- 1Department of Translational Medical Sciences and Center for Basic and Clinical Immunology Research, University of Naples Federico II, Naples, Italy
- 2WAO Center of Excellence, Naples, Italy
- 3Department of Public Health, University of Naples Federico II, Naples, Italy
- 4Monaldi Hospital Pharmacy, Naples, Italy
- 5Institute of Experimental Endocrinology and Oncology “Gaetano Salvatore”, National Research Council (CNR), Naples, Italy
Thymic stromal lymphopoietin (TSLP) is a pleiotropic cytokine originally isolated from a murine thymic stromal cell line. TSLP exerts its biological effects by binding to a high-affinity heteromeric complex composed of thymic stromal lymphopoietin receptor chain and IL-7Rα. TSLP is primarily expressed by activated lung and intestinal epithelial cells, keratinocytes, and fibroblasts. However, dendritic cells (DCs), mast cells, and presumably other immune cells can also produce TSLP. Different groups of investigators have demonstrated the existence of two variants for TSLP in human tissues: the main isoform expressed in steady state is the short form (sf TSLP), which plays a homeostatic role, whereas the long form (lfTSLP) is upregulated in inflammatory conditions. In addition, there is evidence that in pathological conditions, TSLP can be cleaved by several endogenous proteases. Several cellular targets for TSLP have been identified, including immune (DCs, ILC2, T and B cells, NKT and Treg cells, eosinophils, neutrophils, basophils, monocytes, mast cells, and macrophages) and non-immune cells (platelets and sensory neurons). TSLP has been originally implicated in a variety of allergic diseases (e.g., atopic dermatitis, bronchial asthma, eosinophilic esophagitis). Emerging evidence indicates that TSLP is also involved in chronic inflammatory (i.e., chronic obstructive pulmonary disease and celiac disease) and autoimmune (e.g., psoriasis, rheumatoid arthritis) disorders and several cancers. These emerging observations greatly widen the role of TSLP in different human diseases. Most of these studies have not used tools to analyze the expression of the two TSLP isoforms. The broad pathophysiologic profile of TSLP has motivated therapeutic targeting of this cytokine. Tezepelumab is a first-in-class human monoclonal antibody (1) that binds to TSLP inhibiting its interaction with TSLP receptor complex. Tezepelumab given as an add-on-therapy to patients with severe uncontrolled asthma has shown safety and efficacy. Several clinical trials are evaluating the safety and the efficacy of tezepelumab in different inflammatory disorders. Monoclonal antibodies used to neutralize TSLP should not interact or hamper the homeostatic effects of sf TSLP.
Introduction
Thymic stromal lymphopoietin (TSLP) is a pleiotropic cytokine originally isolated from a murine thymic stromal cell line (2) and characterized as a lymphocyte growth factor (3). A human homolog was identified using in silico methods (4, 5). The human TSLP gene is located on chromosome 5q22.1 next to the atopic cytokine cluster on 5q31 (6), while the murine Tslp is mapped on chromosome 18 (3). Human and mouse TSLP exert their biological activities by binding to a high-affinity heteromeric complex composed of thymic stromal lymphopoietin receptor (TSLPR) chain and interleukin 7 receptor-α (IL-7Rα) (7, 8). The sequence homology between mouse and human TSLP is only about 40%, and their biological functions are similar (5), but not identical (9). TSLP is expressed predominantly by gut (10–15) and lung epithelial cells (16–21), skin keratinocytes (15, 22–25), and by dendritic cells (DCs) (26). However, TSLP can be produced also by airway smooth muscle cells (27), human DCs (26) and mast cells (16, 25, 28, 29), human monocytes (26), macrophages and granulocytes (30), synovial (31) and cancer-associated fibroblasts (CAF) (32), murine basophils (33), and cancer cells (34).
For many years, TSLP has been widely studied in the regulation of inflammatory processes occurring at the barrier surfaces (e.g., skin, lung, and gut). In fact, TSLP activates TSLPR+ DCs and plasmacytoid DCs to induce functional Th2 and regulatory T (Treg) cells (35, 36) and human T follicular helper (Tfh) cells (37). Interestingly, TSLP has now emerged as a cytokine with a plethora of pleiotropic properties mediated by the activation of a broad range of immune and non-immune cells. Depending on the immune cells targeted by TSLP, it is reported not only to promote Th2 response but also to be associated with autoimmune disorders (38–40) and cancer (32, 34, 41). Such a broad pathophysiological profile has motivated therapeutic targeting of TSLP- and TSLPR-mediated signaling (42–45).
TSPL—TSLP Receptor Interaction
Thymic stromal lymphopoietin initiates signaling by establishing a ternary complex with its specific receptor, TSLPR, and then with IL-7Rα (7, 8). The latter receptor also serves, together with the common γ-chain (γc) receptor, in signaling complex driven by IL-7 to modulate T cell development (46). First human TSLP, positively charged, binds to TSLPR, which is negatively charged, with high affinity and fast kinetics (44). Then, IL-7Rα binds to the preformed TSLP:TSLPR binary complex with high affinity. The formation of the ternary complex, TSLPR:TSLP: IL-7Rα, initiates signaling in cells co-expressing TSLPR and IL-7Rα (Figure 1). The variable heavy chain of human monoclonal antibody (mAb) anti-TSLP, tezepelumab, binds to TSLP, while the variable light chain fragment does not interact with TSLP (44). Tezepelumab inhibits in vitro human blood DC maturation and chemokine (CCL17) production induced by TSLP (44).
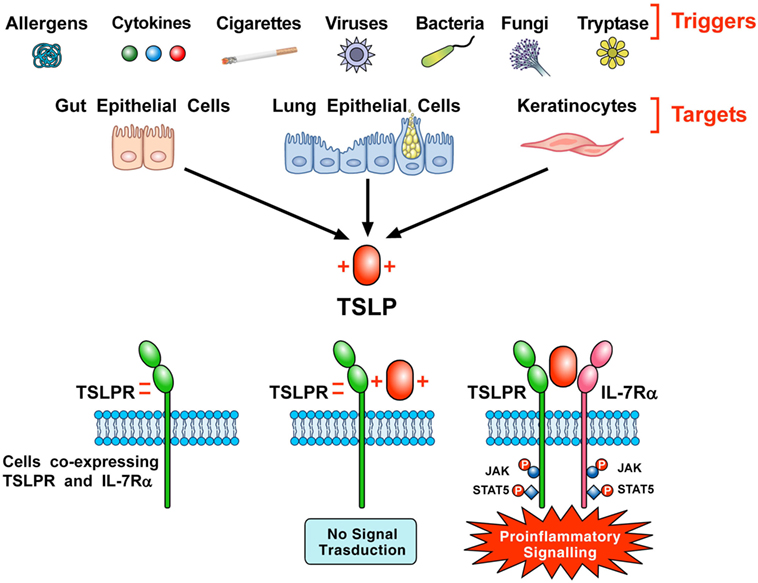
Figure 1. Schematic representation of the production of thymic stromal lymphopoietin (TSLP) and its signaling complex via a cooperative stepwise mechanism on the surface of cellular targets. A plethora of triggers including allergens, cigarette smoke extracts, cytokines, viral, bacterial and fungal products, and tryptase can activate lung and gut epithelial cells and keratinocytes to release TSLP. The latter, which is positively charged, binds to thymic stromal lymphopoietin receptor (TSLPR), which is negatively charged, with high affinity and fast kinetics. Then, IL-7Rα associates with performed TSLPR:TSLP binary complex to form the ternary TSLPR-TSLP-IL-7Rα complex (44). This receptor complex on cells co-expressing TSLPR and IL-7Rα phosphorylates JAK and STAT5 to initiate proinflammatory signaling.
Activators of TSLP Production
Several cytokines (e.g., TNF-α, IL-1β) (13, 14, 16, 20), respiratory viruses (17–19, 21, 47), bacterial (e.g., Staphylococcus aureus) (23) and fungal products (48), mechanical injury (49), allergens (50), cigarette smoke extracts (51, 52), and tryptase (53) can induce the expression of TSLP from different target cells.
Cellular Targets and Biological Properties of TSLP
Several cellular targets of TSLP have been identified, including immune and non-immune cells (Figure 2). DCs have a critical role in promoting Th2 cytokine responses (54). TSLP-stimulated DCs activate CD4+ T cells. Culture of TSLP-activated DCs together with naive syngeneic CD4+ T cells leads to T cell proliferation, which suggests a role for TSLP in CD4+ T cell homeostasis. However, when TSLP-stimulated DCs prime CD4+ T cells in an antigen-specific manner, the resulting T cells show characteristic features of T helper type 2 (Th2)-differentiated cells (production of IL-4, IL-5, and IL-13) (25). These data suggest that TSLP-activated DCs prime for inflammatory Th2 cell differentiation (32).
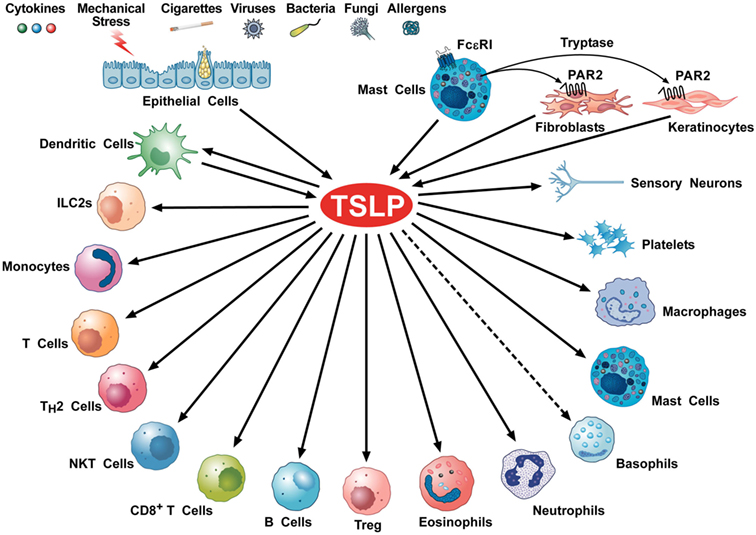
Figure 2. Schematic representation of cellular targets of thymic stromal lymphopoietin (TSLP). Several triggers can activate lung and gut epithelial cells and keratinocytes to release TSLP. This cytokine can also be produced by activated mast cells (28, 55–57) and dendritic cells (DCs) (26, 58). Tryptase, released by mast cell activates the protease-activated receptor 2 receptor on fibroblasts (53, 59) and keratinocytes (53) to release TSLP. TSLP activates DCs (37, 44, 60), ILC2 (61–63), CD4+ T and Th2 cells (64, 65), NKT cells (66), CD8+ T cells (67) and B cells (68, 69), Treg (35, 70), eosinophils (71, 72), neutrophils (73), murine (74), but not human basophils (9), monocytes (75), mast cells (16, 76–78), macrophages (79), platelets (80, 81), and sensory neurons (53).
In addition to its effects on the differentiation of CD4+ Th2 cells potentially via DCs and/or basophils (32), TSLP is able to directly promote the Th2 cell differentiation of naive T cells. The combination of TCR stimulation and TSLP treatment can induce IL-4 transcription and Th2 differentiation (64).
Type-2 immune responses that occur in airways of asthmatics are accompanied by goblet cell hyperplasia and mucus production and are driven by IL-33, TSLP, and IL-25 produced by activated lung epithelial cells (16, 82, 83). These three cytokines target members of group 2 innate lymphoid cells (ILC2s) (61, 84, 85). TSLP is a critical mediator acting on ILC2s in allergen-induced airway inflammation (61, 62) and drives the development of Th2 cells (65, 86) and CD4+ T cells (87). TSLP also provides critical signals for human B cell proliferation (68), Treg expansion (36, 70), and Tfh differentiation (37).
Human peripheral blood monocytes can be divided into three major subsets, classical, intermediate, and non-classical monocytes based on their expression of CD14 and CD16 [CD14high CD16−, CD14+ CD16+, and CD14dim CD16+, respectively (88)]. It was originally demonstrated that TSLPR and IL-7Rα mRNAs are coexpressed in human monocytes and that TSLP induces the production of CCL17 (5). Borriello and co-workers recently demonstrated that freshly isolated monocytes do not express TSLPR and IL-7Rα by flow cytometry, nor they phosphorylate STAT5 in response to TSLP (75). However, stimulation of monocytes with lipopolysaccharide (LPS) induced the expression of TSLPR complex on a small percentage of human monocytes. TSLP enhanced CCL17 production by monocytes induced by LPS and IL-4. The in vivo relevance of this original observation is supported by the demonstration that monocytes from patients with Gram-negative sepsis have a higher expression of TSLPR and IL-7Rα mRNAs compared to healthy controls (89). The elegant study by Borriello and collaborators unravels a previously unknown phenotypic and functional heterogeneity of human monocytes based on TSLPR expression.
Several groups have reported that TSLP activates human eosinophils through the engagement of TSLPR and IL-7Rα expressed on their surface (71, 72, 90, 91). Recently, it has been reported that TSLP acts on mouse and human neutrophils to enhance S. aureus killing in a complement-dependent manner (73).
The relevance of TSLP–TSLPR axis and basophils has been emphasized in several experimental models. Siracusa and collaborators reported that TSLP promotes peripheral basophilia and that TSLPR-expressing basophils can restore Th2-dependent immunity in mice (74). This study was followed by the observation that a mouse model of eosinophilic esophagitis (EoE)-like disease was dependent on TSLP acting on basophils (92). In the same study, the authors reported overexpression of TSLP and increased basophil responses in esophageal biopsies of EoE patients. The group of Gauvreau found that approximately 10% human basophils express TSLPR and that TSLP increases histamine release from basophils (93). By contrast, a collaborative study demonstrated that basophils from allergic patients do not respond to TSLP and do not express IL-7Rα (9). It is well established that human basophils differ from mouse basophils (94), and these differences might explain, at least in part, the different effects of TSLP on human and mouse basophils.
Thymic stromal lymphopoietin–TSLPR interactions appear essential for immunity to Trichuris (95, 96). The importance of the TSLP pathway and basophils in protective immunity to Trichuris, coupled with the demonstration that delivery of recombinant TSLP can augment basophil numbers in the periphery (97), suggests that coordinated TSLP-mediated regulation of DCs and basophils may have an important role in developing Th2 cytokine responses.
The expression of both TSLPR and IL-7Rα has been reported in CD34+ progenitor mast cells and human lung mast cells at the transcript and protein level (16). TSLP, alone or in combination with IL-1β or TNF-α, does not induce mast cell degranulation or lipid mediator release (16, 55). However, TSLP in combination with IL-1β or TNF-α releases several cytokines and chemokines (16, 76, 77). TSLP induces prostaglandin D2 (PGD2) production by human cord blood-derived cultured mast cells and by human peripheral blood-derived mast cells when combined with IL-33 (78).
In vivo administration of TSLP modulates the differentiation of alternatively activated macrophages (79). Interestingly, TSLP synergistically potentiated CCL17 production induced by IL-4 from murine macrophages. The expression of TSLRP and IL-7Rα and the production of TSLP isoforms should be investigated in both human primary macrophages and tumor-associated macrophages.
Human platelets express both TSLPR and IL-7Rα and their activation by TSLP promotes platelet activation (80, 81). Based on these findings, it has been suggested that TSLP could be involved in patients with metabolic syndrome (98) and in atherosclerosis (81, 99).
Wilson and collaborators have demonstrated that activation of protease-activated receptor 2 receptors by serine proteases on keratinocytes can trigger the release of TSLP. This in turn activates TSLP receptor on sensory neurons contributing to itch in patients with atopic dermatitis (AD) (53).
Short and Long Isoforms of TSLP
Harada and collaborators first identified two variants for TSLP in human bronchial epithelial cells (10). The authors demonstrated that a poly I:C, a toll-like receptor 3 (TLR3) ligand, known to upregulate TSLP (16, 18), induced the upregulation of a long isoform of TSLP. A shorter isoform, composed of 63 amino acids, was constitutively expressed in all normal tissues examined, including human lung fibroblasts, and its expression did not change after challenge with LPS or poly I:C. These two isoforms were initially considered the result of alternative splicing (10). Subsequently, the same group identified two distinct 50-untranslated regions resulting in two different open reading frames for TSLP in the human genome (100). The sequence of the 63 amino acids of short TSLP is homologous to the C-terminus of the long form and specific antibodies anti-sf TSLP are not commercially available. Primer pairs specifically targeting one or the other transcript variant should be used to study the two isoforms of TSLP at the mRNA level. It is important to emphasize that none of all previous studies had used tools to analyze the expression or functions of the two TSLP isoforms. Harada and collaborators also identified two polymorphisms upstream the long isoform untranslated region that increase transcription factor binding and, consequently, long TSLP expression. These two polymorphisms correlate positively with asthma susceptibility, whereas this is not true for the polymorphism found in the second intron of the long form (100). The authors suggested that TSLP could be a therapeutic target in asthma.
Xie et al. studied the differential expression and modulation of the two isoforms in primary human keratinocytes (47). They confirmed that TLR3, TLR2, and TLR6 ligands induced long TSLP in the presence of an atopic cytokine milieu (IL-4, IL-13, and TNF-α). They also reported that the constitutive expression of the short isoform by human keratinocytes is greater than the long form. Cultrone et al. showed that activation of human intestinal epithelial cell lines with cytokines (i.e., IL-1, TNF-α) upregulates long TSLP (14). Another group confirmed that TNF-α activation of primary human lung fibroblasts upregulates the long isoforms (101).
Rescigno and collaborators extensively examined the differential expression and biologic activity of the two isoforms in vitro and in vivo (15). They confirmed that the two isoforms are not the result of alternative splicing of the same transcript but are rather controlled by two different promoter regions. They also found that in healthy barrier surfaces, such as human intestinal and skin tissue, short TSLP is the main transcript variant. sf TSLP inhibits in vitro the production of several cytokines (i.e., TNF-α, IL-1β, IL-6), whereas lf TSLP increases the release of IFN-γ. Importantly, they reported that lf TSLP activates a canonical TSLPR on human immune cells, whereas sf TSLP induces or inhibits signaling through an unknown receptor (15). The authors also found that highly immunogenic strains such as Salmonella typhimurium and invasive Escherichia coli (E. coli) upregulate the long isoform and downregulate the short isoform, whereas the opposite is true after challenge with a commensal E. coli strain. The latter observation suggests that the dysbiosis observed in barrier surfaces could impact the expression of TSLP. More recently, Dong et al. reported that inflammatory stimuli upregulate lf TSLP mRNA but not sf TSLP in human bronchial epithelial cells (102). Importantly, administration of sf TSLP decreased airway hyperreactivity and inflammation in a mouse model of asthma. Finally, Kuroda and collaborators found that the constitutive expression of lf TSLP in unstimulated primary human keratinocytes is markeally lower than sf TSLP and that lf TSLP was strongly induced by several allergens (50).
Short TSLP Functions
Despite increasing evidence of a dichotomy for the two isoforms of TSLP in humans, the physiological role of the short isoform was largely unknown. Bjerkan et al. examined the expression and biologic activity of short TSLP on barrier surfaces such as the oral mucosa and the skin (22). The authors found that TSLP on healthy barriers is limited to the short isoform, whereas the long TSLP is upregulated in mucosal lesions after challenge. Recombinant human long TSLP had previously been found to exert antimicrobial activities (103). Using synthetic overlapping peptides, the authors demonstrated that the antimicrobial effect was primarily mediated by the C-terminal region of TSLP. Bjerkan and collaborators assessed the antimicrobial activity of short TSLP and found that the growth of all bacterial strains tested was markedly inhibited (22). They also addressed the biologic activity of the short isoform in vitro by conditioning with short TSLP monocyte-derived DCs, which do not express TSLPR if not activated (104). They found that the anti-inflammatory effect of short TSLP on human monocyte-derived DCs is mediated via an as yet unknown mechanism involving p38 phosphorylation (105) rather than STAT5 phosphorylation through which the long isoform signals (15). The latter findings support the hypothesis that sf TSLP activates an unknown receptor different from TSLPR (15).
TSLP Isoforms in Human Diseases
Rescigno and collaborators have demonstrated the upregulation of long but not short TSLP in patients with AD and ulcerative colitis (15). They also observed a downregulation of the short TSLP transcript in patients with celiac disease (11, 12).
There is compelling evidence that, in human pathologic conditions, TSLP can also be modulated by endogenous proteases. Bianchieri et al. demonstrated that the protease furin, which was upregulated in biopsies from celiac disease patients, can cleave the long isoform producing fragments of 10 and 4 kDa that show different activity on human peripheral blood mononuclear cells compared with the mature TSLP (106). Schleimer and collaborators reported that TSLP is truncated in two fragments (aa 29-124 and aa 131-159) by furin-like and carboxypeptidase N proteases in inflamed tissue. These fragments showed enhanced pro-Th2 activity on mast cells and ILC2 compared with the long TSLP (63, 106).
It would be of great interest to examine the differential expression of the two isoforms in inflammed tissues and peripheral blood from patients with asthma, chronic obstructive pulmonary disease (COPD), EoE, allergic rhinitis, etc. Because of the pathophysiological relevance of long TSLP expression in all these diseases, the cytokine has been repeatedly suggested as a valid target for therapy with antibodies that would target TSLP and/or prevent its binding to TSLPR (42–45). Since the short isoform of TSLP has homeostatic and anti-inflammatory effects, this should be taken into account when designing targeted therapeutic strategies. mAbs used to neutralize TSLP should ideally not interact or hamper the homeostatic functions of short TSLP.
TSLP and Allergic Inflammation
Genetic analysis has shown an association of polymorphisms in TSLP with several allergic diseases, including asthma and airway hyperresponsiveness, IgE concentrations, and eosinophilia (10, 100, 107, 108). Additional evidence for the relevance of TSLP in airway inflammation has been provided by genetic studies of mice. TSLPR-deficient mice are resistant to the development of ovalbumin-induced inflammation in mice (109, 110). Mice overexpressing TSLP in the airway epithelium develop an inflammatory disease with characteristics of asthma (110). Intranasal delivery of TSLP and antigen leads to the onset of severe disease (111). Asthmatic patients have higher concentrations of TSLP in their lungs (30, 56) and in peripheral blood (112).
Asthma and Chronic Rhinosinusitis (CRS)
Genetic variants located in or near TSLP have been detected in genome wide studies for asthma, rhinitis, and atopy (100, 113–118). Polymorphisms of the TSLP gene appear to contribute to Th2-polarized immunity through greater TSLP production by bronchial epithelial cells in response to viral respiratory infections (10, 100, 116). Moreover, TSLP polymorphisms appear to be associated with a higher risk of allergic rhinitis (117).
In a mouse model of asthma microRNA-19b (miR-19b) reduces airway inflammation and remodeling by inhibiting Stat3 signaling through TSLP downregulation (45). Interestingly, anti-TSLP alleviates airway inflammation in a dust mite-induced mouse model of asthma (119). Together, these results suggest that TSLP pathway is a promising target for immunotherapy of bronchial asthma. TSLP mRNA is increased in the airways of severe asthmatic patients (57) and correlates with disease severity (56). TSLP is also increased in bronchoalveolar lavage (BAL) (Liu JACI141:257, 2018), in induced sputum (120), in exhaled breath condensate (121), and in plasma of asthmatic patients (112). Recent evidence suggests that elevated expression of TSLP in the airways is a biomarker of severe refractory asthma (122). Unfortunately, in the above studies, the differential expression of the two isoforms of TSLP was not examined.
Tezepelumab, a human IgG2 mAb (1) anti-TSLP (700 mg i.v. on days 1, 29, and 57) inhibited early and late asthmatic responses, blood and sputum eosinophils, and exhaled nitric oxide in patients with mild, atopic asthma (123). Corren and collaborators reported that tezepelumab (70, 210, or 280 mg s.c. every 4 weeks) reduced asthma exacerbations, blood eosinophils, and Feno and improved FEV1 and ACQ-6 score in patients with different asthma phenotypes (124). These two studies indicate that TSLP is an attractive therapeutic target in asthma (Figure 3). Several studies are evaluating the efficacy and safety of tezepelumab alone (NCT 03347279, NCT 03406078, NCT 02698501) or in combination with allergen immunotherapy (NCT 02037196) in asthmatic patients.
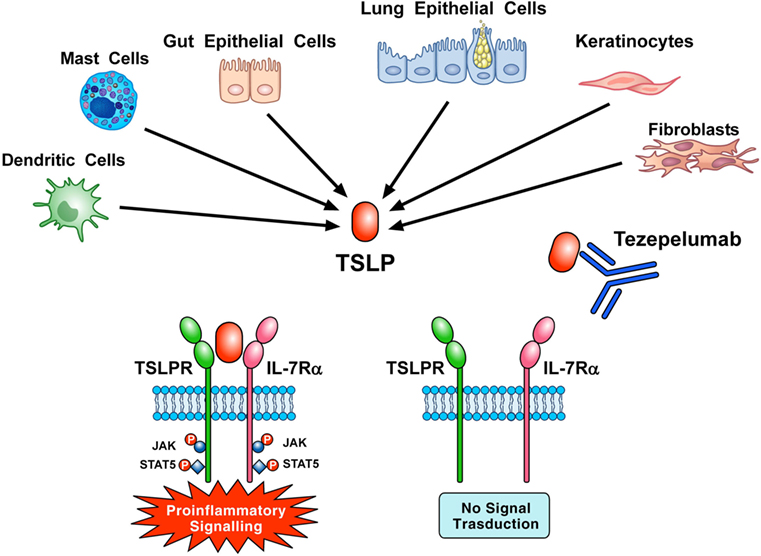
Figure 3. Thymic stromal lymphopoietin (TSLP) produced mainly by gut and epithelial cells and keratinocytes but also by dendritic cells, mast cells, and fibroblasts initiates signaling by establishing a ternary complex with thymic stromal lymphopoietin receptor (TSLPR) and IL-7Rα. Tezepelumab, a human mAb anti-TSLP, binds with high affinity to TSLP and blocks the formation of TSLPR:TSLP: IL-7Rα ternary complex on effector cells. In particular, the variable heavy chain of tezepelumab binds to TSLP, while the variable light chain fragment does not interact with TSLP (44). Tezepelumab inhibits in vitro human DC maturation and chemokine production induced by TSLP (44) and reduced exacerbations and improved quality of life in patients with severe uncontrolled asthma (124).
Chronic rhinosinusitis is a heterogeneous disease characterized by local inflammation of the upper airways and sinuses (125, 126). Genetic analysis has shown an association of TSLP polymorphism with a higher risk for allergic rhinitis (117). TSLP mRNA levels were upregulated in CRS with nasal polyps compared to control subjects and positively correlated with eosinophils and type 2 cytokines (106). The same group of Kato elegantly demonstrated that rh TSLP can be truncated by endogenous serine proteases present in CRS to generate two major peptides which potently activated DCs and ILC2s (63). These results highlight the relevance of posttranslational modifications that control the functional activity of TSLP in human inflammatory disorders. Recent evidence suggests that TSLP controls PGD2 production by human mast cells in patients with aspirin-exacerbated respiratory disease (78).
Eosinophilic Esophagitis
Eosinophilic esophagitis is an emerging disorder distinct from gastroesophageal reflux disease (127, 128). Multiple genome-wide association studies and candidate gene studies have implicated genetic variants of TSLP in genetic susceptibility to EoE (129–132). TSLP is increased in the esophageal tissue of patients with EoE (129, 130). In a mouse model, EoE-like disease developed independently of IgE, but was dependent on TSLP activation of basophils (92). Interestingly, TSLPR and IL-7Rα are not constitutively expressed by human peripheral blood basophils and TSLP does not activate these cells (9). The latter data apparently contrast with the increased density of basophils in esophageal biopsy of pediatric patients with EoE (92).
Atopic Dermatitis
Atopic dermatitis is a chronic, inflammatory skin disease affecting up to 15% of children and 2–10% of adults in industrialized countries (133, 134). Genetic variants in TSLP are associated with AD (118, 135, 136). TSLP is highly expressed in the lesional skin of patients with AD (25, 40, 95, 137) and TSLP-activated DCs prime naïve T cells to differentiate into Th2 cells (25). Serum levels of TSLP are elevated in AD patients compared to controls (138). However, the relationships between the degree of TSLP expression in the skin and the severity, the phenotypes (i.e., extrinsic vs intrinsic), and epidermal barrier function of AD remain to be elucidated (139). Recent evidence indicates that keratinocyte-derived TSLP stimulates pruritus in AD, and perhaps some other dermatologic disorders, by activating TSLP receptor complex on afferent sensory neurons (53). Several clinical trials (NCT02525094, NCT01732510) are evaluating the safety and efficacy of tezepelumab in patients with moderate-to-severe AD.
Basophils are found in skin biopsies of AD patients (134, 140, 141). It has been reported that TSLP elicits basophilia in mice and skin recruitment could be blocked by anti-TSLP antibody suggesting that TSLP promotes skin inflammation through the activation of basophils (74, 141). In a recent study, Voehringer and collaborators have demonstrated in a murine model of AD that skin recruitment of basophils occurred without direct TSLP recognition by basophils (142). Furthermore, basophils did not promote but rather ameliorated skin inflammation, and this effect was dependent on TSLPR expression on murine basophils. Interestingly, human basophils do not express constitutively TSLP and IL-7Rα (9). Together, these results emphasize some of the striking differences between human ad mouse basophils (93, 94).
TSLP and Chronic Inflammatory Diseases
COPD and Idiopathic Pulmonary Fibrosis (IPF)
Chronic obstructive pulmonary disease and asthma are conventionally considered TH1/macrophage/neutrophils and TH/eosinophil/mast cell mediated, respectively (143, 144). TSLP mRNA and protein are overexpressed in the bronchial epithelium of COPD patients compared to controls (30). Several activators of airway epithelial cells such as respiratory viruses (19), double-stranded RNA (17, 18), cigarette smoke extracts (51, 52), and proinflammatory cytokines (16, 20) can stimulate the production of TSLP in COPD patients. TSLP and TSLPR are overexpressed in lung biopsy of patients with IPF (101) and BAL TSLP is increased compared to controls (145).
Celiac Disease
Celiac disease (CD) is a heterogeneous enteropathy caused in genetically susceptible individuals by the ingestion of gluten (146). TSLPR and IL-7Rα are expressed in CD mucosa and both lf TSLP and sf TSLP mRNAs are reduced in CD compared to control subjects (11). Interestingly, the serin protease furin, which is overexpressed in CD mucosa, degrades lf TSLP. The latter intriguing findings extend the observation that proteases can cleave and modulate the functions of TSLP (63, 106). Collectively, these results indicate that in pathological conditions endogenous proteases can regulate TSLP activities. The role of sf and lf TSLP in experimental inflammatory bowel disease is hampered by the absence of sf TSLP in the mouse (147).
TSLP and Autoimmune Disorders
The role of TSLP in autoimmune diseases is largerly unknown, and only very few studies have started to explore the role of this pleiotropic cytokine in Th1- or Th17-driven autoimmune disorders. It has been demonstrated that TSLP induced polyclonal B-cell activation in vitro and development of autoimmune hemolytic anemia in vivo (148). TSLPR-deficient mice showed less severe arthritis in collagen-induced autoimmune arthritis (149). In a model of experimental autoimmune encephalomyelitis (EAE), TSLP-knock-out mice displayed a delayed outset of disease and an attenuated form of EAE (150). These studies suggest that TSLP–TSLPR axis might contribute to the pathogenesis of autoimmune disorders.
Rheumatoid Arthritis (RA)
Rheumatoid arthritis is a systemic autoimmune disease characterized by chronic synovitis (151). TSLP has been implicated as a possible exacerbating mediator in RA (38). Synovial fluid concentrations of TSLP are increased in RA patients compared to osteoarthritis (39). Fibroblasts from RA patients can produce TSLP when activated by several immunologic stimuli (e.g., IL-1β, TNF-α) (152). In addition, mast cells and macrophages, present in RA synovium (151, 153), may contribute to TSLP levels in the RA joint (28, 56, 154). Supportive role of TSLP in arthritis derives also from several mouse models (38, 149). Blockade of TSLP/TSLPR axis warrants further experimental and clinical studies in RA.
Psoriasis
Psoriasis is a common, chronic inflammatory disease that manifests predominantly in the skin (155). Although psoriasis is classified as an organ-specific autoimmune disease (156–158), there is increasing understanding of psoriasis as a systemic inflammatory disease that extends beyond the skin (159, 160). The pathophysiology of psoriasis is characterized by skin DC activation and pathogenetic IL-23 production by blood and skin DCs (155). Volpe and collaborators found that TSLP is overexpressed in human psoriatic skin (40). Moreover, they reported that TSLP induces DC maturation and primes for subsequent CD40L-induced IL-23 production by DCs. These original findings extend the role of TSLP from allergic disorders to IL-23-driven autoimmunity, with possible implication in other forms of autoimmune disorders (e.g., RA).
TSLP and Cancer
Initially shown to promote the growth and activation of B cells and DCs (3, 25), TSLP is now known to have wide-ranging effects on cells of innate and adaptive immune system (Figure 2). These include DCs, ILC2, T and B cells, NKT and Treg cells, eosinophils, neutrophils, basophils, mast cells, and macrophages. All these cells are implicated in tumor initiation and growth, angiogenesis, and lymphangiogenesis (161–163). Therefore, it is not surprising that TSLP plays a direct and/or indirect role in the control of a variety of experimental and human cancers (34).
A pro-tumorigenic role of TSLP is supported by a study using an orthotopic model of breast cancer in the mouse (164). Using a variety of methods, several groups found that genetic rearrangements and mutations in the TSLP gene are present in pediatric acute lymphoblastic leukemia (165–167). TSLP is overexpressed in plasma and lymph nodes from Hodgkin patients (41). Moreover, TSLP acts through the production of Th2 cytokines (e.g., IL-4 and IL-13) to induce cutaneous T-cell lymphoma (168). De Monte and collaborators elegantly demonstrated that human pancreatic CAF release TSLP which activates TSLPR+ DCs to drive Th2 differentiation mediated by the release of IL-4 from basophils (32). It has been reported that human cervical carcinoma cells release TSLP acting on TSLPR+ endothelial cells to promote angiogenesis and cancer growth (169). In another study, it was reported that breast cancer cells can produce TSLP (170). Kuan and Ziegler extended the previous observation demonstrating that TSLP promotes the survival of breast cancer cells through the expression of the antiapoptotic molecule Bcl-2 (171).
In contrast to these studies, two groups have demonstrated a tumor-suppressing role for TSLP in murine models of skin carcinoma (172, 173). Yue and collaborators found decreased TSLP expression in human colon cancer and TSLP levels negatively correlated with the clinical staging score of cancer (174). Moreover, TSLP enhanced apoptosis of colon cancer cells through the engagement of TSLPR. Finally, using a xenograft mouse model, the authors found that peritumoral administration of TSLP reduced tumor growth. In mouse models of breast and pancreatic carcinogenesis, it was found that early administration of TSLP blocked cancer development. The antitumor effect of TSLP was mediated by activation of CD4+ Th2 cells around tumors and in draining LNs (175). Finally, Soumelis and collaborators reported that TSLP was undetectable or expressed at low levels in breast cancer and in several human breast cancer cell lines (176).
In conclusion, the role of TSLP–TSLPR axis in experimental and human cancer is still controversial (34). In certain neoplasias, TSLP plays a pro-tumorigenic role, whereas in others, a protective role. It is obvious that there are many important questions that should be urgently addressed before we understand whether TSLP isoforms and TSLPR+ immune cells are an ally or an adversary in different types of human cancer. This is another fundamental question that should be seriously considered when designing TSLP-targeted therapeutic strategies. Finally, studies are urgently needed to examine the differential expression and functions of sf TSLP and lf TSLP on patients with cancer.
Conclusion and Perspectives
Thymic stromal lymphopoietin is a cytokine originally characterized by its ability to promote DC and B cell activation (177). There is increasing evidence that TSLP can directly and/or indirectly activate a plethora of immune and non-immune cells involved in a wide spectrum of inflammatory disorders and cancer (161–163). These emerging observations greatly widen the role of TSLP in different human diseases.
It is important to emphasize that several groups have demonstrated the existence of two variants (sf and lf TSLP) of TSLP in humans (10, 14, 15, 22, 47, 100, 101). sf TSLP is constitutively expressed in steady state in different human tissues and plays a homeostatic role (15). By contrast, lf TSLP is induced at sites of inflammation and plays a proinflammatory role (14, 15, 47, 50, 100, 102). lf TSLP has a sequence of 159 amino acids, which corresponds to the commercially available recombinant TSLP produced by prokaryotic cells. Moreover, sf TSLP overlaps the lf TSLP in the terminal region and available anti-TSLP antibodies do not distinguish between the two isoforms. Therefore, at present, the use of specific primers is the only way to distinguish the two TSLP variants at the molecular level. Unfortunately, only few studies have examined the expression and functions of the two TSLP variants in different human disorders. The studies examining the two isoforms have already revealed interesting dichotomies in the expression and function of two TSLP variants in different inflammatory diseases (11, 14, 15, 22, 100). Additional studies are urgently needed to evaluate the presence and function of the two isoforms of TSLP in different pathological conditions.
An additional level of complexity derives from the observation that, in pathological conditions, TSLP can be cleaved by several endogenous proteases (11, 63, 106). The latter observation is not unexpected because another epithelial-derived cytokine such as IL-33 can be cleaved by several endogenous (i.e., tryptase) (178) and exogenous proteases (179).
The role of TSLP in different types of cancer is controversial (34, 171, 174). The role of the two TSLP isoforms has not been investigated in most of these studies. In addition, the commercial TSLP assay used in some studies (41) does not distinguish the two TSLP isoforms. The role of the two isoforms of TSLP in human cancers should be taken into account in future studies.
Given the role of TSLP in experimental and clinical asthma (45, 56, 57, 119, 180) and in AD (25, 40, 95, 137), this cytokine soon appeared an attractive therapeutic target (100). Tezepelumab is a first-in-class human mAb that binds to TSLP inhibiting its interaction with TSLP receptor complex (44). Tezepelumab given as an add-on therapy to patients with severe uncontrolled reduced asthma exacerbations and improved quality of life (124). It is unknown whether tezepelumab administration in humans affects plasma levels or the bronchial expression of the two TSLP variants. Several undergoing clinical trials are investigating the efficacy of tezepelumab in AD. Because the short form of TSLP has important homeostatic (15) and antibacterial activities (22), the long-term safety should be taken into account when designing anti-TSLP targeted therapeutic strategies.
Thymic stromal lymphopoietin was initially considered a key cytokine that initiates and promotes type 2 immunity (177). Increasing evidence suggests that TSLP could mediate immune inflammation also in patients with autoimmune disorders such as RA (38, 39) and psoriasis (40). The latter findings add to the complexity of TSLP interactions with several immune cells. We would like to suggest that TSLP and/or perhaps TSLP isoforms might have different immunomodulatory roles, depending on the type of immune environment.
Given the complexity of the interactions of different isoforms and cleavage products of TSLP with a plethora of immune cells in various organs and in different diseases, several fundamental questions remain to be elucidated. A better understanding of the impact of TSLP isoforms and cleavage products on immune cells will be critical for the development of safe and effective TSLP targeted therapies in inflammatory disorders and cancer.
Author Contributions
All authors contributed equally to reviewing the current literature and writing of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The authors apologize to the authors who have contributed importantly to this field and whose work has not been cited due to space and citation restrictions. The authors thank Fabrizio Fiorbianco for the elaboration of figures. This work was supported in part by Regione Campania CISI-Lab and TIMING Project.
Funding
This work was supported in part by Regione Campania CISI-Lab and TIMING Project.
Abbreviations
AD, atopic dermatitis; ALL, acute lymphoblastic leukemia; ASM, airway smooth muscle; BAL, bronchoalveolar lavage; CD, celiac disease; COPD, chronic obstructive pulmonary disease; CRS, chronic rhinosinusitis; DC, dendritic cell; dsRNA, double-stranded RNA; E. coli, Escherichia coli; EoE, eosinophilic esophagitis; γc, γ chain; GERD, gastroesophageal reflux; GWAS, genome-wide association studies; ILC, innate lymphoid cell; lfTSLP, long form of TSLP; LN, lymph node; LPS, lipopolysaccharide; mAb, monoclonal antibody; PAR2, protease- activated receptor 2; Poly I: C, polyinosinic-polycytidylic acid; PGD2, prostaglandin D2; RA, rheumatoid arthritis; sfTSLP, short form of TSLP; Tfh, T follicular helper; Th2, T helper type 2; TLR, toll like receptor; Treg, T regulatory; TSLP, thymic stromal lymphopoietin; TSLPR, thymic stromal lymphopoietin receptor.
References
1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, et al. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet (2012) 380:2095–128. doi:10.1016/S0140-6736(12)61728-0
2. Friend SL, Hosier S, Nelson A, Foxworthe D, Williams DE, Farr A. A thymic stromal cell line supports in vitro development of surface IgM+ B cells and produces a novel growth factor affecting B and T lineage cells. Exp Hematol (1994) 22:321–8.
3. Sims JE, Williams DE, Morrissey PJ, Garka K, Foxworthe D, Price V, et al. Molecular cloning and biological characterization of a novel murine lymphoid growth factor. J Exp Med (2000) 192:671–80. doi:10.1084/jem.192.5.671
4. Ray RJ, Furlonger C, Williams DE, Paige CJ. Characterization of thymic stromal-derived lymphopoietin (TSLP) in murine B cell development in vitro. Eur J Immunol (1996) 26:10–6. doi:10.1002/eji.1830260103
5. Reche PA, Soumelis V, Gorman DM, Clifford T, Liu M, Travis M, et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J Immunol (2001) 167:336–43. doi:10.4049/jimmunol.167.1.336
6. Quentmeier H, Drexler HG, Fleckenstein D, Zaborski M, Armstrong A, Sims JE, et al. Cloning of human thymic stromal lymphopoietin (TSLP) and signaling mechanisms leading to proliferation. Leukemia (2001) 15:1286–92. doi:10.1038/sj.leu.2402175
7. Park LS, Martin U, Garka K, Gliniak B, Di Santo JP, Muller W, et al. Cloning of the murine thymic stromal lymphopoietin (TSLP) receptor: formation of a functional heteromeric complex requires interleukin 7 receptor. J Exp Med (2000) 192:659–70. doi:10.1084/jem.192.5.659
8. Pandey A, Ozaki K, Baumann H, Levin SD, Puel A, Farr AG, et al. Cloning of a receptor subunit required for signaling by thymic stromal lymphopoietin. Nat Immunol (2000) 1:59–64. doi:10.1038/76923
9. Salabert-Le Guen N, Hemont C, Delbove A, Poli C, Braudeau C, Fantou A, et al. Thymic stromal lymphopoietin does not activate human basophils. J Allergy Clin Immunol (2018) 141:1476–9.e6. doi:10.1016/j.jaci.2017.11.012
10. Harada M, Hirota T, Jodo AI, Doi S, Kameda M, Fujita K, et al. Functional analysis of the thymic stromal lymphopoietin variants in human bronchial epithelial cells. Am J Respir Cell Mol Biol (2009) 40:368–74. doi:10.1165/rcmb.2008-0041OC
11. Biancheri P, Di Sabatino A, Rescigno M, Giuffrida P, Fornasa G, Tsilingiri K, et al. Abnormal thymic stromal lymphopoietin expression in the duodenal mucosa of patients with coeliac disease. Gut (2016) 65:1670–80. doi:10.1136/gutjnl-2014-308876
12. Iliev ID, Spadoni I, Mileti E, Matteoli G, Sonzogni A, Sampietro GM, et al. Human intestinal epithelial cells promote the differentiation of tolerogenic dendritic cells. Gut (2009) 58:1481–9. doi:10.1136/gut.2008.175166
13. Collison AM, Sokulsky LA, Sherrill JD, Nightingale S, Hatchwell L, Talley NJ, et al. TNF-related apoptosis-inducing ligand (TRAIL) regulates midline-1, thymic stromal lymphopoietin, inflammation, and remodeling in experimental eosinophilic esophagitis. J Allergy Clin Immunol (2015) 136:971–82. doi:10.1016/j.jaci.2015.03.031
14. Cultrone A, de Wouters T, Lakhdari O, Kelly D, Mulder I, Logan E, et al. The NF-kappaB binding site located in the proximal region of the TSLP promoter is critical for TSLP modulation in human intestinal epithelial cells. Eur J Immunol (2013) 43:1053–62. doi:10.1002/eji.201142340
15. Fornasa G, Tsilingiri K, Caprioli F, Botti F, Mapelli M, Meller S, et al. Dichotomy of short and long thymic stromal lymphopoietin isoforms in inflammatory disorders of the bowel and skin. J Allergy Clin Immunol (2015) 136:413–22. doi:10.1016/j.jaci.2015.04.011
16. Allakhverdi Z, Comeau MR, Jessup HK, Yoon BR, Brewer A, Chartier S, et al. Thymic stromal lymphopoietin is released by human epithelial cells in response to microbes, trauma, or inflammation and potently activates mast cells. J Exp Med (2007) 204:253–8. doi:10.1084/jem.20062211
17. Calven J, Yudina Y, Hallgren O, Westergren-Thorsson G, Davies DE, Brandelius A, et al. Viral stimuli trigger exaggerated thymic stromal lymphopoietin expression by chronic obstructive pulmonary disease epithelium: role of endosomal TLR3 and cytosolic RIG-I-like helicases. J Innate Immun (2012) 4:86–99. doi:10.1159/000329131
18. Kato A, Favoreto S Jr, Avila PC, Schleimer RP. TLR3- and Th2 cytokine-dependent production of thymic stromal lymphopoietin in human airway epithelial cells. J Immunol (2007) 179:1080–7. doi:10.4049/jimmunol.179.2.1080
19. Lee HC, Headley MB, Loo YM, Berlin A, Gale M Jr, Debley JS, et al. Thymic stromal lymphopoietin is induced by respiratory syncytial virus-infected airway epithelial cells and promotes a type 2 response to infection. J Allergy Clin Immunol (2012) 130:1187–96.e5. doi:10.1016/j.jaci.2012.07.031
20. Lee HC, Ziegler SF. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFkappaB. Proc Natl Acad Sci U S A (2007) 104:914–9. doi:10.1073/pnas.0607305104
21. Nagarkar DR, Poposki JA, Comeau MR, Biyasheva A, Avila PC, Schleimer RP, et al. Airway epithelial cells activate TH2 cytokine production in mast cells through IL-1 and thymic stromal lymphopoietin. J Allergy Clin Immunol (2012) 130:225–32.e4. doi:10.1016/j.jaci.2012.04.019
22. Bjerkan L, Schreurs O, Engen SA, Jahnsen FL, Baekkevold ES, Blix IJ, et al. The short form of TSLP is constitutively translated in human keratinocytes and has characteristics of an antimicrobial peptide. Mucosal Immunol (2015) 8:49–56. doi:10.1038/mi.2014.41
23. Vu AT, Baba T, Chen X, Le TA, Kinoshita H, Xie Y, et al. Staphylococcus aureus membrane and diacylated lipopeptide induce thymic stromal lymphopoietin in keratinocytes through the toll-like receptor 2-toll-like receptor 6 pathway. J Allergy Clin Immunol (2010) 126:985–93, 993.e1–3. doi:10.1016/j.jaci.2010.09.002
24. Li M, Hener P, Zhang Z, Kato S, Metzger D, Chambon P. Topical vitamin D3 and low-calcemic analogs induce thymic stromal lymphopoietin in mouse keratinocytes and trigger an atopic dermatitis. Proc Natl Acad Sci U S A (2006) 103:11736–41. doi:10.1073/pnas.0604575103
25. Soumelis V, Reche PA, Kanzler H, Yuan W, Edward G, Homey B, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol (2002) 3:673–80. doi:10.1038/ni805
26. Kashyap M, Rochman Y, Spolski R, Samsel L, Leonard WJ. Thymic stromal lymphopoietin is produced by dendritic cells. J Immunol (2011) 187:1207–11. doi:10.4049/jimmunol.1100355
27. Zhang K, Shan L, Rahman MS, Unruh H, Halayko AJ, Gounni AS. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am J Physiol Lung Cell Mol Physiol (2007) 293:L375–82. doi:10.1152/ajplung.00045.2007
28. Okayama Y, Okumura S, Sagara H, Yuki K, Sasaki T, Watanabe N, et al. FcepsilonRI-mediated thymic stromal lymphopoietin production by interleukin-4-primed human mast cells. Eur Respir J (2009) 34:425–35. doi:10.1183/09031936.00121008
29. Allakhverdi Z, Comeau MR, Jessup HK, Delespesse G. Thymic stromal lymphopoietin as a mediator of crosstalk between bronchial smooth muscles and mast cells. J Allergy Clin Immunol (2009) 123:958–60.e2. doi:10.1016/j.jaci.2009.01.059
30. Ying S, O’Connor B, Ratoff J, Meng Q, Fang C, Cousins D, et al. Expression and cellular provenance of thymic stromal lymphopoietin and chemokines in patients with severe asthma and chronic obstructive pulmonary disease. J Immunol (2008) 181:2790–8. doi:10.4049/jimmunol.181.4.2790
31. Ohba T, Haro H, Ando T, Koyama K, Hatsushika K, Suenaga F, et al. A potential role of thymic stromal lymphopoietin in the recruitment of macrophages to mouse intervertebral disc cells via monocyte chemotactic protein 1 induction: implications for herniated discs. Arthritis Rheum (2008) 58:3510–9. doi:10.1002/art.23965
32. De Monte L, Reni M, Tassi E, Clavenna D, Papa I, Recalde H, et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J Exp Med (2011) 208:469–78. doi:10.1084/jem.20101876
33. Sokol CL, Barton GM, Farr AG, Medzhitov R. A mechanism for the initiation of allergen-induced T helper type 2 responses. Nat Immunol (2008) 9:310–8. doi:10.1038/ni1558
34. Lo Kuan E, Ziegler SF. Thymic stromal lymphopoietin and cancer. J Immunol (2014) 193:4283–8. doi:10.4049/jimmunol.1400864
35. Watanabe N, Wang YH, Lee HK, Ito T, Cao W, Liu YJ. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature (2005) 436:1181–5. doi:10.1038/nature03886
36. Hanabuchi S, Ito T, Park WR, Watanabe N, Shaw JL, Roman E, et al. Thymic stromal lymphopoietin-activated plasmacytoid dendritic cells induce the generation of FOXP3+ regulatory T cells in human thymus. J Immunol (2010) 184:2999–3007. doi:10.4049/jimmunol.0804106
37. Pattarini L, Trichot C, Bogiatzi S, Grandclaudon M, Meller S, Keuylian Z, et al. TSLP-activated dendritic cells induce human T follicular helper cell differentiation through OX40-ligand. J Exp Med (2017) 214:1529–46. doi:10.1084/jem.20150402
38. Koyama K, Ozawa T, Hatsushika K, Ando T, Takano S, Wako M, et al. A possible role for TSLP in inflammatory arthritis. Biochem Biophys Res Commun (2007) 357:99–104. doi:10.1016/j.bbrc.2007.03.081
39. Moret FM, Hack CE, van der Wurff-Jacobs KM, Radstake TR, Lafeber FP, van Roon JA. Thymic stromal lymphopoietin, a novel proinflammatory mediator in rheumatoid arthritis that potently activates CD1c+ myeloid dendritic cells to attract and stimulate T cells. Arthritis Rheumatol (2014) 66:1176–84. doi:10.1002/art.38338
40. Volpe E, Pattarini L, Martinez-Cingolani C, Meller S, Donnadieu MH, Bogiatzi SI, et al. Thymic stromal lymphopoietin links keratinocytes and dendritic cell-derived IL-23 in patients with psoriasis. J Allergy Clin Immunol (2014) 134:373–81. doi:10.1016/j.jaci.2014.04.022
41. Ferretti E, Hohaus S, Di Napoli A, Belmonte B, Cuccaro A, Cupelli E, et al. Interleukin-31 and thymic stromal lymphopoietin expression in plasma and lymph node from Hodgkin lymphoma patients. Oncotarget (2017) 8:85263–75. doi:10.18632/oncotarget.19665
42. Borowski A, Vetter T, Kuepper M, Wohlmann A, Krause S, Lorenzen T, et al. Expression analysis and specific blockade of the receptor for human thymic stromal lymphopoietin (TSLP) by novel antibodies to the human TSLPRalpha receptor chain. Cytokine (2013) 61:546–55. doi:10.1016/j.cyto.2012.10.025
43. Zhang F, Huang G, Hu B, Song Y, Shi Y. A soluble thymic stromal lymphopoietin (TSLP) antagonist, TSLPR-immunoglobulin, reduces the severity of allergic disease by regulating pulmonary dendritic cells. Clin Exp Immunol (2011) 164:256–64. doi:10.1111/j.1365-2249.2011.04328.x
44. Verstraete K, Peelman F, Braun H, Lopez J, Van Rompaey D, Dansercoer A, et al. Structure and antagonism of the receptor complex mediated by human TSLP in allergy and asthma. Nat Commun (2017) 8:14937. doi:10.1038/ncomms14937
45. Ye L, Mou Y, Wang J, Jin ML. Effects of microRNA-19b on airway remodeling, airway inflammation and degree of oxidative stress by targeting TSLP through the Stat3 signaling pathway in a mouse model of asthma. Oncotarget (2017) 8:47533–46. doi:10.18632/oncotarget.17258
46. Mackall CL, Fry TJ, Gress RE. Harnessing the biology of IL-7 for therapeutic application. Nat Rev Immunol (2011) 11:330–42. doi:10.1038/nri2970
47. Xie Y, Takai T, Chen X, Okumura K, Ogawa H. Long TSLP transcript expression and release of TSLP induced by TLR ligands and cytokines in human keratinocytes. J Dermatol Sci (2012) 66:233–7. doi:10.1016/j.jdermsci.2012.03.007
48. Kouzaki H, O’Grady SM, Lawrence CB, Kita H. Proteases induce production of thymic stromal lymphopoietin by airway epithelial cells through protease-activated receptor-2. J Immunol (2009) 183:1427–34. doi:10.4049/jimmunol.0900904
49. Oyoshi MK, Larson RP, Ziegler SF, Geha RS. Mechanical injury polarizes skin dendritic cells to elicit a T(H)2 response by inducing cutaneous thymic stromal lymphopoietin expression. J Allergy Clin Immunol (2010) 126:976–84, 984.e1–5. doi:10.1016/j.jaci.2010.08.041
50. Kuroda Y, Yuki T, Takahashi Y, Sakaguchi H, Matsunaga K, Itagaki H. Long form of thymic stromal lymphopoietin of keratinocytes is induced by protein allergens. J Immunotoxicol (2017) 14:178–87. doi:10.1080/1547691X.2017.1349220
51. Nakamura Y, Miyata M, Ohba T, Ando T, Hatsushika K, Suenaga F, et al. Cigarette smoke extract induces thymic stromal lymphopoietin expression, leading to T(H)2-type immune responses and airway inflammation. J Allergy Clin Immunol (2008) 122:1208–14. doi:10.1016/j.jaci.2008.09.022
52. Smelter DF, Sathish V, Thompson MA, Pabelick CM, Vassallo R, Prakash YS. Thymic stromal lymphopoietin in cigarette smoke-exposed human airway smooth muscle. J Immunol (2010) 185:3035–40. doi:10.4049/jimmunol.1000252
53. Wilson SR, The L, Batia LM, Beattie K, Katibah GE, McClain SP, et al. The epithelial cell-derived atopic dermatitis cytokine TSLP activates neurons to induce itch. Cell (2013) 155:285–95. doi:10.1016/j.cell.2013.08.057
54. Lambrecht BN, Hammad H. Biology of lung dendritic cells at the origin of asthma. Immunity (2009) 31:412–24. doi:10.1016/j.immuni.2009.08.008
55. Allakhverdi Z, Comeau MR, Armant M, Agrawal R, Woodfolk JA, Sehmi R, et al. Mast cell-activated bone marrow mesenchymal stromal cells regulate proliferation and lineage commitment of CD34(+) progenitor cells. Front Immunol (2013) 4:461. doi:10.3389/fimmu.2013.00461
56. Ying S, O’Connor B, Ratoff J, Meng Q, Mallett K, Cousins D, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol (2005) 174:8183–90. doi:10.4049/jimmunol.174.12.8183
57. Shikotra A, Choy DF, Ohri CM, Doran E, Butler C, Hargadon B, et al. Increased expression of immunoreactive thymic stromal lymphopoietin in patients with severe asthma. J Allergy Clin Immunol (2012) 129:104–11.e1–9. doi:10.1016/j.jaci.2011.08.031
58. Spadoni I, Iliev ID, Rossi G, Rescigno M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol (2012) 5:184–93. doi:10.1038/mi.2011.64
59. McLarty JL, Melendez GC, Brower GL, Janicki JS, Levick SP. Tryptase/protease-activated receptor 2 interactions induce selective mitogen-activated protein kinase signaling and collagen synthesis by cardiac fibroblasts. Hypertension (2011) 58:264–70. doi:10.1161/HYPERTENSIONAHA.111.169417
60. Kummola L, Ortutay Z, Chen X, Caucheteux S, Hamalainen S, Aittomaki S, et al. IL-7Ralpha expression regulates murine dendritic cell sensitivity to thymic stromal lymphopoietin. J Immunol (2017) 198:3909–18. doi:10.4049/jimmunol.1600753
61. Halim TY, Krauss RH, Sun AC, Takei F. Lung natural helper cells are a critical source of Th2 cell-type cytokines in protease allergen-induced airway inflammation. Immunity (2012) 36:451–63. doi:10.1016/j.immuni.2011.12.020
62. Kabata H, Moro K, Fukunaga K, Suzuki Y, Miyata J, Masaki K, et al. Thymic stromal lymphopoietin induces corticosteroid resistance in natural helper cells during airway inflammation. Nat Commun (2013) 4:2675. doi:10.1038/ncomms3675
63. Poposki JA, Klingler AI, Stevens WW, Peters AT, Hulse KE, Grammer LC, et al. Proprotein convertases generate a highly functional heterodimeric form of thymic stromal lymphopoietin in humans. J Allergy Clin Immunol (2017) 139:1559–67.e8. doi:10.1016/j.jaci.2016.08.040
64. Omori M, Ziegler S. Induction of IL-4 expression in CD4(+) T cells by thymic stromal lymphopoietin. J Immunol (2007) 178:1396–404. doi:10.4049/jimmunol.178.3.1396
65. Ochiai S, Jagot F, Kyle RL, Hyde E, White RF, Prout M, et al. Thymic stromal lymphopoietin drives the development of IL-13(+) Th2 cells. Proc Natl Acad Sci U S A (2018) 115:1033–8. doi:10.1073/pnas.1714348115
66. Nagata Y, Kamijuku H, Taniguchi M, Ziegler S, Seino K. Differential role of thymic stromal lymphopoietin in the induction of airway hyperreactivity and Th2 immune response in antigen-induced asthma with respect to natural killer T cell function. Int Arch Allergy Immunol (2007) 144:305–14. doi:10.1159/000106319
67. Akamatsu T, Watanabe N, Kido M, Saga K, Tanaka J, Kuzushima K, et al. Human TSLP directly enhances expansion of CD8+ T cells. Clin Exp Immunol (2008) 154:98–106. doi:10.1111/j.1365-2249.2008.03731.x
68. Milford TA, Su RJ, Francis OL, Baez I, Martinez SR, Coats JS, et al. TSLP or IL-7 provide an IL-7Ralpha signal that is critical for human B lymphopoiesis. Eur J Immunol (2016) 46:2155–61. doi:10.1002/eji.201646307
69. Levin SD, Koelling RM, Friend SL, Isaksen DE, Ziegler SF, Perlmutter RM, et al. Thymic stromal lymphopoietin: a cytokine that promotes the development of IgM+ B cells in vitro and signals via a novel mechanism. J Immunol (1999) 162:677–83.
70. Leichner TM, Satake A, Harrison VS, Tanaka Y, Archambault AS, Kim BS, et al. Skin-derived TSLP systemically expands regulatory T cells. J Autoimmun (2017) 79:39–52. doi:10.1016/j.jaut.2017.01.003
71. Wong CK, Hu S, Cheung PF, Lam CW. Thymic stromal lymphopoietin induces chemotactic and prosurvival effects in eosinophils: implications in allergic inflammation. Am J Respir Cell Mol Biol (2010) 43:305–15. doi:10.1165/rcmb.2009-0168OC
72. Morshed M, Yousefi S, Stockle C, Simon HU, Simon D. Thymic stromal lymphopoietin stimulates the formation of eosinophil extracellular traps. Allergy (2012) 67:1127–37. doi:10.1111/j.1398-9995.2012.02868
73. West EE, Spolski R, Kazemian M, Yu ZX, Kemper C, Leonard WJ. A TSLP-complement axis mediates neutrophil killing of methicillin-resistant Staphylococcus aureus. Sci Immunol (2016) 1(5):eaaf8471. doi:10.1126/sciimmunol.aaf8471
74. Siracusa MC, Saenz SA, Hill DA, Kim BS, Headley MB, Doering TA, et al. TSLP promotes interleukin-3-independent basophil haematopoiesis and type 2 inflammation. Nature (2011) 477:229–33. doi:10.1038/nature10329
75. Borriello F, Iannone R, Di Somma S, Vastolo V, Petrosino G, Visconte F, et al. Lipopolysaccharide-elicited TSLPR expression enriches a functionally discrete subset of human CD14(+) CD1c(+) monocytes. J Immunol (2017) 198:3426–35. doi:10.4049/jimmunol.1601497
76. Han NR, Oh HA, Nam SY, Moon PD, Kim DW, Kim HM, et al. TSLP induces mast cell development and aggravates allergic reactions through the activation of MDM2 and STAT6. J Invest Dermatol (2014) 134:2521–30. doi:10.1038/jid.2014.198
77. Kaur D, Doe C, Woodman L, Heidi Wan WY, Sutcliffe A, Hollins F, et al. Mast cell-airway smooth muscle crosstalk: the role of thymic stromal lymphopoietin. Chest (2012) 142:76–85. doi:10.1378/chest.11-1782
78. Buchheit KM, Cahill KN, Katz HR, Murphy KC, Feng C, Lee-Sarwar K, et al. Thymic stromal lymphopoietin controls prostaglandin D2 generation in patients with aspirin-exacerbated respiratory disease. J Allergy Clin Immunol (2016) 137:1566–76.e5. doi:10.1016/j.jaci.2015.10.020
79. Han H, Headley MB, Xu W, Comeau MR, Zhou B, Ziegler SF. Thymic stromal lymphopoietin amplifies the differentiation of alternatively activated macrophages. J Immunol (2013) 190:904–12. doi:10.4049/jimmunol.1201808
80. Dong J, Lin J, Wang B, He S, Wu C, Kushwaha KK, et al. Inflammatory cytokine TSLP stimulates platelet secretion and potentiates platelet aggregation via a TSLPR-dependent PI3K/Akt signaling pathway. Cell Physiol Biochem (2015) 35:160–74. doi:10.1159/000369684
81. Wang B, Peng Y, Dong J, Lin J, Wu C, Su Y, et al. Human platelets express functional thymic stromal lymphopoietin receptors: a potential role in platelet activation in acute coronary syndrome. Cell Physiol Biochem (2013) 32:1741–50. doi:10.1159/000356608
82. Angkasekwinai P, Park H, Wang YH, Chang SH, Corry DB, Liu YJ, et al. Interleukin 25 promotes the initiation of proallergic type 2 responses. J Exp Med (2007) 204:1509–17. doi:10.1084/jem.20061675
83. Drake LY, Kita H. IL-33: biological properties, functions, and roles in airway disease. Immunol Rev (2017) 278:173–84. doi:10.1111/imr.12552
84. Stier MT, Zhang J, Goleniewska K, Cephus JY, Rusznak M, Wu L, et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J Exp Med (2018) 215:263–81. doi:10.1084/jem.20170449
85. Martin NT, Martin MU. Interleukin 33 is a guardian of barriers and a local alarmin. Nat Immunol (2016) 17:122–31. doi:10.1038/ni.3370
86. He R, Oyoshi MK, Garibyan L, Kumar L, Ziegler SF, Geha RS. TSLP acts on infiltrating effector T cells to drive allergic skin inflammation. Proc Natl Acad Sci U S A (2008) 105:11875–80. doi:10.1073/pnas.0801532105
87. Wang Q, Du J, Zhu J, Yang X, Zhou B. Thymic stromal lymphopoietin signaling in CD4(+) T cells is required for TH2 memory. J Allergy Clin Immunol (2015) 135:781–91.e3. doi:10.1016/j.jaci.2014.09.015
88. Wong KL, Yeap WH, Tai JJ, Ong SM, Dang TM, Wong SC. The three human monocyte subsets: implications for health and disease. Immunol Res (2012) 53:41–57. doi:10.1007/s12026-012-8297-3
89. Shalova IN, Lim JY, Chittezhath M, Zinkernagel AS, Beasley F, Hernandez-Jimenez E, et al. Human monocytes undergo functional re-programming during sepsis mediated by hypoxia-inducible factor-1alpha. Immunity (2015) 42:484–98. doi:10.1016/j.immuni.2015.02.001
90. Cook EB, Stahl JL, Schwantes EA, Fox KE, Mathur SK. IL-3 and TNFalpha increase thymic stromal lymphopoietin receptor (TSLPR) expression on eosinophils and enhance TSLP-stimulated degranulation. Clin Mol Allergy (2012) 10:8. doi:10.1186/1476-7961-10-8
91. Noh JY, Shin JU, Park CO, Lee N, Jin S, Kim SH, et al. Thymic stromal lymphopoietin regulates eosinophil migration via phosphorylation of l-plastin in atopic dermatitis. Exp Dermatol (2016) 25:880–6. doi:10.1111/exd.13111
92. Noti M, Wojno ED, Kim BS, Siracusa MC, Giacomin PR, Nair MG, et al. Thymic stromal lymphopoietin-elicited basophil responses promote eosinophilic esophagitis. Nat Med (2013) 19:1005–13. doi:10.1038/nm.3281
93. Salter BM, Oliveria JP, Nusca G, Smith SG, Watson RM, Comeau M, et al. Thymic stromal lymphopoietin activation of basophils in patients with allergic asthma is IL-3 dependent. J Allergy Clin Immunol (2015) 136:1636–44. doi:10.1016/j.jaci.2015.03.039
94. Varricchi G, Raap U, Rivellese F, Marone G, Gibbs BF. Human mast cells and basophils-How are they similar how are they different? Immunol Rev (2018) 282:8–34. doi:10.1111/imr.12627
95. Taylor BC, Zaph C, Troy AE, Du Y, Guild KJ, Comeau MR, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med (2009) 206:655–67. doi:10.1084/jem.20081499
96. Massacand JC, Stettler RC, Meier R, Humphreys NE, Grencis RK, Marsland BJ, et al. Helminth products bypass the need for TSLP in Th2 immune responses by directly modulating dendritic cell function. Proc Natl Acad Sci U S A (2009) 106:13968–73. doi:10.1073/pnas.0906367106
97. Perrigoue JG, Saenz SA, Siracusa MC, Allenspach EJ, Taylor BC, Giacomin PR, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol (2009) 10:697–705. doi:10.1038/ni.1740
98. Turcot V, Bouchard L, Faucher G, Garneau V, Tchernof A, Deshaies Y, et al. Thymic stromal lymphopoietin: an immune cytokine gene associated with the metabolic syndrome and blood pressure in severe obesity. Clin Sci (Lond) (2012) 123:99–109. doi:10.1042/CS20110584
99. Yu K, Dong Q, Mao X, Meng K, Zhao X, Ji Q, et al. Disruption of the TSLP-TSLPR-LAP signaling between epithelial and dendritic cells through hyperlipidemia contributes to regulatory T-Cell defects in atherosclerotic mice. Atherosclerosis (2015) 238:278–88. doi:10.1016/j.atherosclerosis.2014.12.019
100. Harada M, Hirota T, Jodo AI, Hitomi Y, Sakashita M, Tsunoda T, et al. Thymic stromal lymphopoietin gene promoter polymorphisms are associated with susceptibility to bronchial asthma. Am J Respir Cell Mol Biol (2011) 44:787–93. doi:10.1165/rcmb.2009-0418OC
101. Datta A, Alexander R, Sulikowski MG, Nicholson AG, Maher TM, Scotton CJ, et al. Evidence for a functional thymic stromal lymphopoietin signaling axis in fibrotic lung disease. J Immunol (2013) 191:4867–79. doi:10.4049/jimmunol.1300588
102. Dong H, Hu Y, Liu L, Zou M, Huang C, Luo L, et al. Distinct roles of short and long thymic stromal lymphopoietin isoforms in house dust mite-induced asthmatic airway epithelial barrier disruption. Sci Rep (2016) 6:39559. doi:10.1038/srep39559
103. Sonesson A, Kasetty G, Olin AI, Malmsten M, Morgelin M, Sorensen OE, et al. Thymic stromal lymphopoietin exerts antimicrobial activities. Exp Dermatol (2011) 20:1004–10. doi:10.1111/j.1600-0625.2011.01391.x
104. Lu N, Wang YH, Arima K, Hanabuchi S, Liu YJ. TSLP and IL-7 use two different mechanisms to regulate human CD4+ T cell homeostasis. J Exp Med (2009) 206:2111–9. doi:10.1084/jem.20090153
105. Bjerkan L, Sonesson A, Schenck K. Multiple functions of the new cytokine-based antimicrobial peptide thymic stromal lymphopoietin (TSLP). Pharmaceuticals (Basel) (2016) 9(3):41. doi:10.3390/ph9030041
106. Nagarkar DR, Poposki JA, Tan BK, Comeau MR, Peters AT, Hulse KE, et al. Thymic stromal lymphopoietin activity is increased in nasal polyps of patients with chronic rhinosinusitis. J Allergy Clin Immunol (2013) 132:593–600.e12. doi:10.1016/j.jaci.2013.04.005
107. Gudbjartsson DF, Bjornsdottir US, Halapi E, Helgadottir A, Sulem P, Jonsdottir GM, et al. Sequence variants affecting eosinophil numbers associate with asthma and myocardial infarction. Nat Genet (2009) 41:342–7. doi:10.1038/ng.323
108. He JQ, Hallstrand TS, Knight D, Chan-Yeung M, Sandford A, Tripp B, et al. A thymic stromal lymphopoietin gene variant is associated with asthma and airway hyperresponsiveness. J Allergy Clin Immunol (2009) 124:222–9. doi:10.1016/j.jaci.2009.04.018
109. Al-Shami A, Spolski R, Kelly J, Keane-Myers A, Leonard WJ. A role for TSLP in the development of inflammation in an asthma model. J Exp Med (2005) 202:829–39. doi:10.1084/jem.20050199
110. Zhou B, Comeau MR, De Smedt T, Liggitt HD, Dahl ME, Lewis DB, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol (2005) 6:1047–53. doi:10.1038/ni1247
111. Headley MB, Zhou B, Shih WX, Aye T, Comeau MR, Ziegler SF. TSLP conditions the lung immune environment for the generation of pathogenic innate and antigen-specific adaptive immune responses. J Immunol (2009) 182:1641–7. doi:10.4049/jimmunol.182.3.1641
112. Chauhan A, Singh M, Agarwal A, Paul N. Correlation of TSLP, IL-33, and CD4 + CD25 + FOXP3 + T regulatory (Treg) in pediatric asthma. J Asthma (2015) 52:868–72. doi:10.3109/02770903.2015.1026441
113. Hirota T, Takahashi A, Kubo M, Tsunoda T, Tomita K, Doi S, et al. Genome-wide association study identifies three new susceptibility loci for adult asthma in the Japanese population. Nat Genet (2011) 43:893–6. doi:10.1371/journal.pgen.1002170
114. Torgerson DG, Ampleford EJ, Chiu GY, Gauderman WJ, Gignoux CR, Graves PE, et al. Meta-analysis of genome-wide association studies of asthma in ethnically diverse North American populations. Nat Genet (2011) 43:887–92. doi:10.1038/ng.888
115. Ramasamy A, Curjuric I, Coin LJ, Kumar A, McArdle WL, Imboden M, et al. A genome-wide meta-analysis of genetic variants associated with allergic rhinitis and grass sensitization and their interaction with birth order. J Allergy Clin Immunol (2011) 128:996–1005. doi:10.1016/j.jaci.2011.08.030
116. Liu W, Xu LS, Liu QJ, Dong FZ, Qiu RF, Wen MC, et al. Two single nucleotide polymorphisms in TSLP gene are associated with asthma susceptibility in Chinese Han population. Exp Lung Res (2012) 38:375–82. doi:10.3109/01902148.2012.714840
117. Sun Q, Liu Y, Zhang S, Liu K, Zhu X, Liu J, et al. Thymic stromal lymphopoietin polymorphisms and allergic rhinitis risk: a systematic review and meta-analysis with 6351 cases and 11472 controls. Int J Clin Exp Med (2015) 8:15752–8.
118. Wang IJ, Wu LS, Lockett GA, Karmaus WJ. TSLP polymorphisms, allergen exposures, and the risk of atopic disorders in children. Ann Allergy Asthma Immunol (2016) 116:139–45.e1. doi:10.1016/j.anai.2015.11.016
119. Hu Y, Dong H, Zou M, Huang C, Luo L, Yu C, et al. TSLP signaling blocking alleviates E-cadherin dysfunction of airway epithelium in a HDM-induced asthma model. Cell Immunol (2017) 315:56–63. doi:10.1016/j.cellimm.2017.02.003
120. Berraies A, Hamdi B, Ammar J, Hamzaoui K, Hamzaoui A. Increased expression of thymic stromal lymphopoietin in induced sputum from asthmatic children. Immunol Lett (2016) 178:85–91. doi:10.1016/j.imlet.2016.08.004
121. Gluck J, Rymarczyk B, Kasprzak M, Rogala B. Increased levels of interleukin-33 and thymic stromal lymphopoietin in exhaled breath condensate in chronic bronchial asthma. Int Arch Allergy Immunol (2016) 169:51–6. doi:10.1159/000444017
122. Li Y, Wang W, Lv Z, Chen Y, Huang K, Corrigan CJ, et al. Elevated expression of IL-33 and TSLP in the airways of human asthmatics in vivo: a potential biomarker of severe refractory disease. J Immunol (2018) 200:2253–62. doi:10.4049/jimmunol.1701455
123. Gauvreau GM, O’Byrne PM, Boulet LP, Wang Y, Cockcroft D, Bigler J, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med (2014) 370:2102–10. doi:10.1056/NEJMoa1402895
124. Corren J, Parnes JR, Wang L, Mo M, Roseti SL, Griffiths JM, et al. Tezepelumab in adults with uncontrolled asthma. N Engl J Med (2017) 377:936–46. doi:10.1056/NEJMoa1704064
125. Tomassen P, Vandeplas G, Van Zele T, Cardell LO, Arebro J, Olze H, et al. Inflammatory endotypes of chronic rhinosinusitis based on cluster analysis of biomarkers. J Allergy Clin Immunol (2016) 137:1449–56.e4. doi:10.1016/j.jaci.2015.12.1324
126. Schleimer RP. Immunopathogenesis of chronic rhinosinusitis and nasal polyposis. Annu Rev Pathol (2017) 12:331–57. doi:10.1146/annurev-pathol-052016-100401
127. Davis BP, Rothenberg ME. Mechanisms of disease of eosinophilic esophagitis. Annu Rev Pathol (2016) 11:365–93. doi:10.1146/annurev-pathol-012615-044241
128. O’Shea KM, Aceves SS, Dellon ES, Gupta SK, Spergel JM, Furuta GT, et al. Pathophysiology of eosinophilic esophagitis. Gastroenterology (2018) 154:333–45. doi:10.1053/j.gastro.2017.06.065
129. Sherrill JD, Gao PS, Stucke EM, Blanchard C, Collins MH, Putnam PE, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol (2010) 126:160–5.e3. doi:10.1016/j.jaci.2010.04.037
130. Rothenberg ME, Spergel JM, Sherrill JD, Annaiah K, Martin LJ, Cianferoni A, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet (2010) 42:289–91. doi:10.1038/ng.547
131. Kottyan LC, Davis BP, Sherrill JD, Liu K, Rochman M, Kaufman K, et al. Genome-wide association analysis of eosinophilic esophagitis provides insight into the tissue specificity of this allergic disease. Nat Genet (2014) 46:895–900. doi:10.1038/ng.3033
132. Sleiman PM, Wang ML, Cianferoni A, Aceves S, Gonsalves N, Nadeau K, et al. GWAS identifies four novel eosinophilic esophagitis loci. Nat Commun (2014) 5:5593. doi:10.1038/ncomms6593
133. Kuo IH, Yoshida T, De Benedetto A, Beck LA. The cutaneous innate immune response in patients with atopic dermatitis. J Allergy Clin Immunol (2013) 131:266–78. doi:10.1016/j.jaci.2012.12.1563
134. Borriello F, Granata F, Marone G. Basophils and skin disorders. J Invest Dermatol (2014) 134:1202–10. doi:10.1038/jid.2014.16
135. Gao PS, Rafaels NM, Mu D, Hand T, Murray T, Boguniewicz M, et al. Genetic variants in thymic stromal lymphopoietin are associated with atopic dermatitis and eczema herpeticum. J Allergy Clin Immunol (2010) 125:1403–7.e4. doi:10.1016/j.jaci.2010.03.016
136. Margolis DJ, Kim B, Apter AJ, Gupta J, Hoffstad O, Papadopoulos M, et al. Thymic stromal lymphopoietin variation, filaggrin loss of function, and the persistence of atopic dermatitis. JAMA Dermatol (2014) 150:254–9. doi:10.1001/jamadermatol.2013.7954
137. Alysandratos KD, Angelidou A, Vasiadi M, Zhang B, Kalogeromitros D, Katsarou-Katsari A, et al. Increased affected skin gene expression and serum levels of thymic stromal lymphopoietin in atopic dermatitis. Ann Allergy Asthma Immunol (2010) 105:403–4. doi:10.1016/j.anai.2010.09.017
138. Nygaard U, Hvid M, Johansen C, Buchner M, Folster-Holst R, Deleuran M, et al. TSLP, IL-31, IL-33 and sST2 are new biomarkers in endophenotypic profiling of adult and childhood atopic dermatitis. J Eur Acad Dermatol Venereol (2016) 30:1930–8. doi:10.1111/jdv.13679
139. Sano Y, Masuda K, Tamagawa-Mineoka R, Matsunaka H, Murakami Y, Yamashita R, et al. Thymic stromal lymphopoietin expression is increased in the horny layer of patients with atopic dermatitis. Clin Exp Immunol (2013) 171:330–7. doi:10.1111/cei.12021
140. Ito Y, Satoh T, Takayama K, Miyagishi C, Walls AF, Yokozeki H. Basophil recruitment and activation in inflammatory skin diseases. Allergy (2011) 66:1107–13. doi:10.1111/j.1398-9995.2011.02570.x
141. Kim BS, Wang K, Siracusa MC, Saenz SA, Brestoff JR, Monticelli LA, et al. Basophils promote innate lymphoid cell responses in inflamed skin. J Immunol (2014) 193:3717–25. doi:10.4049/jimmunol.1401307
142. Schwartz C, Eberle JU, Hoyler T, Diefenbach A, Lechmann M, Voehringer D. Opposing functions of thymic stromal lymphopoietin-responsive basophils and dendritic cells in a mouse model of atopic dermatitis. J Allergy Clin Immunol (2016) 138:1443–6.e8. doi:10.1016/j.jaci.2016.04.031
143. Varricchi G, Harker J, Borriello F, Marone G, Durham SR, Shamji MH. T follicular helper (Tfh) cells in normal immune responses and in allergic disorders. Allergy (2016) 71:1086–94. doi:10.1111/all.12878
144. Macchia D, Melioli G, Pravettoni V, Nucera E, Piantanida M, Caminati M, et al. Guidelines for the use and interpretation of diagnostic methods in adult food allergy. Clin Mol Allergy (2015) 13:27. doi:10.1186/s12948-015-0033-9
145. Lee JU, Chang HS, Lee HJ, Jung CA, Bae DJ, Song HJ, et al. Upregulation of interleukin-33 and thymic stromal lymphopoietin levels in the lungs of idiopathic pulmonary fibrosis. BMC Pulm Med (2017) 17:39. doi:10.1186/s12890-017-0380-z
146. Di Sabatino A, Corazza GR. Coeliac disease. Lancet (2009) 373:1480–93. doi:10.1016/S0140-6736(09)60254-3
147. Tsilingiri K, Fornasa G, Rescigno M. Thymic stromal lymphopoietin: to cut a long story short. Cell Mol Gastroenterol Hepatol (2017) 3:174–82. doi:10.1016/j.jcmgh.2017.01.005
148. Iseki M, Omori-Miyake M, Xu W, Sun X, Takaki S, Rawlings DJ, et al. Thymic stromal lymphopoietin (TSLP)-induced polyclonal B-cell activation and autoimmunity are mediated by CD4+ T cells and IL-4. Int Immunol (2012) 24:183–95. doi:10.1093/intimm/dxr113
149. Hartgring SA, Willis CR, Dean CE Jr, Broere F, van Eden W, Bijlsma JW, et al. Critical proinflammatory role of thymic stromal lymphopoietin and its receptor in experimental autoimmune arthritis. Arthritis Rheum (2011) 63:1878–87. doi:10.1002/art.30336
150. Eckhardt J, Dobbeler M, Konig C, Kuczera K, Kuhnt C, Ostalecki C, et al. Thymic stromal lymphopoietin deficiency attenuates experimental autoimmune encephalomyelitis. Clin Exp Immunol (2015) 181:51–64. doi:10.1111/cei.12621
151. Rivellese F, Nerviani A, Rossi FW, Marone G, Matucci-Cerinic M, de Paulis A, et al. Mast cells in rheumatoid arthritis: friends or foes? Autoimmun Rev (2017) 16:557–63. doi:10.1016/j.autrev.2017.04.001
152. Ozawa T, Koyama K, Ando T, Ohnuma Y, Hatsushika K, Ohba T, et al. Thymic stromal lymphopoietin secretion of synovial fibroblasts is positively and negatively regulated by toll-like receptors/nuclear factor-kappaB pathway and interferon-gamma/dexamethasone. Mod Rheumatol (2007) 17:459–63. doi:10.1007/s10165-007-0620-9
153. Rivellese F, Mauro D, Nerviani A, Pagani S, Fossati-Jimack L, Messemaker T, et al. Mast cells in early rheumatoid arthritis are associated with synovial lymphoid aggregates and worse disease activity and support autoantibody production by B cells. Ann Rheum Dis (2018).
154. Jeong HJ, Nam SY, Oh HA, Han NR, Kim YS, Moon PD, et al. Interleukin-32-induced thymic stromal lymphopoietin plays a critical role in macrophage differentiation through the activation of caspase-1 in vitro. Arthritis Res Ther (2012) 14:R259. doi:10.1186/ar4104
155. Lowes MA, Suarez-Farinas M, Krueger JG. Immunology of psoriasis. Annu Rev Immunol (2014) 32:227–55. doi:10.1146/annurev-immunol-032713-120225
156. Lande R, Botti E, Jandus C, Dojcinovic D, Fanelli G, Conrad C, et al. The antimicrobial peptide LL37 is a T-cell autoantigen in psoriasis. Nat Commun (2014) 5:5621. doi:10.1038/ncomms6621
157. Arakawa A, Siewert K, Stohr J, Besgen P, Kim SM, Ruhl G, et al. Melanocyte antigen triggers autoimmunity in human psoriasis. J Exp Med (2015) 212:2203–12. doi:10.1084/jem.20151093
158. Fuentes-Duculan J, Bonifacio KM, Hawkes JE, Kunjravia N, Cueto I, Li X, et al. Autoantigens ADAMTSL5 and LL37 are significantly upregulated in active psoriasis and localized with keratinocytes, dendritic cells and other leukocytes. Exp Dermatol (2017) 26:1075–82. doi:10.1111/exd.13378
159. Lockshin B, Balagula Y, Merola JF. Interleukin 17, inflammation, and cardiovascular risk in patients with psoriasis. J Am Acad Dermatol (2018). doi:10.1016/j.jaad.2018.02.040
160. Kim J, Krueger JG. Highly effective new treatments for psoriasis target the IL-23/Type 17 T cell autoimmune axis. Annu Rev Med (2017) 68:255–69. doi:10.1146/annurev-med-042915-103905
161. Staiano RI, Loffredo S, Borriello F, Iannotti FA, Piscitelli F, Orlando P, et al. Human lung-resident macrophages express CB1 and CB2 receptors whose activation inhibits the release of angiogenic and lymphangiogenic factors. J Leukoc Biol (2016) 99:531–40. doi:10.1189/jlb.3HI1214-584R
162. Varricchi G, Galdiero MR, Loffredo S, Marone G, Iannone R, Granata F. Are mast cells MASTers in cancer? Front Immunol (2017) 8:424. doi:10.3389/fimmu.2017.00424
163. Varricchi G, Galdiero MR, Marone G, Granata F, Borriello F. Controversial role of mast cells in skin cancers. Exp Dermatol (2017) 26:11–7. doi:10.1111/exd.13107
164. Olkhanud PB, Rochman Y, Bodogai M, Malchinkhuu E, Wejksza K, Xu M, et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J Immunol (2011) 186:5656–62. doi:10.4049/jimmunol.1100463
165. Perez-Andreu V, Roberts KG, Harvey RC, Yang W, Cheng C, Pei D, et al. Inherited GATA3 variants are associated with Ph-like childhood acute lymphoblastic leukemia and risk of relapse. Nat Genet (2013) 45:1494–8. doi:10.1038/ng.2803
166. Yoda A, Yoda Y, Chiaretti S, Bar-Natan M, Mani K, Rodig SJ, et al. Functional screening identifies CRLF2 in precursor B-cell acute lymphoblastic leukemia. Proc Natl Acad Sci U S A (2010) 107:252–7. doi:10.1073/pnas.0911726107
167. Russell LJ, Capasso M, Vater I, Akasaka T, Bernard OA, Calasanz MJ, et al. Deregulated expression of cytokine receptor gene, CRLF2, is involved in lymphoid transformation in B-cell precursor acute lymphoblastic leukemia. Blood (2009) 114:2688–98. doi:10.1182/blood-2009-03-208397
168. Takahashi N, Sugaya M, Suga H, Oka T, Kawaguchi M, Miyagaki T, et al. Thymic stromal chemokine TSLP Acts through Th2 cytokine production to induce cutaneous T-cell lymphoma. Cancer Res (2016) 76:6241–52. doi:10.1158/0008-5472.CAN-16-0992
169. Xie F, Meng YH, Liu LB, Chang KK, Li H, Li MQ, et al. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am J Reprod Immunol (2013) 70:69–79. doi:10.1111/aji.12104
170. Pedroza-Gonzalez A, Xu K, Wu TC, Aspord C, Tindle S, Marches F, et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J Exp Med (2011) 208:479–90. doi:10.1084/jem.20102131
171. Kuan EL, Ziegler SF. A tumor-myeloid cell axis, mediated via the cytokines IL-1alpha and TSLP, promotes the progression of breast cancer. Nat Immunol (2018) 19:366–74. doi:10.1038/s41590-018-0066-6
172. Di Piazza M, Nowell CS, Koch U, Durham AD, Radtke F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell (2012) 22:479–93. doi:10.1016/j.ccr.2012.08.016
173. Demehri S, Turkoz A, Manivasagam S, Yockey LJ, Turkoz M, Kopan R. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell (2012) 22:494–505. doi:10.1016/j.ccr.2012.08.017
174. Yue W, Lin Y, Yang X, Li B, Liu J, He R. Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor growth by promoting apoptosis of tumor cells. Oncotarget (2016) 7:16840–54. doi:10.18632/oncotarget.7614
175. Demehri S, Cunningham TJ, Manivasagam S, Ngo KH, Moradi Tuchayi S, Reddy R, et al. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J Clin Invest (2016) 126:1458–70. doi:10.1172/JCI83724
176. Ghirelli C, Sadacca B, Reyal F, Zollinger R, Michea P, Sirven P, et al. No evidence for TSLP pathway activity in human breast cancer. Oncoimmunology (2016) 5:e1178438. doi:10.1080/2162402X.2016.1178438
177. He R, Geha RS. Thymic stromal lymphopoietin. Ann N Y Acad Sci (2010) 1183:13–24. doi:10.1111/j.1749-6632.2009.05128.x
178. Lefrancais E, Duval A, Mirey E, Roga S, Espinosa E, Cayrol C, et al. Central domain of IL-33 is cleaved by mast cell proteases for potent activation of group-2 innate lymphoid cells. Proc Natl Acad Sci U S A (2014) 111:15502–7. doi:10.1073/pnas.1410700111
179. Cayrol C, Duval A, Schmitt P, Roga S, Camus M, Stella A, et al. Environmental allergens induce allergic inflammation through proteolytic maturation of IL-33. Nat Immunol (2018) 19:375–85. doi:10.1038/s41590-018-0067-5
Keywords: asthma, atopic dermatitis, cancer, inflammation, thymic stromal lymphopoietin
Citation: Varricchi G, Pecoraro A, Marone G, Criscuolo G, Spadaro G, Genovese A and Marone G (2018) Thymic Stromal Lymphopoietin Isoforms, Inflammatory Disorders, and Cancer. Front. Immunol. 9:1595. doi: 10.3389/fimmu.2018.01595
Received: 24 May 2018; Accepted: 27 June 2018;
Published: 13 July 2018
Edited by:
Fulvio D’Acquisto, University of Roehampton, United KingdomReviewed by:
Alexander A. Shtil, Russian Cancer Research Center NN Blokhin, RussiaGiuseppe Terrazzano, University of Basilicata, Italy
Copyright: © 2018 Varricchi, Pecoraro, Marone, Criscuolo, Spadaro, Genovese and Marone. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Gilda Varricchi, gildanet@gmail.com;
Gianni Marone, marone@unina.it
 Gilda Varricchi
Gilda Varricchi Antonio Pecoraro
Antonio Pecoraro Giancarlo Marone
Giancarlo Marone Gjada Criscuolo
Gjada Criscuolo Giuseppe Spadaro
Giuseppe Spadaro Arturo Genovese1,2
Arturo Genovese1,2 Gianni Marone
Gianni Marone