- 1Department of Prevention Medicine, College of Public health, Shanghai University of Medicine & Health Sciences, Shanghai, China
- 2Jiading Central Hospital, Shanghai University of Medicine & Health Sciences, Shanghai, China
- 3Department of Health, Center for Disease Control and Prevention in Suqian, Suqian, Jiangsu Province, China
- 4Department of Nursing, Shanghai University of Traditional Chinese Medicine, Shanghai, China
- 5Department of Non-communicable Disease, Baoshan District Center for Disease Control and Prevention in Shanghai, Shanghai, China
- 6Department of Epidemiology, School of Public Health, Nantong University, Nantong, Jiangsu Province, China
Objectives: The causal direction and magnitude of the association between total body bone mineral density (TB-BMD) and osteoarthritis (OA) risk is uncertain owing to the susceptibility of observational studies to confounding and reverse causation. The study aimed to explore the relationships between TB-BMD concentration and OA using Mendelian randomization (MR).
Methods: In this study, we used two-sample MR to obtain unconfounded estimates of the effect of TB-BMD on hip and knee OA. Single nucleotide polymorphisms (SNPs) strongly associated with TB-BMD in a large genome-wide association study (GWAS) were identified and selected as instrumental variables (IVs). In addition to the main analysis using inverse-variance weighted (IVW) method, we applied 2 additional methods to control for pleiotropy(MR-Egger regression, weighted median estimator) and compared the respective MR estimates.
Results: MR analyses suggested that genetically predicted higher TB-BMD is associated with risks of hip OA (For IVW: OR=1.199, 95%CI: 1.02-1.42, P=0.032; for WM: OR=1.257, 95%CI: 1.09-1.45, P=0.002). There was no evidence that the observed causal effect between TB-BMD and the risk of hip OA was affected by genetic pleiotropy(P=0.618). Additionally, our study didn’t support causal effects of a genetically increased TB-BMD risk on knee OA risk(OR=1.121, 95%CI: 0.99-1.28, P=0.084 using IVW; OR=1.132, 95%CI: 0.99-1.29, P=0.068 using WM; OR=1.274, 95%CI: 0.88-1.85, P=0.217 using MR-Egger).
Conclusions: Our findings support a causal effect that a genetic predisposition to systematically higher TB-BMD was associated with the risk of OA. And, TB-BMD likely exerts an effect on the risk of hip OA not knee OA.
Introduction
Osteoarthritis (OA) is the most prevalent musculoskeletal disease and the most common form of arthritis (1, 2). The hallmarks of OA are the degeneration of articular cartilage, remodeling of the underlying bone and synovial (3). As the leading cause of disability worldwide, it affects 40% of individuals over 70 years old and carries a substantial public health and health economic burden (4, 5). The health economic burden of OA is continually rising, commensurate with longevity and obesity rates, and current disease-modifying treatment is minimally effective (6). As a highly heterogeneous disease, the pathological features of OA include pain, inflammation, cartilage degradation and bone spurs. The heritability of the disease is over 50%, and previous genetic studies have identified 21 loci in total across hip, knee, and hand sites with limited overlap (5).
Bone mineral density (BMD) is considered to be one of the integral influences in the pathogenesis of OA. The association between BMD and OA was first reported by Foss in 1972 when they observed that higher BMD in femoral heads resected during OA-related hip replacement surgery (7). A cross-sectional study suggested that higher BMD was associated with an increased risk of incident OA defined by osteophyte or Kellgren-Lawrence(KL) grade, suggesting that increased BMD is a risk factor for OA development (8). Women with radiographic hip OA had a 9%-10% higher BMD of the femoral neck compared to those without OA (9). Previous studies indicated that male hand OA patients have reduced radial trabecular density and thickness in the distal radius (10).
Genome-wide association studies (GWAS) have made an important contribution to the identification of genetic variants associated with numerous potential risk factors for health-related outcomes. The GWAS results have facilitated the use of Mendelian randomization(MR) to evaluate causal relationships making use of summary-level data from GWAS between modifiable exposures and outcomes (11). MR uses genetic variants as instrumental variables(IVs) to detect whether the exposure phenotype has a causal effect on the outcome phenotype (12). This approach can overcome common pitfalls of traditional research, such as confounding factors and reverse causality (13). MR method has been applied to estimate the causal associations between parathyroid Hormone (14), insulin-like growth factor-1(IGF-1) (15), and smoking behavior (16), and OA.
Compelling evidence has suggested that a causal effect of high femoral neck BMD on the risk of knee OA and hip OA is predominantly reflecting cortical bone mass (17). However, these studies are susceptible to confounding and reverse causation, and thus it remains unclear whether these associations are accurate. Inferring causality from such studies is challenging because it is difficult to exclude reverse causality, confounding, or measurement error. The relationship between BMD and OA has always been a controversial issue, especially for different sites. The identification of risk factors for primary prevention is therefore of major interest as a method to reduce the incidence and consequences of the disease. Understanding the role of BMD in the pathogenesis of OA may have important therapeutic significance, and this would be of clinical relevance for OA prevention in high-risk individuals since lower total body BMD(TB-BMD) is common and safely correctable in elderly adults.
To the best of our knowledge, the role of TB-BMD in OA risk has not been well established through observational studies. Herein, we used genetic variations strongly associated with TB-BMD traits as unconfounded instruments to investigate the potential causal effect of TB-BMD on the risk of integrating knee OA and hip OA in individuals of similar European origin. In our study, we applied two-sample MR, an approach that combines summary statistics on the genetic variant to exposure and genetic variant to outcome associations from different samples, to provide estimates on the strength of the association between exposure and outcome.
Materials and methods
Study overview
This study determined whether TB-BMD was causally related to OA using MR analysis. First, the genetic variants utilized as instrumental variables (IVs) should not be associated with confounders. Second, genetic variants should not be associated with confounders. Third, the genetic variants should affect the risk of the outcome through the risk factor, not via other pathways. The MR analysis followed strictly the STROBE-MR Statement (18).
As this study is based on existing publications and public databases, no additional ethical approval or consent to participate is required. Further details on the design strategy are shown in Figure 1.
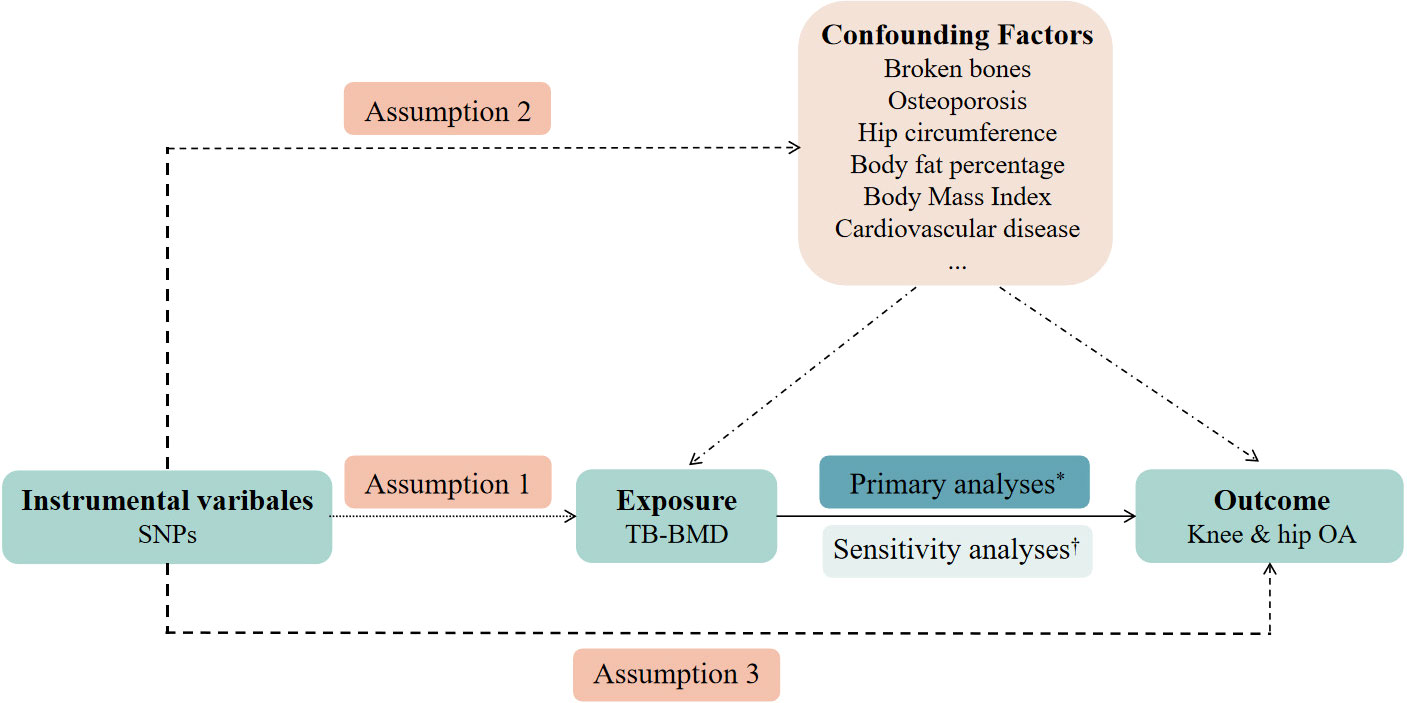
Figure 1 An overview of the study design. SNP, single-nucleotide polymorphism; TB-BMD, total body bone mineral density; OA, osteoarthritis. Assumption 1: the instrumental variables (IVs) must be robustly associated with the exposure. Assumption 2: the IVs must not be associated with any confounding factors of the exposure-outcome relationship. Assumption 3: the IVs must only affect the outcome through the exposure. *Primary analyses: inverse-variance weighted (IVW) method, weighted median estimator, and MR-Egger regression. †Sensitivity analyses: cochran’s Q test and leave-one-out analyses.
Selection of genetic variants
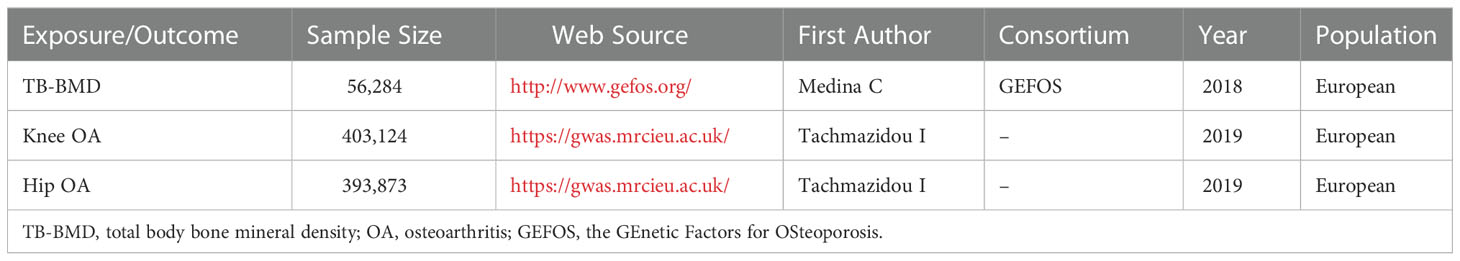
Publicly accessible data for genetic variants associated with TB-BMD were retrieved obtained from the GEnetic Factors for OSteoporosis(GEFOS) consortium (http://www.gefos.org/). Summary data from a previous meta-analysis of 30 GWASs of TB-BMD including 66,628 individuals (56,284 individuals of European ancestry) was performed to investigate genetic determinants of TB-BMD variation (19). The SNPs of TB-BMD were already corrected covariates (age, weight, height and so on) via linear regression models (19).
Screening criteria for TB-BMD-associated SNPs: (i)SNPs have significance in genome-wide studies(P<5×10−8). (ii)All IVs were independent with linkage disequilibrium(LD)(r2<0.001), which validated the independence of selected genetic variants (20).
OA GWAS summary statistics
We searched the NHGRI-EBI GWAS catalog (www.ebi.ac.uk/gwas), which is a manually curated collection of complete GWAS summary datasets. Summary statistics for the effect of TB-BMD-associated SNPs on OA were derived from a genome-wide meta-analysis that included up to 455,221 European individuals (77,052 cases and 378,169 controls), complying with genotype data across 17.5 million variants from the UK Biobank and Arthritis Research UK OA Genetics(arcOGEN) resources (21).
Statistical analysis
The MR approach was used to identify the potential causal link of TB-BMD with knee OA and hip OA. All statistical data analyses were carried out using the package “TwoSampleMR” of the R program (version 4.1.0). The two-sample MR method was estimated for the genetically causal associations were obtained by applying the inverse-variance weighted (IVW) analysis, weighted median(WM) estimator, and MR-Egger regression (22–25). The IVW method examines the causal link by performing a meta-analysis of each Wald ratio for the included SNPs (23). Significantly, the IVW analysis is predicated on the assumption that all of the contained SNPs are genuine variables (23). Unlike the IVW method, the MR-Egger regression can work even if all of the SNPs are invalid. However, MR-Egger may be inaccurate, especially when the correlation coefficient between SNPs and the exposure is similar or the number of genetic instruments is small (22). The WM estimator produced the median when the effect estimations of each single SNP were sorted in order of weight values (24). The estimation of the causal relationship between TB-BMD and OA was expressed as odds ratio(OR) and 95% confidence interval(CI). In addition, P<0.05 was the threshold for a significant difference.
MR analysis assumes that the selected IVs should act on the outcome only through the exposure variable. To eliminate the impact of known confounding factors on causality estimates, potential pleiotropic effects of selected SNPs on BMD were verified via the Ensembl project (http://www.ensembl.org) and the PhenoScanner (http://www.phenoscanner.medschl.cam.ac.uk). We systematically checked each SNP on the both websites and comprehensively aggregated the results. Finally, we excluded one SNP(rs2043230) at-a-time and performed analysis on the remaining SNPs to identify outlying IVs.
Heterogeneity and sensitivity test
In analyses of two-sample MR, pleiotropy represents those genetic variants or SNPs with multiple effects. That is, pleiotropic genetic variants or SNPs may have an effect on the outcome, not the exposure, which could cause a bias in the MR estimate and potential confounding effects; thus, investigating pleiotropy is essential. In the study, we removed SNPs one by one using the “leave-one-out” analysis and calculated the combined effect of the remaining SNPs separately to determine the magnitude of the effect of each SNP on the results. If the results of the “leave-one-out” analysis are inconsistent with the results of the causal effects analysis, it indicates that there may be effect on the estimated causal effects (22). Also, heterogeneity test is essential. We used Cochran’s Q statistics to explore heterogeneity among these SNPs (26). In cases where Cochran’s Q test indicated there was pleiotropy, we adopted the results of a random effects model instead of a fixed effects IVW model. For the sensitivity test, we performed a subgroup analysis utilizing only IV SNPs with genome-wide significance(P<5×10−8).
Results
The detail of studies and datasets was presented in Table 1. The participants were all of European(100%), overcoming the issue of ethnic differences.
Characteristics of instrumental variables
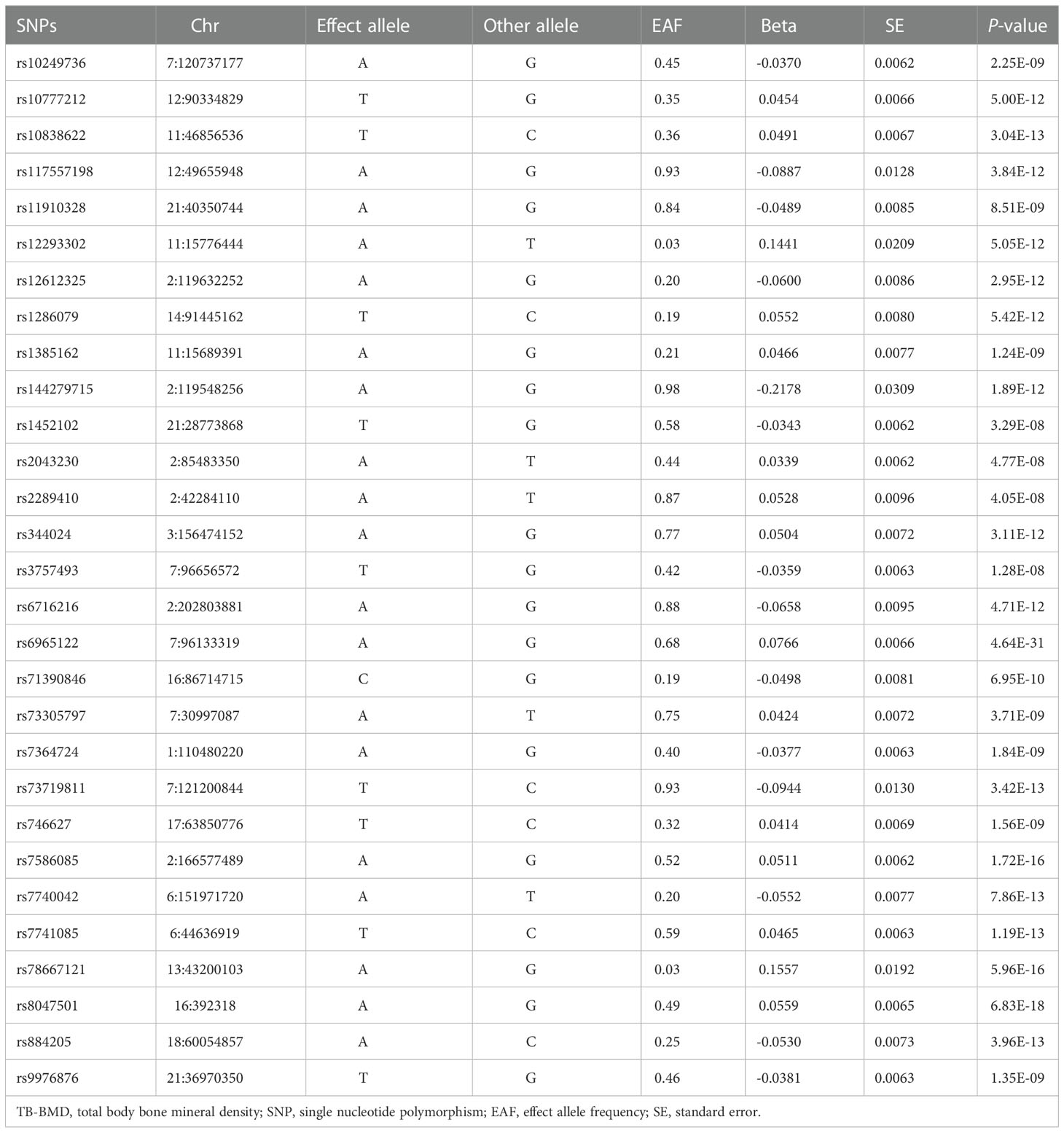
The original data of IVs can be obtained from the link (https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5777980/). Genetic variants were screened according the criteria of screening(P<5×10−8, r2<0.001, kb=10000). Eventually, 29 SNPs were employed as IVs for TB-BMD in the present study (Table 2). The associations between IVs for knee OA and hip OA are summarized in Supplementary Tables 1, 2, including the chromosome location, genes, effect allele(EA), other allele and effect allele frequency(EAF). In addition, estimations of the association between each SNP with TB-BMD and OA, including beta value, standard error(SE) and P value, were also presented.
Causal association with knee OA and hip OA
One palindromic SNPs (rs2043230) with intermediate allele frequencies was removed. Thus, 28 independent SNPs associated with TB-BMD in European ancestry were chosen to perform the MR analysis for the causal link between TB-BMD and knee OA and hip OA. None of the individual 28 SNPs was associated with knee OA in the study(OR: 1.121, 95%CI: 0.99-1.28, P=0.084 using IVW; OR: 1.132, 95%CI: 0.99-1.29, P=0.068 using WM; OR: 1.274, 95%CI: 0.88-1.85, P=0.217 using MR-Egger)(shown in Table 3).
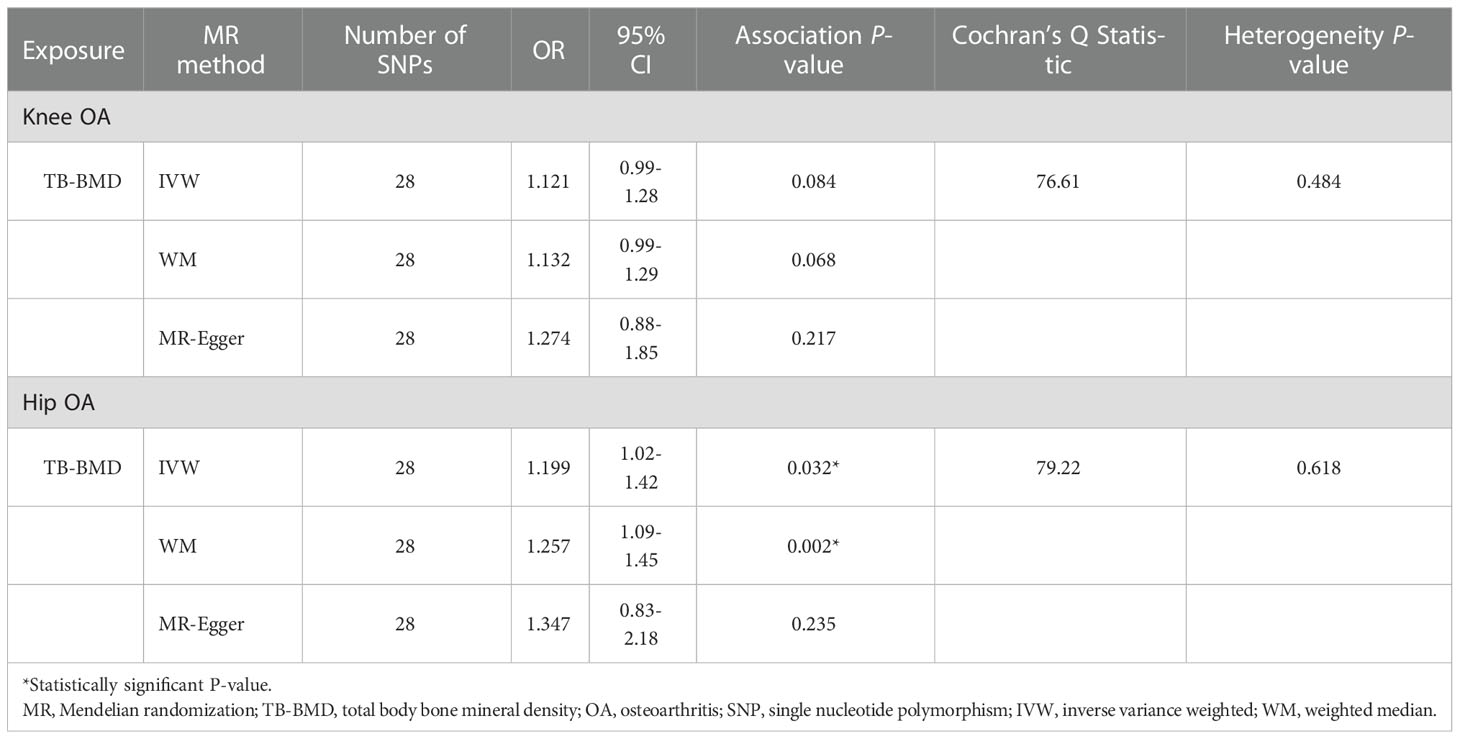
Table 3 MR estimates of associations between TB-BMD with knee and hip OA using various analysis methods.
The association between genetically predicted TB-BMD and hip OA were detected using the IVW method (OR=1.199), although with a wider CI(95%CI: 1.02-1.42, P=0.032). The association between genetically predicted TB-BMD and hip OA was consistent with IVW method using WM method (OR=1.257, 95%CI=1.09-1.45, P=0.002) (Table 3; Figure 2). There was no causal association between TB-BMD and hip OA using MR-Egger analysis(OR: 1.347, 95% CI: 0.83-2.18, P=0.235), and this was inconsistent with WM and IVM methods.
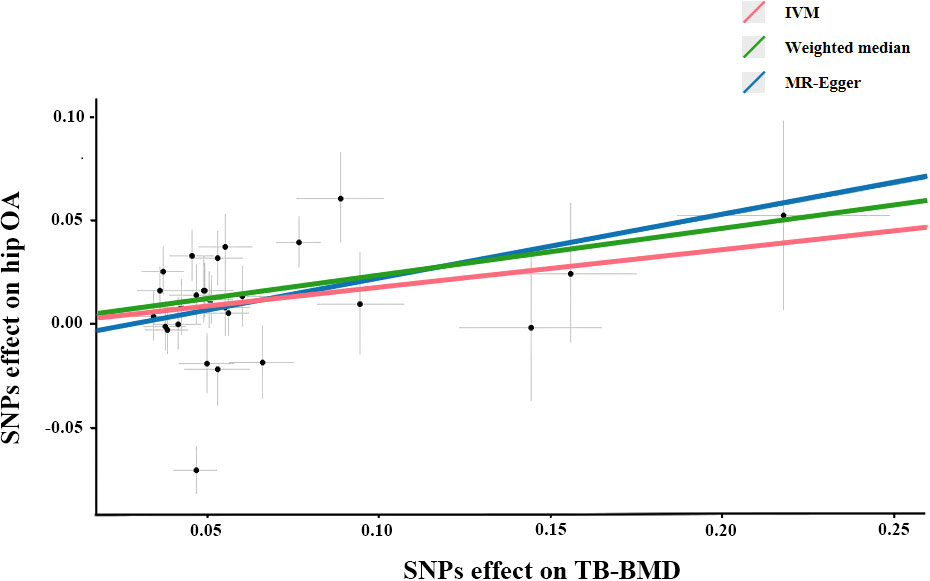
Figure 2 Scatter plots of the Mendelian randomization analyses for the association of TB-BMD with the risk of hip OA. The red line represents the inverse variance weighted estimate, the green line represents the Weighted median estimate, the blue line represents the MR-Egger estimate.
Sensitivity analysis
According to Cochran’s Q test, there was no evidence of heterogeneity between the MR estimates based on individual variants using IVW method (For knee OA, Q value=76.61, P=0.484; for hip OA, Q value=79.22, P=0.618) (Table 3). The “leave one out” analysis revealed that no single SNP play a decisive role in the causal inference (Figure 3).
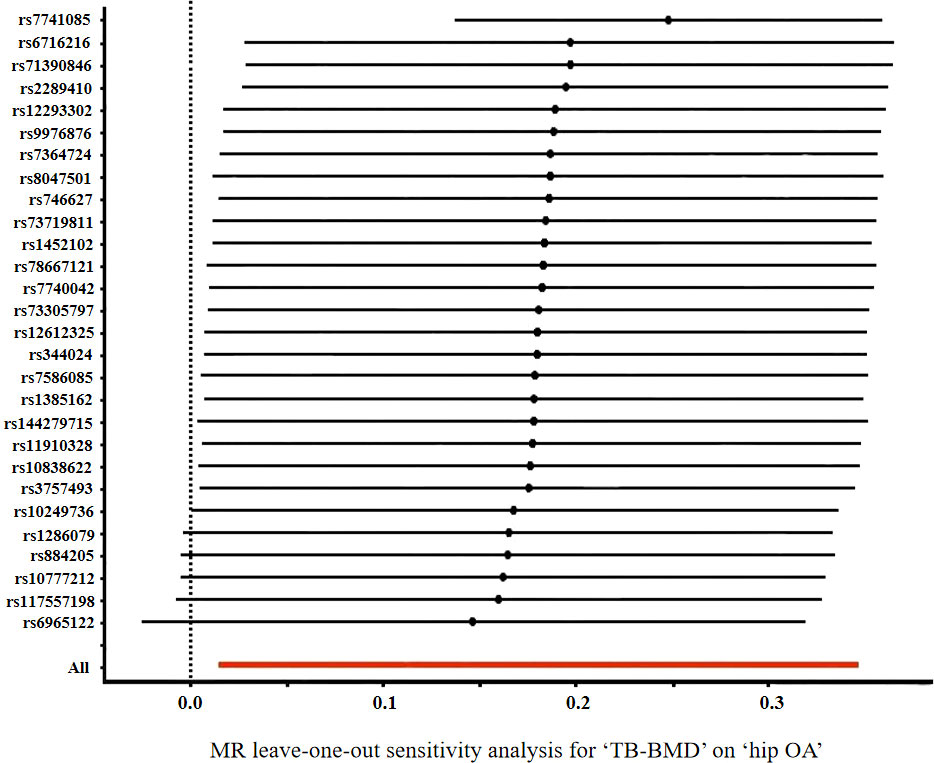
Figure 3 Forrest plot of the causal effects of TB-BMD-associated single nucleotide polymorphisms (SNPs) on hip OA.
Discussion
The causal direction and magnitude of the association between TB-BMD and risk of OA is uncertain, and observational studies make up the majority of previous research. Observational studies are susceptible to demonstrate confounding, reverse causation bias, and measurement error, all of which limit their ability to provide causal estimates of the effect of exposures on outcomes, thereby reducing their ability to inform prevention and treatment strategies against the disease. Unlike observational studies, MR uses exceptional genetic variants that are assumed to satisfy the IVs hypothesis to investigate the question of causality in epidemiological studies, which minimizes the possibility of inherent bias (27). Compared with randomized controlled trials, MR analysis is cost-effective and feasible (28).
In this study, we explored the relationship between TB-BMD and OA risk via a two-sample MR analysis based on 28 SNPs significantly related to TB-BMD as genetic variants. Despite the fact that the MR estimates from IVW, MR-Egger, and WM analysis were inconsistent, the IVW and WM estimators support a causal inverse relationship between TB-BMD and hip OA. The MR analysis suggests that a causal relationship between TB-BMD and hip OA, as the weighted average retains greater precision in the estimates compared to the MR-Egger analysis. However, there is no evidence of a causal association between TB-BMD and knee OA based on these three analyses. MR provide insights into the nature of the genetic aetiology we observed. Large-scale whole genome sequencing studies of well phenotyped individuals across diverse populations will capture the full allele frequency and variation type spectrum, and prompt us to explore the causes of this debilitating disease. This MR approach offers an alternative analytical technique being able to reduce conventional patterns of confounding and reverse causation and re-estimate observations in a framework allowing causal inference. These findings present important novel evidence on the etiology of OA.
OA was previously thought to be a cartilage disease, whereas it is now considered as a disease of the entire joint with other articular components playing a key role in the pathogenesis of OA (29). The central feature of OA is joint cartilage degradation and loss, which is manifested radiographically as a narrowing of the joint space. Because of cartilage loss and metabolic disturbances, the biomechanics of the joint change during the process of OA development result in altered subchondral bone elasticity and stiffness (30). Possible mechanisms whereby changed BMD in the subchondral bone(the region of bone immediately beneath the cartilage that includes the cortical plate and the subchondral cancellous bone) and altered gene expression might be potential players in cartilage degeneration pathogenesis (31). The potential mechanisms underlying the association need to be more thoroughly evaluated.
The reliability of the findings from a MR study depends on 3 key assumptions, which could be violated by population stratification, canalization, pleiotropy and linkage disequilibrium. Since not all genetic variants used as proxies for the exposure of interest may fulfill the “no pleiotropy” assumption, several approaches were undertaken to assess and adjust for potential confounding or pleiotropic effects. Sensitivity analyses using Cochran’s Q statistics and the “leave-one-out” method to explore and adjust for pleiotropy were also conducted. Pleiotropy is defined as the phenomenon in which a single locus affects two or more distinct phenotypic traits, resulting in compromises between trait adaptations because a mutation that benefits one trait may harm another (32, 33). The credibility of MR study results is likely to be affected by whether pleiotropy leads to bias. The results were consistent across these analyses, indicating that confounding is unlikely to explain the observed associations. Linkage disequilibrium with directly causal variants (violating the third assumption) was likely avoided owing to the use of multiple SNPs, most of which were positively associated with hip OA.
Recently, the temporal relationship between BMD and OA has been confirmed. In Johnston County, North Carolina (NC), higher BMD was associated with a lower risk of hip OA among adults aged 45 years old. Intermediate, but not high, BMD levels were linked to an increased risk of knee and hip OA (34). A case-control study provides strong evidence that high baseline femoral neck BMD is significantly related to the incidence of knee and hip OA, but not the incidence of hand OA. Whereas high baseline BMD is not associated with the progression of knee OA (35). Both men and women have presented lower fracture risk when their BMD is higher, particularly in the hip site (36). In middle-aged and older adults, lower fracture risk may be offset by an increased risk of incident knee OA (37). In postmenopausal women, there has been a similar link between higher BMD and incident radiographic hip OA (38). These studies are consistent with the results of the causal analysis.
The strengths of this MR analysis include the availability of larger sample size and the use of multiple uncorrelated SNPs associated with BMD based on published GWAS meta-analysis data, which increase the precision of the estimates. Moreover, a two-sample MR obtains IV-exposure and IV-outcome associations from two different sets of participants. Two-sample MR that uses the effect of IVs on the exposure and the outcome phenotype from two independent studies can greatly increase the power of detecting causality compared with a single sample study (39). Most importantly, population stratification arises when different subgroups of the population vary substantially. This bias can be mitigated by limiting the analysis to ethnically homogenous groups. The participants contributing to this study were composed of European descent populations, and the SNPs have been consistently associated with BMD in populations of different ancestries.
This study has several limitations that need to be considered. First, the overall estimate of the genetic association was based on a publicly available summary meta-analysis, and the study lacked complete information on sex and age. Therefore, subgroup analyses could not be conducted to explore the association separated by study-specific characteristics(e.g. age, gender), and a potential non-linear association between serum BMD levels and OA could not be further evaluated. A potential limitation is that datasets with a larger number of OA cases and non-cases were unavailable in the study. Genetic variants generally have small effects on the exposure and possibly explain only a small proportion of the variance, and large sample sizes are necessary to obtain statistically significant results. Other study limitations include an inability to exclude the existence of smaller associations and a lack of evidence from non-European populations.
In conclusion, our findings provide some support for a causal effect, whereby a systematically higher genetic susceptibility to TB-BMD is associated with an increased risk of OA. The use of multiple SNPs in MR analysis has also established that increased BMD is associated with the risk of hip OA, but not with an increased knee OA risk. Whether the risk of hip OA associated with lifelong genetic exposure to increased BMD can be translated into a risk associated with short-term to medium-term body load is unknown. Given that TB-BMD status is a modifiable trait, these results may have clinical and public health implications that need confirmation by further larger MR studies and clinical trials.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found in the article/Supplementary Material.
Author contributions
LJ drafted the protocol and wrote the final paper. YJ contributed to data analysis and interpretation of results. YS made critical revisions. AW and CW participated in the data collection. All authors contributed to the article and approved the submitted version.
Funding
This study was supported by teacher professional progress project in Shanghai, and Local High-level University (cultivation) project in Shanghai (E1-2602-21-201006-6), and supported by the Shanghai Municipal Health Commission (201840297), and supported by The Training Planned Fund of Academic Leaders from Shanghai Pudong New Area Health System (PWR 12018-09).
Acknowledgments
Summary-level data for genetic associations with OA was collected by Tachmazidou et al., and downloaded from GWAS Catalog website (https://www.ebi.ac.uk/gwas/). The authors thank all investigators for sharing these data.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fendo.2022.1021083/full#supplementary-material
References
1. Sun X, Zhen X, Hu X, Li Y, Gu S, Gu Y, et al. Osteoarthritis in the middle-aged and elderly in China: Prevalence and influencing factors. Int J Environ Res Public Health (2019) 16(23):4701. doi: 10.3390/ijerph16234701
2. Kolasinski SL, Neogi T, Hochberg MC, Oatis C, Guyatt G, Block J, et al. 2019 American College of Rheumatology/Arthritis foundation guideline for the management of osteoarthritis of the hand, hip, and knee. Arthritis Rheumatol (2020) 72(2):220–33. doi: 10.1002/art.41142
3. Zhao L, Huang J, Fan Y, Li J, You T, He S, et al. Exploration of CRISPR/Cas9-based gene editing as therapy for osteoarthritis. Ann Rheum Dis (2019) 78(5):676–82. doi: 10.1136/annrheumdis-2018-214724
4. Cibrian Uhalte E, Wilkinson JM, Southam L, Zeggini E. Pathways to understanding the genomic aetiology of osteoarthritis. Hum Mol Genet (2017) 26(R2):R193–201. doi: 10.1093/hmg/ddx302
5. Zengini E, Hatzikotoulas K, Tachmazidou I, Steinberg J, Hartwig FP, Southam L, et al. Genome-wide analyses using UK biobank data provide insights into the genetic architecture of osteoarthritis. Nat Genet (2018) 50(4):549–58. doi: 10.1038/s41588-018-0079-y
6. Usenbo A, Kramer V, Young T, Musekiwa A. Prevalence of arthritis in Africa: A systematic review and meta-analysis. PloS One (2015) 10(8):e0133858. doi: 10.1371/journal.pone.0133858
7. Foss MV, Byers PD. Bone density, osteoarthrosis of the hip, and fracture of the upper end of the femur. Ann Rheum Dis (1972) 31(4):259–64. doi: 10.1136/ard.31.4.259
8. Zhang Y, Hannan MT, Chaisson CE, McAlindon TE, Evans SR, Aliabadi P, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the framingham study. J Rheumatol (2000) 27(4):1032–7.
9. Nevitt MC, Lane NE, Scott JC, Hochberg MC, Pressman AR, Genant HK, et al. Radiographic osteoarthritis of the hip and bone mineral density. the study of osteoporotic fractures research group. Arthritis Rheumatol (1995) 38(7):907–16. doi: 10.1002/art.1780380706
10. Simon D, Tascilar K, Unbehend S, Bayat S, Berlin A, Liphardt AM, et al. Bone mass, bone microstructure and biomechanics in patients with hand osteoarthritis. J Bone Miner Res (2020) 35(9):1695–702. doi: 10.1002/jbmr.4046
11. Smith GD, Ebrahim S. 'Mendelian randomization': can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol (2003) 32(1):1–22. doi: 10.1093/ije/dyg070
12. Greenland S. Randomization, statistics, and causal inference. Epidemiology (1990) 1(6):421–9. doi: 10.1097/00001648-199011000-00003
13. Burgess S, Thompson SG. Interpreting findings from mendelian randomization using the MR-egger method. Eur J Epidemiol. (2017) 32(5):377–89. doi: 10.1007/s10654-017-0255-x
14. Huang G, Zhong Y, Li W, Liao W, Wu P. Causal relationship between parathyroid hormone and the risk of osteoarthritis: A mendelian randomization study. Front Genet (2021) 12:686939. doi: 10.3389/fgene.2021.686939
15. Hartley A, Sanderson E, Paternoster L, Teumer A, Kaplan RC, Tobias JH, et al. Mendelian randomization provides evidence for a causal effect of higher serum IGF-1 concentration on risk of hip and knee osteoarthritis. Rheumatol (Oxford) (2021) 60(4):1676–86. doi: 10.1093/rheumatology/keaa597
16. Lee YH. Causal association between smoking behavior and the decreased risk of osteoarthritis: a mendelian randomization. Z Rheumatol (2019) 78(5):461–6. doi: 10.1007/s00393-018-0505-7
17. Funck-Brentano T, Nethander M, Moverare-Skrtic S, Richette P, Ohlsson C. Causal factors for knee, hip, and hand osteoarthritis: A mendelian randomization study in the UK biobank. Arthritis Rheumatol (2019) 71(10):1634–41. doi: 10.1002/art.40928
18. Skrivankova VW, Richmond RC, Woolf BAR, Yarmolinsky J, Davies NM, Swanson SA, et al. Strengthening the reporting of observational studies in epidemiology using mendelian randomization: The STROBE-MR statement. JAMA (2021) 326(16):1614–21. doi: 10.1001/jama.2021.18236
19. Medina-Gomez C, Kemp JP, Trajanoska K, Luan J, Chesi A, Ahluwalia TS, et al. Life-course genome-wide association study meta-analysis of total body BMD and assessment of age-specific effects. Am J Hum Genet (2018) 102(1):88–102. doi: 10.1016/j.ajhg.2017.12.005
20. Lawlor DA, Harbord RM, Sterne JA, Timpson N, Davey Smith G. Mendelian randomization: using genes as instruments for making causal inferences in epidemiology. Stat Med (2008) 27(8):1133–63. doi: 10.1002/sim.3034
21. Tachmazidou I, Hatzikotoulas K, Southam L, Esparza-Gordillo J, Haberland V, Zheng J, et al. Identification of new therapeutic targets for osteoarthritis through genome-wide analyses of UK biobank data. Nat Genet (2019) 51(2):230–6. doi: 10.1038/s41588-018-0327-1
22. Hemani G, Zheng J, Elsworth B, Wade KH, Haberland V, Baird D, et al. The MR-base platform supports systematic causal inference across the human phenome. Elife (2018) 7:e34408. doi: 10.7554/eLife.34408
23. Bowden J, Del Greco MF, Minelli C, Davey Smith G, Sheehan N, Thompson J. A framework for the investigation of pleiotropy in two-sample summary data mendelian randomization. Stat Med (2017) 36(11):1783–802. doi: 10.1002/sim.7221
24. Bowden J, Davey Smith G, Haycock PC, Burgess S. Consistent estimation in mendelian randomization with some invalid instruments using a weighted median estimator. Genet Epidemiol. (2016) 40(4):304–14. doi: 10.1002/gepi.21965
25. Hartwig FP, Davey Smith G, Bowden J. Robust inference in summary data mendelian randomization via the zero modal pleiotropy assumption. Int J Epidemiol. (2017) 46(6):1985–98. doi: 10.1093/ije/dyx102
26. Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ (1997) 315(7121):1533–7. doi: 10.1136/bmj.315.7121.1533
27. Bowden J, Holmes MV. Meta-analysis and mendelian randomization: A review. Res Synth Methods (2019) 10(4):486–96. doi: 10.1002/jrsm.1346
28. Emdin CA, Khera AV, Kathiresan S. Mendelian randomization. JAMA (2017) 318(19):1925–6. doi: 10.1001/jama.2017.17219
29. Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheumatol (2012) 64(6):1697–707. doi: 10.1002/art.34453
30. Amini M, Nazemi SM, Lanovaz JL, Kontulainen S, Masri BA, Wilson DR, et al. Individual and combined effects of OA-related subchondral bone alterations on proximal tibial surface stiffness: a parametric finite element modeling study. Med Eng Phys (2015) 37(8):783–91. doi: 10.1016/j.medengphy.2015.05.011
31. Radin EL, Rose RM. Role of subchondral bone in the initiation and progression of cartilage damage. Clin Orthop Relat Res (1986) 213):34–40. doi: 10.1097/00003086-198612000-00005
32. Stearns FW. One hundred years of pleiotropy: a retrospective. Genetics (2010) 186(3):767–73. doi: 10.1534/genetics.110.122549
33. Wagner GP, Zhang J. The pleiotropic structure of the genotype-phenotype map: the evolvability of complex organisms. Nat Rev Genet (2011) 12(3):204–13. doi: 10.1038/nrg2949
34. Barbour KE, Murphy LB, Helmick CG, Hootman JM, Renner JB, Jordan JM. Bone mineral density and the risk of hip and knee osteoarthritis: The Johnston county osteoarthritis project. Arthritis Care Res (Hoboken) (2017) 69(12):1863–70. doi: 10.1002/acr.23211
35. Bergink AP, Rivadeneira F, Bierma-Zeinstra SM, Zillikens MC, Ikram MA, Uitterlinden AG, et al. Are bone mineral density and fractures related to the incidence and progression of radiographic osteoarthritis of the knee, hip, and hand in elderly men and women? the Rotterdam study. Arthritis Rheumatol (2019) 71(3):361–9. doi: 10.1002/art.40735
36. Stone KL, Seeley DG, Lui LY, Cauley JA, Ensrud K, Browner WS, et al. BMD at multiple sites and risk of fracture of multiple types: long-term results from the study of osteoporotic fractures. J Bone Miner Res (2003) 18(11):1947–54. doi: 10.1359/jbmr.2003.18.11.1947
37. Nevitt MC, Zhang Y, Javaid MK, Neogi T, Curtis JR, Niu J, et al. High systemic bone mineral density increases the risk of incident knee OA and joint space narrowing, but not radiographic progression of existing knee OA: the MOST study. Ann Rheum Dis (2010) 69(1):163–8. doi: 10.1136/ard.2008.099531
38. Hochberg MC. Do risk factors for incident hip osteoarthritis (OA) differ from those for progression of hip OA? J Rheumatol Suppl (2004) 70:6–9.
Keywords: osteoarthritis, bone mineral density, single nucleotide polymorphisms, Mendelian randomization, genome-wide association studies
Citation: Jiang L, Jiang Y, Wang A, Wu C and Shen Y (2023) The causal association between bone mineral density and risk of osteoarthritis: A Mendelian randomization study. Front. Endocrinol. 13:1021083. doi: 10.3389/fendo.2022.1021083
Received: 06 September 2022; Accepted: 16 December 2022;
Published: 11 January 2023.
Edited by:
Juan Miguel Díaz Tocados, Lleida Institute for Biomedical Research (IRBLleida), SpainReviewed by:
Hongmei Li, Soochow University, ChinaPeijian Tong, Zhejiang Chinese Medical University, China
Copyright © 2023 Jiang, Jiang, Wang, Wu and Shen. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Shen, sunny@ntu.edu.cn; Cui Wu, 25850569@qq.com
†These authors have contributed equally to this work
 Liying Jiang
Liying Jiang Ying Jiang
Ying Jiang Anqi Wang4
Anqi Wang4