- 1Divisions of Immunology and Molecular Immunology, Walter and Eliza Hall Institute of Medical Research, Parkville, VIC, Australia
- 2Department of Medical Biology, University of Melbourne, Parkville, VIC, Australia
The skin represents a specialized site for immune surveillance consisting of resident, inflammatory and memory populations of lymphocytes. The entry and retention of T cells, B cells, and ILCs is tightly regulated to facilitate detection of pathogens, inflammation and tumors cells. Loss of individual or multiple populations in the skin may break tolerance or increase susceptibility to tumor growth and spread. Studies have significantly advanced our understanding of the role of skin T cells and ILCs at steady state and in inflammatory settings such as viral challenge, atopy, and autoimmune inflammation. The knowledge raised by these studies can benefit to our understanding of immune cell trafficking in primary melanoma, shedding light on the mechanisms of tumor immune surveillance and to improve immunotherapy. This review will focus on the T cells, B cells, and ILCs of the skin at steady state, in inflammatory context and in melanoma. In particular, we will detail the core chemokine and adhesion molecules that regulate cell trafficking to and within the skin, which may provide therapeutic avenues to promote tumor homing for a team of lymphocytes.
Introduction
The skin, the body's largest organ with its 1.8 m2, represents our first line of defense and comprises a wide diversity of innate and adaptive immune cells. It is a complex organ as revealed by its structural organization in layers and by its multiple physiological functions. Indeed, while the skin provides a barrier protection from external environment, it further acts as a physical sensor and a temperature and hydration regulator by communicating with the outside environment. At homeostasis, lymphocytes constantly traffic to and within this complex environment, patrolling for pathogen invasion, inflammatory conditions and malignancy development. Loss of skin immune cell integrity can lead to diverse local diseases like atopic dermatitis, psoriasis, or tumor development (1, 2).
This review intends to map lymphocyte trafficking routes to and within the skin at homeostasis. Furthermore, we point out how lymphocyte trafficking responds to threats and we finally discuss how a better understanding of lymphocyte recruitment, specific location within the skin and interacting partners is essential to identify targets that may be manipulated for therapeutic benefits in cancer.
Skin: Home of a Plethora of Cells Within a Complex Structure
Mammalian skin is composed of a succession of three different layers, the epidermis, the dermis and the hypodermis, comprising nerves, blood vessels, glands, and hair follicles (Figure 1). The outer epidermis is a stratified squamous epithelium (the stratums: corneum, granulosum, spinosum, and basale), mainly populated by keratinocytes (80%), melanocytes, Langerhans cells, and Merkel cells (nerve-ending cells). Below the epidermis, separated by a basement membrane, the dermis is a thicker layer which home various cell types (fibroblasts, immune cells) into a collagen-rich complex matrix. The collagen fibers (thin in the upper stratum papillare and thick in the lower stratum reticulare) offer a structural framework to host cells. Underneath, the hypodermis is an adipocyte rich region. While the epidermis is not vascularized, the dermis is innerved by lymphatic and blood vessels both of them playing important role in outcoming and incoming trafficking of cells, small molecules and pathogens (Figure 1). The dermal lymphatic system represents a major conduit for pathogens and immune cells from the skin to the draining lymph node. This has been recently reviewed elsewhere (3, 4) and will not be described here further.
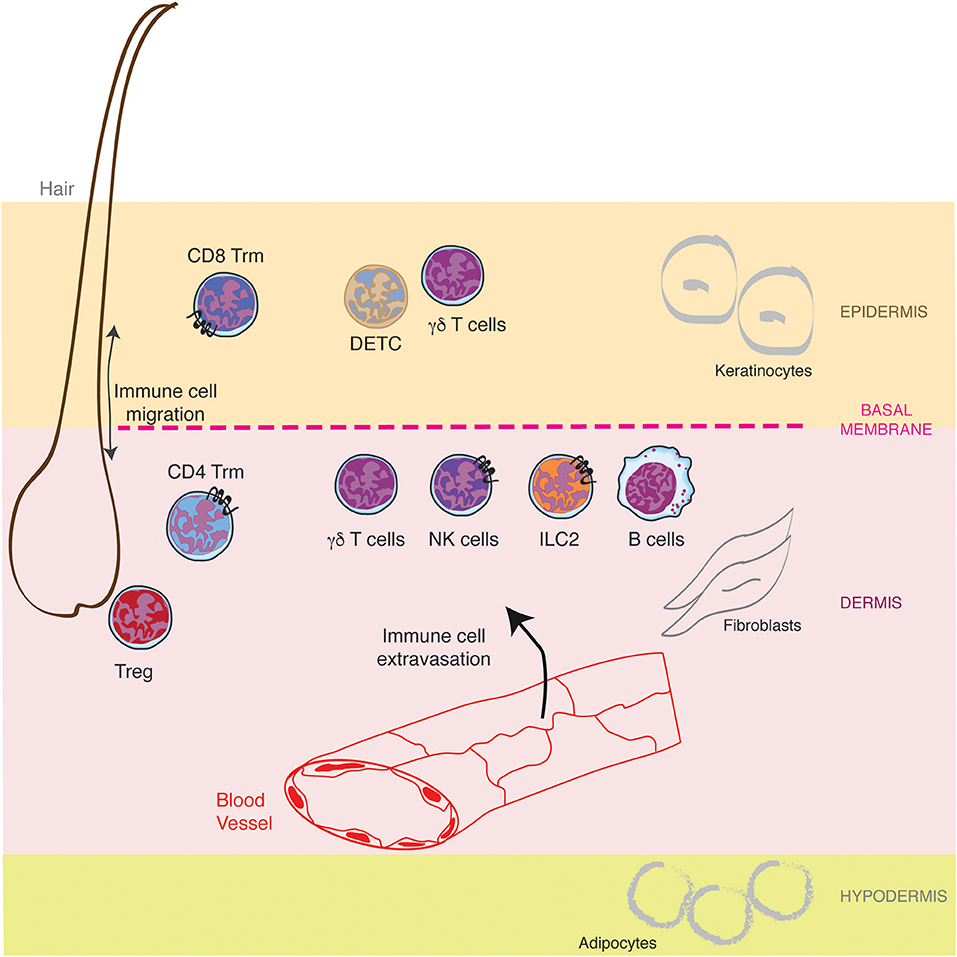
Figure 1. Lymphocytes specific location in healthy skin. The skin is composed of a succession of three different layers, the epidermis, the dermis and the hypodermis populated by resident lymphocytes and lymphocytes recruited from the blood circulation through the cascade of molecular interactions. Furthermore, hair follicles represent a specific niche for some lymphocytes.
The skin harbors a wide variety of immune cells including macrophages, mast cells, dendritic cells, and lymphocytes (3). Some of these subsets seed the skin during embryonic development and become skin resident cells while others continuously recirculate or are replenished from circulating bone-marrow precursors. Our review focuses on innate and adaptive lymphocytes, including T lymphocytes, B lymphocytes, and innate lymphoid cells (ILC). These cells either reside in the skin or are recruited to the skin from the blood circulation at homeostasis and/or following inflammatory signals.
While, it was first assumed that healthy human skin was devoid of B lymphocytes (4), a heterogeneous population of B cells (large B-cells and IgMhigh B-1-like B cells) has been observed in sheep dermis where their expression of MHC-II and CD80/86 was slightly increased compared to lymph node B-cells (5). Recently, immunohistochemical staining revealed the presence of CD22+ B cells in around 30% of healthy human skin samples (6). These cells were mature IgG antibody-expressing B cells distinct from the circulating B cells suggesting that class switched and somatic hypermutated B cells localize to the skin at homeostasis. As opposed to B lymphocytes, T lymphocytes are highly represented in the skin. In healthy individuals, it has been estimated that 20 billion of T lymphocytes populate the entire skin surface, strikingly, this is almost twice the number of T lymphocytes found in the total blood circulation (7). Most of the cutaneous αβ T lymphocytes are resident memory T cells. Both memory CD4+ and CD8+ T cells were observed in the skin with 20–60% of murine cutaneous CD4+ T cells being Treg (8, 9). Unconventional γδT lymphocytes, dendritic epidermal γδ T cells (DETC, Vγ5 Vδ1) and dermal γδ T cells (γδT17 cells) are also skin resident cells under homeostatic conditions. These populations participate in early stage of immune response (9, 10). In human skin, γδ T cells represent <10% of the dermis and epidermis total T cells whereas in mice epidermis, DETC alone constitute 90% of the total T cells (3, 11). γδ T cells recognized self and conserved motifs of microbial-derived antigens.
ILC are non-T, non-B lymphocytes, characterized by a lack of myeloid and dendritic cell phenotype markers and by a lymphoid morphology. Recently, a new nomenclature has been proposed classifying ILC in 5 subgroups: ILC1, ILC2, ILC3, conventional natural killer cells (NK) and lymphoid tissue inducer cells (LTi) (12). While ILC1-3 and NK populations have been observed in healthy human and mouse skin, ILC2 represents more than 90% of them in mice skin (13). In terms of function, ILC2 are an important source of type-2 cytokines and has been associated with allergic inflammation in the skin. Further, healthy human skin is populated by both immature CD56bright CD16− and mature CD56dimCD16+ NK cells (10, 14). Their frequency varies from 1 to 10% of total CD45+ leucocytes between studies, those cells being mainly enriched in the blood circulation. NK cells are cytotoxic to virus-infected cells and tumor cells and cytokine producers.
In addition to immune cells, the skin is also colonized by resident microorganisms (bacteria, fungi, parasites, viruses), feeding on corneocyte debris and sebum. The skin microbiome is essential not only to prevent the development of pathogenic microbes for the organism, but also to educate the immune system (15) and finely tune immune cell functions (16). Recently the skin transcriptome of germ-free mice has been compared to the one of specific pathogen mice (microbiota positive), highlighting more than 2,800 genes modulated in the skin by the microbiota (17). Particularly, 37 genes, including chemokine family gene Ccr2, Ccr5, Ccl5, Ccl6, Cxcl8, and Cxcl9, were upregulated during microbial colonization in the skin and the gut (17, 18). While the Meisel et al. study focussed their bioinformatic analysis to genes co-regulated in the gut and skin microbiota, further datamining of this work would highlight genes specifically regulated in the skin. Indeed, these tissue specific genes may represent interesting therapeutic targets to balance immune cell recruitment in a site-specific manner.
Lymphocyte Entry Within the Skin at Steady State: a Selective System
The Cascade of Molecular Interactions for Circulating Lymphocyte Recruitment
Postcapillary venules composed by a flat endothelium represent the main gateway for lymphocyte recruitment in non-lymphoid tissues. In contrast to other non-lymphoid tissues where leukocyte recruitment is rare or inexistent in the absence of inflammatory stimuli, a constitutive afflux of lymphocytes occurs at steady state in the skin (19, 20). This afflux remains low when compared to the maestro in terms of recruitment: the lymph node high endothelial venules (21, 22). However, similar to that described in lymph nodes, lymphocyte entry in the skin is finely regulated by a cascade of interactions mediating immune cell rolling, adhesion and extravasation (23) (Figure 2). The initial capture of circulating lymphocytes by endothelial cells is achieved by the interaction between members of the selectin family with their ligands, slowing down flowing lymphocytes. Real time intravital microscopy imaging first shed the light on cell rolling, recognizable by a catch bound cell displacement along vessel walls (19, 24). Selectins have a rapid association and dissociation rate constant (Kon/Koff) and a high tensile strength, supporting the observed transient interactions (25). The selectin family which comprises 3 members (E-selectin, L-selectin, and P-selectin), bind to sialyl-Lewisx (sLeX) carbohydrates (26), fucosylated by the fucosyltransferase (FucT)-IV and FucT-VII (20, 27). The rolling phase allows lymphocyte to examine the vessel wall for chemoattractant signals (chemokines and lipids). In turn, chemokine receptor stimulation together with the hemodynamic shear force of blood flow, induce integrin conformational changes from a low-affinity conformation to a high-affinity conformation, within milliseconds, allowing cell firm adhesion on the endothelium (28, 29). Integrins are linked to the actin cytoskeleton by binding to cytoskeleton-associated protein such as talin, inducing lymphocyte morphological changes (30). Lymphocytes take up a polarized morphology forming a leading edge and an uropod to transmigrate between endothelial cells (paracellular transmigration) or through them (transcellular transmigration) (31). The cascade is broadly mechanistically identical for all immune cell recruited across all sites and under physiological or pathological context (23). However, the specificity of the process is controlled by the diversity of the selectin, integrin and chemokine molecules expressed by a tissue and the expression of the matching receptors or ligands on circulating cells (28). Furthermore, as each step of the cascade condition the next one, the combination adds extra specificity to immune cell recruitment at a particular site of the organism.
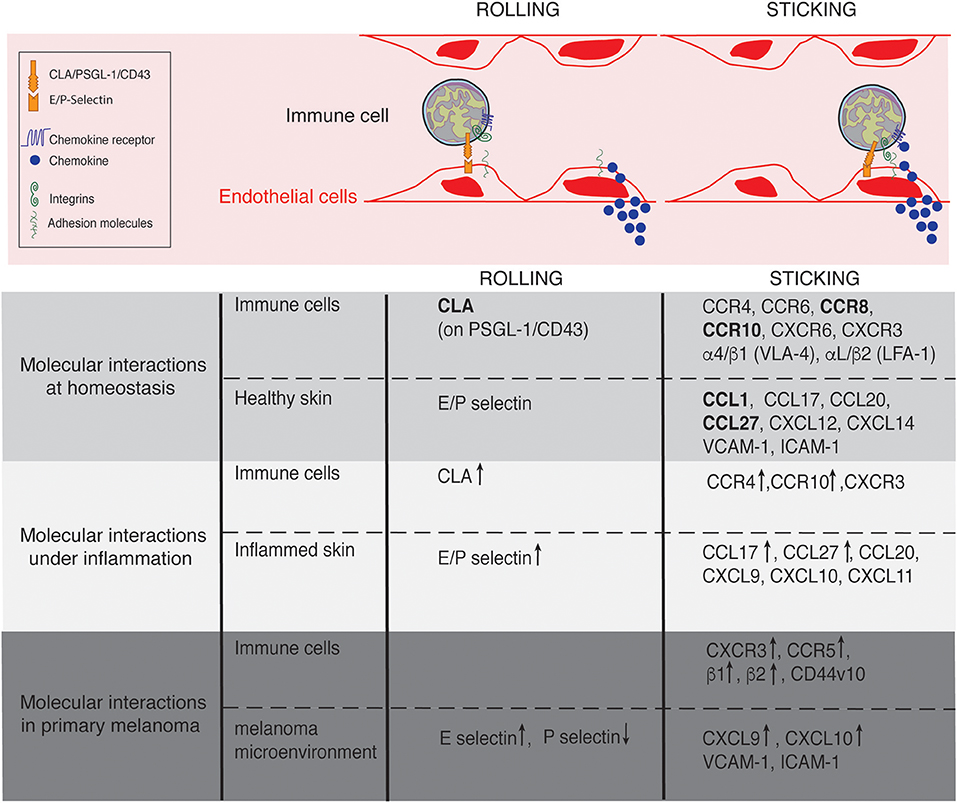
Figure 2. Lymphocyte trafficking into the skin at homeostasis, under inflammation and in primary melanoma. Molecules involved in the rolling and sticking (firm adhesion) steps of lymphocytes recruitment at homeostasis, under inflammatory conditions and in primary melanoma are listed on the figure. Of interest, most of the molecules are shared between conditions. ↑ and ↓ indicate up- and down- regulated expression, respectively in comparison to homeostasis. Skin-specific molecules are in bolt.
Skin-Associated Selectins, Chemokines, and Integrins
The anatomical site of immune cell recruitment is not random but strictly determined by the expression of a tissue-specific set of adhesion molecules and chemokine receptors (Figure 2). Compared to other tissues, the set of adhesion molecules/chemokines expressed in the skin (Table 1) is particularly unique.
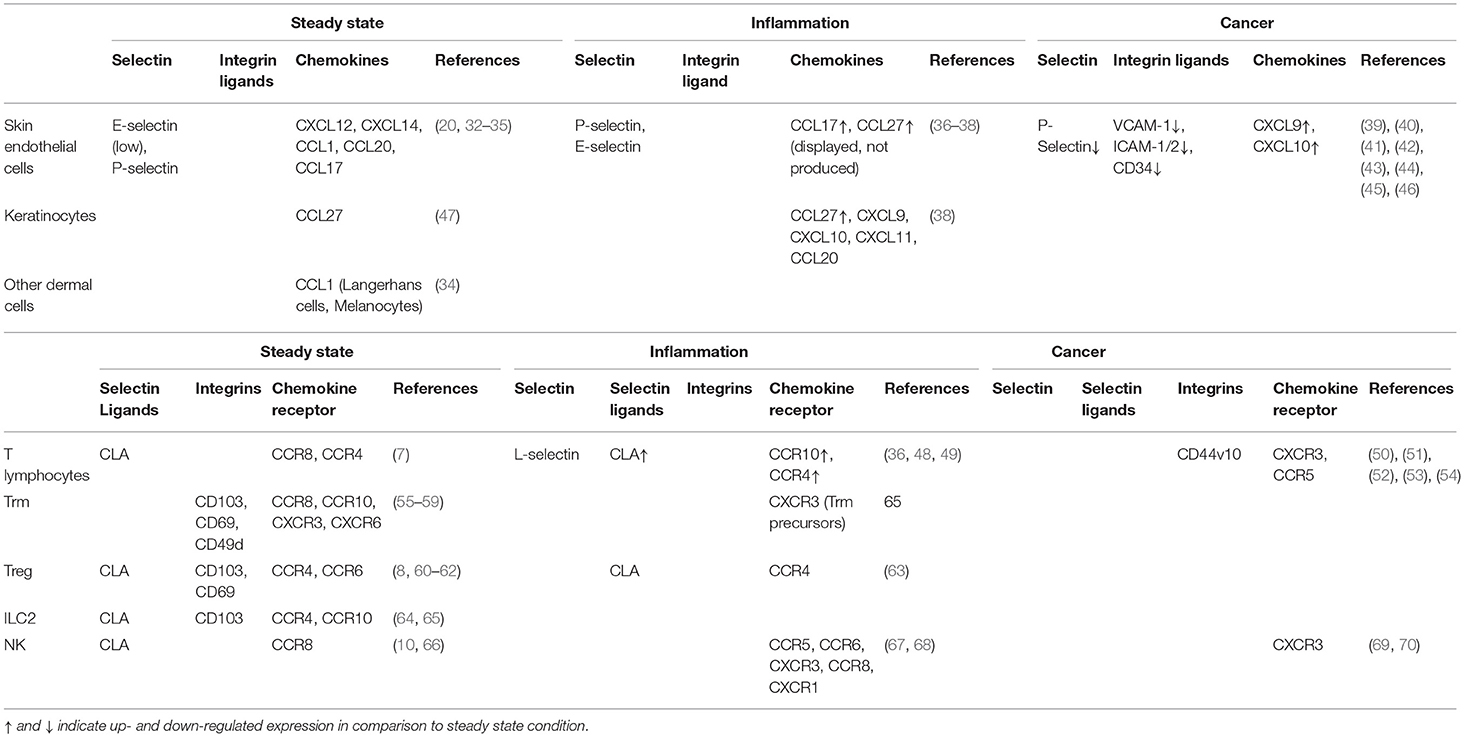
Table 1. Expression of adhesion molecules, chemokines and chemokine receptors at the surface of the indicated cells at steady state, under inflammation and in melanoma.
Three C-type lectins composed the highly conserved Selectin family: the L-Selectin (expressed on leukocytes and hematopoietic stem cells) and the vascular selectins, E-Selectin (expressed on endothelial cells) and P-Selectin (expressed on endothelial cells and platelets) (29). Selectins are type I membrane glycoproteins with an N-terminal C-type lectin domain, an EGF-like motif, a transmembrane domain and a short cytoplasmic tail. Skin endothelial cells constitutively express low level of E-selectin allowing leukocyte rolling at low velocity whereas P-selectin determines the rolling frequency (20). L-selectin is not expressed by endothelial cells but it is expressed at the surface of some subsets of leukocytes. However, it has been shown that L-selectin is not involved in immune cell rolling at homeostasis.
The chemokine family comprises about 50 small chemokine ligands recognized by ~20 seven-transmembrane, G-protein-coupled receptors (71). Most of the chemokine receptors have several ligands and vice-versa. We have previously discussed the implications of a such apparent redundancy, illustrated with CXCR3 and its three ligands CXCL9, CXCL10, and CXCL11 (72). Originating from the Keystone symposium on Chemokines and Chemotactic Receptors in 1999, our current nomenclature of the chemokine and chemokine receptor is based on the arrangement of the two N-terminal cysteine residues forming a disulfate bond and defines 5 groups: CXC, CC, X(C), CX3C, and CX (73). Chemokines can also be segregated in two functional groups: the homeostatic chemokine are constitutively expressed by specific tissues, they retain immune cells within tissues and are involved in their survival and proliferation while the inflammatory chemokine are rapidly induced upon environmental changes allowing a massive on-site recruitment of immune cells. Interestingly, homeostatic chemokines are relatively ancient in the evolution and really well conserved between species compared to the inflammatory chemokines which have rapidly evolved (74). Consequently, homeostatic chemokines represent appealing therapeutic targets, because of the likely transferable knowledge between mouse and human studies and tissue tropism. Chemokines are small molecules which diffuse from their production site forming a gradient that can be sensed by polarized chemokine-receptor expressing lymphocytes. Chemokines can be immobilized on negatively charged scaffold proteins, like heparan sulfates by binding though their positive amino acid (75). The retention and immobilization of soluble chemokines is thought to establish gradients, allowing immune cells to sense differing chemokine concentrations to establish directionality. Skin endothelial cells constitutively display chemokines that are either produced by themselves or passed to them to be presented. This include CXCL12 (SDF-1) (32), CXCL14 (BRAK) (33), CCL1 (34), CCL20 (35), and CCL27 (CTACK) (47). CCL1 and CCL27 are the most skin-associated chemokines and by consequence represents interesting targets for controlling immune cell trafficking within healthy skin. CCL27 was firstly named CTACK for Cutaneous T cell Attracting ChemoKine because of its constitutive and unique expression by cutaneous keratinocytes. CCL27 can be further induced by TNF-α and IL-1β and binds CCR10 (76). CCL1 is constitutively expressed by dermal vessels, epidermal melanocytes and Langerhans cells and binds CCR8 (34).
Integrins are composed of one α- and one β-subunits of noncovalently-linked type I transmembrane glycoproteins (77, 78). 18 α-subunits and 8 β-subunits forming 24 distinct heterodimers has been described in mammals (79). On resting cells, integrins harbor a bent (inactive) conformation offering a low binding affinity for its ligands, that is switched to an extended (active) conformation of high binding affinity. VLA-4 (α4β1), α4β7, LFA-1 (αLβ2) are the major integrins expressed by lymphocytes however, none skin-specific ligands for integrins has been reported under homeostatic conditions in the skin.
Expression of Selectin-Ligands, Chemokine Receptors, and Integrins by Cutaneous Lymphocytes
In the skin-draining lymph nodes, effectors and memory populations receive “imprinting” signals from dendritic cells (DC) to instruct their specific migration to the skin (80). Lymph node effector CD4+ and CD8+ T cell upregulate P-selectin ligands expression and downregulate the gut-associated integrin α4β7 addressing them to the skin (81, 82). One mechanism of such “imprinting” in human involves vitamin D3 metabolization to its active form by DC, leading to an upregulation of CCR10 expression by lymph node T cells, which guides them to the skin (83). More recently it has also been shown that skin Langerin+ CD11b+ migratory DC induce E-selectin expression on naïve CD8+ T cells upon topical immunization (84). Circulating lymphocytes subsets expressing a matching set of receptors/ligands for the skin are constitutively recruited (Table 1). In healthy skin, T lymphocytes express high level of the cutaneous lymphocyte antigen (CLA), a ligand for E- and P-selectin, correlating with high E-selectin-binding activity (7). CLA is an inducible sLeX carbohydrate epitope found on PSGL-1 and CD43 (85, 86). Cutaneous T lymphocytes also largely express CCR4 and CCR6, half of them are positive for CCR8 and CXCR6 expression and about 20% are CXCR3+ (7). By contrast, in the circulation, only around 40–60% of CLA+ T cells express CCR4, CCR6, CCR7, or L-selectin and <2% CCR8, CXCR6, or CXCR3 (7). Combined, this work highlights the predominant role of CCR4 and CCR6 for T cell entry into skin.
Only a low proportion of Treg migrate to the skin under homeostatic conditions in adult (63). However, an important wave of Treg migrate to the skin between day 6 and day 13 after birth in mouse in a CCR6 dependent manner seeding this organ (8). Presumably, this homeostatic recruitment into the skin, coincides with and instructed by the seeding of the cutaneous microbiota after birth, however, this has not formally been tested. Most of human circulating Treg are CLA, L-selectin, CD11a, CCR4, CCR6, and CXCR4 positive (>70%) (60). Treg play a critical role in controlling tissue homeostasis in the skin as CCR4 deficiency in murine Treg lead to impaired Treg homing to the skin and development of severe skin inflammatory disease (61).
Human cutaneous ILC2 expressed CLA, CCR4, and CCR10 (64). Despite expressing specific set of adhesion molecules/chemokine receptors, which theoretically allow their recruitment into the skin from the blood circulation, ILC2 recruitment is still heavily debated and need to be further investigated (as discussed below). Most of the skin NK studies have been conducted in the context of inflammatory disorders or cancer while cutaneous NK cells at homeostasis have been poorly documented in comparison to T cell trafficking. Yet, it has been shown that both human and mouse NK cells mainly locate in skin dermis and that some human cutaneous NK cells expressed CLA and CCR8 but do not expressed CCR7 (10, 66). However, a complete phenotyping of these cells under homeostatic condition is so far lacking.
Recently, it has been observed that a small proportion of circulating B cells express CLA in healthy individuals (6). Sheep skin B cells express α4, β1, and β7 integrin and 16% of them also express E-Selectin ligands (5). In in vitro assays, these B cells migrate toward CCL20 but not CCL17, CCL28, and CCL1, however the minimal required combination of receptors required for cutaneous B cell recruitment is yet to be determined.
Lymphocyte Residency in the Skin at Steady State
A growing number of resident cutaneous lymphocytes has been identified including tissue-resident memory T cells (Trm), Treg but also unconventional T cells and ILCs. Trm provide superior protection against infection and recent studies indicate that they play a crucial role in cancer immunosurveillance (87–89). Indeed, Trm has been associated with a good prognostic value in lung cancer, ovarian carcinoma and more recently in breast cancer (88, 90–92). Furthermore, Trm were required for the efficacy of cancer vaccine in cutaneous melanoma and other cancer (88, 93). These studies underlie the crucial need to better characterize skin-resident populations as their manipulation via therapeutic intervention has clinical potential. The phenotype of the resident populations is highly heterogenous between tissue of residency and the nature/history of pathogen infection, underlying the pivotal influence of the environment on resident-lymphocyte phenotype and functions. However, recent studies had revealed a common transcriptional program for Trm which is distinct from the one of circulating T lymphocytes (94–96). Hobit and Runx3 transcription factors are known master regulators of tissue residency (97). Trm downregulate genes implicated in tissue egress such as S1pr1, S1pr4, S1pr5 and upregulate the chemokine Xcl1 and chemokine receptor Cxcr6 genes. Trm were first discovered in the skin in graft models (98). Indeed, symptomless skin from patients with psoriasis engrafted onto immunodeficient mice gave rise to active skin lesions, thereby demonstrating the presence of a pathogenic population residing in healthy skin. Broadly, Trm lack the lymph node homing molecules CCR7 and CD62L and express CD103 (also known as the integrin α-E subunit), CD69 and CD49d (VLA-1) although these markers do not strictly identify Trm, some of them being either CD103+ or CD69+ or negative for both markers (55, 56). In mice, the dominant pool of Trm, CD8+ CD103+ cells locate in the epidermis while in human, CD4+ CD103− Trm represent the main pool and is located in the dermis (94, 99, 100). Little is known about the mechanisms controlling Trm residency and maintenance within tissues. It has been shown that both environmental signal and transcriptional regulators instruct Trm differentiation from circulating effector T cells or potentially via an unidentified pre-committed precursor recruited via the epithelium (94, 95). While CD69 and CD103 are required for Trm development and maintenance, respectively, CXCR3 is required for Trm precursors entry in skin epithelium in the context of HSV infection (94). The role of CXCR3 has been recently confirmed in non-infected animals, where CXCR3 expression on preimmune mature CD8+ T cell (mature T lymphocytes that have not yet engaged with cognate antigen) controls their recruitment to the skin driven by CXCL10 (57). CXCR6 and CCR10 expression by CD8+ Trm is also required for their optimal formation or maintenance in the skin (58). CCR8 has also been demonstrated to be a marker of skin human Trm (59).
In addition to Trm, recirculating memory T cells (Trcm) has been identified in the skin (101, 102). As opposed to Trm, Trcm do not permanently reside in the skin but can re-enter the circulation and lymph nodes. These cells express intermediate level of L-Selectin and CCR7 required for lymph node homing in addition to E-selectin ligands addressing them the skin.
Skin resident cells also comprises Treg cells, most of them expressing CD103 and CD69 which maintain tissue homeostasis (62). DETC seeds the epidermis during fetal development in a CCR10 dependent manner and are then maintained in a CCR4 dependent manner in situ and not replenished from circulating precursors (103, 104).
At steady state, murine ILC1-3 populations were initially described as tissue resident cells (105) however, although extremely rare, circulating ILC1-3 have also been observed (about 0.01–0.3% of circulating human lymphocytes), most of them being ILC2 (106, 107). Intravital imaging of murine skin has shown that 50–100 CD103+ ILC2 per mm2 continuously patrol skin dermis with an average low speed comparable to the one of migratory DC (65). ILC2 promote proinflammation, epithelial repair and wound healing (108). ILC2 expand under pathologic condition such as allergen challenge (64). Although skin ILC2 increase has largely been attributed to local proliferation, whether ILC2 (or their progenitor) are recruited from the blood circulation to the skin by a cascade of molecular interactions, need to be specifically investigated. Indeed, recent evidences showed that ILC2 develop in the bone marrow from common lymphoid progenitor cells and egress for the blood circulation under IL-33 stimuli or pathogenic conditions to repopulate classical ILC2 niches such as the lung, skin, lymph nodes (106, 107).
Skin Lymphocyte Positioning and Trafficking at Steady State
Recent accumulating evidences suggest that hair follicles (extending through the entire epidermis and terminating in the dermis) provide a conduit system for immune cells, regulating their migration from the epidermis to the dermis and vice-versa, by secreting distinct chemokines along the hair follicles (109). Furthermore, hair follicles have been involved in CD8+ and CD4+ Trm maintenance through chemokine and cytokine production (110, 111). Intravital microscopy experiments revealed that on resolution of infection, memory CD8+ were confined to the epidermis with a predominant slow-moving behavior, adopting a dendrite morphology and did not recirculate (112). This slow motility involved Rho-associated coiled-coil kinases (ROCK) and pertussis toxin (PTX)-sensitive receptors, but do not required CXCR3, CCR8, CXCR6, CCR10, or CD103 (58). Therefore, it remains to be determined which PTX-sensitive receptor principally controls the patrolling migration of skin resident CD8+ T cells. Interestingly, the location and behavior of memory CD4+ in the skin differ strikingly from memory CD8+, suggesting complementary roles of these two populations in cutaneous surveillance. Indeed, memory CD4+ are highly motile cells, localized in the dermis and recirculate (112). Hair follicles also represent a particular cutaneous niche for Treg which form clusters around follicles (63). Intravital 2-photons imaging of Foxp3-GFP mouse showed that Tregs are dominantly non-motile at steady state and are rarely found in the hypodermis but enriched in the dermis with ~2,000 Treg/mm2. A small proportion of Treg (<7%) have migratory behavior while 90% of effector CD4+ T cells were migratory at steady state (63). By contrast, B-cells, NK, and γδT cells where rarely found proximal to hair follicles (113).
Lymphocytes in Inflammatory and Malignant Skin
The crucial role of lymphocytes in cutaneous surveillance is highlighted by the increased frequency and severity of skin malignancies and infections in patients with immunodeficiencies and by the pathogenesis of various acute and chronic inflammatory skin disorders such as psoriasis or atopic dermatitis. Because lymphocytes exploit, at least a partially similar cascade of molecular interactions to home into the skin in various cutaneous pathologies and under homeostasis; our knowledge on cutaneous lymphocytes has potential to inform and benefit therapeutic intervention of these pathologies (Figure 2).
Inflammatory Conditions
Inflammation-driven lymphocyte recruitment into the skin use the same adhesion cascade described above which is boosted by the upregulation of adhesion/chemokine family protein expression both on lymphocytes and tissue side (Figure 2). Lymphocyte recruitment occurs mostly in post-capillary venules, where the shear stress is highly increased in comparison to lymph node high endothelial venules (114). At high shear stress (>6 dyn/cm2), tethers and slings which were firstly discovered in neutrophils, are required to stabilize cell rolling (115). Tethers are formed from pre-existing cell microvilli. When tethers detach and swing around the rolling cell, they can become self-adhesive substrates named slings. While naïve CD4+ T cells cannot form both tethers and slings at high shear stress, it has recently been shown that Th1, Th17, and Treg CD4+ T cell (but not Th2) form tethers and slings under inflammatory conditions (116). In response to pathogenic stimuli, cutaneous expression of homeostatic chemokines CCL27 (CTACK) and CCL17 (TARC) are upregulated and inflammatory chemokines are induced (36–38). Indeed, keratinocytes can produce chemokines and cytokines including CXCL9, CXCL10, CXCL11, CCL20, along with inflammatory cytokines tumor necrosis factor-α (TNF), IL-1α, and IL-1β, IL-6, IL-10, IL-18, and IL-33 (38). Furthermore, CCL27 is also induced down stream of pro-inflammatory cytokines such as TNF-α and IL-1 β (36). In a model of delayed type hypersensitivity, it has been shown that CLA and CCR4 expression are upregulated on lymph node T lymphocytes favoring their recruitment into the skin (48). In addition to CCR4, CCR10 has been shown to be upregulated at the surface of a small proportion of T lymphocytes in delayed type hypersensitivity and bacterial chancroid skin lesions but the latter did not contribute to skin recruitment in comparison to CCR4 which was more highly expressed (49). In contrast, CCR10 is highly expressed by psoriasis, atopic, or allergic dermatitis patient's infiltrating T lymphocytes inducing their recruitment to the skin and the chronic cutaneous inflammation (36). However, skin NK cells isolated from allergic contact dermatitis lesions do not express CCR10 or CCR4 but are instead guided by CXCR3, CCR5, and CCR6 (67). Similarly, dermal NK cells in acute psoriatic plaques express CXCR3, CCR5, CCR6 in addition to CCR8 and CXCR1, however they only express low levels of CCR4 and do not express CLA (68). Upon inflammatory conditions, the number of cutaneous Treg and the proportion of migratory Treg in the skin increase rapidly (63). This Treg recruitment and in tissue migration are P-selectin, E-selectin, and CCR4 dependent. Intravital microscopy experiments have also revealed that CD4+ T cell interstitial migration was αV dependent under diverse inflammatory conditions as opposed to steady state conditions where integrins are not involved (117). Further, pathogenic and inflammatory conditions modify matrix fiber composition and density in the skin, and may assist in immune cell entry (118). While these studies show heterogeneity in the mechanism of lymphocytes recruitment under inflammatory conditions, it is however interesting to note that the molecules involved are restricted to a short list. It is this same list of molecules that may therapeutically benefit recruitment of TILs into melanoma.
Cutaneous Melanoma
Melanoma is the deadliest and most common form of cutaneous cancer. It is also the more investigated cancer in terms of tumor/immune cell relationship due to its exceptional immunogenicity linked to a high mutational burden (119, 120). Studies investigating gene expression profiling of cellular composition of tumors firstly classified tumors in two main subsets: “inflamed” and “non-inflammed” (121). The “inflamed” subset is characterized by the expression of transcripts for chemokines, T- and innate immune cell-lineage specific markers and inhibitory molecules. The “non-inflammed” subset is characterized by transcripts for macrophages and angiogenesis specific markers. A recent study proposed a more complex classification describing 6 profiles based on immune cell composition across 30 type of cancers: “wound healing,” “IFNγ-dominant,” “inflammatory,” “lymphocyte depleted,” “immunologically quiet,” and “TGF-β dominant” (122). Most of cutaneous melanoma fall in the “wound healing,” “IFNγ-dominant,” “inflammatory,” and “lymphocyte depleted” categories. The “inflammatory” subset was associated to the best prognostic value. Thus, tumor-infiltrating lymphocytes (TIL) are generally associated with a favorable prognostic value across multiple cancers, including in primary melanoma (123–127). However, some studies reported no correlation (128–130). Historically TILs referred to lymphocytes who were directly at the contact with tumor cells and/or infiltrate tumor nest (131). In 1989, the following graded system has been adopted to classify TILs in primary melanoma as: “ABSENT” when no lymphocytes were present at all or not at the contact with tumor cells; “BRISK” when lymphocytes were present throughout the vertical growth phase or infiltrating the entire base of the vertical growth phase and “NON-BRISK” when lymphocytes were present in one or more foci of the vertical growth phase (124). In a larger (1241) cohort of primary melanoma patients, Weiss et al. show that only patients with brisk TILs correlates with improved overall survival and relapse free survival but not non-brisk and absent TILs (132). While TILs were first restricted to CD8+ cytotoxic T cells, nowadays, they englobe all immune cells observed in the tumor, comprising effector and regulatory T lymphocytes, B lymphocytes, and NK cells in primary melanoma (133). Furthermore, non-lymphocyte populations such as dendritic cells, myeloid-derived cells, macrophages, mastocytes also infiltrate tumors (134). Beyond their presence, TIL functional status highly impact the prognostic value and response to treatment, explaining the contradictory results mentioned ahead. It is now more evident that integration of measures of TIL infiltration, activation states, and phenotype (immune-suppressor, activator, exhausted, anergic) are required to predict treatment efficiency and disease progression. As examples, the presence of M1 macrophages, N1 neutrophils are associated with a favorable prognosis while the presence of M2 macrophages, N2 neutrophils, or Treg are pro-tumoral (135, 136). While TIL infiltration has a high impact on tumor progression, generally TILs poorly infiltrate most tumors due to strategies established by tumor cells to hijack immune cell afflux. Among them, tumor vessels can be irregularly distributed, disorganized, tortuous, dilated resulting in leaking vessels with high interstitial pressure due to the density of the surrounding tumor mass (137). This probably rends lymphocyte infiltration from blood circulation more difficult than in healthy tissue. Furthermore, the expression of P-Selectin and VCAM-1 is diminished on intratumoral vessels in comparison to non-tumoral adjacent vessels (39, 40). Angiogenic factors like VEGF, FGF, NO, or TGF-b induce ICAM-1, ICAM-2, VCAM-1, and CD34 downregulation on endothelial cells, rending them refractory to immune cell infiltration (41–43). Interestingly, another molecule involved in TILs infiltration is an alternative spliced variant isoform of CD44, CD44v10, expressed on TILs from melanoma patients inducing TILs migration in an selectin and integrin-independent manner (50). Finally, tumoral endothelial cells can express molecules which block T-cell infiltration like the endothelin B receptor and its ligands endothelin-1 (138). Chemokines involved in immune cell recruitment are also deregulated in melanoma. CXCR3 and CCR5 has been shown to be the major chemokine receptor involved in CD8+ T cell infiltration in melanoma (44, 45). CXCR3 along with CCR5 expression correlates with CD8+ T cell recruitment and favorable outcome in melanoma (51–54). Furthermore, it has been shown that a strong CXCL9 and CXCL10 expression within tumors correlate with high CD8+ T cell infiltration in patients with melanoma (46). It has recently been proposed that tumors can inhibit IFNγ-induced CXCL9 by secreting galectin-3, a lectin which block IFNγ diffusion by binding to the glycans of the extracellular matrix (139). The role of CXCR3/CXCL10 axis is not restricted to CD8+ T cell infiltration as it plays an additional role in NK cells, controlling both their infiltration inside tumors and their migration toward their targets (69, 70). While the adhesion molecules and chemokines that favor T cell infiltration in melanoma are known, the precise ones required for the infiltration of pro-tumoral immune cells such as M2 macrophages or Tregs need to be investigated with the intent to control the activation and effector functions of TILs within tumors. Toward this aim, it has been shown in a mouse model that M2 macrophages infiltration required CCR2 ligand expression in tumors (140). Chemokine receptors are also expressed by melanoma cells allowing them to disseminate elsewhere in the organism, preferentially in the draining lymph nodes, lung, liver, gut, and brain where they form secondary lesions (141, 142).
Conclusion and Future Directions
Lymphocyte trafficking to and within the skin is a highly regulated process involving unique and complex combinations of adhesion molecules and chemokine receptors (Figure 2). These combinations are cell-specific thereby controlling the type and location of the recruited cells. Under inflammatory conditions, lymphocyte recruitment is boosted by the increased expression and/or induction of adhesion molecules and chemokines on both cells and tissue side (Table 1). Lymphocytes are effectively recruited in inflamed tumors, but they are inhibited by immunomodulatory mechanisms (Exhaustion, Treg infiltration). However, they poorly infiltrate non-inflamed tumors. CD8+ T cell infiltration in primary melanoma is of favorable prognosis which had lead researchers to mount strategies to boost immune cell trafficking into tumors (137). Mimicking strategies induced by an inflammatory context has shown some successes in cancer. Indeed, the upregulation of adhesion molecules and chemokines on tumor vessels induced by the selective delivery of TNF (NGR-TNF) to these vessels has shown to increase the recruitment of endogenous or adoptively transferred T cells (143). A second illustration of this success is the inhibition of VEGF/VEGFR by the use of inhibitory molecules or antibodies which has also shown to promote lymphocytes infiltration in the tumors (144, 145). As a last example of such mimicking strategy, the induction of inflammatory chemokines through the release of IFN-γ has shown to induced T-cell infiltration in tumors (146, 147). Despite these encouraging successes, future therapeutic strategies may selectively open tumor mass gates to CD8+ T cells while being refractory to Treg or suppressive myeloid cells entry. Uncovering the specific adhesion molecule/chemokine combination for each type of lymphocytes and manipulate them to select lymphocyte types allowed to reach the tumor site may help to improve immunotherapy in cancer. Indeed, similar to that recently performed in breast cancer, single cell RNAseq analysis of melanoma TILs could yield valuable insights into the precise guidance cues required for cutaneous tumor entry (92). Further, there are still gaps in knowledge of the prognostic value in identifying distinct populations within melanoma or how individual lymphocyte populations are altered by interventions such as checkpoint inhibition. In addition, we still fail to fully understand how innate and adaptive lymphocytes work together to influence cancer growth. Recently, NK cells were highlighted as major orchestrators of cDC1 recruitment into melanomas; an axis that provides promising potential (148). However, the role that innate lymphocytes play in the co-ordination of TIL recruitment and function is under studied. We believe that a better understanding of lymphocyte specific location and interacting partners would help future successes in melanoma treatment.
Author Contributions
FL and JG contributed to content conception, wrote, and revised the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
FL is supported by a Walter and Eliza Hall Centenary Fellowship sponsored by CSL. JG is supported by Australian Research Council Future Fellowship (FT130100708). This work was made possible through Victorian State Government Operational Infrastructure Support and Australian Government NHMRC IRIISS.
References
1. Leonard A, Guttman-Yassky E. The unique molecular signatures of contact dermatitis and implications for treatment. Clin Rev Allergy Immunol. (2018). doi: 10.1007/s12016-018-8685-0. [Epub ahead of print].
2. Hawkes JE, Chan TC, Krueger JG. Psoriasis pathogenesis and the development of novel targeted immune therapies. J Allergy Clin Immunol. (2017) 140:645–53. doi: 10.1016/j.jaci.2017.07.004
3. Nestle FO, Di Meglio P, Qin J-Z, Nickoloff BJ. Skin immune sentinels in health and disease. Nat Rev Immunol. (2009) 9:679–91. doi: 10.1038/nri2622
4. Bos JD, Zonneveld I, Das PK, Krieg SR, van der Loos CM, Kapsenberg ML. The Skin Immune System (SIS): distribution and immunophenotype of lymphocyte subpopulations in normal human skin. J Invest Dermatol. (1987) 88:569–73. doi: 10.1111/1523-1747.ep12470172
5. Geherin SA, Fintushel SR, Lee MH, Wilson RP, Patel RT, Alt C, et al. The skin a novel niche for recirculating B cells. J Immunol. (2012) 188:6027–35. doi: 10.4049/jimmunol.1102639
6. Saul L, Ilieva KM, Bax HJ, Karagiannis P, Correa I, Rodriguez-Hernandez I, et al. IgG subclass switching and clonal expansion in cutaneous melanoma and normal skin. Sci Rep. (2016) 6:29736. doi: 10.1038/srep29736
7. Clark RA, Chong B, Mirchandani N, Brinster NK, Yamanaka K-I, Dowgiert RK, et al. The vast majority of CLA+ T cells are resident in normal skin. J Immunol. (2006) 176:4431–9. doi: 10.4049/jimmunol.176.7.4431
8. Scharschmidt TC, Vasquez KS, Truong H-A, Gearty SV, Pauli ML, Nosbaum A, et al. A wave of regulatory t cells into neonatal skin mediates tolerance to commensal microbes. Immunity (2015) 43:1011–21. doi: 10.1016/j.immuni.2015.10.016
9. Ali N, Rosenblum MD. Regulatory T cells in skin. Immunology (2017) 152:372–81. doi: 10.1111/imm.12791
10. Ebert LM, Meuter S, Moser B. Homing and function of human skin gammadelta T cells and NK cells: relevance for tumor surveillance. J Immunol. (2006) 176:4331–36. doi: 10.4049/jimmunol.176.7.4331
11. Sumaria N, Roediger B, Ng LG, Qin J, Pinto R, Cavanagh LL, et al. Cutaneous immunosurveillance by self-renewing dermal gammadelta T cells. J Exp Med. (2011) 208:505–18. doi: 10.1084/jem.20101824
12. Vivier E, Artis D, Colonna M, Diefenbach A, Di Santo JP, Eberl G, et al. Innate lymphoid cells: 10 years on. Cell (2018) 174:1054–66. doi: 10.1016/j.cell.2018.07.017
13. Kim CH, Hashimoto-Hill S, Kim M. Migration and tissue tropism of innate lymphoid cells. Trends Immunol. (2016) 37:68–79. doi: 10.1016/j.it.2015.11.003
14. Batista MD, Ho EL, Kuebler PJ, Milush JM, Lanier LL, Kallas EG, et al. Skewed distribution of natural killer cells in psoriasis skin lesions. Exp Dermatol. (2012) 22:64–6. doi: 10.1111/exd.12060
15. Byrd AL, Belkaid Y, Segre JA. The human skin microbiome. Nat Rev Microbiol. (2018) 16:143–55. doi: 10.1038/nrmicro.2017.157
16. Naik S, Bouladoux N, Wilhelm C, Molloy MJ, Salcedo R, Kastenmuller W, et al. Compartmentalized control of skin immunity by resident commensals. Science (2012) 337:1115–9. doi: 10.1126/science.1225152
17. Meisel JS, Sfyroera G, Bartow-McKenney C, Gimblet C, Bugayev J, Horwinski J, et al. Commensal microbiota modulate gene expression in the skin. Microbiome (2018) 6:20. doi: 10.1186/s40168-018-0404-9
18. Morgun A, Dzutsev A, Dong X, Greer RL, Sexton DJ, Ravel J, et al. Uncovering effects of antibiotics on the host and microbiota using transkingdom gene networks. Gut (2015) 64:1732–43. doi: 10.1136/gutjnl-2014-308820
19. Janssen GH, Tangelder GJ, Oude Egbrink MG, Reneman RS. Spontaneous leukocyte rolling in venules in untraumatized skin of conscious and anesthetized animals. Am J Physiol Heart Circul Physiol. (1994) 267:H1199–204. doi: 10.1152/ajpheart.1994.267.3.H1199
20. Weninger W, Ulfman LH, Cheng G, Souchkova N, Quackenbush EJ, Lowe JB, et al. Specialized contributions by alpha(1,3)-fucosyltransferase-IV and FucT-VII during leukocyte rolling in dermal microvessels. Immunity (2000) 12:665–76. doi: 10.1016/S1074-7613(00)80217-4
21. Girard J-P, Moussion C, Förster R. HEVs, lymphatics and homeostatic immune cell trafficking in lymph nodes. Nat Rev Immunol. (2012) 12:762–73. doi: 10.1038/nri3298
22. Veerman KM, Lafouresse F, Girard J-P. Tumor high endothelial venules and lymphocyte trafficking. In: J-J Feige, G Pagès, F Soncin, editors, Molecular Mechanisms of Angiogenesis. Paris: Springer Paris (2014). p. 339−52.
23. Springer TA. Traffic signals for lymphocyte recirculation and leukocyte emigration: the multistep paradigm. Cell (1994) 76:301–14. doi: 10.1016/0092-8674(94)90337-9
24. Andrian von UH. Intravital microscopy of the peripheral lymph node microcirculation in mice. Microcirculation (1996) 3:287–300.
25. Alon R, Hammer DA, Springer TA. Lifetime of the P-selectin-carbohydrate bond and its response to tensile force in hydrodynamic flow. Nature (1995) 374:539–42. doi: 10.1038/374539a0
26. McEver RP, Moore KL, Cummings RD. Leukocyte trafficking mediated by selectin-carbohydrate interactions. J Biol Chem. (1995) 270:11025–8.
27. Malý P, Thall A, Petryniak B, Rogers CE, Smith PL, Marks RM, et al. The alpha(1,3)fucosyltransferase Fuc-TVII controls leukocyte trafficking through an essential role in L-, E-, and P-selectin ligand biosynthesis. Cell (1996) 86:643–53.
29. McEver RP. Selectins: lectins that initiate cell adhesion under flow. Curr Opin Cell Biol. (2002) 14:581–6. doi: 10.1016/S0955-0674(02)00367-8
30. Lafouresse F, Vasconcelos Z, Cotta-de-Almeida V, Dupré L. Actin cytoskeleton control of the comings and goings of T lymphocytes. Tissue Antigens (2013) 82:301–11. doi: 10.1111/tan.12193
31. Carman CV, Springer TA. A transmigratory cup in leukocyte diapedesis both through individual vascular endothelial cells and between them. J Cell Biol. (2004) 167:377–88. doi: 10.1083/jcb.200404129
32. Pablos JL, Amara A, Bouloc A, Santiago B, Caruz A, Galindo M, et al. Stromal-cell derived factor is expressed by dendritic cells and endothelium in human skin. Am J Pathol. (1999) 155:1577–86. doi: 10.1016/S0002-9440(10)65474-0
33. Frederick MJ, Henderson Y, Xu X, Deavers MT, Sahin AA, Wu H, et al. In vivo expression of the novel CXC chemokine BRAK in normal and cancerous human tissue. Am J Pathol. (2000) 156:1937–50. doi: 10.1016/S0002-9440(10)65067-5
34. Schaerli P, Ebert L, Willimann K, Blaser A, Roos RS, Loetscher P, et al. A skin-selective homing mechanism for human immune surveillance T cells. J Exp Med. (2004) 199:1265–75. doi: 10.1084/jem.20032177
35. Schutyser E, Struyf S, Van Damme J. The CC chemokine CCL20 and its receptor CCR6. Cytokine Growth Factor Rev. (2003) 14:409–26. doi: 10.1016/S1359-6101(03)00049-2
36. Homey B, Alenius H, Müller A, Soto H, Bowman EP, Yuan W, et al. CCL27-CCR10 interactions regulate T cell-mediated skin inflammation. Nat Med. (2002) 8:157–65. doi: 10.1038/nm0202-157
37. Campbell JJ, Haraldsen G, Pan J, Rottman J, Qin S, Ponath P, et al. The chemokine receptor CCR4 in vascular recognition by cutaneous but not intestinal memory T cells. Nature (1999) 400:776–80. doi: 10.1038/23495
38. Albanesi C, Scarponi C, Giustizieri M, Girolomoni G. Keratinocytes in inflammatory skin diseases. CDTIA (2005) 4:329–34. doi: 10.2174/1568010054022033
39. Piali L, Fichtel A, Terpe HJ, Imhof BA, Gisler RH. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J Exp Med. (1995) 181:811–16.
40. Nooijen PT, Westphal JR, Eggermont AM, Schalkwijk C, Max R, de Waal RM, et al. Endothelial P-selectin expression is reduced in advanced primary melanoma and melanoma metastasis. Am J Pathol. (1998) 152:679–82.
41. Dirkx AEM, Oude Egbrink MGA, Kuijpers MJE, van der Niet ST, Heijnen VVT, Bouma-ter Steege JCA, et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. (2003) 63:2322–9.
42. Hellebrekers DMEI, Castermans K, Viré E, Dings RPM, Hoebers NTH, Mayo KH, et al. Epigenetic regulation of tumor endothelial cell anergy: silencing of intercellular adhesion molecule-1 by histone modifications. Cancer Res. (2006) 66:10770–77. doi: 10.1158/0008-5472.CAN-06-1609
43. Hendrix MJC, Seftor EA, Seftor REB, Chao J-T, Chien D-S, Chu Y-W. Tumor cell vascular mimicry: novel targeting opportunity in melanoma. Pharmacol Therap. (2016) 159:83–92. doi: 10.1016/j.pharmthera.2016.01.006
44. Mikucki ME, Fisher DT, Matsuzaki J, Skitzki JJ, Gaulin NB, Muhitch JB, et al. Non-redundant requirement for CXCR3 signalling during tumoricidal T-cell trafficking across tumour vascular checkpoints. Nat Comms. (2015) 6:7458–30. doi: 10.1038/ncomms8458
45. González-Martín A, Gómez L, Lustgarten J, Mira E, Mañes S. Maximal T cell-mediated antitumor responses rely upon CCR5 expression in both CD4(+) and CD8(+) T cells. Cancer Res. (2011) 71:5455–66. doi: 10.1158/0008-5472.CAN-11-1687
46. Kunz M, Toksoy A, Goebeler M, Engelhardt E, Bröcker E, Gillitzer R. Strong expression of the lymphoattractant C-X-C chemokine Mig is associated with heavy infiltration of T cells in human malignant melanoma. J Pathol. (1999) 189:552–58.
47. Morales J, Homey B, Vicari AP, Hudak S, Oldham E, Hedrick J, et al. CTACK, a skin-associated chemokine that preferentially attracts skin-homing memory T cells. Proc Natl Acad Sci USA. (1999) 96:14470–5.
48. Reiss Y, Proudfoot AE, Power CA, Campbell JJ, Butcher EC. CC chemokine receptor (CCR)4 and the CCR10 ligand cutaneous T cell-attracting chemokine (CTACK) in lymphocyte trafficking to inflamed skin. J Exp Med. (2001) 194:1541–47. doi: 10.1084/jem.194.10.1541
49. Soler D, Humphreys TL, Spinola SM, Campbell JJ. CCR4 versus CCR10 in human cutaneous TH lymphocyte trafficking. Blood (2003) 101:1677–82. doi: 10.1182/blood-2002-07-2348
50. Weimann TK, Wagner C, Goos M, Wagner SN. CD44 variant isoform v10 is expressed on tumor-infiltrating lymphocytes and mediates hyaluronan-independent heterotypic cell-cell adhesion to melanoma cells. Exp Dermatol. (2003) 12:204–12. doi: 10.1034/j.1600-0625.2003.00044.x
51. Harlin H, Meng Y, Peterson AC, Zha Y, Tretiakova M, Slingluff C, et al. Chemokine expression in melanoma metastases associated with CD8+ T-cell recruitment. Cancer Res. (2009) 69:3077–85. doi: 10.1158/0008-5472.CAN-08-2281
52. Fridman WH, Pagès F, Sautès-Fridman C, Galon J. The immune contexture in human tumours: impact on clinical outcome. Nat Rev Cancer (2012) 12:298–306. doi: 10.1038/nrc3245
53. Martinet L, Le Guellec S, Filleron T, Lamant L, Meyer N, Rochaix P, et al. High endothelial venules (HEVs) in human melanoma lesions. Oncoimmunology (2014) 1:829–39. doi: 10.4161/onci.20492
54. Mullins IM, Slingluff CL, Lee JK, Garbee CF, Shu J, Anderson SG, et al. CXC chemokine receptor 3 expression by activated CD8+ T cells is associated with survival in melanoma patients with stage III disease. Cancer Res. (2004) 64:7697–701. doi: 10.1158/0008-5472.CAN-04-2059
55. Steinert EM, Schenkel JM, Fraser KA, Beura LK, Manlove LS, Igyártó BZ, et al. Quantifying memory CD8 T Cells Reveals Regionalization of Immunosurveillance. Cell (2015) 161:737–49. doi: 10.1016/j.cell.2015.03.031
56. Mueller SN, MacKay LK. Tissue-resident memory T cells: local specialists in immune defence. Nat Rev Immunol. (2015) 16:79–89. doi: 10.1038/nri.2015.3
57. Alanio C, Barreira da Silva R, Michonneau D, Bousso P, Ingersoll MA, Albert ML. CXCR3/CXCL10 axis shapes tissue distribution of memory phenotype CD8+T cells in nonimmunized mice. J Immunol. (2017) 200:139–46. doi: 10.4049/jimmunol.1700564
58. Zaid A, Hor JL, Christo SN, Groom JR, Heath WR, Mackay LK, et al. Chemokine receptor-dependent control of skin tissue-resident memory T cell formation. J Immunol. (2017) 199:2451–9. doi: 10.4049/jimmunol.1700571
59. McCully ML, Ladell K, Andrews R, Jones RE, Miners KL, Roger L, et al. CCR8 expression defines tissue-resident memory T cells in human skin. J Immunol. (2018) 200:1639–50. doi: 10.4049/jimmunol.1701377
60. Hirahara K, Liu L, Clark RA, Yamanaka K-I, Fuhlbrigge RC, Kupper TS. The majority of human peripheral blood CD4+CD25highFoxp3+ regulatory T cells bear functional skin-homing receptors. J Immunol. (2006) 177:4488–94. doi: 10.4049/jimmunol.177.7.4488
61. Sather BD, Treuting P, Perdue N, Miazgowicz M, Fontenot JD, Rudensky AY, et al. Altering the distribution of Foxp3 +regulatory T cells results in tissue-specific inflammatory disease. J Exp Med. (2007) 204:1335–47. doi: 10.1084/jem.20070081
62. Panduro M, Benoist C, Mathis D. Tissue tregs. Annu Rev Immunol. (2016) 34:609–33. doi: 10.1146/annurev-immunol-032712-095948
63. Chow Z, Mueller SN, Deane JA, Hickey MJ. Dermal regulatory T cells display distinct migratory behavior that is modulated during adaptive and innate inflammation. J Immunol. (2013) 191:3049–56. doi: 10.4049/jimmunol.1203205
64. Salimi M, Barlow JL, Saunders SP, Xue L, Gutowska-Owsiak D, Wang X, et al. A role for IL-25 and IL-33–driven type-2 innate lymphoid cells in atopic dermatitis. J Exp Med. (2013) 210:2939–50. doi: 10.1084/jem.20130351
65. Roediger B, Kyle R, Yip KH, Sumaria N, Guy TV, Kim BS, et al. Cutaneous immunosurveillance and regulation of inflammation by group 2 innate lymphoid cells. Nat Immunol. (2013) 14:564–73. doi: 10.1038/ni.2584
66. Luci C, Reynders A, Ivanov II, Cognet C, Chiche L, Chasson L, et al. Influence of the transcription factor RORγt on the development of NKp46+ cell populations in gut and skin. Nat Immunol. (2008) 10:75–82. doi: 10.1038/ni.1681
67. Carbone T, Nasorri F, Pennino D, Eyerich K, Foerster S, Cifaldi L, et al. CD56highCD16-CD62L- NK cells accumulate in allergic contact dermatitis and contribute to the expression of allergic responses. J Immunol. (2010) 184:1102–10. doi: 10.4049/jimmunol.0902518
68. Ottaviani C, Nasorri F, Bedini C, de Pità O, Girolomoni G, Cavani A. CD56brightCD16- NK cells accumulate in psoriatic skin in response to CXCL10 and CCL5 and exacerbate skin inflammation. Eur J Immunol. (2006) 36:118–28. doi: 10.1002/eji.200535243
69. Wennerberg E, Kremer V, Childs R, Lundqvist A. CXCL10-induced migration of adoptively transferred human natural killer cells toward solid tumors causes regression of tumor growth in vivo. Cancer Immunol Immunother. (2014) 64:225–35. doi: 10.1007/s00262-014-1629-5
70. Kim J, Kim JS, Lee HK, Kim HS, Park EJ, Choi JE, et al. CXCR3-deficient natural killer cells fail to migrate to B16F10 melanoma cells. Int Immunopharmacol. (2018) 63:66–73. doi: 10.1016/j.intimp.2018.07.026
71. Zlotnik A, Yoshie O. The chemokine superfamily revisited. Immunity (2012) 36:705–16. doi: 10.1016/j.immuni.2012.05.008
72. Groom JR, Luster AD. CXCR3 ligands: redundant, collaborative and antagonistic functions. Immunol Cell Biol. (2011) 89:207–15. doi: 10.1038/icb.2010.158
73. Zlotnik A, Yoshie O. Chemokines: a new classification system and their role in immunity. Immunity (2000) 12:121–7. doi: 10.1016/S1074-7613(00)80165-X
74. Nomiyama H, Osada N, Yoshie O. The evolution of mammalian chemokine genes. Cytokine Growth Factor Rev. (2010) 21:253–62. doi: 10.1016/j.cytogfr.2010.03.004
75. Amara A, Lorthioir O, Valenzuela A, Magerus A, Thelen M, Montes M, et al. Stromal cell-derived factor-1alpha associates with heparan sulfates through the first beta-strand of the chemokine. J Biol Chem. (1999) 274:23916–25.
76. Homey B, Wang W, Soto H, Buchanan ME, Wiesenborn A, Catron D, et al. Cutting edge: the orphan chemokine receptor G protein-coupled receptor-2 (GPR-2, CCR10) binds the skin-associated chemokine CCL27 (CTACK/ALP/ILC). J Immunol. (2000) 164:3465–70. doi: 10.4049/jimmunol.164.7.3465
77. Rose DM, Alon R, Ginsberg MH. Integrin modulation and signaling in leukocyte adhesion and migration. Immunol Rev. (2007) 218:126–34. doi: 10.1111/j.1600-065X.2007.00536.x
78. Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Curr Opin Cell Biol. (2011) 23:607–14. doi: 10.1016/j.ceb.2011.08.005
79. Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell (2002) 110:673–87. doi: 10.1016/S0092-8674(02)00971-6
80. Sheridan BS, Lefrançois L. Regional and mucosal memory T cells. Nat Immunol. (2011) 12:485–91. doi: 10.1038/ni.2029
81. Campbell DJ, Butcher EC. Rapid acquisition of tissue-specific homing phenotypes by CD4+T cells activated in cutaneous or mucosal lymphoid tissues. J Exp Med. (2002) 195:135–41. doi: 10.1084/jem.20011502
82. Mora JR, Cheng G, Picarella D, Briskin M, Buchanan N, Andrian von UH. Reciprocal and dynamic control of CD8 T cell homing by dendritic cells from skin- and gut-associated lymphoid tissues. J Exp Med. (2005) 201:303–16. doi: 10.1084/jem.20041645
83. Sigmundsdottir H, Pan J, Debes GF, Alt C, Habtezion A, Soler D, et al. DCs metabolize sunlight-induced vitamin D3 to “program” T cell attraction to the epidermal chemokine CCL27. Nat Immunol. (2007) 8:285–93. doi: 10.1038/ni1433
84. Nizza ST, Campbell JJ. CD11b+ Migratory dendritic cells mediate CD8 T cell cross-priming and cutaneous imprinting after topical immunization. PLoS ONE (2014) 9:e91054–8. doi: 10.1371/journal.pone.0091054
85. Fuhlbrigge RC, Kieffer JD, Armerding D, Kupper TS. Cutaneous lymphocyte antigen is a specialized form of PSGL-1 expressed on skin-homing T cells. Nature (1997) 389:978–81. doi: 10.1038/40166
86. Fuhlbrigge RC, King SL, Sackstein R, Kupper TS. CD43 is a ligand for E-selectin on CLA+ human T cells. Blood (2006) 107:1421–6. doi: 10.1182/blood-2005-05-2112
87. Gebhardt T, Wakim LM, Eidsmo L, Reading PC, Heath WR, Carbone FR. Memory T cells in nonlymphoid tissue that provide enhanced local immunity during infection with herpes simplex virus. Nat Immunol. (2009) 10:524–30. doi: 10.1038/ni.1718
88. Nizard M, Karaki S, Tran T, Voron T, Dransart E, Sandoval F, et al. Induction of resident memory T cells enhances the efficacy of cancer vaccine. Nat Comms. (2017) 8:1–11. doi: 10.1038/ncomms15221
89. Malik BT, Byrne KT, Vella JL, Zhang P, Shabaneh TB, Steinberg SM, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Sci Immunol. (2017) 2:eaam6346–24. doi: 10.1126/sciimmunol.aam6346
90. Djenidi F, Adam J, Goubar A, Durgeau A, Meurice G, de Montpréville V, et al. CD8+CD103+ tumor-infiltrating lymphocytes are tumor-specific tissue-resident memory T cells and a prognostic factor for survival in lung cancer patients. J Immunol. (2015) 194:3475–86. doi: 10.4049/jimmunol.1402711
91. Webb JR, Milne K, Watson P, Deleeuw RJ, Nelson BH. Tumor-infiltrating lymphocytes expressing the tissue resident memory marker CD103 are associated with increased survival in high-grade serous ovarian cancer. Clin Cancer Res. (2014) 20:434–44. doi: 10.1158/1078-0432.CCR-13-1877
92. Savas P, Virassamy B, Ye C, Salim A, Mintoff CP, Caramia F, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nat Med. (2018) 24:986–93. doi: 10.1038/s41591-018-0078-7
93. Gálvez-Cancino F, López E, Menares E, Díaz X, Flores C, Cáceres P, et al. Vaccination-induced skin-resident memory CD8 T cells mediate strong protection against cutaneous melanoma. Oncoimmunology (2018) 7:1–12. doi: 10.1080/2162402X.2018.1442163
94. Mackay LK, Rahimpour A, Ma JZ, Collins N, Stock AT, Hafon M-L, et al. The developmental pathway for CD103+CD8+ tissue-resident memory T cells of skin. Nat Publ Group (2013) 14:1294–301. doi: 10.1038/ni.2744
95. MacKay LK, Kallies A. Transcriptional regulation of tissue-resident lymphocytes. Trends Immunol. (2017) 38:94–103. doi: 10.1016/j.it.2016.11.004
96. Wakim LM, Woodward-Davis A, Liu R, Hu Y, Villadangos J, Smyth G, et al. The molecular signature of tissue resident memory CD8 T cells isolated from the brain. J Immunol. (2012) 189:3462–71. doi: 10.4049/jimmunol.1201305
97. MacKay LK, Minnich M, Kragten NAM, Liao Y, Nota B, Seillet C, et al. Hobit and Blimp1 instruct a universal transcriptional program of tissue residency in lymphocytes. Science (2016) 352:459–63. doi: 10.1126/science.aad2035
98. Boyman O, Hefti HP, Conrad C, Nickoloff BJ, Suter M, Nestle FO. Spontaneous development of psoriasis in a new animal model shows an essential role for resident T cells and tumor necrosis factor-alpha. J Exp Med. (2004) 199:731–6. doi: 10.1084/jem.20031482
99. Watanabe R, Gehad A, Yang C, Scott LL, Teague JE, Schlapbach C, et al. Human skin is protected by four functionally and phenotypically discrete populations of resident and recirculating memory T cells. Sci Transl Med. (2015) 7:279ra39. doi: 10.1126/scitranslmed.3010302
100. Collins N, Jiang X, Zaid A, MacLeod BL, Li J, Park CO, et al. Skin CD4+ memory T cells exhibit combined cluster-mediated retention and equilibration with the circulation. Nat Comms. (2016) 7:1–13. doi: 10.1038/ncomms11514
101. Bromley SK, Yan S, Tomura M, Kanagawa O, Luster AD. Recirculating memory T cells are a unique subset of CD4+ T cells with a distinct phenotype and migratory pattern. J Immunol. (2013) 190:970–6. doi: 10.4049/jimmunol.1202805
102. Carbone FR, MacKay LK, Heath WR, Gebhardt T. Distinct resident and recirculating memory T cell subsets in non-lymphoid tissues. Curr Opin Immunol. (2013) 25:329–3. doi: 10.1016/j.coi.2013.05.007
103. Jin Y, Xia M, Saylor CM, Narayan K, Kang J, Wiest DL, et al. Cutting edge: intrinsic programming of thymic γδT cells for specific peripheral tissue localization. J Immunol. (2010) 185:7156–60. doi: 10.4049/jimmunol.1002781
104. Nakamura K, White AJ, Parnell SM, Lane PJ, Jenkinson EJ, Jenkinson WE, et al. Differential requirement for CCR4 in the maintenance but not establishment of the invariant Vγ5+ dendritic epidermal T-cell pool. PLoS ONE (2013) 8:e74019. doi: 10.1371/journal.pone.0074019
105. Gasteiger G, Fan X, Dikiy S, Lee SY, Rudensky AY. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science (2015) 350:981–85. doi: 10.1126/science.aac9593
106. Stier MT, Zhang J, Goleniewska K, Cephus JY, Rusznak M, Wu L, et al. IL-33 promotes the egress of group 2 innate lymphoid cells from the bone marrow. J Exp Med. (2017) 8:jem.20170449–19. doi: 10.1084/jem.20170449
107. Karta MR, Rosenthal PS, Beppu A, Vuong CY, Miller M, Das S, et al. β2 integrins rather than β1 integrins mediate Alternaria-induced group 2 innate lymphoid cell trafficking to the lung. J Allergy Clin Immunol. (2018) 141:329–38.e12. doi: 10.1016/j.jaci.2017.03.010
108. Rak GD, Osborne LC, Siracusa MC, Kim BS, Wang K, Bayat A, et al. IL-33-dependent group 2 innate lymphoid cells promote cutaneous wound healing. J Invest Dermatol. (2016) 136:487–96. doi: 10.1038/JID.2015.406
109. Heath WR, Mueller SN. Hair follicles: gatekeepers to the epidermis. Nat Immunol. (2012) 13:715–17. doi: 10.1038/ni.2374
110. Nagao K, Kobayashi T, Moro K, Ohyama M, Adachi T, Kitashima DY, et al. Stress-induced production of chemokines by hair follicles regulates the trafficking of dendritic cells in skin. Nat Immunol. (2012) 13:744–52. doi: 10.1038/ni.2353
111. Adachi T, Kobayashi T, Sugihara E, Yamada T, Ikuta K, Pittaluga S, et al. Hair follicle derived IL-7 and IL-15 mediate skin-resident memory T cell homeostasis and lymphoma. Nat Med. (2015) 21:1272–9. doi: 10.1038/nm.3962
112. Gebhardt T, Whitney PG, Zaid A, Mackay LK, Brooks AG, Heath WR, et al. Different patterns of peripheral migration by memory CD4+ and CD8+ T cells. Nature (2011) 477:216–9. doi: 10.1038/nature10339
113. Christoph T, Müller-Röver S, Audring H, Tobin DJ, Hermes B, Cotsarelis G, et al. The human hair follicle immune system: cellular composition and immune privilege. Br J Dermatol. (2000) 142:862–73. doi: 10.1046/j.1365-2133.2000.03464.x
114. Ley K, Gaehtgens P. Endothelial, not hemodynamic, differences are responsible for preferential leukocyte rolling in rat mesenteric venules. Circ Res. (1991) 69:1034–41.
115. Sundd P, Gutierrez E, Koltsova EK, Kuwano Y, Fukuda S, Pospieszalska MK, et al. “Slings” enable neutrophil rolling at high shear. Nature (2012) 488:399–403. doi: 10.1038/nature11248
116. Abadier M, Pramod AB, McArdle S, Marki A, Fan Z, Gutierrez E, et al. Effector and regulatory T cells roll at high shear stress by inducible tether and sling formation. Cell Rep. (2017) 21:3885–99. doi: 10.1016/j.celrep.2017.11.099
117. Overstreet MG, Gaylo A, Angermann BR, Hughson A, Hyun Y-M, Lambert K, et al. Inflammation-induced interstitial migration of effector CD4+ T cells is dependent on integrin αV. Nat Immunol. (2013) 14:949–58. doi: 10.1038/ni.2682
118. Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. (2010) 10:712–23. doi: 10.1038/nri2852
119. Ko JS. The immunology of melanoma. Clin Lab Med. (2017) 37:449–71. doi: 10.1016/j.cll.2017.06.001
120. Passarelli A, Mannavola F, Stucci LS, Tucci M, Silvestris F. Immune system and melanoma biology: a balance between immunosurveillance and immune escape. Oncotarget (2017) 8:106132–42. doi: 10.18632/oncotarget.22190
121. Gajewski TF, Fuertes M, Spaapen R, Zheng Y, Kline J. Molecular profiling to identify relevant immune resistance mechanisms in the tumor microenvironment. Curr Opin Immunol. (2011) 23:286–92. doi: 10.1016/j.coi.2010.11.013
122. Thorsson V, Gibbs DL, Brown SD, Wolf D, Bortone DS, Yang T-HO, et al. The immune landscape of cancer. Immunity (2018) 48:812–30.e14. doi: 10.1016/j.immuni.2018.03.023
123. Azimi F, Scolyer RA, Rumcheva P, Moncrieff M, Murali R, McCarthy SW, et al. Tumor-infiltrating lymphocyte grade is an independent predictor of sentinel lymph node status and survival in patients with cutaneous melanoma. J Clin Oncol. (2012) 30:2678–83. doi: 10.1200/JCO.2011.37.8539
124. Clark WH, Elder DE, Guerry D, Braitman LE, Trock BJ, Schultz D, et al. Model predicting survival in stage I melanoma based on tumor progression. J Natl Cancer Inst. (1989) 81:1893–904.
125. Tuthill RJ, Unger JM, Liu PY, Flaherty LE, Sondak VK Southwest Oncology Group. Risk assessment in localized primary cutaneous melanoma: a southwest oncology group study evaluating nine factors and a test of the Clark logistic regression prediction model. Am J Clin Pathol. (2002) 118:504–11. doi: 10.1309/WBF7-N8KH-71KT-RVQ9
126. Thomas NE, Busam KJ, From L, Kricker A, Armstrong BK, Anton-Culver H, et al. Tumor-infiltrating lymphocyte grade in primary melanomas is independently associated with melanoma-specific survival in the population-based genes, environment and melanoma study. J Clin Oncol. (2013) 31:4252–59. doi: 10.1200/JCO.2013.51.3002
127. Clemente CG, Mihm MC, Bufalino R, Zurrida S, Collini P, Cascinelli N. Prognostic value of tumor infiltrating lymphocytes in the vertical growth phase of primary cutaneous melanoma. Cancer (1996) 77:1303–10.
128. Barnhill RL, Fine JA, Roush GC, Berwick M. Predicting five-year outcome for patients with cutaneous melanoma in a population-based study. Cancer (1996) 78:427–32.
129. Taylor RC, Patel A, Panageas KS, Busam KJ, Brady MS. Tumor-infiltrating lymphocytes predict sentinel lymph node positivity in patients with cutaneous melanoma. J Clin Oncol. (2007) 25:869–75. doi: 10.1200/JCO.2006.08.9755
130. Mandalà M, Imberti GL, Piazzalunga D, Belfiglio M, Labianca R, Barberis M, et al. Clinical and histopathological risk factors to predict sentinel lymph node positivity, disease-free and overall survival in clinical stages I-II AJCC skin melanoma: outcome analysis from a single-institution prospectively collected database. Eur J Cancer (2009) 45:2537–45. doi: 10.1016/j.ejca.2009.05.034
131. Clark WH, From L, Bernardino EA, Mihm MC. The histogenesis and biologic behavior of primary human malignant melanomas of the skin. Cancer Res. (1969) 29:705–27.
132. Weiss SA, Han SW, Lui K, Tchack J, Shapiro R, Berman R. Immunologic heterogeneity of tumor-infiltrating lymphocyte composition in primary melanoma. Hum Pathol. (2016) 57:116–25. doi: 10.1016/j.humpath.2016.07.008
133. Lee N, Zakka LR, Mihm MC Jr, Schatton T. Tumour-infiltrating lymphocytes in melanoma prognosis and cancer immunotherapy. Pathology (2016) 48:177–87. doi: 10.1016/j.pathol.2015.12.006
134. Gajewski TF, Schreiber H, Fu Y-X. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. (2013) 14:1014–22. doi: 10.1038/ni.2703
135. Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. (2013) 35:585–600. doi: 10.1007/s00281-013-0367-7
136. Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-β: “N1” versus ‘N2’ TAN. Cancer Cell (2009) 16:183–94. doi: 10.1016/j.ccr.2009.06.017
137. Bellone M. Ways to enhance lymphocyte trafficking into tumors and fitness of tumor infiltrating lymphocytes. Front Oncol. (2013) 3:231. doi: 10.3389/fonc.2013.00231
138. Buckanovich RJ, Facciabene A, Kim S, Benencia F, Sasaroli D, Balint K, et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat Med. (2008) 14:28–36. doi: 10.1038/nm1699
139. Gordon-Alonso M, Hirsch T, Wildmann C, van der Bruggen P. Galectin-3 captures interferon-gamma in the tumor matrix reducing chemokine gradient production and T-cell tumor infiltration. Nat Commun. (2017)1–15. doi: 10.1038/s41467-017-00925-6
140. Gazzaniga S, Bravo AI, Guglielmotti A, van Rooijen N, Maschi F, Vecchi A, et al. Targeting tumor-associated macrophages and inhibition of MCP-1 reduce angiogenesis and tumor growth in a human melanoma xenograft. J Invest Dermatol. (2007) 127:2031–41. doi: 10.1038/sj.jid.5700827
141. Payne AS, Cornelius LA. The role of chemokines in melanoma tumor growth and metastasis. J Invest Dermatol. (2002) 118:915–22. doi: 10.1046/j.1523-1747.2002.01725.x
142. Nguyen DX, Bos PD, Massagué J. Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer (2009) 9:274–84. doi: 10.1038/nrc2622
143. Calcinotto A, Grioni M, Jachetti E, Curnis F, Mondino A, Parmiani G, et al. Targeting TNF- to neoangiogenic vessels enhances lymphocyte infiltration in tumors and increases the therapeutic potential of immunotherapy. J Immunol. (2012) 188:2687–94. doi: 10.4049/jimmunol.1101877
144. Shrimali RK, Yu Z, Theoret MR, Chinnasamy D, Restifo NP, Rosenberg SA. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. (2010) 70:6171–80. doi: 10.1158/0008-5472.CAN-10-0153
145. Chinnasamy D, Tran E, Yu Z, Morgan RA, Restifo NP, Rosenberg SA. Simultaneous targeting of tumor antigens and the tumor vasculature using T lymphocyte transfer synergize to induce regression of established tumors in mice. Cancer Res. (2013) 73:3371–80. doi: 10.1158/0008-5472.CAN-12-3913
146. Nakajima C, Uekusa Y, Iwasaki M, Yamaguchi N, Mukai T, Gao P, et al. A role of interferon-gamma (IFN-gamma) in tumor immunity: T cells with the capacity to reject tumor cells are generated but fail to migrate to tumor sites in IFN-gamma-deficient mice. Cancer Res. (2001) 61:3399–405.
147. Peng W, Liu C, Xu C, Lou Y, Chen J, Yang Y, et al. PD-1 blockade enhances T-cell migration to tumors by elevating IFN- inducible chemokines. Cancer Res. (2012) 72:5209–18. doi: 10.1158/0008-5472.CAN-12-1187
Keywords: lymphocyte trafficking, skin, adhesion molecule, chemokine, melanoma
Citation: Lafouresse F and Groom JR (2018) A Task Force Against Local Inflammation and Cancer: Lymphocyte Trafficking to and Within the Skin. Front. Immunol. 9:2454. doi: 10.3389/fimmu.2018.02454
Received: 12 August 2018; Accepted: 04 October 2018;
Published: 24 October 2018.
Edited by:
James William Wells, The University of Queensland, AustraliaReviewed by:
Ana Paula Campanelli, Universidade de São Paulo, BrazilCinzia Fionda, Università degli Studi di Roma La Sapienza, Italy
Copyright © 2018 Lafouresse and Groom. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Fanny Lafouresse, lafouresse.f@wehi.edu.au
 Fanny Lafouresse
Fanny Lafouresse Joanna R. Groom
Joanna R. Groom