Characteristics and Prognostic Factors of Pulmonary Fibrosis After COVID-19 Pneumonia
- 1Respiratory Disease Unit, Department of Cardiac, Thoracic, Vascular Sciences and Public Health, University of Padova and Padova City Hospital, Padova, Italy
- 2Department of Medicine, Institute of Radiology, University of Padova and Padova City Hospital, Padova, Italy
- 3Division of Infectious and Tropical Diseases, University of Padova and Padova City Hospital, Padova, Italy
Background: Few is known about the long-term pulmonary sequelae after COVID-19 infection. Hence, the aim of this study is to characterize patients with persisting pulmonary sequelae at follow-up after hospitalization. We also aimed to explore clinical and radiological predictors of pulmonary fibrosis following COVID-19.
Methods: Two hundred and 20 consecutive patients were evaluated at 3–6 months after discharge with high-resolution computed tomography (HRCT) and categorized as recovered (REC) or not recovered (NOT-REC). Both HRCTs at hospitalization (HRCT0), when available, and HRCT1 during follow-up were analyzed semiquantitatively as follows: ground-glass opacities (alveolar score, AS), consolidations (CONS), and reticulations (interstitial score, IS).
Results: A total of 175/220 (80%) patients showed disease resolution at their initial radiological evaluation following discharge. NOT-REC patients (45/220; 20%) were mostly older men [66 (35–85) years vs. 56 (19–87); p = 0.03] with a longer in-hospital stay [16 (0–75) vs. 8 (1–52) days; p < 0.0001], and lower P/F at admission [233 (40–424) vs. 318 (33–543); p = 0.04]. Moreover, NOT-REC patients presented, at hospital admission, higher ALV [14 (0.0–62.0) vs. 4.4 (0.0–44.0); p = 0.0005], CONS [1.9 (0.0–26.0) vs. 0.4 (0.0–18.0); p = 0.0064], and IS [11.5 (0.0– 29.0) vs. 0.0 (0.0–22.0); p < 0.0001] compared to REC patients. On multivariate analysis, the presence of CONS and IS at HRCT0 was independent predictors of radiological sequelae at follow-up [OR 14.87 (95% CI: 1.25–175.8; p = 0.03) and 28.9 (95% CI: 2.17–386.6; p = 0.01, respectively)].
Conclusions: In our population, only twenty percent of patients showed persistent lung abnormalities at 6 months after hospitalization for COVID-19 pneumonia. These patients are predominantly older men with longer hospital stay. The presence of reticulations and consolidation on HRCT at hospital admission predicts the persistence of radiological abnormalities during follow-up.
Background
Coronavirus disease 2019 (COVID-19), which is caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), has infected more than 130 million people worldwide. COVID-19 leads to respiratory manifestations that can range from mild flu-like symptoms such as fever, cough, and fatigue to severe respiratory failure requiring intensive care (1, 2).
Data from previous pandemics caused by coronaviruses suggested that there may be pulmonary sequelae in one-third of patients at 12 weeks after discharge (3, 4).
Some recent studies tried to characterize radiological sequelae after COVID-19 pneumonia (5, 6). This condition, which is referred to as “post-COVID syndrome,” still lacks a universally agreed definition (7). On May 2020, a document of the British Thoracic Society (BTS) proposed an algorithm on post-discharge management of patients with COVID-19 and distinguished two groups of interest: patients with severe pneumonia and patients with mild-to-moderate pneumonia (8). Following up on this document, George and colleagues suggested a structured respiratory follow-up for patients with clinico-radiological confirmation of COVID-19 pneumonia (9). Importantly, they proposed patients with severe pneumonia undergo a full clinical assessment at 12 weeks with a chest X-ray whereas patients with persisting radiological abnormalities should undergo a high-resolution computed tomography (HRCT) scan. In this regard, the role of chest X-ray and HRCT in disease management both during hospitalization and follow-up is well established (10, 11). Han and coauthors recently reported that fibrotic-like changes on CT performed at 6 months during follow-up persist in approximately one-third of patients with COVID-19 (12), but the data on long-term pulmonary sequelae in this patient population remain scarce. The aim of this study is to characterize, among patients hospitalized for COVID-19 pneumonia, those presenting persisting pulmonary sequelae during follow-up, and to define which clinical and radiological features are predictive of persistent radiological abnormalities.
Materials and Methods
Study Population and Study Design
We prospectively collected patients evaluated at the post-COVID clinic of the University Hospital of Padova between June and December 2020. The patients evaluated at the post-COVID clinic were initially admitted to the Division of Infectious and Tropical Diseases of the University Hospital of Padova between February and September 2020 for SARS-CoV-2 infection confirmed by the real-time polymerase chain reaction (RT-PCR) at nasopharyngeal swab.
Among all patients evaluated, we specifically followed up every 3 months those presenting a COVID-19-related severe disease according to the WHO criteria (n = 220) (13). Demographics and clinical data at hospital admission [symptoms, gas exchange values (paO2/FiO2)] and during hospitalization [days of hospital stay, maximal FiO2 (FiO2 max) needed, level of care, treatment] were collected. Comorbidities were categorized as cardiovascular diseases (CVDs), respiratory diseases, metabolic diseases (including diabetes mellitus, obesity, and dyslipidemia), autoimmune diseases, and oncologic diseases (including lung, prostate, pancreatic, breast, and colon cancer). Based on patient's clinical conditions during hospitalization, we distinguished those requiring a low- (LIMC) and high-intensity medical care (HIMC), as previously described (14).
Radiological Evaluation
At follow-up, HRCT was available for the entire study population (HRCT1) whereas at hospital admission, it was available in only a subgroup of patients (HRCT0) (n = 79, 36%). The HRCTs were performed by a 64 slice Siemens Somatom Sensation (Siemens Healthcare, Erlangen, Germany) applying a slice thickness ≤0.5 mm.
According to the presence or absence of radiological abnormalities on HCRT1, the study population was categorized as recovered patients (REC, n = 175) or not recovered patients (NOT-REC, n = 45).
Two expert thoracic radiologists (CG and AG), who were blinded to clinical data and timing of HRCTs, scored the images independently using a composite semiquantitative scale. This represented a modification of the previously reported scoring systems standardized by our group (13). Specifically, ground-glass opacities (GGO) (alveolar score, AS), consolidations (CONS), and reticulations (interstitial score, IS) were analyzed. For each lung lobe, the two radiologists assessed the extent of AS, CONS, and IS using a scale from 0 to 100 and estimated extent to the nearest 2%. The result was expressed as the mean value of the five lobes in AS, CONS, and IS. The level of interobserver agreement was obtained for each patient as a mean of 5 lobes and for each radiological abnormality (AS, CONS, and IS) and expressed as Cohen's k value. Disagreement between radiologists was resolved by consensus.
Statistical Analysis
Categorical variables were described as absolute (n) and relative values (%), whereas continuous variables were described as median and range. To compare demographic and clinical data between REC and NOT-REC patients, chi-square test and Fisher's exact test (n < 5) for categorical variables and Mann–Whitney U tests for continuous variables were used, as appropriate.
To compare radiological scores at HRCT1 in NOT-REC patients, Mann–Whitney U test for continuous variables was used, whereas Wilcoxon signed-rank test was used to compare radiological scores between HRCT0 and HRCT1. A univariate logistic regression analysis, followed by a regression model adjusted for gender, pack-years, paO2/FiO2 at admission, degree of medical care (high or low), and FiO2 max, was performed to detect the predictive factors of radiologic sequelae (NOT-REC) at follow-up. All data were analyzed using SPSS Software version 25.0 (US: IBM Corp., New York, NY, USA). p-Values < 0.05 were considered statistically significant. The graphs were obtained using the statistical package GraphPad Prism 7.0 (GraphPad Software, Inc., La Jolla, CA, USA).
Ethics Statement
The study protocol complies with the ethical guidelines of the 1975 Declaration of Helsinki, and in agreement with national regulation on observational studies, it was notified and approved by the local ethics committee (number: 46430/03.08.2020) and the need for patient's informed consent was waived.
Results
Clinical Evaluation at Hospital Admission and During Hospitalization
Two hundred and 20 patients with COVID-19 pneumonia evaluated at the post-COVID clinic were included in the study (Table 1). A total of 115 patients (52%) were men, with a median age of 59 years (range 19–84) and body mass index (BMI) 26 (18–39). The most prevalent comorbidities were CVDs (n = 98, 45%), followed by the chronic respiratory diseases (18%). Based on the presence of radiological sequelae on HRCT performed at follow-up (HRCT1), 175 (80%) patients were categorized as REC and 45 (20%) as NOT-REC (Figure 1). Baseline demographic and clinical data of REC and NOT-REC patients are summarized in Table 1.
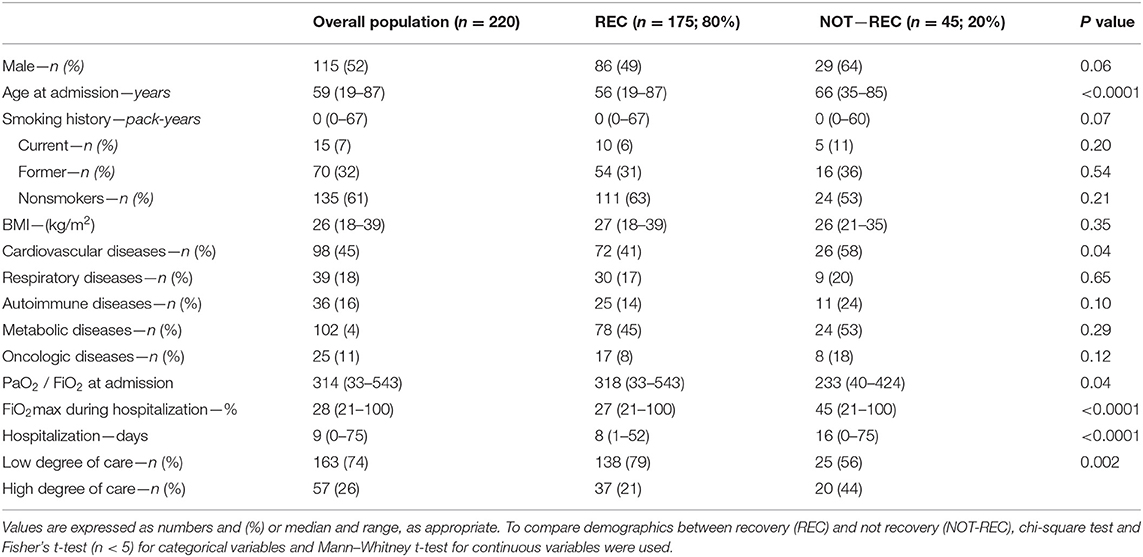
Table 1. Baseline demographics and clinical features of the overall population evaluated at post-COVID clinic, and of the two subgroups categorized according to the presence of radiological recovery during the follow-up period.
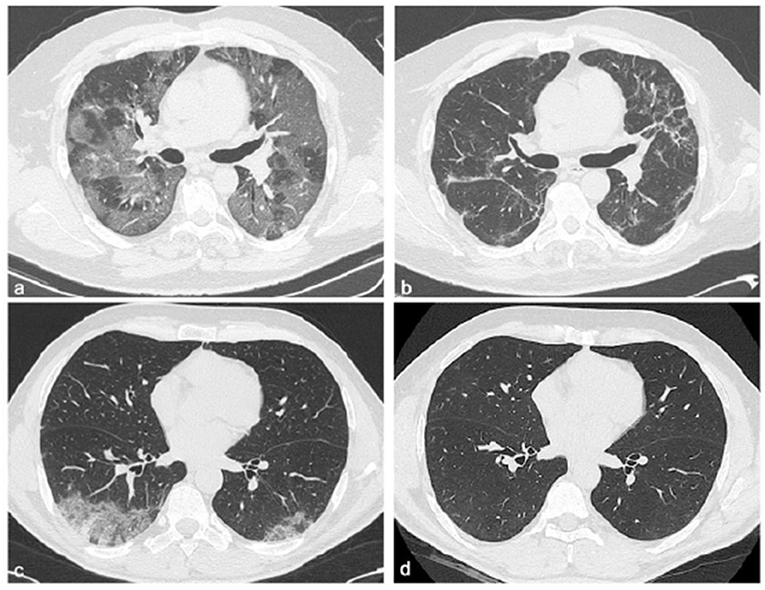
Figure 1. Chest CT features of two patients with COVID-19 pneumonia at different time points: hospitalization and 6 months after discharge. Chest CT images of a 58-year-old male patient with COVID-19, not recovery patient (a,b). The first CT performed at admission shows bilateral areas of ground-glass opacities in a peripheral distribution (a), and after 6 months from discharge, CT shows persistent of interlobular septal thickening with peripheral distribution (b). Chest CT images of a 51-year-old male patient with COVID-19, recovery patient (c,d). The first CT shows, at admission, a small consolidation at the right lower lobe accompanied by ground-glass opacities in both lower lobes (c), and after 6 months from discharge, no residual abnormalities were observed (d).
No differences in sex, smoking history, or BMI were observed between the two groups, with a prevalence of men in NOT-REC compared to REC (64 vs. 49%, respectively). NOT-REC patients were significantly older compared to REC [66 (35–85) vs. 56 (19–87) years; p < 0.0001]. CVDs were significantly more frequent in NOT-REC compared to REC [26 (58%) vs. 72 (41%); p = 0.04] whereas autoimmune, metabolic, and oncologic diseases did not differ between the two groups. Symptoms before hospital admission were also similar, except for a higher proportion of patients presenting with dyspnea in NOT-REC compared to REC group [33 (73%) vs. 64 (37%); p < 0.0001] (Supplementary Table 1).
At hospital admission, NOT-REC had a worse gas exchange with a lower PiO2/FiO2 ratio than REC [233 (40–424) vs. 318 (33543); p = 0.04]. In addition, compared to REC, during hospitalization, NOT-REC required more frequently high-intensity medical care (HIMC) (20, 44 vs. 37, 21%; p = 0.002), higher FiO2 max [45 (21–100) vs. 27 (21–100); p < 0.0001], and longer in-hospital stay [16 (0–75) vs. 8 (1–52) days; p < 0.0001].
The majority of patients were admitted during the first SARS-CoV-2 wave when no standardized protocols existed for treatment of hospitalized patients. NOT-REC patients were more frequently treated with hydroxychloroquine (n = 37, 82 vs. 111, 63%; p = 0.01), antibiotics other than ceftriaxone and azithromycin (n = 25, 56 vs. 44, 25%; p < 0.0001), remdesevir (n = 7, 16 vs. 10, 6%, p = 0.02), tocilizumab (n = 8, 18 vs. 12, 7%; p = 0.02), and steroids (n = 27, 60 vs. 74, 42%; p = 0.03) compared to REC. Conversely, the two groups did not differ with regard to the use of ceftriaxone, azithromycin, lopinovir/ritonavir, and hyperimmune plasma (Supplementary Table 2). At discharge, a similar proportion of patients in both groups were prescribed steroids.
Clinical, Functional, and Radiologic Evaluation at Follow-Up
Patients were evaluated at post-COVID clinic at regular 3-month intervals after discharge. At first evaluation, NOT-REC patients presented more frequently a modified Medical Research Council (mMRC) scores of 1 and 2 compared to REC [15 (33%) vs. 22 (13%), p = 0.0009 and 7 (16%) vs. 3 (2%), p < 0.0001, respectively]. In the overall population, pulmonary function tests (PFTs) revealed a median forced vital capacity (FVC) of 3.40 liters (L) (range 1.40–7.96), 96%pred. and a median total lung capacity (TLC) of 5.36 L (3.63–8.09), 89% pred. within the normal range. Likewise, NOT-REC patients showed preserved lung volumes within normal range (Supplementary Table 3). A number of 32 patients out of 220 (14.5%) had an abnormal diffusing capacity of the lung for carbon monoxide (DLco) at the 6-month follow-up, which occurred in those with persistent interstitial lung abnormalities (NOT-REC patients). At follow-up CT (HRCT1), NOT-REC patients presented higher ALV [2.8 (0.0–40.0)] compared to CONS [0.0 (0.0–2.0); p < 0.0001] and IS [0.6 (0.0–24.0); p < 0.0001] (Supplementary Figure 1). Overall, the interobserver agreement between the two radiologists with regard to change in AS, CONS, and IS was good (Cohen's kappa = 0.79 for AS, k = 0.88 for CONS, and k = 0.81 for IS).
Longitudinal Evaluation of Radiologic Manifestation: From Hospitalization to Follow-Up
At hospital admission, HRCT (HRCT0) was available for 79/220 (36%) patients. ALV [5.0 (0.0–62.0)] was significantly more prevalent compared to CONS [0.8 (0.0–26.0); p < 0.0001] and IS [0.8 (0.0–29.0); p < 0.0001]. When this patient subgroup was stratified in NOT-REC and REC, NOT-REC patients (n = 20) had at hospital admission higher ALV [14.0 (0.0–62.0) vs. 4.4 (0.0–44.0); p = 0.0005] (Figure 2A), CONS [1.9 (0.0–26.0 vs. 0.4 (0.0–18.0); p = 0.0064] (Figure 2B), and IS [11.5 (0.0–29.0) vs. 0.0 (0.0–22.0); p < 0.0001] (Figure 2C) compared to REC patients (n = 59) (Table 2). Finally, when comparing HRCT0 with HRCT1, we observed that in NOT-REC patients, ALV [from 14 (0.0–62.0) to 2.6 (0.0–40.0); p < 0.0001], CONS [from 1.9 (0.0–26.0) to 0.0 (0.0–2.2); p = 0.0001], and IS [1.5 (0.0–29.0) to 1.4 (0.0–24.0)] decreased significantly (Figure 3).
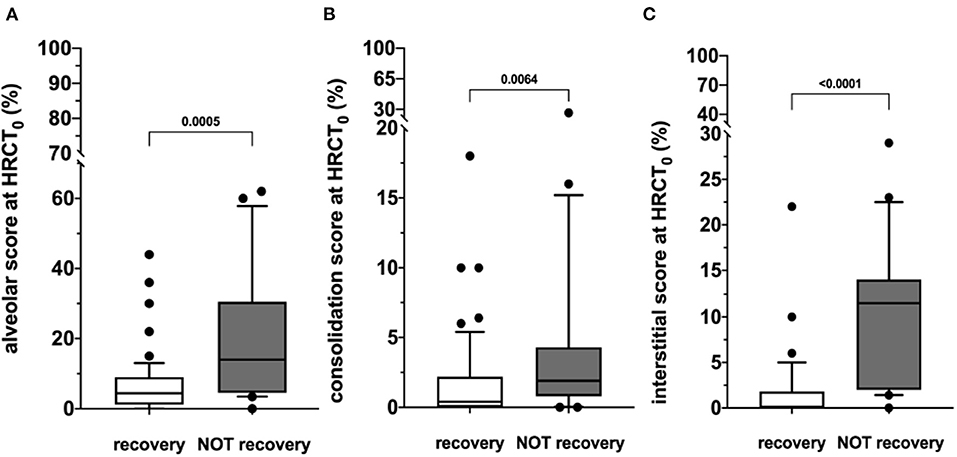
Figure 2. HRCT scores during hospitalization (HRCT0) of the two subgroups categorized according to the presence of radiological recovery [recovery (REC) or NOT-recovery (NOT-REC)] at follow-up period. Horizontal bars represent median values; bottom and top of each box plot 25th and 75th; brackets show 10th and 90th percentiles; and circles represent outliers. White boxes indicate values for recovery group and gray boxes not recovery group. (A) ALV [14.0 (0.0–62.0) vs. 4.4 (0.0–44.0); p = 0.0005]; (B) CONS [1.9 (0.0–26.0 vs. 0.4 (0.0–18.0); p = 0.0064]; (C) INT [11.5 (0.0–29.0) vs. 0.0 (0.0–22.0); p < 0.0001].
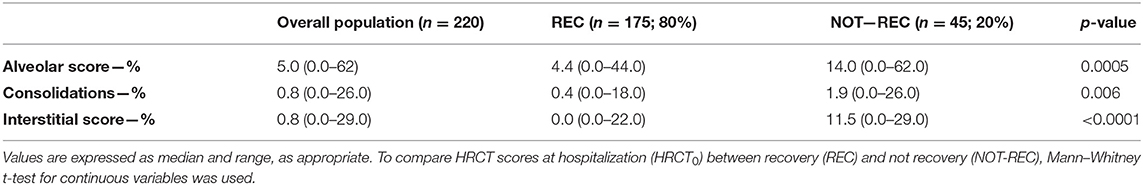
Table 2. HRCT scores during hospitalization (HRCT0) of the overall population evaluated at post-COVID clinic and of the two subgroups categorized according to the presence of radiological recovery during the follow-up period.
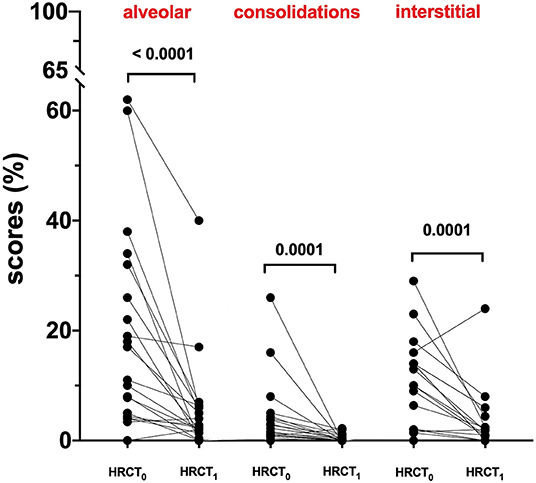
Figure 3. HRCT scores of the not recovery population (NOT-REC) from HRCT0 to HRCT1: ALV. [from 14 (0.0–62.0) to 2.6 (0.0–40.0); p < 0.0001], CONS [from 1.9 (0.0–26.0) to 0.0 (0.0–2.2); p = 0.0001] and INT [1.5 (0.0–29.0) to 1.4 (0.0–24.0)].
Prognostic Factors for Radiological Sequelae at Follow-Up
Univariate analysis showed that older age, a prolonged in-hospital stay, a lower PiO2/FiO2 at hospital admission, cardiovascular comorbidities, a higher degree of medical care, a higher FiO2 max, and higher ALV, CONS, and INT scores at HRCT0, not use of hydroxychloroquine, antibiotics other than azithromycin and ceftriaxone, tocilizumab, remdesevir, and systemic steroids are associated with persistent radiological abnormalities at follow-up. Multivariate analysis revealed that CONS [OR: 20.6 (95%CI: 1. −301.2); p = 0.02] and IS score [23.0 (1.4–377.2); p = 0.02] are independent predictors of radiological sequelae at follow-up (Table 3).
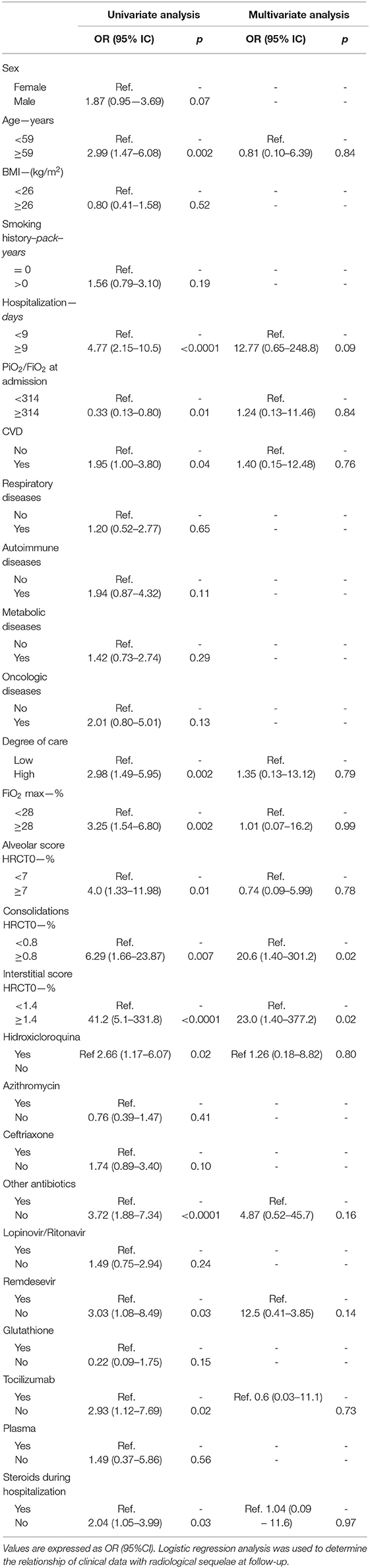
Table 3. Predictive factors of radiological sequelae at follow-up in patients hospitalized for SARS-COV-2-related pneumonia.
Finally, on multivariate analysis adjusted for gender, pack-years, PiO2/FiO2 ratio at admission, degree of care (high or low), and FiO2 max, both CONS and IS at HRCT0 are independent predictors of radiological sequelae at follow-up with an OR of 14.87 (95% CI: 1.25–175.8; p = 0.03) and 28.9 (95% CI: 2.17–386.6; p = 0.01), respectively (Table 4).
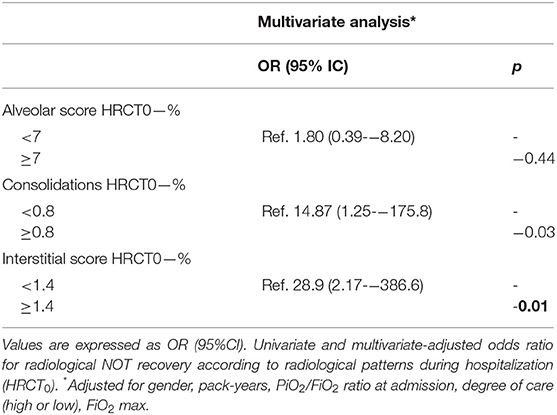
Table 4. Multivariate analysis for factors independently associated with radiological sequelae at follow-up in patients hospitalized for SARS-COV-2-related pneumonia.
Discussion
In our study, we demonstrated that only a significant minority of patients hospitalized for COVID-19 pneumonia has persistent radiological abnormalities at follow-up. Patients who did not recover are mainly elder men, with a more severe gas exchange impairment at hospital admission and a more severe clinical course during hospitalization. Interestingly, the presence of reticulation and consolidation at admission was predictive of persistent interstitial changes at follow-up.
To date, few studies have reported on the follow-up of patients hospitalized for COVID-19 pneumonia (5, 6). Different approaches based on disease severity have been proposed with the aim to standardize patients' follow-up. Specifically, the British Thoracic Society guidelines for management of post-COVID-19 syndrome distinguished patients with severe pneumonia requiring intensive care from patients with mild-to-moderate pneumonia treated in a medical ward or at home (4). However, it is becoming increasingly clear that radiological changes following COVID-19 pneumonia do not resolve completely in a large minority of patients (5, 15). Some studies have started to use CT to assess the presence of long-term lung abnormalities. A recent work from the Chongqing University Three Gorges Hospital evaluated 41 patients and showed that in most patients, the chest CT lesions were no longer present at 7 months after discharge, whereas older patients with severe comorbidities were more prone to develop fibrosis. (16). From the Wuhan cohort, Han and colleagues investigated 114 patients with severe pneumonia according to the WHO criteria (12) and observed fibrotic changes in one-third of them at the 6-month follow-up. Of note, on multivariate analysis, they found that a higher baseline/initial CT lung involvement score (>18 in a score of 25) was independently associated with fibrotic-like changes in the lung (12). Huang and colleagues conducted a cohort study that included 353 patients who were enrolled between January and May 2020 who underwent HRCT at follow-up after discharge. They found that more than 50% of the patients had residual lung abnormalities. Moreover, they found that disease severity in the acute phase was independently associated with the percentage change of CT score in a multivariable analysis (17).
In our hospital, the first patients with COVID-19 pneumonia were admitted in February 2020 and were evaluated in the post-COVID clinic in June 2020. We enrolled prospectively patients diagnosed with COVID-19 pneumonia according to the WHO criteria. Two hundred and 20 patients were evaluated at 3 months after discharge and every 3 months thereafter, according to the current guidelines (8). We found that as many as 20% of our entire patient population had radiological pulmonary sequelae at follow-up. This percentage is lower than that observed in previous studies (12, 17), but our patients' population has been followed up for a longer period of time, thus allowing non-fibrotic pulmonary abnormalities to clear. Patients who did not recover (NOT-REC) were older, mostly men and with worse disease impairment both at admission and during hospitalization compared to patients without radiological sequelae at follow-up. Specifically, NOT-REC patients had a lower PiO2/FiO2 ratio at admission and a more severe clinical course. Moreover, NOT-REC patients who required higher maximal FiO2 during hospital stay were more often treated in a high-intensive care setting and required a longer in-hospital stay, consistent with the findings from the Wuhan cohort (17). Furthermore, we have shown that, in NOT-REC patients, the HRCT performed at hospital admission is more likely to display ground-glass opacities, consolidations, and reticulation. These data suggest that the risk of pulmonary sequelae may be related to the severity of the acute illness and to the intensity of care needed. This is in line with the hypothesis that a cytokine storm might contribute to the pathogenesis of COVID-19 whereas its severity is associated with poor outcomes (18). However, mechanical ventilation and ventilator-induced lung injury, and high-flow oxygen therapy might also have contributed to the development of fibrotic-like changes (19, 20).
The primary aim of our study was to identify predictors of radiological sequelae following COVID-19 pneumonia. Whereas on univariate analysis age, prolonged in-hospital stay, lower PiO2/FiO2 at hospital admission, cardiovascular comorbidities, higher intensity of medical care, and higher FiO2 max, not using hydroxychloroquine, antibiotics other than azithromycin and ceftriaxone, tocilizumab, remdesevir, or systemic steroids were significantly associated with the presence of interstitial changes during follow-up, we found that higher CONS [OR: 20.6 (95%CI: 1.4–301.2); p = 0.02] and IS [23.0 (1.4–377.2); p = 0.02] at hospitalization were the only variables independently associated with the persistence of fibrotic changes at follow-up in multivariate analysis. In particular, this latter observation is consistent with that of Han and colleagues who found that a more extensive baseline or initial CT lung involvement was independently associated with permanent fibrotic-like changes in the lung (12). Additionally, the higher amount of consolidation and reticulation at admission remained significantly associated with persistent radiological abnormalities when adjusted for gender, pack-years of smoking, and PiO2/FiO2 ratio. However, it remains uncertain whether the fibrotic-like changes we observed represent irreversible pulmonary fibrosis, and further monitoring is warranted to answer this question.
The findings of our study should be interpreted in light of some limitations. First, this is a single-center study; however, it is among the first to analyze HRCT changes over time in a large population of patients hospitalized for COVID-19 pneumonia. In addition, we included a large proportion of patients with severe COVID-19, who are at higher risk of developing persistent lung disease. Second, the CT scan at hospital admission was available for only a subset of patients; however, the aim of our study was to characterize the radiological changes occurring over time as previously done in idiopathic pulmonary fibrosis (21) and to identify predictors of persistent radiological abnormalities.
In conclusion, in our study, about 20% of patients with COVID-19 pneumonia had radiological sequelae at follow-up. Patients who did not fully recover showed a more severe impairment at hospital admission and during hospitalization. Moreover, the presence of reticulation and consolidation on the initial chest CT is predictive of persistent radiological interstitial changes at follow-up.
Data Availability Statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics Statement
The studies involving human participants were reviewed and approved by Ethics Committee of the University Hospital of Padova, via Niccolò Giustiniani n.2, 35128 Padova (nr.: 46430/03.08.2020). The patients/participants provided their written informed consent to participate in this study.
Author Contributions
EB, EC, and PS contributed in conceptualization, writing, reviewing and editing, and supervision. EB and EC performed writing original draft—preparation, visualization, and investigation. EC, NB, CG, GC, and AG provided resources and conducted investigation. SP, GC, DL, and AF performed data curation. EB, PS, AC, and MS contributed in resources, visualization, and supervision. All authors have written, read, and approved the final version of the manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Dr. Sara Lococo (Department of Cardiac, Thoracic, Vascular Sciences, and Public Health, University of Padova and Padova City Hospital, Padova, Italy) for data collection.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2021.823600/full#supplementary-material
Abbreviations
IPF, idiopathic pulmonary fibrosis; FVC, forced vital capacity; HRCT, high-resolution computed tomography; REC, recovered; NOT-REC, not recovered; AS, alveolar score; CONS, consolidations; IS, interstitial score; COVID-19, Coronavirus disease 2019; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2; RT-PCR, real- time polymerase chain reaction; LIMC, low-intensity medical care; HIMC, high-intensity medical care; BMI, body mass index; CVDs, cardiovascular diseases; TLC, total lung capacity; DLCO, diffusing capacity of the lung for carbon monoxide.
References
1. Ranard BL, Megjhani M, Terilli K, Doyle K, Claassen J, Pinsky MR, et al. Identification of endotypes of hospitalized COVID-19 patients. Front Med. (2021) 8:770343. doi: 10.3389/fmed.2021.770343
2. Baratella E, Bussani R, Zanconati F, Marrocchio C, Fabiola G, Braga L, et al. Radiological-pathological signatures of patients with COVID-19-related pneumomediastinum: is there a role for the Sonic hedgehog and Wnt5a pathways? ERJ Open Res. (2021) 7:00346–2021. doi: 10.1183/23120541.00346-2021
3. Antonio GE, Wong KT, Hui DS, Wu A, Lee N, Yuen EH, et al. Thin-section CT in patients with severe acute respiratory syndrome following hospital discharge: preliminary experience. Radiology. (2003) 228:810–5. doi: 10.1148/radiol.2283030726
4. Das KM, Lee EY, Singh R, Enani MA, Al Dossari K, Van Gorkom K, et al. Follow-up chest radiographic findings in patients with MERS-CoV after recovery. Indian J Radiol Imaging. 27:342–9. doi: 10.4103/ijri.IJRI_469_16
5. Baratella E, Ruaro B, Marrocchio C, Starvaggi N, Salton F, Giudici F, et al. Interstitial lung disease at high resolution CT after SARS-CoV-2-related acute respiratory distress syndrome according to pulmonary segmental anatomy. J Clin Med. (2021) 10:3985. doi: 10.3390/jcm10173985
6. Korkmaz I, Keleş F. COVID-19-Related lung involvement at different time intervals: evaluation of computed tomography images with semiquantitative scoring system and COVID-19 reporting and data system scoring. Cureus. (2021) 13:e18554. doi: 10.7759/cureus.18554
7. Greenhalgh T, Knight M, A'Court C, Buxton M, Husain L. Management of post-acute covid-19 in primary care. BMJ. (2020) 370:m3026. doi: 10.1136/bmj.m3026
8. British, Thoracic Society. Guidance on Respiratory Follow Up of Patients with a Clinico-Radiological Diagnosis of COVID-19 Pneumonia. Available online at: https://britthoracic.org.uk/about-us/covid-19-information-forthe-respiratory-community/. (accessed: May 7, 2021).
9. George PM, Barratt SL, Condliffe R, Desai SR, Devaraj A, Forrest I, et al. Respiratory follow-up of patients with COVID-19 pneumonia. Thorax. (2020) 75:1009–16. doi: 10.1136/thoraxjnl-2020-215314
10. Fichera G, Stramare R, De Conti G, Motta R, Giraudo C. It's not over until it's over: the chameleonic behavior of COVID-19 over a six-day period. Radiol Med. (2020) 125:514–6. doi: 10.1007/s11547-020-01203-0
11. Kwee TC, Kwee RM. Chest CT in COVID-19: what the radiologist needs to know. Radiographics. (2020) 40:1848–65. doi: 10.1148/rg.2020200159
12. Han X, Fan Y, Alwalid O, Li N, Jia X, Yuan M, et al. Six-month follow-up Chest CT Findings after severe COVID-19 pneumonia. Radiology. (2021) 299:E177–86. doi: 10.1148/radiol.2021203153
13. WHO. Clinical Management of Severe Acute Respiratory Infection When Novel Coronavirus (nCoV) Infection is Suspected: Interim Guidance. (2020). Available online at: https://www.who.int/docs/default-source/coronaviruse/clinical-management-of-novel-cov.pdf (accessec March 13, 2020).
14. Cocconcelli E, Biondini D, Giraudo C, Lococo S, Bernardinello N, Fichera G, et al. Clinical features and chest imaging as predictors of intensity of care in patients with COVID-19. J Clin Med. (2020) 9:2990. doi: 10.3390/jcm9092990
15. Spagnolo P, Balestro E, Aliberti S, Cocconcelli E, Biondini D, Casa GD, et al. Pulmonary fibrosis secondary to COVID-19: a call to arms? Lancet Respir Med. (2020) 8:750–752. doi: 10.1016/S2213-2600(20)30222-8
16. Liu M, Lv F, Huang Y, Xiao K. Follow-up study of the chest CT characteristics of Covid-19 survivors seven months after recovery. Front Med (Lausanne). (2021) 8:636298. doi: 10.3389/fmed.2021.636298
17. Huang C, Huang L, Wang Y, Li X, Ren L, Gu X, et al. 6-month consequences of COVID-19 in patients discharged from hospital: a cohort study. Lancet. (2021) 397:220–32. doi: 10.1016/S0140-6736(20)32656-8
18. Fajgenbaum DC, June CH. Cytokine Storm. N Engl J Med. (2020) 383:2255–73. doi: 10.1056/NEJMra2026131
19. Beitler JR, Malhotra A, Thompson BT. Ventilator-induced Lung Injury. Clin Chest Med. (2016) 37:633–46. doi: 10.1016/j.ccm.2016.07.004
20. Bates JH, Smith BJ, Allen GB. Computational models of ventilator induced lung injury and surfactant dysfunction. Drug Discov Today Dis Models. (2015) 15:17–22. doi: 10.1016/j.ddmod.2014.02.005
Keywords: SARS-CoV-2, coronavirus disease 2019, pulmonary fibrosis, high-resolution computed tomography, pulmonary sequelae
Citation: Cocconcelli E, Bernardinello N, Giraudo C, Castelli G, Giorgino A, Leoni D, Petrarulo S, Ferrari A, Saetta M, Cattelan A, Spagnolo P and Balestro E (2022) Characteristics and Prognostic Factors of Pulmonary Fibrosis After COVID-19 Pneumonia. Front. Med. 8:823600. doi: 10.3389/fmed.2021.823600
Received: 27 November 2021; Accepted: 28 December 2021;
Published: 31 January 2022.
Edited by:
Laurent Pierre Nicod, University of Lausanne, SwitzerlandReviewed by:
Barbara Ruaro, University of Trieste, ItalyAurelien Justet, Centre Hospitalier Universitaire de Caen, France
Copyright © 2022 Cocconcelli, Bernardinello, Giraudo, Castelli, Giorgino, Leoni, Petrarulo, Ferrari, Saetta, Cattelan, Spagnolo and Balestro. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Elisabetta Balestro, elisabetta.balestro@aopd.veneto.it
†These authors share first authorship
 Elisabetta Cocconcelli
Elisabetta Cocconcelli Nicol Bernardinello
Nicol Bernardinello Chiara Giraudo
Chiara Giraudo Gioele Castelli1
Gioele Castelli1  Simone Petrarulo
Simone Petrarulo Annamaria Cattelan
Annamaria Cattelan Paolo Spagnolo
Paolo Spagnolo Elisabetta Balestro
Elisabetta Balestro