- 1HIV Inflammation and Persistence Unit, Institut Pasteur, Paris, France
- 2Vaccine Research Institute, Créteil, France
- 3Yerkes National Primate Research Center, Emory University School of Medicine, Atlanta, GA, United States
- 4Yerkes Nonhuman Primate Genomics Core, Yerkes National Primate Research Center, Atlanta, GA, United States
- 5Center for Virology and Vaccine Research, Beth Israel Deaconess Medical Center (BIDMC), Harvard Medical School, Boston, MA, United States
- 6Ragon Institute of MGH, MIT and Harvard, Cambridge, MA, United States
Combined antiretroviral therapies (cARTs) efficiently control HIV replication leading to undetectable viremia and drastic increases in lifespan of people living with HIV. However, cART does not cure HIV infection as virus persists in cellular and anatomical reservoirs, from which the virus generally rebounds soon after cART cessation. One major anatomical reservoir are lymph node (LN) follicles, where HIV persists through replication in follicular helper T cells and is also trapped by follicular dendritic cells. Natural hosts of SIV, such as African green monkeys and sooty mangabeys, generally do not progress to disease although displaying persistently high viremia. Strikingly, these hosts mount a strong control of viral replication in LN follicles shortly after peak viremia that lasts throughout infection. Herein, we discuss the potential interplay between viral control in LNs and the resolution of inflammation, which is characteristic for natural hosts. We furthermore detail the differences that exist between non-pathogenic SIV infection in natural hosts and pathogenic HIV/SIV infection in humans and macaques regarding virus target cells and replication dynamics in LNs. Several mechanisms have been proposed to be implicated in the strong control of viral replication in natural host’s LNs, such as NK cell-mediated control, that will be reviewed here, together with lessons and limitations of in vivo cell depletion studies that have been performed in natural hosts. Finally, we discuss the impact that these insights on viral dynamics and host responses in LNs of natural hosts have for the development of strategies toward HIV cure.
Introduction
Combined antiretroviral therapy (cART) has transformed HIV infection from a lethal disease into a manageable chronic infection (1). Indeed, cART efficiently controls HIV replication leading to undetectable virus in blood and drastic increases in lifespan of people living with HIV (2). However, cART does not cure HIV infection as virus persists in cellular and anatomical reservoirs, from which the virus most often rapidly rebounds after cART interruption (3, 4). HIV probably rebounds from multiple sources (5). Virus-producing cells can be detected in SIVmac-infected macaques under suppressive cART in nearly every tissue, and in particular in the mucosal tissues and secondary lymphoid organs (6, 7). A major anatomical viral reservoir corresponds to lymph node (LN) B cell follicles, where HIV-1/SIVmac replication persists in follicular helper T cells (TFH) even in Elite controllers and cART-virologically suppressed individuals (8, 9). Surprisingly, TFH cells expand during HIV-1 and SIVmac infections (10). Thus, lymphoid follicles have come to be considered as major sanctuaries for HIV/SIV (9). In parallel, HIV-1 and SIVmac might also persist in some CD4+ T cells within the T zone of LN during cART (11). In this review, we will focus on the viral and host dynamics in LNs of natural hosts and discuss similarities and key differences with regard to HIV and SIVmac infections.
Primary Characteristics of Non-Pathogenic SIV Infection in Natural Hosts
Natural hosts of SIV, such as African green monkeys (AGMs) (Chlorocebus aethiops), sooty mangabeys (SMs) (Cercocebus atys), and mandrills (Papio sphinx), generally do not progress to disease despite displaying persistently high viremia (12–16). The vast majority of the studies carried out on SIV infections in natural hosts have been performed using two species, SMs and AGMs (17). The comparison of the clinical, virological, and immunological parameters of infection in these species with that of HIV/SIVmac infections allowed advances in knowledge on the mechanisms linked to protection against AIDS. In particular, natural hosts rapidly resolve inflammation induced by SIV infection, and unlike pathogenic lentivirus infections do not develop chronic immune activation (see chapter below).
An important aspect of SIV infection in natural hosts is also their ability to preserve the function and structure of their tertiary and secondary lymphoid organs throughout the infection. Indeed, natural hosts avoid the widespread damage to the mucosal immune architecture that is observed in pathogenic infections (Table 1). While acute SIV infection leads to a rapid, near-complete loss of CD4+ T cells in the intestine in both natural hosts and macaques, mucosal CD4+ T cells partially recover in natural hosts, even if not to baseline levels (18–21). Furthermore, cART administration to SIV+ SM induces a rapid and substantial recovery of mucosal CD4+ T cells that is not typically observed in HIV infection (22). Moreover, despite high viremia and high-level replication in the gut (23), natural hosts, in stark contrast to non-natural hosts, preserve intestinal Th17 cells (24, 25), retain the structural integrity of the mucosal barrier (26), and do not exhibit leakage of mucosal lumenal microbiota (i.e., microbial translocation) into systemic circulation (27–29). With regard to LN during SIV infection in natural hosts, there is generally no sign of lymphadenopathia nor fibrosis and LN display a normal follicular dendritic cell (FDC) network (12, 30, 31) (Table 1). Another characteristic of natural hosts is the relatively low infection of central memory T cells (see below) (32). Natural hosts thus seem to have developed ways to protect the sites of education and memory of immune responses.
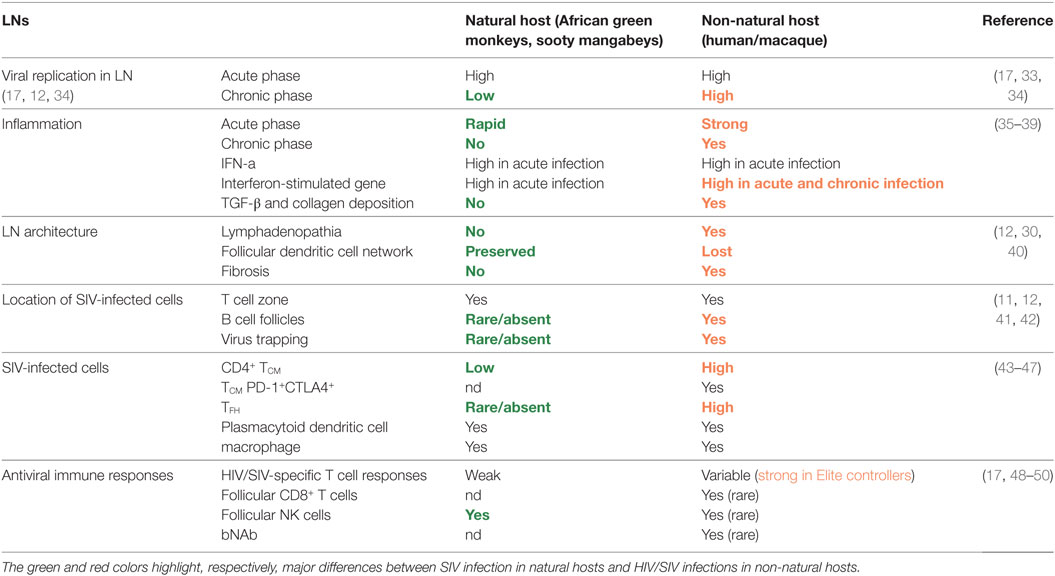
Table 1. Major similarities and differences between HIV/SIVmac infections and SIV infections in natural hosts at the level of lymph nodes (LNs).
Viral Dynamics in LNs during SIV Infection in Natural Hosts
Studies in SIVmac infection have shown that the viral seeding of LN occurs rapidly and progressively. One to three days after infection, some replicative viruses could already be detected in the draining LN and even in systemic LN (51). Of note, during the eclipse phase until peak viremia, productively infected cells are found essentially in the extra-follicular zone of LN (41). Only in later phases of primary infection, and in particular during chronic infection, viral RNA is found inside B cell follicles, where it replicates within TFH cells (45). In addition, virus is trapped within the follicles by FDCs where it remains infectious for 9 months or more (30, 52, 53). The mechanism driving this shift from the T cell zone to the B cell follicles is incompletely understood.
During chronic HIV/SIVmac infections, virus replication in LN exceeds the levels in blood by several orders of magnitude. In ART-naive SIVmac infection, LN are estimated to support ~50% of viral burden, and be reduced to ~1% in the context of suppressive ART, with the remainder supported by mucosal tissue (6). In one SIVmac-infected macaque, the frequency of infected cells in LN was evaluated and appeared to be as high or slightly higher than in the gut (mean frequency ~8.7 × 105 vRNA+ cells/g in LN and ~5.6 × 105 vRNA+ cells/g in the gut) (6). ART administered for >20 weeks decreased the mean frequency of vRNA in LN by approximately 2 log10 in SIVmac251-infected rhesus macaques (6).
The reason of the preservation of the normal architecture of LN in natural hosts might be associated with the significantly lower levels of viral replication in this tissue. Strikingly indeed, AGM and SM mount a strong viral control in LN shortly after peak viremia, which lasts throughout infection (12, 23, 43, 54–56). Thus, while during the first 2 weeks post-infection (p.i.), the number of productively infected cells as well as the copy numbers of cell-associated viral DNA and RNA are similar between SIV infection in natural hosts and macaques, major differences are observed after the viremia peak between natural and non-natural hosts (12, 49, 56). Thus in natural hosts, viral replication levels decrease drastically in LNs after peak viremia, whereas in pathogenic infections, after a moderate decrease, a relatively strong viral replication generally persists throughout the infection in absence of cART, leading to a difference of 2–3 log in the cell-associated viral RNA in LN during chronic infection between macaques and natural hosts. Viral RNA-producing cells as well as cell-associated viral RNA sometimes become even undetectable in LN of AGM, despite continuous high-level plasma viremia (12, 14, 33).
The anatomical distribution of virus replication in chronic infection is also very different between non-pathogenic and pathogenic infection. Indeed, in natural hosts, most virus is detected in the T cell zone, even if at extremely low levels, while in pathogenic HIV/SIV infection, most virus is present in follicles (Figure 1). Strikingly, in natural hosts, such as AGM and to a lesser extent in SM, viral RNA is generally absent in follicles. This is not a matter of the virus, as SIVsm and SIVagm infections of macaques lead to high SIV levels in follicles (57, 58). Natural hosts are thus characterized by a limited or absent replication in TFH cells and frequent lack of FDC deposition of virus (35, 59). Understanding the underlying mechanisms of the strong viral control in LN in natural hosts might yield clues helpful for the development of strategies aiming the elimination of HIV reservoirs in follicles.
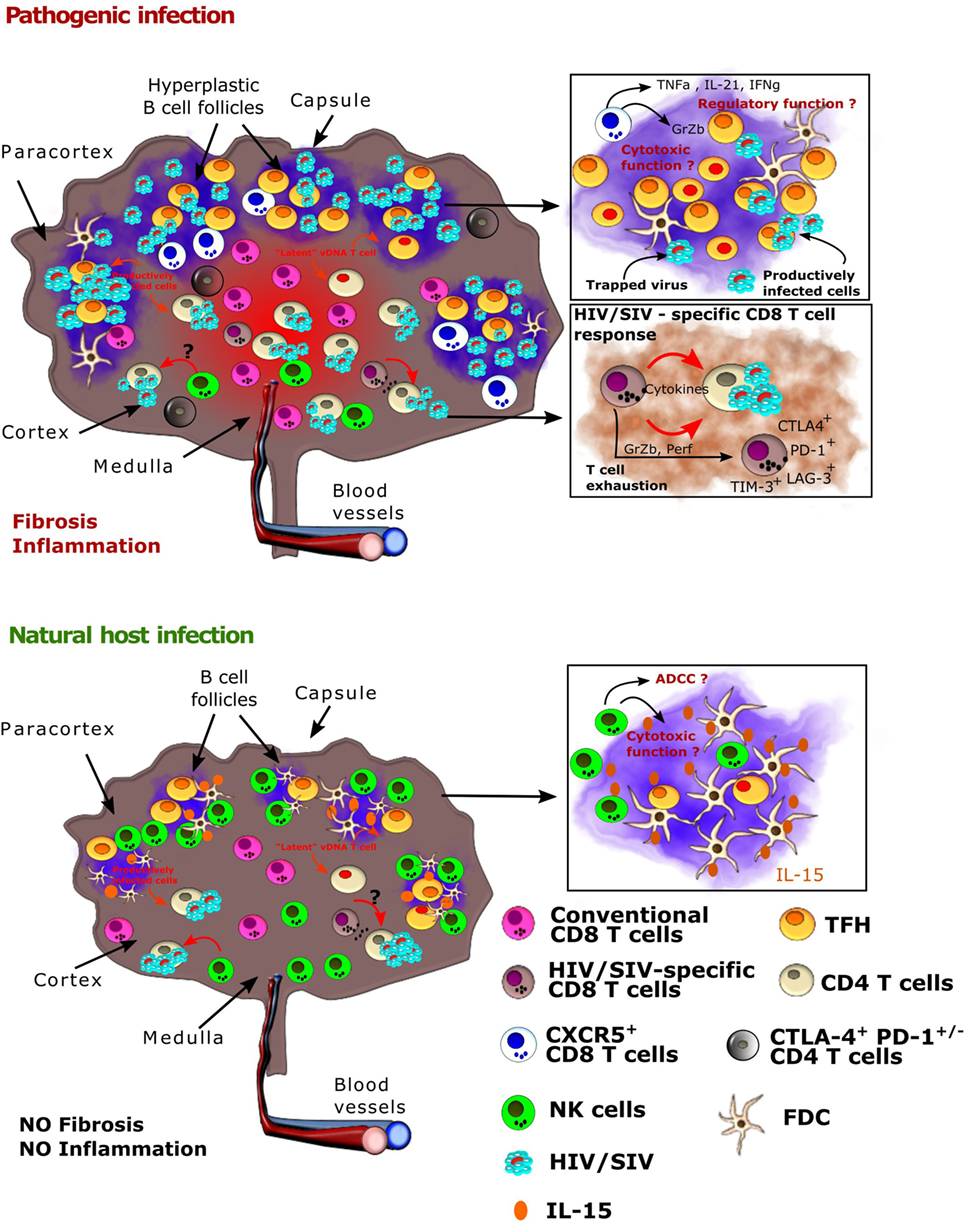
Figure 1. Viral and host immune cell dynamics in lymph nodes (LNs) from natural hosts versus HIV-1/SIVmac infections. Schematic representation of a LN after HIV or SIV infection in pathogenic models (human, macaques, top) and natural hosts [African green monkey (AGM), sooty mangabey, bottom]. (Top) HIV-1 and SIVmac infection in, respectively, humans and macaques result in the formation of hyperplastic germinal centers in LNs with massive B cell proliferation. TFH cells also expand during HIV-1 and SIVmac infections. Inflammation is uncontrolled and leads to collagen deposition and fibrosis. The follicular dendritic cell (FDC) network is disrupted on the long term. HIV-1 and SIVmac replicates in combined antiretroviral therapy (cART)-naïve individuals and animals in both T and B cell zones, but the viral burden is highest in the B cell zones (follicles). In the follicles, virus replication is concentrated within follicular helper T cells (TFH). Virus is also trapped by FDC and remains infectious. On cART, virus persists mostly in TFH cells in the follicles, where it is often outreach of conventional CD8+ T cells and of optimal drug concentrations, as well as in CTLA4+CD4+ T cells within the T zone. The latter cells have a capacity for long survival. NK cells and conventional HIV/SIV-specific CD8+T cells are often expressing immune checkpoint inhibitors. The presence of CXRC5+CD8+T lymphocytes has been described, but their role needs to be further studied. (Bottom) In natural hosts, virus replication is strongly controlled during the chronic phase of infection. Most follicles are exempt of virus. Conventional SIV-specific CD8+ T cell responses are weak. NK cells play a major role in the control of viral replication in AGM LNs. Both the IFN-α and NK cell responses appear earlier than in SIVmac-infected macaques. NK cells accumulate in follicles in SIVagm-infected AGMs, which might be a direct consequence of a high production of IL-15 in the follicles. NK cell migration into B cell follicles in response to SIVagm infection is associated with the acquisition of CXCR5. CXCR5+ NK cells express high levels of Fcγ receptors and of CD107a, which raises the question if they have the capacity to control SIVagm replication through antibody-dependent and/or -independent cellular cytotoxicity.
Regulation of Inflammation in LNs and Impact on SIV Infection in Natural Hosts
The deleterious impact of unabated inflammation in HIV infection has been well documented (35). This immune activation is positively correlated with HIV-1/SIVmac replication in both ART-naïve and ART-treated settings (60, 61). Among the myriad of detrimental manifestations due to the persisting inflammation that have been reported, a handful could be particularly influential in maintaining viral burden, namely: (i) recruitment of target cells, (ii) impairment/exhaustion of adaptive immunity, and (iii) the disruption of lymphoid structures. In this section, we will review existing data on inflammatory pathways differing significantly between natural hosts versus pathogenic SIV infection. These data will be reviewed in the context of the natural host’s low-to-absent SIV burden in LN follicles. Hypotheses concerning the effect of non-natural inflammation in supporting LN SIV replication will be presented.
A longstanding observation in natural hosts is that they are devoid of the pan-lymphocyte activation and chronic inflammation seen in pathogenic HIV/SIV disease (62–64). The molecular and immunological distinctions of these species have been extensively characterized [reviewed in Ref. (35, 44, 65)]. Although natural hosts exhibit levels of immune activation similar to baseline during chronic infection, detailed longitudinal studies have demonstrated that rapid, early immune activation is evident, including elevated levels of IFN-a, CD8+Ki67+ T cells and PD1 expression in LN (63, 66, 67). The most striking confirmation is the massive upregulation of interferon-stimulated gene (ISG) expressions during acute infection in natural hosts (68, 69) in blood, LN, and gut. These ISGs include many antiviral restriction factors, such as MX2 and Tetherin. Of note, the upregulation of ISGs occurs very early, starting from days 1 or 2 p.i. in AGM, concomitantly with a very early transient increase in IFN-α (68, 70). By contrast, during SIVmac infection in macaques, it was reported that the expression of those ISGs encoding antiviral restriction factors was delayed and not upregulated before peak viral replication on day 10 (71). Thus, natural hosts seem to develop a more rapid antiviral innate response to SIV compared to non-natural hosts (66, 68, 70, 71). Subsequently, natural hosts rapidly resolve total ISG expression to baseline before the transition to chronic infection despite prevalent viremia. This downregulation of ISG expression in natural hosts is in stark contrast to HIV/SIVmac infections, in which ISG expression remains elevated indefinitely (72).
The observation that natural hosts resolve IFN-I related responses prompted a series of comparative studies into plasmacytoid dendritic cells (pDCs). pDC trafficking to LN has been reported for both natural and non-natural hosts. A peak of pDC accumulation in LN is observed approximately 7–14 days after SIV infection in macaques, SM, and AGMs concomitant with robust IFN-α and IFN-β in situ production by pDC in LN (66, 73–76). The trafficking of pDC to tissues during SIV infection differs in several aspects between natural hosts and non-natural infections: (i) in AGM, an early first peak of pDC in LN is observed around days 1–3 p.i. (66); (ii) pDC accumulate in the rectal mucosa in infected humans and macaques, but not in SM, which has been attributed to heightened levels of α4β7 in SIVmac infection (77, 78), and (iii) pDC in LN during acute SIVmac infection are prone to apoptosis, while for natural hosts this is not known (39, 73). Both SM and AGM were demonstrated to retain intact sensing and IFN-α production in pDC in response to their native SIV (68, 79–81). Of note, pDC from AGM sense more efficiently SIVagm than SIVmac or HIV-1 viruses (81). Studies in natural hosts have revealed that SIV infection alters the capacity of viral sensing in cells other than pDC, which then can also produce IFN-I during acute infection (80). The contribution of pDC to IFN responses during chronic SIV infection remains unresolved, while some reports have not detected IFN-I in pDC during chronic infection (74), we have observed IFN-α transcripts in LN pDC as far out as 18 months post-infection (Bosinger, unpublished observations).
The consequences of unabated IFN production on immune function and viral reservoirs in HIV infection are under intense study. IFN-induced responses are clearly critical for the control of SIV in LN during acute infection, as antagonism of the IFN-α receptors (IFNAR) from before infection to early time points p.i. in macaques caused elevated levels of LN-associated SIV and plasma viremia (82).
The effects of IFN during chronic HIV infection are less clear. Mouse models have shown that persistent TLR and IFN signaling causes damage to the lymphoid structures (83). Several studies have demonstrated that irreversible fibrosis is evident in the LNs of SIV-infected macaques, but, interestingly, is absent in natural host infection (31, 84). The fibrosis in chronic HIV/SIV infection might be linked to persistent IFN-related inflammation, TGF-β produced by regulatory T cells (Treg) leading to collagen deposition, and/or other yet unknown factors (84). Disruption of IFN-I signaling in chronic infection appears to have indeed a beneficial effect on host immunity in certain settings. In the mouse model of lymphocytic choriomeningitis clone 13 infection, blockade of IFN-β signaling in chronic infection enabled spontaneous clearance of the virus (85–87). In a remarkable set of independent studies using ART-suppressed, HIV-infected humanized mice, disruption of IFNAR signaling reduced latent HIV levels and ameliorated systemic immune activation (88, 89). In both the LCMV and hu-mouse HIV datasets, IFN-blockade reduced expression of co-inhibitory molecules on CD8+ T cells and improved cellular antiviral responses; thus, the mechanism of action was presumed to be alleviation of IFN-mediated exhaustion of T cell responses. These studies provide some rationale for IFN blockade to be applied as a therapy to lower the reservoir, but this hypothesis would first need validation of efficacy and safety in pre-clinical studies. Taken together, the observations that (i) SIV natural host species avoid long-term ISG expression and (ii) in vivo antagonism of type I IFN signaling can improve antiviral immunity and reduce reservoir levels in the hu-mouse model suggest that the overall contribution of IFN in chronic HIV/SIV infection is harmful by maintaining high levels of immune activation and contributing to immune dysfunction. However, exogenous administration of IFN-α to ART-suppressed, HIV-infected patients have shown in some cases clinical benefit in terms of reduced levels of cell-associated HIV DNA (90–92). Thus, the contribution of IFN-α to chronic inflammation and viral persistence during ART-treated HIV/SIV infections is still unclear. Injection of exogenous IFN-α into SIV-infected AGM and SM have not been able to reproduce the phenotype of widespread immune activation observed in non-natural hosts (70, 93). However, the injections of exogenous recombinant IFN-α induced a rapid state of tolerance in vivo to this molecule. It is not excluded that one might need to treat for long periods of time with intermediate breaks to see an effect on chronic inflammation. The other possible explanation is that IFN-I levels are not different between pathogenic and non-pathogenic infections and/or that IFN-I are not the major culprits of the persistent ISG expression (68, 70). Other factors, such as IFN-γ, might contribute to ISG upregulation (68, 94). Collectively these comparative studies in distinct models indicate that IFN-I signaling is (i) beneficial during acute infection, (ii) a major contributor to early immune activation, (iii) alone insufficient to cause chronic immune activation, and (iv) its impact is highly context dependent.
Several other factors have been put forward to explain the absence of chronic inflammation in natural hosts. For example, by sequencing for the first time the genome of the SM, a mutation was uncovered in the gene encoding TLR4, the primary receptor for LPS, that yields a truncated protein and attenuated signaling (95). Intriguingly, this mutation was also observed in the TLR4 gene of the two other natural host species (AGM, mandrills) (95). This mutation might contribute to a lower monocyte/macrophage activation in natural hosts.
The maintenance of viral replication in LNs could impact systemic inflammation, due to the sheer immune “traffic” and recirculatory nature of immune cells. From this point of view, the fact that natural hosts strongly control viral replication in LN might contribute to their capacity to resolve inflammation. In this light, understanding the mechanisms by which HIV/SIV replication could be controlled in the LN is likely to be critical not only for viral eradication strategies but also for therapies aiming at reversing immune activation.
Target Cells for SIV in LNs from Natural Hosts as Compared to Pathogenic HIV and SIVmac Infections
Reducing the persistent HIV/SIV reservoir remains an essential milestone for the achievement of a functional cure for HIV-1 infection; however, this goal has been significantly hindered by poor means for identification of the CD4+ T cell subsets that harbor replication-competent virus, as well as by the anatomic location of these cells in sanctuaries for HIV. Several key differences in the nature of cells targeted by SIV in natural versus non-natural hosts have been identified, raising the fascinating hypothesis that the type of infected CD4+ T cells, even more than the quantity, could contribute to the different capacity to control immune activation and disease progression between the two hosts.
Central Memory CD4+ T Cells (TCM)
In vivo and in vitro comparative studies showed that the frequency of SIV-infected TCM in SM is significantly lower as compared to both CD4+ T effector memory cells of SM and CD4+ TCM of macaques in both blood and LN (32, 59). Thus, SM are partially protecting the important CD4+ TCM cell subset from SIV infection. In line with this relative preservation from viral infection, CD4+ TCM cells are more preserved in SIV-infected SM compared to SIV-infected rhesus macaques (46). CD4+ TCM cells are long-lived, self-renewing cells able to replenish the pool of non-self-renewing, shorter lived CD4+ effector memory cells, thus their maintenance is key for the homeostasis of the overall CD4 T cell compartment and immune memory. Remarkably, a low contribution of infected CD4+ TCM to the overall viral reservoir has similarly been described in (i) long-term non-progressors with protective HLA alleles (96); (ii) viremic non progressors, i.e., rare HIV-infected individuals who maintain high CD4+ T cell levels despite uncontrolled viremia (97); and (iii) post-treatment controllers, i.e., patients with a durable control of viremia after ART-interruption (98). With a distinct strategy, AGMs have also evolved to protect memory CD4+ T cells from viral infection. Indeed, CD4 molecules get downregulated from the surface of the CD4+ T cells when the latter get activated. Of note, these cells maintain their T helper functional activity (99).
The mechanisms of TCM protection are not clear. It has been suggested that CCR5 plays a role. Thus, CD4+ T cells from natural hosts express less CCR5 in blood, LN, and mucosae compared to humans and macaques (100, 101). It also has been shown that in vitro stimulation of SM CD4+ T cells, particularly the TCM, fail to upregulate CCR5 (32). CD4+ TCM cells expressing low levels of cell-surface CCR5 are less susceptible to SIV infection when compared to TCM of macaques both in vivo and in vitro (46, 102). However, SIV from natural hosts can also efficiently use other coreceptors than CCR5 to infect primary CD4+ T cells and other factors might as well be implicated in the relative preservation of TCM to infection in natural hosts (103). LN comprises a higher fraction of TCM compared to mucosal tissues, the latter containing higher proportions of effector cells in mammals (104). Thus it is possible that in natural hosts, the lower ratio of TCM infection is related to the control of viral replication in LN, whereas the predominant virus replication in the gut would explain why most virus infects CD4+ effector T cells in natural hosts. Altogether, the viral tissue distribution could thus in part also explain the lower frequency of infection rate in TCM compared to CD4+ effector T cells in natural hosts.
Follicular Helper T Cells (TFH)
TFH correspond to a subpopulation of memory CD4+ T cells expressing high levels of CXCR5 and PD-1 residing within the follicles of secondary lymphoid organs. They impact the activation, differentiation and survival of B cells. Several studies explored the frequencies, function, and infection rate of TFH cells in HIV-infected humans or SIV-infected macaques. They revealed that TFH cells are infected at high frequencies in chronic infection. Despite the high rate of HIV/SIVmac replication in TFH cells, these cells expand during HIV and SIVmac infections (45, 59, 105, 106). More recently, it was shown that TFH cells constitute an important source of persistent replication-competent virus in ART-treated, aviremic individuals (8). By contrast, a low infection rate of TFH cells has been described during non-pathogenic infection of SM (59) and AGM (49), where follicles often remain virus free. LN TFH cells showed lower levels of Ki-67 expression than non-TFH memory CD4+ T cells and fewer of the TFH cells expressed CCR5, but this was similar between macaques and natural hosts (59). Phenotypic studies on TFH cells in natural hosts are though limited so far and whether TFH cells in LN expand differently during SIV infection in natural hosts needs to be further investigated.
CD4+PD-1+CTLA-4+ T Cells
The contribution of TFH cells to the persistent reservoir progressively decreases with increased length of cART (8, 107), suggesting that other cell subsets, apart from TFH cells, can contribute to the magnitude of the pool of latently infected cells. In a recent study, it was found that PD-1+ cells, the subset that contributes most to TFH cells, were indeed the dominant contributors to the viral DNA pool in the B cell follicles in the LN in ART-treated SIV-infected macaques; however, CTLA-4+PD-1− memory CD4+ T-cells, a subset comprised predominantly of Tregs, were identified as a previously unrecognized component of the SIV reservoir (11). These cells are significantly enriched in SIV DNA in multiple tissue compartments, including the blood, LN, spleen, and gut and have been shown to harbor replication-competent and infectious virus (11). CTLA-4+PD-1− cells localized in the extra-follicular zones of the LN in ART-treated SIV-infected macaques and HIV-infected humans. Therefore, in addition to PD-1+ TFH cells, HIV-1 and SIVmac are able to establish and maintain viral persistence through the specific targeting of another CD4+ T cell subset, CTLA-4+PD-1− cells. These cells seem to have long living capacities (11). Further studies are needed to determine if the rare SIV-producing cells in the T zone of natural host’s LNs correspond, at least partially, to these CTLA-4+PD-1− cells.
Plasmacytoid Dendritic Cell
Unlike humans’ and macaques’ pDC, pDC from natural hosts display substantially lower CD4+ and CCR5+ surface expression (80). The lowered SIV receptor/coreceptor expression however does not affect the ability of SIVagm to infect pDCs. Indeed, high rates of pDC infection were detected in the spleen of AGM, to a similar high rate as pDC infection by HIV in cART-naïve humans (81).
Potential Immune-Mediated Mechanisms for Viral Control in LN: The Role OF CD8+ T and NK Cells
There are several clear lines of evidence that CD8+ T cells play an important role for the overall control of HIV-1/SIVmac replication (108, 109). Some of the most convincing evidences have been obtained in macaques and include a temporal correlation between the rise of SIV-specific CD8+ T cells and post-peak viremia decline, as well as the increase of viremia after in vivo depletion of CD8+ cells (110). Of note, most in vivo depletion studies used monoclonal antibodies that did not discriminate between CD8+ T and NK cells, and thus in some of these studies, the contribution of NK cells remained undetermined. Nonetheless, the role of CD8+ T cells in viral control is undeniable and is evident in HIV controllers and rhesus macaques with specific MHC alleles (111, 112).
CD8+ T cells in LN are generally located in the T cell zones. Early studies have revealed massive infiltrations of activated CD8+ T cells into B cell follicles in progressors, but this could be due to the disruption of the FDC network in late stage disease (113–116). Nevertheless, the magnitude of fully cytolytic CD8+ T cells was significantly higher in LN compared to blood (117), and HIV-1-specific CD8+ T cells are preferentially located in LN compared to blood, including a subset of responses that is present solely in secondary lymphoid organs (118). This preferential location of HIV-1-specific CD8+ T cells in the LN was also observed in chronically infected individuals on cART (118). These migrating CD8+ T cells localize to the extra-follicular zones of the LNs, where most of endogenous HIV-1-specific CTL were also observed, far from sites of virus replication inside the follicles (117). After in vivo depletion of CD8+ cells in SIVmac-infected macaques, the frequency of SIV-infected cells in extra-follicular regions increased and reached levels similar to that in B cell follicles (9) confirming that CD8+ T cells exert control of viral replication predominantly in the T cell zones. Until recently, it was considered that CD8+ T cells generally do not migrate into the B cell follicles and it was further suggested that antiretroviral drugs inefficiently diffuse into or are unequally distributed within LN (84), collectively making follicles a prime sanctuary for HIV/SIV replication. Nonetheless, a small proportion of CD8+ T cells expressing CXCR5+ has been recently described in both SIVmac and HIV-1 infections (119–121). The levels of these CXCR5+CD8+ T cells in LN were higher in HIV-infected individuals compared to healthy donors, and they were detected in close proximity to viral RNA+ cells, probably starting from primary infection on (119, 122). The frequency of SIV-specific CXCR5+CD8+ T cells correlated negatively with that of SIV infection in TFH cells and viremia, suggesting a role of CXCR5+CD8+ T cells in viral control (50). However, other studies highlighted a regulatory phenotype of CXCR5+CD8+ T cells with poor capacity of viral control which could further impair germinal center function in HIV infection (120, 123). Unfortunately, little is known about these recently described follicular CD8+ T cells, and whether the contrasting results are due to the presence of distinct CXCR5+CD8+ T cell subsets, differences in the infection models studied or other yet unknown factors.
In natural hosts, the contribution of CD8+ T cells to controlling SIV replication may be comparatively small. Indeed, although SIV-specific CD8+ T cell responses were observed for SIV-infected SM and AGM, their magnitude and breadth were similar or even lower than those generally observed in HIV-1 and SIVmac progressive infections both in blood and LN (124–127). However, it has been suggested that these responses appear temporally earlier in LN of natural hosts compared to pathogenic species and that this confers an advantage (56). Of note, these cells do not seem to migrate into follicles. CD8+ T cells from natural hosts were indeed found to be exclusively located in the T cell zones both in non-infected and SIV-infected animals (12). In line with this, CD8+ T cells in LN from AGM do not upregulate CXCR5 in response to SIV infection (49). To further address the question of the role(s) played by CD8+ T cells during natural host’s SIV infection, in vivo cell depletion experiments have been conducted. Administration of anti-CD8+ and anti-CD20+ antibodies during the first 2 weeks of SIVagmver90 infection in pig-tailed macaques (pathogenic infection) and AGM (non-pathogenic infection), led to dramatically different results in the two species (128). In pig-tailed macaques, a one-log increase in peak viremia and four-log increase in set-point viremia were observed following antibody administrations. Moreover, these animals rapidly progressed toward disease and displayed CMV reactivation. By strong contrast, in AGM, depletion of CD8+ and CD20+ cells did not modify peak viremia and the animals displayed only a minor delay in post-peak viremia decline compared to control animals, and all animals remained clinically healthy (128). In another study, treatment of SIVsm-infected SM using a CD8α-specific Ab (OKT8F) led to a profound depletion of CD8+ cells in both blood and tissues such as LN, but only minor changes in plasma viremia (129). Similar results were also observed in AGMs in which CD8+ cell depletion during the acute phase led only to a delay of 5–10 days in the post-peak viral decline (130). By contrast, virtually all CD8+ in vivo depletion studies conducted in non-natural host models during acute or chronic SIV infection have reported significant increases in viral loads and rapid disease progression (110, 131–133). Altogether, these data highlight that while CTL responses can play a large role in HIV controllers, they may contribute only modestly to the control of viral replication in LN in natural hosts. Thus, while CD8+ T cells might still be involved to some extent in the control of viral replication in the T cell zone, they most likely do not represent the major cellular component of viral control in LN follicles during SIV infection in natural hosts.
As an alternative to CD8+ T cells, multiple lines of evidence pointed toward a role of NK cells in the control of SIV replication in LN of natural hosts. Upon SIV infection, AGM temporarily display high levels of IFN-α and IL-15 in the plasma (70). These cytokines are known to activate NK cells and enhance their cytotoxic profile (134, 135). Plasma IFN-α levels correlated indeed with activation and cytotoxic activity (CD107a) of NK cells and plasma IL-15 with the proliferation (Ki-67) of NK cells in LN during acute SIVagm infection (70). During the acute phase of SIVagm infection, CD107a+ NK cells increased to higher levels in LN than in blood (70). Studies in SM demonstrated a more rapid activation of NK cells compared to macaques (136, 137). These previous studies raised the hypothesis that NK cells may play a role in LN viral control in natural hosts. It was subsequently shown that upon SIVagm infection, NK cells change their distribution within LN and migrate into follicles, where they accumulate (49). The increase of NK cell numbers in follicles was associated with a high production of IL-15 within follicles, presented in membrane-bound form by FDC and antigen-presenting-like cells (49). By contrast, the number of functionally competent NK cells in LN decrease in macaques in response to SIV infection (49, 138). The pattern of LN homing receptors (CX3CR1, CD62L, CXCR3, CCR7) were similar on NK cells from SIV-infected AGM and MAC and do not explain the higher levels of NK cells in LN of AGM as compared to MAC (49). It is more likely that in SIVagm infection, the IL-15 in the follicles enhances the survival of NK cells. Interestingly, SIVagm-infected AGM showed high levels of CXCR5+ NK cells in LN (49). This suggests that migration of NK cells into AGM follicles was CXCR5-mediated. The presence of CXCR5+ NK cells was observed in secondary lymphoid organs (LN, spleen), but not in blood or gut of SIV-infected AGM. Thus, the CXCR5 expression on NK cells during SIVagm infection was tissue-specific. Of note, this enrichment of CXCR5+ NK cells in secondary lymphoid organs was not observed in SIVmac-infected macaques. Strikingly, IL-15-mediated depletion of NK cells in chronic SIVagm infection led to high viral replication in the follicles as well as in the T zones (49). These results indicate that TFH cells are not resistant to SIV infection in AGM and clearly reveal a crucial role for NK cells in the viral control within LN of a natural host.
Concluding Remarks
Herein, we summarize current knowledge on differences in LN of non-natural versus natural hosts. The remarkable control and clearance of virus from lymphoid follicles in natural hosts is associated with multiple differences compared to pathogenic infection: (1) LN architecture is preserved; (2) inflammation is controlled; (3) FDC network is maintained intact; (4) rapid mobilization of innate antiviral responses; (5) viral replication is strongly controlled; (6) TFH are particularly spared from virus; (7) NK cells migrate into follicles; and (8) high IL-15 production within follicles (Table 1). Collectively, natural hosts have developed mechanisms of protection for the most vulnerable lymphoid CD4+ T cell subsets: CD4+ TCM, TFH cells in LN, and Th17 cells in gut (35, 139). Better preservation of these cells likely influences the preservation of intact lymphoid structures, immune competencies, and immune memory (49, 55). As a control model for lentivirus infections, we must ask how we might exploit the knowledge garnered from natural host research. Given the IL-15-dependent accumulation of NK cells (and potentially CD8+ T cells) in natural hosts into follicles, this could be envisioned therapeutically to recapitulate virus clearance in pathogenic hosts and HIV patients. Multiple oncology studies are now exploring the utility of IL-15 superagonists and heterodimers to expand both CD8+ T cells and NK cells and recent studies evaluated these molecules in the SIV macaque model (140–143). Additional cytokine therapeutics (i.e., IL-21 and IFN-α) could also be attractive targets to mimic or induce the conditions in natural hosts that are conducive to virus clearance in the LNs. Recently, the use of NKG2A inhibitors has also been suggested as an attractive approach in HIV cure strategies (144). Many open questions remain, including delineation of factors responsible for the high IL-15 production in LN follicles, the maintenance of an intact FDC network, the upregulation of CXCR5 on NK cells in LN and the very rapid innate antiviral responses in natural hosts. The remaining gaps in the knowledge base will require future studies to understand how natural hosts reduce inflammation and how they protect LN architecture. Such ongoing studies are hoped to direct future strategies aimed at granting permissive entry of relevant effector cells into the highly restricted lymphoid follicles, thus creating a unique opportunity for reservoir clearance and representing a further step toward HIV remission and cure. Altogether, studies in natural hosts of SIV continue to reveal clues highly relevant for understanding and managing HIV infection in humans.
Author Contributions
NH, SB, MP, RR, and MM-T wrote the review. NH designed the figure and the table. RR and MM-T edited the text. MM-T composed and oversaw the chapters.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The handling Editor declared a shared affiliation, though no other collaboration, with several of the authors SB, MP.
Funding
NH was recipient of a fellowship from the French Vaccine Research Institute funded by the National Agency of Research (ANR) under reference ANR-10-LABX-77. The Infectious Disease Models and Innovative Therapies (IDMIT) center in Fontenay-aux-Roses, France, is funded by the French government’s Investissements d’Avenir program for infrastructures (PIA) under grant ANR-11-INBS-0008 and the PIA grant ANR-10-EQPX-02-01. MM-T received a grant from the French Agency of AIDS Research, ANRS (AO 2017-2), and a donation from the L’OREAL Foundation. SB is supported by NIH grants U24-AI120134, UM1-AI124436, and R21-AI118542. RR is supported by National Institutes of Health (NIH) grants RO1 DE026014 and RO1 AI120828. MP is supported by NIH grants R01AI-110334, R01AI-116379, R33AI-104278, and R33AI116171.
References
1. Broder S. The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res (2010) 85:1. doi:10.1016/j.antiviral.2009.10.002
2. May MT, Gompels M, Delpech V, Porter K, Orkin C, Kegg S, et al. Impact on life expectancy of HIV-1 positive individuals of CD4+ cell count and viral load response to antiretroviral therapy. AIDS (2014) 28:1193–202. doi:10.1097/QAD.0000000000000243
3. Alexaki A, Liu Y, Wigdahl B. Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res (2008) 6:388–400. doi:10.2174/157016208785861195
4. Martinez-Picado J, Deeks SG. Persistent HIV-1 replication during antiretroviral therapy. Curr Opin HIV AIDS (2016) 11:417–23. doi:10.1097/COH.0000000000000287
5. Rothenberger MK, Keele BF, Wietgrefe SW, Fletcher CV, Beilman GJ, Chipman JG, et al. Large number of rebounding/founder HIV variants emerge from multifocal infection in lymphatic tissues after treatment interruption. Proc Natl Acad Sci U S A (2015) 112:E1126–34. doi:10.1073/pnas.1414926112
6. Estes JD, Kityo C, Ssali F, Swainson L, Makamdop KN, Del Prete GQ, et al. Defining total-body AIDS-virus burden with implications for curative strategies. Nat Med (2017) 23(11):1271–6. doi:10.1038/nm.4411
7. Santangelo PJ, Rogers KA, Zurla C, Blanchard EL, Gumber S, Strait K, et al. Whole-body immunoPET reveals active SIV dynamics in viremic and antiretroviral therapy-treated macaques. Nat Methods (2015) 12:427–32. doi:10.1038/nmeth.3320
8. Banga R, Procopio FA, Noto A, Pollakis G, Cavassini M, Ohmiti K, et al. PD-1(+) and follicular helper T cells are responsible for persistent HIV-1 transcription in treated aviremic individuals. Nat Med (2016) 22(7):754–61. doi:10.1038/nm.4113
9. Fukazawa Y, Lum R, Okoye AA, Park H, Matsuda K, Bae JY, et al. B cell follicle sanctuary permits persistent productive simian immunodeficiency virus infection in elite controllers. Nat Med (2015) 21:132–9. doi:10.1038/nm.3781
10. Chowdhury A, Del Rio Estrada PM, Tharp GK, Trible RP, Amara RR, Chahroudi A, et al. Decreased T follicular regulatory cell/T follicular helper cell (TFH) in simian immunodeficiency virus-infected rhesus macaques may contribute to accumulation of TFH in chronic infection. J Immunol (2015) 195:3237–47. doi:10.4049/jimmunol.1402701
11. McGary CS, Deleage C, Harper J, Micci L, Ribeiro SP, Paganini S, et al. CTLA-4(+)PD-1(-) memory CD4(+) T cells critically contribute to viral persistence in antiretroviral therapy-suppressed, SIV-infected rhesus macaques. Immunity (2017) 47:776–88.e5. doi:10.1016/j.immuni.2017.09.018
12. Diop OM, Gueye A, Dias-Tavares M, Kornfeld C, Faye A, Ave P, et al. High levels of viral replication during primary simian immunodeficiency virus SIVagm infection are rapidly and strongly controlled in African green monkeys. J Virol (2000) 74:7538–47. doi:10.1128/JVI.74.16.7538-7547.2000
13. Broussard SR, Staprans SI, White R, Whitehead EM, Feinberg MB, Allan JS. Simian immunodeficiency virus replicates to high levels in naturally infected African green monkeys without inducing immunologic or neurologic disease. J Virol (2001) 75:2262–75. doi:10.1128/JVI.75.5.2262-2275.2001
14. Goldstein S, Ourmanov I, Brown CR, Beer BE, Elkins WR, Plishka R, et al. Wide range of viral load in healthy African green monkeys naturally infected with simian immunodeficiency virus. J Virol (2000) 74:11744–53. doi:10.1128/JVI.74.24.11744-11753.2000
15. Onanga R, Kornfeld C, Pandrea I, Estaquier J, Souquière S, Rouquet P, et al. High levels of viral replication contrast with only transient changes in CD4(+) and CD8(+) cell numbers during the early phase of experimental infection with simian immunodeficiency virus SIVmnd-1 in Mandrillus sphinx. J Virol (2002) 76:10256–63. doi:10.1128/JVI.76.20.10256-10263.2002
16. Rey-Cuillé MA, Berthier JL, Bomsel-Demontoy MC, Chaduc Y, Montagnier L, Hovanessian AG, et al. Simian immunodeficiency virus replicates to high levels in sooty mangabeys without inducing disease. J Virol (1998) 72:3872–86.
17. Sodora DL, Allan JS, Apetrei C, Brenchley JM, Douek DC, Else JG, et al. Toward an AIDS vaccine: lessons from natural simian immunodeficiency virus infections of African nonhuman primate hosts. Nat Med (2009) 15:861–5. doi:10.1038/nm.2013
18. Gordon SN, Klatt NR, Bosinger SE, Brenchley JM, Milush JM, Engram JC, et al. Severe depletion of mucosal CD4+ T cells in AIDS-free simian immunodeficiency virus-infected sooty mangabeys. J Immunol (2007) 179:3026–34. doi:10.4049/jimmunol.179.5.3026
19. Mattapallil JJ, Douek DC, Hill B, Nishimura Y, Martin M, Roederer M. Massive infection and loss of memory CD4+ T cells in multiple tissues during acute SIV infection. Nature (2005) 434:1093–7. doi:10.1038/nature03501
20. Pandrea IV, Gautam R, Ribeiro RM, Brenchley JM, Butler IF, Pattison M, et al. Acute loss of intestinal CD4+ T cells is not predictive of simian immunodeficiency virus virulence. J Immunol (2007) 179:3035–46. doi:10.4049/jimmunol.179.5.3035
21. Veazey RS, DeMaria M, Chalifoux LV, Shvetz DE, Pauley DR, Knight HL, et al. Gastrointestinal tract as a major site of CD4+ T cell depletion and viral replication in SIV infection. Science (1998) 280:427–31. doi:10.1126/science.280.5362.427
22. Calascibetta F, Micci L, Carnathan D, Lawson B, Vanderford TH, Bosinger SE, et al. Antiretroviral therapy in simian immunodeficiency virus-infected sooty mangabeys: implications for AIDS pathogenesis. J Virol (2016) 90:7541–51. doi:10.1128/JVI.00598-16
23. Gueye A, Diop OM, Ploquin MJY, Kornfeld C, Faye A, Cumont M-C, et al. Viral load in tissues during the early and chronic phase of non-pathogenic SIVagm infection. J Med Primatol (2004) 33:83–97. doi:10.1111/j.1600-0684.2004.00057.x
24. Brenchley JM, Paiardini M, Knox KS, Asher AI, Cervasi B, Asher TE, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood (2008) 112:2826–35. doi:10.1182/blood-2008-05-159301
25. Favre D, Lederer S, Kanwar B, Ma Z-M, Proll S, Kasakow Z, et al. Critical loss of the balance between Th17 and T regulatory cell populations in pathogenic SIV infection. PLoS Pathog (2009) 5:e1000295. doi:10.1371/journal.ppat.1000295
26. Estes JD, Harris LD, Klatt NR, Tabb B, Pittaluga S, Paiardini M, et al. Damaged intestinal epithelial integrity linked to microbial translocation in pathogenic simian immunodeficiency virus infections. PLoS Pathog (2010) 6:e1001052. doi:10.1371/journal.ppat.1001052
27. Brenchley JM, Price DA, Schacker TW, Asher TE, Silvestri G, Rao S, et al. Microbial translocation is a cause of systemic immune activation in chronic HIV infection. Nat Med (2006) 12:1365–71. doi:10.1038/nm1511
28. Dinh DM, Volpe GE, Duffalo C, Bhalchandra S, Tai AK, Kane AV, et al. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J Infect Dis (2015) 211:19–27. doi:10.1093/infdis/jiu409
29. Zaunders J, Danta M, Bailey M, Mak G, Marks K, Seddiki N, et al. CD4+T follicular helper and IgA+B cell numbers in gut biopsies from HIV-infected subjects on antiretroviral therapy are similar to HIV-uninfected individuals. Front Immunol (2016) 7:438. doi:10.3389/fimmu.2016.00438
30. Estes JD. Pathobiology of HIV/SIV-associated changes in secondary lymphoid tissues. Immunol Rev (2013) 254:65–77. doi:10.1111/imr.12070
31. Zeng M, Smith AJ, Wietgrefe SW, Southern PJ, Schacker TW, Reilly CS, et al. Cumulative mechanisms of lymphoid tissue fibrosis and T cell depletion in HIV-1 and SIV infections. J Clin Invest (2011) 121:998–1008. doi:10.1172/JCI45157
32. Paiardini M, Cervasi B, Reyes-Aviles E, Micci L, Ortiz AM, Chahroudi A, et al. Low levels of SIV infection in sooty mangabey central memory CD4+ T cells are associated with limited CCR5 expression. Nat Med (2011) 17:830–6. doi:10.1038/nm.2395
33. Müller MC, Barré-Sinoussi F. SIVagm: genetic and biological features associated with replication. Front Biosci (2003) 8:d1170–85. doi:10.2741/1130
34. Pandrea I, Sodora DL, Silvestri G, Apetrei C. Into the wild: simian immunodeficiency virus (SIV) infection in natural hosts. Trends Immunol (2008) 29:419–28. doi:10.1016/j.it.2008.05.004
35. Paiardini M, Müller-Trutwin M. HIV-associated chronic immune activation. Immunol Rev (2013) 254:78–101. doi:10.1111/imr.12079
36. Bosinger SE, Sodora DL, Silvestri G. Generalized immune activation and innate immune responses in simian immunodeficiency virus infection. Curr Opin HIV AIDS (2011) 6:411–8. doi:10.1097/COH.0b013e3283499cf6
37. Mir KD, Gasper MA, Sundaravaradan V, Sodora DL. SIV infection in natural hosts: resolution of immune activation during the acute-to-chronic transition phase. Microbes Infect (2011) 13:14–24. doi:10.1016/j.micinf.2010.09.011
38. Ploquin MJ, Silvestri G, Müller-Trutwin M. Immune activation in HIV infection: what can the natural hosts of simian immunodeficiency virus teach us? Curr Opin HIV AIDS (2016) 11(2):201–8. doi:10.1097/COH.0000000000000238
39. Huot N, Rascle P, Garcia-Tellez T, Jacquelin B, Müller-Trutwin M. Innate immune cell responses in non pathogenic versus pathogenic SIV infections. Curr Opin Virol (2016) 19:37–44. doi:10.1016/j.coviro.2016.06.011
40. Deleage C, Turkbey B, Estes JD. Imaging lymphoid tissues in nonhuman primates to understand SIV pathogenesis and persistence. Curr Opin Virol (2016) 19:77–84. doi:10.1016/j.coviro.2016.07.002
41. Chakrabarti L, Cumont MC, Montagnier L, Hurtrel B. Variable course of primary simian immunodeficiency virus infection in lymph nodes: relation to disease progression. J Virol (1994) 68:6634–43.
42. Hong JJ, Chang K-T, Villinger F. The dynamics of T and B cells in lymph node during chronic HIV infection: TFH and HIV, unhappy dance partners? Front Immunol (2016) 7:522. doi:10.3389/fimmu.2016.00522
43. Brenchley JM, Paiardini M. Immunodeficiency lentiviral infections in natural and non-natural hosts. Blood (2011) 118:847–54. doi:10.1182/blood-2010-12-325936
44. Chahroudi A, Bosinger SE, Vanderford TH, Paiardini M, Silvestri G. Natural SIV hosts: showing AIDS the door. Science (2012) 335:1188–93. doi:10.1126/science.1217550
45. Petrovas C, Yamamoto T, Gerner MY, Boswell KL, Wloka K, Smith EC, et al. CD4 T follicular helper cell dynamics during SIV infection. J Clin Invest (2012) 122:3281–94. doi:10.1172/JCI63039
46. McGary CS, Cervasi B, Chahroudi A, Micci L, Taaffe J, Meeker T, et al. Increased stability and limited proliferation of CD4+ central memory T cells differentiate nonprogressive simian immunodeficiency virus (SIV) infection of sooty mangabeys from progressive SIV infection of rhesus macaques. J Virol (2014) 88:4533–42. doi:10.1128/JVI.03515-13
47. Xu H, Wang X, Malam N, Lackner AA, Veazey RS. Persistent simian immunodeficiency virus infection causes ultimate depletion of follicular Th cells in AIDS. J Immunol (2015) 195:4351–7. doi:10.4049/jimmunol.1501273
48. Norley S, Kurth R. The role of the immune response during SIVagm infection of the African green monkey natural host. Front Biosci (2004) 9:550–64. doi:10.2741/1219
49. Huot N, Jacquelin B, Garcia-Tellez T, Rascle P, Ploquin MJ, Madec Y, et al. Natural killer cells migrate into and control simian immunodeficiency virus replication in lymph node follicles in African green monkeys. Nat Med (2017) 23(11):1277–86. doi:10.1038/nm.4421
50. Mylvaganam GH, Rios D, Abdelaal HM, Iyer S, Tharp G, Mavigner M, et al. Dynamics of SIV-specific CXCR5+ CD8 T cells during chronic SIV infection. Proc Natl Acad Sci U S A (2017) 114:1976–81. doi:10.1073/pnas.1621418114
51. Ma Z-M, Dutra J, Fritts L, Miller CJ. Lymphatic dissemination of simian immunodeficiency virus after penile inoculation. J Virol (2016) 90:4093–104. doi:10.1128/JVI.02947-15
52. Heesters BA, Lindqvist M, Vagefi PA, Scully EP, Schildberg FA, Altfeld M, et al. Follicular dendritic cells retain infectious HIV in cycling endosomes. PLoS Pathog (2015) 11:e1005285. doi:10.1371/journal.ppat.1005285
53. Racz P, Tenner-Racz K, Schmidt H. Follicular dendritic cells in HIV-induced lymphadenopathy and AIDS. APMIS Suppl (1989) 8:16–23.
54. Beer B, Scherer J, zur Megede J, Norley S, Baier M, Kurth R. Lack of dichotomy between virus load of peripheral blood and lymph nodes during long-term simian immunodeficiency virus infection of African green monkeys. Virology (1996) 219:367–75. doi:10.1006/viro.1996.0262
55. Martinot AJ, Meythaler M, Pozzi L-A, Dalecki Boisvert K, Knight H, Walsh D, et al. Acute SIV infection in sooty mangabey monkeys is characterized by rapid virus clearance from lymph nodes and absence of productive infection in germinal centers. PLoS One (2013) 8:e57785. doi:10.1371/journal.pone.0057785
56. Meythaler M, Wang Z, Martinot A, Pryputniewicz S, Kasheta M, McClure HM, et al. Early induction of polyfunctional simian immunodeficiency virus (SIV)-specific T lymphocytes and rapid disappearance of SIV from lymph nodes of sooty mangabeys during primary infection. J Immunol (2011) 186:5151–61. doi:10.4049/jimmunol.1004110
57. Goldstein S, Ourmanov I, Brown CR, Plishka R, Buckler-White A, Byrum R, et al. Plateau levels of viremia correlate with the degree of CD4+-T-cell loss in simian immunodeficiency virus SIVagm-infected pigtailed macaques: variable pathogenicity of natural SIVagm isolates. J Virol (2005) 79:5153–62. doi:10.1128/JVI.79.8.5153-5162.2005
58. Joling P, van Wichen DF, Parmentier HK, Biberfeld P, Böttiger D, Tschopp J, et al. Simian immunodeficiency virus (SIVsm) infection of cynomolgus monkeys: effects on follicular dendritic cells in lymphoid tissue. AIDS Res Hum Retroviruses (1992) 8:2021–30. doi:10.1089/aid.1992.8.2021
59. Brenchley JM, Vinton C, Tabb B, Hao XP, Connick E, Paiardini M, et al. Differential infection patterns of CD4+ T cells and lymphoid tissue viral burden distinguish progressive and nonprogressive lentiviral infections. Blood (2012) 120:4172–81. doi:10.1182/blood-2012-06-437608
60. Klatt NR, Estes JD, Sun X, Ortiz AM, Barber JS, Harris LD, et al. Loss of mucosal CD103+ DCs and IL-17+ and IL-22+ lymphocytes is associated with mucosal damage in SIV infection. Mucosal Immunol (2012) 5:646–57. doi:10.1038/mi.2012.38
61. Pallikkuth S, Micci L, Ende ZS, Iriele RI, Cervasi B, Lawson B, et al. Maintenance of intestinal Th17 cells and reduced microbial translocation in SIV-infected rhesus macaques treated with interleukin (IL)-21. PLoS Pathog (2013) 9:e1003471. doi:10.1371/journal.ppat.1003471
62. Chakrabarti LA, Lewin SR, Zhang L, Gettie A, Luckay A, Martin LN, et al. Normal T-cell turnover in sooty mangabeys harboring active simian immunodeficiency virus infection. J Virol (2000) 74:1209–23. doi:10.1128/JVI.74.3.1209-1223.2000
63. Kornfeld C, Ploquin MJ-Y, Pandrea I, Faye A, Onanga R, Apetrei C, et al. Antiinflammatory profiles during primary SIV infection in African green monkeys are associated with protection against AIDS. J Clin Invest (2005) 115:1082–91. doi:10.1172/JCI23006C1
64. Silvestri G, Sodora DL, Koup RA, Paiardini M, O’Neil SP, McClure HM, et al. Nonpathogenic SIV infection of sooty mangabeys is characterized by limited bystander immunopathology despite chronic high-level viremia. Immunity (2003) 18:441–52. doi:10.1016/S1074-7613(03)00060-8
65. Bosinger SE, Utay NS. Type I interferon: understanding its role in HIV pathogenesis and therapy. Curr HIV/AIDS Rep (2015) 12:41–53. doi:10.1007/s11904-014-0244-6
66. Diop OM, Ploquin MJ-Y, Mortara L, Faye A, Jacquelin B, Kunkel D, et al. Plasmacytoid dendritic cell dynamics and alpha interferon production during Simian immunodeficiency virus infection with a nonpathogenic outcome. J Virol (2008) 82:5145–52. doi:10.1128/JVI.02433-07
67. Estes JD, Gordon SN, Zeng M, Chahroudi AM, Dunham RM, Staprans SI, et al. Early resolution of acute immune activation and induction of PD-1 in SIV-infected sooty mangabeys distinguishes nonpathogenic from pathogenic infection in rhesus macaques. J Immunol (2008) 180:6798–807. doi:10.4049/jimmunol.180.10.6798
68. Jacquelin B, Mayau V, Targat B, Liovat A-S, Kunkel D, Petitjean G, et al. Nonpathogenic SIV infection of African green monkeys induces a strong but rapidly controlled type I IFN response. J Clin Invest (2009) 119:3544–55. doi:10.1172/JCI40093
69. Bosinger SE, Li Q, Gordon SN, Klatt NR, Duan L, Xu L, et al. Global genomic analysis reveals rapid control of a robust innate response in SIV-infected sooty mangabeys. J Clin Invest (2009) 119:3556–72. doi:10.1172/JCI40115
70. Jacquelin B, Petitjean G, Kunkel D, Liovat A-S, Jochems SP, Rogers KA, et al. Innate immune responses and rapid control of inflammation in African green monkeys treated or not with interferon-alpha during primary SIVagm infection. PLoS Pathog (2014) 10:e1004241. doi:10.1371/journal.ppat.1004241
71. Barouch DH, Ghneim K, Bosche WJ, Li Y, Berkemeier B, Hull M, et al. Rapid inflammasome activation following mucosal SIV infection of rhesus monkeys. Cell (2016) 165:656–67. doi:10.1016/j.cell.2016.03.021
72. Hyrcza MD, Kovacs C, Loutfy M, Halpenny R, Heisler L, Yang S, et al. Distinct transcriptional profiles in ex vivo CD4+ and CD8+ T cells are established early in human immunodeficiency virus type 1 infection and are characterized by a chronic interferon response as well as extensive transcriptional changes in CD8+ T cells. J Virol (2007) 81:3477–86. doi:10.1128/JVI.01552-06
73. Brown KN, Wijewardana V, Liu X, Barratt-Boyes SM. Rapid influx and death of plasmacytoid dendritic cells in lymph nodes mediate depletion in acute simian immunodeficiency virus infection. PLoS Pathog (2009) 5:e1000413. doi:10.1371/journal.ppat.1000413
74. Bruel T, Dupuy S, Démoulins T, Rogez-Kreuz C, Dutrieux J, Corneau A, et al. Plasmacytoid dendritic cell dynamics tune interferon-alfa production in SIV-infected cynomolgus macaques. PLoS Pathog (2014) 10:e1003915. doi:10.1371/journal.ppat.1003915
75. Harris LD, Tabb B, Sodora DL, Paiardini M, Klatt NR, Douek DC, et al. Downregulation of robust acute type I interferon responses distinguishes nonpathogenic simian immunodeficiency virus (SIV) infection of natural hosts from pathogenic SIV infection of rhesus macaques. J Virol (2010) 84:7886–91. doi:10.1128/JVI.02612-09
76. Malleret B, Manéglier B, Karlsson I, Lebon P, Nascimbeni M, Perié L, et al. Primary infection with simian immunodeficiency virus: plasmacytoid dendritic cell homing to lymph nodes, type I interferon, and immune suppression. Blood (2008) 112:4598–608. doi:10.1182/blood-2008-06-162651
77. Kwa S, Kannanganat S, Nigam P, Siddiqui M, Shetty RD, Armstrong W, et al. Plasmacytoid dendritic cells are recruited to the colorectum and contribute to immune activation during pathogenic SIV infection in rhesus macaques. Blood (2011) 118:2763–73. doi:10.1182/blood-2011-02-339515
78. Reeves RK, Evans TI, Gillis J, Wong FE, Kang G, Li Q, et al. SIV infection induces accumulation of plasmacytoid dendritic cells in the gut mucosa. J Infect Dis (2012) 206:1462–8. doi:10.1093/infdis/jis408
79. Bosinger SE, Johnson ZP, Folkner KA, Patel N, Hashempour T, Jochems SP, et al. Intact type I Interferon production and IRF7 function in sooty mangabeys. PLoS Pathog (2013) 9:e1003597. doi:10.1371/journal.ppat.1003597
80. Jochems SP, Petitjean G, Kunkel D, Liovat A-S, Ploquin MJ, Barré-Sinoussi F, et al. Modulation of type I interferon-associated viral sensing during acute simian immunodeficiency virus infection in African green monkeys. J Virol (2015) 89:751–62. doi:10.1128/JVI.02430-14
81. Jochems SP, Jacquelin B, Chauveau L, Huot N, Petitjean G, Lepelley A, et al. Plasmacytoid dendritic cell infection and sensing capacity during pathogenic and nonpathogenic simian immunodeficiency virus infection. J Virol (2015) 89:6918–27. doi:10.1128/JVI.00332-15
82. Sandler NG, Bosinger SE, Estes JD, Zhu RTR, Tharp GK, Boritz E, et al. Type I interferon responses in rhesus macaques prevent SIV infection and slow disease progression. Nature (2014) 511:601–5. doi:10.1038/nature13554
83. Heikenwalder M, Polymenidou M, Junt T, Sigurdson C, Wagner H, Akira S, et al. Lymphoid follicle destruction and immunosuppression after repeated CpG oligodeoxynucleotide administration. Nat Med (2004) 10:187–92. doi:10.1038/nm987
84. Estes JD, Haase AT, Schacker TW. The role of collagen deposition in depleting CD4+ T cells and limiting reconstitution in HIV-1 and SIV infections through damage to the secondary lymphoid organ niche. Semin Immunol (2008) 20:181–6. doi:10.1016/j.smim.2008.04.002
85. Teijaro JR, Ng C, Lee AM, Sullivan BM, Sheehan KCF, Welch M, et al. Persistent LCMV infection is controlled by blockade of type I interferon signaling. Science (2013) 340:207–11. doi:10.1126/science.1235214
86. Wilson EB, Yamada DH, Elsaesser H, Herskovitz J, Deng J, Cheng G, et al. Blockade of chronic type I interferon signaling to control persistent LCMV infection. Science (2013) 340(6129):202–7. doi:10.1126/science.1235208
87. Ng CT, Sullivan BM, Teijaro JR, Lee AM, Welch M, Rice S, et al. Blockade of interferon beta, but not interferon alpha, signaling controls persistent viral infection. Cell Host Microbe (2015) 17(5):653–61. doi:10.1016/j.chom.2015.04.005
88. Cheng L, Yu H, Li G, Li F, Ma J, Li J, et al. Type I interferons suppress viral replication but contribute to T cell depletion and dysfunction during chronic HIV-1 infection. JCI Insight (2017) 2:94366. doi:10.1172/jci.insight.94366
89. Zhen A, Rezek V, Youn C, Lam B, Chang N, Rick J, et al. Targeting type I interferon-mediated activation restores immune function in chronic HIV infection. J Clin Invest (2017) 127:260–8. doi:10.1172/JCI89488
90. Azzoni L, Foulkes AS, Papasavvas E, Mexas AM, Lynn KM, Mounzer K, et al. Improved treatment for primary HIV infection by interferon-alfa therapy? Does HCV treatment in HIV/HCV coinfected patients help us to test this hypothesis? Reply to zur Wiesch and van Lunzen. J Infect Dis (2013) 208:363. doi:10.1093/infdis/jit160
91. Hua S, Vigano S, Tse S, Zhengyu O, Harrington S, Negron J, et al. Pegylated IFN-α-induced NK cell activation is associated with HIV-1 DNA decline in ART-treated HIV-1/HCV co-infected patients. Clin Infect Dis (2017). doi:10.1093/cid/cix1111
92. Sun H, Buzon MJ, Shaw A, Berg RK, Yu XG, Ferrando-Martinez S, et al. Hepatitis C therapy with interferon-α and ribavirin reduces CD4 T-cell-associated HIV-1 DNA in HIV-1/hepatitis C virus-coinfected patients. J Infect Dis (2014) 209:1315–20. doi:10.1093/infdis/jit628
93. Vanderford TH, Slichter C, Rogers KA, Lawson BO, Obaede R, Else J, et al. Treatment of SIV-infected sooty mangabeys with a type-I IFN agonist results in decreased virus replication without inducing hyperimmune activation. Blood (2012) 119:5750–7. doi:10.1182/blood-2012-02-411496
94. Echebli N, Tchitchek N, Dupuy S, Bruel T, Peireira Bittencourt Passaes C, Bosquet N, et al. Stage-specific IFN-induced and IFN gene expression reveal convergence of type I and type II IFN and highlight their role in both acute and chronic stage of pathogenic SIV infection. PLoS One (2018) 13:e0190334. doi:10.1371/journal.pone.0190334
95. Palesch D, Bosinger SE, Tharp GK, Vanderford TH, Paiardini M, Chahroudi A, et al. Sooty mangabey genome sequence provides insight into AIDS resistance in a natural SIV host. Nature (2018) 553:77–81. doi:10.1038/nature25140
96. Descours B, Avettand-Fenoel V, Blanc C, Samri A, Mélard A, Supervie V, et al. Immune responses driven by protective human leukocyte antigen alleles from long-term nonprogressors are associated with low HIV reservoir in central memory CD4 T cells. Clin Infect Dis (2012) 54:1495–503. doi:10.1093/cid/cis188
97. Klatt NR, Bosinger SE, Peck M, Richert-Spuhler LE, Heigele A, Gile JP, et al. Limited HIV infection of central memory and stem cell memory CD4+ T cells is associated with lack of progression in viremic individuals. PLoS Pathog (2014) 10:e1004345. doi:10.1371/journal.ppat.1004345
98. Sáez-Cirión A, Bacchus C, Hocqueloux L, Avettand-Fenoel V, Girault I, Lecuroux C, et al. Post-treatment HIV-1 controllers with a long-term virological remission after the interruption of early initiated antiretroviral therapy ANRS VISCONTI study. PLoS Pathog (2013) 9:e1003211. doi:10.1371/journal.ppat.1003211
99. Vinton CL, Ortiz AM, Calantone N, Mudd JC, Deleage C, Morcock DR, et al. Cytotoxic T cell functions accumulate when CD4 is downregulated by CD4+ T cells in African green monkeys. J Immunol (2017) 198:4403–12. doi:10.4049/jimmunol.1700136
100. Pandrea I, Apetrei C, Gordon S, Barbercheck J, Dufour J, Bohm R, et al. Paucity of CD4+CCR5+ T cells is a typical feature of natural SIV hosts. Blood (2007) 109:1069–76. doi:10.1182/blood-2006-05-024364
101. Pandrea I, Onanga R, Souquiere S, Mouinga-Ondéme A, Bourry O, Makuwa M, et al. Paucity of CD4+ CCR5+ T cells may prevent transmission of simian immunodeficiency virus in natural nonhuman primate hosts by breast-feeding. J Virol (2008) 82:5501–9. doi:10.1128/JVI.02555-07
102. Riddick NE, Wu F, Matsuda K, Whitted S, Ourmanov I, Goldstein S, et al. Simian immunodeficiency virus SIVagm efficiently utilizes non-CCR5 entry pathways in African green monkey lymphocytes: potential role for GPR15 and CXCR6 as viral coreceptors. J Virol (2015) 90:2316–31. doi:10.1128/JVI.02529-15
103. Wetzel KS, Yi Y, Elliott ST, Romero D, Jacquelin B, Hahn BH, et al. CXCR6-mediated simian immunodeficiency virus SIVagmSab entry into sabaeus African green monkey lymphocytes implicates widespread use of non-CCR5 pathways in natural host infections. J Virol (2017) 91(4):e01626–16. doi:10.1128/JVI.01626-16
104. Sathaliyawala T, Kubota M, Yudanin N, Turner D, Camp P, Thome JJC, et al. Distribution and compartmentalization of human circulating and tissue-resident memory T cell subsets. Immunity (2013) 38:187–97. doi:10.1016/j.immuni.2012.09.020
105. Lindqvist M, van Lunzen J, Soghoian DZ, Kuhl BD, Ranasinghe S, Kranias G, et al. Expansion of HIV-specific T follicular helper cells in chronic HIV infection. J Clin Invest (2012) 122:3271–80. doi:10.1172/JCI64314
106. Perreau M, Savoye A-L, Crignis ED, Corpataux J-M, Cubas R, Haddad EK, et al. Follicular helper T cells serve as the major CD4 T cell compartment for HIV-1 infection, replication, and production. J Exp Med (2013) 210:143–56. doi:10.1084/jem.20121932
107. Paiardini M, Lichterfeld M. Follicular T helper cells: hotspots for HIV-1 persistence. Nat Med (2016) 22:711–2. doi:10.1038/nm.4138
108. Borrow P, Lewicki H, Hahn BH, Shaw GM, Oldstone MB. Virus-specific CD8+ cytotoxic T-lymphocyte activity associated with control of viremia in primary human immunodeficiency virus type 1 infection. J Virol (1994) 68:6103–10.
109. Koup RA, Safrit JT, Cao Y, Andrews CA, McLeod G, Borkowsky W, et al. Temporal association of cellular immune responses with the initial control of viremia in primary human immunodeficiency virus type 1 syndrome. J Virol (1994) 68:4650–5.
110. Schmitz JE, Kuroda MJ, Santra S, Sasseville VG, Simon MA, Lifton MA, et al. Control of viremia in simian immunodeficiency virus infection by CD8+ lymphocytes. Science (1999) 283:857–60. doi:10.1126/science.283.5403.857
111. Betts MR, Nason MC, West SM, De Rosa SC, Migueles SA, Abraham J, et al. HIV nonprogressors preferentially maintain highly functional HIV-specific CD8+ T cells. Blood (2006) 107:4781–9. doi:10.1182/blood-2005-12-4818
112. Taborda NA, González SM, Alvarez CM, Correa LA, Montoya CJ, Rugeles MT. Higher frequency of NK and CD4+ T-cells in mucosa and potent cytotoxic response in HIV controllers. PLoS One (2015) 10:e0136292. doi:10.1371/journal.pone.0136292
113. Cheynier R, Henrichwark S, Hadida F, Pelletier E, Oksenhendler E, Autran B, et al. HIV and T cell expansion in splenic white pulps is accompanied by infiltration of HIV-specific cytotoxic T lymphocytes. Cell (1994) 78:373–87. doi:10.1016/0092-8674(94)90417-0
114. Kuroda MJ, Schmitz JE, Seth A, Veazey RS, Nickerson CE, Lifton MA, et al. Simian immunodeficiency virus-specific cytotoxic T lymphocytes and cell-associated viral RNA levels in distinct lymphoid compartments of SIVmac-infected rhesus monkeys. Blood (2000) 96:1474–9.
115. Racz P, Tenner-Racz K, van Vloten F, Schmidt H, Dietrich M, Gluckman JC, et al. Lymphatic tissue changes in AIDS and other retrovirus infections: tools and insights. Lymphology (1990) 23:85–91.
116. Tenner-Racz K, Racz P, Thomé C, Meyer CG, Anderson PJ, Schlossman SF, et al. Cytotoxic effector cell granules recognized by the monoclonal antibody TIA-1 are present in CD8+ lymphocytes in lymph nodes of human immunodeficiency virus-1-infected patients. Am J Pathol (1993) 142:1750–8.
117. Connick E, Mattila T, Folkvord JM, Schlichtemeier R, Meditz AL, Ray MG, et al. CTL fail to accumulate at sites of HIV-1 replication in lymphoid tissue. J Immunol (2007) 178:6975–83. doi:10.4049/jimmunol.178.11.6975
118. Altfeld M, van Lunzen J, Frahm N, Yu XG, Schneider C, Eldridge RL, et al. Expansion of pre-existing, lymph node-localized CD8+ T cells during supervised treatment interruptions in chronic HIV-1 infection. J Clin Invest (2002) 109:837–43. doi:10.1172/JCI0214789
119. He R, Hou S, Liu C, Zhang A, Bai Q, Han M, et al. Follicular CXCR5-expressing CD8(+) T cells curtail chronic viral infection. Nature (2016) 537:412–28. doi:10.1038/nature19317
120. Miles B, Miller SM, Folkvord JM, Levy DN, Rakasz EG, Skinner PJ, et al. Follicular regulatory CD8 T cells impair the germinal center response in SIV and ex vivo HIV infection. PLoS Pathog (2016) 12:e1005924. doi:10.1371/journal.ppat.1005924
121. Petrovas C, Ferrando-Martinez S, Gerner MY, Casazza JP, Pegu A, Deleage C, et al. Follicular CD8 T cells accumulate in HIV infection and can kill infected cells in vitro via bispecific antibodies. Sci Transl Med (2017) 9:eaag2285. doi:10.1126/scitranslmed.aag2285
122. Leong YA, Chen Y, Ong HS, Wu D, Man K, Deleage C, et al. CXCR5(+) follicular cytotoxic T cells control viral infection in B cell follicles. Nat Immunol (2016) 17(10):1187–96. doi:10.1038/ni.3543
123. Bronnimann MP, Skinner PJ, Connick E. The B-cell follicle in HIV infection: barrier to a cure. Front Immunol (2018) 9:20. doi:10.3389/fimmu.2018.00020
124. Betts MR, Ambrozak DR, Douek DC, Bonhoeffer S, Brenchley JM, Casazza JP, et al. Analysis of total human immunodeficiency virus (HIV)-specific CD4+ and CD8+ T-cell responses: relationship to viral load in untreated HIV infection. J Virol (2001) 75:11983–91. doi:10.1128/JVI.75.24.11983-11991.2001
125. Lozano Reina J-M, Favre D, Kasakow Z, Mayau V, Nugeyre M-T, Ka T, et al. Gag p27-specific B- and T-cell responses in simian immunodeficiency virus SIVagm-infected African green monkeys. J Virol (2009) 83:2770–7. doi:10.1128/JVI.01841-08
126. Wang Z, Metcalf B, Ribeiro RM, McClure H, Kaur A. Th-1-type cytotoxic CD8+ T-lymphocyte responses to simian immunodeficiency virus (SIV) are a consistent feature of natural SIV infection in sooty mangabeys. J Virol (2006) 80:2771–83. doi:10.1128/JVI.80.6.2771-2783.2006
127. Zahn RC, Rett MD, Korioth-Schmitz B, Sun Y, Buzby AP, Goldstein S, et al. Simian immunodeficiency virus (SIV)-specific CD8+ T-cell responses in vervet African green monkeys chronically infected with SIVagm. J Virol (2008) 82:11577–88. doi:10.1128/JVI.01779-08
128. Schmitz JE, Zahn RC, Brown CR, Rett MD, Li M, Tang H, et al. Inhibition of adaptive immune responses leads to a fatal clinical outcome in SIV-infected pigtailed macaques but not vervet African green monkeys. PLoS Pathog (2009) 5:e1000691. doi:10.1371/journal.ppat.1000691
129. Barry AP, Silvestri G, Safrit JT, Sumpter B, Kozyr N, McClure HM, et al. Depletion of CD8+ cells in sooty mangabey monkeys naturally infected with simian immunodeficiency virus reveals limited role for immune control of virus replication in a natural host species. J Immunol (2007) 178:8002–12. doi:10.4049/jimmunol.178.12.8002
130. Gaufin T, Ribeiro RM, Gautam R, Dufour J, Mandell D, Apetrei C, et al. Experimental depletion of CD8+ cells in acutely SIVagm-infected African green monkeys results in increased viral replication. Retrovirology (2010) 7:42. doi:10.1186/1742-4690-7-42
131. Jin X, Bauer DE, Tuttleton SE, Lewin S, Gettie A, Blanchard J, et al. Dramatic rise in plasma viremia after CD8+ T cell depletion in simian immunodeficiency virus-infected macaques. J Exp Med (1999) 189:991–8. doi:10.1084/jem.189.6.991
132. Metzner KJ, Jin X, Lee FV, Gettie A, Bauer DE, Di Mascio M, et al. Effects of in vivo Cd8+ T cell depletion on virus replication in rhesus macaques immunized with a live, attenuated simian immunodeficiency virus vaccine. J Exp Med (2000) 191:1921–32. doi:10.1084/jem.191.11.1921
133. Veazey RS, Acierno PM, McEvers KJ, Baumeister SHC, Foster GJ, Rett MD, et al. Increased loss of CCR5+ CD45RA− CD4+ T cells in CD8+ lymphocyte-depleted simian immunodeficiency virus-infected rhesus monkeys. J Virol (2008) 82:5618–30. doi:10.1128/JVI.02748-07
134. Ferlazzo G, Pack M, Thomas D, Paludan C, Schmid D, Strowig T, et al. Distinct roles of IL-12 and IL-15 in human natural killer cell activation by dendritic cells from secondary lymphoid organs. Proc Natl Acad Sci U S A (2004) 101:16606–11. doi:10.1073/pnas.0407522101
135. Imamura M, Shook D, Kamiya T, Shimasaki N, Chai SMH, Coustan-Smith E, et al. Autonomous growth and increased cytotoxicity of natural killer cells expressing membrane-bound interleukin-15. Blood (2014) 124:1081–8. doi:10.1182/blood-2014-02-556837
136. Ansari AW, Ahmad F, Meyer-Olson D, Kamarulzaman A, Jacobs R, Schmidt RE. Natural killer cell heterogeneity: cellular dysfunction and significance in HIV-1 immuno-pathogenesis. Cell Mol Life Sci (2015) 72:3037–49. doi:10.1007/s00018-015-1911-5
137. Bostik P, Takahashi Y, Mayne AE, Ansari AA. Innate immune natural killer cells and their role in HIV and SIV infection. HIV Ther (2010) 4:483–504. doi:10.2217/hiv.10.28
138. Schafer JL, Li H, Evans TI, Estes JD, Reeves RK. Accumulation of cytotoxic CD16+ NK cells in simian immunodeficiency virus-infected lymph nodes associated with in situ differentiation and functional anergy. J Virol (2015) 89:6887–94. doi:10.1128/JVI.00660-15
139. Brenchley J, Douek D. HIV infection and the gastrointestinal immune system. Mucosal Immunol (2008) 1:23–30. doi:10.1038/mi.2007.1
140. Ellis-Connell AL, Balgeman AJ, Zarbock KR, Barry G, Weiler A, Egan JO, et al. ALT-803 transiently reduces simian immunodeficiency virus replication in the absence of antiretroviral treatment. J Virol (2018) 92:e01748–17. doi:10.1128/JVI.01748-17
141. Rhode PR, Egan JO, Xu W, Hong H, Webb GM, Chen X, et al. Comparison of the superagonist complex, ALT-803, to IL15 as cancer immunotherapeutics in animal models. Cancer Immunol Res (2016) 4:49–60. doi:10.1158/2326-6066.CIR-15-0093-T
142. Watson DC, Moysi E, Valentin A, Bergamaschi C, Devasundaram S, Fortis SP, et al. Treatment with native heterodimeric IL-15 increases cytotoxic lymphocytes and reduces SHIV RNA in lymph nodes. PLoS Pathog (2018) 14:e1006902. doi:10.1371/journal.ppat.1006902
143. Webb GM, Li S, Mwakalundwa G, Folkvord JM, Greene JM, Reed JS, et al. The human IL-15 superagonist ALT-803 directs SIV-specific CD8+ T cells into B-cell follicles. Blood Adv (2018) 2:76–84. doi:10.1182/bloodadvances.2017012971
Keywords: HIV, SIV, natural hosts, lymph nodes, viral control, T cells, NK cells, inflammation
Citation: Huot N, Bosinger SE, Paiardini M, Reeves RK and Müller-Trutwin M (2018) Lymph Node Cellular and Viral Dynamics in Natural Hosts and Impact for HIV Cure Strategies. Front. Immunol. 9:780. doi: 10.3389/fimmu.2018.00780
Received: 07 February 2018; Accepted: 28 March 2018;
Published: 19 April 2018
Edited by:
Vijayakumar Velu, Emory University, United StatesReviewed by:
Jonah B. Sacha, Oregon Health & Science University, United StatesPhilippe Benaroch, Centre national de la recherche scientifique (CNRS), France
Copyright: © 2018 Huot, Bosinger, Paiardini, Reeves and Müller-Trutwin. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michaela Müller-Trutwin, mmuller@pasteur.fr
 Nicolas Huot
Nicolas Huot Steven E. Bosinger
Steven E. Bosinger Mirko Paiardini
Mirko Paiardini R. Keith Reeves
R. Keith Reeves Michaela Müller-Trutwin
Michaela Müller-Trutwin