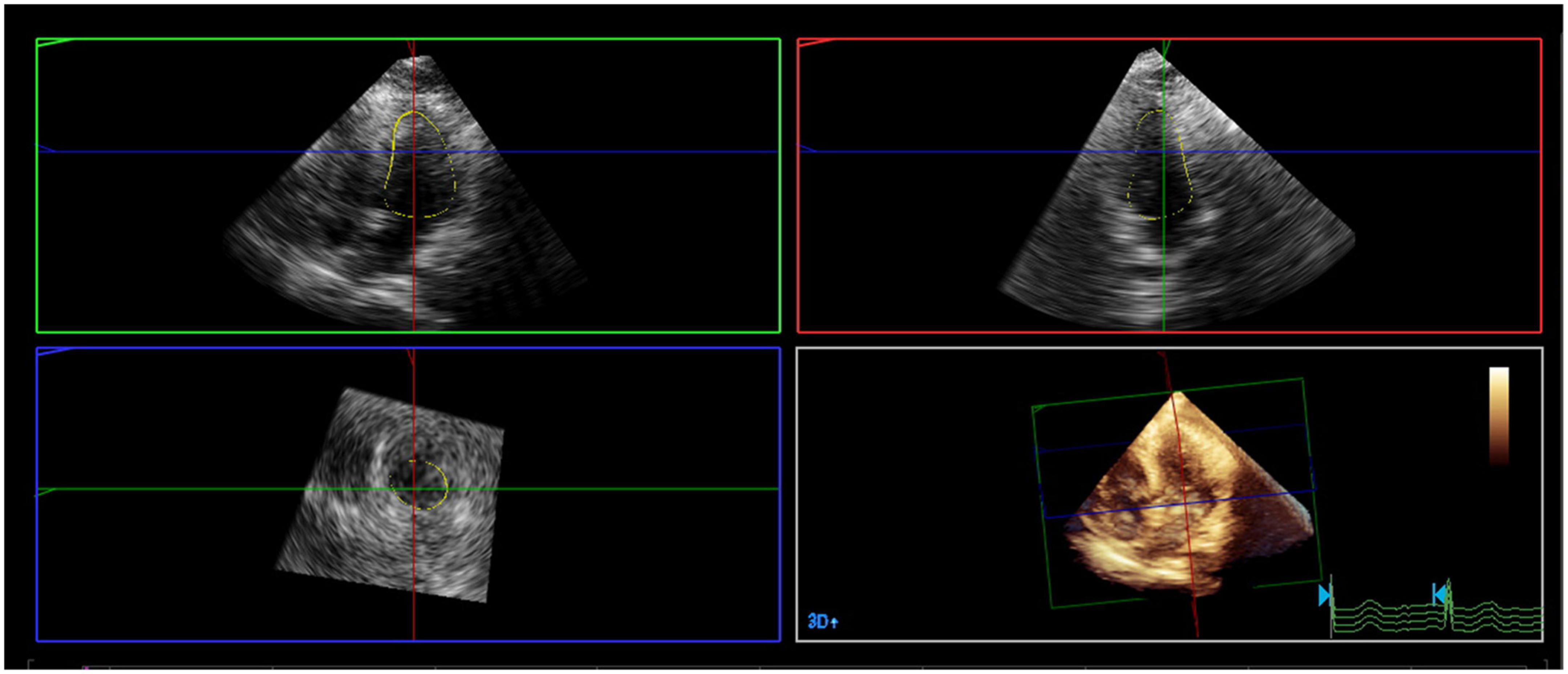
Figure 1. A mathematical full-volume model of the left ventricle by real-time three-dimensional echocardiography.
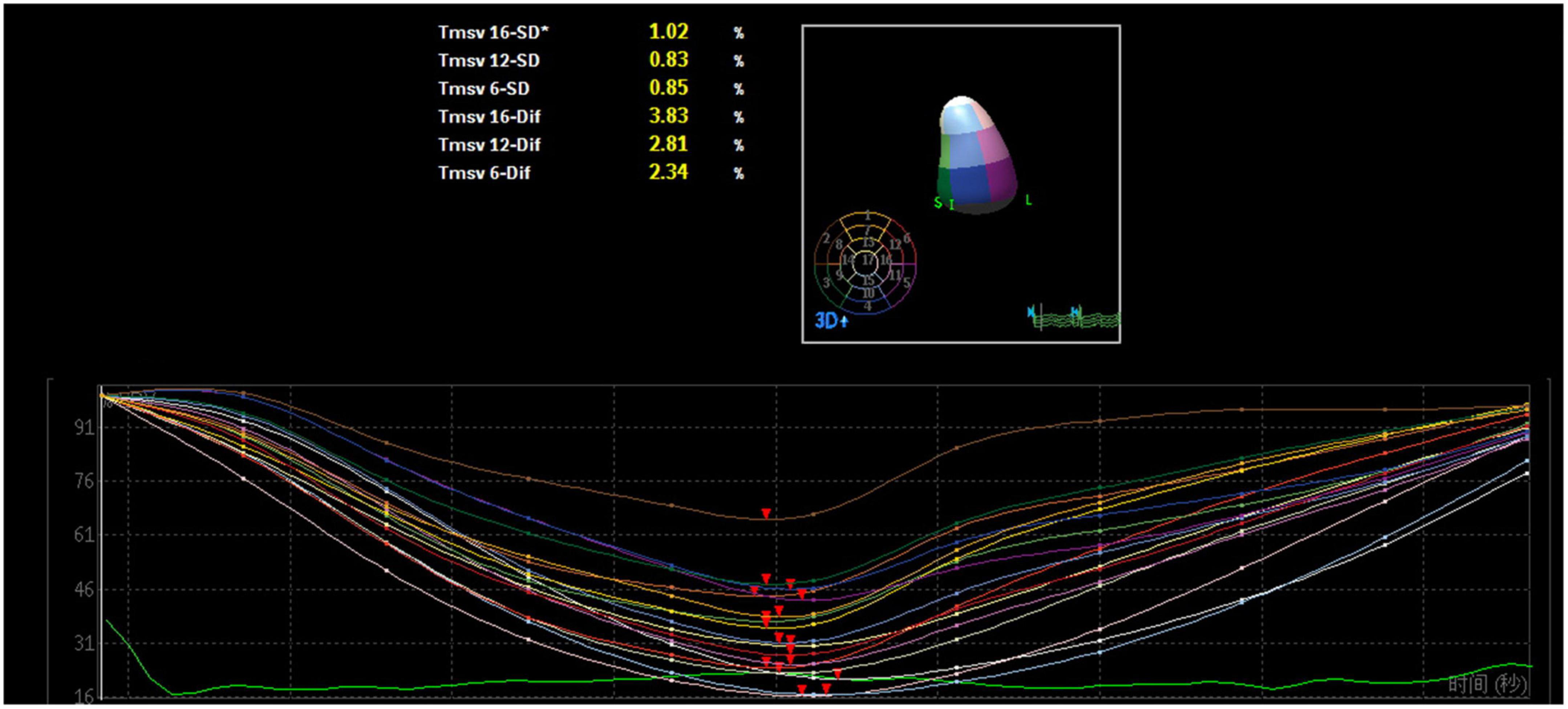
Figure 2. Three-dimensional echocardiography assessment of left ventricular dyssynchrony of a patient with normal intraventricular synchrony. The time–volume curves and the time of reaching the trough are consistent and overlapping.
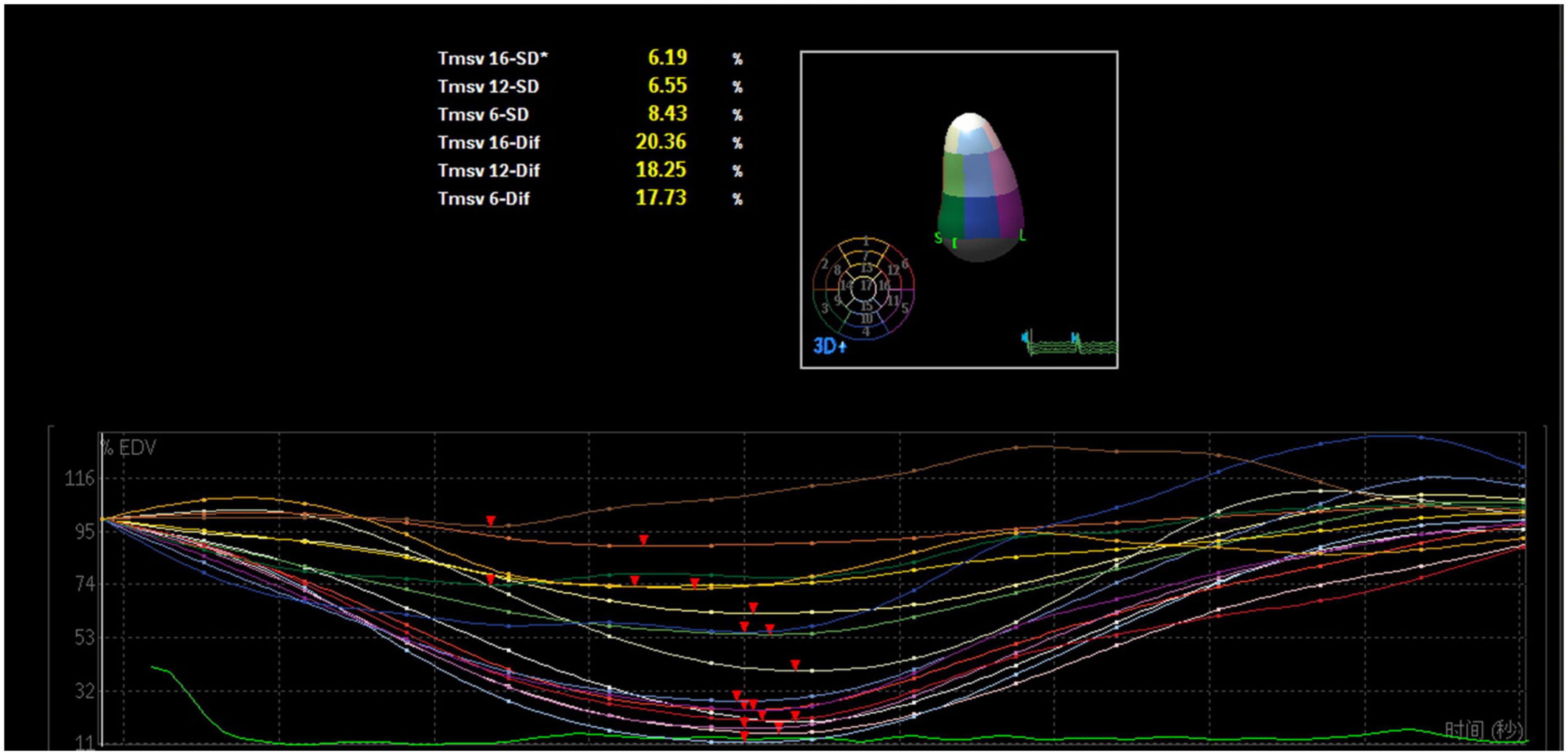
Figure 3. Three-dimensional echocardiography assessment of left ventricular dyssynchrony of a patient with intraventricular dyssynchrony. The time-volume curves are not consistent and lagged, and the disordered time-volume curves signify LV dyssynchrony.
The myocardial perfusion index, CSI, was different between the two groups (P = 0.010).
The correlations among the dyssynchrony parameters were significant (P < 0.001). However, there was no correlation between Tmsv16-SD% and QRS duration (r = 0.073, P = 0.500) (Supplementary Figure 1).
Predictors for postoperative occurrence of short-term adverse outcomes
The univariate logistic regression analysis identified the following parameters with P-value < 0.10: NT-proBNP (P = 0.045), LVDd (P = 0.012), Tmsv16-SD% (P = 0.002), Tmsv12-SD% (P = 0.015), Tmsv6-SD% (P = 0.028), Tmsv16-Dif% (P = 0.015), Tmsv12-Dif% (P = 0.060), Tmsv6-Dif% (P = 0.017), and CSI (P = 0.014) (Table 3). Due to a strong internal correlation between the LV synchronization indexes, we only included Tmsv16-SD%, the most representative synchronization index, (24) and the other variables in the multivariate model. The multivariate logistic regression analysis indicated that Tmsv16-SD% (OR 1.362, 95% CI 1.090–1.702, P = 0.006) was independently associated with the occurrence of postoperative short-term adverse outcomes (Table 4).
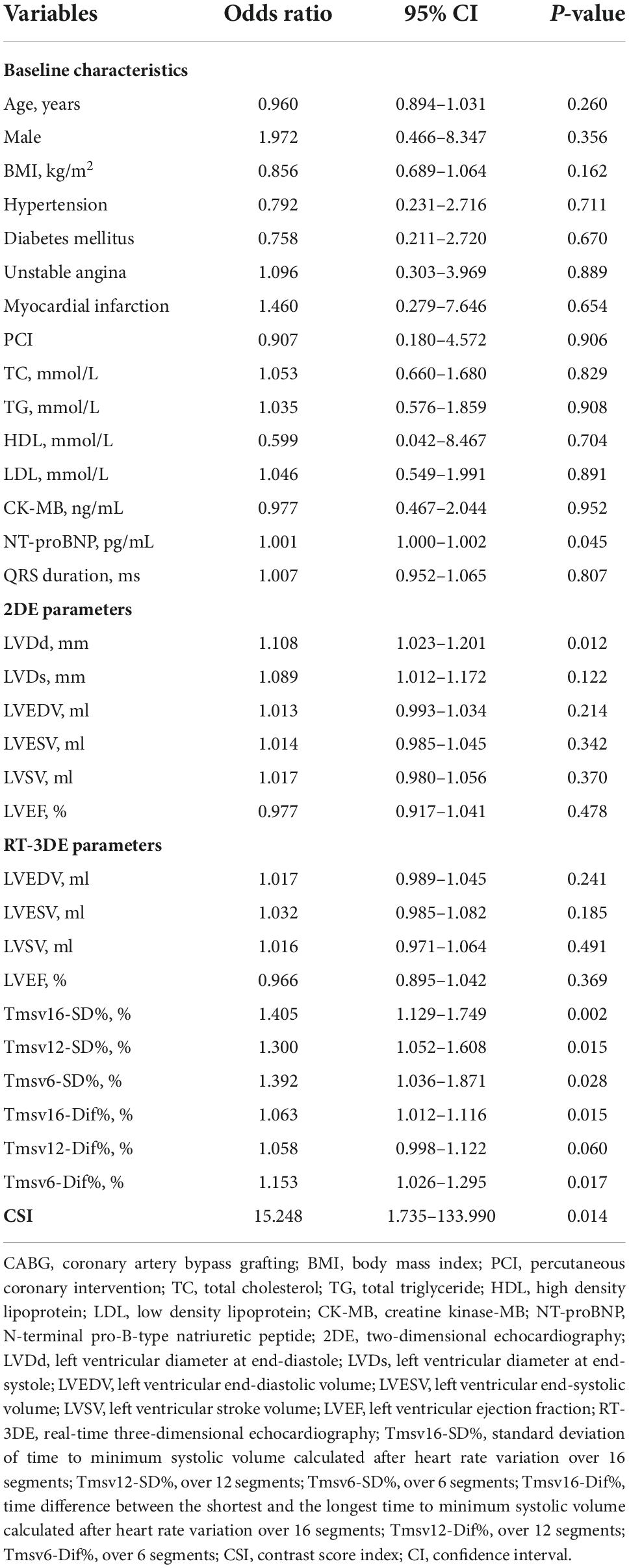
Table 3. Univariate logistic regression analyses of variables predictive of short-term adverse outcome occurrence after CABG.
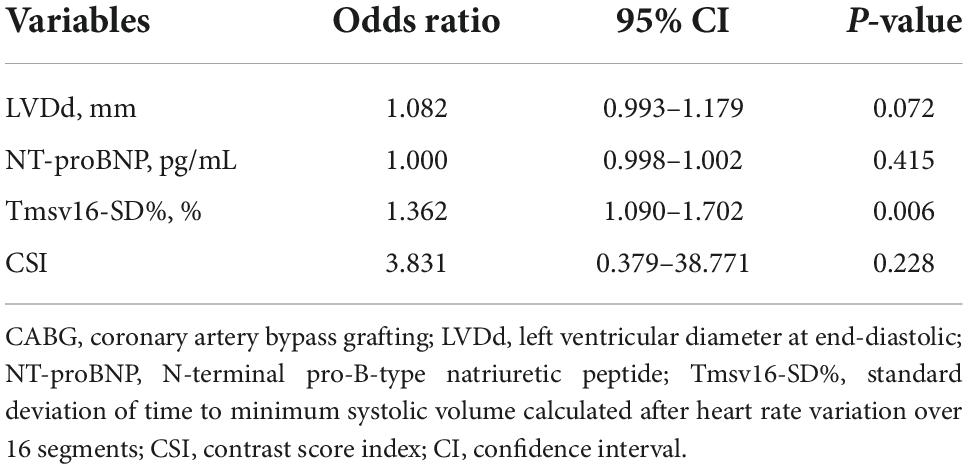
Table 4. Multivariate logistic regression analyses of variables predictive of short-term adverse outcome occurrence after CABG.
The ROC curve analysis of Tmsv16-SD% as a predictor of adverse short-term outcome occurrence is shown in Figure 4. We demonstrated that the optimal Tmsv16-SD% cutoff value for short-term adverse clinical outcomes was 4.1%, with an area under the curve (AUC) of 0.858, the sensitivity of 91.7%, and the specificity of 81.3%.
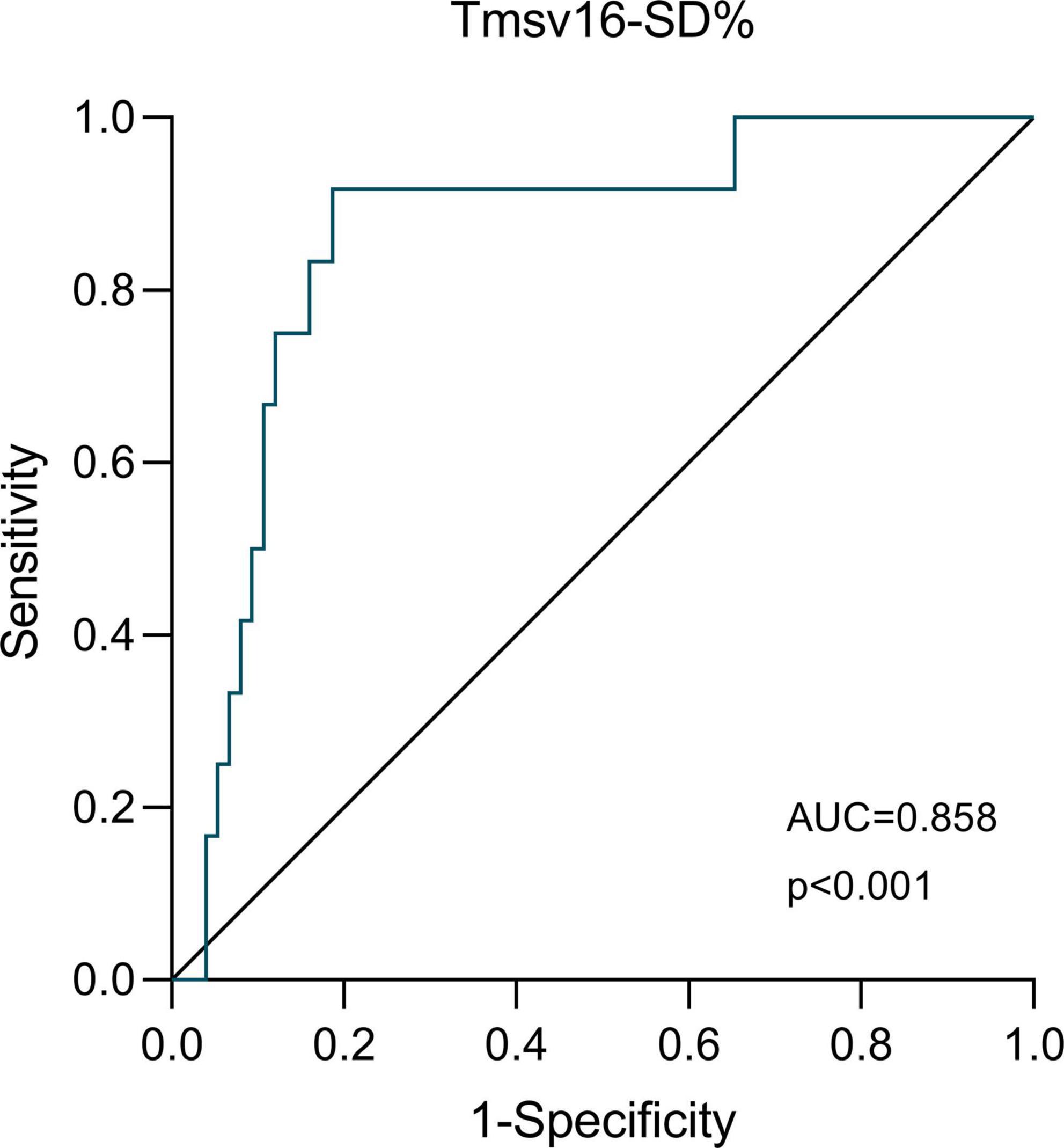
Figure 4. The receiver-operator characteristic (ROC) analysis. ROC analysis for the ability of Tmsv16-SD% to discriminate patients with or without the occurrence of short-term adverse outcomes after coronary artery bypass grafting.
Comparison of postoperative follow-up characteristics according to Tmsv16-SD%
As shown in Table 5, 3VD patients with preserved or mildly reduced LVEF were grouped according to the Tmsv16-SD% cutoff value. Patients with Tmsv16-SD%>4.1% (n = 24) before CABG had a longer postoperative stay in ICU (P < 0.001) and hospital (P = 0.043).
Discussion
This study revealed that 3VD patients with preserved or mildly reduced LVEF undergoing CABG who experienced postoperative short-term adverse outcomes had a higher preoperative LV systolic dyssynchrony. The parameter Tmsv16-SD% obtained from RT-3DE was identified as an independent risk factor. Thus, RT-3DE was a useful additional method for the risk classification in 3VD patients with preserved or mildly reduced LVEF before CABG. To our knowledge, this is the first study which focus on predicting adverse postoperative short-term outcomes occurrence of 3VD patients with preserved or mildly reduced LVEF, based on RT-3DE measurement.
It is crucial to identify individuals at high risk before undergoing CABG surgery. Accordingly, the modified EuroSCORE II has been developed for risk assessment and treatment guidance. However, it still shows defective discrimination ability in terms of sensitivity and accuracy (25). A similar unsatisfactory result was also found in our study, given that the EuroSCORE II was similar between the two groups.
Patients with preserved or mildly reduced LVEF were included in our study, and the measured LVEF in both groups with different outcomes was comparable. This volumetric approach mainly reflecting the change in LV morphology was not able to adequately describe the early change of myocardial dysfunction. It is important to identify additional indicators that can supplement risk stratification. The impairment of LV systolic synchrony is prevalent among CAD patients, and it can also occur in those patients with preserved or mildly reduced LVEF (26–28). In our study, the LV dyssynchrony parameters, including Tmsv16-SD%, Tmsv12-SD%, Tmsv16-Dif%, and Tmsv12-Dif% were higher in the group with short-term adverse outcomes. These LV dyssynchrony parameters might be more sensitive indicators than LVEF in an early stage of LV dysfunction in 3VD patients. Furthermore, LV systolic dyssynchrony is kind of LV mechanical dysfunction (LVMD), which indicates a difference in the timing of mechanical contraction or relaxation between different segments and might precede any changes in overt regional wall motion or global systolic abnormalities (28). There was also no correlation between Tmsv16-SD% and QRS duration in this study, which suggested that the increased dyssynchrony resulted from mechanical disturbance might be more sensitive than QRS duration. The similar findings were reported by Wang H et al. (29). When coronary artery stenosis or blocking occurs progressively, based on reduced myocardial oxygen and blood flow, myocardial hypoperfusion and ischemia occur (16). Cardiomyocytes and myocardial contractile proteins are damaged and myocardial metabolism is disturbed. Thus, myocardial deformation property as reflected by LV synchrony is decreased (30). Another pathological process, the decrease in coronary flow velocity due to the obstructed segments of the coronary artery, may also lead to synchronous changes due to impaired microcirculation. Myocardial microvascular ischemia can result from local inflammation and vascular rarefaction demonstrated in CAD (31). In our study, an accordant result regarding the myocardial perfusion change was found. The CSI was higher, indicating more severe hypoperfusion in the adverse outcome group. Some studies have also advocated that LV mechanical activation is triggered by the rapid propagation of electrical signals via the specialized conduction system, while the early impaired myocardial perfusion in CAD patients may damage the synchronization of LV mechanical activity by reducing electromechanical coupling and LV coordination, resulting in a change of LV myocardial mechanics (30, 32).
Moreover, preoperative LV systolic dyssynchrony appeared to play an important role in the occurrence of post-CABG short-term adverse outcomes. The most representative parameter, Tmsv16-SD%, was significantly associated with the occurrence of short-term adverse outcomes, according to the multivariate regression analysis. This indicated the significance of LVMD in CAD patients with preserved or mildly reduced LVEF. Fudim et al. (33) also found a close link between LV dyssynchrony and adverse clinical outcome by single-photon emission computed tomography. To our knowledge, data on predicting adverse postoperative short-term outcomes occurrence of CAD patients with preserved or mildly reduced LVEF, based on RT-3DE measurement, are still scarce.
The LV dyssynchrony parameters for different segments in this study were higher than those in a community-based population, (24) but lower than those in patients with severe LV dysfunction (34). In addition, our findings indicated that Tmsv16-SD% above 4.1% had a sensitivity of 91.7% and a specificity of 81.3% for predicting post-CABG short-term adverse outcome occurrence. When patients were dichotomized according to Tmsv16-SD%, those patients with inferior synchrony were more likely to spend more time in ICU and hospital after operation. Tmsv16-SD% might serve as a sensitive indicator and may provide an additional diagnostic standard in patients with preserved or mildly reduced LVEF before CABG operation. This indicator will alert clinicians to which patients need more attention and tend to have more postoperative complications. These procedures allow some patients to avoid short-term adverse postoperative outcomes and suffer less physical damage or economic loss.
Echocardiography is one of the cornerstones of cardiac function assessment. Previous studies have reported that conventional echocardiography has some inherent limitations, including the inability to image the whole structure simultaneously, geometric assumptions, and the possibility of imperfect alignment (35, 36). For RT-3DE, the endocardial surface can be automatically detected throughout the cardiac cycle, allowing accurate identification of the timing of the end of ejection; thus, global LV systolic function and mechanical dyssynchrony can be evaluated simultaneously. It is possible to quantify global and regional cardiac deformation non-invasively. An addition of echocardiographic assessment of cardiac function would enhance the predictive accuracy and thus would facilitate clinical decision-making.
Our study had several limitations. First, the limited size of the present study population may have affected the power of the present analysis. The presented study was designed as a single-center study; thus, validation from multiple centers could not be obtained. Therefore, to support the viewpoint from this study, larger studies are needed with a higher number of patients and with multiple centers. Second, although patients with poor image quality were excluded as much as possible, RT-3DE imaging could also have led to some errors in the volume measurement due to its definition of the endocardial border.
Conclusion
The current study demonstrated an independent association between pre-operative Tmsv16-SD% and the occurrence of short-term adverse outcomes after CABG in 3VD patients with preserved or mildly reduced LVEF. Tmsv16-SD% obtained by RT-3DE may be useful for additional evaluation of LV systolic function and can improve echocardiographic evaluation levels in pre-operative risk classification.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by the Institutional Review Board of The First Affiliated Hospital of Sun Yat-sen University. The patients/participants provided their written informed consent to participate in this study.
Author contributions
F-JY, WL, and JL contributed towards the conception and design of the study. JL collected data of clinical trials and drafted the manuscript together with RF and C-LL. Y-QL and D-HL revised and edited the final version of the manuscript for important intellectual content and gave the technical support. JL and RF contributed equally to the acquisition of data, e.g., RF contributed in examination, and JL contributed towards the to patient collection and gave technical support. JL and RF performed data analysis together, including imaging, computation and statistical analysis, and edited the manuscript together. All authors read and approved the final manuscript.
Acknowledgments
We are grateful to the patients and staff who participated in this study at The First Affiliated Hospital, Sun Yat-sen University.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fcvm.2022.1036780/full#supplementary-material
References
1. Roth GA, Johnson C, Abajobir A, Abd-Allah F, Abera SF, Abyu G, et al. Global, regional, and national burden of cardiovascular diseases for 10 causes, 1990 to 2015. J Am Coll Cardiol. (2017) 70:1–25. doi: 10.1016/j.jacc.2017.04.052
2. Mohr FW, Morice MC, Kappetein AP, Feldman TE, Stahle E, Colombo A, et al. Coronary artery bypass graft surgery versus percutaneous coronary intervention in patients with three-vessel disease and left main coronary disease: 5-year follow-up of the randomised, clinical syntax trial. Lancet. (2013) 381:629–38. doi: 10.1016/S0140-6736(13)60141-5
3. Veeger NJ, Panday GF, Voors AA, Grandjean JG, van der Meer J, Boonstra PW. Excellent long-term clinical outcome after coronary artery bypass surgery using three pedicled arterial grafts in patients with three-vessel disease. Ann Thorac Surg. (2008) 85:508–12. doi: 10.1016/j.athoracsur.2007.09.048
4. Fihn SD, Blankenship JC, Alexander KP, Bittl JA, Byrne JG, Fletcher BJ, et al. 2014 ACC/AHA/AATS/PCNA/SCAI/STS focused update of the guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American college of cardiology/American heart association task force on practice guidelines, and the American association for thoracic surgery, preventive cardiovascular nurses association, society for cardiovascular angiography and interventions, and society of thoracic surgeons. J Am Coll Cardiol. (2014) 64:1929–49. doi: 10.1016/j.jacc.2014.07.017
5. Head SJ, Davierwala PM, Serruys PW, Redwood SR, Colombo A, Mack MJ, et al. Coronary artery bypass grafting vs. percutaneous coronary intervention for patients with three-vessel disease: final five-year follow-up of the syntax trial. Eur Heart J. (2014) 35:2821–30. doi: 10.1093/eurheartj/ehu213
6. Alexander JH, Smith PK. Coronary-artery bypass grafting. N Engl J Med. (2016) 375:e22. doi: 10.1056/NEJMc1608042
7. Shroyer AL, Coombs LP, Peterson ED, Eiken MC, DeLong ER, Chen A, et al. The society of thoracic surgeons: 30-day operative mortality and morbidity risk models. Ann Thorac Surg. (2003) 75:1856–64. doi: 10.1016/s0003-4975(03)00179-6
8. Nashef SA, Roques F, Sharples LD, Nilsson J, Smith C, Goldstone AR, et al. EuroSCORE II. Eur J Cardiothorac Surg. (2012) 41:734–44. doi: 10.1093/ejcts/ezs043
9. Biancari F, Vasques F, Mikkola R, Martin M, Lahtinen J, Heikkinen J. Validation of EuroSCORE II in patients undergoing coronary artery bypass surgery. Ann Thorac Surg. (2012) 93:1930–5. doi: 10.1016/j.athoracsur.2012.02.064
10. Shen L, Chen X, Gu J, Xue S. Validation of EuroSCORE II in Chinese patients undergoing coronary artery bypass surgery. Heart Surg Forum. (2018) 21:E36–9. doi: 10.1532/hsf.1710
11. Jakobsen CJ, Torp P, Sloth E. Assessment of left ventricular ejection fraction may invalidate the reliability of euroscore. Eur J Cardiothorac Surg. (2006) 29:978–82. doi: 10.1016/j.ejcts.2006.02.014
12. Chalmers J, Pullan M, Fabri B, McShane J, Shaw M, Mediratta N, et al. Validation of EuroSCORE II in a modern cohort of patients undergoing cardiac surgery. Eur J Cardiothorac Surg. (2013) 43:688–94. doi: 10.1093/ejcts/ezs406
13. Borracci RA, Rubio M, Celano L, Ingino CA, Allende NG, Ahuad Guerrero RA. Prospective validation of EuroSCORE II in patients undergoing cardiac surgery in argentinean centres. Interact Cardiovasc Thorac Surg. (2014) 18:539–43. doi: 10.1093/icvts/ivt550
14. Hongning Y, Ruiqin X, Jing W, Gaojie H, Xuqian Z, Hong Z, et al. Assessment of left atrial function and dyssynchrony by real time three-dimensional echocardiography predicts recurrence of paroxysmal atrial fibrillation after radiofrequency ablation. Eur Rev Med Pharmacol Sci. (2018) 22:3151–9. doi: 10.26355/eurrev_201805_15075
15. Yan GH, Wang M, Yue WS, Yiu KH, Siu CW, Lee SW, et al. Elevated pulmonary artery systolic pressure in patients with coronary artery disease and left ventricular dyssynchrony. Eur J Heart Fail. (2010) 12:1067–75. doi: 10.1093/eurjhf/hfq125
16. Lee PW, Zhang Q, Yip GW, Wu L, Lam YY, Wu EB, et al. Left ventricular systolic and diastolic dyssynchrony in coronary artery disease with preserved ejection fraction. Clin Sci. (2009) 116:521–9. doi: 10.1042/CS20080100
17. Jenkins C, Tsang W. Three-dimensional echocardiographic acquisition and validity of left ventricular volumes and ejection fraction. Echocardiography. (2020) 37:1646–53. doi: 10.1111/echo.14862
18. Tromp J, Shen L, Jhund PS, Anand IS, Carson PE, Desai AS, et al. Age-related characteristics and outcomes of patients with heart failure with preserved ejection fraction. J Am Coll Cardiol. (2019) 74:601–12. doi: 10.1016/j.jacc.2019.05.052
19. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Ernande L, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. Eur Heart J Cardiovasc Imaging. (2015) 16:233–70. doi: 10.1093/ehjci/jev014
20. Li W, Lv XZ, Liu J, Zeng JH, Ye M, Li CL, et al. Assessment of myocardial dysfunction by three-dimensional echocardiography combined with myocardial contrast echocardiography in type 2 diabetes mellitus. Front Cardiovasc Med. (2021) 8:677990. doi: 10.3389/fcvm.2021.677990
21. Rupprecht S, Schultze T, Nachtmann A, Rastan AJ, Doenst T, Schwab M, et al. Impact of sleep disordered breathing on short-term post-operative outcome after elective coronary artery bypass graft surgery: a prospective observational study. Eur Respir J. (2017) 49:1601486. doi: 10.1183/13993003.01486-2016
22. Davierwala PM, Leontyev S, Verevkin A, Rastan AJ, Mohr M, Bakhtiary F, et al. Temporal trends in predictors of early and late mortality after emergency coronary artery bypass grafting for cardiogenic shock complicating acute myocardial infarction. Circulation. (2016) 134:1224–37. doi: 10.1161/CIRCULATIONAHA.115.021092
23. Haller G, Bampoe S, Cook T, Fleisher LA, Grocott MPW, Neuman M, et al. Systematic review and consensus definitions for the standardised endpoints in perioperative medicine initiative: clinical indicators. Br J Anaesth. (2019) 123:228–37. doi: 10.1016/j.bja.2019.04.041
24. Russo C, Jaubert MP, Jin Z, Homma S, Di Tullio MR. Intra- and interobserver reproducibility of left ventricular mechanical dyssynchrony assessment by real time three-dimensional echocardiography. Echocardiography. (2012) 29:598–607. doi: 10.1111/j.1540-8175.2011.01655.x
25. Herreros J, Bustamante-Munguira J. Euroscore II, is its predictive capacity influenced by the patient’s risk profile. Cardiol J. (2015) 22:479–81. doi: 10.5603/CJ.2015.0067
26. Fudim M, Fathallah M, Shaw LK, James O, Samad Z, Piccini JP, et al. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary artery disease and heart failure. J Nucl Cardiol. (2020) 27:1622–32. doi: 10.1007/s12350-019-01843-4
27. Hess PL, Shaw LK, Fudim M, Iskandrian AE, Borges-Neto S. The prognostic value of mechanical left ventricular dyssynchrony defined by phase analysis from gated single-photon emission computed tomography myocardial perfusion imaging among patients with coronary heart disease. J Nucl Cardiol. (2017) 24:482–90. doi: 10.1007/s12350-015-0388-9
28. Wang M, Yan GH, Yue WS, Siu CW, Yiu KH, Lee SW, et al. Left ventricular mechanical dyssynchrony impairs exercise capacity in patients with coronary artery disease with preserved left ventricular systolic function and a qrs duration </= 120 ms. Circ J. (2012) 76:682–8. doi: 10.1253/circj.cj-11-0735
29. Wang H, Song Y, Mu J, Shang J, Wang J, Ruan L. Left ventricular systolic dyssynchrony in patients with Kawasaki disease: a real-time three-dimensional echocardiography study. Int J Cardiovasc Imaging. (2020) 36:1941–51. doi: 10.1007/s10554-020-01909-2
30. Li L, Zhang PY, Ran H, Dong J, Fang LL, Ding QS. Evaluation of left ventricular myocardial mechanics by three-dimensional speckle tracking echocardiography in the patients with different graded coronary artery stenosis. Int J Cardiovasc Imaging. (2017) 33:1513–20. doi: 10.1007/s10554-017-1147-6
31. Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. (2005) 352:1685–95.
32. Li M, Li L, Wu W, Ran H, Zhang P. Left ventricular dyssynchrony in coronary artery disease patients without regional wall-motion abnormality: correlation with gensini score. Echocardiography. (2019) 36:1689–97. doi: 10.1111/echo.14453
33. Fudim M, Fathallah M, Shaw LK, Liu PR, James O, Samad Z, et al. The prognostic value of diastolic and systolic mechanical left ventricular dyssynchrony among patients with coronary heart disease. JACC Cardiovasc Imaging. (2019) 12:1215–26. doi: 10.1016/j.jcmg.2018.05.018
34. Kleijn SA, Aly MF, Knol DL, Terwee CB, Jansma EP, Abd El-Hady YA, et al. A meta-analysis of left ventricular dyssynchrony assessment and prediction of response to cardiac resynchronization therapy by three-dimensional echocardiography. Eur Heart J Cardiovasc Imaging. (2012) 13:763–75. doi: 10.1093/ehjci/jes041
35. Karuzas A, Rumbinaite E, Verikas D, Ptasinskas T, Muckiene G, Kazakauskaite E, et al. Accuracy of three-dimensional systolic dyssynchrony and sphericity indexes for identifying early left ventricular remodeling after acute myocardial infarction. Anatol J Cardiol. (2019) 22:13–20. doi: 10.14744/AnatolJCardiol.2019.02844
Keywords: left ventricular dyssynchrony, coronary artery bypass grafting, three-vessel disease, real-time three-dimensional echocardiography (RT-3DE), short-term outcomes
Citation: Liu J, Fan R, Li C-l, Liu Y-q, Liu D-h, Li W and Yao F-j (2022) Predictive value of left ventricular dyssynchrony for short-term outcomes in three-vessel disease patients undergoing coronary artery bypass grafting with preserved or mildly reduced left ventricular ejection fraction. Front. Cardiovasc. Med. 9:1036780. doi: 10.3389/fcvm.2022.1036780
Received: 05 September 2022; Accepted: 02 November 2022;
Published: 17 November 2022.
Edited by:
Ana Teresa Timoteo, Hospital de Santa Marta, PortugalReviewed by:
Lígia Mendes, Hospital da Luz Setúbal, PortugalPaula Fazendas, Hospital Garcia de Orta, Portugal
Copyright © 2022 Liu, Fan, Li, Liu, Liu, Li and Yao. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Feng-juan Yao, yaofj@mail.sysu.edu.cn; Wei Li, liwei259@mail.sysu.edu.cn
†These authors have contributed equally to this work and share first authorship
 Jia Liu†
Jia Liu†  Dong-hong Liu
Dong-hong Liu Wei Li
Wei Li Feng-juan Yao
Feng-juan Yao