Mediterranean, DASH, and MIND Dietary Patterns and Cognitive Function: The 2-Year Longitudinal Changes in an Older Spanish Cohort
- 1Universitat Rovira i Virgili, Departament de Bioquímica i Biotecnologia, and Hospital Universitari San Joan de Reus, Unitat de Nutrició Humana, Reus, Spain
- 2Institut d’Investigació Sanitária Pere Virgili (IISPV), Reus, Spain
- 3Consorcio CIBER, M.P. Fisiopatología de la Obesidad y Nutrición (CIBERObn), Instituto de Salud Carlos III (ISCIII), Madrid, Spain
- 4Toronto 3D (Diet, Digestive Tract and Disease) Knowledge Synthesis and Clinical Trials Unit, Toronto, ON, Canada
- 5Clinical Nutrition and Risk Factor Modification Centre, St. Michael’s Hospital, Unity Health Toronto, Toronto, ON, Canada
- 6Nutrition Unit, University Hospital of Sant Joan de Reus, Reus, Spain
- 7Department of Preventive Medicine and Public Health, University of Navarra, IdiSNA, Pamplona, Spain
- 8Department of Nutrition, Harvard T.H. Chan School of Public Health, Boston, MA, United States
- 9Department of Endocrinology and Nutrition, Lipid Clinic, Institut d’Investigacions Biomèdiques August Pi Sunyer (IDIBAPS), Hospital Clínic, Barcelona, Spain
- 10Department of Preventive Medicine, University of Valencia, Valencia, Spain
- 11Unit of Cardiovascular Risk and Nutrition, Institut Hospital del Mar de Investigaciones Médicas Municipal d’Investigació Médica (IMIM), Barcelona, Spain
- 12Department of Nutrition, Food Sciences, and Physiology, Center for Nutrition Research, University of Navarra, IdiSNA, Pamplona, Spain
- 13Precision Nutrition and Cardiometabolic Health Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain
- 14Bioaraba Health Research Institute, Cardiovascular, Respiratory and Metabolic Area, Osakidetza Basque Health Service, Araba University Hospital, University of the Basque Country UPV/EHU, Vitoria-Gasteiz, Spain
- 15EpiPHAAN Research Group, School of Health Sciences, Instituto de Investigación Biomédica de Málaga (IBIMA), University of Málaga, Málaga, Spain
- 16CIBER de Epidemiología y Salud Pública (CIBERESP), Instituto de Salud Carlos III, Madrid, Spain
- 17Instituto de Investigación Sanitaria y Biomédica de Alicante, Universidad Miguel Hernández (ISABIAL-UMH), Alicante, Spain
- 18Health Research Institute of the Balearic Islands (IdISBa), Palma de Mallorca, Spain
- 19Department of Internal Medicine, Maimonides Biomedical Research Institute of Cordoba (IMIBIC), Reina Sofia University Hospital, University of Cordoba, Cordoba, Spain
- 20Department of Internal Medicine, Institut d’Investigacions Biomédiques August Pi Sunyer (IDIBAPS), Hospital Clinic, University of Barcelona, Barcelona, Spain
- 21Department of Endocrinology, Virgen de la Victoria Hospital, Instituto de Investigación Biomédica de Málaga (IBIMA), University of Málaga, Málaga, Spain
- 22Department of Family Medicine, Research Unit, Distrito Sanitario Atención Primaria Sevilla, Seville, Spain
- 23Research Institute of Biomedical and Health Sciences (IUIBS), University of Las Palmas de Gran Canaria and Centro Hospitalario Universitario Insular Materno Infantil (CHUIMI), Canarian Health Service, Las Palmas de Gran Canaria, Spain
- 24Department of Preventive Medicine and Public Health, University of Granada, Granada, Spain
- 25Instituto de Investigación Biosanitaria ibs, GRANADA, Granada, Spain
- 26Research Group on Community Nutrition and Oxidative Stress, University of Balearic Islands, Palma de Mallorca, Spain
- 27Institute of Biomedicine (IBIOMED), University of León, León, Spain
- 28Lipids and Vascular Risk Unit, Internal Medicine, Hospital Universitario de Bellvitge, Hospitalet de Llobregat, Barcelona, Spain
- 29Division of Preventive Medicine, Faculty of Medicine, University of Jaén, Jaén, Spain
- 30Department of Endocrinology and Nutrition, Instituto de Investigación Sanitaria Hospital Clínico San Carlos (IdISSC), Madrid, Spain
- 31CIBER Diabetes y Enfermedades Metabólicas (CIBERDEM), Instituto de Salud Carlos III (ISCIII), Madrid, Spain
- 32Department of Endocrinology, Institut d’ Investigacions Biomédiques August Pi Sunyer (IDIBAPS), Hospital Clinic, University of Barcelona, Barcelona, Spain
- 33Department of Endocrinology and Nutrition, Hospital Fundación Jimenez Díaz, Instituto de Investigaciones Biomédicas IISFJD, University Autonoma, Madrid, Spain
- 34Nutritional Control of the Epigenome Group, Precision Nutrition and Obesity Program, IMDEA Food, CEI UAM + CSIC, Madrid, Spain
- 35Department of Computer Languages and Systems, Universitat Jaume I, Castellon, Spain
- 36Integrative Pharmacology and Systems Neurosciences, Instituto Hospital del Mar de Investigaciones Médicas (IMIM), Barcelona, Spain
- 37Division of Pure and Applied Biochemistry, Lund University, Lund, Sweden
Background and Aims: Plant-forward dietary patterns have been associated with cardiometabolic health benefits, which, in turn, have been related to cognitive performance with inconsistent findings. The objective of this study was to examine the relationship between baseline adherence to three a priori dietary patterns (Mediterranean, DASH, and MIND diets) with 2-year changes in cognitive performance in older adults with overweight or obesity and high cardiovascular disease risk.
Methods: A prospective cohort analysis was conducted within the PREDIMED-Plus trial, involving 6,647 men and women aged 55–75 years with overweight or obesity and metabolic syndrome. Using a validated, semiquantitative 143-item food frequency questionnaire completed at baseline, the dietary pattern adherence scores were calculated. An extensive neuropsychological test battery was administered at baseline and 2-year follow-up. Multivariable-adjusted linear regression models were used to assess associations between 2-year changes in cognitive function z-scores across tertiles of baseline adherence to the a priori dietary patterns.
Results: Adherence to the Mediterranean diet at baseline was associated with 2-year changes in the general cognitive screening Mini-Mental State Examination (MMSE, β: 0.070; 95% CI: 0.014, 0.175, P-trend = 0.011), and two executive function-related assessments: the Trail Making Tests Part A (TMT-A, β: −0.054; 95% CI: −0.110, − 0.002, P-trend = 0.047) and Part B (TMT-B, β: −0.079; 95% CI: −0.134, −0.024, P-trend = 0.004). Adherence to the MIND diet was associated with the backward recall Digit Span Test assessment of working memory (DST-B, β: 0.058; 95% CI: 0.002, 0.114, P-trend = 0.045). However, higher adherence to the DASH dietary pattern was not associated with better cognitive function over a period of 2 years.
Conclusion: In older Spanish individuals with overweight or obesity and at high cardiovascular disease risk, higher baseline adherence to the Mediterranean dietary pattern may be associated with better cognitive performance than lower adherence over a period of 2 years.
Introduction
Cognitive decline, associated with aging, is a serious public health concern, given the increasing prevalence of neurodegenerative diseases as people are living longer and the proportion of older persons worldwide continues to rise rapidly (United Nations et al., 2020). Globally, dementia affects an estimated 50 million people, and this prevalence is projected to increase over 130 million by 2050 (World Health Organization [WHO], 2019). Epidemiological studies further suggest a negative interaction of aging and obesity with cognitive dysfunction (Kanaya et al., 2009; Hassing et al., 2010). With the prevalence of overweight and obesity affecting an estimated 30% of the adult population and more, there are additional adverse implications for cognition health (Ng et al., 2014; Balasubramanian et al., 2020). Cognitive decline carries a significant social and economic burden, given cognitive impairment and dementia are strong predictors of functional disability and dependence (Petersen et al., 2001). Cognitive decline is a normal part of the aging process; however, the rate of decline may vary depending on the differences in genetic and lifestyle-related factors (Wu et al., 2020).
The potential of modifiable lifestyle factors is important as there are no effective pharmacological agents identified for the improvement of cognition or delay of the progression of cognitive decline (Petersen et al., 2018). Diet is a key lifestyle risk factor. Individual nutrients and foods have been inconsistently associated with cognitive function, including some vitamins, carotenoids, long-chain n-3 polyunsaturated fatty acids (PUFAs), such as seafood, and whole foods rich in polyphenols, such as fruits and vegetables, nuts, olive oil, and coffee (Rutjes et al., 2018; Ammar et al., 2020; Brainard et al., 2020). As food is consumed as part of a dietary pattern, it is important to consider the interactions and associations of whole dietary approaches. Three dietary patterns, in particular, are hypothesized to have a beneficial impact on cognitive function: the Mediterranean diet (MedDiet), the Dietary Approaches to Stop Hypertension (DASH), and the MedDiet-DASH Intervention for Neurodegenerative Delay (MIND). The MedDiet and DASH are currently promoted for their cardiovascular benefits (Arnett et al., 2019) yet may also be advisable to benefit cognition in themselves and because of the association of vascular risk factors with dementia risk (Gottesman et al., 2017).
Epidemiological studies and clinical trials have shown a relationship between adherence to MedDiet and cognitive function (Loughrey et al., 2017; Wu and Sun, 2017), and the World Health Organization (WHO) has included this dietary pattern in their guidelines for risk reduction of cognitive decline and dementia; however, the strength of the recommendation is considered conditional (World Health Organization [WHO], 2019). A hybrid of the MedDiet and DASH diet, the MIND diet, is also being promoted for brain health, albeit it has been less extensively investigated in relation to cognition and other cardiometabolic health outcomes (van den Brink et al., 2019). At any rate, dietary recommendations for preventing cognitive decline are still not widely accepted in guidelines due to conflicting and limited evidence. The MedDiet, DASH, and MIND dietary patterns each represent a modifiable lifestyle practice that could aid cognitive performance, yet further evidence is needed to inform cognitive guideline recommendations, as well as assess whether changes may be observed in a period of 2 years.
The aim of this study was to prospectively examine the relationship between baseline adherence to a priori dietary patterns, assessed using the MedDiet, DASH, and MIND dietary patterns scores, with 2-year changes in cognitive performance in a large sample of community-dwelling older adults with overweight or obesity at high cardiovascular disease risk.
Materials and Methods
Study Design
The present analyses were conducted within the framework of the PREvención con DIeta MEDiterránea (PREDIMED)-Plus trial, as an observational cohort, assessing the longitudinal (2-year) associations between baseline adherence to prespecified dietary patterns and cognitive performance. The PREDIMED-Plus study is an ongoing 6-year, multicenter, randomized, parallel-group and primary prevention trial conducted in Spain. The aim of the trial is to assess the effect of an intensive weight loss intervention program based on an energy-restricted traditional MedDiet and physical activity promotion and behavioral support, on clinical cardiovascular events, than usual care and dietary counseling intervention only with an energy unrestricted MedDiet (control group). More detailed information about the study protocol can be found at http://predimedplus.com/ and elsewhere (Martínez-González et al., 2019).
Participants
Participants were recruited between October 2013 and December 2016 in 23 Spanish health centers. Eligible participants were community-dwelling adults (55–75 years) with overweight or obesity (BMI: 27–40 kg/m2) who met at least three criteria for metabolic syndrome, namely, without stroke, myocardial infarction, or diagnosis of neurodegenerative disease at baseline, according to the International Diabetes Federation and the American Heart Association (Alberti et al., 2009). Participants who had not completed the baseline dietary questionnaires or had reported energy intakes outside the prespecified limits of ≥800 to ≤4,000 kcal/day for men and ≥500 to ≤3,500 kcal/day for women were excluded from these analyses (Willett, 2012). If a given cognitive function assessment was missing, this test was not included in the analysis for that participant.
Exposure: Dietary Assessments
Trained dietitians assessed dietary intake via face-to-face interviews at baseline using a previously validated semiquantitative 143-item food frequency questionnaire (FFQ) (Fernández-Ballart et al., 2010). For each item, a portion size was established, and nine consumption frequencies were available, ranging from “never or almost never” to “≥6 times/day”. Energy and nutrient intakes were obtained using data from Spanish food composition tables and by multiplying the frequency by the portion size and accounting for the duration of the period assessed (Moreiras et al., 2018).
Dietary pattern adherence scores were computed from responses to the FFQ. In the case of the MedDiet, it was determined based on a Mediterranean Diet Adherence Screener (MEDAS) score, ranging from 0 to 14 points, which has been previously validated (Schröder et al., 2011; García-Conesa et al., 2020). The DASH diet was defined using the score developed by Fung et al. (2008), which ranges from 8 to 40 points. For the MIND diet, the score developed by Morris et al. (2015a; Berendsen et al., 2018), which ranges from 0 to 15 points, was used.
Outcome: Cognitive Assessments
Participants completed a battery of cognitive tasks at baseline and 2 years of follow-up. This battery of neuropsychological tests included Mini-Mental State Examination (MMSE), a commonly used cognitive screening test (Folstein et al., 1975; Blesa et al., 2001); clock-drawing test (CDT) for evaluating visuospatial and visuo-constructive capacity (del Ser Quijano et al., 2004; Aprahamian et al., 2009; Paganini-Hill and Clark, 2011); semantical and phonological verbal fluency tasks (VFT-a and VFT-p, respectively) for assessing verbal ability and executive function (Benton et al., 1994); Trail Making Tests (TMT) parts A and B for executive function assessment, where part A assesses attention and processing speed and part B further examines cognitive flexibility (Llinàs-Reglà et al., 2017); and forward recall and backward recall Digit Span Tests (DST-f and DST-b, respectively) of the Wechsler Adult Intelligence Scale-III (WAIS-III), where DST-f evaluates attention and short-term memory capacity and DST-b tests working memory (Wechsler, 1997; Rossi et al., 2008). Raw scores for each cognitive assessment were standardized using the mean and standard deviation from the baseline population scores, creating z-scores. Global cognitive function (GCF) was determined as a composite score of all eight assessments (Shah et al., 2013; Gómez Martínez et al., 2021), adding or subtracting each individual test value based on whether a higher score indicates higher or lower cognitive performance, respectively, using z-scores according to the following equation:
Covariate Assessment
Trained staff collected information about sociodemographic (i.e., age, sex, education level, and civil status) and lifestyle (i.e., physical activity, dietary intake, and smoking habits) factors via interviewer-administered questionnaires. Physical activity was assessed using a Spanish validated version of the Minnesota leisure-time physical activity questionnaire (Elosua et al., 1994, 2000). Total daily energy intake was estimated according to data from the FFQ. Anthropometric variables, such as weight and height, were measured by trained staff using calibrated scales and wall-mounted stadiometers, respectively. Body mass index (BMI) was calculated as weight in kilograms divided by height in meters squared. Personal history related to chronic diseases (e.g., hypertension, hypercholesterolemia, and type 2 diabetes) was self-reported or collected from the medical records of participants. Depressive symptomology was evaluated based on Beck’s Depression Inventory-II (BDI-II) with the threshold for depressive status risk established as a score ≥ 14 (Beck et al., 1996; Sanz et al., 2003). The intervention group (treatment or control) and center size (<250, 250 to <300, 300 to <400, ≥400) of the PREDIMED-Plus study were also considered as covariates.
Statistical Analyses
All statistical analyses were performed using the latest PREDIMED-Plus study dataset generated on December 22, 2020. Data for dietary adherence scores (exposure variables) are presented as median (range). For the covariates and outcome variables, data are shown as percentages and mean ± standard deviation (SD), for qualitative and quantitative descriptive variables, respectively, and as β [95% confidence interval (CI)] for associations. Participants were classified according to tertiles of dietary pattern adherence, and the lowest tertile was used as the reference category. The chi-squared test and one-way ANOVA were used for qualitative and quantitative variables, respectively, to compare baseline characteristics according to dietary pattern adherence score.
Longitudinal associations between adherences to the a priori dietary patterns of participants who completed each of the neuropsychological function tests were analyzed separately using multivariate linear regression. All analyses were conducted with robust estimates of the variance to correct for intracluster correlation. Crude and two adjusted models were assessed. The first model was minimally adjusted using established non-modifiable risk factor-related confounders for cognitive function (age, sex) along with intervention arm, study center size, respective baseline cognitive function score, and corrected for clusters (to account for couples living in the same household being randomized as a single unit). The second model was further adjusted for baseline education level (i.e., primary school, secondary school, or college), civil status (i.e., single, divorced or separated, married, or widower), smoking status (i.e., former smoker, never smoked, or current smoker), BMI (kg/m2), hypertension (yes/no), hypercholesterolemia (yes/no), diabetes (yes/no), depressive symptomology (yes/no), baseline physical activity (METs min/day), and total energy intake (kcal/day).
The probability P for trend across categories of dietary pattern adherence score was calculated using the median value of each category as a continuous variable, and a two-tailed P-value < 0.05 was considered statistically significant. Several sensitivity analyses were performed to test the robustness of the findings and identify significant exposure factors to aid in developing priorities for risk mitigation. First, the removal of participants with a baseline MMSE score ≤ 23 indicated possible mild dementia (Dementia Care Central, 2020). Second, alcohol was added as a potential confounder in the models (despite being a component of the MedDiet and MIND patterns) as excessive alcohol intake is considered a risk factor for cognitive decline and dementia (Livingston et al., 2020). Third, analyses were conducted assessing the impact of individual food components of each dietary pattern using linear regression accounting for multicollinearity, if present. Statistical analyses were performed using Stata (14.0, StataCorp LP, TX, United States).
Results
Figure 1 provides the flow diagram of participants. This study included a total of 6,647 participants (mean age 65 years, 48% women). Table 1 provides the baseline characteristics of the participants overall and shows the categories representing the lowest and highest adherence to each of the a priori dietary pattern scores at baseline. The median (range) of dietary adherence scores for the lowest and highest tertiles of each of the three assessed dietary patterns were 6 (1–7) and 10 (10–14) for the MedDiet (lowest possible score 0, highest possible score 14), respectively; 19 (8–21) and 30 (27–38) for DASH (lowest possible score 8, highest possible score 40), respectively; and 8 (2.5–8.5) and 10.5 (10.0–13.5) for MIND (lowest possible score 0, highest possible score 15), respectively.
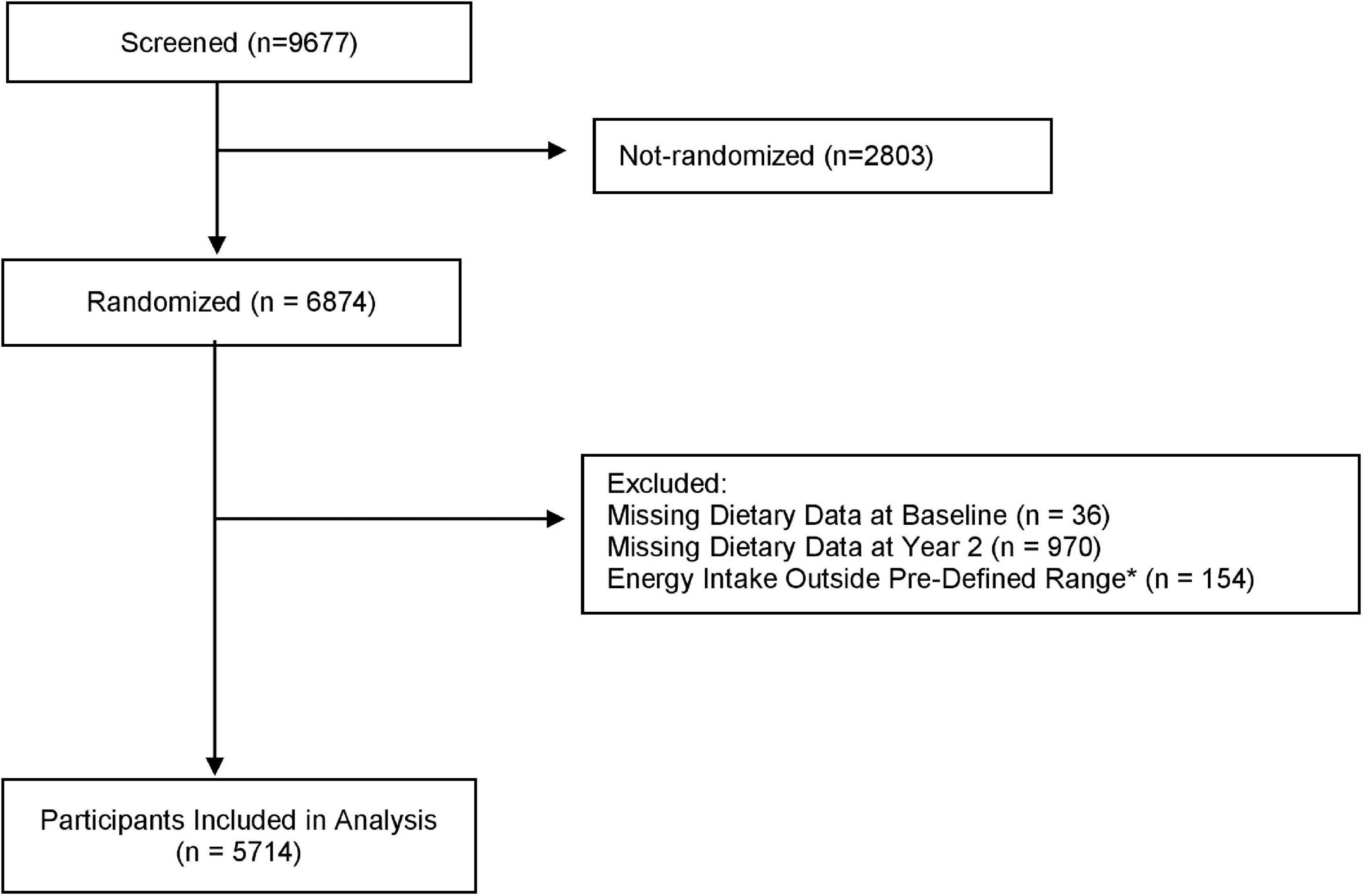
Figure 1. Flow diagram of participants for the analysis of a priori dietary pattern adherence and cognitive performance in the PREDIMED-Plus trial. *Energy intakes outside pre-specified limits were identified as ≤800 to ≥4,000 Kcal/d for men and ≤500 to ≥3,500 Kcal/d for women.
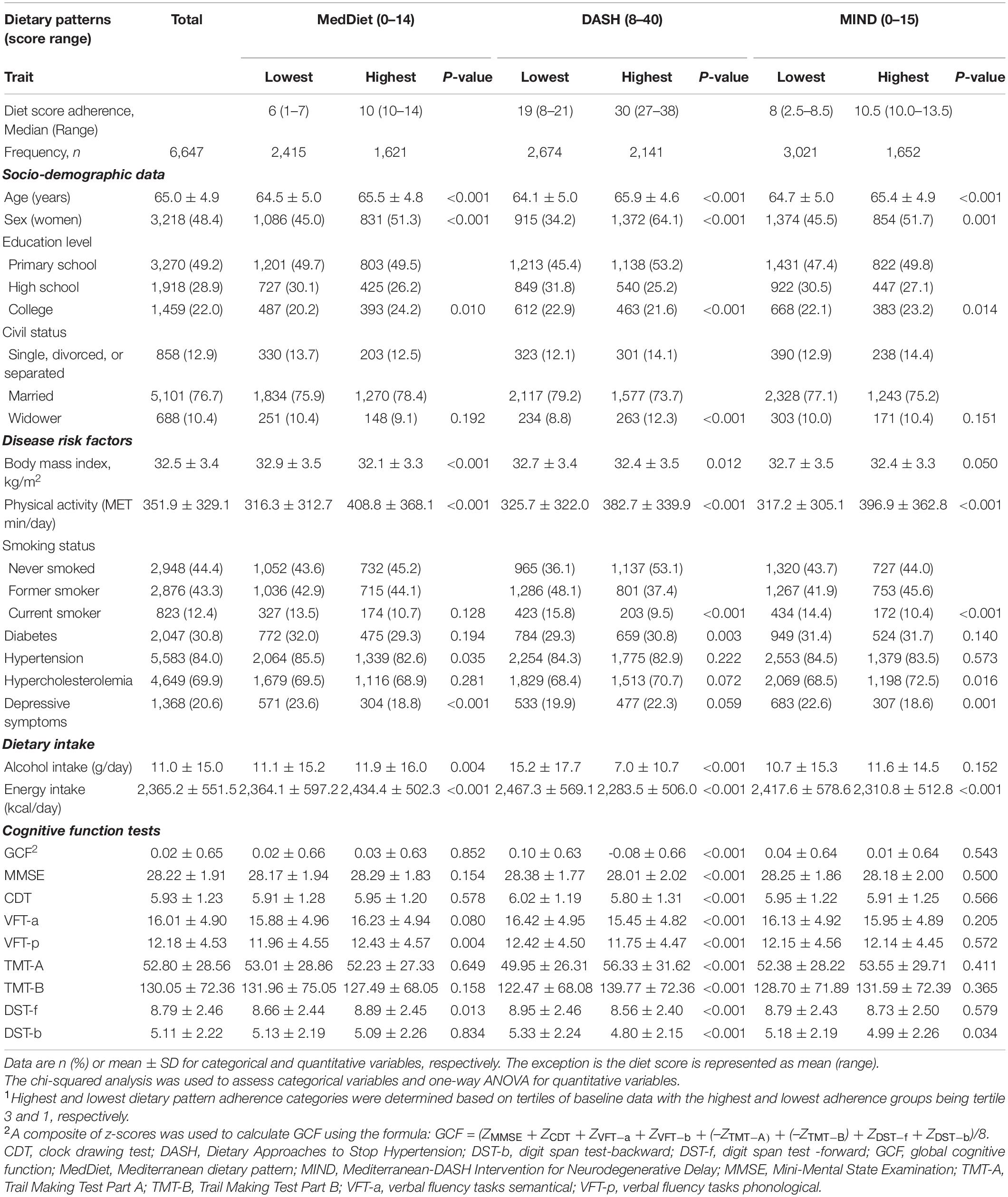
Table 1. Baseline characteristics of the PREDIMED-Plus participants according to categories of highest and lowest baseline adherence (based on tertile categorization1) to a priori dietary patterns.
A higher percentage of women (P ≤ 0.001), older age (P < 0.001), higher physical activity (P < 0.001), and a tendency toward lower BMI, although not clinically significant (P ≤ 0.05), were observed in the highest adherence tertiles for all three dietary patterns. In the DASH and MIND patterns, lower percentages of daily energy intake and current smokers (both P < 0.001) were also observed in the highest adherence tertiles of these patterns. Furthermore, higher adherence to the DASH and MIND diets were associated with less alcohol intake (P < 0.001) and depression (P = 0.001), respectively. For the MedDiet, a lower percentage of participants with depressive symptoms, higher alcohol, and total energy (all P < 0.01) was observed in the highest adherence tertile compared with the lowest adherence tertile.
Figure 2 and Supplementary Table 1 show the β coefficients (95% CIs) associated with 2-year changes in cognitive assessment z-scores across tertiles of a priori dietary pattern adherence scores. Results of the fully adjusted linear regression models show a significant association between highest adherence to the MedDiet and 2-year changes in MMSE (β: 0.070; 95% CI: 0.014, 0.175, P-trend = 0.011), TMT-A (β: −0.054; 95% CI: −0.110, −0.002, P-trend = 0.047), and TMT-B (β: −0.062; 95% CI: −0.116, −0.007, P-trend = 0.024). Adherence to the MIND diet was significantly associated with 2-year changes in DST-B (β: 0.058; 95% CI: 0.002, 0.114, P-trend = 0.045). No other significant beneficial associations with changes in cognitive performance measured by the different neuropsychological test batteries were observed between adherence to the MedDiet, MIND, or DASH dietary patterns. Conversely, significant associations were observed in the crude models with greater 2-year increases in the DASH diet being associated with lower performance in all nine cognitive tests. Sensitivity analyses, which included assessing only participants with baseline MMSE scores above 23 (as scores 23 and below suggest possible mild dementia or worse), or the addition of alcohol as a potential confounder in the model, did not significantly modify the findings (data not shown). The only modification observed was that including total alcohol intake in the model slightly, but non-significantly, mitigated any negative associations observed between adherence to the DASH diet and changes in cognitive function. Figure 3 shows the impact of all 14 food components of the MedDiet on changes of each individual cognitive test. Of these components, olive oil used as the primary oil was found to be positively associated with changes in global cognitive function, as well as changes in the two DSTs (both forward recall P = 0.007 and backward recall P ≤ 0.001). Nut intake was significantly and positively associated with an increase in CDT performance (P = 0.034) and trended toward beneficially impacting changes in MMSE score (P = 0.069). Red wine was also significantly associated with changes in various cognitive function assessments, yet indicating converse findings, where red wine intake may have a beneficial relationship with changes in TMT-A (P = 0.004), TMT-B (P = 0.006), and DST-b (P = 0.020), and a negative association with changes in VFT-a (P = 0.048). Likewise, preferably consuming white meat as opposed to red or processed meat showed conflicting findings where a beneficial association was observed with changes in DST-f (P = 0.001) and negatively associated with changes in CDT (P = 0.001). Fish and shellfish intake was negatively associated with changes in DST-b (P = 0.008). Analyses investigating the DASH and MIND diets showed similar associations with nut consumption and wine intake. Additionally, when assessed within the context of the MIND diet, lower consumption of confectionery products was associated with improvements in 2-year changes in GCF (P = 0.007), VFT-a (P = 0.019), TMT-a (P = 0.011), DST-b (P = 0.042), and higher red meat intake was associated with worsening changes in TMT-a (P = 0.001) and TMT-b (P = 0.014) scores.
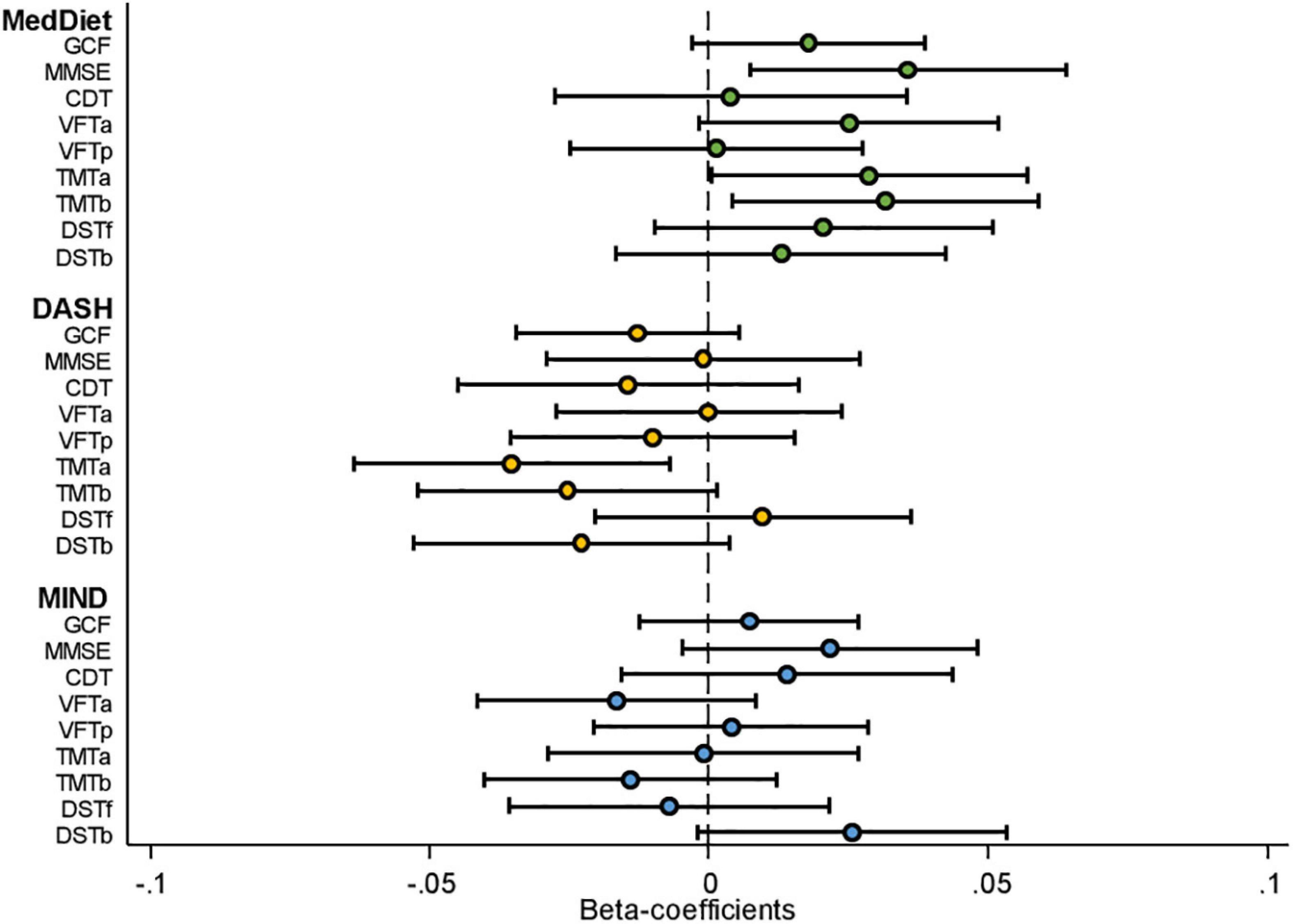
Figure 2. Cognitive function assessment by dietary pattern adherence [standardized beta-coefficients (95% confidence intervals)]. CDT; clock drawing test; DASH; Dietary Approaches to Stop Hypertension; DST-b, digit span test backward; DST-f, digit span test forward; GCF, global cognitive function; MedDiet, Mediterranean dietary pattern; MIND; Mediterranean-DASH Intervention for Neurodegenerative Delay; MMSE, Mini-Mental State Examination; TMT-A, Trail Making Test Part A; TMT-B, Trail Making Test Part B; VFT-a; verbal fluency tasks semantical; VFT-b; verbal fluency tasks phonological. Model presented adjusted for age (in Years), sex, intervention group, centre size (<250, 250 to <300, 300 to <400, ≥400), respective cognitive test score at baseline, baseline education level (primary school, secondary school collage), civil status (single, divorced, or separated, married, and windower), smoking habits (smoker, former smoker, and never smoked), corrected for clusters (to account for couples living in the same household being randomized as a single unit), BMI (kg/m2), hypertension (yes/no), baseline physical activity (MET min/week) and total energy intake (kcal/day). For the neurological tests, a positive beta-coefficient value in the figure indicates better cognitive performance according to the associated test.
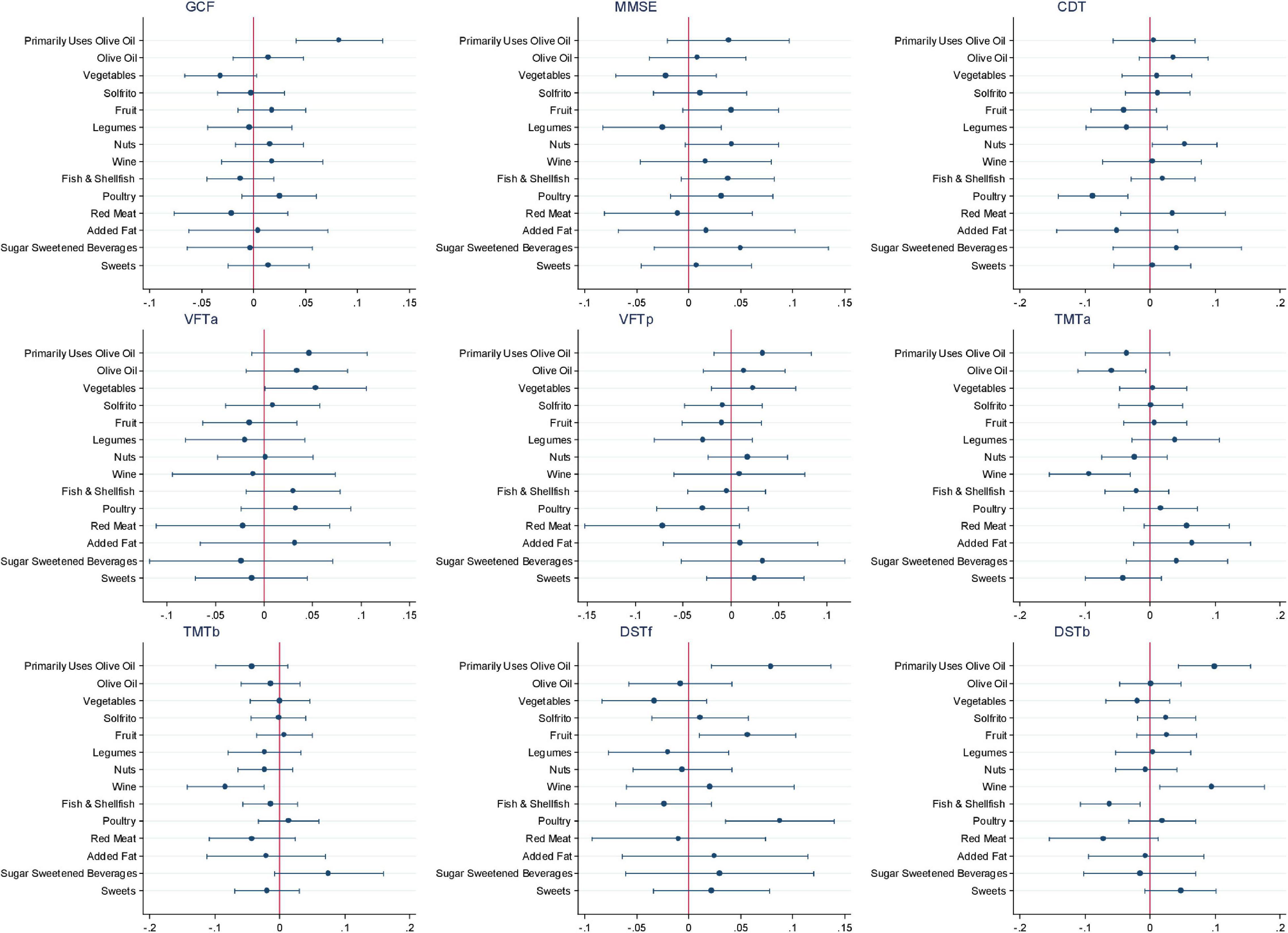
Figure 3. Association of specific dietary components of the Mediterranean dietary pattern (MedDiet) on the cognitive function battery of tests. CDT; clock drawing test; DST-b, digit span test backward; DST-f, digit span test forward; GCF, global cognitive function; MMSE, Mini-Mental State Examination; TMT-A, Trail Making Test Part A; TMT-B, Trail Making Test Part B; VFT-a; verbal fluency tasks semantical; VFT-b; verbal fluency tasks phonological.
Table 2 presents the quantity of intake of various dietary components overall and by tertile of dietary pattern adherence score and shows differences between the dietary patterns. When assessing the MedDiet adherence, 94.7% of participants within the highest adherence tertile used olive oil as their primary oil with a mean intake level of 47.4 ± 14.7 g/day, nut intake was 19.9 ± 18.2 g/day, and red wine consumption was on average 59.4 ± 98.1 g/day.
Discussion
This study examined the PREDIMED-Plus trial as a longitudinal, observational cohort to evaluate the relationship between adherence to a priori dietary patterns and changes in cognitive performance in community-dwelling older adults with overweight or obesity and at high cardiovascular disease risk. Findings suggested that the MedDiet may support cognitive function in older age as significant beneficial associations were observed between greater adherence to the MedDiet with favorable cognitive changes in MMSE, TMT-A, and TMT-B assessments over the follow-up period of 2 years. This represented better general cognitive function, as well as executive function specifically attention and processing speed and cognitive flexibility in those with higher adherence to a MedDiet. Findings also indicated that the MIND diet may be associated with better working memory based on higher adherence being related to higher DST-b assessment. However, the DASH diet was not beneficially associated with 2-year changes in cognitive function in this population with overweight or obesity at high cardiovascular disease risk. The observed advantageous associations between adherence to the MedDiet and cognition align with previous findings presented in systematic reviews and meta-analyses of observational studies suggesting associations between MedDiet adherence with slower cognitive decline, lower risk of dementia (especially Alzheimer’s disease), and reduced conversion of mild cognitive impairment to Alzheimer’s disease (Singh et al., 2014; Loughrey et al., 2017; Wu and Sun, 2017). Recently, a meta-analysis including nine prospective cohort studies reported that high adherence to the MedDiet was associated with a 21% risk reduction in pooled cognitive disorders, in addition to a dose-response with positive findings almost exclusively limited to higher MedDiet adherence (Wu and Sun, 2017). Furthermore, neuroimaging evaluations have found evidence in favor of a protective effect (Karstens et al., 2019). Nevertheless, it is interesting to highlight that previous prospective cohort studies generally had 4 or more years of follow-up, many of which were conducted in relatively healthy, non-Mediterranean populations, with many of the cognitive assessments using screening tests based on criteria to discriminate overall mild cognitive impairment or dementia. Our study also found a positive association between higher adherence to MedDiet and a screening test (MMSE) but also showed specific beneficial associations for executive functioning, including attention and processing speed (TMT-A) and cognitive flexibility (TMT-B). Conversely, a systematic review of randomized controlled trials (nine reports, five unique trials) showed inconsistent findings when comparing a MedDiet with either a waiting list, usual diet, or a low-fat control group for a duration ranging from 10 days to 6.5 years on cognition or brain morphology and function (Radd-Vagenas et al., 2018). However, the authors stated that significant and clinically meaningful effect sizes were found for cognitive composites in the largest and most robust trial, with a duration of 4.1 years, conducted within the context of the PREDIMED trial (Valls-Pedret et al., 2015). Furthermore, in a more recent analysis of a smaller sub-cohort of the PREDIMED-Plus trial evaluating cognition, higher adherence to an energy-reduced MedDiet was associated with greater improvements in memory; however, interpretation of these findings was related to the interplay with weight loss (Soldevila-Domenech et al., 2021). In this study population, we did not find an association for the GCF, which may be explained by the short duration (2 years) and by the broad neuropsychological battery utilized compared with other studies. However, the present findings further support the MedDiet for better cognition while suggesting that beneficial associations may be observable within a shorter timeframe and have applicability for populations at greater risk of cognitive decline (older, with overweight or obesity, and at high risk of cardiovascular disease), which could have implications for improving quality of life. In particular, obesity and its comorbidities are associated with accelerated cognitive decline and impaired cognitive performance including neurodegenerative pathologies, such as dementia, in later life (Dye et al., 2017).
While a significant association was seen in the present analyses with the MedDiet and MIND diet within a shorter follow-up duration with some cognitive function assessments compared with other prospective cohort studies that have been conducted, this relationship was not found with the DASH diet. While MedDiet has been associated with a lower risk of cognitive impairment, it has not always been associated with a slower decline in cognitive function (Keenan et al., 2020). Inconsistent findings have previously been observed with the DASH diet (van den Brink et al., 2019). However, MIND dietary adherence has been associated with better cognitive function across various domains in a systematic review of 13 MIND studies (9 cohorts, 3 cross-sectional, and 1 RCT) evaluating cognitive functioning in older adults (Kheirouri and Alizadeh, 2021). The observed discrepancies with present findings may be related to differences in the types of foods consumed by the study population, and a potential threshold effect related to the amount of each food component consumed, as well as the cognitive tests performed.
The analyzed three dietary patterns each have plant-based foundations, with moderate to high amounts of fish and dairy products, yet they differ in the types and amounts of each dietary component. The MedDiet is typically characterized by high consumption of olive oil, fruits, vegetables, legumes, nuts, cereals, and unsaturated fatty acids; low consumption of meat and saturated fatty acids; low to moderate consumption of dairy products; moderate to high consumption of fish; and a regular, but moderate, intake of wine (Sánchez-Sánchez et al., 2020). The DASH diet shares many similarities yet differs in recommending low-fat dairy and low sodium, besides having fewer specifications (Fung et al., 2008). Based on these two dietary patterns, the MIND diet was developed combining Mediterranean and DASH aspects and incorporating purported neuroprotective foods such as green leafy vegetables and berries (Morris et al., 2015a,b). A potential explanation for our discordant findings may be due to differences in the use of olive oil as the primary oil between tertiles of adherence. In our sample, the use of olive oil was clearly linked with a beneficial association in the GCF composite, as well epidemiological and clinical evidence have suggested improved cognition with olive oil (Millman et al., 2021; Theodore et al., 2021). With any dietary pattern, in addition to observing associations with specific food components, there is also the potential for synergistic effects (Schulz et al., 2021). The present findings suggest such effects given the observed associations for MedDiet with MMSE and TMT-A and TMT-B or the MIND diet with DST-b did not appear to be fully explained by any one component of the dietary pattern; however, further investigation is warranted.
Potential Mechanisms
The antioxidant, vitamin, probiotic, plant protein, and unsaturated fatty acid content along with low glycemic index/load components of the a priori dietary patterns studied have been proposed to possibly affect biological mechanisms of neurocognitive aging (Frisardi et al., 2010). These factors are thought to potentially lead to improved cognition through influencing vascular health and direct promotion of neuroprotection via anti-inflammatory mechanisms and reducing oxidative stress, ameliorating glycemic control, and supporting a favorable microbiome (Caracciolo et al., 2014). Specifically, the observations with the MedDiet and changes in cognitive function may be related to synergistic or individual associations of specific foods, such as olive oil and nuts, due to associations of these foodstuffs with the above-mentioned mechanisms (Viguiliouk et al., 2014; Marcelino et al., 2019; Creedon et al., 2020).
Limitations and Strengths
There are several limitations to this research. First, the demographic profile of the PREDIMED-Plus cohort, which is composed of predominantly white, older Spanish individuals with metabolic syndrome and overweight or obesity, may limit the generalizability of the results to other populations. However, the homogeneity and the large sample size of the cohort increase the internal validity of the findings by avoiding potential confounding effects of socioeconomic status, educational level, and access to health care. Second, FFQs tend to be limited concerning the variety of foods assessed, as compared to 24-h recalls and food records but are often more likely to reflect usual intake (Willett, 2012). The certainty in the dietary pattern scoring systems utilized may also be limited and experience restrictions due to a potential lack of direct alignment of food items and questions noted in the FFQ with each of the diet score components. FFQs are also prone to misclassification and recall bias as they rely on the memory of participants. This is particularly important in a study of cognition when there could be a decline in cognitive function and memory deficits in the population. However, due to the prospective nature of the study, baseline diet recall is unlikely to have been influenced by cognitive outcomes over the follow-up period, and baseline cognitive function was considered as a confounding variable. Another limitation is that the categorization of dietary pattern adherence was based on sample-specific cutoffs, and there are methodological differences among the various dietary scoring systems available, limiting comparability (van den Brink et al., 2019). Likewise, differences in the types of foods consumed by the study population, and the narrow range in scores between lowest and highest tertiles of intake, may have limited the ability to discern a difference, especially for the MIND pattern. Given that optimal or absolute minimum amounts of key foods for cognitive performance are still unclear, threshold amounts of foods for a neuroprotective effect may not have been reached in the present analyses especially in those consuming lower overall energy intake levels. In terms of cognitive assessments, while the use of a composite domain z-score may provide an overall global assessment of cognitive function, the component tests used to create these scores in other studies vary, thus making comparisons difficult. Also, a global screening tool may be less sensitive to detect possible associations due to a potential ceiling effect (Franco-Marina et al., 2010), hence potentially explaining the null associations observed with GCF. Presenting this composite score in addition to the individual cognitive test assessments in this study provides a broader picture of the relationship of these dietary patterns with overall cognition and specific cognitive functions. Additionally, as an observational study, our analysis may be limited by the relatively short duration and is prone to residual confounding from factors not assessed in our models. Specifically, possible genetic interactions, such as with the apolipoprotein E E4 (ApoEε4) genotype, which has been associated with cognition, especially in the presence of hypercholesterolemia (Perna et al., 2016), was not able to be accounted for in the current analyses. Finally, a cause-effect relationship could not be determined due to the nature of the study design, as an observational cohort.
Nonetheless, this study is strengthened by the longitudinal analysis conducted in a large cohort using a comprehensive and thoroughly measured battery of cognitive tests that assess various function areas, as well as the use of an FFQ developed and validated for an older Spanish population. The statistical models were also adjusted for multiple sociodemographic, economic, anthropometric, lifestyle, and biological confounders of the association between diet and cognition, while evaluation of the three distinct a priori dietary patterns within the same study cohort minimizes the effects of population-specific confounders or effect modifiers.
Future Directions
Considering the limitations of the present analyses and inconsistencies observed in the literature, studies, especially randomized controlled trials, accounting for relevant genotypes, use of dietary compliance biomarkers such as via the development of diet-specific metabolomes, and undertaking standardized neuropsychological assessments including biomarkers and neuroimaging would be useful for future research.
Conclusion
In older Spanish adults with overweight or obesity, higher adherence to the MedDiet may help mitigate the risk of cognitive decline, specifically as it relates to general and executive cognitive functioning, even over a short (2-year) period.
Data Availability Statement
Data described in the manuscript, code book, and analytic code will be made available upon request pending application and approval of the PREDIMED-Plus Steering Committee. There are restrictions on the availability of data for the PREDIMED-Plus trial, due to the signed consent agreements around data sharing, which only allow access to external researchers for studies following the project purposes. Requestors wishing to access the PREDIMED-Plus trial data used in this study can make a request to the PREDIMED-Plus trial Steering Committee chair: (JS-S, jordi.salas@urv.cat). The request will then be passed to members of the PREDIMED-Plus Steering Committee for deliberation.
Ethics Statement
The study was conducted in accordance with the principles of the Declaration of Helsinki. The respective Institutional Review Board (IRB) of all study centres approved the study protocol. The trial was registered at the International Standard Randomized Controlled Trial in 2014 (ISRCTNwww.isrctn.com/ISRCTN8989887089898870). All participants provided written informed consent.
Author Contributions
MAM-G, DC, JS-S, MF, JAM, AMA-G, JW, JV, DR, JL-M, RE, FJT, JL, JLS-M, AB-C, JAT, VMS, XP, MD-R, PM-M, JV, CV, LD, and ER (all the principal PREDIMED-Plus investigators) contributed to the study concept and design and to data extraction from the participants in the PREDIMED-Plus trial, with SKN, NB, and JS-S, contributing to the study concept and design of the present analyses. SKN, NB, CG-M, NB-T and JS-S performed the statistical analyses. SKN, NB and JS-S drafted the manuscript. All authors reviewed the manuscript for important intellectual content and approved the final version to be published.
Funding
This work was supported by the official Spanish Institutions for funding scientific biomedical research, CIBER Fisiopatología de la Obesidad y Nutrición (CIBEROBN) and Instituto de Salud Carlos III (ISCIII), through the Fondo de Investigación para la Salud (FIS), which was co-funded by the European Regional Development Fund (six coordinated FIS projects leaded by JS-S and JV, including the following projects: PI13/00673, PI13/00492, PI13/00272, PI13/01123, PI13/00462, PI13/00233, PI13/02184, PI13/00728, PI13/01090, PI13/01056, PI14/01722, PI14/00636, PI14/00618, PI14/00696, PI14/01206, PI14/01919, PI14/00853, PI14/01374, PI14/00972, PI14/00728, PI14/01471, PI16/00473, PI16/00662, PI16/01873, PI16/01094, PI16/00501, PI16/00533, PI16/00381, PI16/00366, PI16/01522, PI16/01120, PI17/00764, PI17/01183, PI17/00855, PI17/01347, PI17/00525, PI17/01827, PI17/00532, PI17/00215, PI17/01441, PI17/00508, PI17/01732, PI17/00926, PI19/00957, PI19/00386, PI19/00309, PI19/01032, PI19/00576, PI19/00017, PI19/01226, PI19/00781, PI19/01560, PI19/01332, PI20/01802, PI20/00138, PI20/01532, PI20/00456, PI20/00339, PI20/00557, PI20/00886, and PI20/01158); the Especial Action Project entitled: Implementation y evaluación de una intervención intensiva sobre la actividad física Cohorte PREDIMED-Plus grant to JS-S; the European Research Council (Advanced Research Grant 2014–2019; agreement #340918) granted to MAM-G; the Recercaixa (number 2013ACUP00194) grant to JS-S; grants from the Consejería de Salud de la Junta de Andalucía (PI0458/2013, PS0358/2016, and PI0137/2018); the PROMETEO/2017/017 grant from the Generalitat Valenciana; the SEMERGEN grant; Juan de la Cierva-Incorporación research grant (IJC2019-042420-I) of the Spanish Ministry of Economy, Industry and Competitiveness and European Social Funds to JK. This research was also partially funded by EU-H2020 Grants [Eat2beNICE/H2020-SFS-2016-2; and the Horizon 2020 PRIME study (Prevention and Remediation of Insulin Multimorbidity in Europe); grant agreement #847879]. SKN was supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR, MFE-171207). None of the funding sources took part in the design, collection, analysis, interpretation of the data, or writing the report, or in the decision to submit the manuscript for publication.
Conflict of Interest
SKN was a volunteer member of the not-for profit group Plant Based Canada. JS-S reported receiving research support from the Instituto de Salud Carlos III, Ministerio de Educación y Ciencia, Departament de Salut Pública de la Generalitat de Catalunya, the European Commission, the United States National Institutes of Health; receiving consulting fees or travel expenses from Danone, California Walnut Commission, Eroski Foundation, Instituto Danone, Nestle, and Abbott Laboratories, receiving non-financial support from Hojiblanca, Patrimonio Comunal Olivarero, the California Walnut Commission, Almond Board of California, La Morella Nuts, Pistachio Growers, and Borges SA; serving on the board of and receiving grant support through his institution from the International Nut and Dried Foundation and the Eroski Foundation; and grants and personal fees from Instituto Danone; Serving in the Board of Danone Institute International. DC reported receiving grants from Instituto de Salud Carlos III. RE reported receiving grants from Instituto de Salud Carlos III, Fundación Dieta Meditarránea and Cerveza y Salud and olive oil for the trial from Fundación Patrimonio Comunal Olivarero and personal fees from Brewers of Europe, Fundación Cerveza y Salud, Interprofesional del Aceite de Oliva, Instituto Cervantes in Albuquerque, Milano and Tokyo, Pernod Ricard, Fundación Dieta Mediterránea (Spain), Wine and Culinary International Forum and Lilly Laboratories; non-financial support from Sociedad Española de Nutrición and Fundación Bosch y Gimpera; and grants from Uriach Laboratories. ER reports grants, personal fees, non-financial support, and others from California Walnut Commission, during the conduct of the study; grants, personal fees, non-financial support and other from Alexion; grants from Amgen and Pfizer; grants, personal fees and other from Sanofi Aventis; personal fees, non-financial support and other from Ferrer International, Danone and Merck Sharp and Dohme, personal fees and other from Amarin, outside the submitted work.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank all PREDIMED-Plus participants and investigators. CIBEROBN, CIBERESP, and CIBERDEM are initiatives of the Instituto de Salud Carlos III (ISCIII), Madrid, Spain. The Hojiblanca (Lucena, Spain) and Patrimonio Comunal Olivarero (Madrid, Spain) food companies donated extra-virgin olive oil. The Almond Board of California (Modesto, CA), American Pistachio Growers (Fresno, CA), and Paramount Farms (Wonderful Company, LLC, Los Angeles, CA) donated nuts.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnagi.2021.782067/full#supplementary-material
References
Alberti, K. G. M. M., Eckel, R. H., Grundy, S. M., Zimmet, P. Z., Cleeman, J. I., Donato, K. A., et al. (2009). Harmonizing the metabolic syndrome: a joint interim statement of the international diabetes federation task force on epidemiology and prevention. national heart, lung, and blood institute. american heart association. world heart federation. International atherosclerosis society. and international association for the study of obesity. Circulation 120, 1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644
Ammar, A., Trabelsi, K., Boukhris, O., Bouaziz, B., Müller, P., Glenn, M. J., et al. (2020). Effects of polyphenol-rich interventions on cognition and brain health in healthy young and middle-aged adults: systematic review and meta-analysis. J. Clin. Med. 9:1598. doi: 10.3390/jcm9051598
Aprahamian, I., Martinelli, J. E., Neri, A. L., and Yassuda, M. S. (2009). O Teste do desenho do relógio: revisão da acurácia no rastreamento de demência. Dementia Neuropsychol. Acad. Brasileira Neurol. 3, 74–80.
Arnett, D. K., Blumenthal, R. S., Albert, M. A., Buroker, A. B., Goldberger, Z. D., Hahn, E. J., et al. (2019). ACC/AHA guideline on the primary prevention of cardiovascular disease: a report of the american college of cardiology/american heart association task force on clinical practice guidelines. J. Am. Coll. Cardiol. 74, e177–e232.
Balasubramanian, P., Kiss, T., Tarantini, S., Nyúl-Tóth, Á, Ahire, C., Yabluchanskiy, A., et al. (2020). Obesity-induced cognitive impairment in older adults: a microvascular perspective. Am. J. Physiol. Heart Circ. Physiol. 320, H740–H761. doi: 10.1152/ajpheart.00736.2020
Beck, A. T., Steer, R. A., Ball, R., and Ranieri, W. F. (1996). Comparison of beck depression inventories -IA and -II in psychiatric outpatients. J. Pers. Assess. 67, 588–597. doi: 10.1207/s15327752jpa6703_13
Benton, A. L., Hamsher, K. D., and Sivan, A. B. (1994). Multilingual Aphasia Examination, 3rd Edn. Iowa City: AJA Associates.
Berendsen, A., Kang, J., Feskens, E., de Groot, C., Grodstein, F., and van de Rest, O. (2018). Association Of long-term adherence to the mind diet with cognitive function & cognitive decline. J. Nutr. Health Aging 22, 222–229.
Blesa, R., Pujol, M., Aguilar, M., Santacruz, P., Bertran-Serra, I., Hernández, G., et al. (2001). Clinical validity of the “mini-mental state” for Spanish speaking communities. Neuropsychologia 39, 1150–1157. doi: 10.1016/s0028-3932(01)00055-0
Brainard, J. S., Jimoh, O. F., Deane, K. H. O., Biswas, P., Donaldson, D., Maas, K., et al. (2020). Omega-3, Omega-6, and polyunsaturated fat for cognition: systematic review and meta-analysis of randomized trials. J. Am. Med. Direct. Assoc. 21, 1439–1450.e21. doi: 10.1016/j.jamda.2020.02.022
Caracciolo, B., Xu, W., Collins, S., and Fratiglioni, L. (2014). Cognitive decline, dietary factors and gut-brain interactions. Mechan. Ageing Dev. 13, 59–69. doi: 10.1016/j.mad.2013.11.011
Creedon, A. C., Hung, E. S., Berry, S. E., and Whelan, K. (2020). Nuts and their effect on gut microbiota, gut function and symptoms in adults: a systematic review and meta-analysis of randomised controlled trials. Nutrients 12:2347. doi: 10.3390/nu12082347
del Ser Quijano, T., García de Yébenes Mj, Sánchez Sánchez, F., Frades Payo, B., Rodríguez Laso, Á, Bartolomé Martínez, M. P., et al. (2004). Evaluación cognitiva del anciano. datos normativos de una muestra poblacional española de más de 70 años. Med. Clín. 122, 727–740.
Dementia Care Central (2020). Mini-Mental State Exam (MMSE) Alzheimer’s / Dementia Test: Administration, Accuracy and Scoring. Available online at: https://www.dementiacarecentral.com/mini-mental-state-exam/ (accessed November 11, 2020)
Dye, L., Boyle, N. B., Champ, C., and Lawton, C. (2017). The relationship between obesity and cognitive health and decline. Proc. Nutrition Soc. 76, 443–454. doi: 10.1017/s0029665117002014
Elosua, R., Garcia, M., Aguilar, A., and Molina, L. (2000). Validation of the minnesota leisure time physical activity questionnaire in spanish women. validation of the minnesota leisure time physical activity questionnaire in spanish women. Med. Sci. Sports Exerc. 32, 1431–1437. doi: 10.1097/00005768-200008000-00011
Elosua, R., Marrugat, J., Molina, L., Pons, S., and Pujol, E. (1994). Validation of the minnesota leisure time physical activity questionnaire in spanish men. Am. J. Epidemiol. 139, 1197–1209. doi: 10.1093/oxfordjournals.aje.a116966
Fernández-Ballart, J. D., Piñol, J. L., Zazpe, I., Corella, D., Carrasco, P., Toledo, E., et al. (2010). Relative validity of a semi-quantitative food-frequency questionnaire in an elderly mediterranean population of Spain. Br. J. Nutrition 103, 1808–1816. doi: 10.1017/S0007114509993837
Folstein, M. F., Folstein, S. E., and Mchugh, P. R. (1975). “MINI-MENTAL STATE” a practical method for grading the cognitive state of patients for the clinician∗. J. Gsychiaf Res. 12, 189–198. doi: 10.1016/0022-3956(75)90026-6
Franco-Marina, F., García-González, J. J., Wagner-Echeagaray, F., Gallo, J., Ugalde, O., Sánchez-García, S., et al. (2010). The Mini-mental state examination revisited: ceiling and floor effects after score adjustment for educational level in an aging Mexican population. Int. Psychogeriatr. 22, 72–81. doi: 10.1017/S1041610209990822
Frisardi, V., Panza, F., Seripa, D., Imbimbo, B. P., Vendemiale, G., Pilotto, A., et al. (2010). Nutraceutical properties of mediterranean diet and cognitive decline: possible underlying mechanisms. J. Alzheimer’s Dis. 22, 715–740. doi: 10.3233/JAD-2010-100942
Fung, T. T., Chiuve, S. E., Mccullough, M. L., Rexrode, K. M., Logroscino, G., and Hu, F. B. (2008). Adherence to a DASH-Style diet and risk of coronary heart disease and stroke in women. Arch. Int. Med. 168, 713–720. doi: 10.1001/archinte.168.7.713
García-Conesa, M. T., Philippou, E., Pafilas, C., Massaro, M., Quarta, S., Andrade, V., et al. (2020). Exploring the validity of the 14-item mediterranean diet adherence screener (Medas): a cross-national study in seven european countries around the mediterranean region. Nutrients 12, 1–18. doi: 10.3390/nu12102960
Gómez Martínez, C., Babio, N., Júlvez, J., Becerra Tomás, N., Martínez González, M. Á, Corella, D., et al. (2021). Glycemic dysregulations are associated with worsening cognitive function in older participants at high risk of cardiovascular disease: two-year follow-up in the PREDIMED-Plus study. Front. Endocrinol. 12:754347. doi: 10.3389/fendo.2021.754347
Gottesman, R. F., Albert, M. S., Alonso, A., Coker, L. H., Coresh, J., Davis, S. M., et al. (2017). Associations between midlife vascular risk factors and 25-year incident dementia in the Atherosclerosis Risk in Communities (ARIC) cohort. JAMA Neurol. Am. Med. Assoc. 74, 1246–1254. doi: 10.1001/jamaneurol.2017.1658
Hassing, L. B., Dahl, A. K., Pedersen, N. L., and Johansson, B. (2010). Overweight in midlife is related to lower cognitive function 30 years later: a prospective study with longitudinal assessments. Dement. Geriatric Cogn. Disord. 29, 543–552. doi: 10.1159/000314874
Kanaya, A. M., Lindquist, K., Harris, T. B., Launer, L., Rosano, C., Satterfield, S., et al. (2009). Total and regional adiposity and cognitive change in older adults: the health, aging and body composition (ABC) study. Arch. Neurol. 66, 329–335. doi: 10.1001/archneurol.2008.570
Karstens, A. J., Tussing-Humphreys, L., Zhan, L., Rajendran, N., Cohen, J., Dion, C., et al. (2019). Associations of the Mediterranean diet with cognitive and neuroimaging phenotypes of dementia in healthy older adults. Am. J. Clin. Nutrition 109, 361–368. doi: 10.1093/ajcn/nqy275
Keenan, T. D., Agrón, E., Mares, J. A., Clemons, T. E., van Asten, F., Swaroop, A., et al. (2020). Adherence to a mediterranean diet and cognitive function in the age-related eye disease studies 1 & 2. Alzheimer’s Dementia 16, 831–842. doi: 10.1002/alz.12077
Kheirouri, S., and Alizadeh, M. (2021). MIND diet and cognitive performance in older adults: a systematic review. Crit. Rev. Food Sci. Nutrition 14, 1–19.
Livingston, G., Huntley, J., Sommerlad, A., Ames, D., Ballard, C., Banerjee, S., et al. (2020). Dementia prevention, intervention, and care: 2020 report of the lancet commission. Lancet 396, 413–446. doi: 10.1016/s0140-6736(20)30367-6
Llinàs-Reglà, J., Vilalta-Franch, J., López-Pousa, S., Calvó-Perxas, L., Torrents Rodas, D., and Garre-Olmo, J. (2017). The trail making test: association with other neuropsychological measures and normative values for adults Aged 55 years and older from a spanish-speaking population-based sample. Assessment 24, 183–196. doi: 10.1177/1073191115602552
Loughrey, D. G., Lavecchia, S., Brennan, S., Lawlor, B. A., and Kelly, M. E. (2017). The impact of the mediterranean diet on the cognitive functioning of healthy older adults: a systematic review and meta-analysis. Adv. Nutrition Am. Soc. Nutrition 8, 571–586. doi: 10.3945/an.117.015495
Marcelino, G., Hiane, P. A., Freitas, K., de, C., Santana, L. F., Pott, A., et al. (2019). Effects of olive oil and its minor components on cardiovascular diseases, inflammation, and gut microbiota. Nutrients 11:1826. doi: 10.3390/nu11081826
Martínez-González, M. A., Buil-Cosiales, P., Corella, D., Bulló, M., Fitó, M., Vioque, J., et al. (2019). Cohort profile: design and methods of the PREDIMED-plus randomized trial. Int. J. Epidemiol. 48, 387–3880. doi: 10.1093/ije/dyy225
Millman, J. F., Okamoto, S., Teruya, T., Uema, T., Ikematsu, S., Shimabukuro, M., et al. (2021). Extra-virgin olive oil and the gut-brain axis: influence on gut microbiota, mucosal immunity, and cardiometabolic and cognitive health. Nutrition Rev. 79, 1362–1374. doi: 10.1093/nutrit/nuaa148
Moreiras, O., Carbajal, Á, and Carmen Cuadrado, L. C. Y. (2018). Tablas de Composicion de Alimentos: guía de Prácticas. Madrid: Universidad Complutense Madrid.
Morris, M. C., Tangney, C. C., Wang, Y., Sacks, F. M., Barnes, L. L., Bennett, D. A., et al. (2015a). MIND diet slows cognitive decline with aging. Alzheimer’s Dementia. 11, 1015–1022. doi: 10.1016/j.jalz.2015.04.011
Morris, M. C., Tangney, C. C., Wang, Y., Sacks, F. M., Bennett, D. A., and Aggarwal, N. T. (2015b). MIND diet associated with reduced incidence of Alzheimer’s disease. Alzheimer’s Dementia 11, 1007–1014.
Ng, M., Fleming, T., Robinson, M., Thomson, B., Graetz, N., Margono, C., et al. (2014). Global, regional, and national prevalence of overweight and obesity in children and adults during 1980-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 384, 766–781. doi: 10.1016/S0140-6736(14)60460-8
Paganini-Hill, A., and Clark, L. J. (2011). Longitudinal assessment of cognitive function by clock drawing in older adults. Dementia Geriatric Cogn. Disord. 1, 75–83. doi: 10.1159/000326781
Perna, L., Mons, U., Rujescu, D., Kliegel, M., and Brenner, H. (2016). Apolipoprotein e e4 and cognitive function: a modifiable association? results from two independent cohort studies. Dementia Geriatric Cogn. Disord. 41, 35–45.
Petersen, R. C., Doody, R., Kurz, A., Mohs, R. C., Morris, J. C., Rabins, P. V., et al. (2001). Current concepts in mild cognitive impairment. Arch. Neurol. 58, 1985–1992.
Petersen, R. C., Lopez, O., Armstrong, M. J., Getchius, T. S. D., Ganguli, M., Gloss, D., et al. (2018). Practice guideline update summary: mild cognitive impairment report of theguideline development, dissemination, and implementation. Neurology 90, 126–135.
Radd-Vagenas, S., Duffy, S. L., Naismith, S. L., Brew, B. J., Flood, V. M., and Fiatarone Singh, M. A. (2018). Effect of the Mediterranean diet on cognition and brain morphology and function: a systematic review of randomized controlled trials. Am. J. Clin. Nutrition 107, 389–404. doi: 10.1093/ajcn/nqx070
Rossi, L., Neer, C.-R., and Lopetegui, S. (2008). Escala de inteligencia para adultos de WECHSLER. WAIS-III Índice de comprensión verbal. Normas para los subtests: vocabulario, analogías e información, para la Ciudad de La Plata Edades: 16 A 24 Años. Revista Psicol. 10, 223–236.
Rutjes, A. W. S., Denton, D. A., di Nisio, M., Chong, L. Y., Abraham, R. P., Al-Assaf, A. S., et al. (2018). Vitamin and mineral supplementation for maintaining cognitive function in cognitively healthy people in mid and late life. Cochrane Database Syst Rev. 12:CD011906. doi: 10.1002/14651858.CD011906.pub2
Sánchez-Sánchez, M. L., García-Vigara, A., Hidalgo-Mora, J. J., García-Pérez, M. Á, Tarín, J., and Cano, A. (2020). Mediterranean diet and health: a systematic review of epidemiological studies and intervention trials. Maturitas 136, 25–37. doi: 10.1016/j.maturitas.2020.03.008
Sanz, J., Luis, A., Carmelo, P., and Resumen, V. (2003). CLÍNICA Y SALUD 249 ARTÍCULOS adaptación española del Inventario para la Depresión de Beck-II (BDI-II): 2. propiedades psicométricas en población general the Spanish adaptation of beck’s depression Inventory-II (BDI-II): 2. Psychometric Properties General Population 14, 249–280.
Schröder, H., Fitó, M., Estruch, R., Martínez-González, M. A., Corella, D., Salas-Salvadó, J., et al. (2011). A Short screener is valid for assessing mediterranean diet adherence among older spanish men and women. J. Nutrition 141, 1140–1145. doi: 10.3945/jn.110.135566
Schulz, C. A., Oluwagbemigun, K., and Nöthlings, U. (2021). Advances in dietary pattern analysis in nutritional epidemiology. Eur. J. Nutrition 60, 4115–4130. doi: 10.1007/s00394-021-02545-9
Shah, R. C., Janos, A. L., Kline, J. E., Yu, L., Leurgans, S. E., Wilson, R. S., et al. (2013). Cognitive decline in older persons initiating anticholinergic medications. PLoS One 8:e64111. doi: 10.1371/journal.pone.0064111
Singh, B., Parsaik, A. K., Mielke, M. M., Erwin, P. J., Knopman, D. S., Petersen, R. C., et al. (2014). Association of Mediterranean diet with mild cognitive impairment and Alzheimer’s disease: a systematic review and meta-analysis. J. Alzheimer’s Dis. 39, 271–282.
Soldevila-Domenech, N., Forcano, L., Vintró-Alcaraz, C., Cuenca-Royo, A., Pintó, X., Jiménez-Murcia, S., et al. (2021). Interplay between cognition and weight reduction in individuals following a Mediterranean diet: three-year follow-up of the PREDIMED-Plus trial. Clin. Nutrition 40, 5221–5237. doi: 10.1016/j.clnu.2021.07.020
Theodore, L. E., Kellow, N. J., Mcneil, E. A., Close, E. O., Coad, E. G., and Cardoso, B. R. (2021). Nut consumption for cognitive performance: a systematic review. Adv. Nutrition 12, 777–792. doi: 10.1093/advances/nmaa153
United Nations, Department of Economic, and Social Affairs and Population Division (2020). World Population Ageing 2020 Highlights: Living Arrangements of Older Persons. New York, NY: United Nations.
Valls-Pedret, C., Sala-Vila, A., Serra-Mir, M., Corella, D., de La Torre, R., Martínez-González, M. Á, et al. (2015). Mediterranean diet and age-related cognitive decline: a randomized clinical trial. JAMA Int. Med. 175, 1094–1103.
van den Brink, A. C., Brouwer-Brolsma, E. M., Berendsen, A. A. M., and van de Rest, O. (2019). The Mediterranean, Dietary Approaches to Stop Hypertension (DASH), and Mediterranean-DASH Intervention for Neurodegenerative Delay (MIND) diets are associated with less cognitive decline and a lower risk of Alzheimer’s disease-a review. Adv. Nutrition 10, 1040–1065. doi: 10.1093/advances/nmz054
Viguiliouk, E., Kendall, C. W. C., Mejia, S. B., Cozma, A. I., Ha, V., Mirrahimi, A., et al. (2014). Effect of tree nuts on glycemic control in diabetes: a systematic review and meta-analysis of randomized controlled dietary trials. PLoS One 9:e103376. doi: 10.1371/journal.pone.0103376
Wechsler, D. (1997). Wechsler Adult Intelligence Scale-III. San Antonio, TX: Psychological Corporation.
World Health Organization [WHO] (2019). Risk Reduction of Cognitive Decline and Dementia: WHO Guidelines. Geneva: World Health Organization.
Wu, L., and Sun, D. (2017). Adherence to Mediterranean diet and risk of developing cognitive disorders: an updated systematic review and meta-analysis of prospective cohort studies. Sci. Rep. 7:41317. doi: 10.1038/srep41317
Keywords: cognition, dietary pattern, Mediterranean diet (MedDiet), DASH diet, MIND diet
Citation: Nishi SK, Babio N, Gómez-Martínez C, Martínez-González MÁ, Ros E, Corella D, Castañer O, Martínez JA, Alonso-Gómez ÁM, Wärnberg J, Vioque J, Romaguera D, López-Miranda J, Estruch R, Tinahones FJ, Lapetra J, Serra-Majem JL, Bueno-Cavanillas A, Tur JA, Martín Sánchez V, Pintó X, Delgado-Rodríguez M, Matía-Martín P, Vidal J, Vázquez C, Daimiel L, Razquin C, Coltell O, Becerra-Tomás N, De La Torre Fornell R, Abete I, Sorto-Sanchez C, Barón-López FJ, Signes-Pastor AJ, Konieczna J, Garcia-Rios A, Casas R, Gomez-Perez AM, Santos-Lozano JM, García-Arellano A, Guillem-Saiz P, Ni J, Trinidad Soria-Florido M, Zulet MÁ, Vaquero-Luna J, Toledo E, Fitó M and Salas-Salvadó J (2021) Mediterranean, DASH, and MIND Dietary Patterns and Cognitive Function: The 2-Year Longitudinal Changes in an Older Spanish Cohort. Front. Aging Neurosci. 13:782067. doi: 10.3389/fnagi.2021.782067
Received: 23 September 2021; Accepted: 08 November 2021;
Published: 13 December 2021.
Edited by:
Yashi Mi, University of Arizona, United StatesReviewed by:
Thomas Holland, Rush University, United StatesNazanin Moslehi, Shahid Beheshti University of Medical Sciences, Iran
Christy Tangney, Rush University Medical Center, United States
Copyright © 2021 Nishi, Babio, Gómez-Martínez, Martínez-González, Ros, Corella, Castañer, Martínez, Alonso-Gómez, Wärnberg, Vioque, Romaguera, López-Miranda, Estruch, Tinahones, Lapetra, Serra-Majem, Bueno-Cavanillas, Tur, Martín Sánchez, Pintó, Delgado-Rodríguez, Matía-Martín, Vidal, Vázquez, Daimiel, Razquin, Coltell, Becerra-Tomás, De La Torre Fornell, Abete, Sorto-Sanchez, Barón-López, Signes-Pastor, Konieczna, Garcia-Rios, Casas, Gomez-Perez, Santos-Lozano, García-Arellano, Guillem-Saiz, Ni, Trinidad Soria-Florido, Zulet, Vaquero-Luna, Toledo, Fitó, Salas-Salvadó. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Stephanie K. Nishi, stephanie.nishi@urv.cat; Nancy Babio, nancy.babio@urv.cat
 Stephanie K. Nishi
Stephanie K. Nishi Nancy Babio
Nancy Babio Carlos Gómez-Martínez
Carlos Gómez-Martínez Miguel Ángel Martínez-González
Miguel Ángel Martínez-González Emilio Ros
Emilio Ros Dolores Corella
Dolores Corella Olga Castañer3,11
Olga Castañer3,11  J. Alfredo Martínez
J. Alfredo Martínez Ramon Estruch
Ramon Estruch Francisco J. Tinahones
Francisco J. Tinahones José Lapetra
José Lapetra Aurora Bueno-Cavanillas
Aurora Bueno-Cavanillas Josep A. Tur
Josep A. Tur Xavier Pintó
Xavier Pintó Lidia Daimiel
Lidia Daimiel Cristina Razquin
Cristina Razquin Oscar Coltell
Oscar Coltell Rafael De La Torre Fornell
Rafael De La Torre Fornell Jadwiga Konieczna
Jadwiga Konieczna Rosa Casas
Rosa Casas Ana Maria Gomez-Perez
Ana Maria Gomez-Perez Estefanía Toledo
Estefanía Toledo Montserrat Fitó
Montserrat Fitó Jordi Salas-Salvadó
Jordi Salas-Salvadó