Nutritional compositions, pathogenic microorganisms and heavy metal concentration in green turtle eggs (Chelonia mydas) from Terengganu and Sabah, Malaysia
- 1Department of Veterinary Preclinical Sciences, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia
- 2School of Marine and Environmental Sciences, Universiti Malaysia Terengganu, Kuala Terengganu, Terengganu, Malaysia
- 3Institute of Oceanography and Environment, Universiti Malaysia Terengganu, Kuala Terengganu, Terengganu, Malaysia
- 4Department of Veterinary Pathology and Microbiology, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia
- 5Department of Veterinary Clinical Studies, Faculty of Veterinary Medicine, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia
- 6Laboratory of Sustainable Animal Production and Biodiversity, Institute of Tropical Agriculture and Food Security, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia
- 7Aquatic Animal Health and Therapeutics Laboratory, Institute of Bioscience, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia
- 8Institute of Bioscience, Universiti Putra Malaysia (UPM), Serdang, Selangor, Malaysia
A total of 60 green turtle eggs were obtained from sampling sites in Terengganu and Sabah, Malaysia. Isolation and identification of bacteria from these eggs resulted in 12 genera of Gram-negative bacteria with 12 different species. The most frequently isolated bacteria were Salmonella (30.9%) and Proteus (23.7%). The rest of the microorganisms were Aeromonas, Escherichia, Citrobacter, Enterobacter, Klebsiella, Morganella, Pseudomonas, Shigella, Serratia and Photobacterium. A slight difference in total crude protein content was recorded in the samples from Terengganu and Sabah, at 16.3% Dry matter (DM) and 15.8% DM, respectively. Meanwhile, the crude fat content found in the samples from Terengganu was 9.88% DM and 7.79% DM from Sabah. In this study, α-linolenic acid, C18:3 n-3 (Terengganu: 16.87% DM) and linoleic acid, C18:2 n-6 (Sabah: 15.19% DM) were the most prevalent fatty acids in both samples. The green turtle egg samples from Terengganu showed higher value of total saturated fatty acid, total C18:3 n-3 and C18:2 n-6 and total polyunsaturated fatty acids as compared to green turtle eggs from Sabah. The results also revealed that majority of the essential amino acids (EAA) recorded in samples from both sampling sites was lysine (9.67% DM), with higher value recorded in green turtle egg from Terengganu. However, there were no significant differences (p>0.05) in the nutritional compositions, fatty acid contents and amino acid compositions of the samples between the two sampling sites. Meanwhile, statistical analysis showed significant differences in heavy metal concentrations between the two sampling sites for all the six heavy metals detected in this study (Hg, Cd, Pb, Mn, Zn and Cu). The ranking of heavy metals concentration in turtle eggs from both sampling sites, in decreasing order is as follows: Zn > Cu > Mn > Pb > Cd > Hg. Overall, these results reveal the contents and contaminations of a green turtle egg, which may raise concern on public health risks. Findings from this study will also be beneficial for the future conservation of green turtle population when the consumption of their eggs should be stopped.
Introduction
Peninsular Malaysia has historically supported large nesting populations of green turtles (Chelonia mydas), predominantly found in the east-coast state of Terengganu (Redang and Perhentian Islands, Kemaman and Kerteh) and surrounding islands. Green turtles recorded the highest nesting density, with between 2,000 and 2,500 nests annually in that state. The species can also be found in Melaka and some parts of Pahang (Chendor and Cherating) and Perak (Pantai Remis), as well as the states of Sabah and Sarawak in East Malaysia Borneo (Chan, 2006). The green turtle was added to the IUCN (International Union for Conservation of Nature) Red List of threatened species in 1982 and it is also listed in Appendix I of the Convention on International Trade in Endangered Species of Wild Fauna and Flora (CITES). This program basically prevents any marine turtle parts or products from being misused or traded for commercial purposes, as their population faces a high risk of extinction.
In Malaysia, one of the most significant threats contributing to the decline of green turtle nesting is the commercial harvest for food and excessive collection of eggs for local consumption (Aguirre et al., 2006; TRAFFIC Southeast Asia for WWF-Malaysia, 2009; Jolis et al., 2015; Brei et al., 2016; Azlina et al., 2019). The long-standing problem of local demand for turtle eggs in the states of Sabah and Terengganu remains active. In Terengganu, other species of marine turtle eggs are allowed to be marketed except the eggs of leatherback turtle (Abd Mutalib et al., 2013). Furthermore, state laws of different states differ with regard to the sale and consumption of turtle eggs (TRAFFIC Southeast Asia for WWF-Malaysia, 2009). Only Sabah and Sarawak have total protection for these endangered marine animals because they are totally prohibited from any trade in marine turtles, parts and products due to the implementation of the Sabah Wildlife Conservation Enactment 1997 and Sarawak Wildlife Protection Ordinance 1998, respectively. Yet, illegal collection and consumption of turtle eggs are still uncontrollable due to insufficient man-power to monitor the areas.
Consumption of turtle products (eggs, meat, adipose tissue, organs and blood) is typical in some regions. Therefore, green turtles are harvested and exploited for their parts, such as their meat, eggs and organs, which are consumed as an important source of protein for coastal communities, for religious and cultural reasons as well as for traditional medicine purposes (TRAFFIC Southeast Asia for WWF-Malaysia, 2009). The consumption is believed to be related to fertility that works as a natural aphrodisiac, although there is no scientific evidence to prove it. Thus, turtles (meat and eggs) are among the many species of reptiles that are most intensely abused worldwide (Magnino et al., 2009; Hochberg and Bhadelia, 2016). Human perceptions might be one of the challenges to extensively publicizing the idea of marine turtle conservation. According to a survey, majority of the respondents consume marine turtle eggs due to its delicacy and the belief of nutritious and rare diet (TRAFFIC Southeast Asia for WWF-Malaysia, 2009). Some of them consume the egg just out of curiosity, especially the first-timers. In addition, some older generation consumers are unaware that marine turtle populations are declining as they believe that marine turtles produce a large number of eggs in each season (Abd Mutalib et al., 2013).
There are relatively few studies conducted on the quality of these products, raising concerns about public health risks due to pathogenic and environmental contaminants. Marine turtles are known as an excellent biological indicators of heavy metals because they can accumulate contaminants in the ecosystems they live in (Al-Rawahy et al., 2007; Yipel et al., 2017). Some factors that influence the toxicity of heavy metals, whether essential or not, include dosage, type of chemical, route of exposure, along with gender, genetics, age and nutritional status of the exposed individuals. According to a previous study, persistent organic pollutants (POPs) were found deposited in the turtle egg during vitellogenesis or development of follicles, which later become eggs (Stewart et al., 2011). It shows that pollutants can be transferred to the egg through maternal transfer and contaminate the reproductive organs where eggs are developed (Sinaei and Bolouki, 2017). Lots of studies have been done and suggested that consumption of sea turtle products (tissues, eggs, and blood) might pose a number of public health concerns because of their high lipid content and the presence of bacteria, parasites, and environmental contaminants (Aguirre et al., 2006; Van de Merwe et al., 2009; Warwick et al., 2013; Al-Musharafi et al., 2015). Nursing women and children are most likely to be affected following consumption of turtle eggs (Aguirre et al., 2006). It is unacceptable to assume that consumption of these products carries zero risk to health, even if well cooked, and possibly creates potential health hazards if consumed raw. It is vital to analyse the quality of green turtle eggs as a food source. Apparently, egg consumption may pose a health threat to the consumer and lead a population decline of green turtles. Therefore, the present study was conducted to determine the nutritional composition, pathogenic bacteria and heavy metal contaminations of green turtle eggs from Terengganu and Sabah, Malaysia.
Materials and methods
Sample collection and preparation
A total of 60 fresh green turtle eggs were collected from nesting sites in Terengganu and Sabah, Malaysia during the nesting season between April and September 2019. The samples were placed in sterile plastic bags, stored in an icebox, transported and analyzed within 24 h in the laboratory. Sample processing for determination of pathogenic microorganisms was done as soon as the arrival of egg samples to the laboratory. To analyze the sample of content, the egg shell was sterilized using 70% alcohol before the content was collected into a sterile plastic bag and labelled accordingly. A food stomacher was used to homogenize the egg yolk and albumen of each sample. The homogenized samples were placed in Scott bottles and stored in a freezer overnight prior to the 48-h freeze-drying process. The freeze-dried samples were then finely ground using a mortar. Then, the samples were stored in desiccators at room temperature and were ready to be used to determine the nutritional composition, fatty acid and amino acid profiles, as well as the heavy metal composition of turtle eggs.
Determination of nutritional composition
Dry matter and ash, moisture, crude protein and crude fat of green turtle eggs were measured according to the procedures of the Association of Official Analytical Chemists (Association of Official Analytical Chemists – International, 2012). In brief, dry matter and ash were measured after burning the sample in a furnace at 550°C for 4 h. Moisture content was determined using the air-dried oven method at 105°C for 24 h. The crude protein in green turtle eggs was estimated by measuring the total nitrogen via the Kjeldahl method and multiplying it by 6.25 protein factor. Meanwhile, crude fat from the sample was extracted using the Gerhardt Soxhlet apparatus, and the weight of the fat recovered was estimated. The energy content (M/J kg) of green turtle eggs was determined by combusting the samples in a bomb calorimeter (IKA C5003). All protocols were carried out in triplicate.
Determination of pathogenic microorganism
Bacterial isolations from the outer green turtle egg shell were done using a sterile cotton swab that was scrubbed over the surface of the eggs. To get samples of egg content, the shell was sterilized using 70% alcohol before the content was collected into a sterile plastic bag. The collected samples were then cultured onto pre-enrichment culture (non-selective liquid media), buffered peptone water and incubated at 37°C for 24 h. Buffered peptone water was used for pre-enrichment to promote the recovery of Salmonella spp. in the food sample prior to selective enrichment medium. The pre-enrichment culture was then sub-cultured into selective enrichment medium, the Rappaport Vassiliadis soy broth (RVS) and incubated at 37°C for 24 h. One ml of the RVS was then inoculated onto two selective agar media; the Xylose-Lysine Desoxycholate (XLD) agar and Brilliant Green agar for the isolation of Salmonella and incubated at 37°C for 24 h. Salmonella colonies were identified on each agar media and results were recorded. On the other hand, the pre-enrichment culture of buffered peptone water was also sub-cultured onto selective media of MacConkey agar to isolate the Gram-negative bacteria and onto blood agar for growth of wide range of microbes as well as detection certain bacteria through haemolytic pattern. The bacterial morphology was identified, including size, shape, colour and changes on various media. Pure isolates were phenotypically characterized by Gram staining and examined using light microscope at x100 with oil immersion. They were further identified by using biochemical tests including TSI, Citrate Utilization, oxidase, Urease, SIM and Indole test. Meanwhile, for identification of Salmonella sp, polyvalent O test was added to the method of identification for initial identification of the primary group of salmonella.
Determination of fatty acid composition
Green turtle eggs were analyzed for the fatty acid composition according to the one-step method (Abdulkadir and Tsuchiya, 2008) whereby fatty acid methyl esters (FAMEs) were prepared without prior lipid extraction. Three replicates of each sample (200–300 mg) were mixed with 4 ml of hexane and 1 ml of internal standard solution in a 15 ml Teflon-lined screw-cap. The internal standard solution was prepared earlier by dissolving 100 mg of 19:0 in 100 ml of hexane to obtain a final concentration of 1 mg/ml of 19:0. Then, the head space of Teflon-lined screw-cap was flushed with nitrogen gas after adding 2 ml of 14% boron trifluoride (BF3) in methanol. The tube was closed tightly with a Teflon-lined screw-cap and heated on a hot plate at 100°C for 120 min under continuous stirring. After cooling to room temperature, 1 ml of hexane was added, followed by 2 ml of distilled water. The tube was then shaken vigorously for 1 min and centrifuged for 3 min at 2500 rpm. Finally, 1 ml of the hexane layer containing the FAMEs was transferred using a Pasteur pipette into a clean sample vial and injected to the GC for FAME analysis. The FAMEs were separated and quantified using a gas chromatography (GC-FID Agilent) equipped with a flame ionization detector. Separation was performed with an Zebron ZB-Fame GC capillary column (30 m × 0.25 mm internal diameter, 0.20 μm film thickness) using hydrogen as a carrier gas. After injection at 80°C, the oven temperature was raised to 150°C at a rate of 40°C min-1, then to 270°C at 3°C min-1, and finally held constant for 30 min. The flame ionization was held at 270°C. The Shimadzu Labsolutions software was used to manage the system, data and analysis of reports. The FAME peaks were identified by comparing their retention times with those of authentic standards (Supelco Inc.),. The fatty acid contents of the samples were expressed as percentage of total component detected.
Determination of amino acid composition
Amino acid profiles were determined using Waters AccQ-Tag method. Approximately 0.2 g of sample was transferred into a glass-stoppered test tube and hydrolysed with 5 mL of 6 M HCl at 110°C for 24 h. Samples were cooled to room temperature before being added to a 4 mL internal standard of 2.5 mM L-2-Aminobutyric acid (AABA). Then, the mixtures were transferred into a 100 mL volumetric flask, made up to 100 mL with ultrapure water and mixed well. The hydrolysate solution of 1.5 mL was filtered using 0.45 µm polytetrafluoroethylene syringe filter and transferred into Eppendorf tubes. As for the derivatization process, 10 µl of hydrolysate sample or standard solution was transferred into a 1.5 mL Eppendorf tube. The hydrolysate sample or standard was buffered to pH 8.8 with the addition of 70 µl of borate buffer and vortex for several seconds. Then, 20 µl of AccQ Fluor reagent was added and thoroughly mixed by vortex for several seconds. The mixture stood for one minute at room temperature before 10 µL of sample was transferred into glass vials and heated at 55°C for 10 min. Then, samples and standards were injected into HPLC of Waters (Alliance e2695) equipped with a fluorescence detector (2475-waters). Separation of all amino acids was carried out using AccQ Taq (3.9 x 150 mm) and the flow rate was set at 1 mL min-1. Preparation of mobile phases that consisted of mobile phase A was Eluent A (100 mL AccQ Taq with 1 L of ultrapure water), mobile phase B (acetonitrile) and mobile phase C (Water). Reference standards consisted of 16 amino acids at 2.5 mM certified concentration, Cystine at 1.25 mM certified concentration, L – Hydroxyproline at ≥ 99%, 2.5 mM (MW = 131.13 g/mol) and L-2-Aminobutyric acid at ≥ 99%, 2.5 mM (MW = 103.12 g/mol) as internal standard.
Determination of heavy metal content
Analysis of heavy metals in turtle eggs was performed according to AOAC Official Method, 16th Edition Official Method 985.01 (1995). Approximately 0.3 g of each freeze-dried sample was added into a separate digestion vessel of tetrafluorrmethaxil (TFM) vessel with 3 ml of nitric acid (65%) and 2 ml of hydrogen peroxide (30%). The TFM vessels were closed and placed in the polypropylene rotor body, in pairs opposite each other. The screws on the polypropylene rotor body were tightened with a wrench to ensure the vessels were locked in their niche. The digestion of samples was carried out for about 30 min using Anton Paar microwave digestion. Following digestion, the samples were left to cool and the pressure was allowed to be released completely by loosening the screw. The samples were collected from the vessels, transferred into 50 ml volumetric flasks, then diluted up to volume with deionized water. Element determination was accomplished by ICP-MS (ELAN 9000). Calibration of the instrument was done before proceeded with the samples.
Statistical analysis
All Statistical analyses were performed using statistical analysis software package SPSS (Statistical Package for the Social Science 25.0, Inc., Chicago, IL, USA) and were analyzed by independent T-tests. Experiments were carried out in triplicate and the results were presented as means. The level of significance among the two different groups, which involves samples from Terengganu and Sabah was set at p<0.05.
Results
Pathogenic bacteria of green turtle egg
According to the geographic site of sampling, 64 (42.1%) and 88 (57.8%) bacterial isolates from a total of 152 isolates were detected in the samples from Terengganu and Sabah, respectively. A total of 74 isolates, which represented 10 different genera of bacteria with 10 species were identified from the outer surface of green turtle eggs (Figure 1). The dominant genera found in samples from Terengganu were Salmonella (48%), followed by Escherichia (15%) and Morganella (15%). Meanwhile, the most frequent isolates found in samples from Sabah were Salmonella (44.2%), followed by Aeromonas (14.7%) and Citrobacter (11.7%). Both sampling locations showed high percentage of Salmonella, and Salmonella enterica was the most common bacterial isolate of both sites. Total percentage of bacterial isolation from Terengganu was 26.3%, which was higher than the 22.4% from Sabah (Figure 2).
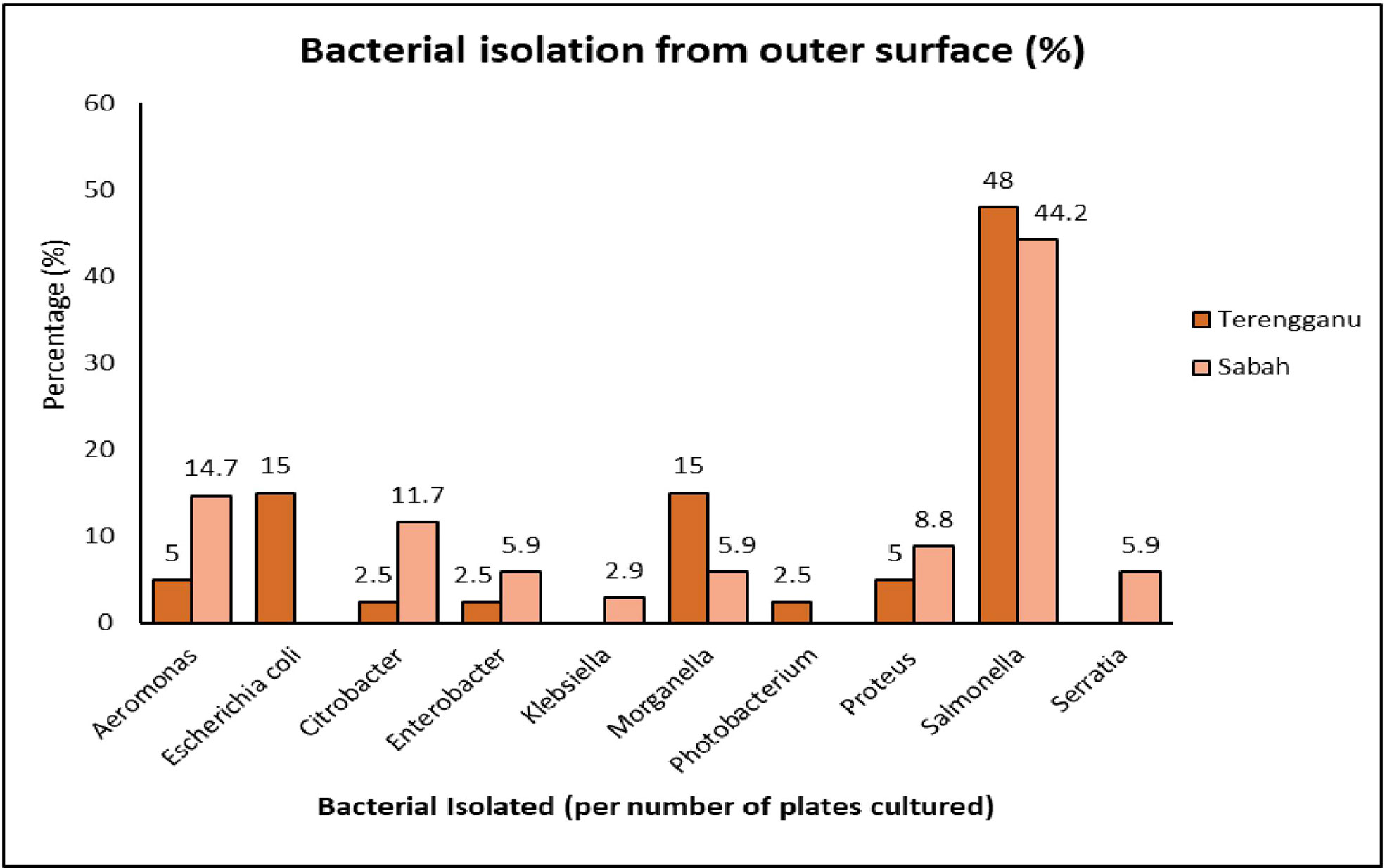
Figure 1 The percentage of bacterial isolation identified on the outer surface of green turtle eggs collected from Terengganu and Sabah.
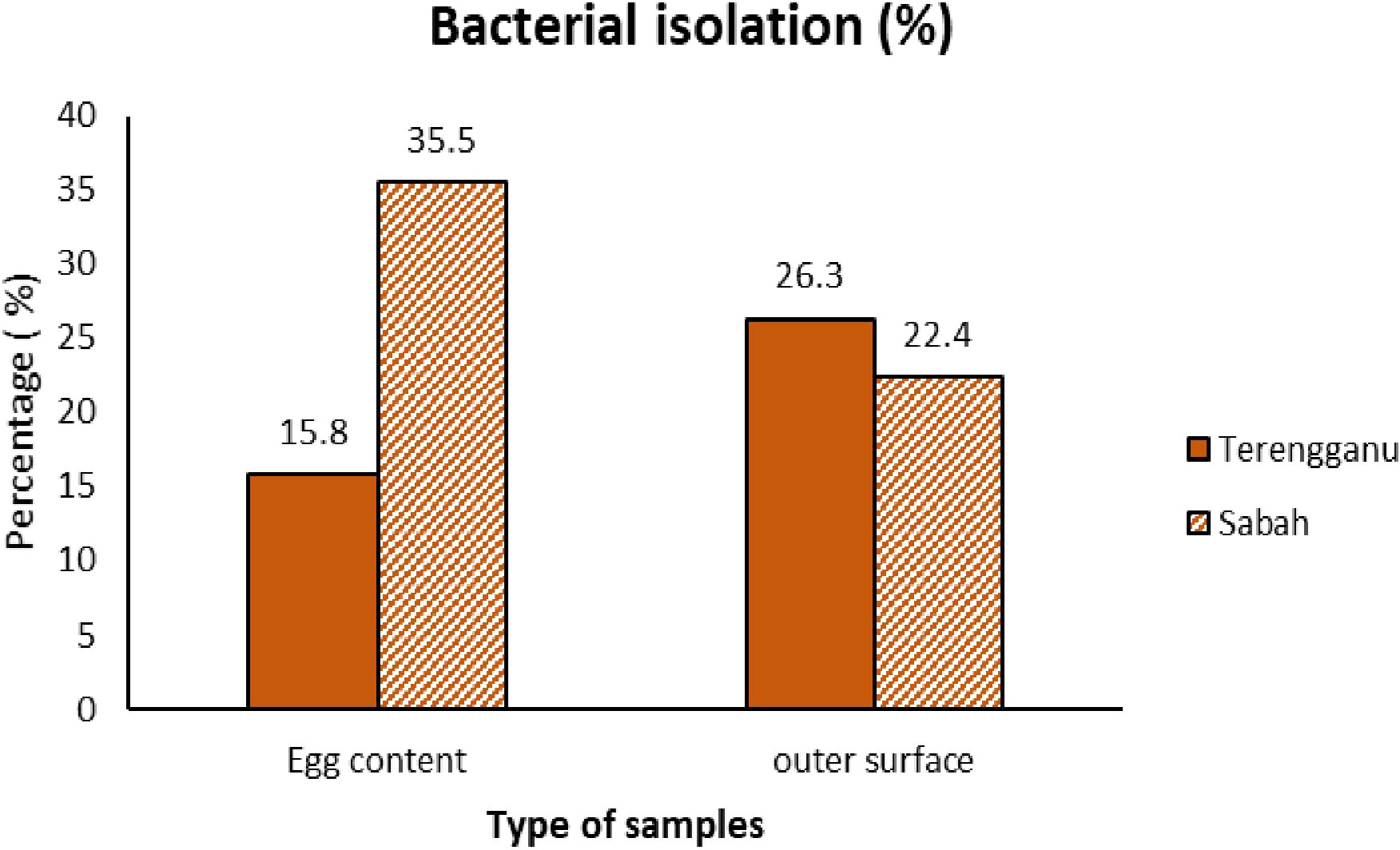
Figure 2 The comparison of bacterial isolation percentages between samples of green turtle egg contents and the outer surface of the eggs from Terengganu and Sabah.
Figure 3 summarizes the percentage of bacteria identified from the green turtle egg contents collected from Terengganu and Sabah. A total of 7 different bacterial species were identified that represented 9 genera of bacteria. The most frequent isolates in samples from Terengganu were Proteus (45.8%), followed by Salmonella (29.1%), Aeromonas (8.3%) and Pseudomonas (8.3%), while samples from Sabah also recorded Proteus (36.6%) as the most frequent isolates, followed by Citrobacter (18.5%) and Salmonella (11.2%).

Figure 3 The percentage of bacterial isolation identified in green turtle egg contents collected from Terengganu and Sabah.
Nutritional composition of green turtle egg
The compositions of moisture, dry matter, ash, crude protein, crude fat and energy value of green turtle eggs are presented in Table 1 along with the characteristics of green turtle eggs. All parameters of the green turtle eggs were from samples taken from the two different sampling sites. The average weight of green turtle eggs ranged between 31.81 g and 29.52 g, with heavier samples from Terengganu. Meanwhile, the average height and diameter of the egg yolk were dominated by samples from Sabah, which ranged between 30.01 mm and 41.63 mm. Overall, the values of crude protein and crude fat were higher in the samples from Terengganu (16.33% DM and 9.88% DM, respectively) and similar patterns were also detected for moisture and ash contents. Meanwhile, slightly higher value of dry matter content was recorded in the samples from Sabah. However, there were no significant differences (p>0.05) between the mean values of samples from Terengganu and Sabah for all measured parameters.
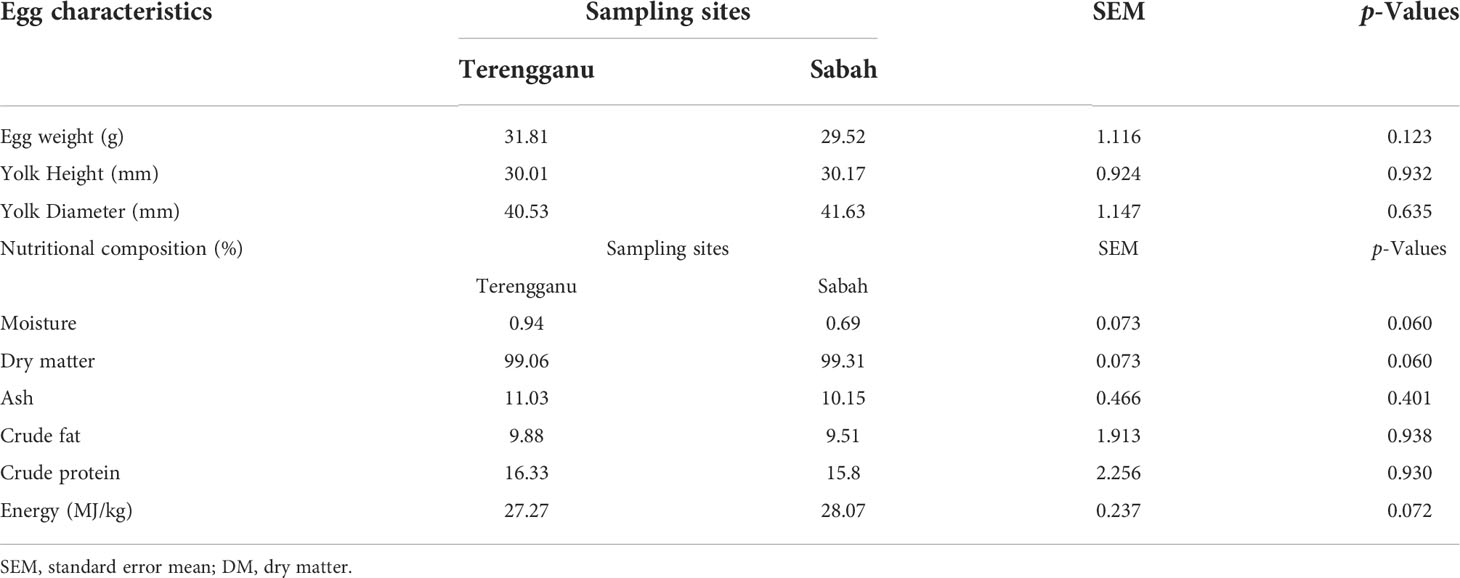
Table 1 The characteristics of green turtle eggs and the mean concentrations of nutritional composition (% DM) in green turtle eggs collected from two different sampling sites, Terengganu and Sabah, Malaysia.
Fatty acid composition of green turtle eggs
Table 2 shows the fatty acid composition of green turtle eggs from different sampling sites of Terengganu and Sabah. The compositions were classified according to different fatty acid length and saturation, namely saturated fatty acid (SFA), monounsaturated fatty acid (MUFA) and polyunsaturated fatty acid (PUFA). The total fatty acid contents in green turtle eggs were higher in the sample from Terengganu (2827.3 mg/kg DM) than in the sample from Sabah (1782 mg/kg DM). The most abundant fatty acids in samples from Terengganu was α-linolenic acid, C18:3 n-3 (16.87% DM) while linoleic acid, C18:2 n-6 was recorded for samples from Sabah (15.19% DM). Both sampling sites reported C22:6 n-3, known as docosahexaenoic acid (DHA), as the lowest value of fatty acid components (Terengganu: 0.01% DM, Sabah: 0.13% DM). Turtle egg samples collected from Terengganu recorded a higher value of myristic acid, C14:0 (3.48% DM) from the total saturated fatty acid of 15.57% DM, which was higher than the sample from Sabah at 9.92% DM. The major component of polyunsaturated fatty acid (29.55% DM) was α-linolenic acid, C18:3 n-3 (16.87% DM), which was the highest fatty acid value. Meanwhile, Sabah recorded a higher total value of monounsaturated fatty acids (31.12% DM) with the value of palmitoleic acid, C16:1 (12.33% DM) being the highest monounsaturated fatty acid in the sample. There were no significant differences (p > 0.05) of fatty acids between the two sampling sites except for linoleic acid, C18:2 n-6. Overall, the green turtle egg samples collected from Terengganu showed higher value of total saturated fatty acid, total n-3 PUFAs and n-6 PUFAs and total polyunsaturated fatty acids.
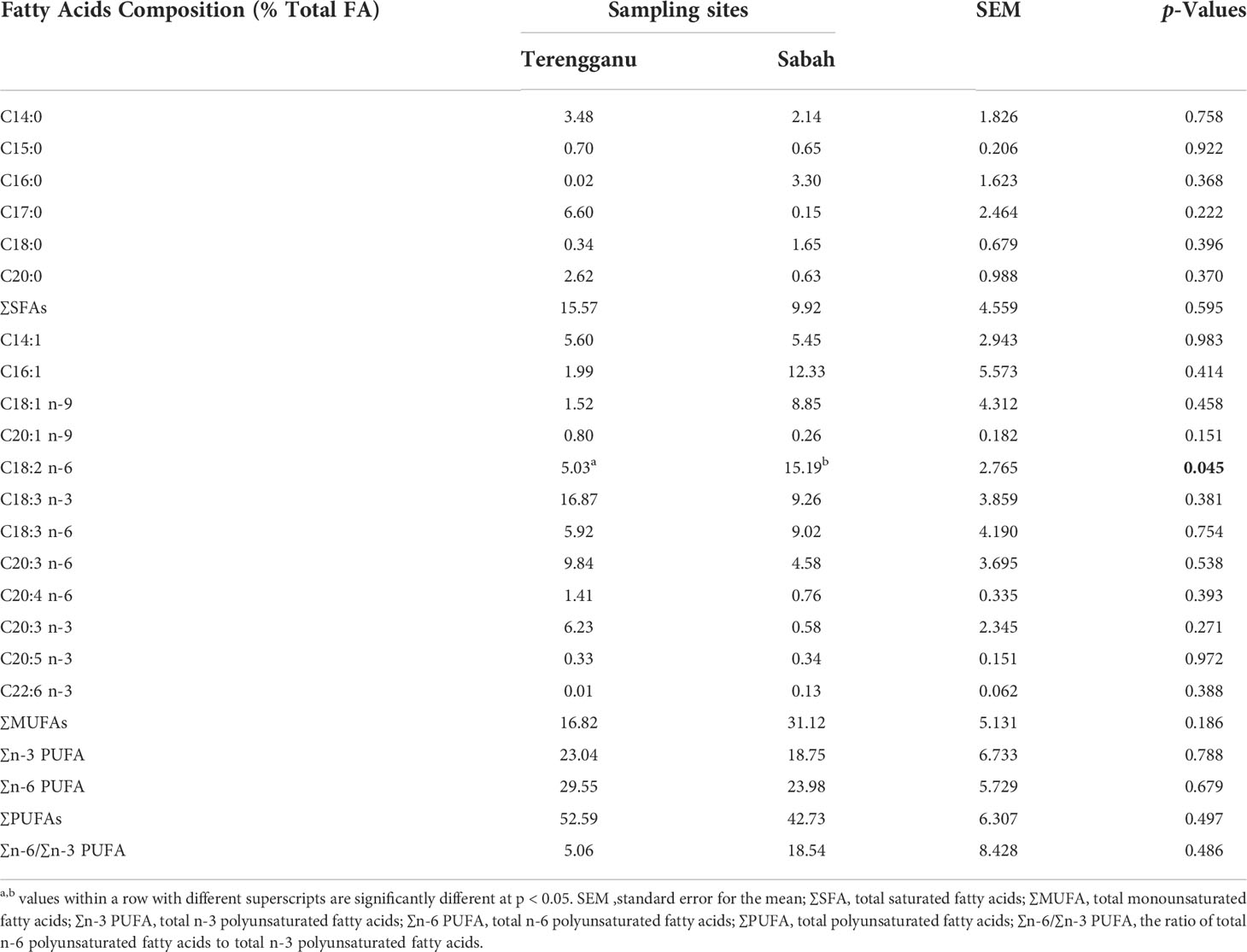
Table 2 The mean concentrations of fatty acid (% DM) in green turtle eggs collected from Terengganu and Sabah, Malaysia.
Amino acid composition of green turtle eggs
The amino acid compositions of green turtle eggs from Terengganu and Sabah are summarised in Table 3. The results revealed that the majority of essential amino acids (EAA) recorded in samples from both sampling sites was lysine, with higher value being recorded in green turtle eggs from Terengganu. Lysine (9.67% DM) and isoleucine (7.63% DM) were major components of the essential amino acid (EAA) in the sample from Terengganu, followed by threonine (4.97% DM), valine (4.95% DM) and leucine (4.63% DM), which made up the total EAA of 40.73% DM. All the listed components were higher in the samples from Terengganu except threonine and valine. Moreover, there were significantly (p<0.05) higher concentrations of threonine (5.03% DM) and valine (5.03% DM) in the samples from Sabah than Terengganu (threonine, 4.97% DM and valine, 4.95% DM; p < 0.05). Meanwhile, the concentration of histidine was recorded significantly (p<0.05) higher in the samples from Terengganu (2.64% DM) than Sabah (2.58% DM; p < 0.05). Among the non-essential amino acids (NEAA), glutamic acid was the highest in samples from both sites (Terengganu: 12.66% DM; Sabah: 12.65% DM). Comparatively, all components of NEAA detected in the samples from Sabah were higher except for glutamic acid, alanine, tyrosine and hydroxyproline. In addition, a significantly higher concentration of serine was observed in the samples from Sabah (9.94% DM) than Terengganu (9.18% DM; p < 0.05). The results showed that samples from Sabah contained higher values of total NEAA and EAA, while the total NEAA values were more dominant in both samples.
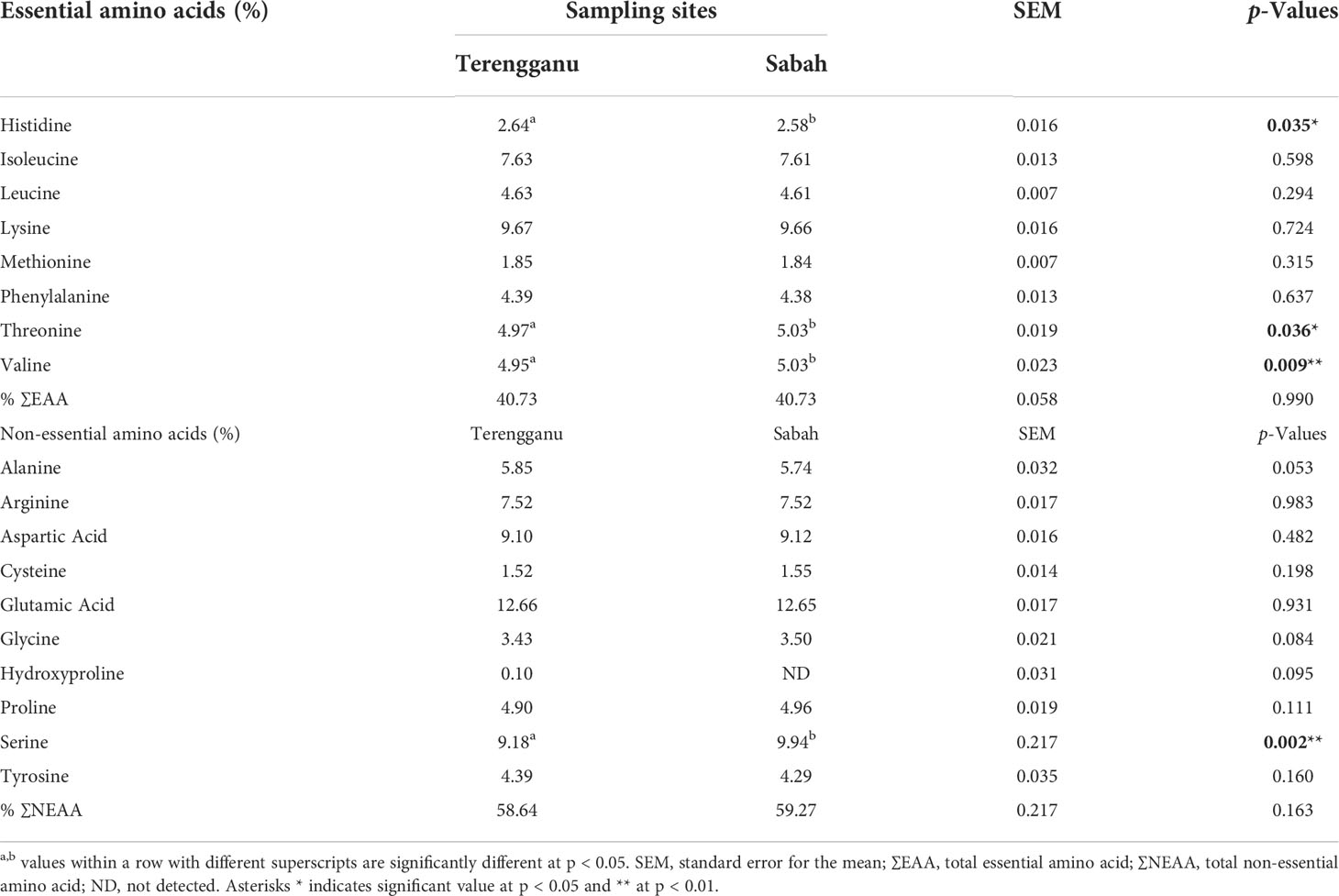
Table 3 The mean concentrations of amino acid composition (% DM) in green turtle eggs collected from two different sampling sites, Terengganu and Sabah, Malaysia.
Heavy metal concentrations in green turtle eggs
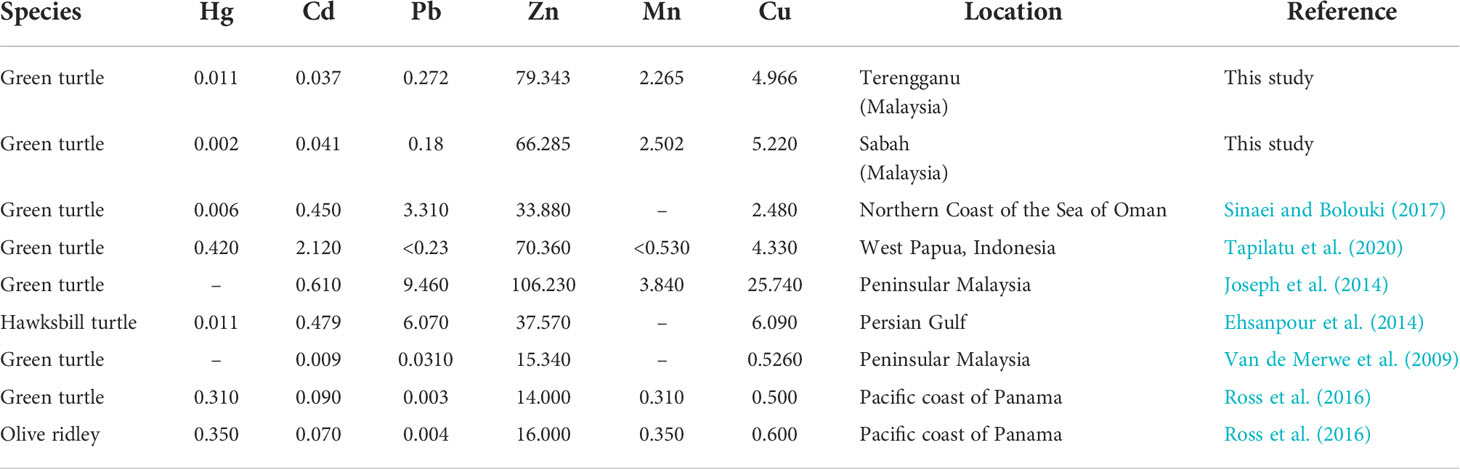
Concentrations of heavy metals in turtle eggs collected from Terengganu and Sabah are presented in Table 4. The differences in mean concentrations of heavy metals (Hg, Cd, Pb, Zn, Mn and Cu) between the two sampling sites were found to be statistically significant (p < 0.05). A higher concentration of mercury, Hg was recorded in turtle eggs from Terengganu (0.011 mg/kg DM) than Sabah (0.002 mg/kg DM). Meanwhile, cadmium (Cd), which is categorized as toxic metal together with mercury (Hg) and lead (Pb) was found in relatively low concentrations (Terengganu: 0.037 mg/kg DM; Sabah: 0.041 mg/kg DM). Zinc concentrations were the highest in the turtle eggs from both sampling sites, and relatively higher in Terengganu (79.343 mg/kg DM) than Sabah (66.285 mg/kg DM). The ranking of heavy metal concentration in turtle eggs from Terengganu in decreasing order is as follows: Zn > Cu > Mn > Pb > Cd > Hg. The trend was similar to heavy metal concentrations found in turtle eggs from Sabah.
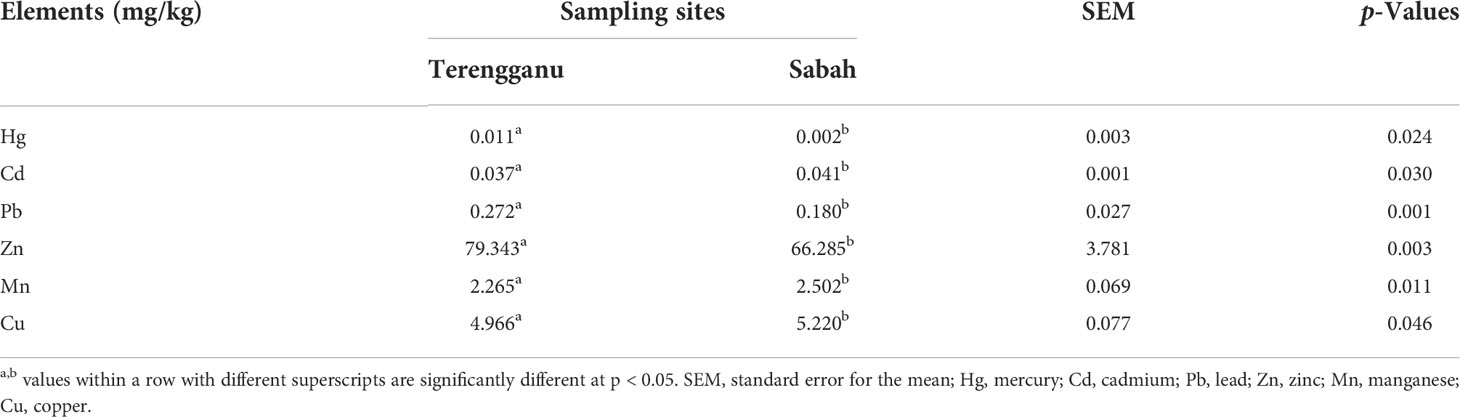
Table 4 The mean concentrations of heavy metal composition (mg/kg) in green turtle eggs collected from two different sampling sites, Terengganu and Sabah, Malaysia.
Discussion
Typically, green turtle egg is a soft-shelled egg with the size of a ping-pong ball. However, the size of turtle eggs varies amongst marine turtle species, with the Hawksbill turtle having the smallest egg size of 3.8 cm in diameter and weighing approximately 28 g, while the largest egg size belongs to the Leatherback turtle at 5.3 cm in diameter and weighing 90 g. The development of green turtle eggs can be influenced by both biotic and abiotic factors (Wallace et al., 2006). For example, the dietary intake of female green turtles is essential for the production of eggs, especially the egg yolk that contains proteins, fatty acids, fat-soluble antioxidants (vitamins A, E and carotenoids) and lipids. In fact, fatty acids and carotenoids are vital for the development of embryonic tissues of the green turtle egg. Other than seagrass, some green turtle populations in certain habitats depend on marine algae, invertebrates, and fish for their diets. Apparently, green turtle egg is an amniotic egg, which consists of membranes that protect the embryo while the eggshell allows for gas, heat and moisture exchanges with the external environment (Ackerman, 1997; Robinson and Paladino, 2013). Therefore, any microbial or heavy metal contamination will probably affect the composition of the eggs.
Green turtle eggs are not commercially used for human consumption as chicken eggs are. Chicken eggs are more suitable as primary food for humans and are more preferred to consume than turtle eggs due to the high nutritional quality (Réhault-Godbert et al., 2019). Meanwhile, green turtle eggs have to be protected solely for the continuity of the species. However, this endangered species is getting popular for consumption, especially the eggs. This raised a concern that the phenomenon might trigger a public health risk due to insufficient data on its nutritional properties. The present study revealed that there was not much difference in the overall turtle egg parameters collected from different sampling sites of Terengganu and Sabah, although the average weight of the green turtle egg was higher in Terengganu but lower in height and diameter of the eggs.
Protein and fat contents were slightly higher in Terengganu, while moisture content was the lowest composition recorded in the turtle eggs. This shows that protein is a major component of the nutritional composition in green turtle eggs, likely due to the consumption of seagrass as the main diet of female green turtles (Wyneken et al., 2013). In fact, seagrass contains high protein, especially during summer (Pradheeba et al., 2011) and contributes to the nutritional composition of its consumers. A previous study revealed similar protein and fat contents but different moisture content in Hermann’s tortoise egg (Stvarnik et al., 2017). On the other hand, moisture and protein contents are the highest components in Olive ridley (Lepidochelys olivacea) eggs (Castro-González and Romo, 2011). The differences in nutritional compositions between different species of turtle could be due to species variations in dietary intake (Kabir et al., 2015). In fact, almost 95% of the mean gut content biomass of green turtle male and non-breeding females and 58% of gravid females were dominated by seagrass (Stokes et al., 2019). However, diets of green turtles are different between the Western South Atlantic and the tropical Pacific since there are tropical reefs that become habitats for macroalgae and turf algae (Goatley et al., 2012; Santos et al., 2015). Therefore, nutritional compositions of green turtle eggs coming from different regions might be different from the present study due to disparities in food sources consumed by the gravid females. Furthermore, the olive ridley turtle is a carnivorous species that mainly feeds on shellfish and small crabs, and this may influence the findings. Thus, the differences in nutritional values of these reptiles depend on the diet, genetics, age, management and feeding of the source animal (Yang et al., 2020). In this study, the energy content of turtle eggs was around 25 - 29 MJ/kg, which is similar to a previous study (Booth, 2003). The lipid and protein contents in egg yolk act as sources of energy for the development of the embryo (Thompson and Speake, 2002). Nevertheless, the relatively low energy content found in these samples was probably influenced by the low protein intake by female green turtles.
In this study, 12 genera of Gram-negative bacteria consisted of 12 species were identified. They are mainly represented by the phylum of proteobacteria, and the most prevalent bacteria were Salmonella and Proteus. Green turtle eggs can be contaminated by potentially pathogenic microorganisms prior to oviposition or even before the eggshell construction (Al-Bahry et al., 2009). They are prone to being exposed to pathogenic microorganisms due to the fact that their marine environment is subjected to a variety of contaminants, including oil spills, chemical waste and other debris. Furthermore, green turtles undergo dietary changes throughout the ontogenetic shift. During their juvenile stage, they are mostly omnivorous that commonly found in surface-pelagic habitats, feeding on algae, seagrass, invertebrates and terrestrial plants (Nagaoka et al., 2012). However, they eventually become herbivorous once they grow into adulthood and move to neritic habitats that consist of huge sources of seagrass that become the major component of green turtles diets (Howell et al., 2016; Price et al., 2017). This long-term exposure to contaminated feed leads to an accumulation of poisons in the blood and organs of green turtles. Similarly, pathogenic microorganisms like Salmonella are ingested with contaminated feed or water, infecting the digestive system that subsequently contaminate the eggs (Alkindi et al., 2006). Other than that, the gut microbiome of green turtles and other marine mammals can also be influenced by age, diet, habitat and health status (Nelson et al., 2013). Since green turtles have high mobility and their movement patterns vary during migration between breeding and foraging habitats, this facilitates long-term exposure to environmental toxicants along their migratory routes.
Proteus is commonly found in nature, particularly in soil, stagnant water and sewage, and in the gastrointestinal tract. This study revealed that Proteus vulgaris and Proteus mirabilis dominated the turtle egg contents collected from Terengganu and Sabah, respectively. Keene (2012) suggested that the bacterial contamination could affect the reproductive organs, mainly the ovary and uterus (Keene, 2012). Therefore, it is also possible that harmful microorganisms are transmitted to the egg through maternal transmission and through cloacal passage during oviposition (Gantois et al., 2009). However, a low percentage of Proteus sp was isolated from oral and cloacal samples of healthy loggerhead sea turtles (Gantois et al., 2009), since cloacal microbial community is commonly dominated by Citrobacter freundii, Edwardsiella tarda and Serratia odorifer (Keene et al., 2014; Ahasan et al., 2017). More studies should be conducted to understand the relationship between a female’s infection status and the infection status of her eggs.
This study revealed that both Salmonella enterica spp arizonae and Salmonella enterica dominated the turtle egg surface. Green turtle eggs can be contaminated by a wide spectrum of bacterial species while being incubated in the sand since the nest chamber is also a potential source of microbial infection of eggs. In fact, proteobacteria is the predominant bacterial phylum commonly found in nesting sea turtles (Scheelings et al., 2020), which includes Bacillus sp., Salmonella sp., Citrobacter freundii and Mucor sp. (Estika, 2013). It should be noted that the condition of the sand in the nest chamber, such as high humidity, nutrients and warm temperature influence bacterial growth in the sand that subsequently contaminate the green turtle eggs. These environmental factors promote the filtration of microorganisms into the eggs (Al-Bahry et al., 2009). It is easier for the microorganisms to penetrate the egg when there are damages on the eggshells (Al-Bahry et al., 2004). Furthermore, the longer an egg remains in direct contact with contaminated sand, the more likely bacteria penetrate the egg. Similarly, other environmental variables such as rain and runoff, wave action and the presence of organics provide significantly impact on the prevalence of microorganisms in sand. A recent study showed that the diversity of eggshell bacterial communities was correlated with the bacteria found in the egg content. In this study, Aeromonas, Citrobacter sp, Citrobacter freundii, Enterobacter spp, Klebsiella pneumonia, Proteus vulgaris and Salmonella enterica were found in both egg content and eggshell. This indicates that microorganisms on the outer shells had succeeded in penetrating the eggs through the eggshell pores (Al-Bahry et al., 2011). Originally, the function of eggshell pores is to modulate the movement of water and gases into and out of the egg. However, certain conditions such as low temperatures and high humidity promote the filtration of microorganisms into the eggs (Al-Bahry et al., 2011).
Some proteobacteria have been associated poor percentage of hatching success (Santoro et al., 2006; Elshafie et al., 2007; Sarmiento-Ramirez et al., 2017). However, a recent study found that microbiomes that are vertically transmitted to the eggs of oviparous animals can decrease egg failure and inhibit fungal growth on the eggshells (Bunker et al., 2021). Nevertheless, this does not change the fact that being associated with the animal and consuming green turtle products on a regular basis can endanger human health (Aguirre et al., 2006). Coastal communities of Mexico prefer sea turtles as a source of food, medicine and decoration (Delgado, 2005). They usually consume the entire animal, including blood and eggs as a remedy for anaemia and asthma. Subsequently, there is an issue related to sea turtle and chelonitoxin that leads to chelonitoxism to the consumers who consume contaminated sea turtles. It is harmful to infants and fetuses following maternal poisoning through breast milk and transplacental transfer (Fussy et al., 2007). In fact, a total of sixty-eight cases were reported, with four deaths in association with the illness due to consumption turtle meat and turtle meat soup (Ventura et al., 2015). Therefore, marine turtles are one of the sources of foodborne toxicity in humans.
Salmonella sp., Proteus sp., Aeromonas, Escherichia coli, Citrobacter sp., Enterobacter sp., Klebsiella sp., Morganella morganii, Pseudomonas aeruginosa, Salmonella sp. and Shigella sp. are some of the pathogenic microorganisms found in this study. Some of these microorganisms are common bacterial flora of nesting green turtles (Santoro et al., 2006). In this study, Salmonella sp. and Proteus sp. were the most common Gram-negative microbes, but Salmonella infection in green turtles can cause symptoms ranging from mild disruption of the gastrointestinal tract to death. According to a group of researchers, Salmonella is commonly isolated from poultry (De Freitas et al., 2010). However, a study reported cases of human salmonellosis in Japan that occurred due to the consumption of raw meat, blood and cooked meat of snapping turtles (Fukushima et al., 2008). Meanwhile, Proteus species are parts of human intestinal flora. However, it can cause severe infections in humans along with Escherichia coli and Klebsiella sp. such as urinary tract infections and gastrointestinal disease. Marine turtle products are one of the typical routes of human contamination either through consumption or contact. Numerous studies have reported them as a significant reservoir of potential pathogenic microbes and toxin contaminations (Fussy et al., 2007; Soslau et al., 2011; Domiciano et al., 2017; Mashkour et al., 2018). In lined with this, this study was able to identify the potential pathogenic microorganisms in the green turtle eggs that might be harmful to consumers. The high demand for green turtle eggs for human consumption has inhibited the growth of turtle population for the past few years. Although conservation efforts have been conducted thoroughly by responsible people, it is not enough due to limited human resources and infrastructure.
There were no significant differences in fatty acid composition between the two sampling sites except for linoleic acid, C18:2 n-6. The current study revealed that among the polyunsaturated fatty acids, (PUFAs), α-linolenic acid (ALA; C18:3 n-3) was the dominant fatty acid of omega-3 (n-3) PUFAs in Terengganu, while linoleic acid (LA; C18:2 n-6) of omega-6 (n-6) PUFAs in Sabah. This might be the outcomes of consumptions of seagrass and algae, which are known sources of ALA and LA. In fact, studies have shown that the sources of ALA and LA are principally plant-based essential fatty acids, and algae have proven as a good source of n-3 PUFAs (Peltomaa et al., 2018; Harwood, 2019). Due to its nutritional value, algae has been described as a “functional food” that benefits human health (Wells et al., 2017). Sources of n-3 fatty acids include water plants of algae, fish oil, vegetable oil and marine animals (Rajaram, 2014). Alpha-linolenic acid acts as a precursor in the human and animal body by converting into EPA and DHA through desaturation and elongation reactions (Barcelo´-Coblijn and Murphy, 2009). However, synthesis of these long-chain n-3 PUFA is inefficient. This explains the low levels of EPA and DHA detected in the current study. Besides, the sources of EPA and DHA mostly come from marine animals. On the contrary, a previous study exhibited different fatty acid results where α-linolenic acid was not detected in the eggs of green turtles caught from the wild but showed a low value of LA (Craven et al., 2008). Both n-3 and n-6 PUFAs are known as essential fatty acids, which are required only in small amounts in the human diet. However, an imbalance of n-6 and n-3 PUFAs (n-6/n-3) in the diet can influence human health (Ristic-Medic et al., 2013). Consequently, excessive dietary intake of PUFAs contributes to the development of a variety of chronic diseases. Therefore, the balanced ratio concept of n-6/n-3 was developed as a nutritional indicator in food (Simopoulos, 2008). It is recommended to reduce the ratio of n-6/n-3 PUFAs to as close to a 1:1 ratio, which is ideal for a healthy balanced diet (Bhardwaj et al., 2016). However, this study has revealed contradicting results for n-6/n-3 PUFAs when samples from Terengganu recorded three times higher ratio than Sabah, and exceeded the recommended ratios. The imbalanced n-6/n-3 PUFAs can trigger cholesterol levels in humans, increase the tendency for blood clotting and lead to the potential risk of having coronary heart disease (Swanson et al., 2012; Sokoła-Wysoczańska et al., 2018). Saturated fatty acid (SFA) has been known to have the potential of causing cardiovascular disease (CVD) (DiNicolantonio et al., 2016; Ruiz-Núñez et al., 2016). However, there is not much debate about SFA of marine turtle eggs, especially when the total saturated fatty acids reported in this study was low compared to unsaturated fatty acid, similar to the eggs of snail-eating turtle (Malayemys macrocephala) and quail (Coturnic coturnix japonica) (Tunsaringkarn et al., 2013a; Tunsaringkarn et al., 2013b). The current finding has reported that both myristic acids (C14:0) and palmitic acids (C16:0) are the major components of SFA, which apparently could lead to the increase of low-density lipoprotein (LDL) cholesterol as one of the risk factors for cardiovascular disease (Grundy, 2012; Mensink, 2016). Therefore, saturated fatty acid should be consumed in low quantities as it is not an essential fatty acid and replacing SFA with PUFA and proteins, especially plant proteins could probably reduce risks of CVD (Siri-tarino et al., 2010; Briggs et al., 2017).
The amino acid profiles of green turtle eggs from Terengganu and Sabah revealed that lysine was the most abundant essential amino acids, which is in agreement with the eggs of Hermann’s tortoise and snail-eating turtle (Tunsaringkarn et al., 2013b). In fact, lysine was among the highest amino acid found in chicken eggs (Attia et al., 2020). The highest essential amino acid composition in an egg would be the combination of phenylalanine and tyrosine (Sakanaka et al., 2000), with tryptophan at the lowest value (Attia et al., 2020). The main functions of lysine are to support the immune system, prevent osteoporosis through involvement in calcium absorption, and enhance the release of growth hormone that increases muscle mass. Meat, poultry, soy-based and dairy-based products are some of the food sources that are rich in lysine. A study has shown that valine is the highest amino acid in beef meat, followed by lysine and leucine, which are higher than lamb and pork (Ahmad et al., 2018). Meanwhile, among the ten non-essential amino acids measured in this study, glutamic acid was the dominant amino acid recorded in both sampling sites, followed by serine and aspartic acid, similar to previous studies (Tunsaringkarn et al., 2013b; Stvarnik et al., 2017). These could be due to the dietary intake of female green turtles that affect the composition of eggs (Craven et al., 2008; Weber, 2010). Glutamic acid or its ionic form, glutamate has some important roles in amino acid metabolism and physiological processes in humans, mainly within the intestines and the nervous system of the brain (Brosnan and Brosnan, 2013). In general, this study revealed that the total non-essential amino acids were more dominant that essential amino acids in turtle eggs of both sites, which is similar to other consumable eggs like Hermanns tortoise and snail-eating turtle. Since relatively low levels of amino acids were recorded in turtle eggs, it is perhaps not worth to consume turtle eggs on daily basis. Insufficient or excessive intake of certain foods is not recommended on a daily basis because it may cause certain dysfunctional processes in the body and probably lead to some diseases. The public is advised to have a moderate consumption of nutrient intake and follow the guidelines of the food pyramid in order to maintain good health.
The present study revealed that accumulations of heavy metals do happen in turtle eggs other than the blood and tissue of the animals. However, a study reported that there were relatively low levels of trace elements and organic contaminants in the tissues and blood samples of green turtles than in vertebrates (D’ilio et al., 2011). Additionally, different heavy metals were acquired in different samples obtained from different locations (Hostetler et al., 2003). Toxic contaminants in marine green turtles are most probably dependent on the availability of food resources, particularly from the polluted foraging grounds and through trophic transfer (Van de Merwe, 2008). Trace elements normally accumulate in water and sediment as well as algae and seagrass that become the main diet of marine turtles (Wyneken et al., 2013). Therefore, they are unintendedly more exposed to pollutions. In other words, they are susceptible to any contamination coming from their surroundings.
Numerous studies have suggested that female marine turtles have the potential to excrete their toxic contaminants through reproductive tract and is transferred to the egg (Guirlet et al., 2008; Páez-Osuna et al., 2010; Stewart et al., 2011; Ehsanpour et al., 2014; De Andrés et al., 2016). This process is called vitellogenesis that takes place during the development of follicles in female marine turtles where ingested nutrients from the foraging ground as well as the contaminants that are present in the lipid store of the animals are deposited into the follicles to form the yolk of an individual egg (Van de Merwe, 2008). Therefore, vitellogenesis usually occurs during breeding seasons and is completed before the first nesting (Hamann et al., 2002). However, it also depends on the species of turtle, for example, Dermochelys coriacea has the highest reproduction output among reptiles, so they transfer more energy, nutrients and trace elements to the egg. Furthermore, their longevity gives them the ability to accumulate diverse contaminants in a wide range of migration areas.
The comparison of heavy metal concentration in eggs of marine turtles of previous studies from different location is presented in Table 5. The current findings revealed a high lead concentration in samples from Terengganu but lower than green turtle eggs collected from the Northern Coast of the Sea of Oman (Sinaei and Bolouki, 2017), moderately higher than Hong Kong, Peninsular Malaysia and the Pacific Coast of Panama (Fussy et al., 2007; Warwick et al., 2013; Rajaram, 2014). The concentrations of Pb vary in different places and could be influenced by the environment, especially those close to anthropogenic sources like urban areas (Burger and Gochfeld, 2000). Zinc (Zn) was the highest level of heavy metal found in this study, but the concentrations were similar to the green turtle eggs collected from West Papua, Indonesia (Tapilatu et al., 2020) and from other places in Peninsular Malaysia (Joseph et al., 2014). However, some studies reported lower Zinc levels (Van de Merwe et al., 2009; Ross et al., 2016), including in hawksbill turtle eggs (Ehsanpour et al., 2014) and olive ridley turtle eggs (Ross et al., 2016). Nevertheless, Zn is an essential heavy metal other than magnesium (Mn) and copper (Cu) that is needed in an adequate amount for the growth and metabolism of cells as well as the development of foetus (Hostetler et al., 2003). Besides, the differences might be caused by the different amounts of heavy metals being discharged into the foraging ground by natural or anthropogenic sources (Richir and Gobert, 2016). Moreover, the chemical levels may vary depending on the geographical area and the natural enrichment of trace elements in that area (Ahmadi and Rahimi, 2011).
Mercury (Hg) and cadmium (Cd) are non-essential heavy metals that are toxic, even at low concentration. Any foodstuff containing Hg concentration higher than the permissible limit of 0.01 mg/kg could initiate potential toxicity in humans and animals. In this study revealed low Hg concentration, well below the detection limit of 0.02 mg/kg fresh weight of mercury in food (World Health Organization. Regional Office for Europe, 2000). Generally, the concentration of Hg tends to be low in marine turtle eggs (Kaska and Furness, 2001; Lam et al., 2006; Guirlet et al., 2008; Ehsanpour et al., 2014; Sinaei and Bolouki, 2017) and a similar pattern was detected in current studies. On the other hand, some of the previous reports contradict this observation (Ross et al., 2016; Tapilatu et al., 2020). They investigated selected heavy metals in green turtle eggs and found Hg levels exceeded the maximum permissible limit. This toxicant element is also hazardous for fish when exposed to considerable amounts and might easily enter the nervous system of humans in the form of methyl mercury present in food (Tchounwou et al., 2012). Meanwhile, slightly low levels of Cd were measured, well within the 0.01 to 0.05 mg/kg acceptable levels and are comparable with previous studies (Van de Merwe et al., 2009; Ehsanpour et al., 2014; Sinaei and Bolouki, 2017). Besides, kidney and liver are the target organs for Cd (D’ilio et al., 2011; Yipel et al. (2017). In fact, highest bio-concentration of Cd was found in kidney of green turtles collected from Australia, Brazil, Hawaii, Japan and the Gulf of Mexico (Fraga et al., 2018). Similarly, kidney and liver tissues are also the target tissues for heavy metals in humans as they consume contaminated food products, which are then distributed through the blood (Gall et al., 2015; Engwa et al., 2019).
Many studies have been conducted on sea turtles in relation to the concentration of heavy metals in muscles, kidneys, and liver (Barbieri, 2009; Ross et al., 2017; Yipel et al., 2017). Once a contaminant enters the body, it is difficult to dissipate it from the body, but it was concluded that excretion of contaminants via eggs is not the main pathway for removing trace elements. However, during the nesting season, concentrations of heavy metals in the blood of female green turtles increased and to reduce the amount of contaminants, parts of the contaminant are transferred to the eggs (Van De Merwe et al., 2010). Usually, essential metals are passed on into the yolk and available for development of embryo. On the other hand, transfer of non-essential metals is limited, hence they persistently accumulate in the muscle, kidney, liver and pancreas of the mother (Kaska and Furness, 2001). Indeed, heavy metals are harmful to mammals, reptiles, fish and birds by disrupting the growth, immune systems and reproduction (Gall et al., 2015).
The metal concentrations in the present study were considerably different as there were low concentrations compared to previous study on green turtle eggs in Peninsular Malaysia (Joseph et al., 2014). It is probably due to the short period of time spent by those female marine turtles in polluted places and the different diets in the habitats. Besides, the green turtles are freely on the move to different foraging grounds that are often remote, expansive and inaccessible. Thus, it is quite challenging to trace the source of contamination. Their potential traits of having high mobility and their movement patterns during migration between breeding and foraging habitats facilitate long-term exposure to environmental toxicants along their migratory route. In fact, marine turtles have a long lifespan of up to several decades. Consequently, it poses a health risk to these animals due to their life pattern. Aside from that, contamination by trace elements in their blood and tissues has raised concern about public health risk, particularly for the consumer of green turtle. Nevertheless, this made them an excellent organism for contaminant biomonitoring of overall health status of marine ecosystem. Further study on this topic should be considered because the knowledge on this issue is applicable to the management and conservation of endangered species.
Conclusions
This study reveals the minor nutritional differences between green turtle eggs collected from Terengganu and Sabah. Nutrient contents including fatty acid and amino acid compositions varied slightly. However, the heavy metal concentrations might raise a concern about the public health risks. Furthermore, pathogenic bacteria of human concern were also isolated from turtle eggs, particularly Salmonella spp. The data contribute to public awareness of consuming green turtle eggs. Instead of purchasing green turtle eggs, the public has many options for healthier, more nutritious foods that are readily available. Further research is required on how the consumption of green turtle eggs affects the health of consumers. Perhaps this study is also helpful as a guideline for the future conservation of the green turtle population.
Data availability statement
The original contributions presented in the study are included in the article/supplementary material. Further inquiries can be directed to the corresponding author.
Author contributions
HH contributed to the original idea of manuscript writing. NK performed the data collection, analyses and wrote the first draft of manuscript. All authors contributed to the article and approved the submitted version.
Acknowledgments
The authors were thankful to Prof Zainudin bin Bachok from INOS, UMT for his invaluable support in providing some of the lab equipment. The authors would like to acknowledge the laboratory Centre of Research and Field Service - CRAFS, UMT and Nutrition’s laboratory, UPM in providing the facilities for conducting the experiments. The authors would like to acknowledge all person involved in this study.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
Abd Mutalib A. H., Fadzly N., Foo R. (2013). Striking a balance between tradition and conservation: General perceptions and awareness level of local citizens regarding turtle conservation efforts based on age factors and gender. Ocean Coast. Manage. 78, 56–63. doi: 10.1016/j.ocecoaman.2013.03.015
Abdulkadir S., Tsuchiya M. (2008). One-step method for quantitative and qualitative analysis of fatty acids in marine animal samples. J. Exp. Mar. Biol. Ecol. 354 (1), 1–8. doi: 10.1016/j.jembe.2007.08.024
Ackerman R. A. (1997). “The nest environment and the embryonic development ofsea turtles,” in The biology of Sea turtles. Eds. Lutz P. L., Musick J. A. (Boca Raton, FL, USA: CRC Press), 83–106.
Aguirre A. A., Gardner S. C., Marsh J. C., Delgado S. G., Limpus C. J., Nichols W. J. (2006). Hazards associated with the consumption of sea turtle meat and eggs: A review for health care workers and the general public. EcoHealth 3 (3), 141–53. doi: 10.1007/s10393-006-0032-x
Ahasan M. S., Picard J., Elliott L., Kinobe R., Owens L., Ariel E. (2017). Evidence of antibiotic resistance in enterobacteriales isolated from green sea turtles, Chelonia mydas on the great barrier reef. Mar. pollut. Bull. 120 (1–2), 18–27. doi: 10.1016/j.marpolbul.2017.04.046
Ahmad R. S., Imran A., Hussain M. B. (2018). “Nutritional composition of meat” in Meat science and nutrition. Ed. Arshad M. S. (IntechOpen). doi: 10.5772/intechopen.71954
Ahmadi F., Rahimi F. (2011). Factors affecting quality and quantity of egg production in laying hens: A review. World Appl. Sci. J. 12 (3), 372–84.
Al-Bahry S. N., Mahmoud I. Y., Al-Harthy A., Elshafie A. E., Al-Ghafri S., Alkindi A. Y. A., et al. (2004). “Bacterial contamination in freshly laid egg green turtles chelonia mydas at ras Al hadd reserve, Oman,” in 24th international symposium for Sea turtles (San Jose, Costa Rica: Elsevier).
Al-Bahry S., Mahmoud I., Elshafie A., Al-Harthy A., Al-Ghafri S., Al-Amri I., et al. (2009). Bacterial flora and antibiotic resistance from eggs of green turtles Chelonia mydas: An indication of polluted effluents. Mar. pollut. Bull. 58 (5), 720–25. doi: 10.1016/j.marpolbul.2008.12.018
Al-Bahry S. N., Mahmoud I. Y., Elshafie A. E., Al-Harthy A., Al-Zadjali M., Al- Alawi W. (2011). Antibiotic resistant bacteria as bioindicator of polluted effluent in the green turtle chelonia mydas in Oman. Mar. Environ. Res. 71 (1), 139–49. doi: 10.1016/j.marenvres.2010.12.005
Alkindi A. Y. A., Mahmoud I. Y., Woller M. J., Plude J. L. (2006). Oviductal morphology in relation to hormonal levels in the snapping turtle, chelydra serpentine. Tiss. Cell 38, 19–33. doi: 10.1016/j.tice.2005.10.001
Al-Musharafi S. K., Mahmoud I. Y., Al-Bahry S. N. (2015). Heavy metals and antibiotic resistant bacteria in green turtles are indicators of environmental pollution. international. J. Biological Food Veterinary Agric. Eng. 9 (4), 374–77. doi: 10.5281/zenodo.1100569
Al-Rawahy S. H., AlKindi A. Y., Elshafie A., Ibrahim M., Al-Bahry S. N., Al Siyabi S. S., et al. (2007). Accumulation of metal in the egg yolk and liver of hatcling of green turtles chelonia mydas at ras Al hadd, sultanate of Oman. J. Biol. Sci. 7, 925–30. doi: 10.3923/jbs.2007.925.930
Attia Y. A., Al-Harthi M. A., Korish M. A., et al. (2020). Protein and amino acid content in four brands of commercial table eggs in retail markets in relation to human requirements. Animals 10 (3), 1–11. doi: 10.3390/ani10030406
Association of Official Analytical Chemists – International (2012). “Official method 982.30 – protein efficiency ratio,” in Official methods of analysis, 19th ed (Gaithersburg, MD, USA: AOAC).
Azlina A. A., Kamaludin M., Hassan M. A. N. (2019). “Assessing public willingness to pay for Sea turtle conservation in terengganu, malaysia” in Environmetal impacts and conservation evaluation. Ed. Samad A. R. A. (Universiti Putra Malaysia Press), 33–53.
Barbieri E. (2009). Concentration of heavy metals in tissues of green turtles (Chelonia mydas) sampled in the cananéia estuary, Brazil. Braz. J. Oceanogr. 57 (3), 243–48. doi: 10.1590/S1679-87592009000300007
Barcelo´-Coblijn G., Murphy E. J. (2009). Alpha-linolenic acid and its conversion to longer chain n-3 fatty acids: benefits for human health and a role in maintaining tissue n-3 fatty acid levels. Prog. Lipid Res. 48, 355–74. doi: 10.1016/j.plipres.2009.07.002
Bhardwaj K., Verma N., Trivedi R. K., Bhardwaj S., Shukla N. (2016). Significance of ratio of omega-3 and omega-6 in human health with special reference to flaxseed oil. Int. J. Biol. Chem. 10 (1), 1–6. doi: 10.3923/ijbc.2016.1.6
Booth D. T. (2003). Composition and energy density of eggs from two species of freshwater turtle with twofold ranges in egg size. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 134 (1), 129–37. doi: 10.1016/S1095-6433(02)00216-7
Brei M., Pérez-Barahona A., Strobl E. (2016). Environmental pollution and biodiversity: Light pollution and sea turtles in the Caribbean. J. Environ. Economics Manage. 77, 95–116. doi: 10.1016/j.jeem.2016.02.003
Briggs M., Petersen K., Kris-Etherton P. (2017). Saturated fatty acids and cardiovascular disease: Replacements for saturated fat to reduce cardiovascular risk. Healthcare 5 (2), 29. doi: 10.3390/healthcare5020029
Brosnan J. T., Brosnan M. E. (2013). Glutamate: A truly functional amino acid. Amino Acids 45 (3), 413–8. doi: 10.1007/s00726-012-1280-4
Bunker M. E., Elliott G., Heyer-Gray H., Martin M. O., Arnold A. E., Weiss S. L. (2021). Vertically transmitted microbiome protects eggs from fungal infection and egg failure. Anim. Microbiome 3 (1), 1–13. doi: 10.1186/s42523-021-00104-5
Burger J., Gochfeld M. (2000). Metal levels in eggs of common terns (Sterna hirundo) in new Jersey: temporal trends from 1971 to 2002. Environ. Res. 94, 336–43. doi: 10.1016/S0013-9351(03)00081-1
Castro-González M. I., Romo F. P. (2011). Chemical composition of eggs of the olive ridley Lepidochelys olivacea (Testudines: Cheloniidae) and it's potential as a food source Revista de biologia tropical 59 (4), 1729–42.
Chan E. H. (2006). Marine turtles in Malaysia: On the verge of extinction? Aquat. Ecosystem Health Manage. 9 (2), 175–84. doi: 10.1080/14634980600701559
Craven K. S., Parsons J., Taylor S. A., Belcher C. N., Owens D. W. (2008). The influence of diet on fatty acids in the egg yolk of green sea turtles, Chelonia mydas. J. Comp. Physiol. B: Biochem. Systemic Environ. Physiol. 178 (4), 495–500. doi: 10.1007/s00360-007-0242-8
De Andrés E., Gómara B., González-Paredes D., Ruiz-Martín J., Marco A. (2016). Persistent organic pollutant levels in eggs of leatherback turtles (Dermochelys coriacea) point to a decrease in hatching success. Chemosphere 146, 354–61. doi: 10.1016/j.chemosphere.2015.12.021
De Freitas C. G., Santana A. P., da Silva P. H., Gonçalves V. S., Barros M. A., Torres F. A., et al. (2010). PCR multiplex for detection of Salmonella enteritidis, typhi and typhimurium and occurrence in poultry meat. Int. J. Food Microbiol. 39 (1-2), 15–22. doi: 10.1016/j.ijfoodmicro.2010.02.007
Delgado S. (2005). Local perceptions and ocean conservation: Human consumption, exploitation, and conservation of endangered Sea turtles in Baja California sur, Mexico (Madison (WI: University of Wisconsin). [dissertation/master’s thesis].
D’ilio S., Mattei D., Blasi M. F., Alimonti A., Bogialli S. (2011). The occurrence of chemical elements and POPs in loggerhead turtles (Caretta caretta): An overview. Mar. pollut. Bull. 62 (8), 1606–15. doi: 10.1016/j.marpolbul.2011.05.022
DiNicolantonio J. J., Lucan S. C., O’Keefe J. H. (2016). The evidence for saturated fat and for sugar related to coronary heart disease. Prog. Cardiovasc. Dis. 58 (5), 464–72. doi: 10.1016/j.pcad.2015.11.006
Domiciano I. G., Domit C., Rodrigues A. P. F., Bracarense L. (2017). The green turtle Chelonia mydas as a marine and coastal environmental sentinels: Anthropogenic activities and diseases. Semina:Ciencias Agrar. 38 (5), 3417–34. doi: 10.5433/1679-0359.2017v38n5p3417
Ehsanpour M., Afkhami M., Khoshnood R., Reich K. J. (2014). Determination and maternal transfer of heavy metals (Cd, Cu, zn, Pb and Hg) in the hawksbill sea turtle (Eretmochelys imbricata) from a nesting colony of qeshm island, Iran. Bull. Environ. Contamination Toxicol. 92 (6), 667–73. doi: 10.1007/s00128-014-1244-3
Elshafie A., Al-Bahry S. N., AlKindi A. Y., Ba-Omar T., Mahmoud I. (2007). Mycoflora and aflatoxins in soil, eggshells, and failed eggs of Chelonia mydas at ras Al-jinz, Oman. Chelonian Conserv. Biol. 6 (2), 267–70. doi: 10.2744/1071-8443(2007)6[267:MAAISE]2.0.CO;2
Engwa G. A., Ferdinand P. U., Nwalo F. N., Unachukwu M. N. (2019). “Mechanism and health effects of heavy metal toxicity in humans” in Poisoning in the modern world - new tricks for an old dog? (IntechOpen), 1–23. doi: 10.5772/intechopen.82511
Estika A. (2013). “Analisis mikroorganisme pada penyu hijau (Chelonia mydas) dari pulau bilang-bilangan, kalimantan Timur,” in Proceeding of national seminar on biology (Bandung: Universitas Pendidikan Indonesia).
Fraga N. S., Martins A. S., Faust D. R., Sakai H., Bianchini A., da Silva C. C., et al. (2018). Cadmium in tissues of green turtles (Chelonia mydas): A global perspective for marine biota. Sci. Total Environ. 637–638, 389–97. doi: 10.1016/j.scitotenv.2018.04.317
Fukushima H., Okuno J., Fujiwara Y., Hosoda T., Kurazono T., Ohtsuka K., et al. (2008). An outbreak of Salmonella food poisoning at a snapping turtle restaurant. J. Japanese Assoc. Infect. Dis. 61, 328.
Fussy A., Pommier P., Lumbroso C., de Haro L. (2007). Chelonitoxism: New case reports in French Polynesia and review of the literature. Toxicon 49 (6), 827–32. doi: 10.1016/j.toxicon.2006.12.002
Gall J. E., Boyd R. S., Rajakaruna N. (2015). Transfer of heavy metals through terrestrial food webs : a review. Environ. Monit. Assess. 187 (201), 1–21. doi: 10.1007/s10661-015-4436-3
Gantois I., Ducatelle R., Pasmans F., Haesebrouck F., Gast R., Humphrey T. J., et al. (2009). Mechanisms of egg contamination by Salmonella enteritidis. FEMS Microbiol. Rev. 33 (4), 718–38. doi: 10.1111/j.1574-6976.2008.00161.x
Goatley C. H. R., Hoey A. S., Bellwood D. R. (2012). The role of turtles as coral reef macroherbivores. PloS One 7 (6), 1–7. doi: 10.1371/journal.pone.0039979
Grundy S. M. (2012). “Cholesterol: Factors determining blood levels,” in Encyclopedia of human nutrition, vol. Vol. 1–4. (University of Texas Southwestern Medical Center: Elsevier Ltd).
Guirlet E., Das K., Girondot M. (2008). Maternal transfer of trace elements in leatherback turtles (Dermochelys coriacea) of French Guiana. Aquat. Toxicol 88 (4), 267–276. doi: 10.1016/j.aquatox.2008.05.004
Hamann M., Limpus C. J., Whittier J. M. (2002). Patterns of lipid storage and mobilisation in the female green sea turtle (Chelonia mydas). J. Comp. Physiol. B: Biochem. Systemic Environ. Physiol. 172 (6), 485–93. doi: 10.1007/s00360-002-0271-2
Harwood J. L. (2019). Algae: Critical sources of very long-chain polyunsaturated fatty acids. Biomolecules 9 (11), 708. doi: 10.3390/biom9110708
Hochberg N. S., Bhadelia N. (2016). Infections associated with exotic cuisine: The dangers of delicacies. Microbiol. Spectr. 3 (5), 355–74. doi: 10.1128/microbiolspec.IOL5-0010-2015
Hostetler C. E., Kincaid R. L., Mirando M. A. (2003). The role of essential trace elements in embryonic and fetal development in livestock. Veterinary J. 166 (2), 125–39. doi: 10.1016/s1090-0233(02)00310-6
Howell L. N., Reich K. J., Shaver D. J., Landry A. M., Gorga C. C. (2016). Ontogenetic shifts in diet and habitat of juvenile green sea turtles in the northwestern gulf of Mexico. Mar. Ecol. Prog. Ser. 559, 217–29. doi: 10.3354/meps11897
Jolis G., Min L. M., Mustafa S. R. S., Sumampouw M., Rajan S. G., Jumin R., et al. (2015). “Sea Turtle conservation in Malaysia : Issues , challenges and recommendations,” in Seminar and workshop on Sea turtle conservation in Malaysia 2015 At: Kuala terengganu, terengganu (Terengganu, Malaysia: Seminar and workshop on Sea turtle conservation in Malaysia).
Joseph J., Ali S. N., Hing L. S. (2014). Heavy metal composition in green turtle (Chelonia mydas) eggs from nesting beaches in peninsular Malaysia. Asian J. Conserv. Biol. 3, 83–7.
Kabir M., Nkeonye U. K., Adamu H. Y., Umar U. A., Badmus K. A. (2015). “Comparison of proximate composition, internal and external qualities of eggs from four species of poultry marketed in samaru zaria, Nigeria,” in Proceedings of 40th Annual conference of Nigerian society for Animal production (NSAP) ( Zaria Nigeria: Ahmadu Bello University), 32–5.
Kaska Y., Furness R. W. (2001). Heavy metals in marine turtle eggs and hatchlings in the Mediterranean. Zoology Middle East 24 (1), 127–32. doi: 10.1080/09397140.2001.10637891
Keene E. L. (2012). Microorganisms from sand, cloacal fluid, and eggs of Lepidochelys olivacea and standard testing of cloacal fluid antimicrobial properties. Purdue University Fort Wayne, 1–124.
Keene E., Soule T., Paladino F. (2014). Microbial isolations from olive ridley ( Lepidochelys olivacea ) and East pacific green ( Chelonia mydas agassizii ) Sea turtle nests in pacific Costa Rica, and testing of cloacal fluid antimicrobial properties. Chelonian Conserv. Biol. 13 (1), 49–55. doi: 10.2744/CCB-1051.1
Lam J. C. W., Tanabe S., Chan S. K. F., Lam M. H. W., Martin M., Lam P. K. S. (2006). Levels of trace elements in green turtle eggs collected from Hong Kong: Evidence of risks due to selenium and nickel. Environ. pollut. 144 (3), 790–801. doi: 10.1016/j.envpol.2006.02.016
Magnino S., Colin P., Dei-Cas E., Madsen M., McLauchlin J., Nöckler K., et al. (2009). Biological risks associated with consumption of reptile products. Int. J. Food Microbiol. 134 (3), 163–75. doi: 10.1016/j.ijfoodmicro.2009.07.001
Mashkour N., Maclaine A., Burgess G. W., Ariel E. (2018). Discovery of an Australian chelonia mydas papillomavirus via green turtle primary cell culture and qPCR. J. Virological Methods 258 (November 2017), 13–23. doi: 10.1016/j.jviromet.2018.04.004
Mensink R. P. (2016). Effects of saturated fatty acids on serum lipids and lipoproteins: a systematic review and regression analysis. World Health Organ., 1–63.
Nagaoka S. M., Martins A. S., dos Santos R. G., Tognella M. M. P., de Oliveira Filho E. C., Seminoff J. A. (2012). Diet of juvenile green turtles (Chelonia mydas) associating with artisanal fishing traps in a subtropical estuary in Brazil. Mar. Biol. 159 (3), 573–81. doi: 10.1007/s00227-011-1836-y
Nelson T. M., Rogers T. L., Brown M. V. (2013). The gut bacterial community of mammals from marine and terrestrial habitats. PloS One 8 (12), 1–8. doi: 10.1371/journal.pone.0083655
Páez-Osuna F., Calderón-Campuzano M. F., Soto-Jiménez M. F., Ruelas-Inzunza J. R. (2010). Trace metals (Cd, Cu, Ni, and zn) in blood and eggs of the Sea turtle lepidochelys olivacea from a nesting colony of oaxaca, Mexico. Arch. Environ. Contamination Toxicol. 59 (4), 632–41. doi: 10.1007/s00244-010-9516-3
Peltomaa E., Johnson M. D., Taipale S. J. (2018). Marine cryptophytes are great sources of EPA and DHA. Mar. Drugs 16 (1), 1–11. doi: 10.3390/md16010003
Pradheeba M., Dilipan E., Nobi E. P., Thangaradjou T., Sivakumar K. (2011). Evaluation of seagrasses for their nutritional value. Indian J. Mar. Sci. 40 (1), 105–11.
Price J. T., Paladino F. V., Lamont M. M., Witherington B. E., Bates S. T., Soule T. (2017). Characterization of the juvenile green turtle (Chelonia mydas) microbiome throughout an ontogenetic shift from pelagic to neritic habitats. PloS One 12 (5), 1–13. doi: 10.1371/journal.pone.0177642
Rajaram S. (2014). Health benefits of plant-derived a -linolenic acid. Am. J. Clin. Nutr. 3, 1–6. doi: 10.3945/ajcn.113.071514
Réhault-Godbert S., Guyot N., Nys Y. (2019). The golden egg: Nutritional value, bioactivities, and emerging benefits for human health. Nutrients 11 (3), 1–26. doi: 10.3390/nu11030684
Richir J., Gobert S. (2016). Trace elements in marine environments: Occurrence, threats and monitoring with special focus on the coastal Mediterranean. J. Environ. Analytical Toxicol. 06 (01), 1–19. doi: 10.4172/2161-0525.1000349
Ristic-Medic D., Vucic V., Takic M., Karadzic I., Marija G. (2013). Polyunsaturated fatty acids in health and disease. J. Serb. Chem. Soc 78 (9), 1269–89. doi: 10.2298/JSC130402040R
Robinson N. J., Paladino F. V. (2013). Sea Turtles. reference module in earth systems and environmental sciences Elsevier 457–69. doi: 10.1016/B978-0-12-409548-9.04352-9
Ross D. A. N., Guzmán H. M., Potvin C., Van Hinsberg V. J. (2017). A review of toxic metal contamination in marine turtle tissues and its implications for human health. Regional Stud. Mar. Science 15, 1–9. doi: 10.1016/j.rsma.2017.06.003
Ross D. A., Guzmán H. M., Van Hinsberg V. J., Potvin C. (2016). Metal contents of marine turtle eggs (Chelonia mydas; lepidochelys olivacea) from the tropical eastern pacific and the implications for human health. J. Environ. Sci. Health - Part B Pesticides Food Contaminants Agric. Wastes 51 (10), 675–87. doi: 10.1080/03601234.2016.1191888
Ruiz-Núñez B., Dijck-Brouwer D. A. J., Muskiet F. A. J. (2016). The relation of saturated fatty acids with low-grade inflammation and cardiovascular disease. J. Nutr. Biochem. 36, 1–20. doi: 10.1016/j.jnutbio.2015.12.007
Sakanaka S., Kitahata K., Mitsuya T., Gutierrez M. A., Juneja L. R. (2000). Protein quality determination of delipidated egg-yolk. J. Food Composition Anal. 13, 773–81. doi: 10.1006/jfca.2000.0914
Santoro M., Hernández G., Caballero M., García F. (2006). Aerobic bacterial flora of nesting green turtles (Chelonia mydas) from tortuguero national park, Costa Rica. J. Zoo Wildlife Med. 37 (4), 549–52. doi: 10.1638/05-118.1
Santos R. G., Martins A. S., Batista M. B., Horta P. A. (2015). Regional and local factors determining green turtle Chelonia mydas foraging relationships with the environment. Mar. Ecol. Prog. Ser. 529 (1981), 265–77. doi: 10.3354/meps11276
Sarmiento-Ramirez J. M., Sim J., Van West p., Dieguez-uribeondo J. (2017). Isolation of fungal pathogens from eggs of the endangered sea turtle species Chelonia mydas in ascension island. J. Mar. Biol. Assoc. United Kingdom 97 (4), 661–7. doi: 10.1017/S0025315416001478
Scheelings T. F., Moore R. J., Van T. T. H., Klaassen M., Reina R. D. (2020). Microbial symbiosis and coevolution of an entire clade of ancient vertebrates: the gut microbiota of sea turtles and its relationship to their phylogenetic history. Anim. Microbiome 2 (1), 1–12. doi: 10.1186/s42523-020-00034-8
Simopoulos A. P. (2008). The importance of the omega-6/Omega-3 fatty acid ratio in cardiovascular disease and other chronic diseases. Exp. Biol. Med. 233, 674–88. doi: 10.3181/0711-MR-311
Sinaei M., Bolouki M. (2017). Metals in blood and eggs of green Sea turtles (Chelonia mydas) from nesting colonies of the northern coast of the Sea of Oman. Arch. Environ. Contamination Toxicol. 73, 552–61. doi: 10.1007/s00244-017-0421-x
Siri-tarino P. W., Sun Q., Hu F. B., Krauss R. M. (2010). Saturated fatty acids and risk of coronary heart Disease : Modulation by replacement nutrients. Curr. Atheroscl. Rep. 12, 384–90. doi: 10.1007/s11883-010-0131-6
Sokoła-Wysoczańska E., Wysoczański T., Wagner J., Czyż K., Bodkowski R., Lochyński S., et al. (2018). Polyunsaturated fatty acids and their potential therapeutic role in cardiovascular system disorders–a review. Nutrients 10 (10), 1–21. doi: 10.3390/nu10101561
Soslau G., Spotila J. R., Chun A., Yi S., Weber K. T. (2011). Potentially lethal bacteria in leatherback turtle eggs in the wild threaten both turtles and conservationists. J. Exp. Mar. Biol. Ecol. 410, 101–6. doi: 10.1016/j.jembe.2011.10.018
Stewart K. R., Keller J. M., Templeton R., Kucklick J. R., Johnson C. (2011). Monitoring persistent organic pollutants in leatherback turtles (Dermochelys coriacea) confirms maternal transfer. Mar. pollut. Bull. 62, 1396–409. doi: 10.1016/j.marpolbul.2011.04.042
Stokes H. J., Mortimer J. A., Hays G. C., Unsworth R. K. F., Laloë J. O., Esteban N. (2019). Green turtle diet is dominated by seagrass in the Western Indian ocean except amongst gravid females. Mar. Biol. 166 (135), 1–12. doi: 10.1007/s00227-019-3584-3
Stvarnik M., Bajc Z., Gačnik K.Š., Dovč A. (2017). Evaluation of different chemical compositions in eggs of the hermann’s tortoise ( Testudo hermanni ) Slovenian Veterinary research. 54 (1), 11–20.
Swanson D., Block R., Mousa S. A. (2012). Omega-3 fatty acids EPA and DHA: Health benefits throughout life. Adv. Nutr. 3 (1), 1–7. doi: 10.3945/an.111.000893
Tapilatu R. F., Wona H., Siburian R. H., Saleda S. T. (2020). Heavy metals contaminants in the eggs and temperatures of nesting beaches of sea turtles in kaimana, West Papua, Indonesia. Biodiversitas 21 (10), 4582–90. doi: 10.13057/biodiv/d211016
Tchounwou P. B., Yedjou C. G., Patlolla A. K., Sutton D. J. (2012). Heavy metal toxicity and the environment. Molecular Clin. Environ. Toxicol. 101, 133–64. doi: 10.1007/978-3-7643-8340-4
Thompson M. B., Speake B. K. (2002). Energy and nutrient utilisation by embryonic reptiles. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 133 (3), 529–38. doi: 10.1016/S1095-6433(02)00188-5
TRAFFIC Southeast Asia for WWF-Malaysia (2009). Survey of marine turtle egg consumption and trade in Malaysia. (Petaling Jaya, Malaysia: World Wide Fund for Nature). p. 1–24.
Tunsaringkarn T., Tungjaroenchai W., Siriwong W. (2013a). Nutrient benefits of quail (Coturnix coturnix japonica) eggs. Int. J. Sci. Res. Publications 3 (5), 1–8.
Tunsaringkarn T., Tungjaroenchai W., Siriwong W. (2013b). Determination of chemical compositions of snail-eating turtle ( Malayemys macrocephala ) eggs. Agric. Sci. Developments 2 (4), 31–9.
Van de Merwe J. P. (2008). Persistent organic pollutants and heavy metals in the green sea turtle , chelonia mydas (Griffith University). dissertation/PhD’s thesis.
Van de Merwe J. P., Hodge M., Olszowy H. A., Whittier J. M., Ibrahim K., Lee S. Y. (2009). Chemical contamination of green turtle (Chelonia mydas) eggs in peninsular Malaysia: Implications for conservation and public health. Environ. Health Perspect. 117 (9), 1397–401. doi: 10.1289/ehp.0900813
Van De Merwe J. P., Hodge M., Whittier J. M., Ibrahim K., Lee S. Y. (2010). Persistent organic pollutants in the green sea turtle chelonia mydas: Nesting population variation, maternal transfer, and effects on development. Mar. Ecol. Progress. 403, 269–78. doi: 10.3354/meps08462
Ventura R. J., Ching P. K., de los Reyes V. C., Sucaldito M. N., Tayag E. (2015). Chelonitoxism outbreak caused from consuming turtle, Eastern samar, Philippines, august 2013. Western Pac. surveillance response journal : WPSAR 6, 12–6. doi: 10.5365/WPSAR.2015.6.1.003
Wallace B. P., Sotherland P. R., Tomillo P. S., Bouchard S. S., Reina R. D., Spotila J. R., et al. (2006). Egg components, egg size, and hatchling size in leatherback turtles. Comp. Biochem. Physiol. - A Mol. Integr. Physiol. 145 (4), 524–32. doi: 10.1016/j.cbpa.2006.08.040
Warwick C., Arena P. C., Steedman C. (2013). Health implications associated with exposure to farmed and wild sea turtles. J. R. Soc. Med. Short Rep. People 4 (8), 1–7. doi: 10.1177/2042533313475574
Weber S. B. (2010). Maternal effects in the green turtle ( chelonia mydas ) (England: University of Exeter ). dissertation/PhD’s thesis.
Wells M. L., Potin P., Craigie J. S., Raven J. A., Merchant S. S., Helliwell K. E., et al. (2017). Algae as nutritional and functional food sources: revisiting our understanding. J. Appl. Phycol. 29 (2), 949–82. doi: 10.1007/s10811-016-0974-5
World Health Organization. Regional Office for Europe (2000). “Air quality guidelines for Europe,”2nd ed. in World Health Organization. Regional Office for Europe, https://apps.who.int/iris/handle/10665/107335
Wyneken J., Lohmann K. J., Musick J. A. (Eds.) (2013). The biology of Sea turtles, volume III (Boca Raton: CRC Press), 447.
Yang E. J., Seo Y. S., Dilawar M. A., Mun H. S., Park H. S., Yang C. J. (2020). Physico-chemical attributes, sensory evaluation and oxidative stability of leg meat from broilers supplemented with plant extracts. J. Anim. Sci. Technol. 62, 730–40. doi: 10.5187/jast.2020.62.5.730
Keywords: fatty acid, green turtle egg, heavy metal, nutritional composition, public health risk
Citation: Katni NH, Azmi AFM, Abdullah MM, Rusli MU, Zakaria Z, Azizan TRPT, Amat AC, Saad MZ, Yasin ISM, Nazarudin MF and Hassim HA (2022) Nutritional compositions, pathogenic microorganisms and heavy metal concentration in green turtle eggs (Chelonia mydas) from Terengganu and Sabah, Malaysia. Front. Mar. Sci. 9:948427. doi: 10.3389/fmars.2022.948427
Received: 19 May 2022; Accepted: 08 August 2022;
Published: 30 August 2022.
Edited by:
Salvatore Siciliano, Fundação Oswaldo Cruz (Fiocruz), BrazilReviewed by:
Paula Baldassin, BW Consultoria Veterinária, BrazilEduardo Reséndiz, Universidad Autónoma de Baja California Sur, Mexico
Copyright © 2022 Katni, Azmi, Abdullah, Rusli, Zakaria, Azizan, Amat, Saad, Yasin, Nazarudin and Hassim. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Hasliza Abu Hassim, haslizaabu@upm.edu.my
 Nor Hashikin Katni
Nor Hashikin Katni Amirul Faiz Mohd Azmi1
Amirul Faiz Mohd Azmi1  Zunita Zakaria
Zunita Zakaria Mohd Zamri Saad
Mohd Zamri Saad Ina Salwany Md. Yasin
Ina Salwany Md. Yasin Muhammad Farhan Nazarudin
Muhammad Farhan Nazarudin