Small Hydropower—Small Ecological Footprint? A Multi-Annual Environmental Impact Analysis Using Aquatic Macroinvertebrates as Bioindicators. Part 1: Effects on Community Structure
- 1Institute for Alpine Environment, Eurac Research, Bozen, Italy
- 2Freshwater Biology Section, Department of Biology, University of Copenhagen, Copenhagen, Denmark
- 3Department of Community Ecology, Helmholtz Centre for Environmental Research—UFZ, Halle (Saale), Germany
- 4German Centre for Integrative Biodiversity Research (iDiv) Halle‐Jena‐Leipzig, Leipzig, Germany
- 5Institute of Ecology, University of Innsbruck, Innsbruck, Austria
The increasing incentives stemming from many international initiatives that target sustainable energy production have led to the global success of small hydropower plants. However, there is a growing debate about the actual environmental impact these structures have on riverine ecosystems, to the extent that some researchers argue that they could have a proportionally greater impact than large hydropower plants; nevertheless, an empirical evaluation applying a long-term true “Before-After-Control-Impact (BACI)” approach has never been conducted. In a true “BACI” experiment applying generalized linear mixed models, we assessed changes in benthic macroinvertebrate communities—taxonomic composition, spatial and temporal β-diversity, and possible indicator taxa—along 6 sites located in a glacier-fed stream in the Italian Alps before and after the installation of a small “run-of-river” hydropower plant. The “BACI” results over the 5-year study showed no significant variation in the benthic macroinvertebrate communities stemming from the activity of the hydropower plant. Moreover, spatial β-diversity showed, in relation to the “control” site, a constant dissimilarity pattern throughout all the sampling years, exhibiting a constant increase proceeding downstream. On the other hand, temporal β-diversity showed changes in the benthic macroinvertebrate communities, but these changes were largely caused by variation in densities and not by the appearance or disappearance of new taxa. In summary, we were unable to detect a significant impact of the small “run-of-river” hydropower plant on the benthic macroinvertebrate communities of the glacier-fed stream under study. Despite the need of further studies that consider the different features and operational measures of small hydropower plants, our findings illustrate that, when correctly managed, small hydropower plants and the energy they produce may represent an added resource for strategic debates on energy planning processes, especially in light of the commitments at the international level of many countries in developing strategies toward a “carbon-neutral” energy sector.
Introduction
Since the beginning of human history, rivers have supported human development, since they act—among many other functions—as communication routes, direct and indirect food providers (i.e., fish stocks and agricultural water, respectively) and, in more recent times, as large-scale industrialization and power generation sources (Grill et al., 2019; Baird and Plummer, 2021).
Hydropower energy represents approximately 16% of the worldwide electricity production and approximately 80% of the global energy derived from renewable sources (Zarfl et al., 2015; Gernaat et al., 2017). However, despite these already remarkable contribution levels, these percentages and the global hydropower capacity are expected to increase in the coming years (IRENA, 2019) as several countries have committed to environmental targets set by a manifold of international initiatives for sustainable energy production. These initiatives include the Paris Agreement at the global level (UNFCCC, 2015), the EU Directive 2018/2001 (European Parliament and Council, 2018), the proposal of the European Climate Law (European Parliament and Council, 2020), and the United States Renewable Portfolio Standard legislations (Kosnik, 2010). In this scenario, hydropower is currently, and will be in the future, a key-factor in developing concrete strategies to reach these environmental targets (Hermoso, 2017).
Nonetheless, due to the high social and ecological impact of large hydropower plants (LHPs) that entail the construction of large dams (Rosenberg et al., 1995), worldwide investments are substantially favoring the construction of small hydropower plants (SHPs, installed capacity of 1–10 MW) (Lange et al., 2019), as one of the key technologies to achieve the targets set by the abovementioned international environmental initiatives. Indeed, from the patch to landscape scale, the adverse ecological effects of dams on riverine ecosystems have been widely reported, such as the loss of habitats and ecosystem services (Schöngart et al., 2021), disruption of biogeochemical cycles (Friedl and Wüest, 2002), alteration of hydrological regimes (Hecht et al., 2019), and/or geomorphological changes in the regulated river (Petts and Gurnell, 2005). Thus, mainly supported by ad hoc national policies, feed-in tariffs, and low environmental compliance standards, SHPs are experiencing an unprecedented global boom (Lange et al., 2019): 7% of the energy produced through renewable sources worldwide is produced by SHPs, and their global installed capacity has increased by approximately 10% from 2013 to 2019, whereas the world’s SHP potential capacity remains unexploited by 66% (Liu et al., 2019), thus indicating that this positive trend will continue in the coming years (Couto and Olden, 2018).
A crucial additional reason that justifies the worldwide success of SHPs is the belief that they have fewer adverse ecological impacts than LHPs (Anderson et al., 2015; Venus et al., 2020). However, the supposition that SHPs generally constitute a low-footprint technology (Kosnik, 2008) is not backed by any strong empirical evidence (Abbasi and Abbasi, 2011), to the extent that some authors argue that the social and environmental costs per megawatt produced by SHPs may be higher than those of LHPs (Bakken et al., 2012; Ziv et al., 2012; Kibler and Tullos, 2013). Therefore, the expected global expansion of the hydropower sector may potentially contribute to a decline in the ecological status of riverine ecosystems worldwide (Winemiller et al., 2016; Hermoso, 2017; Zarfl et al., 2019). Despite these contrasting opinions and the expansion trends of the SHP sector, knowledge gaps in scientific literature concerning SHP effects on the ecological, social, or economic sphere remain remarkable (Kuriqi et al., 2021): whereas SHPs constitute approximately 91% of the currently installed power plants, only 5% of the published studies regarding the impact of hydropower energy focus on them (Couto and Olden, 2018). Additionally, in most of these studies, the SHP capacities and schemes—which vary considerably in size and design—are not described (Kelly-Richards et al., 2017). Consequently, it has been difficult to gather thorough scientific knowledge that could contribute to an unbiased, urgently needed debate on strategic hydropower planning (Couto and Olden, 2018). Moreover, long-term data for evaluating the effects of SHPs—and, more generally, of small impoundments—on riverine ecosystems are notoriously lacking (Mbaka and Wanjiru Mwaniki, 2015). Therefore, available studies are generally limited to spatial comparisons with “control” reaches, i.e., upstream vs. downstream on the same stream with no temporal perspective (Mueller et al., 2011; Wang et al., 2016) or to large geographical scale where “control” sites are located on different streams (Bilotta et al., 2017), while a true, multiannual, Before-After-Control-Impact (BACI) analysis on SHPs has, to the best of our knowledge, never been applied (Anderson et al., 2015; Kuriqi et al., 2021).
In this study, using aquatic macroinvertebrates as a proxy for changes in the running-water environment, we aimed to narrow this scientific gap by performing a long-term environmental impact analysis of a “run-of-river” SHP (Anderson et al., 2015)—applying a BACI design—in a high-gradient, glacier-fed stream. Aquatic macroinvertebrates are widely recognized as effective bioindicators for stream health due to their diversity, widespread presence, long generation time albeit limited mobility, easy sampling effort requirements, and crucial ecological roles (Resh, 2008). In fact, they are directly involved in providing ecosystem services (i.e., pollination, crop pest control, see Raitif et al., 2019), and important ecological functions such as litter decomposition, nutrient retention, and riverbeds stabilization (Suter and Cormier, 2015). Furthermore, they have already been successfully used in several studies to detect both subtle and more obvious ecological impacts of hydropower plants (e.g., Armanini et al., 2014; Quadroni et al., 2017). More specifically, we intended to 1) assess the actual environmental impact of an SHP on the riverine ecosystem over a 5-year period by sampling aquatic macroinvertebrates before and after the implementation of the SHP at sampling sites divided into control and impacted sites (BACI design); 2) identify macroinvertebrates potentially sensitive to SHP activities that may be useful for further monitoring assessments in scenarios similar to the one presented in this study; and 3) disentangle the variability in the macroinvertebrate communities potentially introduced by the SHP from the spatial and temporal community fluctuations that are naturally present in a glacier-fed stream.
For the first objective, we hypothesized that, given the variety of habitats that the installation of an SHP may create due to new hydrologic and sediment regimes (Csiki and Rhoads, 2010; Mueller et al., 2011), the BACI analysis would result in a significant difference between “control” and “impact” sites and between “before” and “after” temporal windows. Consequently, for the second objective, we expected to find that macroinvertebrates species associated with the specific habitats created by SHP structures would indicate the new hydrology and sediment regime and/or the unimpacted environmental conditions of the control site. Finally, concerning the third objective, we expected that the heterogeneity of the new habitats created by the SHP would increase the spatial and temporal variability of the macroinvertebrate communities in comparison to a scenario without the presence of the hydropower plant (Scotti et al., 2019a).
Materials and Methods
Experimental Design
The Saldur stream (Figure 1A), approximately 21.5 km long, originates from the Matscher glacier (N 46°, E 10°, Italian Central-Eastern Alps) and is a fish-free tributary of the Adige River, the second longest Italian river. Its whole catchment (101 km2) has been part of the International Long Term Ecological Research (ILTER) network since 2014 (site code IT-25). The climate in the catchment—based on the Köpper Geiger climate classification (Rubel et al., 2017)—spans from Et to Dfc following a decreasing elevational gradient (3,738 m a.s.l.—921 m a.s.l.). Saldur stream water is abstracted or exploited for aquaculture, household and tourism sectors and, predominantly, agriculture and hydropower production (Scotti and Bottarin, 2021). Indeed, at the beginning of 2016, a small “run-of-river” hydropower plant—whose technical features are reported in Table 1—commenced its operations on the stream (Figures 1B,C).
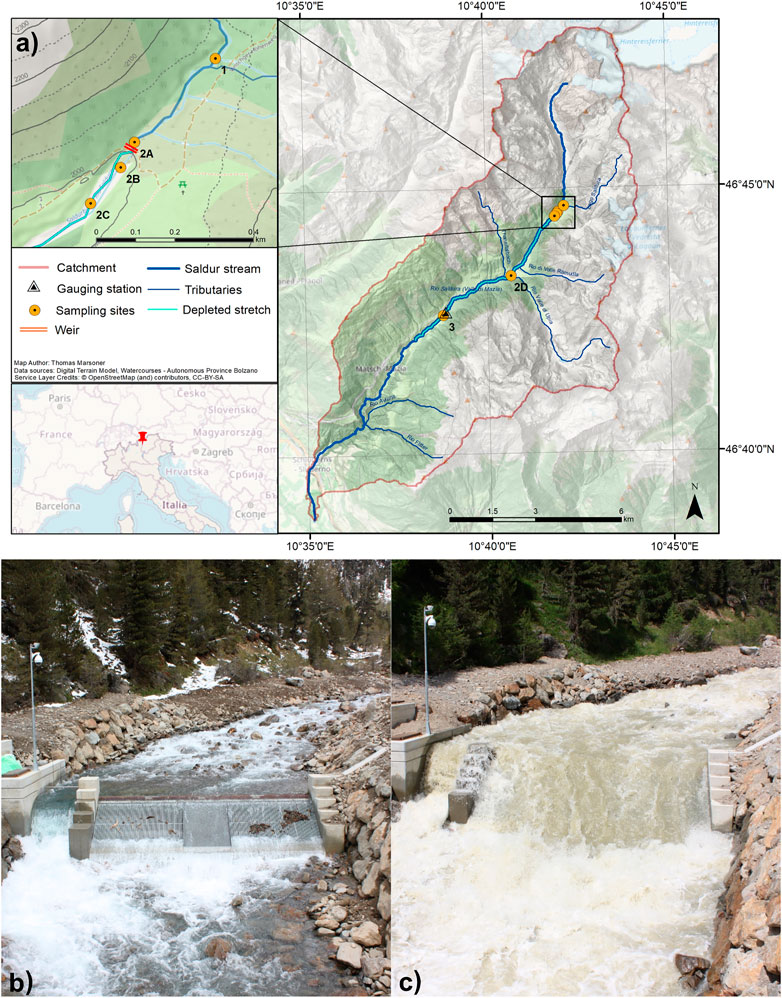
FIGURE 1. (A) Map showing the geographical framework of the study area and the location of the sampling sites along the Saldur stream. The upper left map shows a magnification of the geographical area close to the Tyrolean weir of the “run-of-river” hydropower plant; (B) photo of the hydropower plant taken in the “early” sampling period (April); (C) photo of the hydropower plant taken at the apex of the glacial melting period (July).
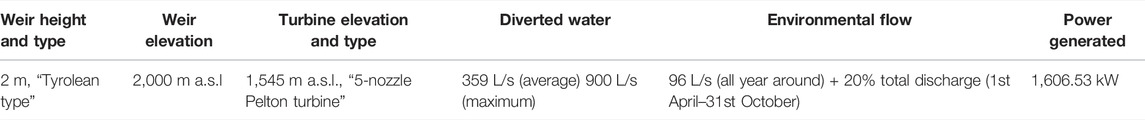
TABLE 1. Summary of the technical features of the “run-of-river” small hydropower plant located on the Saldur stream, South Tyrol, Italy (source: Environment and Climate Agency of the Autonomous Province of Bolzano/Bozen).
The design for this study was conceived in 2014, when the construction operations of the “run-of-river” SHP were planned. Aquatic macroinvertebrates have been collected from the Saldur stream since 2010 for different research purposes and sampling designs (Scotti et al., 2019b). Thus, to assure continuity and comparability with past and future ILTER activities, the site nomenclature adopted from sampling season 2015 onward followed that of the prior seasons with some adaptations, i.e., a split of site 2 into four different new sites (Figure 1A; Scotti et al., 2019a). We collected aquatic macroinvertebrates at 6 sites along the stream (Figure 1A): site 1 acted as the control site, while the sites in the water diversion area of the SHP, i.e., from 2A to 2C (Supplementary Figure S1), were selected based on their relative position to the weir—the water abstraction structure, see Figures 1B,C and to the outlet pipe of the desilting tank—a construction that conveys sediments, after desiltation of the water headed to the turbine, approximately 150 m downstream of the weir, just upstream of site 2C (Supplementary Figure S1). Sites 2D and 3 were located approximately 3 and 6 km downstream of the weir, respectively. Thus, sampling sites 2A (just upstream of the weir), 2B (just downstream of the weir), 2C (downstream of the weir, just downstream of the outlet pipe of the desilting tank), 2D and 3 are considered impacted sites potentially influenced by the SHP activities and/or located in the depleted stretch. Further details about the catchment and the sampling sites are retrievable in Scotti et al. (2019a) and Scotti and Bottarin (2021).
Fieldwork and Laboratory Activities
The sites were sampled from 2015 to 2019 twice a year, in late April/mid-May (“early” sampling period) and in late September/mid-October (“late” sampling period). Each sampling site was represented by a 20–50 m segment of the stream from which we collected a total of 12 quantitative Surber samples (22 × 23 cm, mesh-size 500 µm) at each sampling event. Three subsamples were collected at each site, each consisting of 4 pooled Surber samples. Each subsample was processed independently and pooled only for the specific analyses described below. This method was motivated by the highly uniform substrate of the stream, mainly composed of cobbles of two sizes (macrolithal, 20–40 cm; and mesolithal, 6–20 cm). Once the substrates to be targeted for each sampling had been identified, the subsamples were distributed proportionally to the area covered by each substrate. The sampling effort consisted of perturbing the substrate upstream of the Surber sampler for approximately 1 min. Thus, the final sampled area for every subsample was approximately 0.20 and 0.60 m2 for the entire sample. Each subsample was then filtered in the field using a 100 µm sieve net, preserved in plastic sealed bottles containing 70% ethanol, labeled and brought to the laboratory within the same day. A gauging station located at site 3—maintained by the Hydrographical Office of the Autonomous Province of Bolzano/Bozen—provided water discharge data throughout the entire length of the study to assess potential variations in discharge introduced by the activity of the SHP.
In the laboratory, samples were sorted without applying subsampling, and organisms were separated from the debris. All organisms were then counted and identified to the lowest possible taxonomic level (mostly genus) under a stereoscopic microscope at 50× magnification, referring to the appropriate literature (Olmi, 1978; Consiglio, 1980; Ferrarese and Rossaro, 1981; Rossaro, 1982; Belfiore, 1983; Nicolai, 1983; Rivosecchi, 1983; Lechthaler and Car, 2005; Lechthaler and Stockinger, 2005). We considered the issue of taxonomic ambiguities in our dataset, but given that ambiguous taxa are equally present in all our sites and their proportion in taxa richness and abundance is extremely low (see Scotti and Bottarin, 2021), we did not apply any correction methods. In this way, we could preserve true rarity and abundance patterns across sampling sites (Cuffney et al., 2007; Meredith et al., 2019).
Data Analysis Path
As a first step, we used the iNEXT R package (Hsieh et al., 2016) to check the relationship between sample coverage vs. number of individuals retrieved and their diversity for Hill numbers of order 0, 1, 2 to assess whether our sampling depth was adequate. Then, for each subsample, we calculated, using the diversity function in the R package vegan (Oksanen et al., 2019), five faunal descriptors to characterize the community of macroinvertebrates: taxonomic richness (N0), density (individuals/m2), percent Ephemeroptera–Plecoptera–Tricoptera, the macroinvertebrates most sensitive to weir presence (% EPT) (Mueller et al., 2011), Shannon evenness (Hill’s ratio, E10) and Simpson evenness (Hill’s ratio, E20).
Applying an approach resembling that of Pardini et al. (2018), we used generalized linear mixed models (GLMMs) to apply a BACI analysis for each of the five formerly identified faunal indices. GLMMs are robust to unbalanced datasets (Pinheiro and Bates, 2006) and thus appropriate for our data, for which our BACI design consisted of a single “before” year (2015), multiple “after” years (2016, 2017, 2018, 2019), a single “control” site (site 1), and multiple “impact” sites (2A, 2B, 2C, 2D, 3). For each faunal descriptor, using the glmmTMB function in the glmmTMB R package (Brooks et al., 2017), we ran a GLMM to determine the proportion of variance explained by the fixed effects of period (before-after) and treatment (control-impact) and their interaction (the actual BACI effect). The error distribution and link functions of the glmmTMB function were set accordingly to the numerical characteristics of the specific faunal descriptor (see Table 2 and “Data availability statement” section for the used code), whereas for the five GLMMs, we included site and year, and we tested their interaction term as random effects to generalize our inferences. Moreover, the procedure followed for each GLMM included the choice of the best-fitting model through Akaike’s information criterion (AIC), use of a possible observation-level random effect to account for overdispersion, inspection of residual and random effects, and test of significance through likelihood ratio test (Harrison et al., 2018).
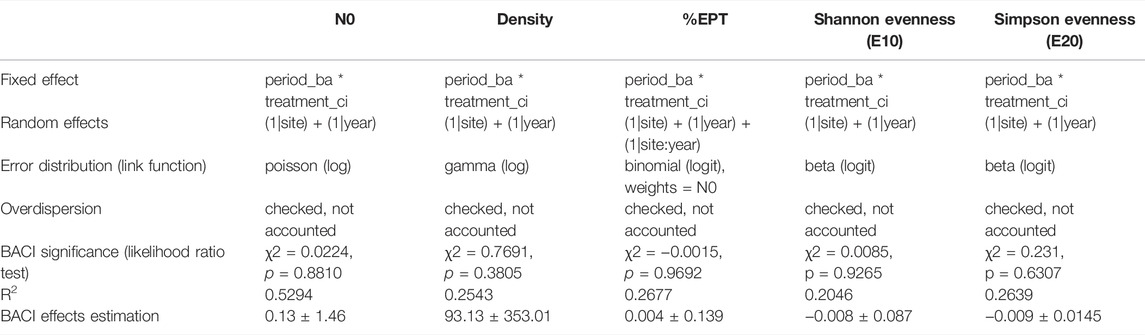
TABLE 2. Summary of GLMMs used for BACI analysis: each of the five faunal descriptors was an input of five different GLMMs. None of the five models showed significance through the likelihood ratio test, thus indicating that the macroinvertebrate communities are not influenced by the presence of the small hydropower plant. For each model, we summarize the chosen fixed and random effects, the applied error distribution, and the GLMM fitting results. The BACI effects estimation was calculated as (control after—control before)—(impact after—impact before). For further methodological details, refer to the main text and to the code included in the “Data availability statement” section.
Subsequently, to disentangle the inter- and intra-site and annual variability of the macroinvertebrate community, we performed an analysis of similarities (ANOSIM), corroborated by 9,999 permutations of class membership, that was based on a percentage difference distance matrix calculated from raw abundance data. Using the anosim function in the vegan R package (Oksanen et al., 2019), each site and year was tested separately, with either year or site being the grouping variable. Thus, a total of 11 different analyses (6 sites + 5 years) were conducted. To further differentiate and quantify the macroinvertebrate dissimilarities between sites and years, we ran a db-RDA (Legendre and Anderson, 1999) on Canoco5 (Šmilauer and Lepš, 2014) based on a percentage difference matrix—calculated from raw abundance data of samples (aggregating subsamples)—and on a factor representing the five sampling years of the study.
Moreover, spatial β-diversity—related to the “control” site, using subsamples—was partitioned into richness differences and replacement and was calculated to better disentangle the macroinvertebrate community dissimilarities identified thus far. We applied the beta.div.comp function in the R package adespatial (Dray et al., 2020) to the raw abundance data using the quantitative equivalent of the Sørensen’s index of the Podani group (Podani et al., 2013) since this form of the index is generally preferable in studies carried out within small spatial extents where species are largely the same (Legendre, 2014). Afterward, we again aggregated the subsamples and investigated the temporal β-diversity (Legendre, 2019) between each “after” sampling year and the “before” year, given the space-time dimension of our study. Using the TBI function included in adespatial (Dray et al., 2020), we derived the contribution over time of species gains and losses to the total dissimilarity, calculated as percentage difference and Sørensen dissimilarity on raw abundance and presence/absence data, respectively, to better understand whether dissimilarity stemmed from the actual appearance of new taxa or solely from differences in the abundances of the taxa. Summary and site-specific TBI output indices were tested for significance with a permutation test (9,999 permutations), and through the additional adespatial (Dray et al., 2020) function tpaired.krandtest, we tested whether the changes in each macroinvertebrate taxon between two different sampling years were significant (9,999 permutations). Since several taxa were tested simultaneously, the resulting p-values were corrected for multiple tests with Benjamini & Hochberg’s false discovery rate (Benjamini and Hochberg, 1995) with the p.adjust function of the stats R package (R Core Team, 2020).
Finally, to avoid limiting the search of possible indicator/sensitive macroinvertebrate species to a specific pair of sampling years or sites, we ran an indicator species analysis first for all the possible combinations of years and then for all the possible combinations of sites, applying the multipatt function (9,999 permutations)—“IndVal.g” argument—of indicspecies (De Cáceres and Legendre, 2009) R package. Again, the resulting p-values were corrected for multiple tests with Benjamini & Hochberg’s false discovery rate (Benjamini and Hochberg, 1995) with the p.adjust function of the stats (R Core Team, 2020) R package.
Apart from where otherwise explicitly indicated, all analyses were conducted using Microsoft R Open software, version 4.0.2 (Microsoft R Core Team, 2020). Graphics produced with ggplot2 (Wickham, 2011).
We provide the entire R code of this data analysis path in the “Data availability statement” section.
Results
Faunal Descriptors & Before-After-Control-Impact Analysis
We collected 34,836 benthic macroinvertebrates, mainly from the families Nemouridae (28%), Chironomidae (21%), Baetidae (19%), Taeniopterygidae (13%), and Leuctridae (5%). The sampling depth was adequate for both the number and the diversity of the organisms retrieved. (Supplementary Figure S2). In general terms, taxonomic richness (N0), individual density, and Shannon evenness (E10) showed some important general patterns (Figure 2): 1) these faunal descriptors showed variability both in their intra-annual (between periods of the same year) and interannual (among years) components; 2) high values of evenness were generally coupled to low values of taxonomic richness and density, and vice versa; 3) a reduction of richness and density of benthic macroinvertebrates between the “before” and “after” study period was not detected; 4) sites from 1 to 2C (Figures 2A–D) differentiated clearly from sites 2D and 3 (Figures 2E,F), especially in terms of taxonomic richness and density; and 5) both the “before” year (2015) and the “control” site (site 1) were not distinctly different from the “after” years and the “impact” sites, respectively. When testing this last pattern with GLMMs, no significant BACI effect was found, and none of the five community descriptors shifted due to the presence of the hydropower plant (p ≫ 0.05, Table 2; see also BACI interaction plots in Supplementary Figure S3). Moreover, the water discharge, measured at site 3, continued to show a constant water regime pattern throughout the whole study period (Figure 3).
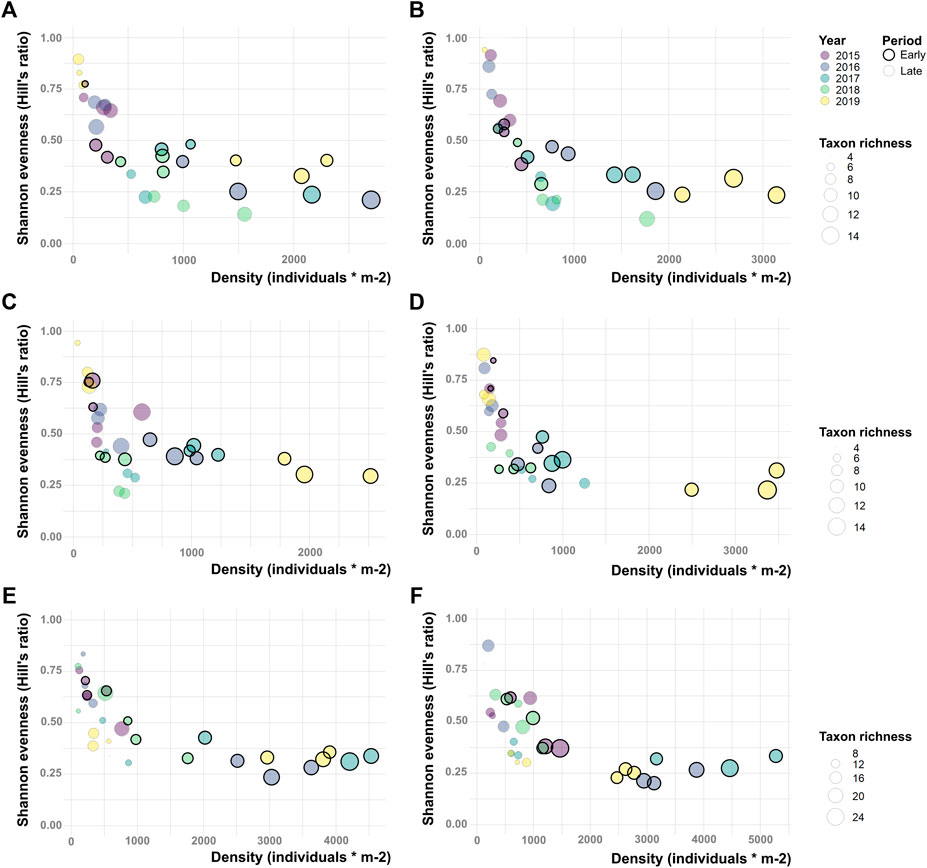
FIGURE 2. Shannon evenness (Hill’s ratio), density and taxon richness variation among years and sampling periods at (A) site 1; (B) site 2A; (C) site 2B; (D) site 2C; (E) site 2D; and (F) site 3. Overall, despite evident patterns of intra- and interannual variability among sites and descriptors, “before” year 2015 and “control” site 1 are not distinctly different from the “after” years and the “impact” sites.
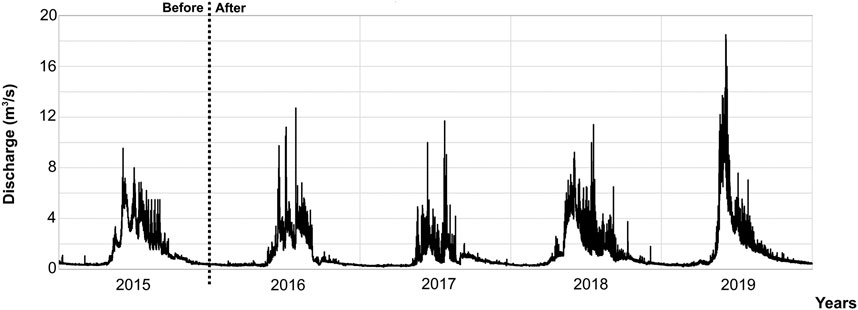
FIGURE 3. Hydrograph representing the water discharge in the Saldur stream, measured at site 3, throughout the whole study period. “before” and “after” refer to the start-up of the small hydropower plant. Despite being measured in the depleted stretch, the discharge did not show any pattern variation comparing “before” and “after” situations.
Spatial and Temporal β-Diversity
When testing the macroinvertebrate communities of each site and year separately, with either year or site being the grouping variable, we found a higher intersite and interannual dissimilarity compared to the intra-site and intra-annual dissimilarities (except for 2019, ANOSIM, R ˃ 0, p < 0.05, see Supplementary Table S1). The amount of the macroinvertebrate community variance explained by the sampling years was 36.7% (db-RDA, first axis: pseudo-F = 8.6, p = 0.0001; all axes: pseudo-F = 3.6, p = 0.0001, Figure 4). The results shown in Figure 4 demonstrate that macroinvertebrate communities differed across years, but the proximity of control site 1 to the other geographically close “impact” sites (i.e., 2A, 2B, 2C), even in 2016–2019, once again indicated no overall effect caused by the presence of the SHP (Figure 4).
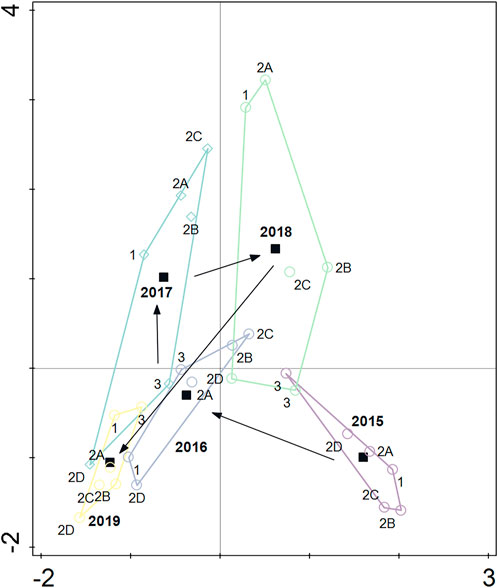
FIGURE 4. Distance-based redundancy analysis (db-RDA) where the centroid of each sampling year (black squares) is surrounded by the respective six sampling sites (empty, colored circles or squares). Black arrows indicate how the centroids of the community are moving through years. The analysis highlights the presence of an interannual variation that does not appear to be influenced by the small hydropower plant.
To better analyze the identified macroinvertebrate community dissimilarities, we calculated spatial β-diversity related to the “control” site partitioned into richness difference and replacement (Figure 5). While these two components of dissimilarity did not show a clear trend through years and sites, the total dissimilarity was consistent throughout all the sampling years, showing a constant increase proceeding downstream (Figure 5): the average dissimilarity among control site 1 and the nearby “impact” sites 2A, 2B, 2C was quite low (0.24) but increased almost linearly when compared to sites 2D (0.55) and 3 (0.75).
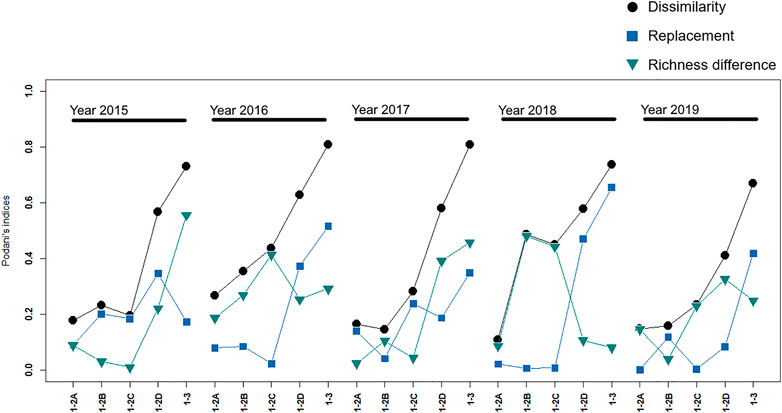
FIGURE 5. Dissimilarity in spatial β-diversity between the pairs of sites—against “control” site 1—throughout the study period, partitioned in replacement and richness difference. Total dissimilarity is consistent throughout all the sampling years, showing a constant increase proceeding downstream, while richness difference and replacement do not show a persistent pattern.
Additionally, given the space-time dimension of our study, we investigated the temporal β-diversity between each “after” sampling year paired with the “before” year 2015 (Figure 6). When performed on abundance data (Figure 6A), macroinvertebrate community dissimilarity quantification showed values between 0.81 and 0.31, with the component representing taxa increase—C/(2A + B + C)—significantly accounting for approximately 88% of the mean dissimilarity calculated among all the pairs of sampling years at each site (TBI function tests: p < 0.05). Remarkably, trends of decreasing and increasing dissimilarity, despite differing in magnitude, were shared by the sampling sites altogether (both “control” and “impact”): for instance, 2018 represented the lowest community dissimilarity for all sites when compared to 2015. In contrast, the dissimilarity values on presence/absence data (Figure 6B) were between 0.39 and 0.16, and the taxa increase component represented only approximately 53% of the mean dissimilarity calculated among all the pairs sampling years at each site. As a consequence, the overall variation in taxa increase or decrease was not significant for any sampling-year pair, and the general trend of dissimilarity—despite being low in magnitude—was fairly variable among the different sites (tpaired.krandtest, p > 0.05).
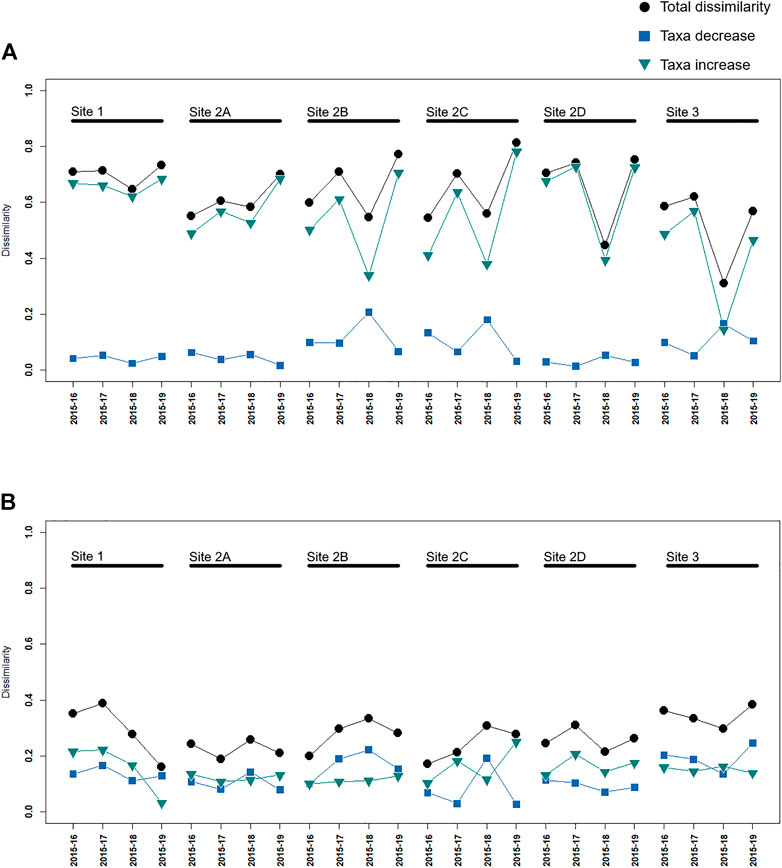
FIGURE 6. Dissimilarity in temporal β-diversity between the pairs of years—compared to “before” year 2015—for all the study sites, partitioned in taxa decrease—B/(2A + B + C)—and increase—C/(2A + B + C)—calculated on (A) raw abundance data; (B) presence/absence data. The comparison of the two graphs shows that the total dissimilarity is mainly caused by differences in the abundance of the taxa and only marginally by the appearance or disappearance of specific taxa.
However, when performing the IndVal analysis, 26 taxa were classified as “indicator taxa” (p < 0.01): 4 in relation to a year or temporal combination and 22 in relation to a site or a combination of sites. Nevertheless, none was associated with a specific habitat potentially introduced by the presence of the weir (Supplementary Table S2).
Discussion
The main finding of our study is that we could not detect an impact by the run-of-river SHP of the Saldur stream on benthic macroinvertebrates, which were used as a proxy for changes in the ecological conditions and stream health of the riverine environment. Indeed, our first hypothesis of finding a significant BACI effect could not be confirmed. The main reason is potentially that both the hydrologic and sediment regimes—which we expected to change—were not influenced by the SHP activities: the water discharge continued to show the typical regime pattern of a glacier-fed stream (Huss and Hock, 2018; Figures 1A, 3). It must be noted that although the gauging station is located in the depleted stretch, some tributaries are present between it and the weir, but they are intermittent and have very limited discharge (Scotti et al., 2019a). Thus, this highlights that the longitudinal connectivity of the stream—even in the water-depleted stretch—was always present (see Figures 1B,C). Indeed, the longitudinal connectivity, the flow regime and its alterations are widely recognized as key drivers in shaping aquatic biodiversity; they directly influence the physical habitats of streams, shape the life history characteristics of riverine fauna and flora, and may also determine the success or failure of new exotic species (Bunn and Arthington, 2002).
The trends illustrated in Figure 2, which show an increase in dissimilarity and diversity of macroinvertebrate communities with increasing distance from the glacial source, are commonly recorded in glacier-fed streams worldwide (Milner et al., 2001), and they were found in the Saldur stream itself when the SHP was not yet operating and in other glacier-fed streams of the region (Lencioni, 2018; Scotti et al., 2019a). In fact, benthic macroinvertebrate communities living in glacier-fed streams comprise an extremely specialized pool of species (Muhlfeld et al., 2020). Following the outcome of several studies conducted worldwide, Milner et al. (2001) identified water temperature and channel stability of stream/riverbed as the key environmental parameters that contribute the most to shaping the communities of benthic macroinvertebrates in glacier-fed streams. The combination of these two components allows us to define where along a gradient of glacial meltwater, the appearance, disappearance, or replacement of species may be expected. This conceptual model of faunal distribution, despite not explicitly investigated in this paper, was tested in the Saldur stream under natural conditions by Scotti et al. (2019a) (i.e., before the “run-of-river” hydropower plant was established on the stream); the benthic macroinvertebrate communities fitted the habitat template model of Milner et al. (2001), and higher values of diversity and dissimilarity of the macroinvertebrate communities were found where glacial influence was reduced (sites 2D and 3). Here, at the same sampling sites as Scotti et al. (2019a), the identical pattern was registered even after the start of operations of the hydropower plant in terms of several faunal descriptors (taxonomic richness N0, Shannon evenness E10, Simpson evenness E20, see Figure 2) and spatial β-diversity (Figure 5).
Moreover, in glacier-fed streams, the water flow is tightly linked to the transport of sediments, especially during the months of glacier ablation (Milner et al., 2017). Concerning the effect of a potentially new sediment regime caused by the weir, we were unable to detect a diminishing trend of % Ephemeroptera-Plecoptera-Trichoptera (% EPT)—a reported direct consequence of the weir presence (Mueller et al., 2011)—and, among other species, we even found two plecopterans broadly present in all the sites upstream and downstream of the weir area (sites 1–2C, Supplementary Table S2). This indicates that a possible deposition of fine sediments upstream of the weir (site 2A) and at the outlet pipe of the desilting tank (site 2C) did not have an impact on the macroinvertebrate community. Our results are supported by other studies that, despite not referring to macroinvertebrates, found no evidence of long-term fine sediment accumulation caused by “run-of-river” dams (Csiki and Rhoads, 2014). In addition, we could not observe any decrease in the density and taxonomic richness of benthic macroinvertebrates caused by the presence of the SHP (Figure 2), although this phenomenon is reported to happen more often in highland rivers than in lowland rivers (Kuriqi et al., 2021).
As a consequence, our second expectation of finding species associated with the habitats created by the weir must also be rejected, since we could not detect any specific macroinvertebrate whose presence or absence could be directly linked to the presence or activities of the SHP (Supplementary Table S2).
Furthermore, the temporal β-diversity analysis of presence/absence data (Figure 6B) clarified that community dissimilarities were mainly caused by, despite not being statistically significant (tpaired.krandtest, p > 0.05), variations in the density of the organisms and did not originate from the permanent appearance or disappearance of species. This result is supported by other studies conducted in comparable streams, where interannual climate variations (e.g., differences in snowpack) greatly influenced the densities and life-cycle behaviors of some benthic macroinvertebrates (Finn and Poff, 2008).
In summary, even if the choice of sampling time (April/May, and September/October) was made to minimize the effect of glacial meltwater (Engel et al., 2016) and consequently to maximize the possibility of detecting an effect of the SHP on the benthic macroinvertebrates, the community patterns remained clearly driven by the influencing dynamics of glacial melting, and the overlapping of a potential disrupting factor caused by the activities of the SHP had no detectable effects on the macroinvertebrate assemblages, leading us to again reject our third hypothesis.
The results of this study gain additional value when placed into the global context. Indeed, since the start of the twenty-first century, we have been living in a period of the “global water crisis” (Bunn, 2016). Thus, we are moving increasingly toward an era where trade-off policies regarding water resource management are becoming the rule, rather than the exception (Tickner et al., 2017): an example is the necessity to balance energy production with biodiversity sustainability (Kareiva, 2012), since biodiversity conservation is considered one of the most powerful tools to achieve sustainability from a long-term perspective (Naeem et al., 2016). In this regard, strategic planning at the river basin scale is an effective tool to reach this balance and to select an optimal portfolio of dams—with neither SHPs nor LHPs precluded—able to meet both energy and conservation needs worldwide (Ziv et al., 2012). This approach could be part of the solution to the “inherent contradiction” regarding hydropower plants when related, for example, to the UN Sustainable Development Goals (SDGs): on one hand, SDG 7 advocates to “ensure access to affordable, reliable, sustainable and modern energy for all”; on the other hand, SDG 14 recommends to “conserve and sustainably use life below water” (Kuriqi et al., 2021). Moreover, planning at this level would allow us to better consider crucial issues such as the social acceptance of new hydropower plants (Rygg et al., 2021), their weight in terms of the creation of a new, so-called “energy landscape” (Ferrario and Castiglioni, 2017), and the benefits of locally produced energy (Rygg et al., 2021).
Concerning the present study, we recognize the potential limitations of our BACI (Smokorowski and Randall, 2017), mainly because of the presence of only one “before” year and because our study was conducted in a fish-free, glacier-fed, high-gradient stream—where catchment slope contributes to minimizing the potential impact of a weir. However, most “run-of-river” SHPs in mountain areas are placed in this type of stream, and our results show that the presence of an SHP does not automatically imply an increase in habitat fragmentation and deterioration between the upstream and downstream stretches of a stream/river, especially when environmental flows are correctly managed and multiple- cascade systems of SHPs are not overdeveloped (Kuriqi et al., 2021). In addition, as advocated by some authors (Anderson et al., 2015; Kuriqi et al., 2021), our study is among the first to consider potential environmental impacts even upstream of diversion structures.
Nevertheless, to allow a broad generalization of our results, further studies considering the different sizes, designs, operational measures, and cumulative impacts of SHP—as well as the sharing of good design principles and practices among professionals—are vital for supporting both informed scientific debates and policy decisions (Couto and Olden, 2018; Lange et al., 2018). This study represents a first step in this direction, empirically demonstrating that SHPs represent an opportunity in the abovementioned energy planning process; they may indeed constitute an important resource for pursuing the climate and sustainability target plans set by the international community, especially at the local level, given the limitations of LHPs related to carbon neutrality (Wehrli, 2011).
Data Availability Statement
Dataset used for this study is permanently stored by PANGAEA - Data Publisher for Earth and Environmental Science, and it is publicly accessible at this web address: https://doi.pangaea.de/10.1594/PANGAEA.922524. The R code and related data used to perform the statistical analyses can be accessed through this web address: https://github.com/valentinitnelav/Saldur-ROR-analysis.
Author Contributions
AS: conceptualisation, methodology, software, formal analysis, investigation, resources, data curation, writing—original draft, writing—review and editing, visualisation, supervision; DJ: conceptualisation, methodology, resources, writing—review and editing, supervision; VȘ: methodology, software, validation, formal analysis, resources, data curation, writing—review and editing, visualisation; UT: conceptualisation, investigation, funding acquisition; RB: conceptualisation, methodology, investigation, resources, writing—review and editing, supervision, project administration, funding acquisition.
Funding
Eurac Research internal funds, with the support of the Autonomous Province of Bolzano/Bozen.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We thank the Hydrographical Office and the Environment and Climate Agency of the Autonomous Province of Bolzano/Bozen for providing data of discharge and the technical details of the small hydropower plant under study. Moreover, the authors thank Thomas Marsoner for producing the map in Figure 1 and Christian Brida for guidance with ggplot2. In addition, we thank Johannes Klotz, Agnese Martinelli, Arianna Peron, Karin Plank, Alexia Rottensteiner, Igor Zanvettor for providing help during the fieldwork and in the lab. The site Matsch|Mazia belongs to the national and international long term ecological research networks (LTER-Italy, LTER Europe, and ILTER). The authors thank the Department of Innovation, Research and University of the Autonomous Province of Bozen/Bolzano for covering the Open Access publication costs.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fenvs.2022.902603/full#supplementary-material
References
Abbasi, T., and Abbasi, S. A. (2011). Small Hydro and the Environmental Implications of its Extensive Utilization. Renew. Sustain. Energy Rev. 15 (4), 2134–2143. doi:10.1016/j.rser.2010.11.050
Anderson, D., Moggridge, H., Warren, P., and Shucksmith, J. (2015). The Impacts of 'run-Of-River' Hydropower on the Physical and Ecological Condition of Rivers. Water Environ. J. 29 (2), 268–276. doi:10.1111/wej.12101
Armanini, D. G., Chaumel, A. I., Monk, W. A., Marty, J., Smokorowski, K., Power, M., et al. (2014). Benthic Macroinvertebrate Flow Sensitivity as a Tool to Assess Effects of Hydropower Related Ramping Activities in Streams in Ontario (Canada). Ecol. Indic. 46, 466–476. doi:10.1016/j.ecolind.2014.07.018
Baird J., and Plummer R. (Editors) (2021). Water Resilience: Management and Governance in Times of Change (Cham, Switzerland: Springer International Publishing).
Bakken, T. H., Sundt, H., Ruud, A., and Harby, A. (2012). Development of Small Versus Large Hydropower in Norway- Comparison of Environmental Impacts. Energy Procedia 20, 185–199. doi:10.1016/j.egypro.2012.03.019
Benjamini, Y., and Hochberg, Y. (1995). Controlling the False Discovery Rate: A Practical and Powerful Approach to Multiple Testing. J. R. Stat. Soc. Ser. B Methodol. 57 (1), 289–300. doi:10.1111/j.2517-6161.1995.tb02031.x
Bilotta, G. S., Burnside, N. G., Turley, M. D., Gray, J. C., and Orr, H. G. (2017). The Effects of Run-Of-River Hydroelectric Power Schemes on Invertebrate Community Composition in Temperate Streams and Rivers. PloS one 12 (2), e0171634. doi:10.1371/journal.pone.0171634
Brooks, M. E., Kristensen, K., Benthem, K. J. v., Magnusson, A., Berg, C. W., Nielsen, A., et al. (2017). glmmTMB Balances Speed and Flexibility Among Packages for Zero-Inflated Generalized Linear Mixed Modeling. R J. 9 (2), 378–400. doi:10.32614/rj-2017-066
Bunn, S. E., and Arthington, A. H. (2002). Basic Principles and Ecological Consequences of Altered Flow Regimes for Aquatic Biodiversity. Environ. Manag. 30 (4), 492–507. doi:10.1007/s00267-002-2737-0
Bunn, S. E. (2016). Grand Challenge for the Future of Freshwater Ecosystems. Front. Environ. Sci. 4, 21. doi:10.3389/fenvs.2016.00021
Couto, T. B., and Olden, J. D. (2018). Global Proliferation of Small Hydropower Plants - Science and Policy. Front. Ecol. Environ. 16 (2), 91–100. doi:10.1002/fee.1746
Csiki, S. J., and Rhoads, B. L. (2014). Influence of Four Run-Of-River Dams on Channel Morphology and Sediment Characteristics in Illinois, USA. Geomorphology 206, 215–229. doi:10.1016/j.geomorph.2013.10.009
Csiki, S., and Rhoads, B. L. (2010). Hydraulic and Geomorphological Effects of Run-Of-River Dams. Prog. Phys. Geogr. Earth Environ. 34 (6), 755–780. doi:10.1177/0309133310369435
Cuffney, T. F., Bilger, M. D., and Haigler, A. M. (2007). Ambiguous Taxa: Effects on the Characterization and Interpretation of Invertebrate Assemblages. J. North Am. Benthol. Soc. 26 (2), 286–307. doi:10.1899/0887-3593(2007)26[286:ateotc]2.0.co;2
De Cáceres, M., and Legendre, P. (2009). Associations between Species and Groups of Sites: Indices and Statistical Inference. Ecology 90 (12), 3566–3574. doi:10.1890/08-1823.1
Dray, S., Blanchet, G., Borcard, D., Guenard, G., Jombart, T., Larocque, G., et al. (2020). Package ‘adespatial’. R package version 0.3-8.
Engel, M., Penna, D., Bertoldi, G., Dell'Agnese, A., Soulsby, C., and Comiti, F. (2016). Identifying Run-Off Contributions during Melt-Induced Run-Off Events in a Glacierized Alpine Catchment. Hydrol. Process. 30 (3), 343–364. doi:10.1002/hyp.10577
European Parliament and Council (2020). COM(2020) 80 Final. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?qid=1588581905912&uri=CELEX:52020PC0080 (accessed March 22, 2022).
European Parliament and Council (2018). Directive 2018/2011. Available at: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A02018L2001-20181221 (accessed March 22, 2022).
Ferrarese, U., and Rossaro, B. (1981). Chironomidi, 1 (Diptera, Chironomidae: Generalità, Diamesinae, Prodiamesinae). Italy: Consiglio Nazionale delle Ricerche.
Ferrario, V., and Castiglioni, B. (2017). Visibility/Invisibility in the 'Making' of Energy Landscape. Strategies and Policies in the Hydropower Development of the Piave River (Italian Eastern Alps). Energy Policy 108, 829–835. doi:10.1016/j.enpol.2017.05.012
Finn, D. S., and Poff, N. L. (2008). Emergence and Flight Activity of Alpine Stream Insects in Two Years with Contrasting Winter Snowpack. Arct. Antarct. Alp. Res. 40 (4), 638–646. doi:10.1657/1523-0430(07-072)[finn]2.0.co;2
Friedl, G., and Wüest, A. (2002). Disrupting Biogeochemical Cycles-Consequences of Damming. Aquat. Sci. 64 (1), 55–65. doi:10.1007/s00027-002-8054-0
Gernaat, D. E. H. J., Bogaart, P. W., Vuuren, D. P. V., Biemans, H., and Niessink, R. (2017). High-Resolution Assessment of Global Technical and Economic Hydropower Potential. Nat. Energy 2 (10), 821–828. doi:10.1038/s41560-017-0006-y
Grill, G., Lehner, B., Thieme, M., Geenen, B., Tickner, D., Antonelli, F., Babu, S., Borrelli, P., Cheng, L., Crochetiere, H., Ehalt Macedo, H., Filgueiras, R., Goichot, M., Higgins, J., Hogan, Z., Lip, B., McClain, M. E., Meng, J., Mulligan, M., Nilsson, C., Olden, J. D., Opperman, J. J., Petry, P., Reidy Liermann, C., Sáenz, L., Salinas-Rodríguez, S., Schelle, P., Schmitt, R. J. P., Snider, J., Tan, F., Tockner, K., Valdujo, P. H., van Soesbergen, A., and Zarfl, C. (2019). Mapping the World's Free-Flowing Rivers. Nature 569 (7755), 215–221. doi:10.1038/s41586-019-1111-9
Harrison, X. A., Donaldson, L., Correa-Cano, M. E., Evans, J., Fisher, D. N., Goodwin, C. E. D., et al. (2018). A Brief Introduction to Mixed Effects Modelling and Multi-Model Inference in Ecology. PeerJ 6, e4794. doi:10.7717/peerj.4794
Hecht, J. S., Lacombe, G., Arias, M. E., Dang, T. D., and Piman, T. (2019). Hydropower Dams of the Mekong River Basin: A Review of Their Hydrological Impacts. J. Hydrology 568, 285–300. doi:10.1016/j.jhydrol.2018.10.045
Hermoso, V. (2017). Freshwater Ecosystems Could Become the Biggest Losers of the Paris Agreement. Glob. Change Biol. 23 (9), 3433–3436. doi:10.1111/gcb.13655
Hsieh, T. C., Ma, K. H., and Chao, A. (2016). iNEXT: An R Package for Rarefaction and Extrapolation of Species Diversity (H Ill Numbers). Methods Ecol. Evol. 7 (12), 1451–1456. doi:10.1111/2041-210x.12613
Huss, M., and Hock, R. (2018). Global-Scale Hydrological Response to Future Glacier Mass Loss. Nat. Clim. Change 8 (2), 135–140. doi:10.1038/s41558-017-0049-x
IRENA (2019). Global Energy Transformation: A Roadmap to 2050 (2019 Edition). Abu Dhabi, United Arab States: International Renewable Energy Agency.
Kareiva, P. M. (2012). Dam Choices: Analyses for Multiple Needs. Proc. Natl. Acad. Sci. U. S. A. 109 (15), 5553–5554. doi:10.1073/pnas.1203263109
Kelly-Richards, S., Silber-Coats, N., Crootof, A., Tecklin, D., and Bauer, C. (2017). Governing the Transition to Renewable Energy: A Review of Impacts and Policy Issues in the Small Hydropower Boom. Energy Policy 101, 251–264. doi:10.1016/j.enpol.2016.11.035
Kibler, K. M., and Tullos, D. D. (2013). Cumulative Biophysical Impact of Small and Large Hydropower Development in Nu River, China. Water Resour. Res. 49 (6), 3104–3118. doi:10.1002/wrcr.20243
Kosnik, L. (2010). The Potential for Small Scale Hydropower Development in the US. Energy Policy 38 (10), 5512–5519. doi:10.1016/j.enpol.2010.04.049
Kosnik, L. (2008). The Potential of Water Power in the Fight against Global Warming in the US. Energy Policy 36 (9), 3252–3265. doi:10.1016/j.enpol.2008.05.009
Kuriqi, A., Pinheiro, A. N., Sordo-Ward, A., Bejarano, M. D., and Garrote, L. (2021). Ecological Impacts of Run-Of-River Hydropower Plants-Current Status and Future Prospects on the Brink of Energy Transition. Renew. Sustain. Energy Rev. 142, 110833. doi:10.1016/j.rser.2021.110833
Lange, K., Meier, P., Trautwein, C., Schmid, M., Robinson, C. T., Weber, C., et al. (2018). Basin-Scale Effects of Small Hydropower on Biodiversity Dynamics. Front. Ecol. Environ. 16 (7), 397–404. doi:10.1002/fee.1823
Lange, K., Wehrli, B., Åberg, U., Bätz, N., Brodersen, J., Fischer, M., et al. (2019). Small Hydropower Goes Unchecked. Front. Ecol. Environ. 17 (5), 256–258. doi:10.1002/fee.2049
Lechthaler, W., and Car, M. (2005). Simuliidae: Key to Larvae and Pupae from Central and Western Europe. Austria: Eutaxa ‐ Technisches Büro für Biologie.
Lechthaler, W., and Stockinger, W. (2005). Trichoptera: Key to Larvae from Central Europe. Austria: Eutaxa ‐ Technisches Büro für Biologie.
Legendre, P. (2019). A Temporal Beta‐Diversity Index to Identify Sites that Have Changed in Exceptional Ways in Space-Time Surveys. Ecol. Evol. 9 (6), 3500–3514. doi:10.1002/ece3.4984
Legendre, P., and Anderson, M. J. (1999). Distance-Based Redundancy Analysis: Testing Multispecies Responses in Multifactorial Ecological Experiments. Ecol. Monogr. 69 (1), 1–24. doi:10.1890/0012-9615(1999)069[0001:dbratm]2.0.co;2
Legendre, P. (2014). Interpreting the Replacement and Richness Difference Components of Beta Diversity. Glob. Ecol. Biogeogr. 23 (11), 1324–1334. doi:10.1111/geb.12207
Lencioni, V. (2018). Glacial Influence and Stream Macroinvertebrate Biodiversity under Climate Change: Lessons from the Southern Alps. Sci. Total Environ. 622-623, 563–575. doi:10.1016/j.scitotenv.2017.11.266
Liu, D., Liu, H., Wang, X., and Kremere, E. (2019). World Small Hydropower Development Report 2019. China: United Nations Industrial Development Organization; International Center on Small Hydro Power.
Mbaka, J. G., and Wanjiru Mwaniki, M. (2015). A Global Review of the Downstream Effects of Small Impoundments on Stream Habitat Conditions and Macroinvertebrates. Environ. Rev. 23 (3), 257–262. doi:10.1139/er-2014-0080
Meredith, C. S., Trebitz, A. S., and Hoffman, J. C. (2019). Resolving Taxonomic Ambiguities: Effects on Rarity, Projected Richness, and Indices in Macroinvertebrate Datasets. Ecol. Indic. 98, 137–148. doi:10.1016/j.ecolind.2018.10.047
Milner, A. M., Brittain, J. E., Castella, E., and Petts, G. E. (2001). Trends of Macroinvertebrate Community Structure in Glacier-Fed Rivers in Relation to Environmental Conditions: A Synthesis. Freshw. Biol. 46 (12), 1833–1847. doi:10.1046/j.1365-2427.2001.00861.x
Milner, A. M., Khamis, K., Battin, T. J., Brittain, J. E., Barrand, N. E., Füreder, L., et al. (2017). Glacier Shrinkage Driving Global Changes in Downstream Systems. Proc. Natl. Acad. Sci. U. S. A. 114 (37), 9770–9778. doi:10.1073/pnas.1619807114
Mueller, M., Pander, J., and Geist, J. (2011). The Effects of Weirs on Structural Stream Habitat and Biological Communities. J. Appl. Ecol. 48 (6), 1450–1461. doi:10.1111/j.1365-2664.2011.02035.x
Muhlfeld, C. C., Cline, T. J., Giersch, J. J., Peitzsch, E., Florentine, C., Jacobsen, D., et al. (2020). Specialized Meltwater Biodiversity Persists Despite Widespread Deglaciation. Proc. Natl. Acad. Sci. U. S. A. 117 (22), 12208–12214. doi:10.1073/pnas.2001697117
Naeem, S., Chazdon, R., Duffy, J. E., Prager, C., and Worm, B. (2016). Biodiversity and Human Well-Being: an Essential Link for Sustainable Development. Proc. R. Soc. B 283 (1844), 20162091. doi:10.1098/rspb.2016.2091
Nicolai, P. (1983). Blefariceridi (Diptera: Blephariceridae). Italy: Consiglio Nazionale delle Ricerche.
Oksanen, J., Blanchet, F. G., Kindt, R., Legendre, P., Minchin, P. R., O’hara, R. B., et al. (2019). Vegan: Community Ecology Package. R package version 2.5-6.
Olmi, M. (1978). Driopidi, Elmintidi (Coleoptera Dryopidae, Elminthidae). Italy: Consiglio Nazionale delle Ricerche.
Pardini, E. A., Parsons, L. S., Ştefan, V., and Knight, T. M. (2018). GLMM BACI Environmental Impact Analysis Shows Coastal Dune Restoration Reduces Seed Predation on an Endangered Plant. Restor. Ecol. 26 (6), 1190–1194. doi:10.1111/rec.12678
Petts, G. E., and Gurnell, A. M. (2005). Dams and Geomorphology: Research Progress and Future Directions. Geomorphology 71 (1-2), 27–47. doi:10.1016/j.geomorph.2004.02.015
Pinheiro, J., and Bates, D. (2006). Mixed-effects Models in S and S-PLUS. New York, NY: Springer Science & Business Media.
Podani, J., Ricotta, C., and Schmera, D. (2013). A General Framework for Analyzing Beta Diversity, Nestedness and Related Community-Level Phenomena Based on Abundance Data. Ecol. Complex. 15, 52–61. doi:10.1016/j.ecocom.2013.03.002
Quadroni, S., Crosa, G., Gentili, G., and Espa, P. (2017). Response of Stream Benthic Macroinvertebrates to Current Water Management in Alpine Catchments Massively Developed for Hydropower. Sci. Total Environ. 609, 484–496. doi:10.1016/j.scitotenv.2017.07.099
R Core Team (2020). R: A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing.
Raitif, J., Plantegenest, M., and Roussel, J.-M. (2019). From Stream to Land: Ecosystem Services Provided by Stream Insects to Agriculture. Agric. Ecosyst. Environ. 270-271, 32–40. doi:10.1016/j.agee.2018.10.013
Resh, V. H. (2008). Which Group is Best? Attributes of Different Biological Assemblages Used in Freshwater Biomonitoring Programs. Environ. Monit. Assess. 138 (1), 131–138. doi:10.1007/s10661-007-9749-4
Rosenberg, D., Bodaly, R. A., and Usher, P. J. (1995). Environmental and Social Impacts of Large Scale Hydroelectric Development: Who is Listening? Glob. Environ. Change 5 (2), 127–148. doi:10.1016/0959-3780(95)00018-j
Rossaro, B. (1982). Chironomidi, 2 (Diptera Chironomidae: Orthocladiinae). Italy: Consiglio Nazionale delle Ricerche.
Rubel, F., Brugger, K., Haslinger, K., and Auer, I. (2017). The Climate of the European Alps: Shift of Very High Resolution Köppen-Geiger Climate Zones 1800-2100. Meteorol. Z. 26 (2), 115–125. doi:10.1127/metz/2016/0816
Rygg, B. J., Ryghaug, M., and Yttri, G. (2021). Is Local Always Best? Social Acceptance of Small Hydropower Projects in Norway. Int. J. Sustain. Energy Plan. Manag. 31, 161–174. doi:10.5278/ijsepm.6444
Schöngart, J., Wittmann, F., Faria de Resende, A., Assahira, C., de Sousa Lobo, G., Rocha Duarte Neves, J., et al. (2021). The Shadow of the Balbina Dam: A Synthesis of over 35 Years of Downstream Impacts on Floodplain Forests in Central Amazonia. Aquatic Conserv. Mar. Freshw. Ecosyst. 31 (5), 1117–1135. doi:10.1002/aqc.3526
Scotti, A., and Bottarin, R. (2021). Fine-Scale Multiannual Survey of Benthic Invertebrates in a Glacier-Fed Stream Used for Hydropower Generation. Sci. Data 8 (1), 105. doi:10.1038/s41597-021-00887-x
Scotti, A., Tappeiner, U., and Bottarin, R. (2019b). Stream Benthic Macroinvertebrates Abundances over a 6-year Monitoring Period of an Italian Glacier-Fed Stream. Biodivers. Data J. 7, e33576. doi:10.3897/BDJ.7.e33576
Scotti, A., Jacobsen, D., Tappeiner, U., and Bottarin, R. (2019a). Spatial and Temporal Variation of Benthic Macroinvertebrate Assemblages during the Glacial Melt Season in an Italian Glacier-Fed Stream. Hydrobiologia 827 (1), 123–139. doi:10.1007/s10750-018-3731-8
Šmilauer, P., and Lepš, J. (2014). Multivariate Analysis of Ecological Data Using CANOCO 5. New York, USA: Cambridge University Press.
Smokorowski, K. E., and Randall, R. G. (2017). Cautions on Using the Before-After-Control-Impact Design in Environmental Effects Monitoring Programs. Facets 2 (1), 212–232. doi:10.1139/facets-2016-0058
Suter, G. W., and Cormier, S. M. (2015). Why Care about Aquatic Insects: Uses, Benefits, and Services. Integr. Environ. Assess. Manag. 11 (2), 188–194. doi:10.1002/ieam.1600
Tickner, D., Parker, H., Moncrieff, C. R., Oates, N. E. M., Ludi, E., and Acreman, M. (2017). Managing Rivers for Multiple Benefits-A Coherent Approach to Research, Policy and Planning. Front. Environ. Sci. 5, 4. doi:10.3389/fenvs.2017.00004
UNFCCC (2015). The Paris Agreement (United Nations Framework Convention on Climate Change). Available at: https://unfccc.int/sites/default/files/english_paris_agreement.pdf (accessed March 22, 2022).
Venus, T. E., Hinzmann, M., Bakken, T. H., Gerdes, H., Godinho, F. N., Hansen, B., et al. (2020). The Public's Perception of Run-Of-The-River Hydropower across Europe. Energy Policy 140, 111422. doi:10.1016/j.enpol.2020.111422
Wang, H., Chen, Y., Liu, Z., and Zhu, D. (2016). Effects of the "Run-Of-River" Hydro Scheme on Macroinvertebrate Communities and Habitat Conditions in a Mountain River of Northeastern China. Water 8 (1), 31. doi:10.3390/w8010031
Winemiller, K. O., McIntyre, P. B., Castello, L., Fluet-Chouinard, E., Giarrizzo, T., Nam, S., et al. (2016). Balancing Hydropower and Biodiversity in the Amazon, Congo, and Mekong. Science 351 (6269), 128–129. doi:10.1126/science.aac7082
Zarfl, C., Berlekamp, J., He, F., Jähnig, S. C., Darwall, W., and Tockner, K. (2019). Future Large Hydropower Dams Impact Global Freshwater Megafauna. Sci. Rep. 9 (1), 18531. doi:10.1038/s41598-019-54980-8
Zarfl, C., Lumsdon, A. E., Berlekamp, J., Tydecks, L., and Tockner, K. (2015). A Global Boom in Hydropower Dam Construction. Aquat. Sci. 77 (1), 161–170. doi:10.1007/s00027-014-0377-0
Keywords: glacier-fed stream, run-of-river (RoR) hydropower plant, BACI, energy policies, small hydropower (SHP), green energies, biodiversity
Citation: Scotti A, Jacobsen D, Ștefan V, Tappeiner U and Bottarin R (2022) Small Hydropower—Small Ecological Footprint? A Multi-Annual Environmental Impact Analysis Using Aquatic Macroinvertebrates as Bioindicators. Part 1: Effects on Community Structure. Front. Environ. Sci. 10:902603. doi: 10.3389/fenvs.2022.902603
Received: 23 March 2022; Accepted: 14 June 2022;
Published: 14 July 2022.
Edited by:
Alberto Doretto, Università del Piemonte Orientale, ItalyReviewed by:
Laura Gruppuso, University of Turin, ItalyCourtney Elizabeth Larson, University of Minnesota Duluth, United States
Copyright © 2022 Scotti, Jacobsen, Ștefan, Tappeiner and Bottarin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Alberto Scotti, alberto.scotti@eurac.edu
 Alberto Scotti
Alberto Scotti Dean Jacobsen
Dean Jacobsen Valentin Ștefan3,4
Valentin Ștefan3,4