Treatment of COVID-19: Perspective on Convalescent Plasma Transfusion
- 1College of Medicine Phoenix, University of Arizona, Phoenix, AZ, United States
- 2Division of Pulmonary Critical Care and Sleep Medicine, Department of Medicine, University of Arizona College of Medicine-Phoenix, Phoenix, AZ, United States
The novel coronavirus (COVID-19) has continued its global spread since the first documented case in late 2019 in Wuhan, China. With over 10 million cases and 500 thousand deaths reported worldwide, the need for an effective treatment regimen is evident. Historically, convalescent plasma (CP) has been utilized in the treatment of viral respiratory pathogens. Critically ill patients with COVID-19 in China and South Korea have been treated with CP given the ineffectiveness of experimental therapies with antivirals alone. This commentary explores the importance of published experience and the pending establishment of efficacy to facilitate an informed decision regarding the therapeutic use of CP. With increasing mortality around the world from COVID-19 infection, the need for alternative, effective treatment regimens is critical.
The Novel Coronavirus Outbreak
As of June 29, 2020, there have been over 10 million reported cases and over 500,000 deaths from the COVID-19 infection (1). Although many clinical trials are in progress, there is a lack of data supporting any treatments including favipiravir, remdesivir, hydroxychloroquine, and lopinavir (2–4). Historically, convalescent plasma (CP) has been utilized in the treatment of viral respiratory pathogens (5). Patients have undergone transfusion of pathogen specific antibodies from donors who recovered from the virus, providing them with passive immunization (6–9). With this historical perspective, critically ill patients with COVID-19 in China and South Korea have been treated with CP given the ineffectiveness of experimental therapies with antivirals alone (10–14). There are ongoing randomized, double-blind trials, with one study showing insignificant improvement after 28 days using CP along with standard treatment, although the study was determined to be underpowered and terminated early (15). As of June 29, 2020, the Food and Drug Administration (FDA) has not approved the use of CP for use in COVID-19 patients. This commentary explores the importance of published experience and the pending establishment of efficacy to facilitate an informed decision regarding the therapeutic use of CP. With increasing mortality around the world from COVID-19 infection, the need for alternative, effective treatment regimens is critical.
Advantages of CP Against SARS-CoV-2
Studies using CP during SARS, influenza pandemic, and MERS demonstrate potential for clinical efficacy during the COVID-19 pandemic. A meta-analysis of 1,700 patients during the 1918 Spanish influenza revealed a 16% fatality rate in patients treated with CP compared to 37% in those not treated with CP. Mortality was 20% in patients treated with CP compared to 54.8% in those not treated with CP in a study in patients with severe H1N1. Patients in the treatment group had a lower viral load on days 3, 5, and 7 after transfusion and had lower levels of inflammatory cytokines such as IL6, IL10, and TNF (16). Another study in patients with H1N1 also reported similar clinical outcomes (17). Shorter hospital stays and lower mortality were found in patients with SARS who received CP than patients treated with methylprednisolone alone (6). Patients treated with neutralizing antibody achieving titers (NAT) of at least 1:80 during the MERS outbreak showed clinical improvement compared to lower titers (18). There were no adverse effects noted in the aforementioned studies.
Due to these promising results in patients with SARS and H1N1 infections, CP has been studied as a treatment option in COVID-19 (10–15) (Table 1). The titers of CP are based on recommendations from the FDA of at least 1:160 or 1:80 when a more concentrated alternative is unavailable (19). Five critically ill patients with COVID-19-related pneumonia who failed corticosteroids and antiviral treatments alone were given 2 doses of between 200 and 250 mL of CP therapy between days 10 and 22. The NAT of the donors were <40. Viral loads became undetectable, pulmonary infiltrates decreased by day 3, and CRP and procalcitonin levels also decreased (10). In a separate study, ten patients with severe COVID-19 were treated with 200 mL of CP after an average of 16.5 days after symptom onset. The NAT of the donors was 1:640. Seven patients showed improved oxyhemoglobin saturation, decreased CRP, improved lymphocyte counts, and undetectable viral load after transfusion (11). Patients treated as late as 4 weeks after symptom onset with 200 mL of CP in Wuhan, China, showed clinical and radiological improvement, although NAT were unknown (12). A single report demonstrated that mechanical ventilation was not required 11 days after a transfusion containing titers >1:320 of IgG to COVID-19 (14).
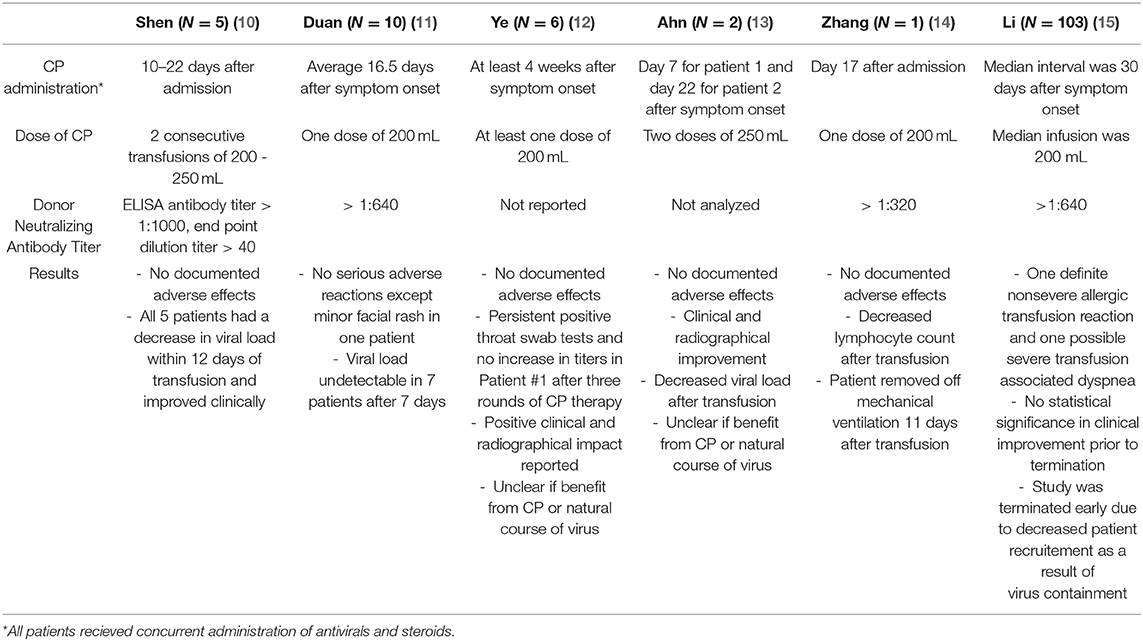
Table 1. Summary of recently published studies of convalscent plasma transfusion in COVID-19 patients.
Limitations of CP Implementation
There are several significant concerns and disadvantages of CP in the treatment of patients with COVID-19. The transfusion of blood products, specifically plasma, is associated with a few rare adverse effects. Severe effects include transfusion-related acute lung injury (TRALI), transfusion-associated circulatory overload (TACO), and anaphylactic reactions. Minor effects include transmission of disease and hemolytic and non-hemolytic transfusion reactions (20). Two cases of patients with Ebola virus and MERS-CoV who received CP developed acute respiratory distress syndrome (ARDS) thought to be a result of TRALI (21, 22). Pathogen reduction technology during the apheresis of CP has been demonstrated to mitigate viral activity in blood products. Currently, the efficacy of inactivating SARS-CoV-2 in collected plasma using similar technology has not been tested (5).
There are also limitations in the use of CP not directly associated with clinical outcomes. Donor eligibility requirements account for factors such as age, sex, weight, and symptom course. Specifically, plasma donors in the United States must pass a medical examination and be 18 years of age weighing at least 50 kg (23). Additional pre-donation requirements include assessment of microbiology and anti-HLA Ab. The current recommended timeline for CP use calls for plasma donation 14 to 28 days following symptom resolution5 coupled with the recommendation to initiate treatment by day 5 at the latest to improve survival, prevent clinical deterioration, and shorten hospitalization (24). The NAT threshold in over one hundred COVID-19 CP investigations ranges between 1:160 and 1:640, presenting an additional consideration prior to transfusion. Treatment includes both antiviral and CP use, rendering it difficult to identify the individual effects of CP therapy (11). The possibility of negative clinical outcomes and limitations to implementation combined with the lack of randomized controlled trials (RCT) constitute the potential downsides of CP treatment in patients with COVID-19 infection.
Interpretation and Recommendation
The lack of a successful treatment for SARS-CoV-2 coupled with a devastating number of cases supports the consideration of CP as a treatment. Reviews of recently published studies and historical use of CP suggest that convalescent plasma can be considered as an experimental treatment in critically ill patients as supported by the FDA (19). A recent open-label RCT showed no statistically significant effect of CP (1:160) in addition to standard treatment on mortality at 28 days post treatment or time to discharge (15). Limitations of this study included early termination, a small sample size, and lack of standardization of methods and procedures. A recent review underscores the lack of randomized, double-blind trials and highlights the importance of considering a “scale up” study in order to utilize CP once efficacy is established (25).
There are many parameters of plasma collection and transfusion that must be considered to maintain the safety of both donors and patients. Current national requirements for plasma donation in the United States should be followed including age and weight restrictions. Plasma should be collected from confirmed positive patients who are asymptomatic for between 10 and 28 days and subsequently test negative for SARS-CoV-2 and other viral illnesses. Treatment should be initiated by Day 5 of symptomatic presentation in critically ill patients to decrease length of hospital stay and mortality. NAT threshold should follow the FDA recommendation of 1:160 or 1:80 in situations with no alternatives (19). Improvements in diagnosing patients with COVID-19 has resulted in greater identification of those with the disease, which can help aid a greater number of patients with CP transfusion (26).
With total mortality still on the rise, convalescent plasma should be considered as an experimental therapeutic approach. The published cases suggest positive clinical outcomes in patients receiving CP in addition to antiviral therapy +/– corticosteroids (27). The increasing availability of reliable testing worldwide should facilitate the identification of potential donors. Additional studies to determine titers and establish long-term clinical outcomes are needed to confirm any potential benefit of CP therapy.
CP shows promise as an effective therapeutic option for patients with COVID-19. There are currently multiple randomized trials in progress purportedly measuring the therapeutic benefit of CP (clinicaltrials.gov, accessed June 29, 2020). Determination of “responders” and “non-responders” in robustly conducted clinical trials will establish optimal dosing and efficacy and support the use of CP as standard treatment instead of experimental therapy for COVID-19 infection.
Data Availability Statement
All datasets presented in this study are included in the article/supplementary material.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. World Health Organization. Coronavirus Disease (COVID-2019) Situation Reports. Geneva: World Health Organization (2020).
2. McKee DL, Sternberg A, Stange U, Laufer S, Naujokat C. Candidate drugs against SARS-CoV-2 and COVID-19. Pharmacol Res. (2020) 157:104859. doi: 10.1016/j.phrs.2020.104859
3. Sanders JM, Monogue ML, Jodlowski TZ, Cutrell JB. Pharmacologic treatments for coronavirus disease 2019 (COVID-19): a review. JAMA. (2020) 323:1824–36. doi: 10.1001/jama.2020.6019
4. Ahn DG, Shin HJ, Kim MH, Lee S, Kim HS, Myoung J, et al. Current status of epidemiology, diagnosis, therapeutics, and vaccines for Novel Coronavirus Disease 2019 (COVID-19). J Microbiol Biotechnol. (2020) 30:313–24. doi: 10.4014/jmb.2003.03011
5. Tiberghien P, Lamballerie X, Morel P, Gallian P, Lacombe K, Yazdanpanah Y. Collecting and evaluating convalescent plasma for COVID-19 treatment: why and how? Vox Sang. (2020). doi: 10.1111/vox.12926. [Epub ahead of print].
6. Soo YO, Cheng Y, Wong R, Hui DS, Lee CK, Tsang KK, et al. Retrospective comparison of convalescent plasma with continuing high-dose methylprednisolone treatment in SARS patients. Clin Microbiol Infect. (2004) 10:676–8. doi: 10.1111/j.1469-0691.2004.00956.x
7. Lai ST. Treatment of severe acute respiratory syndrome. Eur J Clin Microbiol Infect Dis. (2005) 24:583–91. doi: 10.1007/s10096-005-0004-z
8. Marano G, Vaglio S, Pupella S, Facco G, Catalano L, Liumbruno GM, et al. Convalescent plasma: new evidence for an old therapeutic tool? Blood Transfus. (2016) 14:152–7. doi: 10.2450/2015.0131-15
9. Rajam G, Sampson JM., Carlone G, W. Ades E. An augmented passive immune therapy to treat fulminant bacterial infections. Recent Pat Antiinfect Drug Discov. (2010) 5:157–67. doi: 10.2174/157489110791233496
10. Shen C, Wang Z, Zhao F, Yang Y, Li J, Yuan J, et al. Treatment of 5 critically Ill patients with COVID-19 with convalescent plasma. JAMA. (2020) 323:1582–9. doi: 10.1001/jama.2020.4783
11. Duan K, Liu B, Li C, Zhang H, Yu T, Qu J, et al. Effectiveness of convalescent plasma therapy in severe COVID-19 patients. Proc Natl Acad Sci USA. (2020) 117:9490–6. doi: 10.1073/pnas.2004168117
12. Ye M, Fu D, Ren Y, Wang F, Wang D, Zhang F, et al. Treatment with convalescent plasma for COVID-19 patients in Wuhan, China. J Med Virol. (2020). doi: 10.1002/jmv.25882. [Epub ahead of print].
13. Ahn JY, Sohn Y, Lee SH, Cho Y, Hyun JH, Baek YJ, et al. Use of convalescent plasma therapy in two covid-19 patients with acute respiratory distress syndrome in Korea. J Korean Med Sci. (2020) 35:e149. doi: 10.3346/JKMS.2020.35.E149
14. Zhang L, Pang R, Xue X, Bao J, Ye S, Dai Y, et al. Anti-SARS-CoV-2 virus antibody levels in convalescent plasma of six donors who have recovered from COVID-19. Aging. (2020) 12:6536–42. doi: 10.18632/aging.103102
15. Li L, Zhang W, Hu Y, Tong X, Zheng S, Yang J, et al. Effect of convalescent plasma therapy on time to clinical improvement in patients with severe and life-threatening COVID-19: a randomized clinical trial. JAMA. (2020) e2010044. doi: 10.1001/jama.2020.10044. [Epub ahead of print].
16. Luke TC, Kilbane EM, Jackson JL, Hoffman SL. Meta-analysis: convalescent blood products for Spanish influenza pneumonia: a future H5N1 treatment? Ann Intern Med. (2006) 145:599–609. doi: 10.7326/0003-4819-145-8-200610170-00139
17. Hung IF, To KK, Lee CK, Lee KL, Chan K, Yan WW, et al. Convalescent plasma treatment reduced mortality in patients with severe pandemic influenza A (H1N1) 2009 virus infection. Clin Infect Dis. (2011) 52:447–56. doi: 10.1093/cid/ciq106
18. Ko JH, Seok H, Cho SY, Ha YE, Baek JY, Kim SH, et al. Challenges of convalescent plasma infusion therapy in Middle East respiratory coronavirus infection: a single centre experience. Antivir Ther. (2018) 23:617–22. doi: 10.3851/IMP3243
19. Food and Drug Administration. Investigational COVID-19 Convalescent Plasma - Guidance for Industry. (2020). Available online at: https://www.fda.gov/regulatory- (accessed May 11, 2020).
20. Pandey S, Vyas GN. Adverse effects of plasma transfusion. Transfusion. (2012) 52(Suppl. 1):65S−79S. doi: 10.1111/j.1537-2995.2012.03663.x
21. Mora-Rillo M, Arsuaga M, Ramírez-Olivencia G, de la Calle F, Borobia AM, Sánchez-Seco P, et al. Acute respiratory distress syndrome after convalescent plasma use: treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir Med. (2015) 3:554–62. doi: 10.1016/S2213-2600(15)00180-0
22. Chun S, Chung CR, Ha YE, Han TH, Ki CS, Kang ES, et al. Possible transfusion-related acute lung injury following convalescent plasma transfusion in a patient with middle east respiratory syndrome. Ann Lab Med. (2016) 36:393–5. doi: 10.3343/alm.2016.36.4.393
23. IQPP Qualified Donor Standard. Plasma Protein Therapeutics Association. (2014). Available online at: https://www.pptaglobal.org/images/IQPP/QualifiedDonorStdFinal1.pdf (accessed May 5, 2020).
24. Woelfel R, Corman VM, Guggemos W, Michael Seilmaier, Sabine Zange, Marcel A Mueller, et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv. (2020). doi: 10.1101/2020.03.05.20030502
25. Bloch EM, Shoham S, Casadevall A, Sachais BS, Shaz B, Winters JL, et al. Deployment of convalescent plasma for the prevention and treatment of COVID-19. J Clin Invest. (2020) 130:2757–65. doi: 10.1172/JCI138745
26. Sethuraman N, Jeremiah SS, Ryo A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA. (2020) 323:2249–51. doi: 10.1001/jama.2020.8259
Keywords: coronavirus (2019-nCoV), SARS–CoV-2, transfusion safety, neutralizing antibody titers (NAT), convalescent plasma for COVID-19 therapy
Citation: Farhat RM, Mousa MA, Daas EJ and Glassberg MK (2020) Treatment of COVID-19: Perspective on Convalescent Plasma Transfusion. Front. Med. 7:435. doi: 10.3389/fmed.2020.00435
Received: 22 May 2020; Accepted: 06 July 2020;
Published: 28 July 2020.
Edited by:
Argyrios Tzouvelekis, Alexander Fleming Biomedical Sciences Research Center, GreeceReviewed by:
Paschalis Ntolios, Democritus University of Thrace, GreeceEleni Papakonstantinou, Aristotle University of Thessaloniki, Greece
Copyright © 2020 Farhat, Mousa, Daas and Glassberg. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Mohammad A. Mousa, momousa22@email.arizona.edu
 Ryan M. Farhat
Ryan M. Farhat Mohammad A. Mousa
Mohammad A. Mousa Eshaan J. Daas
Eshaan J. Daas Marilyn K. Glassberg
Marilyn K. Glassberg