- 1CAS Key Laboratory for Agro-Ecological Processes in Subtropical Region, National Engineering Laboratory for Pollution Control and Waste Utilization in Livestock and Poultry Production, Hunan Provincial Key Laboratory of Animal Nutritional Physiology and Metabolic Process, Institute of Subtropical Agriculture, The Chinese Academy of Sciences, Changsha, China
- 2University of Chinese Academy of Science, Beijing, China
- 3School of Animal Science, Inner Mongolia Agricultural University, Hohhot, China
- 4Inner Mongolia Academy of Agricultural and Animal Husbandry Sciences, Hohhot, China
Silybum marianum meal is a by-product that remains silymarin complex and is perceived as a potential-protein source. The potential and its mechanism of silybum marianum meal as a protein supplement in ruminants were evaluated by testing the growth performance, biochemical parameters, cytokine levels, gut transcriptome and microbial community profiles. Forty-two male Hulunbeier growing lambs (aged about 3-month-old; averaged body weight of 21.55 kg) were randomly divided into the CON (with 10% soybean meal) and SIL groups (with 10% silybum marianum meal). There was no significant difference in growth performance, feed intakes, or serum biochemical parameters between CON and SIL. The serum levels of IL-1β, TNF-α, TGF-β, HGF, and VEGF were all increased (p < 0.05) in the SIL group as compared with the CON group. Transcriptome gene set enrichment analysis (GSEA) revealed that the core genes in the rumen from SIL group were enriched with fructose and mannose metabolism, while the core genes in the ileum were enriched for three biological process, including digestive tract development, positive regulation of MAPK cascade, and regulation of I-kappaB kinase/NF-kappaB signaling. The 16S rDNA results showed that the relative abundance of Bacteroidetes, Firmicutes, Synergistetes, and Verrucomicrobia in the rumen from SIL group was significantly higher than that in CON group (p < 0.05), whereas Proteobacteria was significantly lower than that in CON group (p < 0.05). The LEfSe analysis showed that the genera Pyramidobacter, Saccharofermentans, Anaerovibrio, Oscillibacter and Barnesiella were enriched in the rumen from SIL group, whereas Sharpea was enriched in the CON group (LDA > 2). In the ileum, there were no significant differences in the phylum-level classification of microbes observed. At the genus level, the relative abundances of Bifidobacterium and Ruminococcus in the ileum from SIL group were significantly higher than that in the CON group (p < 0.05), whereas the relative abundance of Clostridium_XI was lower (p < 0.05). Correlation analysis showed that Clostridium_XI was negatively correlated with VEGF, TGF-β, TNF-α and HGF (p < 0.05). Core genes BMP4 and CD4 were negatively correlated with Clostridium_XI (p < 0.05). Our results indicated that supplementing silybum marianum meal as a replacement for soybean meal resulted in increased cytokines production without affecting growth performance in growing lambs, and the enrichment of immune-related genes and altered microbial community in the ileum were contributed to the increased immune responses.
1. Introduction
The livestock industry is struggling to access quality protein feed that could satisfy animal body requirements at an affordable price, in particular in developing countries. With the current trends of rising prices of soybean meal, it is essential to develop locally available low-cost protein supplements. Silybum marianum is a kind of medicinal herb with potential health effects, which is widely grown in Asia and Latin America. The silymarin, a main bioactive component of silybum marianum, contains an isomeric mixture of unique flavonoid complexes and has hepatic protection, detoxification and anti-oxidation functions (Tajmohammadi et al., 2018). The application of silymarin in livestock has received considerable attention due to the advancement of extraction technology and its role in promoting growth. Silymarin has been shown to have a positive effect on the production and carcass plasma profiles in laying hens (Šťastník et al., 2019), horse (Dockalova et al., 2021), and pigs (Grela et al., 2020). In addition, silybum marianum meal is a by-product of silymarin production (Dehghan et al., 2011). Due to its high protein content, it has a potential to use as an unconventional feed resource (Fan et al., 2019). However, mature silybum marianum contains nitrite, which is supposed to have a toxic effect in animal (Arviv et al., 2016), resulting in increased inflammatory responses. In addition, silybum marianum meal contains a high fiber level (Stastnik et al., 2020), which may be beneficial for ruminants theoretically. The potential and its mechanism of silybum marianum meal as a protein supplement in ruminants remain uncovered.
With the development of high-throughput sequencing technology, multi-omics approach was widely used to explore the interaction between microbiota and host in response to variable phenotypes at a molecular level. The objectives of this study were: (1) to evaluate the effects of substituting soybean meal with silybum marianum meal on the growth performance, biochemical parameters and cytokine levels, (2) to explore the potential mechanism in response to altered phenotypes by using an integrated transcriptome and microbial community analysis.
2. Materials and methods
2.1. Animals, diets, and experimental design
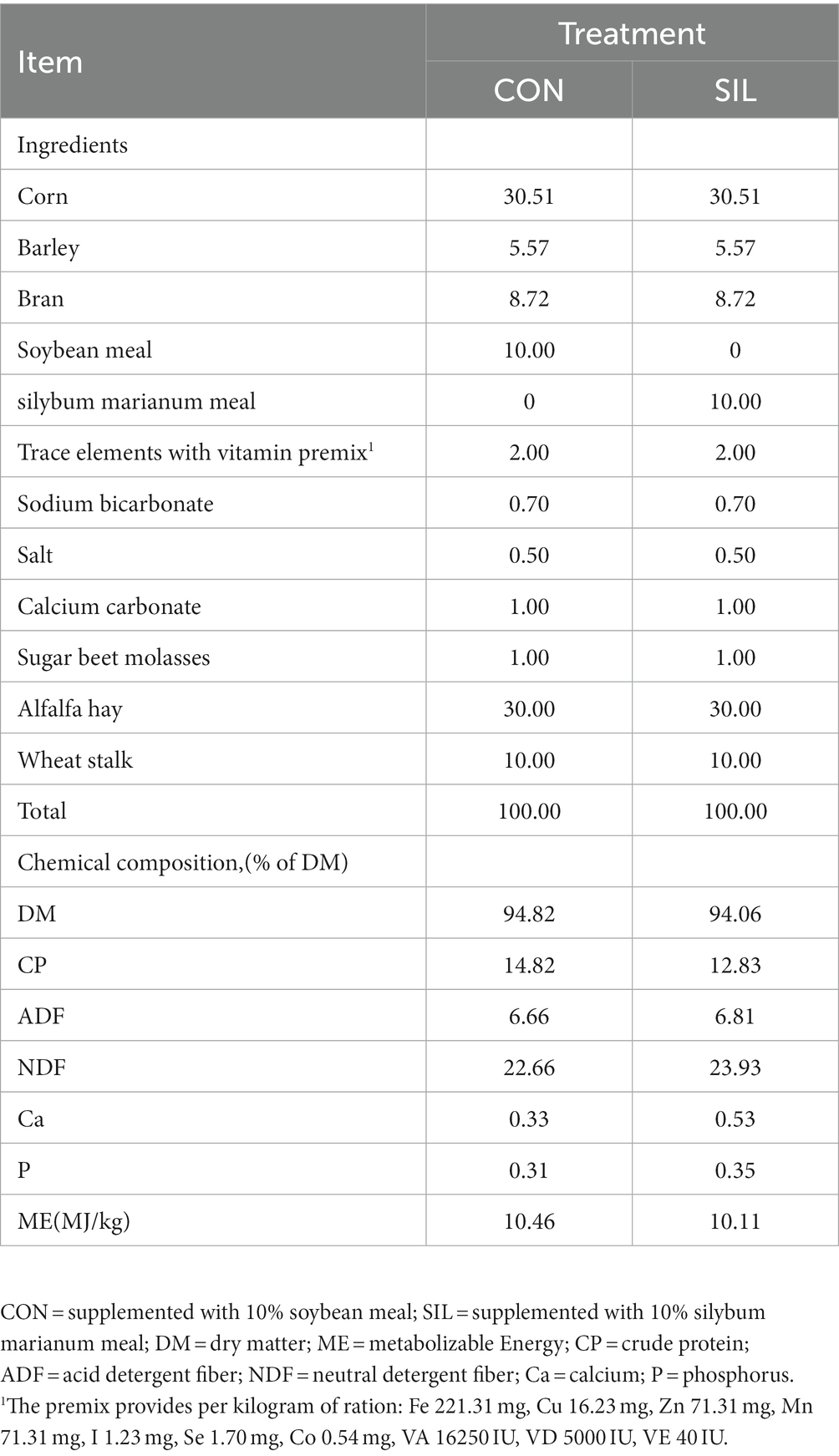
The experimental procedures of this study were carried out in accordance with the guidelines of the Animal Ethics Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences. A total of 42 healthy 3-month-old Hulunbeier lambs (averaged with 21.55 kg) with similar body weight were selected and randomly divided into two groups (initial weight was tested for non-significant differences between groups), with 21 sheep in each group. The experimental animals were divided into the CON (supplemented with 10% soybean meal) and SIL groups (supplemented with 10% silybum marianum meal). The compositions and nutrient levels of the experimental diets are shown in Table 1. The experiment lasted for 90 days, including the pre-feeding period of 15 days and the trial period of 75 days. During the pre-feeding period, the experimental animals were ear tagged, dewormed, weighed, and vaccinated according to the normal immunization program for lamb fattening. The sheep were fed and watered three times a day, at 6:00, 11:00 and 18:00, respectively, to ensure that there was a surplus of diet and water. The same diet was fed during the pre-feeding period and the trial period.
2.2. Sample collection
All the experimental sheep were weighed every 2 weeks before morning feeding from the beginning of the experimental period, the data were recorded, and the daily weight gain was calculated. The amount of feed was recorded daily, and the leftover was recorded before feeding the following day. The remaining feed was cleared afterward, and a new feed was added. Average daily feed intake = total feed intake/number of experimental days; Feed weight ratio (F/g) = average daily intake / average daily gain.
At the end of the animal test, seven tested sheep in each group were randomly selected for blood collection by jugular venipuncture before morning feeding. The blood was collected with a 10 ml BD Vacutainer serum tube and stored at 4°C for 30 min, then centrifuged at 3,000 rpm/min for 15 min to separate the serum. The collected supernatant was placed in a 1.5 ml centrifuge tube and stored at −20°C.
After dissection, the rumen was opened, and the rumen digesta collected. Then, rumen abdominal sac (5 × 5 cm) was collected. The ileum digesta and ileum tissue were collected from 50 cm proximal to the junction of the ileum and the cecum. Tissue samples were washed with pre-cooled phosphate buffer during collection. After collection, digesta and tissue samples were snap-frozen in liquid nitrogen and stored in an ultra-low temperature freezer at −80°C. Tissue samples were used for RNA extraction and sequencing, and digesta samples were used for 16S rDNA gene sequencing and bioinformatics analysis. Since the rumen and ileum were vital organs for feed digestion and immune functions (Mowat and Agace, 2014; Na and Guan, 2022), their tissue and digest were selected for further transcriptome and microbial community analysis.
2.3. Analysis of biochemical parameters and cytokine levels in blood
Serum biochemical parameters, including total protein (TP), high-density lipoprotein (HDL), glutamic aminotransferase (ALT), glutamic oxalacetic aminotransferase (AST), lactate dehydrogenase (LDH), glucose (GLU), triglycerides (TG), total cholesterol (CHOL), amylase (AMS), blood ammonia (NH3L), total bilirubin (TBIL), and c-reactive protein (CRP), were measured using an automated biochemical instrument (Cobasc311, Roche, Basel, Switzerland). Serum cytokine indicators, including interleukin 1β (IL-1β), interleukin 2 (IL-2), tumor necrosis factor (TNF-α), transforming growth factor beta (TGF-β), hepatocyte growth factor (HGF), vascular endothelial growth factor (VEGF), insulin-like growth factor 1 (IGF-1) were measured using commercial sheep-specific ELISA kits(Jiangsu Meimian industrial Co., Ltd., Yancheng, China).
2.4. Rumen and ileum RNA sequencing and bioinformatic analysis
Total RNA was extracted from 14 rumen and 14 ileum tissues using Trizol (Invitrogen, Carlsbad, CA, United States) according to the manual. Subsequently, total RNA was characterized and quantified using a NanoDrop and Agilent 2,100 Bioanalyzer (Thermo Fisher Scientific, MA, United States).
Oligo (dT)-linked magnetic beads were used to purify mRNA. The purified mRNA was split into small pieces at the appropriate temperature using a fragmentation buffer. First-strand cDNA was then generated using random hexamer-triggered reverse transcription, followed by the synthesis of second-strand cDNA. After which, A-Tailing Mix and RNA Index Adapters were added by incubation to conclude the repair. The cDNA fragment obtained in the previous step was amplified by PCR and the product was purified by Ampure XP Beads and then dissolved in EB solution. The product was validated for quality control on an Agilent Technologies 2,100 Bioanalyzer. The double-stranded PCR products from the previous step were heat denatured and cyclized by splinting oligonucleotide sequences to obtain the final library. Single-stranded circular DNA (ssCirDNA) was formatted as the final library. The final library was amplified with phi29 to make DNA nanoballs (DNBs) with more than 300 copies of one molecule, DNBs were loaded into patterned nanoarrays, and single-end 50-base reads were generated on the BGIseq500 platform (BGI-Shenzhen, China).
The sequencing data was filtered with SOAPnuke (v1.5.2) (Li et al., 2008): (1) to remove reads containing sequencing adapters; (2) to remove reads with a low-quality base ratio (base quality less than or equal to 5) greater than 20%; (3) Remove reads with a ratio of unknown bases (‘N’ bases) greater than 5%, after which clean reads are obtained and saved in FASTQ format. These clean reads were mapped onto the sheep reference genome (GCF_002742125.1_Oar_rambouillet_v1.0) using HISAT2 (v2.0.4) (Kim et al., 2015). Bowtie2 (v2.2.5) (Langmead and Salzberg, 2012) was applied to align the clean reads to the reference coding genome, and then RSEM (v1.2.12) (Li and Dewey, 2011) was used to calculate gene expression levels. Transcripts Per Kilobase of exon model per Million mapped reads (TPM) were used to estimate the amount of gene expression in each sample. The Gene Set Enrichment Analysis (GSEA) algorithm was used to identify the pathways, biological processes, or functional components of genes with the most significant changes in expression in the CON and SIL samples (|NES| > 1, Nominal value of p:<0.05, FDR q-value:<0.25, https://www.gsea-msigdb.org/gsea/index.jsp).
2.5. Sequencing and bioinformatics analysis of 16SrDNA gene of rumen and ileum microorganisms
Microbial DNA was extracted from 14 rumen digest samples and 14 ileum digest samples using MagPure Stool DNA KF kit B (Magen, China) according to the manufacturer’s instructions. DNA was quantified with a Qubit Fluorometer by using Qubit dsDNA BR Assay kit (Invitrogen, United States) and the quality was checked by running aliquot on 1% agarose gel. The variable region V3-V4 of the bacterial 16S rRNA gene was amplified using the condensed PCR primers 341F (5’-ACTCCTACGGGAGGCAGCAG-3′) and 806R (5’-GGACTACHVGGGTWTCTAAT-3′). Forward and reverse primers were labeled with Illumina adapters, pads and splice sequences. PCR enrichment was performed in a 50 μ L reaction containing a 30 ng template, fusion PCR primers and PCR master mix. PCR cycling conditions were as follows: 94°C for 3 min, 94°C for 30 s, 56°C for 45 s, 72°C for 45 s, 30 cycles, 72°C for 10 min final extension for 10 min. PCR products were purified with AmpureXP beads and eluted in elution buffer. Libraries were characterized by Agilent 2,100 Bioanalyzer (Agilent, United States). Validated libraries were used for sequencing on the Illumina MiSeq platform (BGI, Shenzhen, China) following Illumina’s standard procedure and generating 2 × 300 bp double-end reads.
The raw reads were filtered to remove splices and low quality and ambiguous bases, and then double-ended reads were added to the tags by the Fast Length Adjustment of Short reads program (FLASH, v1.2.11) (Magoc and Salzberg, 2011) to obtain the tags. The tags were clustered into OTUs with a cutoff of 97% using UPARSE software (v7.0.1090) (Edgar, 2013) and the chimeric sequences were compared to the Gold database for detection using UCHIME (v4.2.40) (Edgar et al., 2011). Then, OTU representative sequences were classified using the Ribosome Database Project (RDP) classifier v.2.2 with a minimum confidence threshold of 0.6 and trained on the Greengenes database v201305 using QIIME v1.8.0 (Caporaso et al., 2010). All Tags were compared with OTUs using USEARCH_global (Edgar, 2010) to obtain a statistical table of OTU abundance for each sample. The data were analyzed for diversity indices based on OTUs, including Chao1, ACE, Simpson and Shannon indices. Beta diversity analysis was performed using principal coordinate analysis (PCoA) with weighted UniFrac distances. Linear discriminant analysis (LDA) effect size (LEfSe) tools were applied to identify different taxa between CON and SIL groups by using an online software package (LC Bio-Technology Co., Ltd., Hangzhou, China) with thresholds (p < 0.05 and LDA > 2).
2.6. Statistical analysis
Firstly, Shapiro–Wilk and Levene’s tests were employed to confirm the normality and homoscedasticity of data, respectively. The serum biochemical and immune indices, and alpha-diversity index results were analyzed using the independent-sample t-test in SPSS software (SPSS version 25.0, SPSS, Inc.). The relative abundance bacteria at the phylum and genus levels was assessed using Wilcoxon rank-sum test. Data are presented as means ± SEM. p < 0.05 was regarded as statistically significant, and 0.05 ≤ p < 0.10 was regarded as a statistical tendency. The correlation analysis was performed using Spearman’s rank correlation coefficient in R package and the network was constructed on the Omics-studio (LC-Bio Technology Co., Ltd., Hangzhou, China).
3. Results
3.1. Effects of replacing soybean meal with silybum marianum meal on growth performance, biochemical parameters, and cytokine levels in growing lambs
No difference (p > 0.05) was observed between SIL and CON for final body weight, feed average daily gain, or feed intake (Table 2). Most of the biochemical parameters were not affected by replacing soybean meal with silybum marianum meal except that the TP tended to decrease (p = 0.08) and the AST tended to increase (p = 0.06; Table 3).
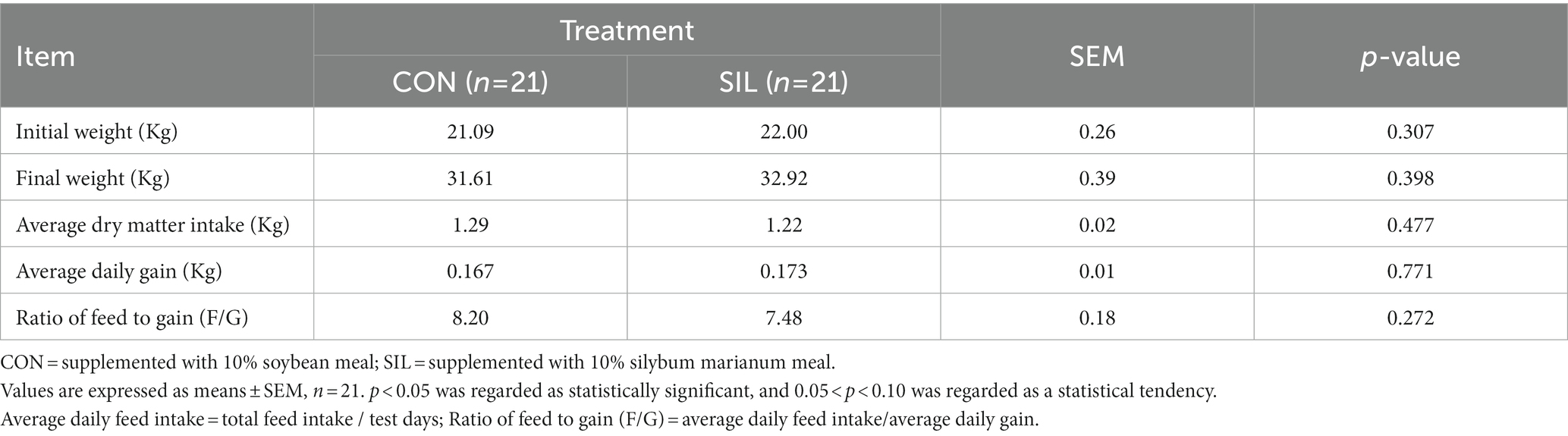
Table 2. Effect of silybum marianum meal as a replacement for soybean meal on feed intake and growth performance of lambs.
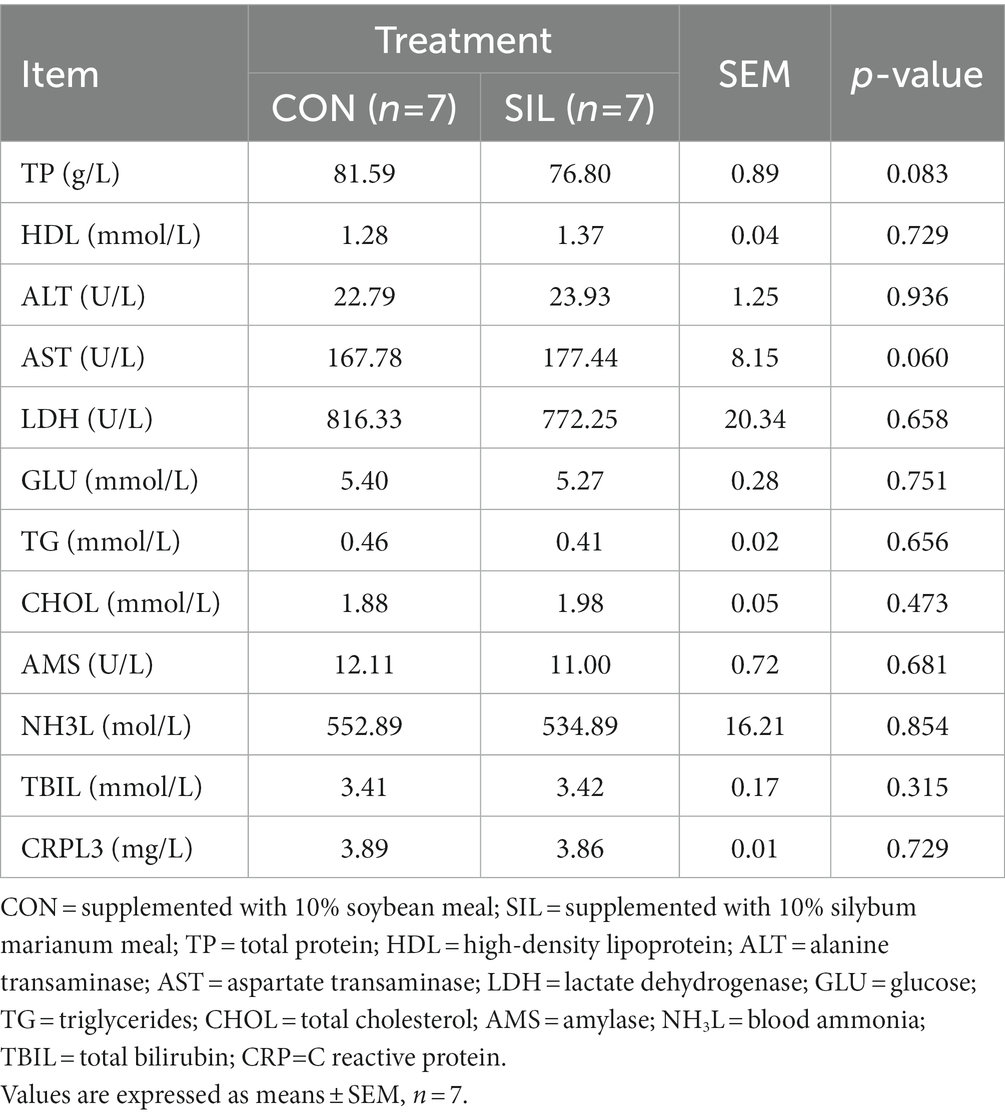
Table 3. Effect of silybum marianum meal as a replacement for soybean meal on serum biochemical parameters in lambs.
Serum cytokines were determined to assess the effects of the immune response when replacing silybum marianum meal (Table 4). The levels of IL-1β and TNF-α were significantly increased in SIL as compared with CON (p < 0.05). Similarly, lambs in SIL had higher concentrations (p < 0.05) of TGF-β, HGF and VEGF than those in CON. The concentrations of IL-2 (p = 0.083) and IGF-1 (p = 0.075) tended to be higher in SIL as compared with CON.
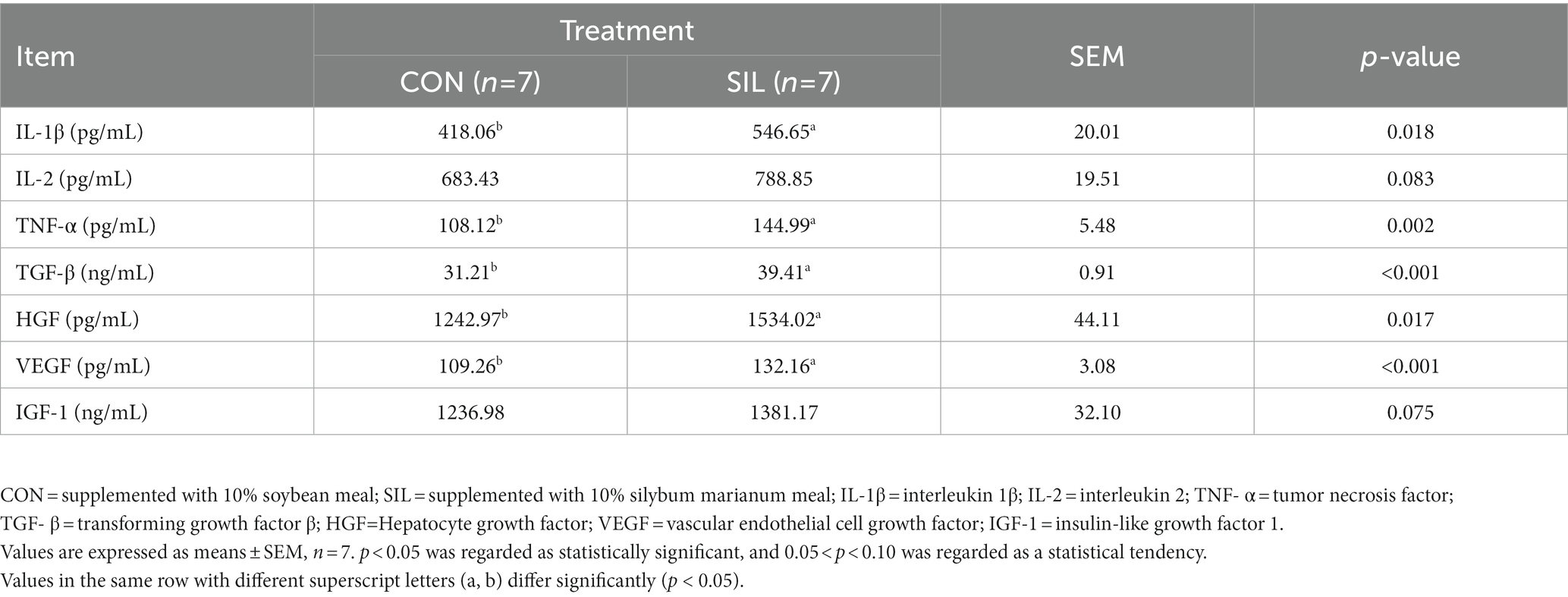
Table 4. Effect of silybum marianum meal as a replacement for soybean meal on serum cytokine levels in lambs.
3.2. Effects of replacing soybean meal with silybum marianum meal on transcriptome profiles in rumen and ileum of growing lambs
The transcriptomes of the rumen and ileum tissues were evaluated to explore the potential mechanism in response to the altered cytokines in the blood. RNA-seq of the rumen tissue in the CON and SIL groups yielded approximately 23.95 and 23.38 million clean reads, of which 95.67 and 95.49% were mapped to the sheep reference genome (Supplementary file 2; Sheet 1). About 17,682 and 17,958 expressed genes were detected in the rumen of the CON and SIL groups, respectively (Supplementary file 2; Sheet 2). RNA-seq of the ileum tissue from the CON and SIL groups yielded approximately 23.55 and 23.75 million clean reads, 94.55 and 94.37% of which were mapped to the sheep reference genome (Supplementary file 2; Sheet 3). 16,127 and 16,092 expressed genes were detected in ileum tissue from the CON and SIL groups, respectively (Supplementary file 2; Sheet 4).
Since the number of differential expressed genes observed in the rumen or ileum between groups was small (Supplementary file 4), GSEA algorithm was used to further identify the pathways, biological processes or functional components of genes with the most significant changes in expression. Results showed that the rumen of the SIL was enriched with fructose and mannose metabolism-related genes in the Kyoto Encyclopedia of Genes and Genomes (KEGG) (NES: 1.875, Nominal value of p: 0.007, FDR q-value: 0.178) (Figure 1A; Supplementary file 3; Sheet 1). Ileum of the SIL was enriched for three biological process-related genes in the Gene Ontology (GO), including digestive tract development (NES: 1.883, Nominal value of p: <0.001, FDR q-value: 0.186) (Figure 1B; Supplementary file 3; Sheet 2), positive regulation of MAPK cascade (NES: 1.667, Nominal value of p: <0.001, FDR q-value: 0.211) (Figure 1C; Supplementary file 3; Sheet 3) and regulation of I-kappaB kinase/NF-kappaB signaling (NES: 1.763, Nominal value of p: 0.002, FDR q-value: 0.236) (Figure 1D; Supplementary file 3; Sheet 4).
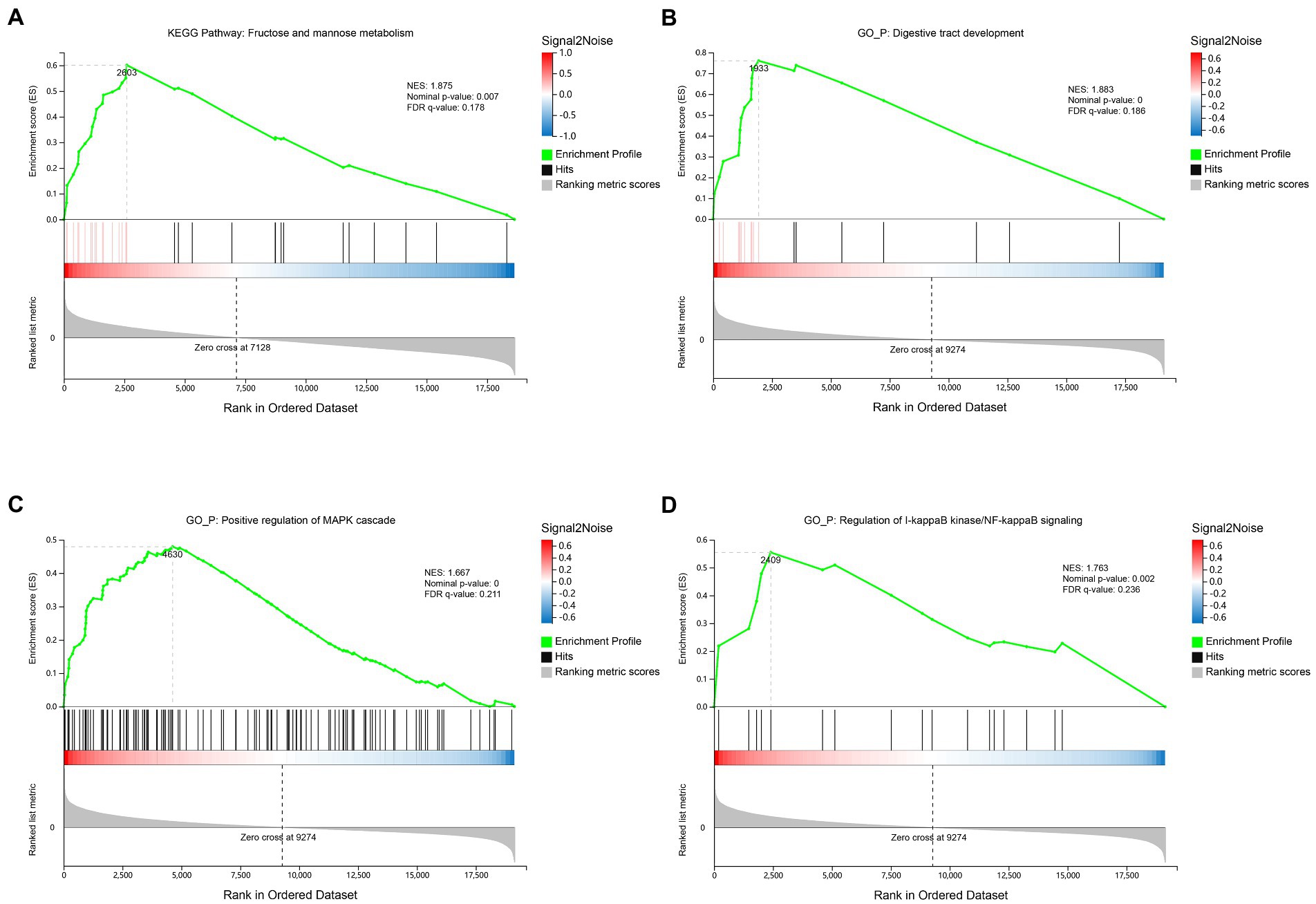
Figure 1. Transcriptome gene set enrichment analysis (GSEA) of rumen and ileum tissue of lambs in the CON and SIL groups. (A): Gene set enriched in Fructose and mannose metabolism pathway of rumen; (B): Gene set enriched in digestive tract development biological process of ileum; (C): Gene set enriched in positive regulation of MAPK cascade biological process of ileum; (D): Gene set enriched in regulation of I-kappaB kinase/NF-kappaB signaling biological process of ileum. Screening criteria for significant gene sets included adj. p-value <0.05 and FDR < 0.25. NES: normalized enrichment score.
3.3. Effects of replacing soybean meal with silybum marianum meal on microbial community in rumen and ileum of growing lambs
16SrDNA gene sequencing was used to determine the microbial community composition in the rumen and ileum. As shown in Table 5, an average of 511.13 and 658.86 OTUs with 97% similarity per sample were obtained in the rumen of CON and SIL groups, respectively, which was significantly higher in SIL as compared to CON (p < 0.05). As shown by the Coverage index of the two groups, the coverage of each sample was more than 99%, thus accurately reflecting the diversity of microbial community species and structure in the rumen. Based on the OTU clustering results for α-diversity analysis, the ACE index, Chao1 index, and Shannon index of the SIL group were significantly higher than those of the CON group (p < 0.05). However, the Simpson index of SIL group was significantly lower than that of the CON group (p < 0.05). The results of PCoA analysis with weighted distance metric showed that the rumen community composition of the CON group was clearly separated (p < 0.05 and R > 0.25) from the SIL group (Figure 2A).
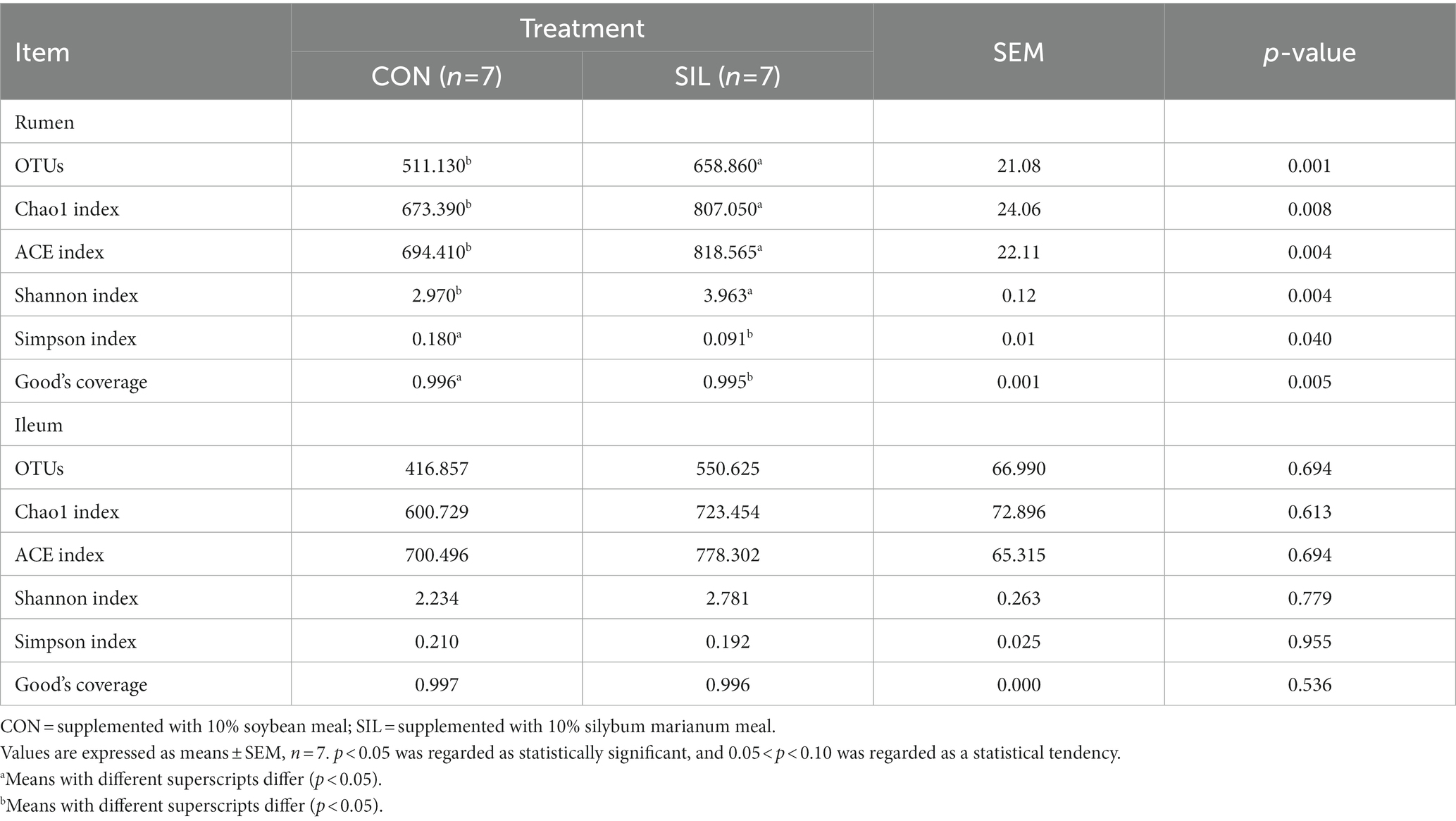
Table 5. Effect of silybum marianum meal as a replacement for soybean meal on the alpha diversity index of the rumen and ileum digesta of lambs.
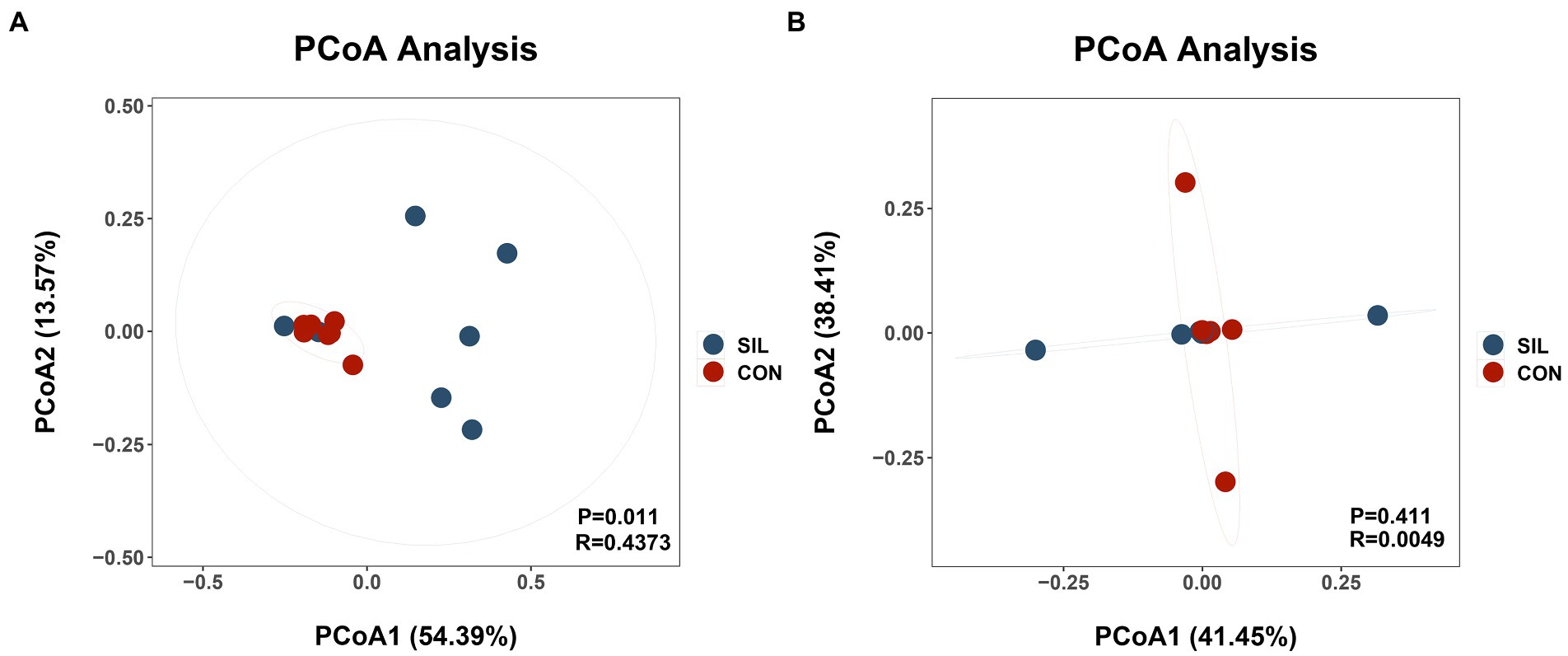
Figure 2. Beta diversity of the microbial communities in the rumen digesta of lambs in the CON and SIL groups. Principal coordinate analysis (PCoA) of weighted UniFrac distance metric of rumen (A) and ileum (B). CON = supplemented with 10% soybean meal; SIL = supplemented with 10% silybum marianum meal. p < 0.05 was regarded as statistically significant.
Taxonomic analysis revealed 14 phyla co-identified by the two groups in the rumen (Figure 3A). The predominant phyla in the rumen digesta were Bacteroidetes, Proteobacteria, and Firmicutes. The relative abundances of those top three phyla were 39.71, 39.77 and 16.33% in the CON group and 52.27, 14.10, and 25.61% in the SIL group, respectively. The relative abundances of Bacteroidetes and Firmicutes in the SIL group were significantly higher than those in the CON group (p < 0.05), but Proteobacteria were significantly lower than those in the CON group (p < 0.05) (Supplementary file 1; Table 1). In addition, the relative abundances of Synergistetes and Verrucomicrobia in the SIL group were also significantly higher than those in the CON group (p < 0.05) (Supplementary file 1; Table 1). At the genus level, the relative abundances of Pyramidobacter and Saccharofermentans were higher in the SIL group as compared with the CON group (p < 0.05). Conversely, the relative abundance of Sharpea was lower (p < 0.05) in the SIL group than in the CON group (Supplementary file 1; Table 2). The results of LEfSe analysis showed that the genera Pyramidobacter, Saccharofermentans, Anaerovibrio, Oscillibacter and Barnesiella were enriched in the SIL group, whereas Sharpea was enriched in the CON group (LDA > 2) (Figure 3B).
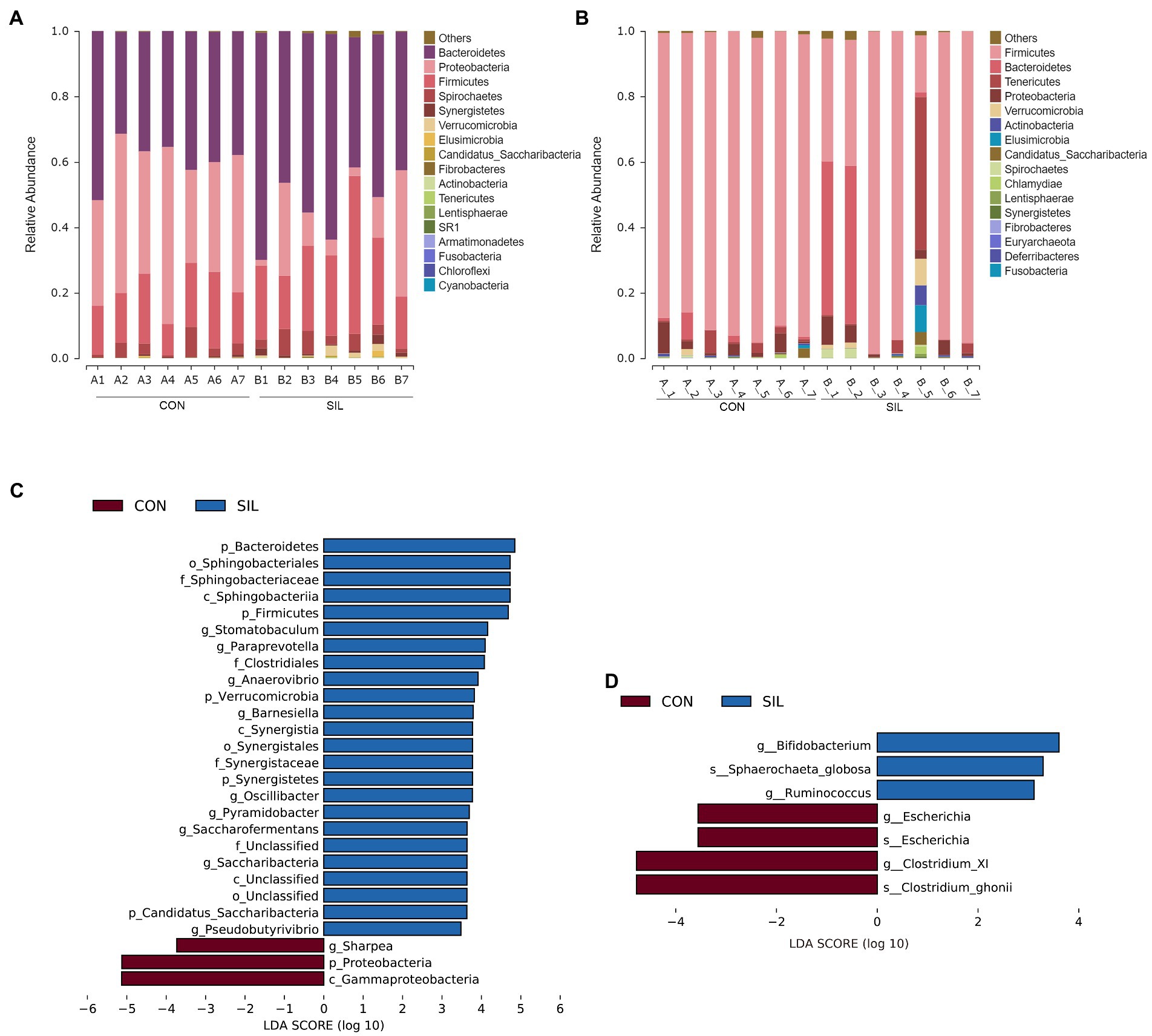
Figure 3. Taxonomic analysis of the microbial communities in the rumen and ileum digesta samples of lambs in the CON and SIL groups. The relative abundance of microbial composition at the phylum level in the rumen (A) and ileum (B); Linear discriminant analysis (LDA) effect size linear discriminant analysis (LEfSe) of the rumen (C) and ileum (D) CON = supplemented with 10% soybean meal; SIL = supplemented with 10% silybum marianum meal.
In the ileum, an average of 416.85 and 550.62 OTUs with 97% similarity per sample were obtained in the CON and SIL groups, respectively. The coverage of each sample was more than 99%, as shown by the Coverage index of both groups, thus accurately reflecting the diversity of microbial community species and structures in the ileum. Based on the OTU clustering results for α-diversity analysis, no significant differences in alpha and β diversity indexes were observed between the two groups (Figure 2B).
Taxonomic analysis revealed that ileum microbes were co-annotated into 15 phyla between the two groups (Figure 3C). Firmicutes was the most dominant phylum followed by Bacteroidetes. There were no significant differences at the phylum level between the two groups (Supplementary file 1; Table 3). At the genus level, at least one group of genera with a relative abundance greater than 0.1% in both groups was annotated to 43 (Supplementary file 1; Table 4). The relative abundances of Bifidobacterium and Ruminococcus in the SIL group were significantly higher than in the CON group (p < 0.05) (Supplementary file 1; Table 4). Conversely, the relative abundance of Clostridium_XI was lower (p < 0.05) in the SIL group than in the CON group (Supplementary file 1; Table 4). In addition, the LEfSe analysis results showed that the genera Bifidobacterium and Ruminococcus were dominant in the SIL group, and Clostridium_XI also was enriched in the CON group (LDA > 2) (Figure 3D).
3.4. Correlation between serum cytokines, transcriptional core genes and relative abundance of Top 20 genera in the ileum
Since the above GSEA algorithm of transcriptomes results releveled potential core genes (enriched in positive regulation of MAPK cascade and regulation of I-kappaB kinase/NF-kappaB signaling) associated with immune responses only in the ileum, rather than the rumen, the spearman’s correlation coefficient was used to investigate the relationship between serum cytokines and dominant bacterial genera (top 20) in the ileum. Correlation analysis showed that Clostridium_XI was negatively correlated with VEGF, TGF-β, TNF-α, and HGF (p < 0.05). Likewise, Escherichia showed a negative correlation with IL-2, VEGF, TGF-β, TNF-α, and HGF (p < 0.05) (Figure 4). Moreover, the correlation between the core genes enriched by positive regulation of MAPK cascade and regulation of I-kappaB kinase/NF-kappaB signaling in GSEA and the dominant bacterial genera in ileum were analyzed. The results showed that the core genes BMP4 and CD4 were negatively correlated with Clostridium_XI (p < 0.05) (Figure 5).
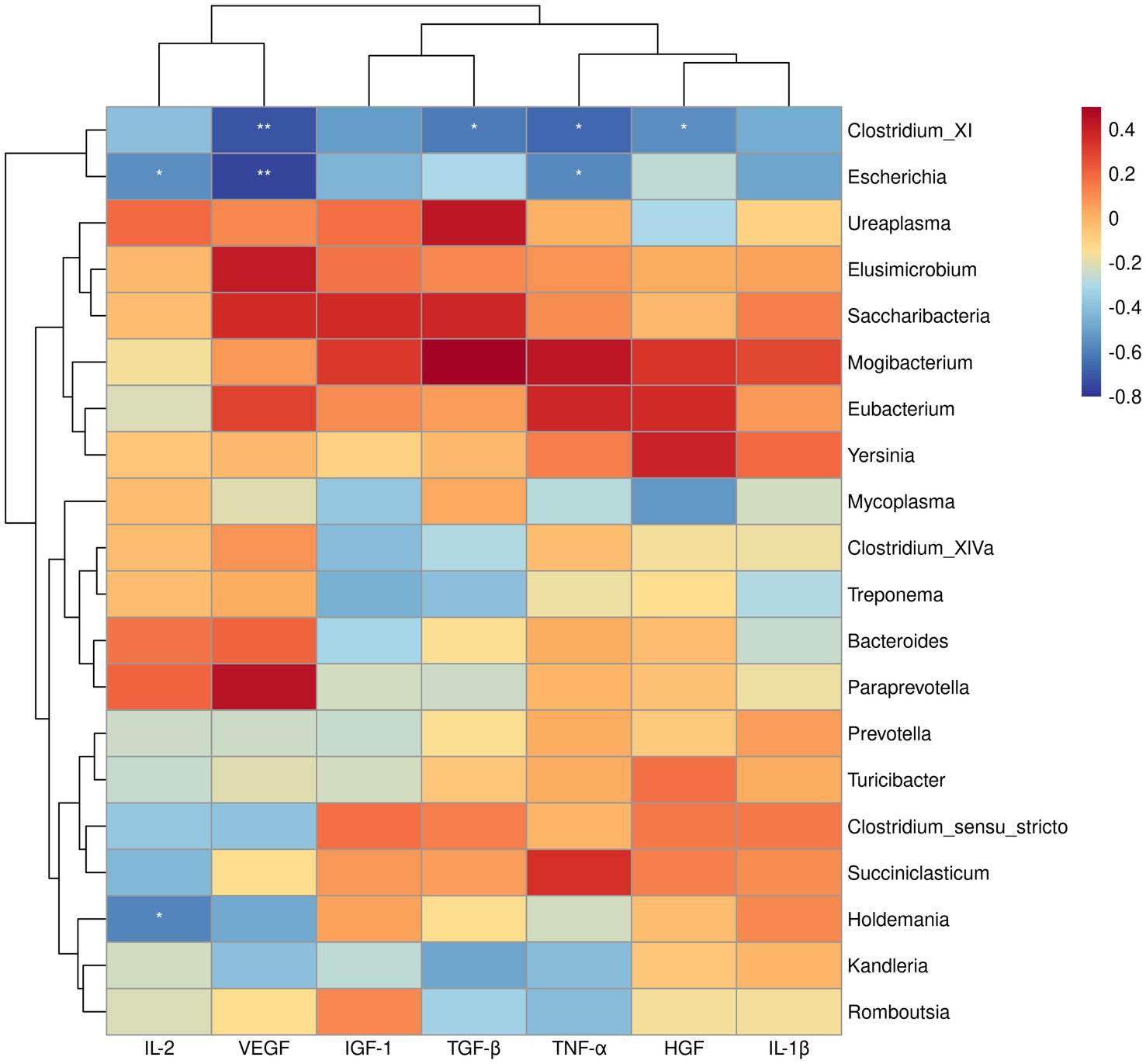
Figure 4. Heatmap of spearman’s correlation between serum cytokines and the relative abundance of top 20 bacteria at genus level in the ileum. The blue suggests a negative correlation, and the red suggests a positive correlation. *p < 0.05, **p < 0.01.
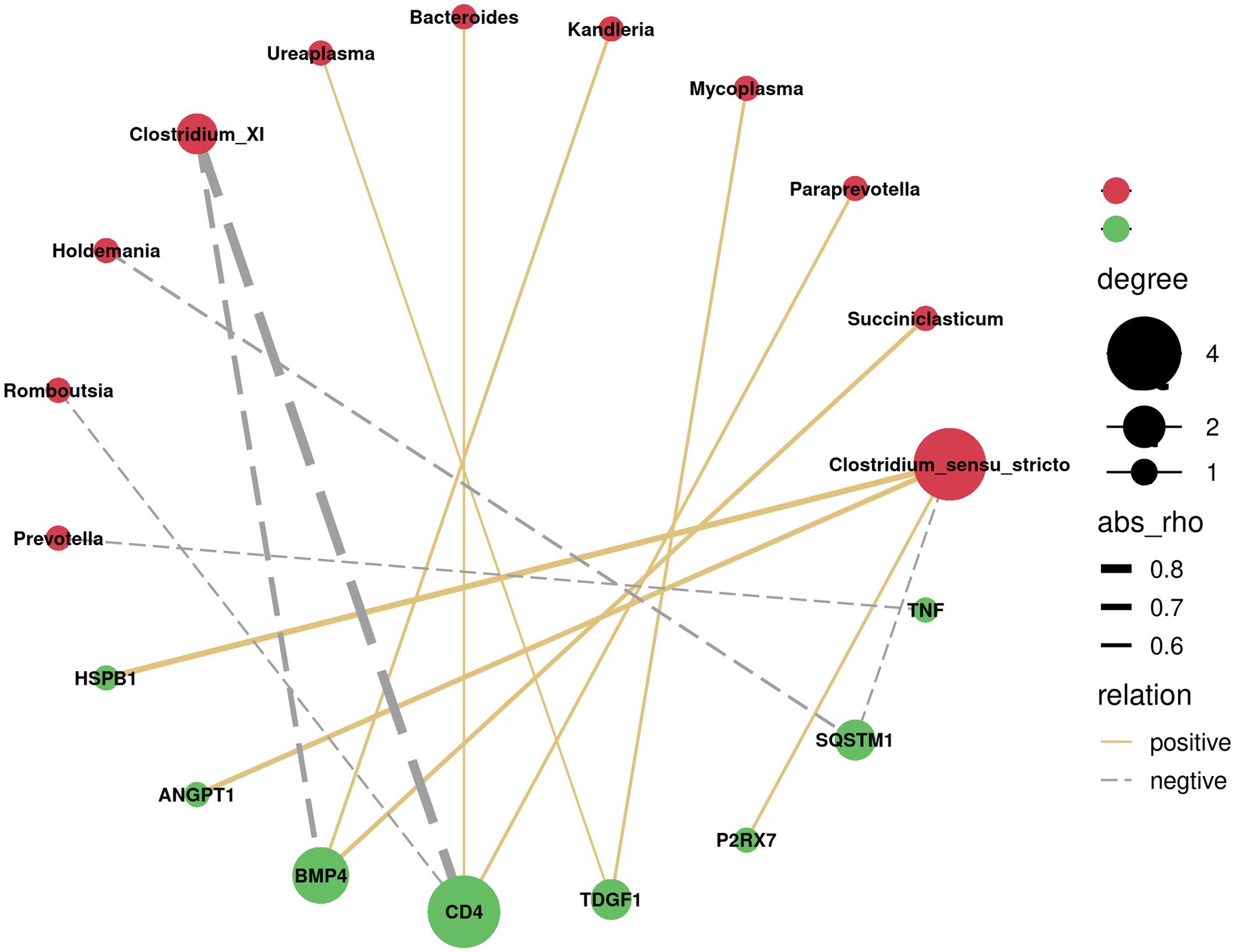
Figure 5. Correlation network analysis between the core genes (green circles) of positive regulation of MAPK cascade and regulation of I-kappaB kinase/NF-kappaB signalling and the predominant bacteria at genus level (red circles) in the ileum. Silver lines, negative correlation (p < 0.05); Golden lines, positive correlation (p < 0.05).
4. Discussion
The rapidly growing livestock sector and the increasing demand for livestock feeds have led to a substantial upsurge in feedstuff prices, especially protein feed resources. Finding alternative protein feed resources that could replace commercial protein supplements without profoundly affecting growth performance and health is required. Herein, we performed a comprehensive evaluation of silybum marianum meal as a potential replacement for soybean meal in growing lambs using a multi-omics approach. In this study, neither growth performance nor feed intakes were affected by the replacement of soybean meal with silybum marianum meal, suggesting that silybum marianum meal could be used in the diets of growing lambs. The inclusion of a higher percentage of silybum marianum meal in the diets of broilers has been reported to limit the body weight gain of broilers due to the high content of fiber and nitrite in silybum marianum meal (Suchý et al., 2008). However, ruminants can effectively utilize the high fiber and nitrite content of silybum marianum meal (Niyas et al., 2019) without adversely affecting their growth. In this regard, it has been reported that adding 20% silymarin flour to the diet of buffaloes improved the digestibility of NDF to some extent (Nikzad et al., 2017).
The silybum marianum meal usually contains 2–4% active substances on a dry matter basis (Stastnik et al., 2020). It was expected that the silymarin complex or flavonoid in the silybum marianum meal could induce the immune responses of animals. Cytokines are proteins or small polypeptides with immunoregulatory and effector functions, including lymphokines, monokines, various growth factors and others (Matthews, 2002). The stimulating and releasing of pro-inflammatory cytokines are essential steps for the activation of innate host defenses and subsequently regulating adaptive immune responses (Netea et al., 2010). In this study, the elevated pro-inflammatory factors IL-1β and TNF-α in SIL group were mainly resulted from its low crude protein content, low palatability (Stastnik et al., 2020) and high nitrite (Arviv et al., 2016). However, if there is enough energy feed in the rumen (such as corn), nitrite can be easily converted into ammonia and eventually used for microbial protein synthesis for ruminants (Niyas et al., 2019). TGF- β has been reported to suppress the immune system by inhibiting the immune function of inflammatory cells and promoting the differentiation and function of Treg cells (Pourgholaminejad et al., 2016). In our study, the increased level of TGF- βin the SIL group may inhibit excessive increase of proinflammatory factors and maintain the balance of the immune function. Moreover, it has been reported that silymarin has anti-inflammatory properties (Hadolin et al., 2001) and enhances cell growth factors (Vahabzadeh et al., 2018). The unregulated HGF in the SIL group in our study was expected, since TGF-β can exhibit immunosuppressive effects in synergy with HGF (Okunishi et al., 2005). We found that both the pro-inflammatory and anti-inflammatory cytokines were upregulated in lambs fed silybum marianum meal, which may indicate an immune system balance and result in minimal effects in growth performance and biochemical parameters of growing lambs.
Since the observed number of differential genes in the rumen and ileum was small (Supplementary file 4), a further GSEA was conducted to detect subtle gene expression changes. Fructose and mannose metabolisms are vital in cellular productivity, complex cellular processes (e.g., glycosylation) and cellular health (Lieu et al., 2021). In the present study, the GSEA analysis showed that feeding silybum marianum meal to growing lambs upregulated some genes in the fructose and mannose metabolism pathway in the rumen. This implies that the use of silybum marianum meal as a component in the growing lamb is beneficial for rumen carbohydrate metabolism. In the ileum, it was found that three biological process-related genes, including digestive tract development, positive regulation of MAPK cascade, and regulation of I-kappaB kinase/NF-kappaB signaling, were upregulated in the SIL group. MAPK cascade activation is central to various signaling pathways that regulate various important cellular physiological processes such as cell growth, differentiation, adaptation to environmental stress, and inflammatory responses (Sun et al., 2015; Kirk et al., 2020). NF-kappaB has a normal physiological function in mediating the immune response. The inflammatory response of the organism, after infection, requires the initiation of NF-kappaB signaling pathway to transcribe some cytokines to mediate the immune response to clear the invading pathogenic bacteria (Sun, 2017; Wang and Shen, 2022). Our results indicated that the ileum was the more important section for immune functions in response to feeding silybum marianum meal as compared with the rumen.
Ruminal microorganisms play a crucial role in feed degradation and production of volatile fatty acids, lipids, amino acids and hydrogen, which are essential for ruminants to maintain growth and productive performance (Kim et al., 2009; Lopes et al., 2015; Salami et al., 2021). Changes in diet composition or type can alter the microbial structure and composition in the rumen (Ramos et al., 2021). Our study found a higher microbial diversity in the rumen of lambs from the SIL group, as evidenced by the alpha and beta diversity indexes. These changes might be associated with the high fiber content in the silybum marianum meal (Koh et al., 2016). In ruminants, Bacteroidetes are mainly responsible for the degradation of cellulose, hemicellulose and pectin, and Firmicutes are mainly responsible for the decomposition of exogenous peptides and amino acids (Cholewińska et al., 2021). In our study, the relative abundance of Bacteroidetes and Firmicutes phyla in the rumen of lambs from the SIL group was higher which was in line with reports for the gut of healthy lambs (Gebeyew et al., 2021). However, the relative abundance of Sharpea belongs to Firmicutes was decreased in the SIL group, which was reported to be enriched in the low-methane producing sheep (Kamke et al., 2016). Studies have found a positive association of Proteobacteria with rumen biohydrogenation in ruminants (Salami et al., 2021). The lower relative abundance of Proteobacteria in the SIL group suggested that replacing soybean meal with silymarin marianum meal may alter the ruminal biohydrogenation. The phylum Synergistetes is generally found in the gut as a minor component, where it co-metabolizes secondary phytocompounds (Jumas-Bilak et al., 2009). In addition, Kang et al. (2020) found that Pyramidobacter from Synergistetes phylum can degrade natural toxic substances in plants. Interestingly, the relative abundance of Synergistetes phylum and Pyramidobacter genera in the SIL group was higher in the present study, which may be associated with the presence of some secondary compound in the silybum marianum meal. Saccharofermentans, as sugar fermenters, are able to ferment glucose, D-fructose, esculin, sucrose, starch, galactitol, mannitol, myo inositol and adonitol (Chen et al., 2010). In our study, the LEfSe analysis showed that Bacteroidetes, Firmicutes, Synergistetes, Verrucomicrobia, Pyramidobacter, and Saccharofermentans were enriched in the rumen of the SIL group compared with the CON group, whereas the Proteobacteria and Sharpea were depleted. These differential microorganisms further suggested that silybum marianum meal can maintain the nutrient and growth needs for growing lambs.
The ileum is an important site for the digestion and absorption of nutrients such as lipids, bile salts, and vitamins (Wang et al., 2020). In addition, the ileum also gathers a relatively large number of immune-related cells and tissues, such as Paneth cells (producing antimicrobial peptides, such as lysozyme, defensins and other antibacterial substances) and Peyer’s patches (gut-associated lymphoid tissue) (Mowat and Agace, 2014), making it as an important part of the body’s resistance to pathogens. The innate and acquired immune function of the ileum have been reported to be significantly affected by gut microorganisms (Martinez-Guryn et al., 2019). It has been reported that the addition of 3% silybum marianum meal was able to reduce the count of E. coli in the ileum of laying hens (Hashemi Jabali et al., 2018). In our study, the abundance of Clostridium_XI was lower in the ileum of lambs from the SIL group, which was probably due to the higher fiber content in the silybum marianum meal. In this regard, Zheng et al. (2018) have indicated that feeding fermentable fiber reduced the presence of Clostridium_XI in the intestine of mice. In addition, our enriched abundances of Bifidobacterium and Ruminococcus genera in SIL group implied a healthier ileum environment because of the positive effect of Bifidobacterium and Ruminococcus on intestine health (Belkaid and Hand, 2014; Bi et al., 2019; Ding et al., 2020).
Integrated transcriptome and microbial community analysis in the ileum was further conduced to explore the potential mechanisms for increased blood cytokines in SIL group. Initially, it was speculated that silybum marianum meal resulted in stress in lambs, as evidenced by the enrichment of genes related to MAPK cascade and NF-kappaB signaling identified using GSEA and the production of cytokines. The enriched core genes related to MAPK cascade and NF-kappaB signaling in the ileum, including BMP4 (Hogg et al., 2010), P2RX7, CD4 (Blanc et al., 2018), HSPB1 (Lee et al., 2012), were expected to be involved in cytokine production. Moreover, it was observed that Clostridium_XI was as the dominant genus in the ileum in the present study. It has been reported that Clostridium_XI produces toxins that could induce intestinal damage (Wan et al., 2022). In the present study, the abundance of Clostridium_XI was depleted in the SIL group compared to the CON group. It would be explainable that the increases in cytokines levels may be associated with the colonization of Clostridium_XI. It has been reported that toxins produced by Clostridium_XI triggered circulating immunity to increase the expression of some interleukins, such as IL-1β, prompting immune cells to secrete antimicrobial peptides in an attempt to clear Clostridium_XI from the gut (Hernández Del Pino et al., 2021; Nibbering et al., 2021). This statement coincided with our negative correlation between Clostridium_XI and blood cytokines. It was speculated that feeding silybum marianum meal to lambs may drive MAPK cascade and NF-kappaB signaling-related gene enrichment in the ileum, which may in turn produce cytokines that mediate a decrease in the abundance of the harmful bacterium, such as Clostridium_XI.
5. Conclusion
Replacement of soybean meal with silybum marianum meal in the diet of lambs resulted in increased cytokines production without affecting growth performance, feed intakes or blood biochemical parameters in growing lambs. Feeding silybum marianum meal as a replacement for soybean meal differently altered the transcriptome profiles and microbial community in the rumen and ileum. Further integrated transcriptome and microbial community analysis in the ileum revealed that silybum marianum meal promoted the enrichment of immune-related genes (such as BMP4 and CD4), which were supposed to trigger the production of cytokines and suppress the abundance of harmful bacteria, such as Clostridium_XI.
Data availability statement
The data presented in the study are deposited in the NCBI Sequence Read Archive (SRA) database repository, accession number PRJNA919487 (ileal tissue transcriptome), accession number PRJNA918960 (rumen tissue transcriptome), accession number PRJNA917096 (ileal digesta 16s rDNA), accession number PRJNA917525 (rumen digesta 16s rDNA).
Ethics statement
The experimental protocols were reviewed and approved by the Animal Care and Use Committee of the Institute of Subtropical Agriculture, Chinese Academy of Sciences (ISA-202020) and all applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Funding
This study was funded by Chinese Academy of Sciences (Strategic Priority Research Program Grant Nos. XDA26040304 and XDA26050102), the National Natural Science Foundation of China (32072760), and the Natural Science Foundation of Hunan Province of China (2022JJ10054), and Innovation Province Project (2019RS3021).
Author contributions
TZ: conceptualization, methodology, data acquisition, data analysis, and writing–original draft. YR: and TZ: animal experiments and data acquisition. CY, KG, and MG: review and editing. ZH: conceptualization, methodology, project administration, supervision, and review and editing. ZT: conceptualization, methodology, and project administration. All authors contributed to the article and approved the submitted version.
Acknowledgments
Thanks to the Institutional Center for Shared Technologies and Facilities of Institute of Subtropical Agriculture, CAS, for support.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fmicb.2023.1093129/full#supplementary-material
References
Arviv, A., Muklada, H., Kigel, J., Voet, H., Glasser, T., Dvash, L., et al. (2016). Targeted grazing of milk thistle (Silybum marianum) and Syrian thistle (Notobasis syriaca) by goats: preference following preconditioning, generational transfer, and toxicity. Appl. Anim. Behav. Sci. 179, 53–59. doi: 10.1016/j.applanim.2016.03.008
Belkaid, Y., and Hand, T. W. (2014). Role of the microbiota in immunity and inflammation. Cells 157, 121–141. doi: 10.1016/j.cell.2014.03.011
Bi, Y., Cox, M. S., Zhang, F., Suen, G., Zhang, N., Tu, Y., et al. (2019). Feeding modes shape the acquisition and structure of the initial gut microbiota in newborn lambs. Environ. Microbiol. 21, 2333–2346. doi: 10.1111/1462-2920.14614
Blanc, F., Créchet, F., Bruneau, N., Piton, G., Leplat, J.-J., Andréoletti, F., et al. (2018). Impact of a CD4 gene haplotype on the immune response in minipigs. Immunogenetics 70, 209–222. doi: 10.1007/s00251-017-1037-z
Caporaso, J. G., Kuczynski, J., Stombaugh, J., Bittinger, K., Bushman, F. D., Costello, E. K., et al. (2010). QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 7, 335–336. doi: 10.1038/nmeth.f.303
Chen, S., Niu, L., and Zhang, Y. (2010). Saccharofermentans acetigenes gen. Nov., sp. nov., an anaerobic bacterium isolated from sludge treating brewery wastewater. Int. J. Syst. Evol. Microbiol. 60, 2735–2738. doi: 10.1099/ijs.0.017590-0
Cholewińska, P., Wołoszyńska, M., Michalak, M., Czyż, K., Rant, W., Smoliński, J., et al. (2021). Influence of selected factors on the Firmicutes, Bacteroidetes phyla and the Lactobacillaceae family in the digestive tract of sheep. Sci. Rep. 11:23801. doi: 10.1038/s41598-021-03207-w
Dehghan, A., Rowshan Ghasrodashti, A., Esfandiari, A., Mohebbi-Fani, M., Hoshyar, M. B., and Nayeri, K. (2011). Hepatoprotective effect of silymarin during negative energy balance in sheep. Comp. Clin. Pathol. 20, 233–238. doi: 10.1007/s00580-010-0984-7
Ding, S., Hu, C., Fang, J., and Liu, G. (2020). The protective role of probiotics against colorectal cancer. Oxidative Med. Cell. Longev. 2020, 1–10. doi: 10.1155/2020/8884583
Dockalova, H., Zeman, L., Baholet, D., Batik, A., Skalickova, S., and Horky, P. (2021). Dose effect of milk thistle (Silybum marianum) seed cakes on the digestibility of nutrients, flavonolignans and the individual components of the silymarin complex in horses. Animals 11:1687. doi: 10.3390/ani11061687
Edgar, R. C. (2010). Search and clustering orders of magnitude faster than BLAST. Bioinforma. Oxf. Engl. 26, 2460–2461. doi: 10.1093/bioinformatics/btq461
Edgar, R. C. (2013). UPARSE: highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 10, 996–998. doi: 10.1038/nmeth.2604
Edgar, R. C., Haas, B. J., Clemente, J. C., Quince, C., and Knight, R. (2011). UCHIME improves sensitivity and speed of chimera detection. Bioinforma. Oxf. Engl. 27, 2194–2200. doi: 10.1093/bioinformatics/btr381
Fan, H., Wang, J., Meng, Q., and Jin, Z. (2019). Extraction optimization, preliminary characterization, and bioactivities of polysaccharides from silybum marianum meal. J. Food Meas. Charact. 13, 1031–1039. doi: 10.1007/s11694-018-0018-8
Gebeyew, K., Yang, C., He, Z., and Tan, Z. (2021). Low-protein diets supplemented with methionine and lysine alter the gut microbiota composition and improve the immune status of growing lambs. Appl. Microbiol. Biotechnol. 105, 8393–8410. doi: 10.1007/s00253-021-11620-4
Grela, E. R., Świątkiewicz, M., Florek, M., and Wojtaszewska, I. (2020). Impact of milk thistle (Silybum marianum L.) seeds in fattener diets on pig performance and carcass traits and fatty acid profile and cholesterol of meat, backfat and liver. Livest. Sci. 239:104180. doi: 10.1016/j.livsci.2020.104180
Hadolin, M., S̆kerget, M., Knez, Z., and Bauman, D. (2001). High pressure extraction of vitamin E-rich oil from Silybum marianum. Food Chem. 74, 355–364. doi: 10.1016/S0308-8146(01)00152-2
Hashemi Jabali, N. S., Mahdavi, A. H., Ansari Mahyari, S., Sedghi, M., and Kakhki, A. M. (2018). Effects of milk thistle meal on performance, ileal bacterial enumeration, jejunal morphology and blood lipid peroxidation in laying hens fed diets with different levels of metabolizable energy. J. Anim. Physiol. Anim. Nutr. 102, 410–420. doi: 10.1111/jpn.12747
Hernández Del Pino, R. E., Barbero, A. M., Español, L. Á., Morro, L. S., and Pasquinelli, V. (2021). The adaptive immune response to Clostridioides difficile: a tricky balance between immunoprotection and immunopathogenesis. J. Leukoc. Biol. 109, 195–210. doi: 10.1002/JLB.4VMR0720-201R
Hogg, K., Etherington, S. L., Young, J. M., McNeilly, A. S., and Duncan, W. C. (2010). Inhibitor of differentiation (id) genes are expressed in the steroidogenic cells of the ovine ovary and are differentially regulated by members of the transforming growth factor-β family. Endocrinology 151, 1247–1256. doi: 10.1210/en.2009-0914
Jumas-Bilak, E., Roudiere, L., and Marchandin, H. (2009). Description of “Synergistetes” phyl. Nov. and emended description of the phylum “Deferribacteres” and of the family Syntrophomonadaceae, phylum “Firmicutes.”. Int. J. Syst. Evol. Microbiol. 59, 1028–1035. doi: 10.1099/ijs.0.006718-0
Kamke, J., Kittelmann, S., Soni, P., Li, Y., Tavendale, M., Ganesh, S., et al. (2016). Rumen metagenome and metatranscriptome analyses of low methane yield sheep reveals a Sharpea-enriched microbiome characterised by lactic acid formation and utilisation. Microbiome 4:56. doi: 10.1186/s40168-016-0201-2
Kang, S., Khan, S., Webb, R., Denman, S., and McSweeney, C. (2020). Characterization and survey in cattle of a rumen Pyrimadobacter sp. which degrades the plant toxin fluoroacetate. FEMS Microbiol. Ecol. 96:fiaa077. doi: 10.1093/femsec/fiaa077
Kim, E. J., Huws, S. A., Lee, M. R. F., and Scollan, N. D. (2009). Dietary transformation of lipid in the rumen microbial ecosystem. Asian-Aust. J. Anim. Sci. 22, 1341–1350. doi: 10.5713/ajas.2009.r.11
Kim, D., Langmead, B., and Salzberg, S. L. (2015). HISAT: a fast spliced aligner with low memory requirements. Nat. Methods 12, 357–360. doi: 10.1038/nmeth.3317
Kirk, S. G., Samavati, L., and Liu, Y. (2020). MAP kinase phosphatase-1, a gatekeeper of the acute innate immune response. Life Sci. 241:117157. doi: 10.1016/j.lfs.2019.117157
Koh, A., De Vadder, F., Kovatcheva-Datchary, P., and Bäckhed, F. (2016). From dietary fiber to host physiology: short-chain fatty acids as key bacterial metabolites. Cells 165, 1332–1345. doi: 10.1016/j.cell.2016.05.041
Langmead, B., and Salzberg, S. L. (2012). Fast gapped-read alignment with bowtie 2. Nat. Methods 9, 357–359. doi: 10.1038/nmeth.1923
Lee, Y.-J., Lee, H.-J., Choi, S.-H., Jin, Y. B., An, H. J., Kang, J.-H., et al. (2012). Soluble HSPB1 regulates VEGF-mediated angiogenesis through their direct interaction. Angiogenesis 15, 229–242. doi: 10.1007/s10456-012-9255-3
Li, B., and Dewey, C. N. (2011). RSEM: accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 12:323. doi: 10.1186/1471-2105-12-323
Li, R., Li, Y., Kristiansen, K., and Wang, J. (2008). SOAP: short oligonucleotide alignment program. Bioinforma. Oxf. Engl. 24, 713–714. doi: 10.1093/bioinformatics/btn025
Lieu, E. L., Kelekar, N., Bhalla, P., and Kim, J. (2021). Fructose and mannose in inborn errors of metabolism and cancer. Meta 11:479. doi: 10.3390/metabo11080479
Lopes, L. D., de Souza Lima, A. O., Taketani, R. G., Darias, P., da Silva, L. R. F., Romagnoli, E. M., et al. (2015). Exploring the sheep rumen microbiome for carbohydrate-active enzymes. Antonie Van Leeuwenhoek 108, 15–30. doi: 10.1007/s10482-015-0459-6
Magoc, T., and Salzberg, S. L. (2011). FLASH: fast length adjustment of short reads to improve genome assemblies. Bioinformatics 27, 2957–2963. doi: 10.1093/bioinformatics/btr507
Martinez-Guryn, K., Leone, V., and Chang, E. B. (2019). Regional diversity of the gastrointestinal microbiome. Cell Host Microbe 26, 314–324. doi: 10.1016/j.chom.2019.08.011
Matthews, B. G. (2002). “Cytokines, cytokine antagonists, and growth factors for treating infections” in Immunotherapy for Infectious Diseases. ed. J. M. Jacobson (Totowa, NJ: Humana Press)
Mowat, A. M., and Agace, W. W. (2014). Regional specialization within the intestinal immune system. Nat. Rev. Immunol. 14, 667–685. doi: 10.1038/nri3738
Na, S. W., and Guan, L. L. (2022). Understanding the role of rumen epithelial host-microbe interactions in cattle feed efficiency. Anim. Nutr. 10, 41–53. doi: 10.1016/j.aninu.2022.04.002
Netea, M., Simon, A., Van De Veerdonk, F. L., Kullberg, B., Van Der Meer, J. V. D., and Joosten, L. (2010). IL-1β processing in host defense: beyond the inflammasomes. PLoS Pathog. 6:e1000661. doi: 10.1371/journal.ppat.1000661
Nibbering, B., Gerding, D. N., Kuijper, E. J., Zwittink, R. D., and Smits, W. K. (2021). Host immune responses to clostridioides difficile: toxins and beyond. Front. Microbiol. 12:804949. doi: 10.3389/fmicb.2021.804949
Nikzad, Z., Chaji, M., Mirzadeh, K., Mohammadabadi, T., and Sari, M. (2017). Effect of different levels of milk thistle (Silybum Marianum) in diets containing cereal grains with different ruminal degradation rate on rumen bacteria of Khuzestan buffalo. Iran. J. Appl. Anim. Sci. 7, 401–409.
Niyas, E., Simon, S., Banglavan, S. J., Mathew, J. J., Das, A. S., John, R., et al. (2019). Successful management of nitrite poisoning in crossbred dairy calves. Indian J. Anim. Nutr. 36:419. doi: 10.5958/2231-6744.2019.00068.9
Okunishi, K., Dohi, M., Nakagome, K., Tanaka, R., Mizuno, S., Matsumoto, K., et al. (2005). A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 175, 4745–4753. doi: 10.4049/jimmunol.175.7.4745
Pourgholaminejad, A., Aghdami, N., Baharvand, H., and Moazzeni, S. M. (2016). Is TGFβ as an anti-inflammatory cytokine required for differentiation of inflammatory T H 17 cells? J. Immunotoxicol. 13, 775–783. doi: 10.1080/1547691X.2016.1193574
Ramos, S. C., Jeong, C. D., Mamuad, L. L., Kim, S. H., Kang, S. H., Kim, E. T., et al. (2021). Diet transition from high-forage to high-concentrate alters rumen bacterial community composition, epithelial transcriptomes and ruminal fermentation parameters in dairy cows. Animals 11:838. doi: 10.3390/ani11030838
Salami, S. A., Valenti, B., Luciano, G., Lanza, M., Umezurike-Amahah, N. M., Kerry, J. P., et al. (2021). Dietary cardoon meal modulates rumen biohydrogenation and bacterial community in lambs. Sci. Rep. 11:16180. doi: 10.1038/s41598-021-95691-3
Šťastník, O., Mrkvicová, E., Pavlata, L., Roztočilová, A., Umlášková, B., and Anzenbacherová, E. (2019). Performance, biochemical profile and antioxidant activity of hens supplemented with addition of milk thistle (Silybum marianum) seed cakes in diet. Acta Univ. Agric. Silvic. Mendel. Brun. 67, 993–1003. doi: 10.11118/actaun201967040993
Stastnik, O., Pavlata, L., and Mrkvicova, E. (2020). The milk thistle seed cakes and hempseed cakes are potential feed for poultry. Animals 10:1384. doi: 10.3390/ani10081384
Suchý, P., Straková, E., Kummer, V., Herzig, I., Písaříková, V., Blechová, R., et al. (2008). Hepatoprotective effects of milk thistle (Silybum marianum) seed cakes during the chicken broiler fattening. Acta Vet. Brno 77, 31–38. doi: 10.2754/avb200877010031
Sun, S.-C. (2017). The non-canonical NF-κB pathway in immunity and inflammation. Nat. Rev. Immunol. 17, 545–558. doi: 10.1038/nri.2017.52
Sun, Y., Liu, W.-Z., Liu, T., Feng, X., Yang, N., and Zhou, H.-F. (2015). Signaling pathway of MAPK/ERK in cell proliferation, differentiation, migration, senescence and apoptosis. J. Recept. Signal Transduct. 35, 600–604. doi: 10.3109/10799893.2015.1030412
Tajmohammadi, A., Razavi, B. M., and Hosseinzadeh, H. (2018). Silybum marianum (milk thistle) and its main constituent, silymarin, as a potential therapeutic plant in metabolic syndrome: a review: silybum marianum and metabolic syndrome. Phytother. Res. 32, 1933–1949. doi: 10.1002/ptr.6153
Vahabzadeh, M., Amiri, N., and Karimi, G. (2018). Effects of silymarin on metabolic syndrome: a review: silymarin and metabolic syndrome. J. Sci. Food Agric. 98, 4816–4823. doi: 10.1002/jsfa.9115
Wan, J., Zhang, Y., He, W., Tian, Z., Lin, J., Liu, Z., et al. (2022). Gut microbiota and metabolite changes in patients with ulcerative colitis and Clostridioides difficile infection. Front. Microbiol. 13:802823. doi: 10.3389/fmicb.2022.802823
Wang, B., and Shen, J. (2022). NF-κB inducing kinase regulates intestinal immunity and homeostasis. Front. Immunol. 13:895636. doi: 10.3389/fimmu.2022.895636
Wang, Y., Song, W., Wang, J., Wang, T., Xiong, X., Qi, Z., et al. (2020). Single-cell transcriptome analysis reveals differential nutrient absorption functions in human intestine. J. Exp. Med. 217:e20191130. doi: 10.1084/jem.20191130
Keywords: silybum marianum meal, soybean meal, lambs, cytokines, microbial community, transcriptome
Citation: Zhang T, Ren Y, Yang C, Gebeyew K, Gao M, He Z and Tan Z (2023) An integrated transcriptome and microbial community analysis reveals potential mechanisms for increased immune responses when replacing silybum marianum meal with soybean meal in growing lambs. Front. Microbiol. 14:1093129. doi: 10.3389/fmicb.2023.1093129
Edited by:
Naifeng Zhang, Institute of Feed Research of Chinese Academy of Agricultural Sciences, ChinaReviewed by:
Hui Yan, China Agricultural University, ChinaJintao Wei, Hubei Academy of Agricultural Sciences, China
Copyright © 2023 Zhang, Ren, Yang, Gebeyew, Gao, He and Tan. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Zhixiong He, zxhe@isa.ac.cn
 Tianxi Zhang
Tianxi Zhang Yanbo Ren1,3
Yanbo Ren1,3 Chao Yang
Chao Yang Kefyalew Gebeyew
Kefyalew Gebeyew Zhixiong He
Zhixiong He Zhiliang Tan
Zhiliang Tan