Non-valvular Atrial Fibrillation in CKD: Role of Vitamin K Antagonists and Direct Oral Anticoagulants. A Narrative Review
- 1Departament de Medicina, Universitat de Barcelona, Institut d'Investigacions Biomèdiques August Pi i Sunyer (IDIBAPS), Barcelona, Spain
- 2Unidad de Factores de Riesgo Vascular, Servicio de Nefrología, Hospital Universitario de Jerez, Jerez de la Frontera, Spain
- 3Departament de Medicina, Universitat de Valencia, Valencia, Spain
- 4Hospital Clínico Universitario de Valencia, Valencia, Spain
- 5Servicio de Cardiología, Hospital Universitario Virgen del Rocío, Sevilla, Spain
- 6Hospital Universitario Puerta de Hierro, Madrid, Spain
- 7Instituto de Investigación del Hospital Clinico Universitario, Valencia (INCLIVA), Valencia, Spain
Atrial fibrillation (AF) is the most common arrhythmia in chronic kidney disease (CKD), with a close bidirectional relationship between the two entities. The presence of CKD in AF increases the risk of thromboembolic events, mortality and bleeding. Vitamin K antagonists (VKA) have been the mainstay of treatment for the prevention of thromboembolic events in AF until recently, with confirmed benefits in AF patients with stage 3 CKD. However, the risk-benefit profile of VKA in patients with AF and stages 4–5 CKD is controversial due to the lack of evidence from randomized controlled trials. Treatment with VKA in CKD patients has been associated with conditions such as poorer anticoagulation quality, increased risk of bleeding, faster progression of vascular/valvular calcification and higher risk of calciphylaxis. Direct oral anticoagulants (DOACs) have shown equal or greater efficacy in stroke/systemic embolism prevention, and a better safety profile than VKA in post-hoc analysis of the pivotal randomized controlled trials in patients with non-valvular AF and stage 3 CKD, yet evidence of its risk-benefit profile in more advanced stages of CKD is scarce. Observational studies associate DOACs with a good safety/effectiveness profile compared to VKA in non-dialysis CKD patients. Further, DOACs have been associated with a lower risk of acute kidney injury and CKD development/progression than VKA. This narrative review summarizes the evidence of the efficacy and safety of warfarin and DOACs in patients with AF at different CKD stages, as well as their effects on renal function, vascular/valvular calcification and bone health.
Introduction
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is associated with an increased risk of stroke or systemic embolism (S/SE) (1). Moreover, AF-related stroke is usually more severe, associated with greater disability, increased mortality and longer hospital stays (2).
Chronic kidney disease (CKD) is also common, with increasing prevalence worldwide (3, 4). Atrial fibrillation is more prevalent in CKD, in both non-dialysis (ND) (16–21% and increasing with higher CKD stages), and particularly end-stage kidney disease (ESKD) patients on dialysis (17–27%) (5). In fact, the relationship between AF and CKD is bidirectional: AF is associated with an increased risk of CKD [reduced glomerular filtration rate (GFR) or albuminuria], while CKD is linked to increased incidence/prevalence of AF (6–9). Atrial fibrillation and CKD share several risks factors (older age, hypertension, diabetes, prevalent cardiovascular disease); while inflammation, frequent in CKD, has been involved in initiation/perpetuation of AF (10). Patients with severe CKD frequently have cardiac abnormalities favoring AF development (left ventricular hypertrophy, myocardial fibrosis or left atrial enlargement) (11). Furthermore, rapid shifts in serum pH and electrolytes during hemodialysis (HD) sessions may be a trigger for AF.
Oral anticoagulation therapy (OAT) with vitamin K antagonists (VKA) and more recently direct oral anticoagulants (DOACs) have been the cornerstone of S/SE prevention in patients with non-valvular AF (NVAF) at risk for stroke. However, the presence of CKD in AF further heightens the risk of S/SE (46–49% in ND-CKD or 83% in ESKD) and death (60–65% higher risk in CKD or ESKD) (5), as well as risk of bleeding in patients under OAT, raising doubts about the risk-benefit profile (5, 9, 12). Atrial fibrillation is associated with a prothrombotic state through numerous pathophysiological pathways, which is further aggravated by CKD, via changes in the left atrium, endothelial dysfunction or activation of coagulation and platelets (9). However, there is no clear evidence of a significant increase in S/SE risk in ESKD patients with AF. Possible explanations for this include: the high prevalence/incidence of non-AF-related stroke in ESKD, uremic platelet dysfunction, use of intradialytic heparin and the paroxysmal and self-limiting nature of hemodialysis-associated AF bursts, which should be associated with a low risk of S/SE, in contrast to the persistent/permanent AF associated with chronic structural atrial changes (13, 14). Thus, if no association between AF and S/SE in hemodialysis patients was proven, there would not be a need for OAT in this population (13).
Vitamin K Antagonists in Patients With AF and CKD
Vitamin K antagonists reduce S/SE incidence in patients with NVAF in the general population (15). However, VKA have several drawbacks: slow onset of action and cessation of effects, a narrow therapeutic range requiring frequent monitoring and dose adjustments, and significant drug-drug and food-drug interactions (16), which may be aggravated by dietary restrictions in CKD (17).
Evidence of the efficacy and safety of VKA in CKD patients with AF from randomized controlled trials (RCT) is scarce. Warfarin targeted at an international normalized ratio (INR) of 2–3 vs. aspirin plus fixed low-dose of warfarin, reduced the risk of S/SE by 76% without increasing the risk of major bleeding in the AF patient subset with stage 3 CKD (18). However, there is no RCT evidence of the risk/benefit ratio of VKA in patients with AF and stage ≥ stage 4 CKD.
In a meta-analysis of observational studies, warfarin reduced the risk of S/SE and mortality (30 and 35%, respectively) without increasing the risk of major bleeding in the ND-CKD group; in ESKD patients, however, warfarin did not decrease the risk of S/SE or mortality, yet increased the risk of major bleeding by 30%, suggesting that VKAs have a favorable risk/benefit profile in ND-CKD patients, but not in ESKD (19). This agrees with an updated meta-analysis of 15 observational studies in ESKD patients with AF, in which those receiving warfarin, had a 49% increased rate of hemorrhagic stroke with no benefits on ischemic stroke (IS), major bleeding or mortality vs. those not receiving warfarin (20), and also with the results of two recent studies; an observational study (21) and a retrospective study of patients with advanced CKD and incident AF before initiating dialysis (22). Despite the limitations of these studies (observational, results subject to confounding, moderate to high heterogeneity, no data on quality of anticoagulation), they suggest that VKA may not be effective for S/SE prevention in patients with AF and ESKD, and indeed may be harmful (20, 23). In addition, the time in therapeutic range (TTR) is lower in CKD patients; the worse the renal function, the lower the TTR; while poor anticoagulation quality is associated with increased risk of thromboembolic and bleeding events (24–26). INR variability has also been associated with bleeding and mortality in ESKD patients (27). Further, patients with moderate-severe CKD usually require lower doses of VKA, and show a higher bleeding risk with supra-therapeutic INR (28, 29). Further information on the net clinical benefit of VKA in this population is forthcoming from two ongoing RCTs comparing the hemorrhagic and thrombotic risk of VKA vs. no anticoagulation in hemodialysis patients with AF: the Oral Anticoagulation in Hemodialysis Patients (AVKDIAL) (NCT02886962), and the Danish Warfarin-Dialysis Study - Safety and Efficacy of Warfarin in Patients With Atrial Fibrillation on Dialysis (DANWARD) (NCT03862859).
Direct Oral Anticoagulants in Patients With AF and CKD
The efficacy of DOACs (dabigatran, rivaroxaban, apixaban and edoxaban) in S/SE prevention and their safety profile vs. VKA have been demonstrated in pivotal trials (30–33). A meta-analysis of these trials found that DOACs have greater efficacy in reducing S/SE, mainly driven by reducing hemorrhagic stroke, lowering the risk of mortality, and conferring a non-significant lower risk of major bleeding (significant for intracranial hemorrhage) (34).
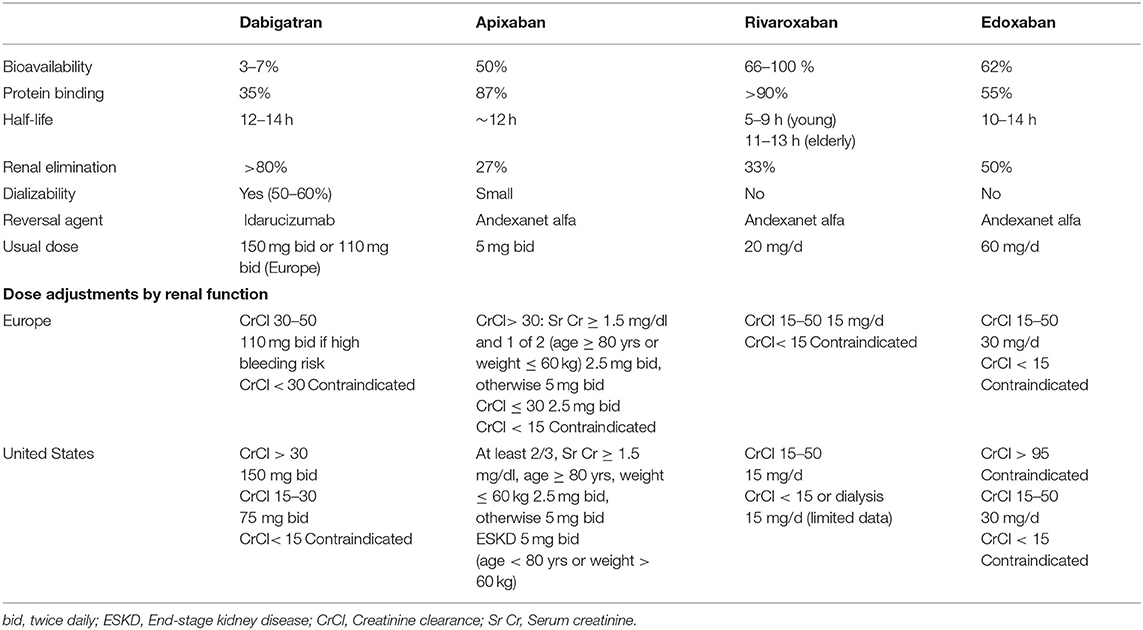
However, DOAC rely on the kidney, to variable extent, for drug elimination. Renal clearance is about 80% with dabigatran, 50% with edoxaban, 33% with rivaroxaban and 27% with apixaban (35), and these may require dose adjustments in moderate-severe CKD (Table 1). In this regard, it could be useful to measure the anticoagulant effect of DOACs in advanced CKD or in patients with deteriorating renal function (36).
Among patients with moderate CKD post-hoc analysis of pivotal RCTs of DOACs vs. warfarin in patients with NVAF (37–41), showed a favorable risk-benefit profile for DOACs, which has been confirmed in several meta-analyses (42–44). In the Cochrane meta-analysis including 12,545 AF patients with CKD (390 patients in stage 4), DOACs reduced the incidence of S/SE (RR 0.81, 95% CI 0.65–1.00) and non-significantly reduced the incidence of major bleeding (RR 0.79, 95% CI 0.59–1.04) vs. warfarin (42). However, these studies included patients with creatinine clearances (CrCl) > 30 ml/min [the Apixaban for the Prevention of Stroke in Subjects with Atrial Fibrillation (ARISTOTLE) trial included patients with CrCl > 25 ml/min]. Therefore, the risk/benefit profile of DOACs in patients with AF and stages 4–5D CKD is uncertain. A post-hoc analysis of the ARISTOTLE trial confirmed the safety profile of apixaban with a 66% lower risk of major bleeding among patients with CrCl 25–30 ml/min (45).
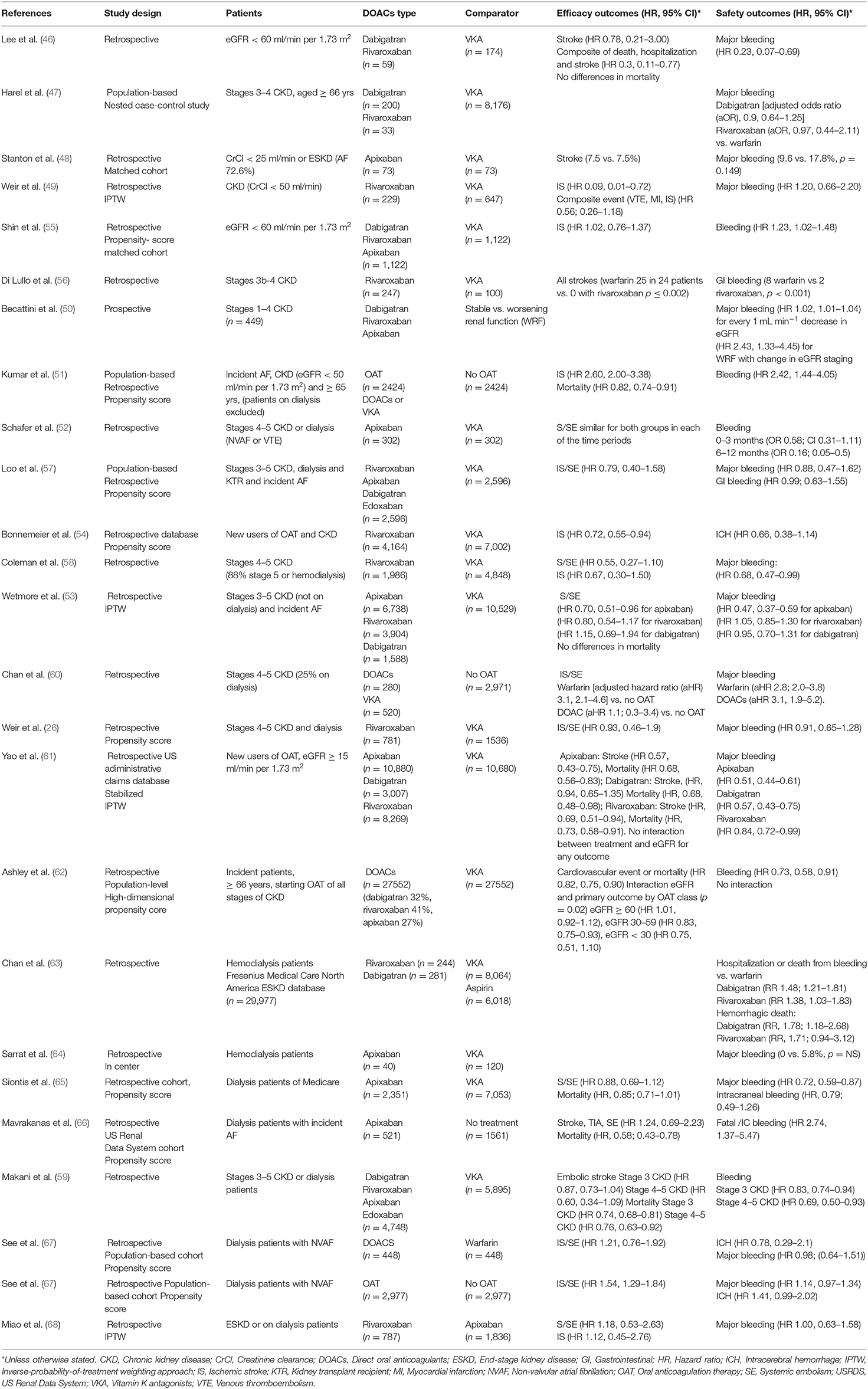
Several observational studies have evaluated the effectiveness and safety of DOACs vs. warfarin, covering a range of different CKD stages (26, 46–62) (see Table 2). Despite the low quality of the evidence, due to heterogeneity or retrospective study designs, they suggest that DOACs are a viable alternative to VKA in patients with ND-CKD, with a net clinical benefit observed in a meta-analysis (69).
In ESKD patients observational studies comparing DOACs vs. VKA show high heterogenicity (63–68, 70), with initial studies showing safety concerns with dabigatran or rivaroxaban vs. warfarin (63). In contrast, a retrospective study comparing apixaban vs. warfarin showed similar event rates for IS/SE but, significantly lower rates of major bleeding events. Furthermore, 5 mg/12 h apixaban was associated with lower risk for IS/SE and mortality vs. warfarin (65). However, recent studies have not confirmed these benefits (67), or the benefit of DOACs vs. non-OAT in this population (66).
A RCT in hemodialysis patients with NVAF compared the effect of VKA, rivaroxaban at 10 mg/d and rivaroxaban at 10 mg/d plus vitamin K2 at 2,000 μg/thrice weekly for 18 months on the progression of cardio-aortic calcium deposits (primary endpoint). After the initial follow-up, only severe bleeding events were lower in rivaroxaban-treated patients (71). However, after a follow-up extension of the trial for at least 18 additional months, a reduction was observed in the composite outcome of fatal and non-fatal cardiovascular events and of life-threatening or major bleeding complications compared with VKA, although the risk of stroke or death were not different between groups (72).
In a recent meta-analysis in ESKD patients with AF, DOACs showed comparable effectiveness and safety to VKA, while OAT vs. no anticoagulation was associated with a higher risk of IS/SE and a similar risk of major bleeding (67). Thus, there is no evidence of a net clinical benefit of DOACs in ESKD patients with AF, despite an urgent need. Several ongoing RCT are assessing OAT use and comparing the safety and efficacy of DOACs vs. VKAs in this population [Compare Apixaban and vitamin-K antagonists in patients with AF and ESKD (AXADIA); NCT02933697] [Strategies for the Management of Atrial Fibrillation in patiEnts Receiving Dialysis (SAFE-D); NCT03987711] which will likely yield useful data.
Control of Renal Function During Oral Anticoagulation
Worsening of kidney function (WRF) is common among AF patients (73, 74) and is associated with an increased risk of cardiovascular events, all-cause mortality and bleeding (41, 73–75). This worsening is also common in observational studies (50, 76, 77), especially among CKD patients, and is a better predictor of IS/SE and bleeding than renal dysfunction per se (50, 78). Together with changes in renal function over time because of concomitant treatments (e.g., RAAS blockade and diuretics) or acute events, WRF during follow-up requires monitoring to adjust DOAC dosage. Discordance in DOAC prescription with drug labeling is common in clinical practice, and may result in overdosing or underdosing. Overdosing of DOAC due to inadequate adjustment increases the risk of bleeding, while underdosing is associated with an increased risk of stroke, especially with apixaban (79, 80).
Dose adjustment of DOAC according to drug labeling is based on estimated CrCl using the Cockroft-Gault (CG) formula, while laboratories usually report eGFR (MDRD or CKD-EPI equations), which provide a better estimation of measured GFR, especially for bed-ridden patients whose weight is difficult to estimate (81, 82). Discrepancies in results between different equations may result in inappropriate dosing and patient misclassification (e.g., as eligible for DOACs when they are contraindicated) (83–86). This issue will likely be addressed in the near future, but until more data are available it is reasonable to use the CG-eCrCl formula to adjust DOAC dose.
Guidelines recommend measuring renal function at DOAC treatment initiation and annually thereafter, but suggest more frequent monitoring in CKD patients > 75 years-old, with frailty, and those treated with DOAC with higher renal clearance (87). In CKD patients the interval for monitoring kidney function (in months) can be calculated by dividing eGFR/10. Monitoring renal function in patients under DOAC treatment should also be considered when WRF is suspected, in contexts such as acute events or when prescribing drugs that affect renal hemodynamics (87).
Complications During OAT: DOACs vs. VKA
Effects on Kidney Function
Acute Kidney Injury/Anticoagulant-Related Nephropathy
Warfarin-related nephropathy (WRN) is an episode of acute kidney injury (AKI) associated with warfarin and was first reported in 2009 (88). It is characterized by an increase in serum creatinine, with micro or macrohematuria, in the absence of other causes of AKI, often following supra-therapeutic INR values. Subsequent studies have reported further cases of WRN, most associated with an INR > 3 (89). Kidney biopsies showed signs of acute tubular injury and glomerular hemorrhage with red blood cells in Bowman's space and renal tubular obstruction by red blood cell casts. Although all cases had underlying renal disease in the initial study (88), later studies reported WRN in both CKD and non-CKD patients, and it was also associated with increased mortality risk (89) and faster CKD progression (90).
Several cases have also been reported of AKI in patients treated with DOACs, particularly with dabigatran. Pathological findings were similar to those found in WRN, and this entity is now known as anticoagulant-related nephropathy (ARN). In a recent histologic study of ARN most cases had underlying renal disease (91), suggesting that excessive anticoagulation aggravates an underlying glomerular disease and increases glomerular hematuria. Other possible mechanisms of AKI during OAT include oxidative stress due to free iron release and hemoglobin tubular toxicity.
The risk of AKI in patients with AF is high. In an observational study, one in seven had an AKI episode within 2 years (76). The risk of this injury was higher among patients with CKD and those receiving VKA vs. DOAC-treated patients in several observational studies (76, 92–95).
CKD Development and Progression
Post-hoc analysis of the Rivaroxaban Once Daily Oral Direct Factor Xa Inhibition Compared with Vitamin K Antagonism for Prevention of Stroke and Embolism Trial in Atrial Fibrillation (ROCKET) (73) and Randomized Evaluation of Long-Term Anticoagulant Therapy with Dabigatran etexilate (RE-LY) (96) trials suggest that DOACs have a more beneficial effect on renal function decline than warfarin, which has been confirmed in multiple observational studies (76, 94, 95, 97–99). The renal benefit of DOACs was more apparent with rivaroxaban and dabigatran, and was notably better than in VKA-treated patients with supra-therapeutic INR levels.
The renal benefit of DOACs vs. VKA may be owing to its more predictable dose-response effect and lower risk of hemorrhages; the lower risk of AKI which could influence progressive deterioration of renal function, and its potential renal anti-inflammatory effect, as observed in experimental models (100). In contrast, VKA can promote vascular calcification (as discussed below), increasing arterial stiffness, favoring systolic hypertension and a pulsatile flow at the microvasculature, which may be deleterious for the kidney (101).
Lastly, the Factor XA Inhibition in REnal Patients with Non-valvular Atrial Fibrillation-Observational Registry (XARENO) (NCT02663076) is an observational prospective study that will compare CKD progression with rivaroxaban, VKA or no anticoagulation in patients with CKD stages 3–4 and NVAF.
Vascular and Valvular Calcification and Risk of Calciphylaxis
A common complication in CKD patients, vascular calcification (VC) is inversely associated with renal function and positively associated with worse prognosis in this population (102). Further, vascular medial calcification favors arterial stiffness, which is associated with systolic hypertension, left ventricular hypertrophy and reduced coronary flow reserve, and is a risk factor for cardiovascular events, and CKD progression (101). Vitamin K antagonists impede the -carboxylation of coagulation factors, as well as other vitamin K-dependent proteins which inhibit VC, such as matrix Gla protein, Gla-rich protein, and growth arrest–specific protein 6, preventing their activation (103).
In fact, VKA have been associated with increased progression of VC (104), and arterial stiffness (105), which may be especially relevant in CKD patients, and supporting that DOACs are safer than VKA as regards these two conditions, although the differential effect was not demonstrated in a recent RCT (71). Although switching from warfarin to rivaroxaban improved arterial stiffness in a prospective study (106), this finding was not substantiated in the above mentioned RCT (71). Further, rivaroxaban was also found to reduce the progression of aortic or mitral valve calcification vs. VKA in an observational, retrospective study in stage 3b-4 CKD patients (107).
Calciphylaxis is a rare but severe complication in dialysis patients, characterized by skin necrosis and ulceration, with panniculitis, calcification and luminal occlusion of small arterioles and subcutaneous capillaries (108, 109). Calciphylaxis is associated with high morbidity and mortality. Treatment with VKA increases several fold the risk of calciphylaxis in both uremic and non-uremic patients (110), suggesting a beneficial effect for DOACs in preventing this complication.
OAT and Bone Health
Patients with ND-CKD and ESKD are prone to bone fractures and osteoporosis due to factors such as bone mineral disease (111). Vitamin K antagonists inhibit the γ-carboxylation of osteocalcin which plays an important role in bone matrix formation (17). Nonetheless, the role of VKA on bone fractures and bone density is controversial. In a recent meta-analysis, VKA for >1 year was not associated with increased risk of fracture vs. controls or compared to DOACs, although the risk was increased in females and elderly patients vs. controls (112).
The available evidence suggests that DOACs are associated with a lower impact on bone metabolism and potentially with lower risk of fractures than VKA (113–120), although this warrants corroboration in properly designed studies. Interestingly, switching from warfarin to rivaroxaban was associated with an improvement in bone metabolism markers (106).
Left Atrial Appendage Occlusion
Given the increased risk of bleeding among patients with severe CKD, left atrial appendage occlusion (LAAO) may be an attractive alternative to reduce the risk of S/SE events while avoiding the need for OAT. All the RCTs showing LAAO as non-inferior to OAT in preventing stroke and bleeding events had excluded patients with severe CKD; however a recent prospective study showed that, at 2 years' follow-up, LAAO in dialysis patients was associated with no thromboembolic events and lower bleeding incidence compared to a cohort treated with oral anticoagulants (121), similar results were found in a small study (122). Besides this study, a prospective single arm study of the Watchman device in dialysis patients is currently ongoing (NCT03446794).
Key Concepts
1. Atrial fibrillation prevalence and incidence is higher in CKD and rises with the degree of kidney dysfunction. The presence of CKD in AF confers an increased risk of stroke/systemic embolism, mortality, and bleeding.
2. Oral anticoagulation with VKA in mild-moderate CKD patients with AF results in a net clinical benefit, although there are no conclusive data on their risk/benefit ratio in more advanced stages of CKD, especially among ESKD patients. Vitamin K antagonist treatment in CKD is associated with poorer anticoagulation quality and increased risk of bleeding.
3. Pivotal RCTs have demonstrated a net clinical benefit for DOACs vs. warfarin in NVAF with mild-moderate CKD, but there is little evidence in patients with AF and stages ≥ 4 CKD. Observational studies suggest that the benefit remains in ND-CKD patients, but is controversial in ESKD.
4. Vitamin K antagonists are associated with a higher risk of anticoagulant-related nephropathy, acute kidney injury and faster CKD progression than DOACs, especially among CKD patients.
5. In patients with NVAF, worsening renal function is common and associated with poorer outcomes. Renal function should be periodically monitored and DOAC dose adjusted to avoid the risk of over or underdosing. Renal function should be measured using the CG-eCrCl, rather than eGFR.
6. Vitamin K antagonists have been associated with an increased risk of vascular/valvular calcification in CKD and likely with higher risk of osteoporosis/fractures, which may be particularly relevant in CKD.
Author Contributions
AC and PG conceived and drafted the manuscript. AC, PG, JB, and EP carried out the literature search. JA, JP, and JG provided a critical revision of the manuscript. All authors contributed to the article and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We are grateful for the support of the CERCA program of the Generalitat de Catalunya.
References
1. Lip GYH. Stroke in atrial fibrillation: epidemiology and thromboprophylaxis. J Thromb Haemost. (2011) 9:344–51. doi: 10.1111/j.1538-7836.2011.04302.x
2. Ali AN, Abdelhafiz A. Clinical and economic implications of AF related stroke. J Atr Fibrillation. (2016) 8:1279. doi: 10.4022/jafib.1279
3. Webster AC, Nagler EV, Morton RL, Masson P. Chronic kidney disease. Lancet. (2017) 389:1238–52. doi: 10.1016/S0140-6736(16)32064-5
4. Bikbov B, Purcell CA, Levey AS, Smith M, Abdoli A, Abebe M, et al. Global, regional, and national burden of chronic kidney disease, 1990–2017: a systematic analysis for the global burden of disease study 2017. Lancet. (2020) 395:709–33. doi: 10.1016/S0140-6736(20)30045-3
5. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, et al. Chronic kidney disease and arrhythmias: conclusions from a kidney disease: improving global outcomes (KDIGO) controversies conference. Eur Heart J. (2018) 39:2314–25. doi: 10.1093/eurheartj/ehy060
6. Watanabe H, Watanabe T, Sasaki S, Nagai K, Roden DM, Aizawa Y. Close bidirectional relationship between chronic kidney disease and atrial fibrillation: the Niigata preventive medicine study. Am Heart J. (2009) 158:629–36. doi: 10.1016/j.ahj.2009.06.031
7. Bansal N, Fan D, Hsu CY, Ordonez JD, Marcus GM, Go AS. Incident atrial fibrillation and risk of end-stage renal disease in adults with chronic kidney disease. Circulation. (2013) 127:569–74. doi: 10.1161/CIRCULATIONAHA.112.123992
8. Odutayo A, Wong CX, Hsiao AJ, Hopewell S, Altman DG, Emdin CA. Atrial fibrillation and risks of cardiovascular disease, renal disease, and death: systematic review and meta-analysis. BMJ. (2016) 354:i4482. doi: 10.1136/bmj.i4482
9. Lau YC, Proietti M, Guiducci E, Blann AD, Lip GYH. Atrial fibrillation and thromboembolism in patients with chronic kidney disease. J Am Coll Cardiol. (2016) 68:1452–64. doi: 10.1016/j.jacc.2016.06.057
10. Engelmann MDM, Svendsen JH. Inflammation in the genesis and perpetuation of atrial fibrillation. Eur Heart J. (2005) 26:2083–92. doi: 10.1093/eurheartj/ehi350
11. Wang X, Shapiro JI. Evolving concepts in the pathogenesis of uraemic cardiomyopathy. Nature Rev Nephrol. (2019) 15:159–75. doi: 10.1038/s41581-018-0101-8
12. Olesen JB, Lip GYH, Kamper A-L, Hommel K, Køber L, Lane DA, et al. Stroke and bleeding in atrial fibrillation with chronic kidney disease. N Engl J Med. (2012) 367:625–35. doi: 10.1056/NEJMoa1105594
13. De Vriese AS, Heine G. Anticoagulation management in hemodialysis patients with atrial fibrillation: evidence and opinion. Nephrol Dial Transplant. (2021) gfab060. doi: 10.1093/ndt/gfab060
14. Vincenti A, Passini E, Fabbrini P, Luise MC, Severi S, Genovesi S. Recurrent intradialytic paroxysmal atrial fibrillation: hypotheses on onset mechanisms based on clinical data and computational analysis. Europace. (2014) 16:396–404. doi: 10.1093/europace/eut346
15. Hart RG, Pearce LA, Aguilar MI. Meta-analysis: antithrombotic therapy to prevent stroke in patients who have nonvalvular atrial fibrillation. Ann Intern Med. (2007) 146:857–67. doi: 10.7326/0003-4819-146-12-200706190-00007
16. Riva N, Ageno W. Pros and cons of vitamin K antagonists and non-vitamin K antagonist oral anticoagulants. Semin Thromb Hemost. (2015) 41:178–87. doi: 10.1055/s-0035-1544231
17. Cozzolino M, Mangano M, Galassi A, Ciceri P, Messa P, Nigwekar S. Vitamin K in chronic kidney disease. Nutrients. (2019) 11:168. doi: 10.3390/nu11010168
18. Hart RG, Pearce LA, Asinger RW, Herzog CA. Warfarin in atrial fibrillation patients with moderate chronic kidney disease. Clin J Am Soc Nephrol. (2011) 6:2599–604. doi: 10.2215/CJN.02400311
19. Dahal K, Kunwar S, Rijal J, Schulman P, Lee J. Stroke, major bleeding, and mortality outcomes in warfarin users with atrial fibrillation and chronic kidney disease: a meta-analysis of observational studies. Chest. (2016) 149:951–9. doi: 10.1378/chest.15-1719
20. Randhawa MS, Vishwanath R, Rai MP, Wang L, Randhawa AK, Abela G, et al. Association between use of warfarin for atrial fibrillation and outcomes among patients with end-stage renal disease: a systematic review and meta-analysis. JAMA Netw Open. (2020) 3:e202175. doi: 10.1001/jamanetworkopen.2020.2175
21. Pokorney SD, Black-Maier E, Hellkamp AS, Friedman DJ, Vemulapalli S, Granger CB, et al. Oral anticoagulation and cardiovascular outcomes in patients with atrial fibrillation and end-stage renal disease. J Am Coll Cardiol. (2020) 75:1299–308. doi: 10.1016/j.jacc.2020.01.019
22. Agarwal MA, Potukuchi PK, Sumida K, Naseer A, Molnar MZ, George LK, et al. Clinical outcomes of warfarin initiation in advanced chronic kidney disease patients with incident atrial fibrillation. JACC Clin Electrophysiol. (2020) 6:1658–68. doi: 10.1016/j.jacep.2020.06.036
23. Burlacu A, Genovesi S, Ortiz A, Combe C, Basile C, Schneditz D, et al. Pros and cons of antithrombotic therapy in end-stage kidney disease: a 2019 update. Nephrol Dial Transplant. (2019) 34:923–33. doi: 10.1093/ndt/gfz040
24. Szummer K, Gasparini A, Eliasson S, Ärnlöv J, Qureshi AR, Bárány P, et al. Time in therapeutic range and outcomes after warfarin initiation in newly diagnosed atrial fibrillation patients with renal dysfunction. J Am Heart Assoc. (2017) 6:e004925. doi: 10.1161/JAHA.116.004925
25. Bonde AN, Lip GYH, Kamper AL, Staerk L, Torp-Pedersen C, Gislason G, et al. Renal function, time in therapeutic range and outcomes in warfarin-treated atrial fibrillation patients: a retrospective analysis of nationwide registries. Thromb Haemost. (2017) 117:2291–99. doi: 10.1160/TH17-03-0198
26. Weir MR, Ashton V, Moore KT, Shrivastava S, Peterson ED, Ammann EM. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and stage IV-V chronic kidney disease. Am Heart J. (2020) 223:3–11. doi: 10.1016/j.ahj.2020.01.010
27. Rebora P, Moia M, Carpenedo M, Valsecchi MG, Genovesi S. Best quality indicator of vitamin K antagonist therapy to predict mortality and bleeding in haemodialysis patients with atrial fibrillation. Blood Transfus. (2020). doi: 10.2450/2020.0217-20
28. Limdi NA, Beasley TM, Baird MF, Goldstein JA, McGwin G, Arnett DK, et al. Kidney function influences warfarin responsiveness and hemorrhagic complications. J Am Soc Nephrol. (2009) 20:912–21. doi: 10.1681/ASN.2008070802
29. Limdi NA, Nolin TD, Booth SL, Centi A, Marques MB, Crowley MR, et al. Influence of kidney function on risk of supratherapeutic international normalized ratio-related hemorrhage in warfarin users: a prospective cohort study. Am J Kidney Dis. (2015) 65:701–9. doi: 10.1053/j.ajkd.2014.11.004
30. Patel MR, Mahaffey KW, Garg J, Pan G, Singer DE, Hacke W, et al. Rivaroxaban versus warfarin in nonvalvular atrial fibrillation. N Engl J Med. (2011) 365:883–91. doi: 10.1056/NEJMoa1009638
31. Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, et al. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. (2009) 361:1139–51. doi: 10.1056/NEJMoa0905561
32. Granger CB, Alexander JH, McMurray JJV, Lopes RD, Hylek EM, Hanna M, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2011) 365:981–92. doi: 10.1056/NEJMoa1107039
33. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. (2013) 369:2093–104. doi: 10.1056/NEJMoa1310907
34. Ruff CT, Giugliano RP, Braunwald E, Hoffman EB, Deenadayalu N, Ezekowitz MD, et al. Comparison of the efficacy and safety of new oral anticoagulants with warfarin in patients with atrial fibrillation: a meta-analysis of randomised trials. Lancet. (2014) 383:955–62. doi: 10.1016/S0140-6736(13)62343-0
35. Potpara TS, Ferro CJ, Lip GYH. Use of oral anticoagulants in patients with atrial fibrillation and renal dysfunction. Nature Rev Nephrol. (2018) 14:337–51. doi: 10.1038/nrneph.2018.19
36. Baglin T, Hillarp A, Tripodi A, Elalamy I, Buller H, Ageno W, et al. Measuring oral direct inhibitors of thrombin and factor Xa: a recommendation from the subcommittee on control of anticoagulation of the scientific and standardization committee of the international society on thrombosis and haemostasis. J Thromb Haemost. (2013) 11:756–60. doi: 10.1111/jth.12149
37. Fox KAA, Piccini JP, Wojdyla D, Becker RC, Halperin JL, Nessel CC, et al. Prevention of stroke and systemic embolism with rivaroxaban compared with warfarin in patients with non-valvular atrial fibrillation and moderate renal impairment. Eur Heart J. (2011) 32:2387–94. doi: 10.1093/eurheartj/ehr342
38. Hohnloser SH, Hijazi Z, Thomas L, Alexander JH, Amerena J, Hanna M, et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: insights from the ARISTOTLE trial. Eur Heart J. (2012) 33:2821–30. doi: 10.1093/eurheartj/ehs274
39. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in relation to baseline renal function in patients with atrial fibrillation: a RE-LY (Randomized evaluation of long-term anticoagulation therapy) trial analysis. Circulation. (2014) 129:961–70. doi: 10.1161/CIRCULATIONAHA.113.003628
40. Bohula EA, Giugliano RP, Ruff CT, Kuder JF, Murphy SA, Antman EM, et al. Impact of renal function on outcomes with edoxaban in the ENGAGE AF-TIMI 48 trial. Circulation. (2016) 134:24–36. doi: 10.1161/CIRCULATIONAHA.116.022361
41. Hijazi Z, Hohnloser SH, Andersson U, Alexander JH, Hanna M, Keltai M, et al. Efficacy and safety of apixaban compared with warfarin in patients with atrial fibrillation in relation to renal function over time: insights from the ARISTOTLE randomized clinical trial. JAMA Cardiol. (2016) 1:451–60. doi: 10.1001/jamacardio.2016.1170
42. Kimachi M, Furukawa TA, Kimachi K, Goto Y, Fukuma S, Fukuhara S. Direct oral anticoagulants versus warfarin for preventing stroke and systemic embolic events among atrial fibrillation patients with chronic kidney disease. Cochrane Database Syst Rev. (2017) 11:CD011373. doi: 10.1002/14651858.CD011373.pub2
43. Ha JT, Neuen BL, Cheng LP, Jun M, Toyama T, Gallagher MP, et al. Benefits and harms of oral anticoagulant therapy in chronic kidney disease. Ann Intern Med. (2019) 171:181–9. doi: 10.7326/M19-0087
44. Andò G, Capranzano P. Non-vitamin K antagonist oral anticoagulants in atrial fibrillation patients with chronic kidney disease: a systematic review and network meta-analysis. Int J Cardiol. (2017) 231:162–9. doi: 10.1016/j.ijcard.2016.11.303
45. Stanifer JW, Pokorney SD, Chertow GM, Hohnloser SH, Wojdyla DM, Garonzik S, et al. Apixaban versus warfarin in patients with atrial fibrillation and advanced chronic kidney disease. Circulation. (2020) 141:1384–92. doi: 10.1161/CIRCULATIONAHA.119.044059
46. Lee KH, Park HW, Cho JG, Yoon NS, Kim SS, Kim MR, et al. Comparison of non-Vitamin K antagonist oral anticoagulants and warfarin on clinical outcomes in atrial fibrillation patients with renal dysfunction. Europace. (2015) 17 (Suppl. 2):ii69–75. doi: 10.1093/europace/euv198
47. Harel Z, Mamdani M, Juurlink DN, Garg AX, Wald R, Yao Z, et al. Novel oral anticoagulants and the risk of major hemorrhage in elderly patients with chronic kidney disease: a nested case-control study. Can J Cardiol. (2016) 32:986.e17–22. doi: 10.1016/j.cjca.2016.01.013
48. Stanton BE, Barasch NS, Tellor KB. Comparison of the safety and effectiveness of apixaban versus warfarin in patients with severe renal impairment. Pharmacother. (2017) 37:412–9. doi: 10.1002/phar.1905
49. Weir MR, Berger JS, Ashton V, Laliberté F, Brown K, Lefebvre P, et al. Impact of renal function on ischemic stroke and major bleeding rates in nonvalvular atrial fibrillation patients treated with warfarin or rivaroxaban: a retrospective cohort study using real-world evidence. Curr Med Res Opin. (2017) 33:1891–900. doi: 10.1080/03007995.2017.1339674
50. Becattini C, Giustozzi M, Ranalli MG, Bogliari G, Cianella F, Verso M, et al. Variation of renal function over time is associated with major bleeding in patients treated with direct oral anticoagulants for atrial fibrillation. J Thromb Haemost. (2018) 16:833–41. doi: 10.1111/jth.13985
51. Kumar S, De Lusignan S, McGovern A, Correa A, Hriskova M, Gatenby P, et al. Ischaemic stroke, haemorrhage, and mortality in older patients with chronic kidney disease newly started on anticoagulation for atrial fibrillation: a population based study from UK primary care. BMJ. (2018) 360:k342. doi: 10.1136/bmj.k342
52. Schafer JH, Casey AL, Dupre KA, Staubes BA. Safety and efficacy of apixaban versus warfarin in patients with advanced chronic kidney disease. Ann Pharmacother. (2018) 52:1078–84. doi: 10.1177/1060028018781853
53. Wetmore JB, Roetker NS, Yan H, Reyes JL, Herzog CA. Direct-acting oral anticoagulants versus warfarin in medicare patients with chronic kidney disease and atrial fibrillation. Stroke. (2020) 51:2364–73. doi: 10.1161/STROKEAHA.120.028934
54. Bonnemeier H, Huelsebeck M, Kloss S. Comparative effectiveness of rivaroxaban versus a vitamin K antagonist in patients with renal impairment treated for non-valvular atrial fibrillation in Germany — A retrospective cohort study. Int J Cardiol Heart Vasc. (2019) 23:100367. doi: 10.1016/j.ijcha.2019.100367
55. Shin JI, Secora A, Caleb Alexander G, Inker LA, Coresh J, Chang AR, et al. Risks and benefits of direct oral anticoagulants across the spectrum of GFR among incident and prevalent patients with atrial fibrillation. Clin J Am Soc Nephrol. (2018) 13:1144–52. doi: 10.2215/CJN.13811217
56. Di Lullo L, Tripepi G, Ronco C, De Pascalis A, Barbera V, Granata A, et al. Safety and effectiveness of rivaroxaban and warfarin in moderate-to-advanced CKD: real world data. J Nephrol. (2018) 31:751–6. doi: 10.1007/s40620-018-0501-7
57. Loo SY, Coulombe J, Dell'Aniello S, Brophy JM, Suissa S, Renoux C. Comparative effectiveness of novel oral anticoagulants in UK patients with non-valvular atrial fibrillation and chronic kidney disease: a matched cohort study. BMJ Open. (2018) 8:e019638. doi: 10.1136/bmjopen-2017-019638
58. Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. (2019) 132:1078–83. doi: 10.1016/j.amjmed.2019.04.013
59. Makani A, Saba S, Jain SK, Bhonsale A, Sharbaugh MS, Thoma F, et al. Safety and efficacy of direct oral anticoagulants versus warfarin in patients with chronic kidney disease and atrial fibrillation. Am J Cardiol. (2020) 125:210–4. doi: 10.1016/j.amjcard.2019.10.033
60. Chang SH, Wu CCV, Yeh YH, Kuo CF, Chen YL, Wen MS, et al. Efficacy and Safety of oral anticoagulants in patients with atrial fibrillation and stages 4 or 5 chronic kidney disease. Am J Med. (2019) 132:1335–43.e6. doi: 10.1016/j.amjmed.2019.06.006
61. Yao X, Inselman JW, Ross JS, et al. Comparative effectiveness and safety of oral anticoagulants across kidney function in patients with atrial fibrillation. Circ Cardiovasc Qual Outcomes. (2020) 13:e006515. doi: 10.1161/CIRCOUTCOMES.120.006515
62. Ashley J, McArthur E, Bota S, Harel Z, Battistella M, Molnar AO, et al. Risk of cardiovascular events and mortality among elderly patients with reduced gfr receiving direct oral anticoagulants. Am J Kidney Dis. (2020) 76:311–20. doi: 10.1053/j.ajkd.2020.02.446
63. Chan KE, Edelman ER, Wenger JB, Thadhani RI, Maddux FW. Dabigatran and rivaroxaban use in atrial fibrillation patients on hemodialysis. Circulation. (2015) 131:972–9. doi: 10.1161/CIRCULATIONAHA.114.014113
64. Sarratt SC, Nesbit R, Moye R. Safety outcomes of apixaban compared with warfarin in patients with end-stage renal disease. Ann Pharmacother. (2017) 51:445–50. doi: 10.1177/1060028017694654
65. Siontis KC, Zhang X, Eckard A, Bhave N, Schaubel DE, He K, et al. Outcomes associated with apixaban use in patients with end-stage kidney disease and atrial fibrillation in the United States. Circulation. (2018) 138:1519–29. doi: 10.1161/CIRCULATIONAHA.118.035418
66. Mavrakanas TA, Garlo K, Charytan DM. Apixaban versus no anticoagulation in patients undergoing long-term dialysis with incident atrial fibrillation. Clin J Am Soc Nephrol. (2020) 15:1146–54. doi: 10.2215/CJN.11650919
67. See LC, Lee HF, Chao TF, Li PR, Liu JR, Wu LS, et al. Effectiveness and safety of direct oral anticoagulants in an asian population with atrial fibrillation undergoing dialysis: a population-based cohort study and meta-analysis. Cardiovasc Drugs Ther. (2020) doi: 10.1007/s10557-020-07108-4
68. Miao B, Sood N, Bunz TJ, Coleman CI. Rivaroxaban versus apixaban in non-valvular atrial fibrillation patients with end-stage renal disease or receiving dialysis. Eur J Haematol. (2020) 104:328–35. doi: 10.1111/ejh.13383
69. Malhotra K, Ishfaq MF, Goyal N, Katsanos AH, Parissis J, Alexandrov AW, et al. Oral anticoagulation in patients with chronic kidney disease: a systematic review and meta-analysis. Neurology. (2019) 92:e2421–31. doi: 10.1212/WNL.0000000000007534
70. Sy J, Hsiung JT, Edgett D, Kalantar-Zadeh K, Streja E, Lau WL. Cardiovascular and bleeding outcomes with anticoagulants across kidney disease stages: analysis of a national US cohort. Am J Nephrol. (2021) 52:199–208. doi: 10.1159/000514753
71. de Vriese AS, Caluwé R, Pyfferoen L, de Bacquer D, de Boeck K, Delanote J, et al. Multicenter randomized controlled trial of Vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie study. J Am Soc Nephrol. (2020) 31:186–96. doi: 10.1681/ASN.2019060579
72. de Vriese AS, Caluwé R, Van Der Meersch H, De Boeck K, De Bacquer D. Safety and efficacy of vitamin K antagonists versus rivaroxaban in hemodialysis patients with atrial fibrillation: a multicenter randomized controlled trial. J Am Soc Nephrol. (2021) 32:1474–83. doi: 10.1681/ASN.2020111566
73. Fordyce CB, Hellkamp AS, Lokhnygina Y, Lindner SM, Piccini JP, Becker RC, et al. On-treatment outcomes in patients with worsening renal function with rivaroxaban compared with warfarin. Circulation. (2016) 134:37–47. doi: 10.1161/CIRCULATIONAHA.116.021890
74. Hijazi Z, Hohnloser SH, Oldgren J, Andersson U, Connolly SJ, Eikelboom JW, et al. Efficacy and safety of dabigatran compared with warfarin in patients with atrial fibrillation in relation to renal function over time—A RE-LY trial analysis. Am Heart J. (2018) 198:169–77. doi: 10.1016/j.ahj.2017.10.015
75. Posch F, Ay C, Stöger H, Kreutz R, Beyer-Westendorf J. Longitudinal kidney function trajectories predict major bleeding, hospitalization and death in patients with atrial fibrillation and chronic kidney disease. Int J Cardiol. (2019) 282:47–52. doi: 10.1016/j.ijcard.2019.01.089
76. Yao X, Tangri N, Gersh BJ, Sangaralingham LR, Shah ND, Nath KA, et al. Renal outcomes in anticoagulated patients with atrial fibrillation. J Am Coll Cardiol. (2017) 70:2621–32. doi: 10.1016/j.jacc.2017.09.1087
77. Fanola CL, Mooney D, Cowan AJ, Ko D, Sisson EK, Henault LE, et al. Incidence of severe renal dysfunction among individuals taking warfarin and implications for non–vitamin K oral anticoagulants. Am Heart J. (2017) 184:150–5. doi: 10.1016/j.ahj.2016.08.017
78. Fauchier L, Bisson A, Clementy N, Vourc'h P, Angoulvant D, Babuty D, et al. Changes in glomerular filtration rate and outcomes in patients with atrial fibrillation. Am Heart J. (2018) 198:39–45. doi: 10.1016/j.ahj.2017.12.017
79. Yao X, Shah ND, Sangaralingham LR, Gersh BJ, Noseworthy PA. Non–Vitamin K Antagonist Oral Anticoagulant Dosing in Patients With Atrial Fibrillation and Renal Dysfunction. J Am Coll Cardiol. (2017) 69:2779–90. doi: 10.1016/j.jacc.2017.03.600
80. Nielsen PB, Skjøth F, Søgaard M, Kjældgaard JN, Lip GYH, Larsen TB. Effectiveness and safety of reduced dose non-Vitamin K antagonist oral anticoagulants and warfarin in patients with atrial fibrillation: propensity weighted nationwide cohort study. BMJ. (2017) 356:j510. doi: 10.1136/bmj.j510
81. Fernandez-Prado R, Castillo-Rodriguez E, Velez-Arribas FJ, Gracia-Iguacel C, Ortiz A. Creatinine clearance is not equal to glomerular filtration rate and cockcroft-gault equation is not equal to CKD-EPI collaboration equation. Am J Med. (2016) 129:1259–63. doi: 10.1016/j.amjmed.2016.08.019
82. Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group. KDIGO Clinical practice guideline for the evaluation and Management of Chronic Kidney Disease. Kidney Int. (2013) (Suppl. 2012):1–150. doi: 10.1038/kisup.2012.77
83. Andrade JG, Hawkins NM, Fordyce CB, Deyell MW, Er L, Djurdjev O, et al. Variability in non–vitamin K antagonist oral anticoagulants dose adjustment in atrial fibrillation patients with renal dysfunction: the influence of renal function estimation formulae. Can J Cardiol. (2018) 34:1010–8. doi: 10.1016/j.cjca.2018.04.019
84. Manzano-Fernández S, Andreu-Cayuelas JM, Marín F, Orenes-Piñero E, Gallego P, Valdés M, et al. Comparison of estimated glomerular filtration rate equations for dosing new oral anticoagulants in patients with atrial fibrillation. Rev Esp Cardiol. (2015) 68:497–504. doi: 10.1016/j.recesp.2014.06.027
85. Nabiee M, Dashti-Khavidaki S, Khajeh B. Dose discordance of direct acting oral anticoagulants using different equations for estimating GFR: a literature review. Expert Rev Clin Pharmacol. (2020) 13:857–63. doi: 10.1080/17512433.2020.1798759
86. Cemin R, Foco L, Zoccali C, De Caterina R. Should we continue assessing glomerular filtration rate with the cockroft–gault formula in NOAC-treated patients? The Magnitude of the Problem. J Clin Med. (2020) 9:1893. doi: 10.3390/jcm9061893
87. Hughes S, Szeki I, Nash MJ, Thachil J. Anticoagulation in chronic kidney disease patients - the practical aspects. Clin Kidney J. (2014) 7:442–449. doi: 10.1093/ckj/sfu080
88. Brodsky SV, Satoskar A, Chen J, Nadasdy G, Eagen JW, Hamirani M, et al. Acute Kidney injury during warfarin therapy associated with obstructive tubular red blood cell casts: a report of 9 cases. Am J Kidney Dis. (2009) 54:1121–6. doi: 10.1053/j.ajkd.2009.04.024
89. De Aquino Moura KB, Behrens PMP, Pirolli R, Sauer A, Melamed D, Veronese FV, et al. Anticoagulant-related nephropathy: systematic review and meta-analysis. Clin Kidney J. (2019) 12:400–7. doi: 10.1093/ckj/sfy133
90. Brodsky SV, Collins M, Park E, Rovin BH, Satoskar AA, Nadasdy G, et al. Warfarin therapy that results in an international normalization ratio above the therapeutic range is associated with accelerated progression of chronic kidney disease. Nephron Clin Pract. (2010) 115:c142–6. doi: 10.1159/000312877
91. Brodsky SV, Satoskar A, Hemminger J, Rovin B, Hebert L, Ryan MS, et al. Anticoagulant-related nephropathy in kidney biopsy: a single-center report of 41 cases. Kidney Med. (2019) 1:51–6. doi: 10.1016/j.xkme.2019.03.002
92. Shin JI, Luo S, Alexander GC, Inker LA, Coresh J, Chang AR, et al. Direct Oral Anticoagulants and Risk of Acute Kidney Injury in Patients With Atrial Fibrillation. J Am Coll Cardiol. (2018) 71:251–2. doi: 10.1016/j.jacc.2017.10.089
93. Chan YH, Yeh YH, Hsieh MY, Chang CY, Tu HT, Chang SH, et al. The risk of acute kidney injury in Asians treated with apixaban, rivaroxaban, dabigatran, or warfarin for non-valvular atrial fibrillation: a nationwide cohort study in Taiwan. Int J Cardiol. (2018) 265:83–9. doi: 10.1016/j.ijcard.2018.02.075
94. Coleman CI, Kreutz R, Sood N, Bunz TJ, Meinecke AK, Eriksson D, et al. Rivaroxaban's Impact on Renal Decline in Patients With Nonvalvular Atrial Fibrillation: A US MarketScan Claims Database Analysis. Clin Appl Thromb. (2019) 132:1078–83. doi: 10.1177/1076029619868535
95. Hernandez AV, Bradley G, Khan M, Fratoni A, Gasparini A, Roman YM, et al. Rivaroxaban vs. warfarin and renal outcomes in non-valvular atrial fibrillation patients with diabetes. Eur Heart J Qual Care Clin Outcomes. (2020) 6:301–7. doi: 10.1093/ehjqcco/qcz047
96. Böhm M, Ezekowitz MD, Connolly SJ, Eikelboom JW, Hohnloser SH, Reilly PA, et al. Changes in renal function in patients with atrial fibrillation: an analysis from the RE-LY trial. J Am Coll Cardiol. (2015) 65:2481–93. doi: 10.1016/j.jacc.2015.03.577
97. Pastori D, Ettorre E, Lip GYH, Sciacqua A, Perticone F, Melillo F, et al. Association of different oral anticoagulants use with renal function worsening in patients with atrial fibrillation: a multicentre cohort study. Br J Clin Pharmacol. (2020) 86:2455–63. doi: 10.1111/bcp.14350
98. Posch F, Ay C, Stöger H, Kreutz R, Beyer-Westendorf J. Exposure to vitamin K antagonists and kidney function decline in patients with atrial fibrillation and chronic kidney disease. Res Pract Thromb Haemost. (2019) 3:207–16. doi: 10.1002/rth2.12189
99. Wetmore JB, Yan H, Herzog CA, Weinhandl E, Reyes JL, Roetker NS. CKD progression in medicare beneficiaries with nonvalvular atrial fibrillation treated with apixaban versus warfarin. Am J Kidney Dis. (2021) 78:180–9. doi: 10.1053/j.ajkd.2020.12.004
100. Ichikawa H, Shimada M, Narita M, Narita I, Kimura Y, Tanaka M, et al. Rivaroxaban, a direct factor Xa inhibitor, ameliorates hypertensive renal damage through inhibition of the inflammatory response mediated by protease-activated receptor pathway. J Am Heart Assoc. (2019) 8:e012195. doi: 10.1161/JAHA.119.012195
101. Townsend RR. Arterial stiffness in CKD: a review. Am J Kidney Dis. (2019) 73:240–247. doi: 10.1053/j.ajkd.2018.04.005
102. Nelson AJ, Raggi P, Wolf M, Gold AM, Chertow GM, Roe MT. Targeting vascular calcification in chronic kidney disease. JACC Basic Transl Sci. (2020) 5:398–412. doi: 10.1016/j.jacbts.2020.02.002
103. Andrews J, Psaltis PJ, Bayturan O, Shao M, Stegman B, Elshazly M, et al. Warfarin use is associated with progressive coronary arterial calcification: insights from serial intravascular ultrasound. JACC Cardiovasc Imaging. (2018) 11:1315–23. doi: 10.1016/j.jcmg.2017.04.010
104. Tantisattamo E, Han KH, Charles O'Neill W. Increased vascular calcification in patients receiving warfarin. Arterioscler Thromb Vasc Biol. (2015) 35:237–42. doi: 10.1161/ATVBAHA.114.304392
105. Mac-Way F, Poulin A, Utescu MS, De Serres SA, Marquis K, Douville P, et al. The impact of warfarin on the rate of progression of aortic stiffness in hemodialysis patients: a longitudinal study. Nephrol Dial Transplant. (2014) 29:2113–20. doi: 10.1093/ndt/gfu224
106. Namba S, Yamaoka-Tojo M, Kakizaki R, Nemoto T, Fujiyoshi K, Hashikata T, et al. Effects on bone metabolism markers and arterial stiffness by switching to rivaroxaban from warfarin in patients with atrial fibrillation. Heart Vessels. (2017) 32:977–82. doi: 10.1007/s00380-017-0950-2
107. Di Lullo L, Tripepi G, Ronco C, D'Arrigo G, Barbera V, Russo D, et al. Cardiac valve calcification and use of anticoagulants: preliminary observation of a potentially modifiable risk factor. Int J Cardiol. (2019) 278:243–9. doi: 10.1016/j.ijcard.2018.11.119
108. Gaisne R, Péré M, Menoyo V, Hourmant M, Larmet-Burgeot D. Calciphylaxis epidemiology, risk factors, treatment and survival among French chronic kidney disease patients: a case-control study. BMC Nephrol. (2020) 21:63. doi: 10.1186/s12882-020-01722-y
109. Rogers NM, Coates PTH. Calcific uraemic arteriolopathy: an update. Curr Opin Nephrol Hypertens;. (2008) 17:629–4. doi: 10.1097/MNH.0b013e32830f4566
110. Jeong HS, Dominguez AR. Calciphylaxis: controversies in pathogenesis, diagnosis and treatment. Am J Med Sci. (2016) 351:217–27. doi: 10.1016/j.amjms.2015.11.015
111. Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, et al. Executive summary of the 2017 KDIGO chronic kidney disease–mineral and bone disorder (CKD-MBD) guideline update: what's changed and why it matters. Kidney Int. (2017) 92:26–36. doi: 10.1016/j.kint.2017.04.006
112. Fiordellisi W, White K, Schweizer M. A systematic review and meta-analysis of the association between vitamin k antagonist use and fracture. J Gen Intern Med. (2019) 34:304–11. doi: 10.1007/s11606-018-4758-2
113. Steffel J, Giugliano RP, Braunwald E, Murphy SA, Mercuri M, Choi Y, et al. Edoxaban versus warfarin in atrial fibrillation patients at risk of falling: ENGAGE AF–TIMI 48 analysis. J Am Coll Cardiol. (2016) 68:1169–78. doi: 10.1016/j.jacc.2016.06.034
114. Lutsey PL, Norby FL, Ensrud KE, Maclehose RF, Diem SJ, Chen LY, et al. Association of anticoagulant therapy with risk of fracture among patients with atrial fibrillation. JAMA Intern Med. (2020) 180:245–53. doi: 10.1001/jamainternmed.2019.5679
115. Lau WCY, Chan EW, Cheung CL, Sing CW, Man KKC, Lip GYH, et al. Association between Dabigatran vs Warfarin and risk of osteoporotic fractures among patients with nonvalvular atrial fibrillation. JAMA. (2017) 317:1151–58. doi: 10.1001/jama.2017.1363
116. Lau WCY, Cheung CL, Man KKC, Chan EW, Sing CW, Lip GYH, et al. Association between treatment with apixaban, dabigatran, rivaroxaban, or warfarin and risk for osteoporotic fractures among patients with atrial fibrillation: a population-based cohort study. Ann Intern Med. (2020) 173:1–9. doi: 10.7326/M19-3671
117. Binding C, Bjerring Olesen J, Abrahamsen B, Staerk L, Gislason G, Nissen Bonde A. Osteoporotic fractures in patients with atrial fibrillation treated with conventional versus direct anticoagulants. J Am Coll Cardiol. (2019) 74:2150–8. doi: 10.1016/j.jacc.2019.08.1025
118. Huang HK, Liu PPS, Hsu JY, Lin SM, Peng CCH, Wang JH, et al. Fracture risks among patients with atrial fibrillation receiving different oral anticoagulants: a real-world nationwide cohort study. Eur Heart J. (2020) 41:1100–8. doi: 10.1093/eurheartj/ehz952
119. Huang HK, Liu PPS, Hsu JY, Lin SM, Peng CCH, Wang JH, et al. Risk of Osteoporosis in Patients With Atrial Fibrillation Using Non–Vitamin K Antagonist Oral Anticoagulants or Warfarin. J Am Heart Assoc. (2020) 9:e013845. doi: 10.1161/JAHA.119.013845
120. Gu ZC, Zhou LY, Shen L, Zhang C, Pu J, Lin HW, et al. Non-vitamin K antagonist oral anticoagulants vs. Warfarin at risk of fractures: A systematic review and meta-analysis of randomized controlled trials. Front Pharmacol. (2018) 9:348. doi: 10.3389/fphar.2018.00348
121. Genovesi S, Porcu L, Slaviero G, Casu G, Bertoli S, Sagone A, et al. Outcomes on safety and efficacy of left atrial appendage occlusion in end stage renal disease patients undergoing dialysis. J Nephrol. (2021) 34:63–73. doi: 10.1007/s40620-020-00774-5
122. Xipell M, Flores-Umanzor E, Ojeda R, Arias M, Cepas-Guillén PL, Regueiro A, et al. Percutaneous left atrial appendage closure, a safe alternative to anticoagulation for patients with nonvalvular atrial fibrillation and end-stage renal disease on hemodialysis: a single center experience. Artif Organs. (2020) 44:513–21. doi: 10.1111/aor.13603
Keywords: chronic kidney disease, atrial fibrillation, Direct oral anticoagulants, Vitamin K antagonists, anticoagulant-related nephropathy
Citation: Cases A, Gomez P, Broseta JJ, Perez Bernat E, Arjona Barrionuevo JD, Portolés JM and Gorriz JL (2021) Non-valvular Atrial Fibrillation in CKD: Role of Vitamin K Antagonists and Direct Oral Anticoagulants. A Narrative Review. Front. Med. 8:654620. doi: 10.3389/fmed.2021.654620
Received: 16 January 2021; Accepted: 23 August 2021;
Published: 17 September 2021.
Edited by:
Simonetta Genovesi, University of Milano Bicocca, ItalyReviewed by:
Zbigniew Kalarus, Medical University of Silesia, PolandSilvio Borrelli, Università Della Campania Luigi Vanvitelli, Italy
Copyright © 2021 Cases, Gomez, Broseta, Perez Bernat, Arjona Barrionuevo, Portolés and Gorriz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jose Maria Portolés, josem.portoles@salud.madrid.org
†These authors share first authorship
‡ORCID: Aleix Cases orcid.org/0000-0002-6962-8184
Jose Maria Portolés orcid.org/0000-0002-2114-1385
Jose Luis Gorriz orcid.org/0000-0002-1134-9051
 Aleix Cases
Aleix Cases Pablo Gomez2†
Pablo Gomez2†