Photodynamic therapy for skin carcinomas: A systematic review and meta-analysis
- 1Department of Information, Affiliated Cancer Hospital and Institute of Guangzhou Medical University, Guangzhou, China
- 2Guangdong Nuohui Hospital Management LLC, Guangzhou, China
- 3Department of Science and Technology, University of Canberra, Bruce, ACT, Australia
Background: Photodynamic therapy (PDT) is increasingly used for the treatment of basal cell carcinoma (BCC) and squamous cell carcinoma (SCC). However, it is unknown whether photodynamic therapy is more effective than other commonly used treatment modalities for these cancers.
Purpose: The aim of this study was to determine the relative efficacy and safety of PDT compared with placebo or other interventions for the treatment of skin carcinomas.
Methods: Searches were performed in PubMed, Web of Science, Embase, and the Cochrane Central Register of Controlled Trials databases. We included randomized controlled trials comparing the PDT with other interventions in adults skin BCC or SCC that reported on lesion response, recurrence, cosmetic appearance, or safety outcomes.
Results: Seventeen unique randomized controlled trials, representing 22 study arms from 21 publications were included. The included trials included 2,166 participants, comparing methyl aminolevulinic (MAL) PDT (six studies) or aminolevulinic acid (ALA) PDT (two studies). Comparators included placebo, surgery, hexaminolevulinic (HAL) PDT, erbium: yttrium-aluminum-garnet ablative factional laser (YAG-AFL) PDT, fluorouracil, and imiquimod. There were few studies available for each comparison. Mantel-Haenszel fixed effects risk ratios were calculated for response, recurrence, cosmetic outcomes, and adverse events. MAL-PDT had similar response rates to surgery, ALA-PDT, fluorouracil and imiquimod at 3- and 12 months post-intervention. The rate of recurrence was similar, showing few differences at 12 months, but at later time points (24–60 months), fewer lesions recurred with surgery and imiquimod than with PDT. PDT also caused more adverse events and pain than other interventions. However, PDT treatment was more likely to receive a “good” or “excellent” rating for cosmetic appearance than surgery or cryotherapy.
Conclusion: This systematic review and meta-analysis demonstrates that the choice of treatment modality for BCC or SCC is best chosen in the context of the location and size of the lesion, the socioeconomic circumstances of the patient, as well as the patient’s preferences. We call for more high quality studies to be done, in order to enable more reliable interpretations of the data.
Systematic review registration: https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=368626, identifier CRD42022368626.
Introduction
Non-melanoma skin cancers are the most commonly occurring cancer in Caucasian populations, and the incidence of both basal and squamous cell carcinomas in Europe have been increasing (1). From 1969 to 2000, 61% of the deaths associated with non-melanoma skin cancer occurred as a result of primary tumors arising on non-genital skin, and the age-adjusted mortality rate among men and women with non-genital non-melanoma skin cancer was 0.69 and 0.30 deaths per 105 at-risk individuals per year, respectively (2). For genital non-melanoma skin cancers, the rate was 0.30 per 105 at-risk individuals per year for men and 0.54 per 105 at-risk individuals per year for women (2).
The conventional treatments for non-melanoma skin cancers include surgical procedures, radiation, cryotherapy, fluorouracil, and imiquimod. Although surgery is the most common treatment for basal and squamous cell carcinomas, other less invasive methods such as fluorouracil or cryotherapy are sometimes used. Radiation may be considered as treatment for patients in whom surgery is contraindicated (3). Imiquimod has commonly been used in the treatment of various forms of basal cell carcinoma, such as nodular basal cell carcinoma and sclerodermiform basal cell carcinoma, and for various forms of squamous cell carcinoma, such as Bowen’s disease and keratoacanthoma (4, 5). Although the current treatments are effective to varying degrees, they tend to lack specificity and often do not target the tumor itself or the environment in which it exists (6). They are also associated with a high incidence of adverse effects and yield undesirable cosmetic results (7, 8). As such, alternative treatments options for patients with these conditions are needed.
Photodynamic therapy represents a safe alternative for treatment of these conditions. Photodynamic therapy uses a source of visible light to activate a photosensitizing agent (commonly aminolaevulinic acid or methyl aminolevulinic acid) applied on the skin, which releases reactive oxygen species that destroy the lesions (9). The safety and efficacy profile of photodynamic therapy in treating basal cell carcinoma and squamous cell carcinoma is well known (5). However, the relative efficacy of different modes of treatment for basal cell or squamous cell carcinomas is unknown. As such, we undertook a systematic review and meta-analysis of photodynamic therapies compared with each other, placebo, surgery, cryotherapy, imiquimod, or fluorouracil.
Materials and methods
This systematic review and meta-analysis was performed according to the guidelines given in the Preferred Reporting Items for Systematic Review and Meta-analyses (PRISMA) (10). The protocol for this review was registered in the International Prospective Register of Systematic Review (PROSPERO) under the registration number CRD42022368626.
Search strategy
We searched PubMed, Web of Science, Embase, and the Cochrane Library from inception to October 10, 2022. We had no limitations on the date of publication or language. The search strategy used for PubMed is given in Supplementary Table 1 and was altered for use in the other databases. The citations of included studies were searched manually to identify studies that were not returned using the search strategies.
Inclusion/exclusion criteria
Studies were included if they met the follow criteria: randomized, controlled trial (RCT) in patients with basal cell carcinoma (BCC) or squamous cell carcinoma (SCC), who were treated with any form of photodynamic therapy (PDT), compared with another form of PDT, other therapies or placebo, followed for a period of at least 3 months in an outpatient setting. At least one of the following outcomes must have been reported: treatment success, cosmetic acceptability, pain, or adverse events.
Studies were excluded if the trial was not an RCT, the skin cancers were not BCC or SCC, if the treatments did not include PDT, if the treatment duration was too short, or if none of the required outcomes was reported. Comparisons of different protocols within a single technology (e.g., cycle number, wavelength, light source comparisons within MAL-PDT) were excluded, as were trials of recurrent cancers, and protocols that involved substantial debulking of the tumors prior to treatment. Trials adding PDT to other treatments were also excluded.
Inclusion and exclusion at the title and abstract level were carried out by two authors (YY and YZ) independently. In the case of disagreements between two authors, the article was discussed between the authors and a consensus decision was taken. After inclusion of potentially relevant abstracts, full texts of the articles were obtained and subjected to the inclusion and exclusion criteria independently by two authors (YY and YZ) using. Disagreements were resolved by consensus. Data collection was performed by one author (YY) using electronic data collection forms and extracted data was then cross checked with the articles by a second author (KM) to ensure the accuracy of the information.
Quality assessment
The risk of bias of the included studies was carried out using the Cochrane Collaboration’s tool for assessing risk of bias in randomized trials (11). The risk of bias was assessed over seven domains: sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting bias, and other bias. The quality assessment was carried out by YY and checked by KM. Conflicts were resolved by consensus.
Data extraction and data synthesis
The study characteristics and outcome data were extracted into a pre-designed Excel spreadsheet by YZ and checked by KM. Where data were reported by both the clinician and the patient (e.g., cosmetic appearance), we extracted the patient-reported data. Response rates were calculated at the end of the treatment period, regardless of the number of treatments each group received. If data were available at the patient level, we extracted this; if data were only available at the lesion level, we extracted lesion-level data.
All outcomes were reported as dichotomous data; we therefore calculated Mantel-Haenszel risk ratios with 95% confidence intervals using a random effects model. A random effects model was chosen as we expected the heterogeneity between studies to arise mostly by factors other than chance, such as differences in carcinoma size, location, or depth, treatment protocols, and differences in the populations. Where a study reported on more than two arms and both were used in a single analysis, the arms were separated into subgroups, and the overall meta-analyzed result was removed from the analysis.
Subgroup analysis was used to differentiate between the location of the lesion. Meta-regression was undertaken using type of carcinoma (SCC or BCC) and comparators as covariates. Meta-regression was carried out using OpenMetaAnalyst (12) on outcomes that had nine or more study arms comparing any type of PDT with a non-PDT comparator.
Results
Studies
The database searches identified 621 citations, of which 123 were duplicates (Figure 1). The remaining 498 citations were subjected to inclusion and exclusion at the abstract level. This resulted in the exclusion of 412 abstracts. The remaining full texts for the remaining 86 abstracts were sourced and subjected to the inclusion and exclusion criteria. Of these, 21 publications reporting on 22 study arms from 17 individual RCTs were included (13–33).
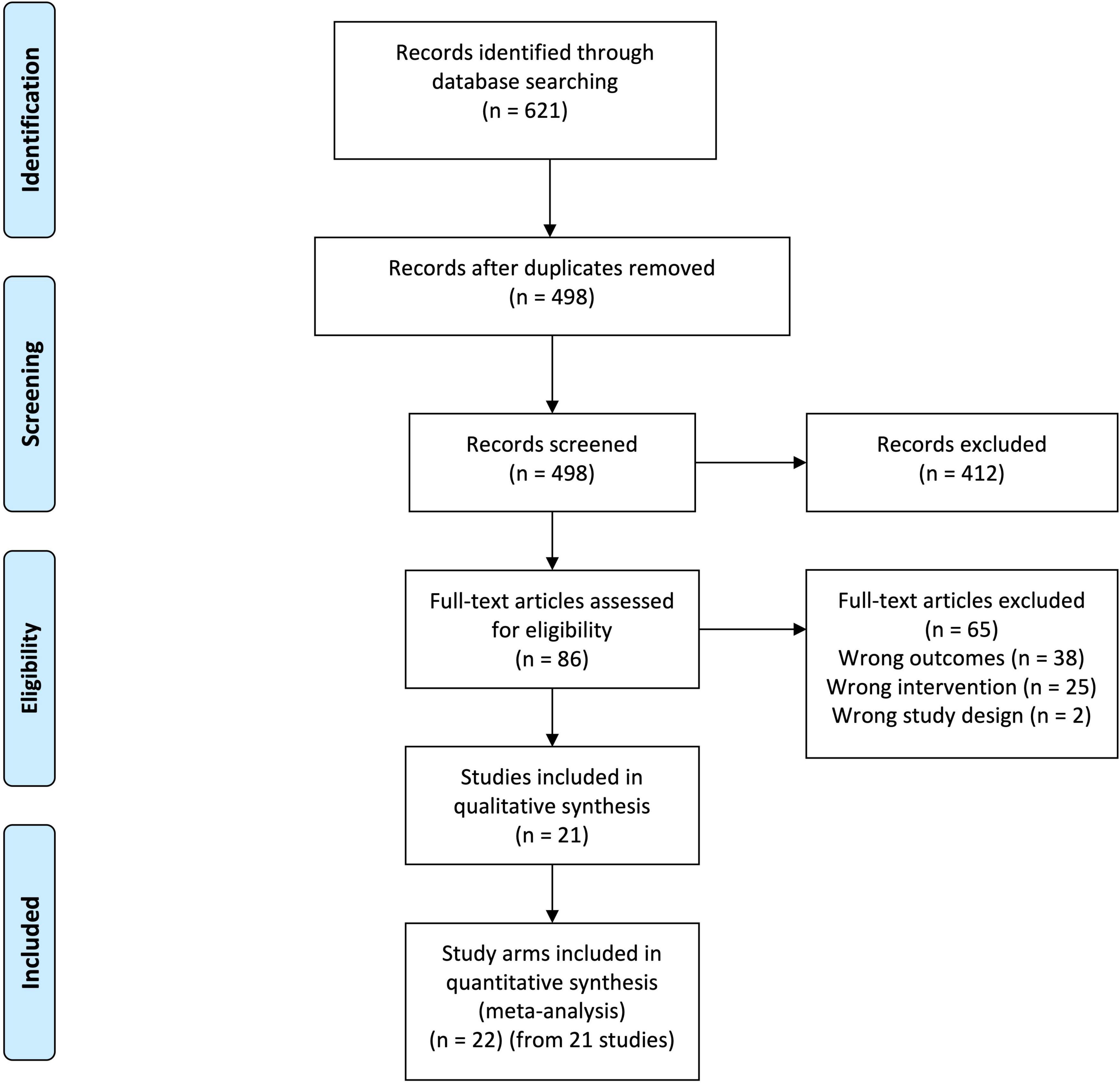
Figure 1. Preferred reporting items for systematic review and meta-analyses (PRISMA) diagram. From 621 records identified from database searching, 123 were duplicates. Screening of these records at the title and abstract level resulted in the exclusion of 412 records. The 86 remaining records were obtained as full texts and submitted to inclusion and exclusion. This resulted in the exclusion of 65 articles. Twenty-one articles representing 22 study arms from 17 separate randomized controlled trials were included in the final analysis.
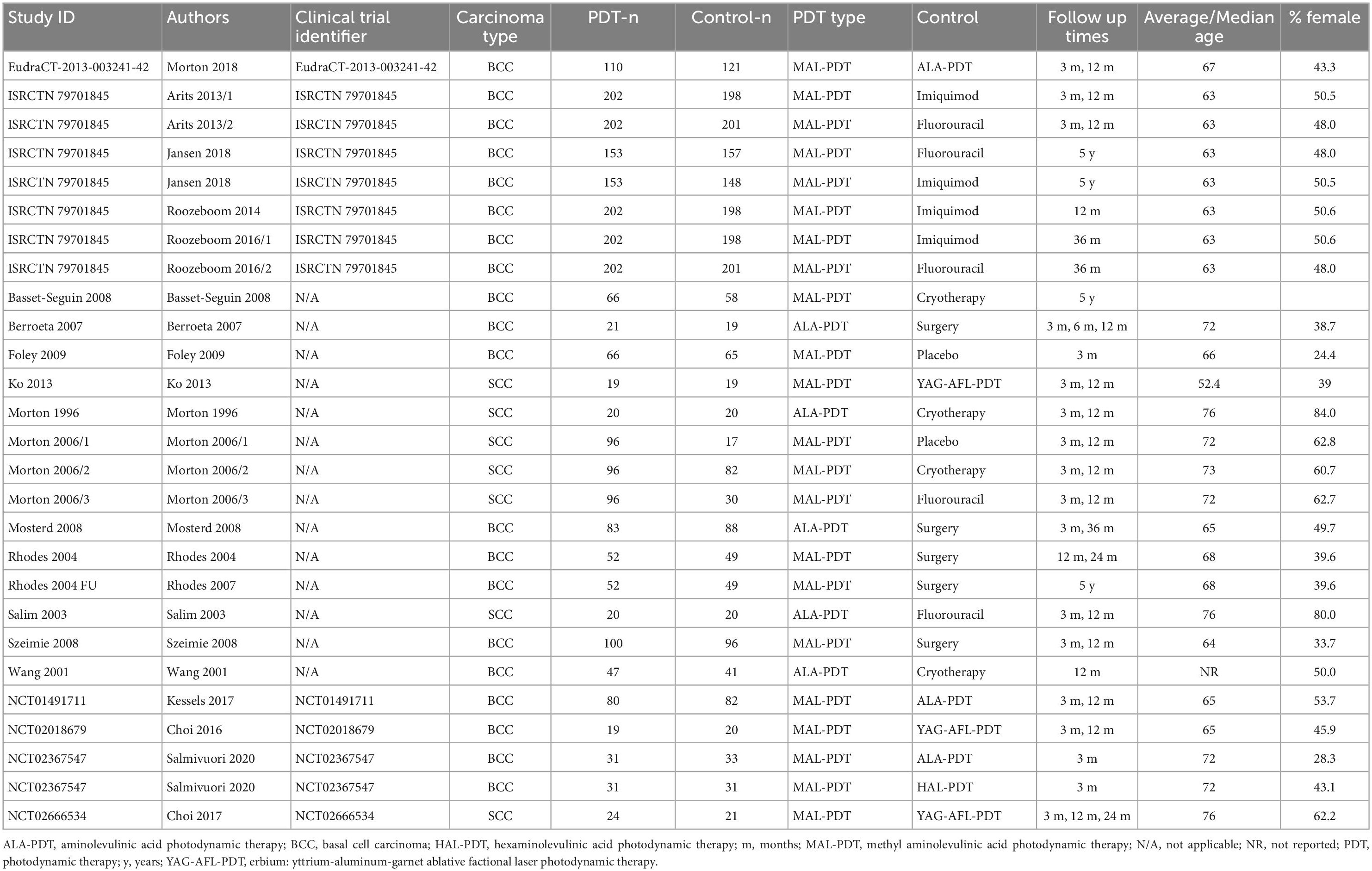
The included studies involved 2,166 participants (Table 1). Twelve trials were in people with basal cell carcinomas, and five trials involved people with squamous cell carcinomas. Most studies used methyl aminolevulinic acid (MAL) as the photosensitizing agent in PDT. These studies compared MAL-PDT with aminolevulinic acid (ALA) PDT, erbium: yttrium-aluminum-garnet ablative fractional laser-assisted (YAG-AFL) PDT, hexaminolevulinic (HAL) PDT, surgical excision, cryotherapy, imiquimod, fluorouracil, and placebo. Five studies compared ALA-PDT with surgical excision, cryotherapy, and fluorouracil. The mean age of the participants was 67.3 years, and on average 49.7% of participants were female.
Study quality as determined by the Cochrane Collaboration’s tool for the assessment of risk of bias in interventions studies was generally good (Supplementary Figure 1). However, due to the nature of the interventions, it was often impossible to blind the participants to their intervention. To compensate for this, the outcome assessors were frequently blinded to the allocation of the patients they assessed. Five of the clinical trials were funded by companies with a financial interest in the outcomes; these trials usually had at least one company employee on the author list of the publication(s).
Response
All clinical trials reported on response at 3 months post-treatment (Figure 2). MAL-PDT was significantly superior to placebo (two studies; RR: 3.00 (95% CI: 2.05 to 4.39); P < 0.00001), but statistically less effective than surgery (one study) and YAG-AFL-PDT (three studies). No other comparisons were statistically significant.
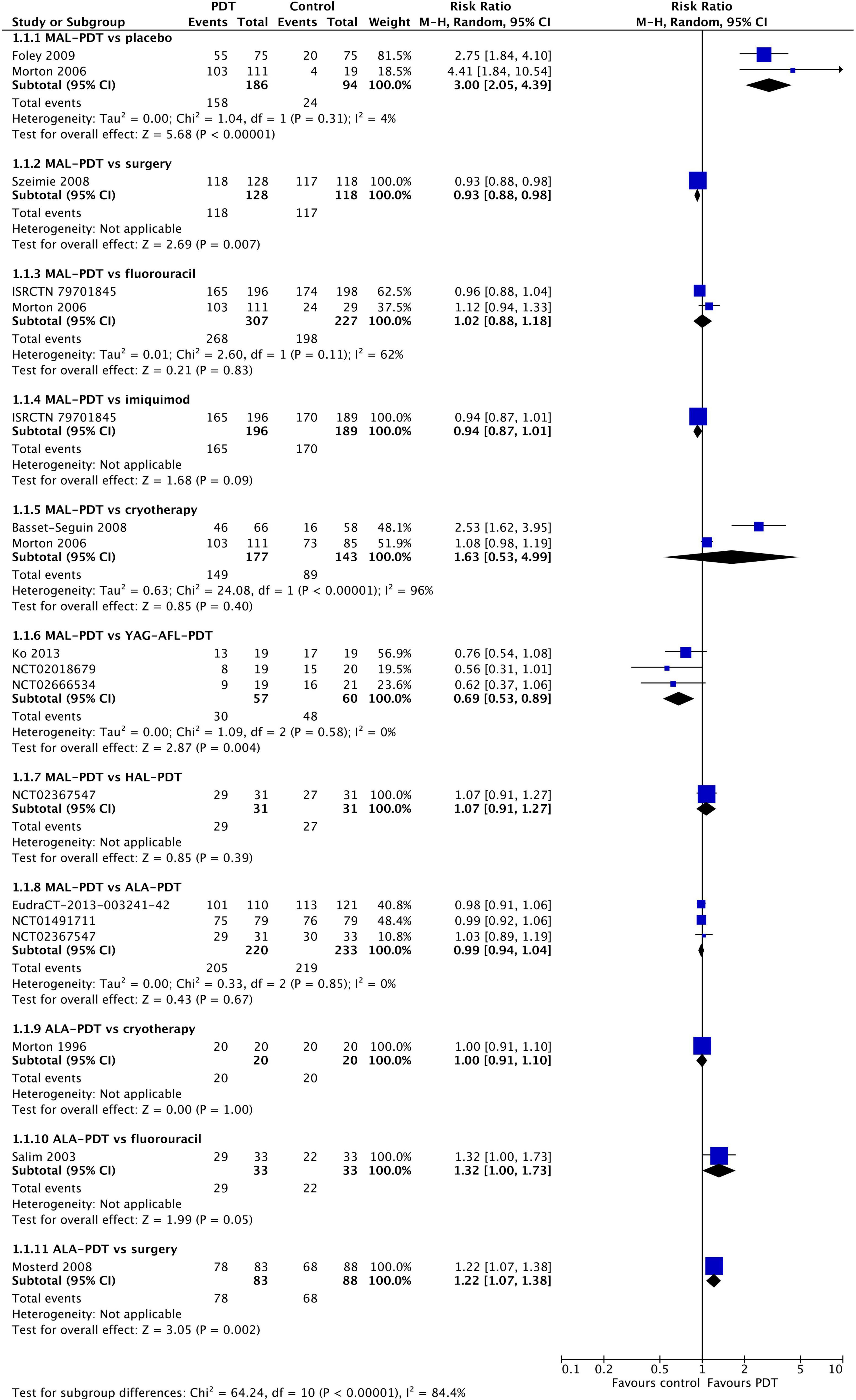
Figure 2. Meta-analysis of the risk of response at 3 months post-treatment by comparator. Data are risk ratios with 95% confidence intervals.
At 12 months post-treatment, similar results were observed (Figure 3). MAL-PDT was less effective than surgery (one study) and YAG-AFL-PDT (three studies). At 24–60 months post-intervention (Supplementary Figure 2), MAL-PDT was statistically inferior to surgery and YAG-AFL-PDT at 24 m (one study), and imiquimod at 36 m (one study).
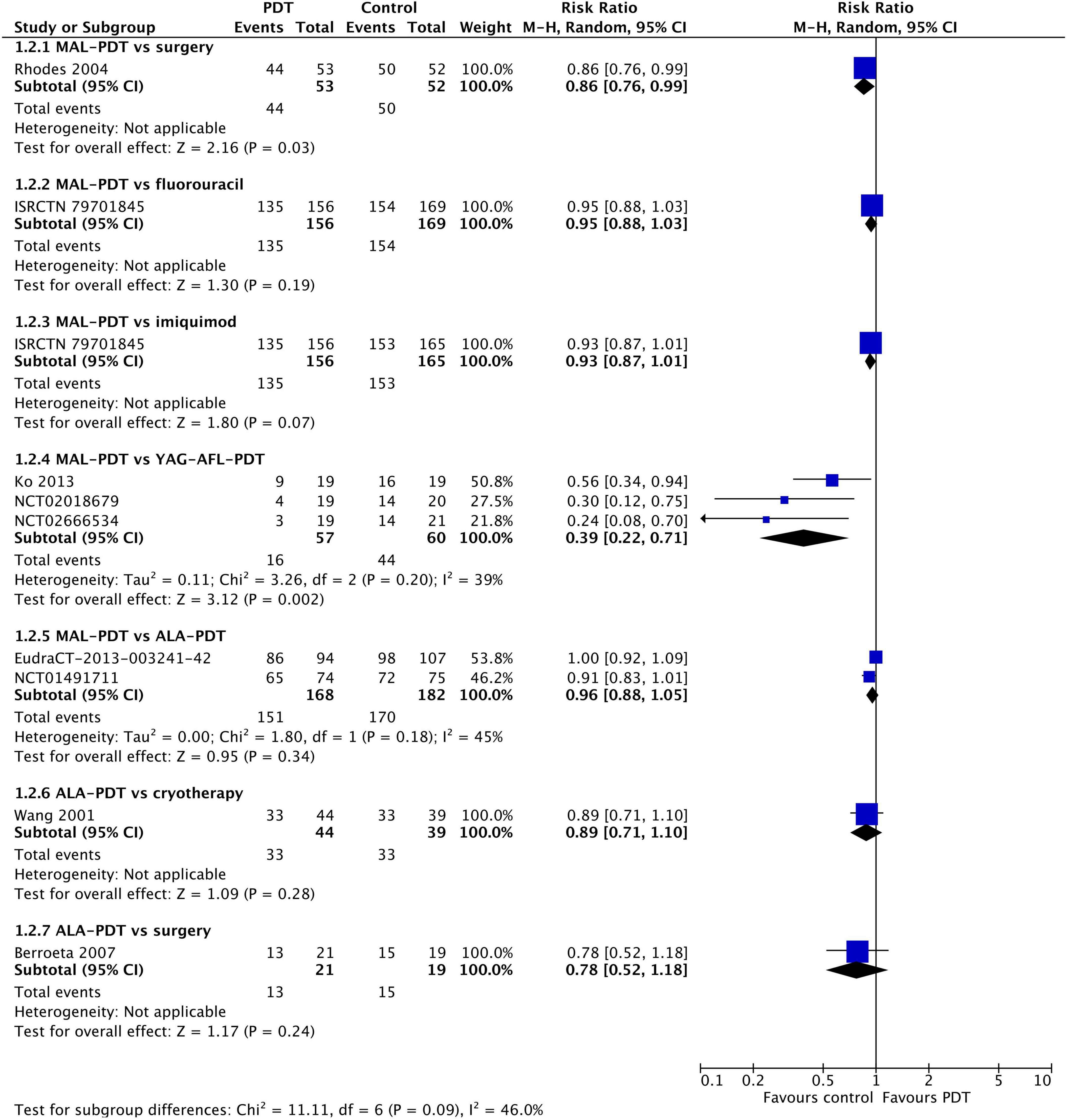
Figure 3. Meta-analysis of the risk of response at 12 months post-treatment by comparator. Data are risk ratios with 95% confidence intervals.
Analyses by location of the original lesion found few differences at 3 months (Supplementary Figure 3). MAL-PDT was more effective than placebo on the extremities, but less effective than surgery on the neck/trunk. At 12 months, imiquimod was statistically superior to MAL-PDT for lesions on the trunk, whereas MAL-PDT was superior to imiquimod for lesions on the extremities (Supplementary Figure 4). However, each of these findings resulted from a single trial, so interpretations should be made with caution.
Meta-regression of all active control studies revealed no significant differences between imiquimod, cryotherapy, fluorouracil, and surgery (Supplementary Table 2). We therefore undertook a meta-regression of all active control studies by type of carcinoma (Supplementary Table 3). There were no significant differences between basal cell and squamous cell carcinomas for this outcome, suggesting that the treatments are similarly effective for both carcinoma types.
Recurrence
At 12 months post-intervention, MAL-PDT was more effective than placebo (one study), but YAG-AFL-PDT was more effective than MAL-PDT (three studies; Figure 4). The risk of recurrence after MAL-PDT was 5.66 times (95% CI: 2.38, 13.46) that of YAG-AFL-PDT (P < 0.00001). At 24 months, this increased risk of recurrence remained (one study per comparison; Figure 5). In addition, both MAL-PDT and ALA-PDT were inferior to surgery at 24- and 36-month post-intervention, respectively, and MAL-PDT was inferior to YAG-AFL-PDT and imiquimod at 24- and 36-month post-intervention, however there was only one study per comparison, so these results should be interpreted with caution.

Figure 4. Meta-analysis of the risk of recurrence at 12 months post-treatment by comparator. Data are risk ratios with 95% confidence intervals.
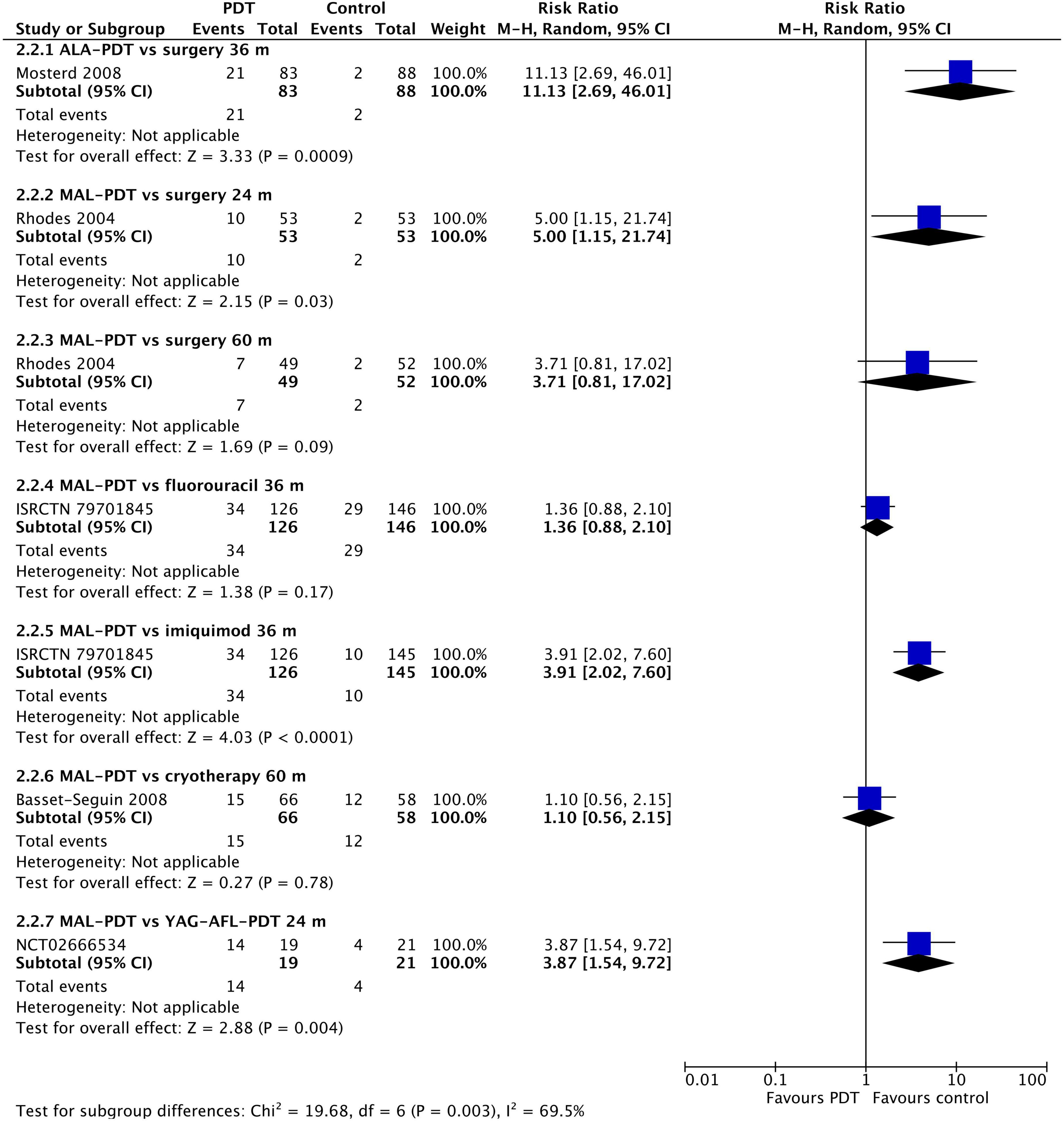
Figure 5. Meta-analysis of the risk of recurrence at 24 to 60 months post-treatment by comparator. Data are risk ratios with 95% confidence intervals.
Analyses by location of the original lesion showed few differences (Supplementary Figure 5). MAL-PDT was superior to cryotherapy on the face/scalp, superior to placebo on the neck/trunk, and superior to imiquimod on the extremities. In contrast, imiquimod was significantly less likely than MAL-PDT to result in lesion recurrence on the body trunk. However, each of these findings resulted from a single trial, so interpretations should be made with caution. There were too few studies to undertake meta-regression.
Cosmetic outcomes
After 3 months, there were no differences in the chance of good/excellent ratings for cosmetic appearance of lesions between MAL-PDT and ALA-PDT (two studies), HAL-PDT, imiquimod, fluorouracil, and placebo (one study per comparison; Figure 6A). The only exception to this was the comparison of MAL-PDT with surgery, which significantly favored MAL-PDT (two studies; RR: 1.12 (95% CI: 1.03, 1.22); p = 0.01). At 12 months, ALA-PDT showed superiority over cryotherapy (one study), and MAL-PDT was superior to surgery (two studies) and cryotherapy (one study) for this outcome (Figure 6B). There were too few studies to undertake meta-regression.
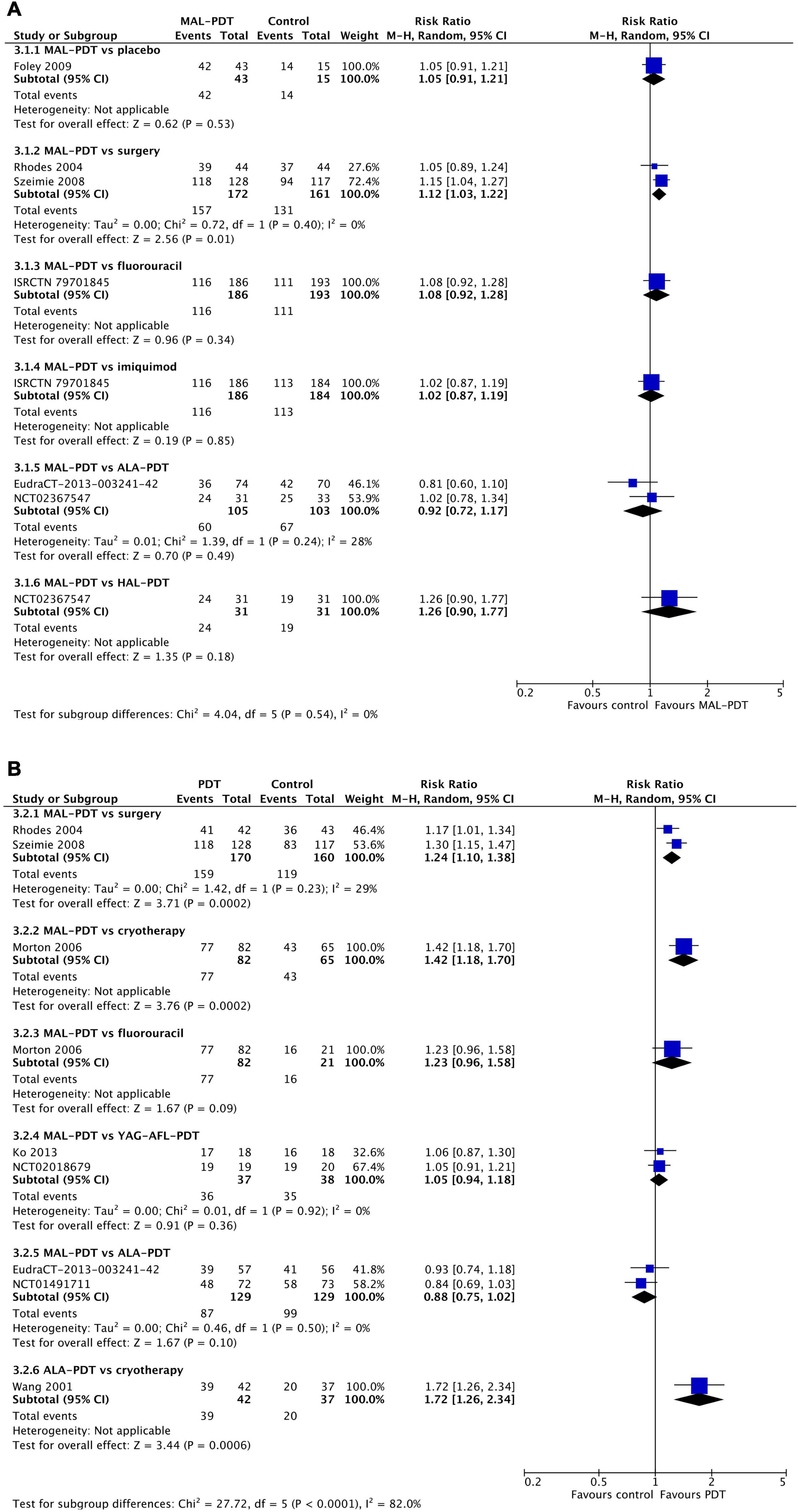
Figure 6. Meta-analysis of the risk of a cosmetic rating of “good” or “excellent” at 3 months (A) or 12 months (B) post-intervention by comparator. Data are risk ratios with 95% confidence intervals.
Adverse events
The rates of any adverse event were relatively high in all studies (Figure 7). There were no significant differences between the different forms of PDT (MAL vs. ALA (one study) and MAL vs. YAG-AFL (three studies)). However, MAL-PDT caused significantly more adverse events than surgery (two studies; RR: 2.12; 95% CI: 1.46 to 3.09; P < 0.0001).
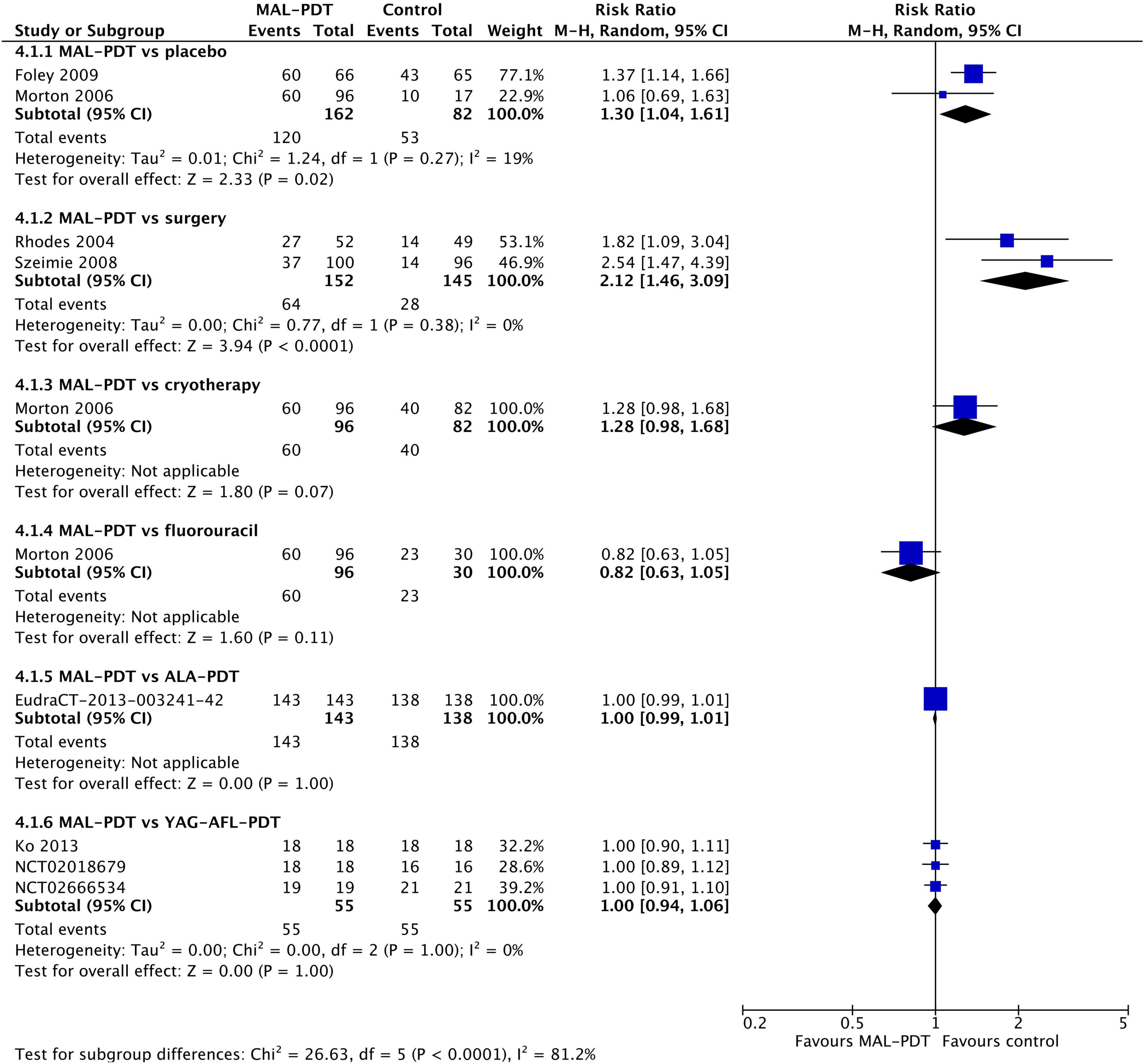
Figure 7. Meta-analysis of the risk of adverse events by comparator. Data are risk ratios with 95% confidence intervals.
The incidence of pain during the procedure was higher in people who had MAL-PDT than imiquimod, fluorouracil, or placebo (one study per comparison; Figure 8), and lower in those undergoing ALA-PDT than cryotherapy (one study). There was no difference in the incidence of pain between MAL-PDT and ALA-PDT (one study), between ALA-PDT and fluorouracil (one study), and between MAL-PDT and surgery (two studies). There were too few studies to undertake meta-regression. Peak pain severity showed a slightly different picture. The only statistically significant difference in pain intensity was between ALA-PDT and surgery (one study; Supplementary Figure 6).
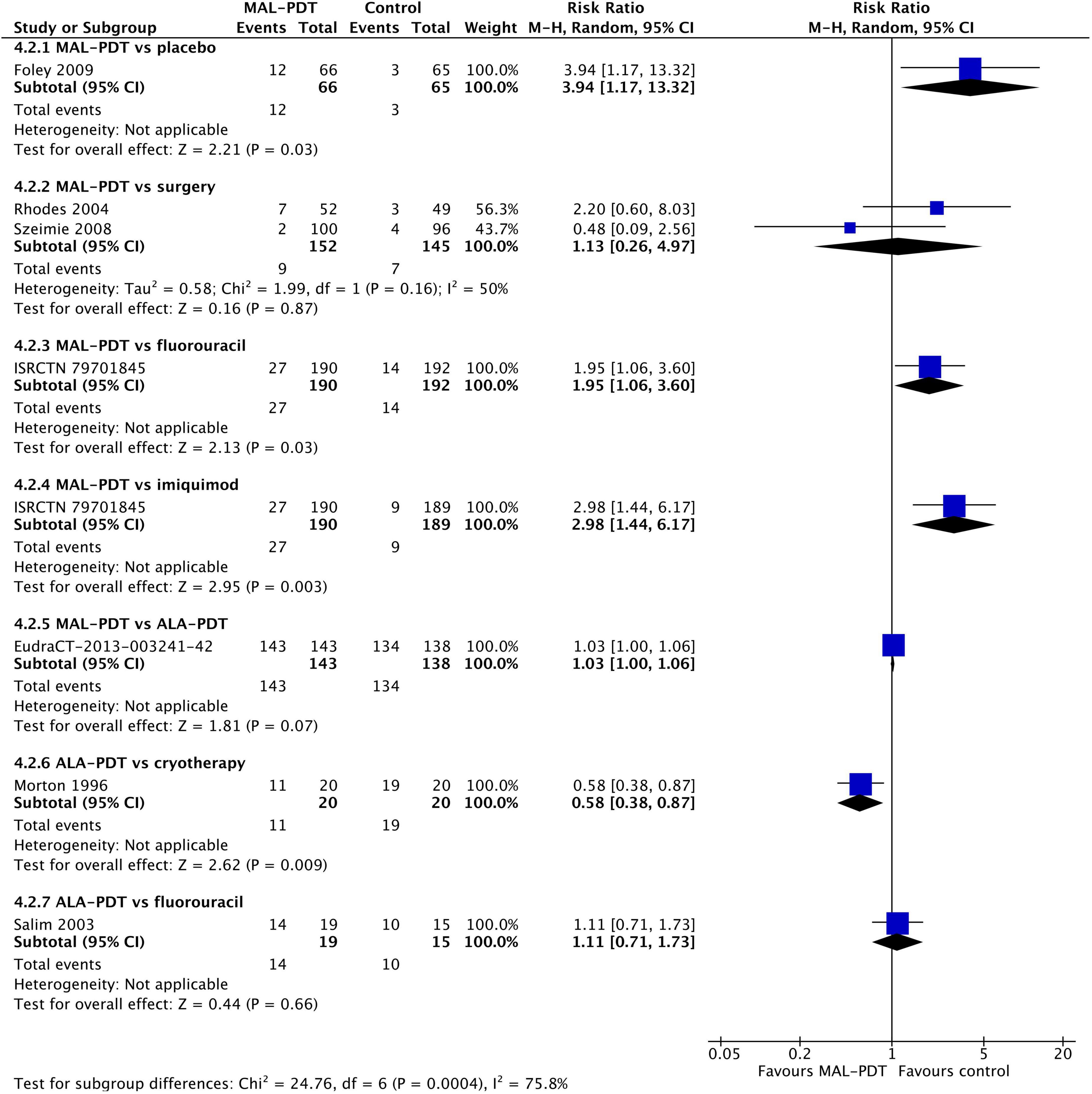
Figure 8. Meta-analysis of the risk of pain during the procedure by comparator. Data are risk ratios with 95% confidence intervals.
Publication bias
The potential for publication bias was examined by visual examination of funnel plots produced for response, recurrence, cosmetic outcomes, and adverse events (Supplementary Figure 7). All plots appeared symmetrical, with little obvious skew in data. We attempted to undertake an analysis of bias by comparing industry-funded with government/university funded studies (Supplementary Figure 8). We used studies reporting on response at 3 months for comparisons that showed a statistically significant advantage toward PDT in the overall meta-analysis. We found no statistically significant differences between the two subgroups, however, heterogeneity was extremely high (96 and 85% for industry-funded and non-industry-funded, respectively). This heterogeneity was likely a result of combing different control types.
Discussion
Our systematic review and meta-analysis demonstrates for the first time the evidence for the relative efficacy and safety of PDT compared with other potential treatments. We found that MAL-PDT was superior to placebo, and ALA-PDT was superior to surgery, but PDT, surgery, fluorouracil, and imiquimod have similar rates of response and recurrence over the short and medium term. However, over the longer term (24–60 m), PDT-treated lesions are significantly more likely to recur than those that were surgically resected, or those treated with imiquimod. PDT is also more likely to cause intra-procedural pain and adverse events. However, PDT is more likely to be rated “good” or “excellent” compared with cryotherapy and surgery. Given the small number of available studies, the choice of treatment for any particular lesion is best left to the discretion of the treating physician, taking into account the individual patient’s preferences.
Interestingly, differences between the photosensitizing agents used with PDT emerged. Whereas MAL-PDT was significantly less likely than surgery to result in response of a lesion at 3 months (32), ALA-DT was significantly more likely than surgery to result in response (25). Whether these differences are real and robust, however, is unclear, as Szeimie et al. (32) treated small superficial lesions, whereas Mosterd et al. (25) treated nodular BCCs. Furthermore, three head-to-head trials failed to show any differences between MAL-PDT and ALA-PDT (20, 24, 31). Intriguingly, the meta-analysis indicates that YAG-AFL-PDT may be superior to MAL-PDT for response and recurrence, without compromising on cosmetic outcomes or risk of adverse events (16, 17, 21). However, larger studies will be required to confirm this result.
Although cosmetic concerns are sometimes minimized or dismissed, they can have serious effects on patients. Patients with visible and unpleasant scarring can suffer from embarrassment, isolation and a modification of social activities (34), along with self-consciousness, unhappiness and insecurity (35). Nevertheless, excision, especially of melanomas and SCCs, does lead to an increase in quality of life, probably due to a reduction in the anxiety associated with a diagnosis of skin cancer (34). It is thus vital that the location of the lesion, the likely size and visibility of the resulting scar, and the personal circumstances, activities, and preferences of the individual patient are considered when choosing the treatment for any particular lesion.
Conclusion
Basal cell carcinoma and SCC lesions cause significant economic burdens for health organizations all around the world. This disease also leads to chronic discomfort and a reduction in quality of life for patients, as it can cause poor cosmetic effects. Finding an appropriate method for treatment of these lesions has remained a challenge as the number of treatment methods increases. Our results suggest that PDT is can be an effective method for treatment of these lesions. However, further studies are required before any strong recommendations can be made.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Author contributions
YO-Y and YZ did the database searching, citation management, and inclusion and exclusion of records. YZ did the data extraction, study quality assessment, which was checked by KM. KM did the meta-analysis and meta-regression in consultation with YO-Y and YZ. KM wrote the manuscript in consultation with YO-Y and YZ. All authors contributed to the article and approved the submitted version.
Conflict of interest
YZ was employed by Guangdong Nuohui Hospital Management LLC.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmed.2023.1089361/full#supplementary-material
References
1. Lomas A, Leonardi-Bee J, Bath-Hextall F. A systematic review of worldwide incidence of nonmelanoma skin cancer. Br J Dermatol. (2012) 166:1069–80. doi: 10.1111/j.1365-2133.2012.10830.x
2. Lewis KG, Weinstock MA. Trends in nonmelanoma skin cancer mortality rates in the United States, 1969 through 2000. J Invest Dermatol. (2007) 127:2323–7. doi: 10.1038/sj.jid.5700897
3. Marzuka AG, Book SE. Basal cell carcinoma: pathogenesis, epidemiology, clinical features, diagnosis, histopathology, and management. Yale J Biol Med. (2015) 88:167–79.
4. Algarin YA, Jambusaria-Pahlajani A, Ruiz E, Patel VA. Advances in topical treatments of cutaneous malignancies. Am J Clin Dermatol. (2022). doi: 10.1007/s40257-022-00731-x
5. Neubert T, Lehmann P. Bowen’s disease–a review of newer treatment options. Ther Clin Risk Manag. (2008) 4:1085–95.
6. Balkrishnan R, Cayce KA, Kulkarni AS, Orsagh T, Gallagher JR, Richmond D, et al. Predictors of treatment choices and associated outcomes in actinic keratoses: results from a national physician survey study. J Dermatolog Treat. (2006) 17:162–6. doi: 10.1080/09546630600765081
7. Jia H-X, He Y-L. Efficacy and safety of imiquimod 5% cream for basal cell carcinoma: a meta-analysis of randomized controlled trial. J Dermatolog Treat. (2020) 31:831–8. doi: 10.1080/09546634.2019.1638883
8. Drucker A, Adam GP, Langberg V, Gazula A, Smith B, Moustafa F, et al. Treatments for Basal Cell and Squamous Cell Carcinoma of the Skin. Rockville, MD: Agency for Healthcare Research and Quality (US) (2017).
9. Morton CA, McKenna KE, Rhodes LE, British Association of Dermatologists Therapy Guidelines and Audit Subcommittee and the British Photodermatology Group. Guidelines for topical photodynamic therapy: update. Br J Dermatol. (2008) 159:1245–66. doi: 10.1111/j.1365-2133.2008.08882.x
10. Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. (2009) 6:e1000097. doi: 10.1371/journal.pmed.1000097
11. Higgins JPT, Altman DG, Gøtzsche PC, Jüni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. BMJ. (2011) 343:d5928. doi: 10.1136/bmj.d5928
12. Wallace BC, Dahabreh IJ, Trikalinos TA, Lau J, Trow P, Schmid CH. Closing the gap between methodologists and end-users: R as a computational back-end. J Stat Softw. (2012) 49:1–15. doi: 10.18637/jss.v049.i05
13. Arits AHMM, Mosterd K, Essers BA, Spoorenberg E, Sommer A, De Rooij MJM, et al. Photodynamic therapy versus topical imiquimod versus topical fluorouracil for treatment of superficial basal-cell carcinoma: a single blind, non-inferiority, randomised controlled trial. Lancet Oncol. (2013) 14:647–54. doi: 10.1016/S1470-2045(13)70143-8
14. Basset-Seguin N, Ibbotson SH, Emtestam L, Tarstedt M, Morton C, Maroti M, et al. Topical methyl aminolaevulinate photodynamic therapy versus cryotherapy for superficial basal cell carcinoma: a 5 year randomized trial. Eur J Dermatol. (2008) 18:547–53. doi: 10.1684/ejd.2008.0472
15. Berroeta L, Clark C, Dawe RS, Ibbotson SH, Fleming CJ. A randomized study of minimal curettage followed by topical photodynamic therapy compared with surgical excision for low-risk nodular basal cell carcinoma. Br J Dermatol. (2007) 157:401–3. doi: 10.1111/j.1365-2133.2007.07996.x
16. Choi SH, Kim KH, Song KH. Er:YAG ablative fractional laser-primed photodynamic therapy with methyl aminolevulinate as an alternative treatment option for patients with thin nodular basal cell carcinoma: 12-month follow-up results of a randomized, prospective, comparative trial. J Eur Acad Dermatol Venereol. (2016) 30:783–8. doi: 10.1111/jdv.13453
17. Choi S-H, Kim K-H, Song K-H. Effect of methyl aminolevulinate photodynamic therapy with and without ablative fractional laser treatment in patients with microinvasive squamous cell carcinoma: a randomized clinical trial. JAMA Dermatol. (2017) 153:289–95. doi: 10.1001/jamadermatol.2016.4463
18. Foley P, Freeman M, Menter A, Siller G, El-Azhary RA, Gebauer K, et al. Photodynamic therapy with methyl aminolevulinate for primary nodular basal cell carcinoma: results of two randomized studies. Int J Dermatol. (2009) 48:1236–45. doi: 10.1111/j.1365-4632.2008.04022.x
19. Jansen MHE, Koekelkoren FHJ, Nelemans PJ, Arits AHMM, Roozeboom MH, Kelleners-Smeets NWJ, et al. Comparison of long-term cosmetic outcomes for different treatments of superficial basal cell carcinoma. J Am Acad Dermatol. (2018) 79:961–4. doi: 10.1016/j.jaad.2018.04.053
20. Kessels JPHM, Kreukels H, Nelemans PJ, Roozeboom MH, van Pelt H, Mosterd K, et al. Treatment of superficial basal cell carcinoma by topical photodynamic therapy with fractionated 5-aminolaevulinic acid 20% vs. two-stage topical methyl aminolaevulinate: results of a randomized controlled trial. Br J Dermatol. (2018) 178:1056–63. doi: 10.1111/bjd.15967
21. Ko DY, Kim KH, Song KH. A randomized trial comparing methyl aminolaevulinate photodynamic therapy with and without Er:YAG ablative fractional laser treatment in Asian patients with lower extremity Bowen disease: results from a 12-month follow-up. Br J Dermatol. (2014) 170:165–72. doi: 10.1111/bjd.12627
22. Morton CA, Whitehurst C, Moseley H, McColl JH, Moore JV, Mackie RM. Comparison of photodynamic therapy with cryotherapy in the treatment of Bowen’s disease. Br J Dermatol. (1996) 135:766–71.
23. Morton C, Horn M, Leman J, Tack B, Bedane C, Tjioe M, et al. Comparison of topical methyl aminolevulinate photodynamic therapy with cryotherapy or fluorouracil for treatment of squamous cell carcinoma in situ: results of a multicenter randomized trial. Arch Dermatol. (2006) 142:729–35. doi: 10.1001/archderm.142.6.729
24. Morton CA, Dominicus R, Radny P, Dirschka T, Hauschild A, Reinhold U, et al. A randomized, multinational, noninferiority, phase III trial to evaluate the safety and efficacy of BF-200 aminolaevulinic acid gel vs. methyl aminolaevulinate cream in the treatment of nonaggressive basal cell carcinoma with photodynamic therapy. Br J Dermatol. (2018) 179:309–19. doi: 10.1111/bjd.16441
25. Mosterd K, Thissen MRTM, Nelemans P, Kelleners-Smeets NWJ, Janssen RLLT, Broekhof KGME, et al. Fractionated 5-aminolaevulinic acid-photodynamic therapy vs. surgical excision in the treatment of nodular basal cell carcinoma: results of a randomized controlled trial. Br J Dermatol. (2008) 159:864–70. doi: 10.1111/j.1365-2133.2008.08787.x
26. Rhodes LE, de Rie M, Enström Y, Groves R, Morken T, Goulden V, et al. Photodynamic therapy using topical methyl aminolevulinate vs surgery for nodular basal cell carcinoma: results of a multicenter randomized prospective trial. Arch Dermatol. (2004) 140:17–23. doi: 10.1001/archderm.140.1.17
27. Rhodes LE, de Rie MA, Leifsdottir R, Yu RC, Bachmann I, Goulden V, et al. Five-year follow-up of a randomized, prospective trial of topical methyl aminolevulinate photodynamic therapy vs surgery for nodular basal cell carcinoma. Arch Dermatol. (2007) 143:1131–6. doi: 10.1001/archderm.143.9.1131
28. Roozeboom MH, Nelemans PJ, Mosterd K, Steijlen PM, Arits AHMM, Kelleners-Smeets NWJ. Photodynamic therapy vs. topical imiquimod for treatment of superficial basal cell carcinoma: a subgroup analysis within a noninferiority randomized controlled trial. Br J Dermatol. (2015) 172:739–45. doi: 10.1111/bjd.13299
29. Roozeboom MH, Arits AHMM, Mosterd K, Sommer A, Essers BAB, de Rooij MJM, et al. Three-year follow-up results of photodynamic therapy vs. imiquimod vs. fluorouracil for treatment of superficial basal cell carcinoma: a single-blind, noninferiority, randomized controlled trial. J Invest Dermatol. (2016) 136:1568–74. doi: 10.1016/j.jid.2016.03.043
30. Salim A, Leman JA, McColl JH, Chapman R, Morton CA. Randomized comparison of photodynamic therapy with topical 5-fluorouracil in Bowen’s disease. Br J Dermatol. (2003) 148:539–43. doi: 10.1046/j.1365-2133.2003.05033.x
31. Salmivuori M, Grönroos M, Tani T, Pölönen I, Räsänen J, Annala L, et al. Hexyl aminolevulinate, 5-aminolevulinic acid nanoemulsion and methyl aminolevulinate in photodynamic therapy of non-aggressive basal cell carcinomas: a non-sponsored, randomized, prospective and double-blinded trial. J Eur Acad Dermatol Venereol. (2020) 34:2781–8. doi: 10.1111/jdv.16357
32. Szeimies RM, Ibbotson S, Murrell DF, Rubel D, Frambach Y, de Berker D, et al. A clinical study comparing methyl aminolevulinate photodynamic therapy and surgery in small superficial basal cell carcinoma (8-20 mm), with a 12-month follow-up. J Eur Acad Dermatol Venereol. (2008) 22:1302–11. doi: 10.1111/j.1468-3083.2008.02803.x
33. Wang I, Bendsoe N, Klinteberg CA, Enejder AM, Andersson-Engels S, Svanberg S, et al. Photodynamic therapy vs. cryosurgery of basal cell carcinomas: results of a phase III clinical trial. Br J Dermatol. (2001) 144:832–40. doi: 10.1046/j.1365-2133.2001.04141.x
34. Caddick J, Stephenson J, Green L, Spyrou G. The psychological impact of facial skin cancer. Proceedings of the Association of Plastic Reconstructive and Aesthetic Surgeons (BAPRAS) Summer Scientific Meeting, 11th - 13th July 2012. Gateshead (2012).
Keywords: basal cell carcinoma, squamous cell carcinoma, photodynamic therapy, skin cancer, systematic review, meta-analysis
Citation: Ou-Yang Y, Zheng Y and Mills KE (2023) Photodynamic therapy for skin carcinomas: A systematic review and meta-analysis. Front. Med. 10:1089361. doi: 10.3389/fmed.2023.1089361
Received: 01 December 2022; Accepted: 02 January 2023;
Published: 19 January 2023.
Edited by:
Paulo Filipe, Centro Hospitalar Lisboa Norte (CHLN), PortugalReviewed by:
Maria Catorze, Hospital de Egas Moniz, PortugalShengqing Hu, Huazhong University of Science and Technology, China
Copyright © 2023 Ou-Yang, Zheng and Mills. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Kerry E. Mills,  Kerry.Mills@canberra.edu.au; Yun Ou-Yang,
Kerry.Mills@canberra.edu.au; Yun Ou-Yang,  ouyang@gzhmu.edu.cn
ouyang@gzhmu.edu.cn
 Yun Ou-Yang1*
Yun Ou-Yang1*  Yaowu Zheng
Yaowu Zheng Kerry E. Mills
Kerry E. Mills