- 1Biosciences Division, Oak Ridge National Laboratory, Oak Ridge, TN, United States
- 2Department of Civil and Environmental Engineering, University of Michigan, Ann Arbor, MI, United States
- 3Department of Environmental Engineering and Earth Sciences, Clemson University, Clemson, SC, United States
Plants are colonized by numerous microorganisms serving important symbiotic functions that are vital to plant growth and success. Understanding and harnessing these interactions will be useful in both managed and natural ecosystems faced with global change, but it is still unclear how variation in environmental conditions and soils influence the trajectory of these interactions. In this study, we examine how nitrogen addition alters plant-fungal interactions within two species of Populus - Populus deltoides and P. trichocarpa. In this experiment, we manipulated plant host, starting soil (native vs. away for each tree species), and nitrogen addition in a fully factorial replicated design. After ~10 weeks of growth, we destructively harvested the plants and characterized plant growth factors and the soil and root endosphere fungal communities using targeted amplicon sequencing of the ITS2 gene region. Overall, we found nitrogen addition altered plant growth factors, e.g., plant height, chlorophyll density, and plant N content. Interestingly, nitrogen addition resulted in a lower fungal alpha diversity in soils but not plant roots. Further, there was an interactive effect of tree species, soil origin, and nitrogen addition on soil fungal community composition. Starting soils collected from Oregon and West Virginia were dominated by the ectomycorrhizal fungi Inocybe (55.8% relative abundance), but interestingly when P. deltoides was grown in its native West Virginia soil, the roots selected for a high abundance of the arbuscular mycorrhizal fungi, Rhizophagus. These results highlight the importance of soil origin and plant species on establishing plant-fungal interactions.
1. Introduction
Over the last century, increases in atmospheric nitrogen (N) concentrations have resulted in significant N deposition within soils across the globe (Canfield et al., 2010). Increased levels of this limiting nutrient within soils result in significant changes in ecosystem structure and function. Belowground, N addition has significant effects on soil microbial diversity, biomass, and growth (Zhang et al., 2018). Root associated microbial communities contain up to 1011 microbial cells per gram plant root and are primarily comprised of bacteria and fungi (White et al., 2017). These communities regulate organic matter decomposition, nutrient cycling, and are key players in plant health and growth, thus changes in these communities may significantly impact plant physiology and ecosystem function.
Soil fungi (the soil mycobiome) are vital members in belowground microbial communities. These fungi include prevalent decomposers and beneficial plant symbionts. Interactions between plants and fungi belowground are essential components for plant health, growth, and response to abiotic stress (Hacquard and Schadt, 2015; Vandenkoornhuyse et al., 2015; Cregger et al., 2021). Understanding factors that regulate and alter these interactions is imperative if we aim to leverage these interactions to develop sustainable ecosystems and influence the way in which carbon and nutrients are cycled and stored within soils (de Vries et al., 2018). Because many plant processes rely on or are improved by associations with soil fungi, it has become increasingly important to study the plant and its microbiota as a joint system (Hacquard and Schadt, 2015).
Within the myriad of possible plant-fungal interactions occurring, plant-mycorrhizal associations are widespread with the two most common mycorrhizal types being ectomycorrhizae (ECM) and arbuscular mycorrhizae (AM). Belowground plant interactions with these fungi have been shown to increase water uptake and nutrient acquisition, and shift soil carbon storage (Gehring and Connell, 2006; Bonfante and Genre, 2010; Miransari, 2011; Liu et al., 2015). Numerous studies have highlighted that global change factors influence mycorrhizal communities (Compant et al., 2010; Bellgard and Williams, 2011; Pickles et al., 2012; Bennett and Classen, 2020; Yang et al., 2021), but it is unclear how variation in the diversity and abundance of these communities alter plant host traits. Most plants associate with one type of mycorrhizal fungi, and individual plant species are less likely to associate with both AM and ECM fungal species (Teste et al., 2020). Contrary to this, trees within the genus Populus have the unique feature that they associate with both AM and ECM simultaneously in natural settings (Gehring and Connell, 2006; Cregger et al., 2018).
Populus has become a model tree species for studying plant-microbe interactions due to its fast growth, clonal propagation, and vast genomic resources (Cregger et al., 2021). Populus species are globally distributed and are often keystone members within forested ecosystems (Suarez-Gonzalez et al., 2016; Kivinen et al., 2020). Populus is commonly found in riparian environments at the interface between terrestrial and aquatic ecosystems, serving a unique role within these environments (Cronk, 2005). Further, Populus is economically important to industries such as lumber, paper, and potentially cellulose-derived biofuels (Sannigrahi et al., 2010; Happs et al., 2021), largely due to their fast growth rate and ability to be grown on marginal lands.
Previous research has demonstrated that fungal communities differ between Populus species and genotypes, and Populus clones have varying degrees of AM and ECM colonization (Gehring and Connell, 2006; Martin et al., 2008; Bonito et al., 2014; Cregger et al., 2018). Plant genetic factors, starting soil inoculum, and abiotic variables such as soil moisture influence fungal community diversity and composition (Shakya et al., 2013; Veach et al., 2020) as well as rates of AM vs. ECM colonization and abundance on Populus (Gehring and Connell, 2006). Interestingly, when grown in a common environment and exposed to variation in soil nitrogen content, both ECM and AM colonization and diversity decreases with increasing levels of soil N (Hodge and Storer, 2015; Yang et al., 2021), but it is unclear if this pattern is consistent across Populus species. Further it is unclear how soil N concentration alters broader Populus-fungal interactions across Populus species, and if changes in these interactions are contingent upon soil origin.
The overarching goal of this study was to determine the impact of increased soil N concentration on belowground plant-fungal interactions within two Populus species, and to identify if soil origin altered the impact of N on Populus-fungal interactions. Specifically, we ask: (1) Does N addition result in convergence of the mycobiome across two Populus species and across two soil types with distinct initial microbial communities, (2) Does N addition alter core fungal taxa present across two Populus species, (3) Does N addition change plant-mycorrhizal associations consistently within two Populus species, and finally (4) Do changes in the fungal community correlate with alterations in plant traits? We hypothesize N addition will result in convergence of the mycobiome within roots and soils regardless of plant species or soil origin, and N addition will result in a decoupling of beneficial Populus-fungal interactions. Specifically, we hypothesize that N addition will result in decreased diversity and relative abundance of both AM and ECM in plant roots and plant associated soils.
2. Materials and methods
2.1. Experimental design
Cuttings of P. trichocarpa (genotype GW9791) and P. deltoides (genotype WV94; Coyle et al., 2006) and their accompanying field soils were obtained from existing common garden experiments located in Oregon and West Virginia where these Populus species are each native. Three soil subsamples were collected from Oregon and West Virginia for initial chemical and mycobiome analyses as described below. A total of 160 cuttings (80 P. deltoides/80 P. trichocarpa) were propagated on March 28, 2019 in autoclaved potting mix in a greenhouse at Oak Ridge National Laboratory (Oak Ridge, TN) to establish growth. Prior to onset of experimental treatments, field-collected soils from Oregon and West Virginia were mixed with sterile sand (50/50 mixture) to induce N-limiting conditions. Forty cuttings from each species (P. trichocarpa/P. deltoides) were grown in each soil mixture (Oregon/West Virginia) for ~10 weeks in 1.6-liter tree pots (Figure 1). During the experiment, Hoagland’s fertilizer (Basal mix #2, Caisson Labs, Smithfield, UT) was applied weekly in 400 mL increments. Half of the plants received Hoagland’s fertilizer with N, while the other half received Hoagland’s fertilizer without N (henceforth referred to as N+ and N− treatments, respectively).
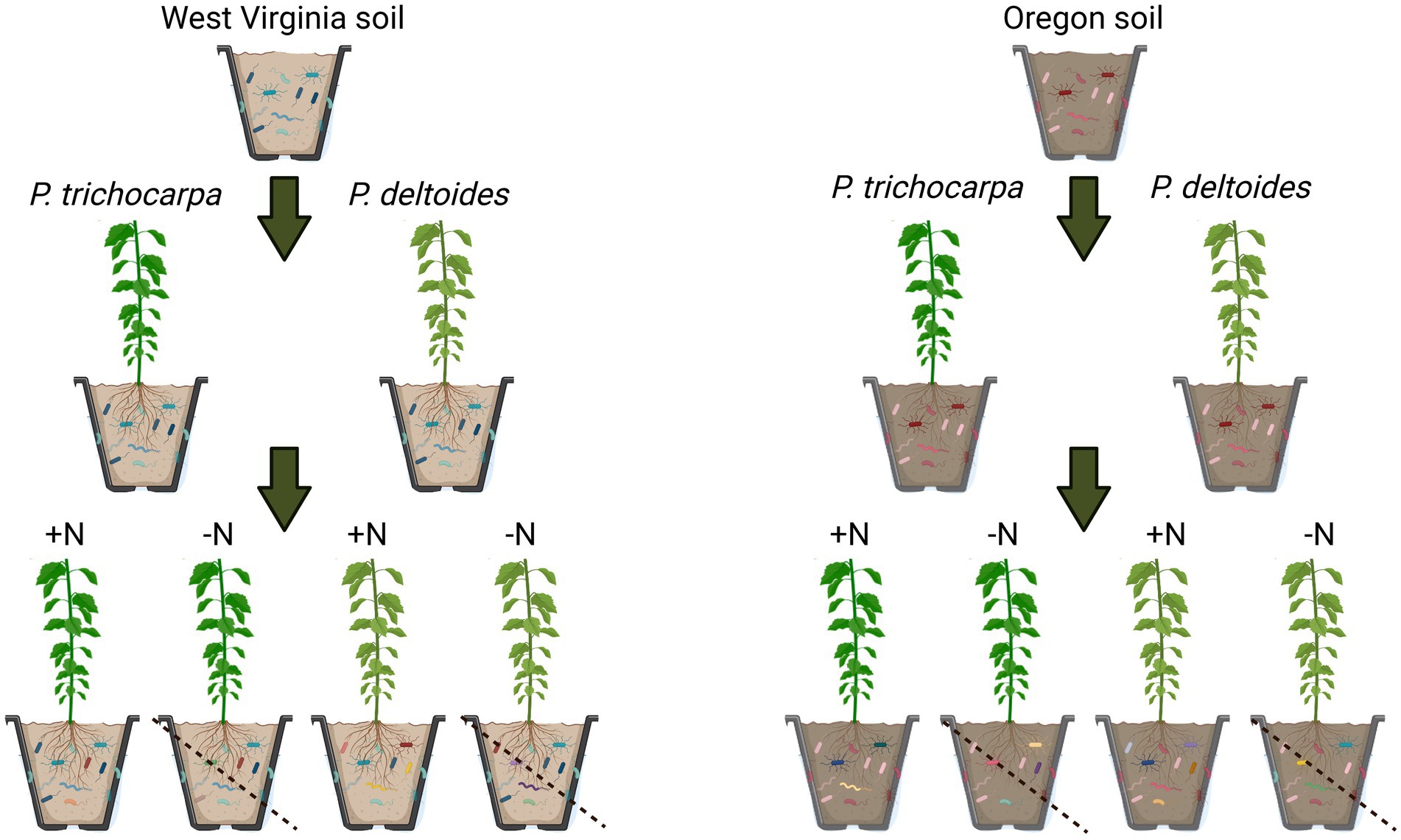
Figure 1. Experimental design. Cuttings of P. trichocarpa and P. deltoides and their accompanying field soils were obtained from Oregon and West Virginia. A total of 160 cuttings (80 P. deltoides/80 P. trichocarpa) were propagated and 40 cuttings from each species (P. trichocarpa/P. deltoides) were grown in each soil mixture (Oregon/West Virginia) for ~10 weeks and half of the plants received Hoagland’s fertilizer with nitrogen, while the other half received Hoagland’s fertilizer without nitrogen (referred to as N+ and N− treatments, respectively).
2.2. Plant and soil characterization
We assessed plant characteristics (number of leaves, plant height, stem diameter at two locations, and leaf chlorophyll content) upon the onset of the experiment in May and in July (~ 8 weeks into the experiment). Leaf chlorophyll content was measured using a SPAD-502 Meter (Spectrum Technologies, Inc., Aurora, IL, United States). For SPAD measurements, three leaves per plant were measured and the mean SPAD content was calculated per plant as performed previously (Veach et al., 2019). Plants were destructively harvested when they became root bound (root production was evident at the bottom of the pot), which differed slightly between N+ and N− by 2 weeks (late July for N+ and mid-August for N−), due to differences in growth rates between groups. Harvesting plants based on when they became root bound allow assessments to be made when plants were at similar physiological states. At harvest, a subset of leaves, stems, and roots were removed to assess percent plant carbon (C) and N. A second subset of roots were collected for ITS2 amplicon sequencing (see section “Root and soil microbiome characterization” below). Soil was homogenized during the destructive harvest and a subset was collected in a 50 mL sterile tube, flash frozen in liquid N2 and stored at −80°C until further processing. Given roots occurred continuously throughout the pot at harvest, soil was not partitioned into bulk soil/rhizosphere samples and this compartment will be referred to as ‘soil’ throughout the manuscript.
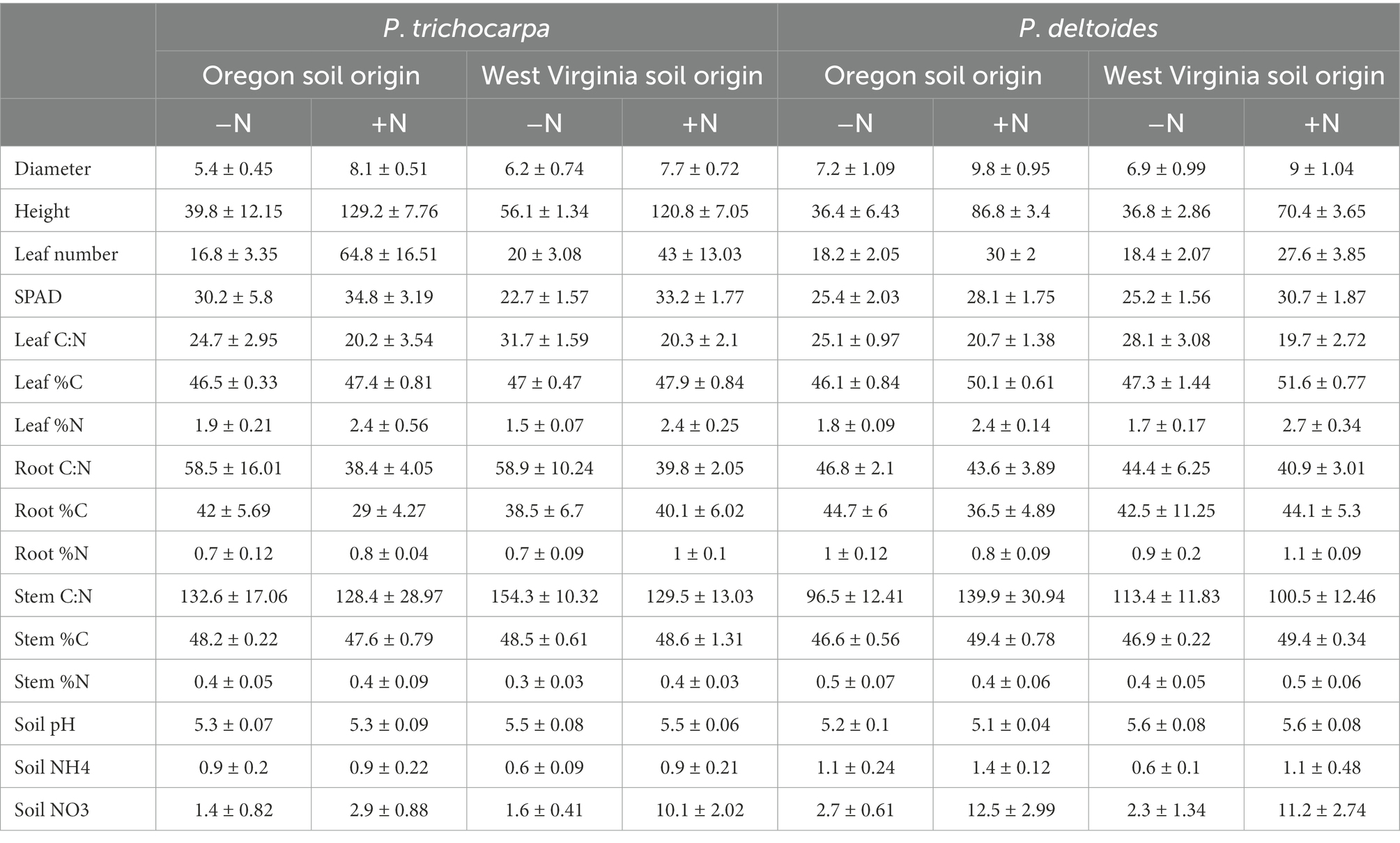
Initial characterization of field soils collected from Oregon and West Virginia was performed at the University of Georgia Agricultural and Environmental Services Laboratories - Soil, Plant, and Water Laboratory (Athens, GA, United States). For triplicate field soil subsamples, soil extractable P, K, Ca, Mg, Mn, and Zn were extracted using the Mehlich-1 method and measured using an inductively coupled plasma spectrograph (Supplementary Table S1). At the completion of the experiment, we assessed soil pH (LabFit AS-3000 pH Analyzer), NH4-N, and NO3-N (the phenate method for NH4+ and the cadmium reduction method for NO3− (Robertson et al., 1999) within 10 replicate pots per experimental group. Additionally, we assessed %C and %N for stem, leaf, and root subsamples across the same 10 replicate trees using the Dumas Method (Kirsten and Hesselius, 1983). In brief, a representative sample (~0.5 g) was loaded into a ceramic sample boat and combusted in an oxygen atmosphere at 1,350°C in a Elementar Vario Max Total Combustion Analyzer. Elemental C and N were converted into CO2, NOx, and N2. These gases were then passed through the IR (infrared) cells to determine the carbon content and a TC (thermal conductivity) cell to determine N2. Results are reported as a ratio between the two percentages.
2.3. Root and soil microbiome characterization
Roots from five replicates across each experimental group (randomly chosen from the same subset of 10 that were used for plant and soil characterization above), were surface sterilized with three 30 s water wash cycles, followed by a 30 s wash of 70% ethanol, and another set of three 30 s water wash cycles. Fine roots were then dissected with a razor blade, pulverized in liquid N2 frozen blocks with the Qiagen TissueLyser at 30 rpm for 3 min with a 5-mm steel bead and DNA isolated with the Qiagen DNeasy PowerPlant Pro Kit (Qiagen, Venlo, Netherlands). To remove possible PCR inhibitors and ethanol carry over, DNA was purified with the Qiagen DNA cleanup kit and Agencourt AMPure XP beads (Beckman Coulter, Brea, CA, United States).
Five replicates from each experimental group, as well as triplicate subsamples from each of the preserved field soils, were used for soil DNA extraction. Soil DNA was extracted with the Qiagen DNeasy PowerSoil Pro Kit, using a Precellys 24 (Bertin Instruments, France) for lysis. For each sample, duplicate extractions of 0.25 g were performed. Duplicate extracts were quantified individually using a Qubit fluorometer and Qubit dsDNA Broad Range Assay Kit (Life Technologies, Carlsbad, CA, United States) and then combined. DNA extracts were purified with the DNeasy PowerClean Pro Cleanup Kit followed by Agencourt AMPure XP beads following the standard DNA cleanup protocol.
2.4. Amplicon library preparation
Amplicons were prepared following the 16S Metagenomic Sequencing Library preparation protocol (Part# 15044223 RevB, Illumina Inc. Hayward CA). Amplicons were amplified by polymerase chain reaction (PCR) with a mixture of custom ITS2 gene region primers for fungi designed to increase phylogenetic coverage within amplicon libraries (Dove et al., 2021), and then uniquely barcoded in a subsequent PCR with Illumina Nextera XT v2 indexes. Amplicon PCR contained 12.5 μL of 2x KAPA HiFi HotStart Ready Mix, 5 μM each of amplicon specific primers, 25–50 ng of template DNA, and water to a final reaction volume of 25 μL. Amplification parameters for amplicon PCR included initial denaturation at 95°C for 3 min, followed by 25 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min. Following the initial amplification using target specific primers, 2 μL of the PCR reaction was visualized by gel electrophoresis (1% agarose). The remainder of the PCR reaction was cleaned with AMPure beads using a 0.8x ratio of beads to PCR reaction volume (23 μL) and resuspended in 50 μL of 10 mM Tris pH 8.5. To uniquely tag each amplicon, a second round of PCR (Index PCR) was performed in a 50 μL reaction volume with 25 μL of 2x KAPA HiFi HotStart Ready Mix, 5 μL of Nextera XT Index primer 1 (N7xx), 5 μL of Nextera XT index primer 2 (S5xx), 5 μL of cleaned DNA from the amplicon amplification and 10 μL water. Index PCR amplification parameters included initial denaturation at 95°C for 3 min, followed by 8 cycles of 95°C for 30 s, 55°C for 30 s, 72°C for 30 s, and a final extension at 72°C for 5 min. After the Index PCR, the amplified products were cleaned using AMPure beads (1x volume ratio) and resuspended in 25 μL of 10 mM Tris pH 8.5. The cleaned amplicons were quantified on a NanoDrop 1,000 (Thermo Fisher Scientific, Wilmington, DE, United States), pooled equimolarly, and validated on an Agilent Bioanalyzer (Agilent, Santa Clara, CA, United States) using a DNA7500 chip. The final pool was again AMPure bead purified (0.7× volume ratio) to remove any remaining small DNA fragments. Lastly, the sample concentration was quantified on a Qubit fluorometer with the broad range dsDNA assay (Life Technologies). Libraries were prepared for sequencing following the Illumina MiSeq denature and dilute libraries guide. PhiX control DNA was included in the sequencing run due to low base diversity associated with amplicon samples. Libraries were loaded into the sequencing cassette (v2 chemistry) and a paired end (2×251×8×8) sequencing was completed on an Illumina MiSeq Instrument (Illumina, San Diego, CA, United States).
2.5. Sequence data processing
Fungal sequences were processed with the QIIME 2 v 2019.10 platform (Bolyen et al., 2019). Paired sequences were demultiplexed with the plugin demux and quality filtered (denoised, dereplicated, chimera filtered, and pair-end merged) and processed into Sequence Variants (SVs) with the dada2 plugin (Callahan et al., 2016). Taxonomy was assigned using a pre-trained Naive Bayes classifier based on the Unite (ITS) database (Nilsson et al., 2019) and mitochondria, chloroplast and unassigned sequences were removed, and samples were rarefied to 12,000 reads. SVs were classified into functional guild using the FUNguild database (Nguyen et al., 2016), filtered to include only assignments greater than “probable” and subset to include AM and ECM fungi.
2.6. Statistical approaches
All statistical analyses were performed in R version 4.2.0 and visualized with ggplot2. For alpha diversity, Hill numbers (or effective numbers of species) were calculated on the full dataset with the package hillR (Chao et al., 2014) to control the contribution of rare taxa to the diversity metrics. In general, the diversity measures are weighted differently by the diversity order (qD), which calculates Hill numbers weighted differently by species abundance distributions (Hill, 1973; Chao et al., 2012). For example, at q = 0, all species are equally weighted, at q = 1, species are proportionally weighted to relative abundance, and at q = 2, rare species are down weighted. A three-way type II ANOVA was performed with the “car” package to test the effect of soil origin, tree species, and/or N addition on Hill numbers for overall fungal communities. Additionally, we performed an ANOVA and Tukey’s honest significant difference (HSD) post hoc analyses on the AM and ECM portion of the fungal communities. Beta diversity was visualized with non-metric multidimensional scaling (NMDS) based on Bray-Curtis distances. A PERMANOVA was performed to test the role of soil origin, tree species, and/or N addition on overall fungal community structure as well as AM and ECM fungal communities. We defined core taxa within the total mycobiome that uniquely occurred within soils, roots, P. trichocarpa, and P. deltoides at the genus level with the core_members (Shetty and Lahti, 2019) function from the microbiome package in R. We defined core taxa has having a prevalence of 95% with a relative abundance >0.001 in each considered dataset.
The relationship of plant and soil characteristics and tree species, soil origin, and N treatment was assessed with general linear models (GLM). Plant and soil characteristic correlations with microbiome taxa were assessed with the Pearson correlation test, with p values corrected for multiple comparisons by the false-discovery rate method. Statistical significance for all analyses was determined using p-value cutoff ≤0.05.
3. Results
3.1. Variation in fungal community alpha and beta diversity
The addition of N impacted microbial alpha and beta diversity differently in soil compared to roots. There was an interactive effect of N addition, tree species, and soil origin on soil fungal alpha diversity at q1 (Shannon diversity; p < 0.05) and q2 (Simpson diversity; p < 0.05). Contrary to this, there were no interactive effects of N addition, tree species, and soil origin on root fungal alpha diversity. There were significant main effects of soil origin, tree species and N addition on soil alpha diversity (Table 1). For example, on average, N addition decreased fungal alpha diversity in soils at q1 and q2 (p < 0.05) but did not alter alpha diversity in plant roots at any level of q (p > 0.05). Soil origin was the only predictor of root fungal community alpha diversity at q0 (p < 0.05).
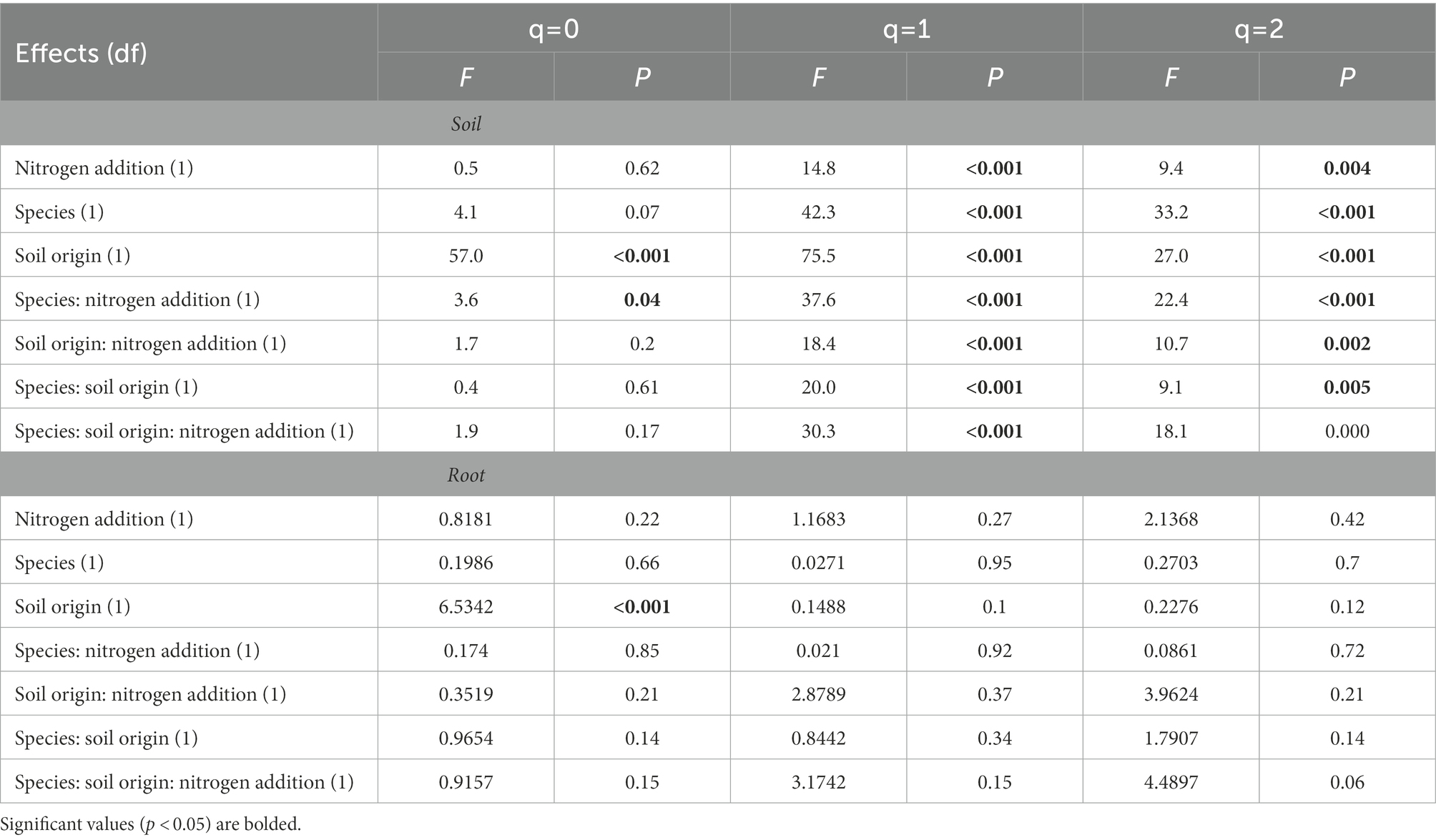
Table 1. Alpha diversity results from analysis of variance tests of the relationship between Hill numbers (q = 0, q = 1, q = 2) and nitrogen addition, tree species, and soil origin.
There was an interactive effect of tree species, soil origin, and N treatment on soil fungal community composition (Figure 2; R2 = 0.02, p < 0.001), but there was not an interaction between tree species, soil origin, and N treatment in roots (Figure 2; R2 = 0.02, p = 0.07). Soil origin was the largest factor impacting both soil and root fungal beta diversity explaining 30 and 35% of the variation in community composition, respectively (Supplementary Figure S2; p < 0.001) followed by tree species (R2 = 0.14 - soil and R2 = 0.09 - root, p < 0.001), and N treatment (R2 = 0.04 - soil and R2 = 0.03 root, p < 0.001).
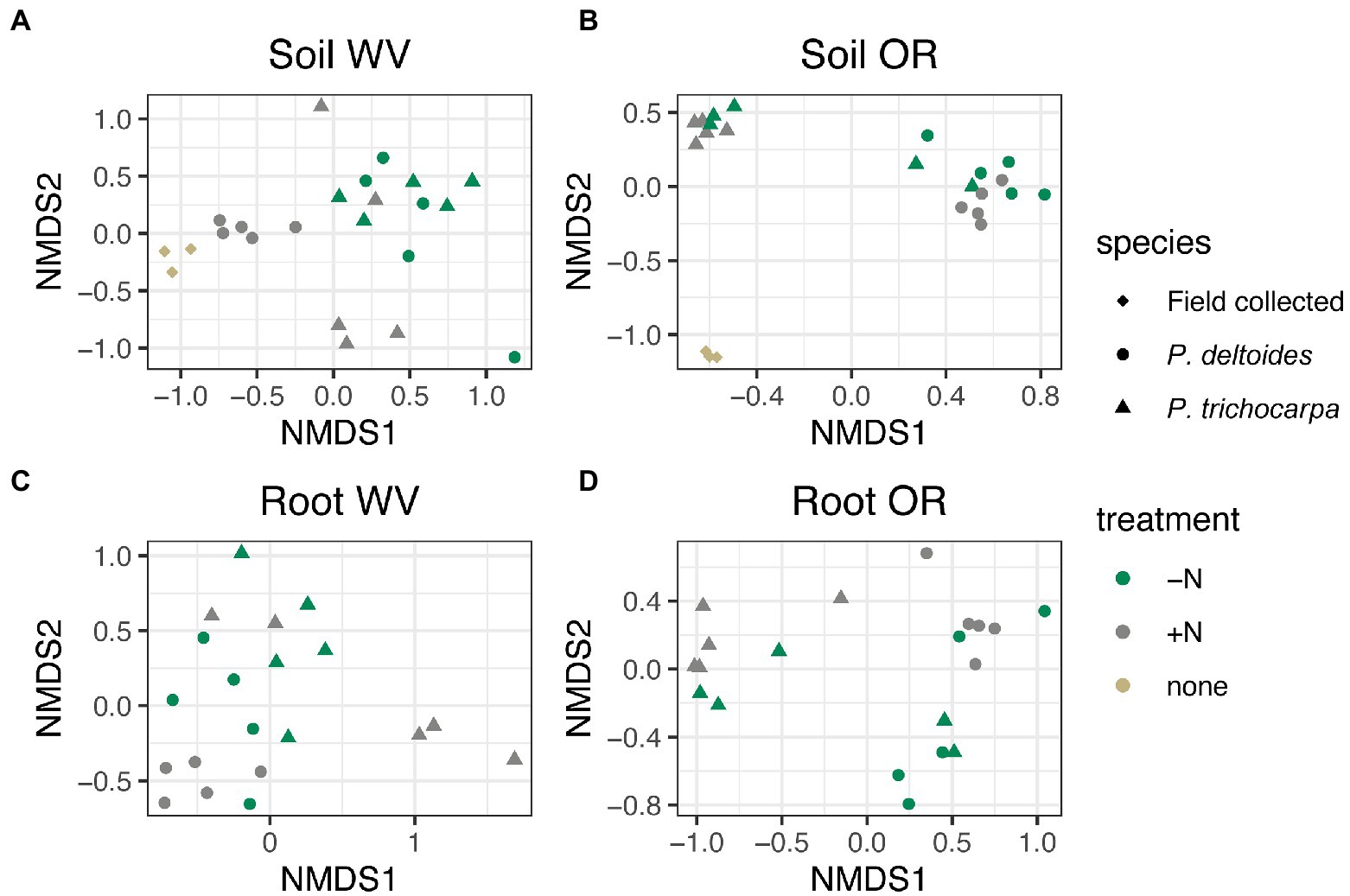
Figure 2. NMDS of Bray Curtis distance matrices for soil (A,B) and root (C,D) samples grown in soil collected from West Virginia (WV; A,C) or Oregon (OR; B,D). Each point corresponds to a sample, shape corresponds to tree species and color corresponds to nitrogen addition. Samples designated as “field collected” for species and “none” for treatment reflect starting soil communities collected from the field sites.
3.2. Fungal core taxa
We identified one core taxa assigned to the genus Rhizophagus that accounted for 8.6% of reads in all root samples and 13 core taxa in all soil samples that accounted for 41.9% of reads and consisted of Trichoderma, Tetracladium, Gibellulopsis, Metacordyceps, Inocybe, Metarhizium, Solicoccozyma, Talaromyces, Saitozyma, and other taxa unable to be classified to the genus level (Supplementary Table S2). A single core fungal genus, the AM fungi Rhizophagus, occupied the roots of P. deltoides with the highest abundance when grown in soil collected from West Virginia (25.2% relative abundance) compared to P. deltoides roots grown in Oregon soil (0.6% relative abundance). P. trichocarpa root samples contained the single fungal core genus Inocybe that dominated the roots of P. trichocarpa grown in Oregon soils (52.4%) but decreased in relative abundance in roots of P. trichocarpa when grown in West Virginia soils (11.6%). Soil samples of P. trichocarpa and P. deltoides contained 10 and 7 core genera, respectively (Supplementary Table S3). Soil samples from both species were dominated by Inocybe (55.8% relative abundance) except for both P. trichocarpa and P. deltoides grown in +N West Virginia soils (8.2% relative abundance).
3.3. Response of arbuscular mycorrhizal and ectomycorrhizal fungi
Soil origin strongly influenced AM and ECM fungal alpha diversity in roots and ECM alpha diversity in soil samples (p < 0.001 – Figure 3; Table 2). Nitrogen addition reduced ECM alpha diversity in the soil (Supplementary Table S4; p < 0.001) but not in the roots (p = 0.77). Ectomycorrhizal diversity was on average higher in P. deltoides than P. trichocarpa (Supplementary Tables S4, S5; p < 0.01). The addition of N decreased AM fungal relative abundance in P. trichocarpa roots compared to P. deltoides roots (Supplementary Table S6).
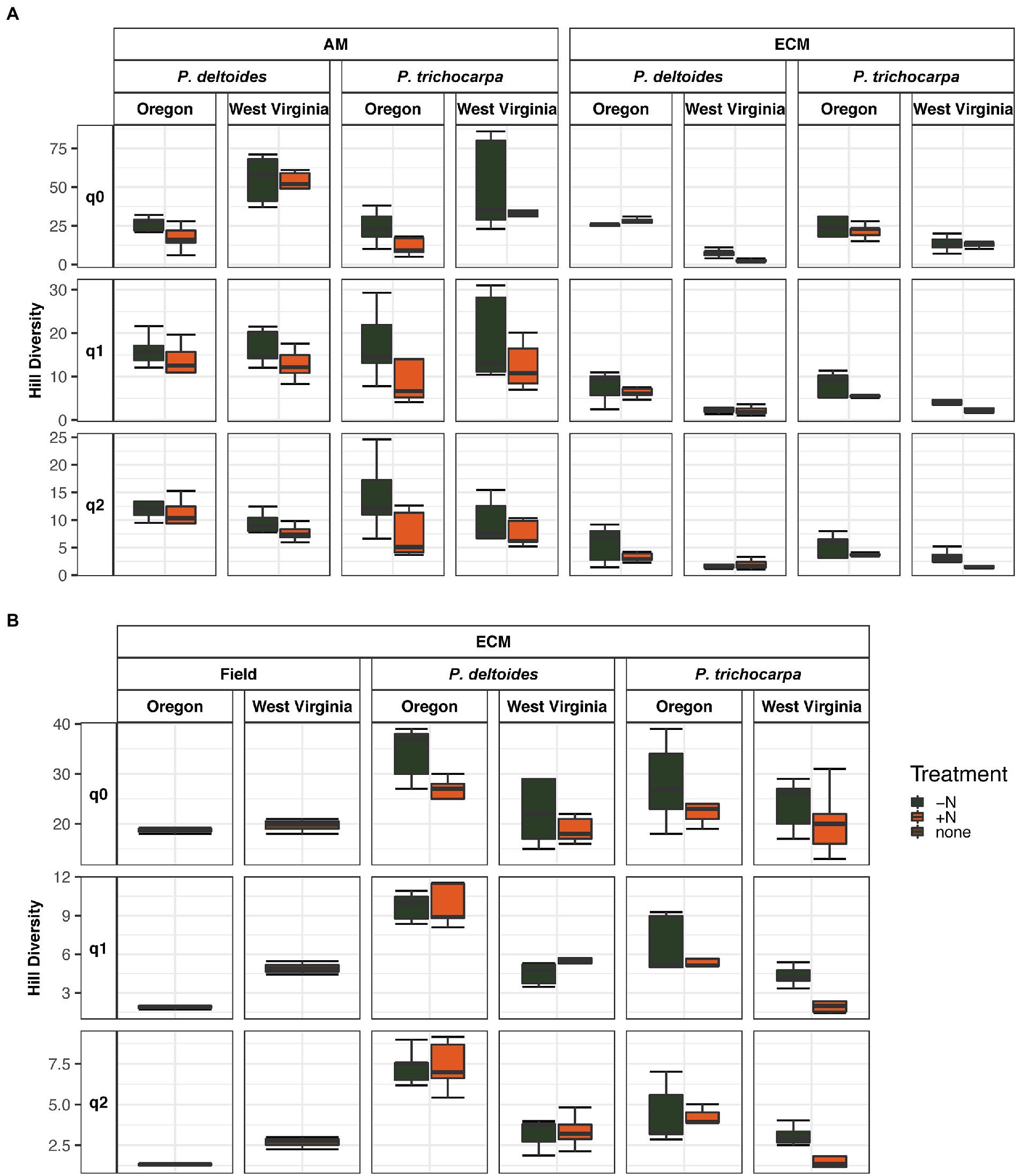
Figure 3. Boxplot of alpha diversity of FUNguild assigned arbuscular and ectomycorrhizal fungi measured by Hill numbers (q = 0, q = 1, q = 2) across nitrogen addition, tree species and soil origin. Lines within the boxplots represent median, 25th and 75th percentile values, while whiskers are defined by the largest value not greater than 1.5× the interquartile range (IQR) and the smallest value not less than 1.5× the IQR.
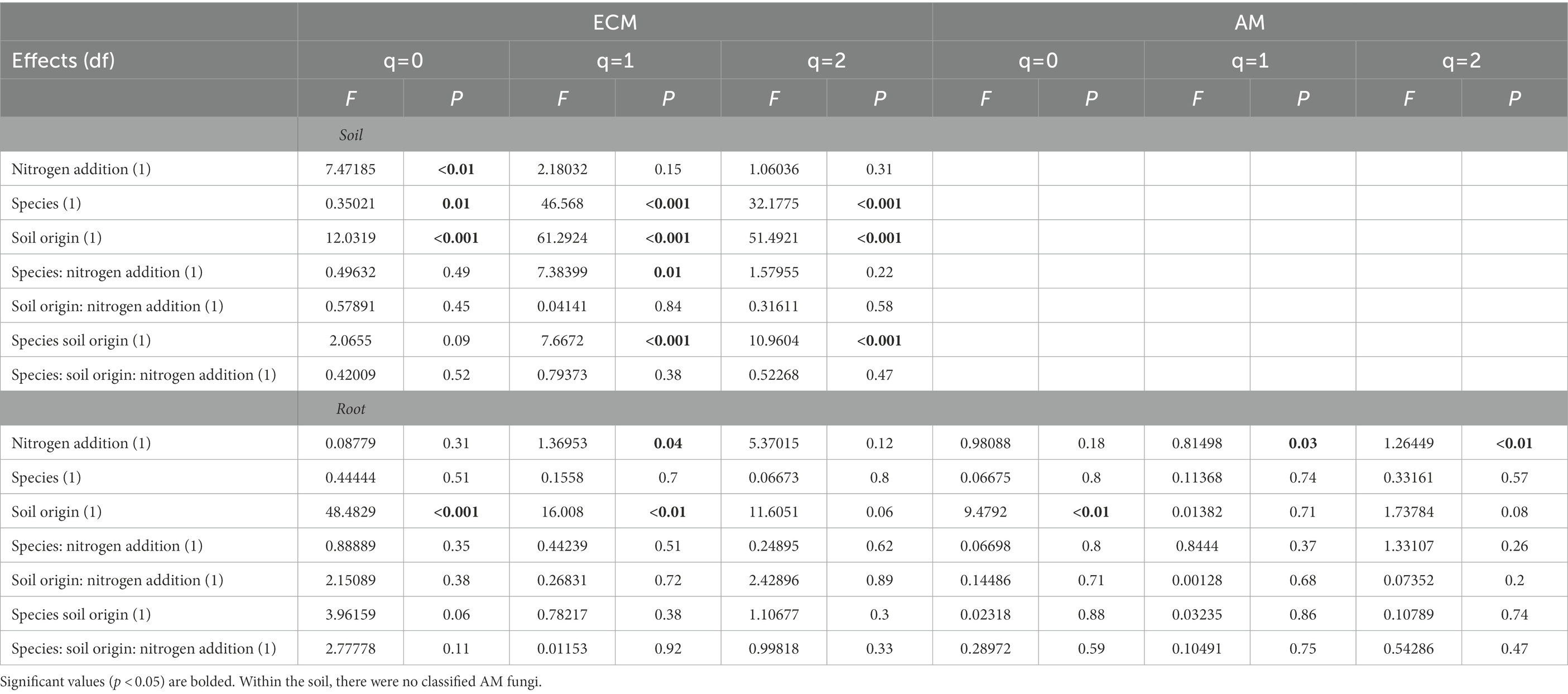
Table 2. Alpha diversity results from analysis of variance tests of the relationship between of FUNguild assigned arbuscular (AM) and ectomycorrhizal (ECM) fungal community Hill numbers (q = 0, q = 1, q = 2) and nitrogen addition, tree species and soil origin.
The ECM fungal class Agaricomycetes dominated all root and soil samples of P. trichocarpa (Figure 2; 46.8% - soil, and 59.6% average relative abundance - root), but interestingly did not dominate the original soil samples collected from Oregon and West Virginia (Figure 4). The Agaricomycetes also dominated P. deltoides soils and roots (Figure 4; 57.2 and 32.3% average relative abundance respectively) except for P. deltoides when grown in its native West Virginia soil with N addition which resulted in a drastic decrease in this fungal class down to 1.3% root relative abundance and 6.3% soil relative abundance. When grown in W. Virginia soil, P. deltoides roots were instead dominated by the AM fungi Glomeromycetes (31.2% relative abundance) which is higher than all other samples (3.2% average relative abundance across other samples).
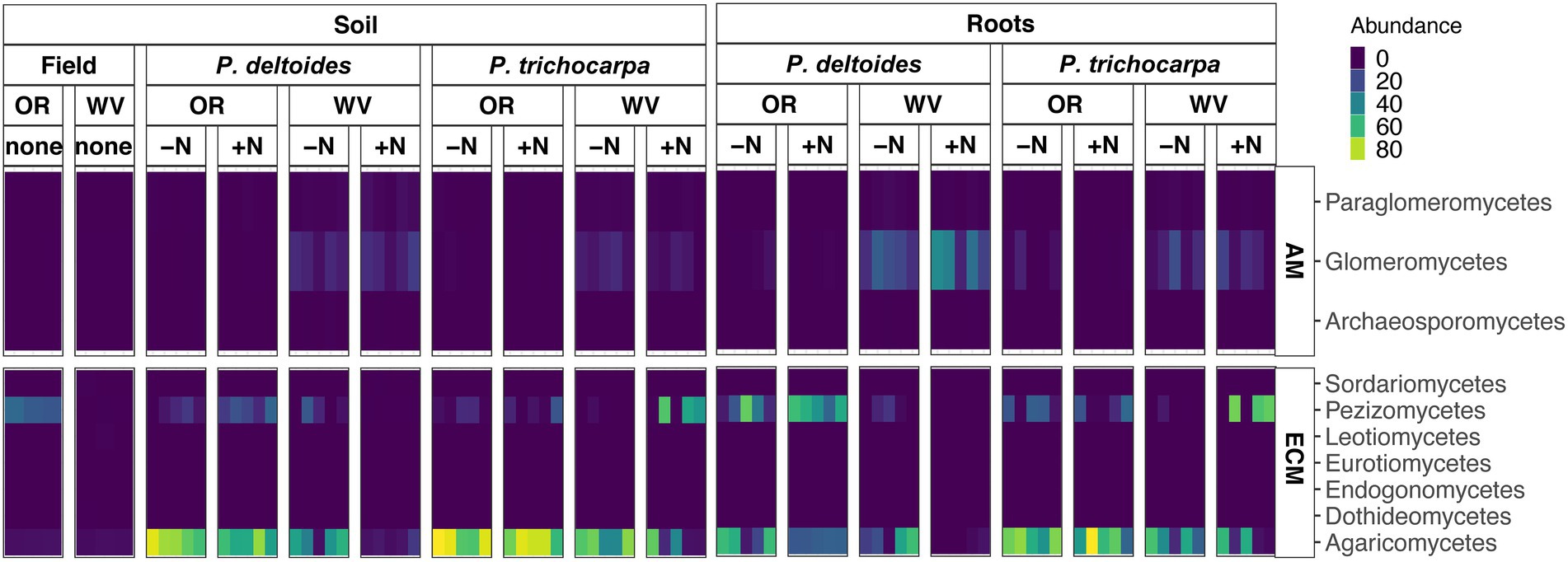
Figure 4. (A) Heatmap of FUNguild assigned arbuscular mycorrhizae (AM) and (B) ectomycorrhizae (ECM) fungal class relative abundance of P. trichocarpa and P. deltoides root and soil samples with nitrogen addition and different initial soils (WV, West Virginia; OR, Oregon).
There was an interactive effect of soil origin, tree species, and N addition on ECM community composition in the soil (R2 = 0.04, p = 0.01). Root ECM and AM fungal beta diversity varied by soil origin (R2 = 0.60 - ECM and R2 = 0.31 - AM, p < 0.001) followed by tree species (R2 = 0.03 - ECM, R2 = 0.07 - AM, p < 0.05). The addition of N did not alter AM or ECM root fungal community composition (PERMANOVA, p > 0.05) but did alter ECM fungal composition in the soil (R2 = 0.04, p < 0.05).
3.4. Soil chemistry and plant characteristics correlation with taxa
Soil origin had only minor effects on plant growth characteristics, such as leaf number, leaf C:N, root %N, and stem %C (Supplementary Table S7). P. trichocarpa grew more rapidly than P. deltoides and had 10% higher leaf SPAD content, 50% greater height, 54% more leaves, and a higher C:N ratio in roots, leaves, and stems (p < 0.001; Table 3). Nitrate and ammonium were 80 and 29% higher in P. deltoides than P. trichocarpa soils at the end of the experiment (p < 0.001; Table 3). Nitrogen significantly increased plant growth rate for both species grown in both soil types. For instance, plant height was, on average, 133 and 111% greater for P. trichocarpa and P. deltoides, respectively, and leaf number was 193 and 49% greater for greater for P. trichocarpa and P. deltoides, respectively, when N was added (Table 3). Leaf number, stem %N, and soil nitrate varied by Populus species, soil origin, and N addition (p < 0.001; Table 3).
Within roots, the relative abundance three fungal genera, Peziza, Alnicola, and Pezoloma, negatively correlated with soil pH (p < 0.001) but did not significantly correlate with plant characteristics (Figure 5). Within the soil, the relative abundance of 11 fungal genera negatively correlated with soil pH, while the relative abundance of 13 fungal taxa positively correlated with soil pH (Figure 5; p < 0.05). Soil NH4 was positively correlated with the relative abundance of eight fungal taxa in the soil while the relative abundance of only one fungal taxon positively correlated to NO3 in the roots. The percentage of leaf carbon positively correlated to 12 fungal taxa in the soil (Figure 5; p < 0.05), but there were no other plant characteristics that correlated with fungal taxa in the roots.
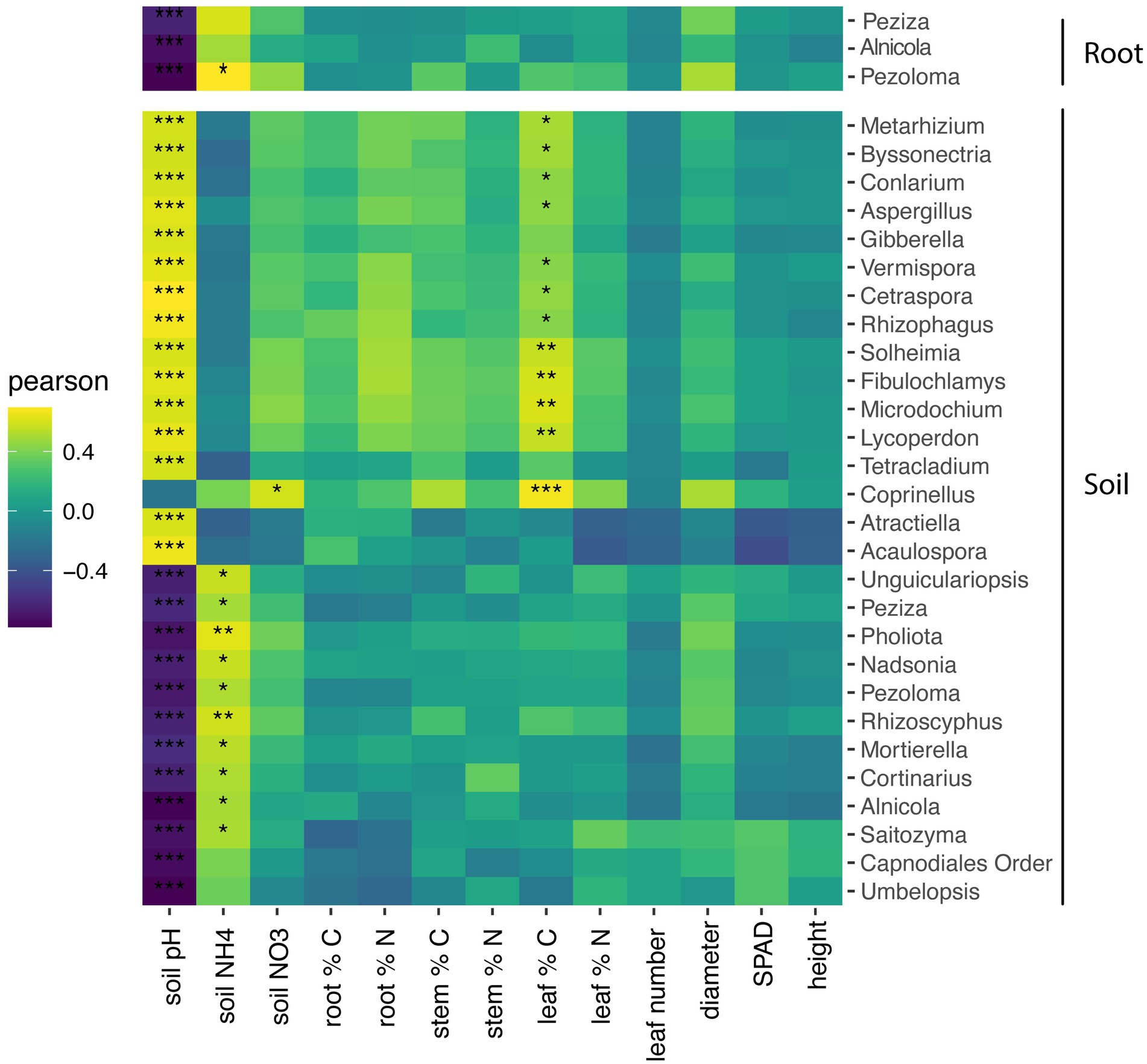
Figure 5. Heatmap of soil and root fungal genera correlations with soil and plant characteristics. Only taxa with significance in at least one environmental measurement is displayed. Pearson correlation test of the relationship between fungal genera and plant and soil characteristics with p values corrected for multiple comparisons by the false-discovery rate method. *P < 0.05; **P < 0.01; ***p < 0.001.
4. Discussion
Management practices within ecosystems, like the addition of nutrients, have been shown to decrease beneficial symbiotic plant-fungal interactions (Wei et al., 2013; Johnson and Gibson, 2021). Consistent with this, we found that N addition significantly altered soil fungal communities, but the addition of N had a smaller impact on root endophytic fungal communities. Instead, within roots, we demonstrated that plant host and soil origin had a larger impact on fungal community diversity, composition, and taxa present, highlighting the dynamic nature of these interactions with implications for the functioning of these symbioses within both managed and natural ecosystems under global change.
Contrary to our hypothesis that N addition would result in convergence of the microbiome across plant species and soil types, we found that the addition of N had minimal impacts on the root mycobiome of P. deltoides and P. trichocarpa, but there were significant impacts of N addition on both soil fungal diversity and community composition. Fungal communities residing within plant roots are often well buffered to short term environmental perturbations (Gdanetz and Trail, 2017; Somenahally et al., 2018). Within roots, soil origin and tree species had the largest effect on fungal alpha diversity, community composition, and core taxa within this plant associated compartment. This result highlights the role plant selection may play on structuring the endospheric mycobiome, and how variation in fungal traits and life history strategy may influence their response to short term increases in soil N. Given the length of our experiment, it is possible that we have not reached a tipping point in soil N concentrations that will drive shifts in overall root fungal diversity and community composition (Lilleskov et al., 2019). Instead, this perturbation may impact distinct taxa present within roots and soils.
Core taxa are often hypothesized to have increased functional importance within the microbiome (Shade and Handelsman, 2012). Interestingly, the only core taxa we found in both P. deltoides and P. trichocarpa roots was the AM fungal taxa, Rhizophagus. Prior studies have demonstrated that Populus readily associate with Rhizophagus spp., and interactions with these fungi can influence plant nutrient uptake (Calabrese et al., 2019). Interestingly, we did not see an impact of N addition on the relative abundance of this core taxon within roots. Within our study, the addition of N profoundly accelerated plant growth relative to plants that were given no extra N. Nitrogen limitation in N− plants was evident by a greater C:N ratio in leaves and roots and lower chlorophyll density. Over the course of the greenhouse experiment, soils were generally depleted in NH4+ for both N+ and N− samples, with NH4+ being only slightly higher in N+ pots. Soil NO3− was also lower in greenhouse endpoint samples relative to field soils, but the difference between N+ and N− was more remarkable than with NH4+ (Table 3). Thus, associations with Rhizophagus under both N treatments may reflect an overall nutrient limitation regardless of N treatment during this greenhouse study when plants were actively growing.
Nitrogen and phosphorus (P) are essential nutrients for plant growth. Within plant-mycorrhizal symbioses, plants provide C to the mycorrhizal community via root exudation and the mycorrhizal fungi directly exchange N and P with their plant hosts. Under N or P limitations, plants invest more C in mycorrhizal fungi (Treseder, 2004) because of the essential role these fungi contribute to nutrient uptake (Marschner and Dell, 1994; George et al., 1995). Conversely, if N or P availability increases, mycorrhizal-plant interactions often decline as the plants allocate carbohydrates elsewhere, thereby limiting C availability to soil fungi. In our study, N addition decreased overall mycorrhizal diversity and altered mycorrhizal fungal communities in the soil. Within the roots, we saw no effect of N addition on mycorrhizal diversity or relative abundance of core mycorrhizal taxa. We hypothesize that N addition did not affect C availability in roots, but available C in the soil may be altered. While there are clear changes in soil mycorrhizal communities with N addition, it is not clear if these shifts in mycorrhizal communities will lead to altered fungal community function or if these changes will persist if N levels return to ambient.
Mismatches between host plant species and soil inoculum may result in decreased host performance especially in response to abiotic stress. Within the soil, we identified 14 unique core taxa that accounted for ~42% of reads. Two of these taxa, Trichoderma (Guzmán-Guzmán et al., 2019) and Inocybe (Bonito et al., 2016) are known plant beneficial fungi. Within our soils, these taxa make up 23.4 and 55.8% relative abundance, respectively. Interestingly, the ECM fungi Inocybe, was a core taxon detected in P. trichocarpa roots. This taxon had a greater relative abundance within P. trichocarpa roots when they were grown in their native Oregon soil (52.4%) but decreased in relative abundance in roots of P. trichocarpa when grown in non-native West Virginia soils (11.6%). Similarly, P. deltoides roots had greater relative abundance of the AM fungi Rhizophagus when grown in its native W. Virginia soil relative to the non-native Oregon soil. Surprisingly, this mismatch of soil origin only had minor effects on plant growth, but as plants mature, these effects could become more pronounced. Future studies should examine the long-term outcomes of mismatches between plant host and soil fungal symbionts. Given the desire to grow Populus for pulp, paper, and biofuel, and the role climatic change may play in shifting the range of these trees in temperate forests, mismatches between plant hosts and symbiotic fungi could have long term consequences within both managed and natural ecosystems (Argüelles-Moyao and Garibay-Orijel, 2018; Peterson and Bode, 2021).
5. Conclusion
Overall, our results highlight the importance of plant species and soil origin on plant-fungal interactions. Nitrogen addition had large effects on fungal diversity, community composition, and the relative abundance and composition of symbiotic mycorrhizal fungal communities, but these effects were most pronounced within soils, not roots, and often contingent upon plant species and soil origin. Future endeavors should extend investigations to understand the long-term impacts of nutrient addition mediated shifts in fungal communities on plant establishment, plant growth and health, nutrient uptake, and subsequent changes in ecosystem function.
Data availability statement
The datasets presented in this study can be found in online repositories. The names of the repository/repositories and accession number(s) can be found at: https://www.ncbi.nlm.nih.gov/, PRJNA875147.
Author contributions
AAC and MAC contributed to the conception and design of the study and wrote the manuscript. DMK, ERJ, MAC, MMC, and SSJ conducted the study, measured plant phenotypes, and contributed to laboratory analyses. AAC, BBH, and ES provided statistical analyses for the manuscript. All authors contributed to the article and approved the submitted version.
Funding
This research was funded as part of the Center for BioEnergy Innovation (CBI) at ORNL. CBI is a U.S. Department of Energy Bioenergy Research Center supported by the Office of Biological and Environmental Research in the DOE Office of Science. This research used resources of the Compute and Data Environment for Science (CADES) at the Oak Ridge National Laboratory, which is supported by the Office of Science of the U.S. Department of Energy under Contract No. DE-AC05-00OR22725. ES was supported in part by the U.S. Department of Energy, Office of Science, Office of Workforce Development for Teachers and Scientists (WDTS) under the Science Undergraduate Laboratory Internships program. BH was supported in part by the GEM Fellowship Program.
Acknowledgments
We thank the editor and reviewers for their careful review and constructive feedback. We also thank Greenwood Resources, Inc. for providing Populus trichocarpa cuttings and Oregon soil. Additionally, we are grateful to Stephen DiFazio at West Virginia University for providing Populus deltoides cuttings and West Virginia soil. Figure 1 was created with BioRender.com.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2022.1033631/full#supplementary-material
References
Argüelles-Moyao, A., and Garibay-Orijel, R. (2018). Ectomycorrhizal fungal communities in high mountain conifer forests in Central Mexico and their potential use in the assisted migration of Abies religiosa. Mycorrhiza 28, 509–521. doi: 10.1007/s00572-018-0841-0
Bellgard, S., and Williams, S. (2011). Response of mycorrhizal diversity to current climatic changes. Diversity 3, 8–90. doi: 10.3390/d3010008
Bennett, A. E., and Classen, A. T. (2020). Climate change influences mycorrhizal fungal–plant interactions, but conclusions are limited by geographical study bias. Ecology 101:e02978. doi: 10.1002/ecy.2978
Bolyen, E., Rideout, J. R., Dillon, M. R., Bokulich, N. A., Abnet, C. C., Al-Ghalith, G. A., et al. (2019). Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 37, 852–857. doi: 10.1038/s41587-019-0209-9
Bonfante, P., and Genre, A. (2010). Mechanisms underlying beneficial plant–fungus interactions in mycorrhizal symbiosis. Nat. Commun. 1:48. doi: 10.1038/ncomms1046
Bonito, G., Hameed, K., Ventura, R., Krishnan, J., Schadt, C. W., and Vilgalys, R. (2016). Isolating a functionally relevant guild of fungi from the root microbiome of Populus. Fungal Ecol. 22, 35–42. doi: 10.1016/j.funeco.2016.04.007
Bonito, G., Reynolds, H., Robeson, M. S., Nelson, J., Hodkinson, B. P., Tuskan, G., et al. (2014). Plant host and soil origin influence fungal and bacterial assemblages in the roots of woody plants. Mol. Ecol. 23, 3356–3370. doi: 10.1111/mec.12821
Calabrese, S., Cusant, L., Sarazin, A., Niehl, A., Erban, A., Brulé, D., et al. (2019). Imbalanced regulation of fungal nutrient transports according to phosphate availability in a Symbiocosm formed by poplar, sorghum, and Rhizophagus irregularis. Front. Plant Sci. 10:1617. doi: 10.3389/fpls.2019.01617
Callahan, B. J., McMurdie, P. J., Rosen, M. J., Han, A. W., Johnson, A. J. A., and Holmes, S. P. (2016). DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583. doi: 10.1038/nmeth.3869
Canfield, D. E., Glazer, A. N., and Falkowski, P. G. (2010). The evolution and future of Earth’s nitrogen cycle. Science 330, 192–196. doi: 10.1126/science.1186120
Chao, A., Chiu, C.-H., and Hsieh, T. C. (2012). Proposing a resolution to debates on diversity partitioning. Ecology 93, 2037–2051. doi: 10.1890/11-1817.1
Chao, A., Chiu, C.-H., and Jost, L. (2014). Unifying species diversity, phylogenetic diversity, functional diversity, and related similarity and differentiation measures through Hill numbers. Annu. Rev. Ecol. Evol. Syst. 45, 297–324. doi: 10.1146/annurev-ecolsys-120213-091540
Compant, S., Van Der Heijden, M. G. A., and Sessitsch, A. (2010). Climate change effects on beneficial plant-microorganism interactions: climate change and beneficial plant-microorganism interactions. FEMS Microbiol. Ecol. 73, 197–214. doi: 10.1111/j.1574-6941.2010.00900.x
Coyle, D., Coleman, M., Durant, J., and Newman, L. (2006). Survival and growth of 31 Populus clones in South Carolina. Biomass Bioenergy 30, 750–758. doi: 10.1016/j.biombioe.2005.08.005
Cregger, M. A., Carper, D. L., Christel, S., Doktycz, M. J., Labbé, J., Michener, J. K., et al. (2021). Plant–microbe interactions: from genes to ecosystems using Populus as a model system. Phytobiomes J. 5, 29–38. doi: 10.1094/PBIOMES-01-20-0009-FI
Cregger, M. A., Veach, A. M., Yang, Z. K., Crouch, M. J., Vilgalys, R., Tuskan, G. A., et al. (2018). The Populus holobiont: dissecting the effects of plant niches and genotype on the microbiome. Microbiome 6:31. doi: 10.1186/s40168-018-0413-8
Cronk, Q. C. B. (2005). Plant eco-devo: the potential of poplar as a model organism. New Phytol. 166, 39–48. doi: 10.1111/j.1469-8137.2005.01369.x
de Vries, F. T., Griffiths, R. I., Bailey, M., Craig, H., Girlanda, M., Gweon, H. S., et al. (2018). Soil bacterial networks are less stable under drought than fungal networks. Nat. Commun. 9:3033. doi: 10.1038/s41467-018-05516-7
Dove, N. C., Veach, A. M., Muchero, W., Wahl, T., Stegen, J. C., Schadt, C. W., et al. (2021). Assembly of the Populus microbiome is temporally dynamic and determined by selective and stochastic factors. mSphere 6:20. doi: 10.1128/mSphere.01316-20
Gdanetz, K., and Trail, F. (2017). The wheat microbiome under four management strategies, and potential for endophytes in disease protection. Phytobiomes J. 1, 158–168. doi: 10.1094/PBIOMES-05-17-0023-R
Gehring, C. A., and Connell, J. H. (2006). Arbuscular mycorrhizal fungi in the tree seedlings of two Australian rain forests: occurrence, colonization, and relationships with plant performance. Mycorrhiza 16, 89–98. doi: 10.1007/s00572-005-0018-5
George, E., Marschner, H., and Jakobsen, I. (1995). Role of arbuscular mycorrhizal fungi in uptake of phosphorus and nitrogen from soil. Crit. Rev. Biotechnol. 15, 257–270. doi: 10.3109/07388559509147412
Guzmán-Guzmán, P., Porras-Troncoso, M. D., Olmedo-Monfil, V., and Herrera-Estrella, A. (2019). Trichoderma species: versatile plant symbionts. Phytopathology 109, 6–16. doi: 10.1094/PHYTO-07-18-0218-RVW
Hacquard, S., and Schadt, C. W. (2015). Towards a holistic understanding of the beneficial interactions across the Populus microbiome. New Phytol. 205, 1424–1430. doi: 10.1111/nph.13133
Happs, R. M., Bartling, A. W., Doeppke, C., Harman-Ware, A. E., Clark, R., Webb, E. G., et al. (2021). Economic impact of yield and composition variation in bioenergy crops: POPULUS TRICHOCARPA. Biofuels Bioprod. Biorefin. 15, 176–188. doi: 10.1002/bbb.2148
Hill, M. O. (1973). Diversity and evenness: a unifying notation and its consequences. Ecology 54, 427–432. doi: 10.2307/1934352
Hodge, A., and Storer, K. (2015). Arbuscular mycorrhiza and nitrogen: implications for individual plants through to ecosystems. Plant Soil 386, 1–19. doi: 10.1007/s11104-014-2162-1
Johnson, N. C., and Gibson, K. S. (2021). Understanding multilevel selection may facilitate Management of Arbuscular Mycorrhizae in sustainable agroecosystems. Front. Plant Sci. 11:627345. doi: 10.3389/fpls.2020.627345
Kirsten, W. J., and Hesselius, G. U. (1983). Rapid, automatic, high capacity dumas determination of nitrogen. Microchem. J. 28, 529–547. doi: 10.1016/0026-265X(83)90011-5
Kivinen, S., Koivisto, E., Keski-Saari, S., Poikolainen, L., Tanhuanpää, T., Kuzmin, A., et al. (2020). A keystone species, European aspen (Populus tremula L.), in boreal forests: ecological role, knowledge needs and mapping using remote sensing. For. Ecol. Manag. 462:118008. doi: 10.1016/j.foreco.2020.118008
Lilleskov, E. A., Kuyper, T. W., Bidartondo, M. I., and Hobbie, E. A. (2019). Atmospheric nitrogen deposition impacts on the structure and function of forest mycorrhizal communities: a review. Environ. Pollut. 246, 148–162. doi: 10.1016/j.envpol.2018.11.074
Liu, T., Sheng, M., Wang, C. Y., Chen, H., Li, Z., and Tang, M. (2015). Impact of arbuscular mycorrhizal fungi on the growth, water status, and photosynthesis of hybrid poplar under drought stress and recovery. Photosynthetica 53, 250–258. doi: 10.1007/s11099-015-0100-y
Marschner, H., and Dell, B. (1994). Nutrient uptake in mycorrhizal symbiosis. Plant Soil 159, 89–102. doi: 10.1007/BF00000098
Martin, F., Aerts, A., Ahrén, D., Brun, A., Danchin, E. G. J., Duchaussoy, F., et al. (2008). The genome of Laccaria bicolor provides insights into mycorrhizal symbiosis. Nature 452, 88–92. doi: 10.1038/nature06556
Miransari, M. (2011). Arbuscular mycorrhizal fungi and nitrogen uptake. Arch. Microbiol. 193, 77–81. doi: 10.1007/s00203-010-0657-6
Nguyen, N. H., Song, Z., Bates, S. T., Branco, S., Tedersoo, L., Menke, J., et al. (2016). FUNGuild: an open annotation tool for parsing fungal community datasets by ecological guild. Fungal Ecol. 20, 241–248. doi: 10.1016/j.funeco.2015.06.006
Nilsson, R. H., Larsson, K.-H., Taylor, A. F. S., Bengtsson-Palme, J., Jeppesen, T. S., Schigel, D., et al. (2019). The UNITE database for molecular identification of fungi: handling dark taxa and parallel taxonomic classifications. Nucleic Acids Res. 47, D259–D264. doi: 10.1093/nar/gky1022
Peterson, K., and Bode, M. (2021). Using ensemble modeling to predict the impacts of assisted migration on recipient ecosystems. Conserv. Biol. 35, 678–687. doi: 10.1111/cobi.13571
Pickles, B. J., Egger, K. N., Massicotte, H. B., and Green, D. S. (2012). Ectomycorrhizas and climate change. Fungal Ecol. 5, 73–84. doi: 10.1016/j.funeco.2011.08.009
Robertson, G.P., Coleman, D.C., Bledsoe, C.S., and Sollins, P. (1999). Standard soil methods for long-term ecological research, long-term ecological research network series. Oxford university press, New York Oxford.
Sannigrahi, P., Ragauskas, A. J., and Tuskan, G. A. (2010). Poplar as a feedstock for biofuels: a review of compositional characteristics. Biofuels Bioprod. Biorefin. 4, 209–226. doi: 10.1002/bbb.206
Shade, A., and Handelsman, J. (2012). Beyond the Venn diagram: the hunt for a core microbiome: the hunt for a core microbiome. Environ. Microbiol. 14, 4–12. doi: 10.1111/j.1462-2920.2011.02585.x
Shakya, M., Gottel, N., Castro, H., Yang, Z. K., Gunter, L., Labbé, J., et al. (2013). A multifactor analysis of fungal and bacterial community structure in the root microbiome of mature Populus deltoides trees. PLoS One 8:e76382. doi: 10.1371/journal.pone.0076382
Shetty, S. A., and Lahti, L. (2019). Microbiome data science. J. Biosci. 44:115. doi: 10.1007/s12038-019-9930-2
Somenahally, A., DuPont, J. I., Brady, J., McLawrence, J., Northup, B., and Gowda, P. (2018). Microbial communities in soil profile are more responsive to legacy effects of wheat-cover crop rotations than tillage systems. Soil Biol. Biochem. 123, 126–135. doi: 10.1016/j.soilbio.2018.04.025
Suarez-Gonzalez, A., Hefer, C. A., Christe, C., Corea, O., Lexer, C., Cronk, Q. C. B., et al. (2016). Genomic and functional approaches reveal a case of adaptive introgression from Populus balsamifera (balsam poplar) in P. trichocarpa (black cottonwood). Mol. Ecol. 25, 2427–2442. doi: 10.1111/mec.13539
Teste, F. P., Jones, M. D., and Dickie, I. A. (2020). Dual-mycorrhizal plants: their ecology and relevance. New Phytol. 225, 1835–1851. doi: 10.1111/nph.16190
Treseder, K. K. (2004). A meta-analysis of mycorrhizal responses to nitrogen, phosphorus, and atmospheric CO 2 in field studies. New Phytol. 164, 347–355. doi: 10.1111/j.1469-8137.2004.01159.x
Vandenkoornhuyse, P., Quaiser, A., Duhamel, M., Le Van, A., and Dufresne, A. (2015). The importance of the microbiome of the plant holobiont. New Phytol. 206, 1196–1206. doi: 10.1111/nph.13312
Veach, A. M., Chen, H., Yang, Z. K., Labbe, A. D., Engle, N. L., Tschaplinski, T. J., et al. (2020). Plant hosts modify belowground microbial community response to extreme drought. mSystems 5, e00092–e00020. doi: 10.1128/mSystems.00092-20
Veach, A. M., Morris, R., Yip, D. Z., Yang, Z. K., Engle, N. L., Cregger, M. A., et al. (2019). Rhizosphere microbiomes diverge among Populus trichocarpa plant-host genotypes and chemotypes, but it depends on soil origin. Microbiome 7:76. doi: 10.1186/s40168-019-0668-8
Wei, C., Yu, Q., Bai, E., Lü, X., Li, Q., Xia, J., et al. (2013). Nitrogen deposition weakens plant-microbe interactions in grassland ecosystems. Glob. Change Biol. 19, 3688–3697. doi: 10.1111/gcb.12348
White, R. A., Rivas-Ubach, A., Borkum, M. I., Köberl, M., Bilbao, A., Colby, S. M., et al. (2017). The state of rhizospheric science in the era of multi-omics: a practical guide to omics technologies. Rhizosphere 3, 212–221. doi: 10.1016/j.rhisph.2017.05.003
Yang, N., Wang, B., Liu, D., Wang, X., Li, X., Zhang, Y., et al. (2021). Long-term nitrogen deposition alters ectomycorrhizal community composition and function in a poplar plantation. J. Fungi 7:791. doi: 10.3390/jof7100791
Keywords: mycorrhizal fungi, nitrogen addition, plant-microbe interactions, Populus, symbiosis
Citation: Carrell AA, Hicks BB, Sidelinger E, Johnston ER, Jawdy SS, Clark MM, Klingeman DM and Cregger MA (2023) Nitrogen addition alters soil fungal communities, but root fungal communities are resistant to change. Front. Microbiol. 13:1033631. doi: 10.3389/fmicb.2022.1033631
Edited by:
Marc Gregory Dumont, University of Southampton, United KingdomReviewed by:
Mengting Maggie Yuan, University of California, Berkeley, United StatesTessa E. Reid, Rothamsted Research, United Kingdom
Copyright © 2023 Carrell, Hicks, Sidelinger, Johnston, Jawdy, Clark, Klingeman and Cregger. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Melissa A. Cregger ✉ creggerma@ornl.gov
†Present address: Eric R. Johnston,Schneider National, Green Bay, WI, United States
 Alyssa A. Carrell
Alyssa A. Carrell Brittany B. Hicks
Brittany B. Hicks Emilie Sidelinger3
Emilie Sidelinger3 Sara S. Jawdy
Sara S. Jawdy Melissa A. Cregger
Melissa A. Cregger