- 1Developmental Neuromotor and Cognition Lab, School of Psychology and Public Health, La Trobe University, Melbourne, VIC, Australia
- 2School of Psychology and Public Health, La Trobe University, Melbourne, VIC, Australia
- 3Department of Radiology, St. Vincent's Hospital Melbourne, Fitzroy, VIC, Australia
- 4Centre for Molecular, Environmental, Genetic and Analytic Epidemiology, University of Melbourne, Melbourne, VIC, Australia
- 5VCGS Cytogenetics Laboratory, Murdoch Children's Research Institute, Royal Children's Hospital, Melbourne, VIC, Australia
- 6Department of Biochemistry and Molecular Medicine, University of California, Davis, Davis, CA, United States
- 7School of Medicine, MIND Institute, University of California Davis Medical Center, Davis, CA, United States
- 8Department of Medicine (Neuroscience), Central Clinical School, Monash University, Melbourne, VIC, Australia
This study explores the relationships between hemispheric and cerebellar white matter lesions and motor and cognitive impairments in male carriers of Fragile-X Mental Retardation 1 (FMR1) premutation alleles, and in a subgroup of these carriers affected with Fragile X-Associated Tremor/Ataxia syndrome (FXTAS). Regional and total white matter hyperintensities (wmhs) on MRI, assessed using semiquantitative scores, were correlated with three motor rating scales (ICARS, UPDRS, Tremor), and neuropsychological measures of non-verbal reasoning, working memory and processing speed, in a sample of 30 male premutation carriers aged 39–81 years, and separately in a subsample of 17 of these carriers affected with FXTAS. There were significant relationships between wmhs in the infratentorial region and all three motor scales, as well as several cognitive measures—Prorated IQ, Matrix Reasoning, Similarities, and the Symbol Digit Modalities Test (SDMT), in the total sample of carriers, as well as in the FXTAS group separately. This shows that whms within the infratentorial region correlates across the categories of clinical status with a range of motor and cognitive impairments. In the FXTAS group, there was a highly significant relationship between supratentorial (periventricular) lesions and parkinsonism, and between both periventricular and supratentorial deep white matter and ICARS ataxia score. These findings further support the relevance of white matter changes in different brain regions to the motor and cognitive deficits across the spectrum of premutation involvement. Future longitudinal studies using larger sample sizes will be necessary to examine the factors that lead to conversion to a greater extent of neurological involvement as seen in the progression across the FXTAS spectrum.
Introduction
The most severe form of clinical disorder associated with premutation expansions (55–200 CGG repeats) in the 5′ untranslated region of the Fragile X Mental Retardation 1 (FMR1) gene is a late-onset progressive neurodegenerative condition: Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) (1). This syndrome usually occurs after, and progresses from, the age of 55 years, and is more prevalent in male (45%) than in female (8–16%) carriers of the premutation allele. The clinical features include intention and/or postural tremor, cerebellar ataxia, dementia, and parkinsonism in some cases. Major neuroradiological features include white matter hyperintensities (wmhs) in the middle cerebellar peduncles (“MCP sign”) and/or in the splenium of the corpus callosum (2). Occasionally, a milder version of this syndrome has been reported in carriers of “gray zone” FMR1 alleles with CGG expansions ranging between 41 and 54 CGGs (3–5).
The mechanisms involved in neural pathology associated with premutation alleles (PM) are not fully understood. However, evidence suggests several possible pathomechanisms: a “toxic” gain of function of the elevated and expanded FMR1 mRNA resulting in sequestration of proteins needed for normal neuronal functioning (6); the cellular aggregation of “toxic” peptides as a result of repeat-associated non-ATG (RAN) translation of the CGG repeat; and cellular dysregulation involved in the DNA damage response to hairpin R-loops formed by FMR1 mRNA expanded CGG repeats (7).
Further neuroradiological and neuropathological studies in FXTAS patients have provided important data concerning the neuropathological underpinnings of the spectrum of premutation involvement, with evidence suggesting preferential white matter lesions within cerebral and cerebellar hemispheres on magnetic resonance imaging (MRI), associated with general cerebral and cerebellar atrophy and enlargement of the lateral ventricles (8, 9). Neuropathological studies have also revealed cerebral and cerebellar white matter degeneration, with widespread loss of myelin and axons alongside preserved frontal cortical thickness and neuronal density (10). More recent studies have shown a significant prevalence of wmhs in the splenium of the corpus callosum (2, 11), which is now one of the major radiological diagnostic features, or in the basis points (12) in FXTAS patients, in addition to the most characteristic MCP sign. Although gray matter volume loss has also been demonstrated in patients with FXTAS in both cortical and subcortical regions (13, 14), the observation of white matter abnormalities in PM carriers with and without FXTAS (8, 15) suggests that white matter pathology is the primum movens. Moreover, the cognitive profile of executive dysfunction, slowed processing speed, deficits in working memory and declarative memory alongside preserved language, supports the notion that FXTAS is primarily a white matter disease (16). Diffusion tensor imaging (DTI) studies have shown loss of white matter integrity extending beyond the cerebellar peduncles and the corpus callosum (14, 17, 18). These white matter abnormalities affect the structural connectivity of numerous fiber tracts relevant to motor control, as well as specific cognitive abilities. Notably, changes in diffuse white matter within cerebral hemispheric and cerebellar regions, as well as volume changes in the cerebellum, brain stem, and whole brain, have been reported in PM carriers with and without FXTAS (15, 17). This suggests that white matter alterations may serve as sensitive markers of incipient decline to FXTAS, or to asyndromic neurological manifestations not meeting the diagnostic criteria for FXTAS. This aspect was discussed in our earlier publication (19), where we reported an example of the presence of the MCP sign in some non-FXTAS PM carriers, consistent with similar observations in five such individuals most recently reported by Famula et al. (20).
The existing evidence from neuroradiological and clinical observations, combined with the recent report of the presence of intranuclear inclusions in both FXTAS and non-FXTAS (including asymptomatic) PM carriers (21), further suggests the continuity of underlying structural pathology within the CNS associated with the FMR1 premutation carriage. This white matter pathology may correspond to the severity and type of neurological manifestations, with the most severe form represented by clinical and structural brain changes as seen in FXTAS.
The current study aimed to examine the relationships between severity of white matter lesions and motor and cognitive impairments in older males carrying the PM allele with and without FXTAS. All three motor scores, assessing ataxia, tremor and parkinsonism (see methods for details), and a limited range of cognitive impairments, were shown to be associated with the extent of white matter lesions in the infratentorial region in the total sample of males with the PM. This suggests that the varying degree and extent of neuropathological processes, unites all carriers of the premutation allele, ranging from asymptomatic to non-syndromic features to the full manifestation of FXTAS.
Methods and Materials
Participants
The study was approved by the La Trobe University Human Ethics Committee (No. 01/85). All participants gave both written and informed consent for their involvement. The sample comprised 30 older men with small CGG repeat expansions: 29 with the FMR1 PM, and one borderline PM/gray-zone carrier with 54 repeats (referred to collectively as “PM carriers”). Seventeen of these 30 participants had a diagnosis of FXTAS, of whom one participant was classified as FXTAS solely on the basis of the MCP sign associated with dementia. Amongst 13 carriers without FXTAS, seven participants manifested other disorders or non-syndromic changes outside the FXTAS spectrum, including: fibromyalgia (1); isolated dementia (2); isolated intention tremors that were associated with minor wmh changes but no MCP sign (3); and orthostatic tremor (4). Six carriers were not affected. Altogether, we included 17 FXTAS and 13 non-FXTAS participants totalling 30 PM carriers.
The sample was drawn from two studies occurring between the years 2004–2008 (5 carriers with FXTAS), and in the years 2012–2016 inclusive (25 carriers, 12 with FXTAS and 13 non-FXTAS). The ages ranged from 50 to 81 years (M = 63.0) for the FXTAS group, and from 55 to 69 years (M = 57.7 years), with one outstanding younger individual aged 39, for PM carriers without FXTAS. CGG repeat sizes in the PM carriers ranged from 56 to 160, and the participant with a PM/gray zone borderline value had 54 repeats. The FMR1 mRNA levels ranged from 2.32 to 5.38 units, compared with the baseline (control) value of 1.0 (6). All participants were recruited from families that were identified through clinical diagnoses of children with Fragile X syndrome (FXS: > 200 CGG repeats) as determined by the Victorian Clinical Genetic Service at the Royal Children's Hospital in Melbourne.
Neuroimaging
Brain MRI scans were acquired using a 1.5 Tesla Siemens scanner located at St. Vincent's Hospital Melbourne. Scans were captured using turbo spin-echo 2 dimensional (i) proton-density with T2 weighting (TR 3500, TE 13/103) and/or (ii) fluid-attenuated inversion recovery (FLAIR) (TR 9000, TE 90, TI 2500) axial images with a 5 mm slice thickness or 3D FLAIR (TR 5800, TE 315, TI 2200) with 1 mm voxel size.
Visual White Matter Hyperintensity Rating
The extent and severity of wmhs in the infratentorial region (infra-DWMH), and the deep and the periventricular supratentorial regions (Supra-DWMH and PV-WMH, respectively) were evaluated from the proton-density/T2 and/or the FLAIR images by one experienced neuroradiologist (NT) using a visual semi-quantitative method. The evaluation was performed blinded to clinical data and repeat size, and was repeated in a subsample of 10 participants after 6 months by the same neuroradiologist, with a consistency approaching 100%. The DWMH rating was based on the method described by Wahlund et al. (22). Since DWMH and PV-WMH are likely to result from different pathological processes and vary in extent and severity between different clinical scenarios (23) and a review of previous studies (24, 25) demonstrated increased PV-WMH in FXTAS subjects compared with normal controls, they were separately rated as described by other authors (23, 26).
DWMHs were defined as areas of increased T2 signal > 3 mm in diameter, and were rated, in four supratentorial regions, for each side of the brain: frontal; parieto-occipital; deep temporal; and subcortical white matter. In infratentorial regions, DWMHs included the MCP T2 hyperintensities that constitute one of the major criteria for the diagnosis of FXTAS, as well as T2 hyperintensities in adjacent deep white matter of the cerebellar hemispheres. The basal ganglia (BG) regions, which encompassed the deep nuclei and internal and external capsules, were not included in the ratings.
PV-WMHs were defined as confluent hyperintensities adjacent to the frontal or occipital horns (caps) or the bodies (bands) of the lateral ventricles. When PV-WMH extended >10 mm from the ventricular surface they were given a score of two, and any excess was included in the DWMH score. Respective scores for individual regions listed above were totalled to give: total supratentorial DWMH (“Total Supra-DWMH”), total infratentorial DWMH (“Total Infra-DWMH”), and total periventricular WMH (“Total PV-WMH”) scores, and the sum of these three regional summary scores was labeled as “Total-WMH”. Chronic lacunes, which were identified as well-defined areas >3 mm with signal characteristics similar to cerebrospinal fluid, were rare and were not included in either the wmhs rating or in other analyses. A detailed description of the measures used, together with illustration of the spectrum of changes, has been provided in a previous study (27). Figure 1 shows the wmhs in the supratentorial (left) and infratentorial (right) regions as well as changes in the corpus callosum in two representative patients with FXTAS.
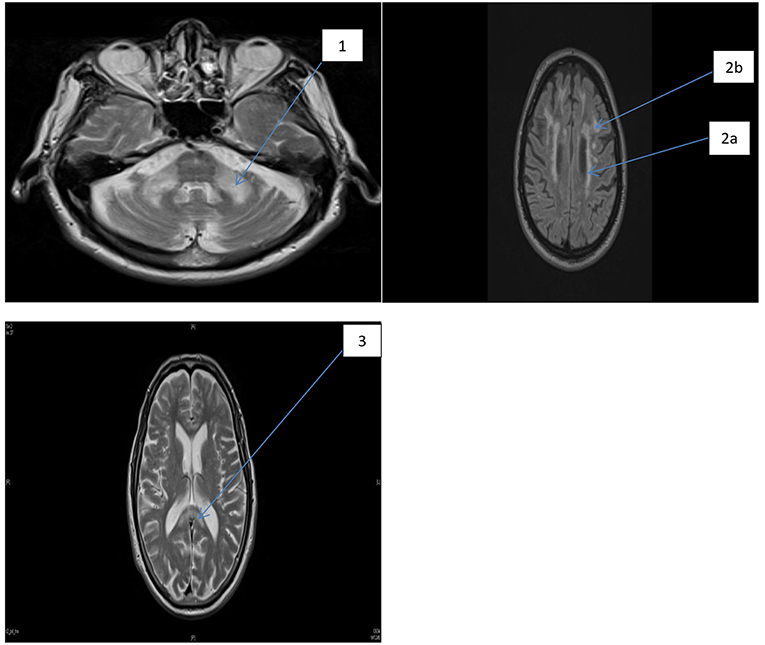
Figure 1. FLAIR MRI axial section of two representative patients with FXTAS. MCP sign and foci of wmhs (1), the periventricular wmhs (2a), deep wmhs (2b), and wmhs in the splenium of the corpus callosum (3).
Neuropsychological and Motor Rating Assessments
A battery of tests were selected to evaluate cognitive status with special emphasis on working memory and processing speed, which are domains predominantly affected in FXTAS (16, 28). General cognitive ability was assessed by the Vocabulary and Matrix Reasoning subtests of the Wechsler Adult Intelligence Scale (Third Edition; WAIS-III), which were used to calculate a prorated Full Scale IQ score (29). Verbal and non-verbal reasoning were evaluated using the Similarities and Matrix Reasoning subtests of the WAIS-III. The Digit Span forward and backward scores from the WAIS-III Digit Span subtest were selected as measures of short-term verbal memory and working memory, respectively. Information processing speed was assessed with the Symbol Digit Modalities Test (SDMT) (30). Severity of motor dysfunction was evaluated using the International Cooperative Ataxia Rating Scale (ICARS) (31), the Unified Parkinson's Disease Rating Scale (UPDRS) Part III-Motor (32), and the Clinical Rating Scale for Tremor (33). The assessments and scoring were conducted by two experienced neurologists (ES & DZL).
Molecular Analyses
CGG repeat expansion size was derived from previous diagnostic testing of fragile X families at the Victorian Clinical Genetic Services, Melbourne. PCRs and Southern Blot analyses were used and all assays were fully validated by internal and external quality assessment to provide a precision of ± one repeat (34, 35). Total RNA was isolated from 3 mL of blood collected in Tempus tubes (Applied Biosystems, Foster City, California, USA) or from 1 × 106 cells using Trizol (Life Technologies, Carlsbad, California, USA). The measurement of FMR1 mRNA expression levels was carried out by quantitative Real Time qRT-PCR on total RNA using custom-designed Taqman gene expression assays (Applied Biosystems) as previously described (36).
Statistical Analysis
Descriptive statistics were presented as means and standard deviations for the total sample of premutation carriers, and separately for FXTAS and Non-FXTAS groups. The relationship between each neuropsychological or motor score, and each wmh score, was assessed using the least square regression method if outliers were not present, otherwise robust regression was used to downweight the influential effect of outliers. Age was included as a covariate in the final regression for all the measures. The results of regression analysis are presented for the FXTAS and Non-FXTAS samples combined if there was no significant group interaction, with 2-sided p-value of <0.1 considered significant. The interaction term was incorporated in the multiple linear regression model, where each motor score or cognitive score were outcome variables, and predictors were individual wmhs scores, FXTAS group (binary), age (if significant) and interaction between FXTAS group and wmhs scores. The significance of the interaction term implies that estimated regression coefficients are different between the two groups, and the results presented for FXTAS group alone have been based on analysis using parameters extracted from the interaction model. Significance of regression was assessed by using the one-tailed test in cases where the direction of the relationship was predictable, with the p-value <0.0125 considered significant after adjustment for multiple testing (of the four wmh scores) conducted using the Bonferroni correction method. All analyses were carried out using STATA statistical software (version 13, STATA Corporation, 2013).
Results
Table 1 shows the descriptive statistics on age, CGG repeat number and the levels of FMR1 mRNA for FXTAS and Non-FXTAS groups separately and combined.
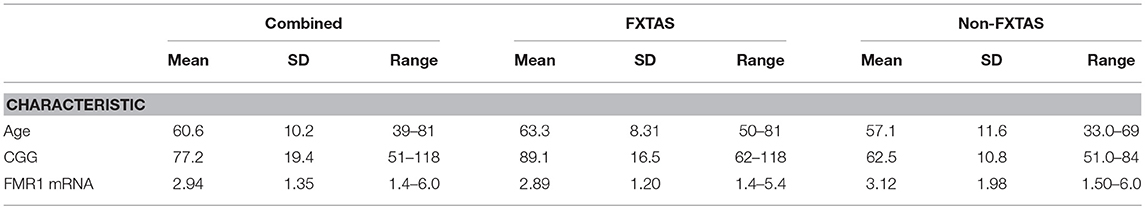
Table 1. Descriptive statistics for sample characteristics for the total sample of PM carriers and separately for FXTAS and non-FXTAS groups.
Tables 2A,B presents the results of regression analyses showing relationships between wmhs scores and motor or neuropsychological scores, with motor or cognitive score as outcome measures, and wmhs scores as predictors. The results for motor scores (in Table 2A) are shown for the FXTAS group alone, and for the total sample of premutation carriers only in cases where the interaction term from regression analysis was not significant.
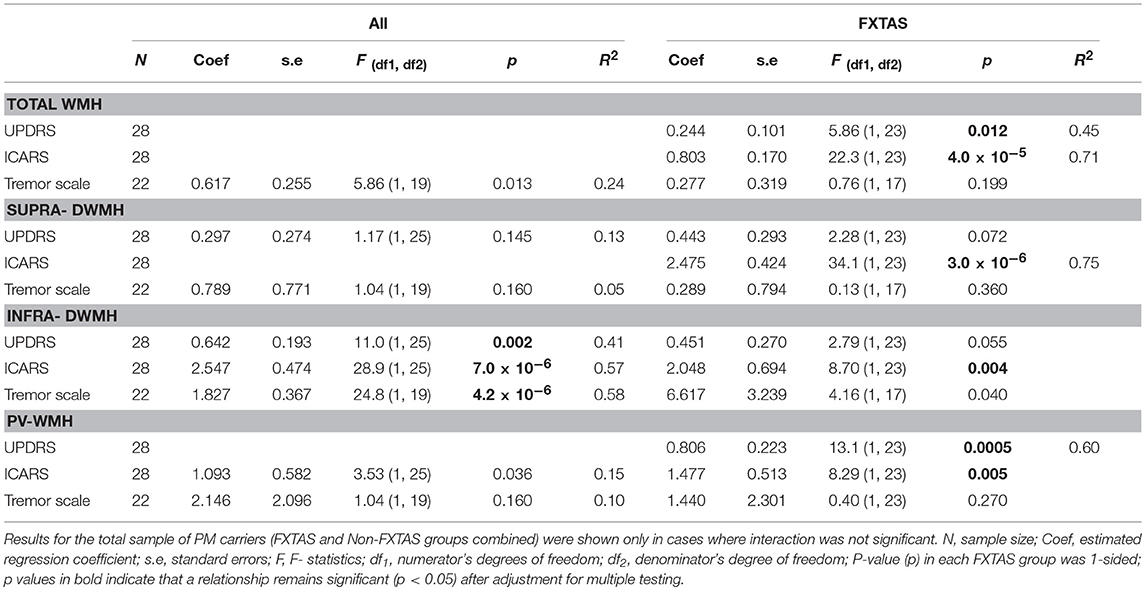
Table 2A. Relationships between each motor score (outcome) and each wmhs score (predictor), adjusted for age, and the FXTAS group.
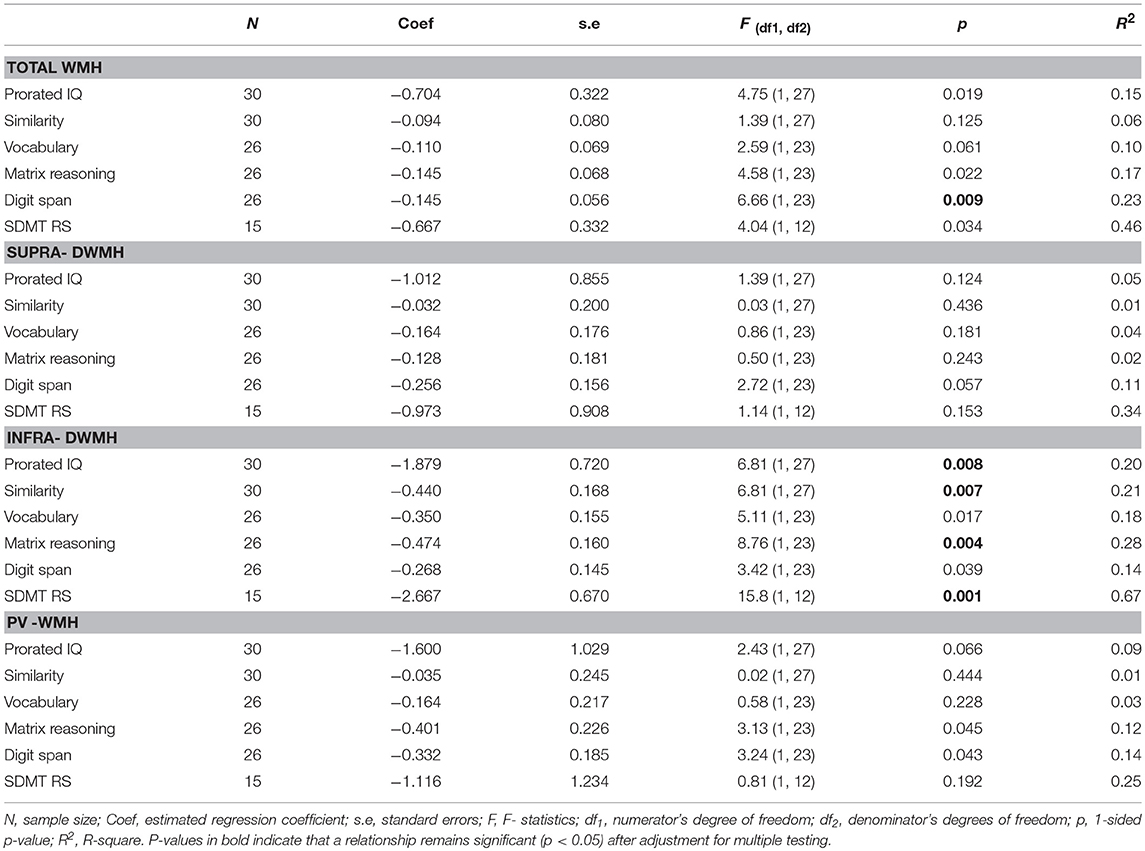
Table 2B. Relationship between each cognitive score (outcome) and each wmhs score (predictor), adjusted for age, in the total sample of PM carriers.
In the FXTAS group, the ICARS (ataxia score)—the measure most closely reflecting the neurological spectrum of FXTAS- was significantly associated with wmhs scores from all supratentorial, as well as infratentorial regions. The UPDRS showed a significant association with the Total WMH score, but the association was exceptionally high with the periventricular wmhs (PV-WMH), with the p value reaching 0.0005, compared with p = 0.012 for the total WMH, which includes all the regions.
The results from the total sample show highly significant associations between all three motor scale scores (including Tremor score) and Infra-DWMH representing the infratentorial locations of wmhs (see Table 2A). The relationships between the two supratentorial wmhs (Supra-DWMH and PV-WMH) with ICARS and Tremor scale, and between Supra-DWMH and UPDRS were not significant in the total sample.
The results of regression for the cognitive test scores are presented for the FXTAS and Non-FXTAS groups combined (Table 2B) considering that the interaction term did not reach significance for all the relationships involving these scores (p values ranging from 0.119 to 0.943).
As shown in Table 2B, there were strong relationships of Prorated IQ (p = 0.008), SDMT (p = 0.001), Matrix Reasoning (p = 0.004), and Similarity (p = 0.007) with Infra-DWMH, but all remaining correlations did not survive adjustment for multiple testing. There was also a highly significant (negative) correlation between Digit Span and Total WMH (p = 0.009) (Figure 2). The scatterplot of this correlation illustrates a continuity of the relationships across the total sample of PM carriers, ranging from apparently asymptomatic throughout asyndromic to affected FXTAS carriers.
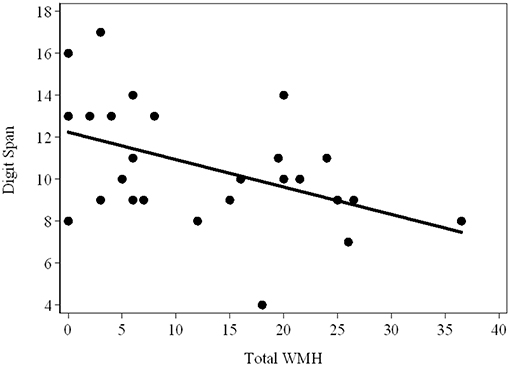
Figure 2. Scatterplot showing the (raw) Total Digit Span scores against the WMH scores in the total sample (FXTAS and Non FXTAS combined) of PM carriers.
Discussion
This is the first study to explore relationships of white matter lesions (whms) in several defined areas of the brain with the wide range of clinical measures including motor features of tremor, ataxia and parkinsonism, and neuropsychological measures of working memory and processing speed in older male carriers of the FMR1 premutation. We have shown that whms within the infratentorial region is associated with impairments across the categories of clinical status for all the three motor rating scores, and a range of neuropsychological cognitive measures. This is supportive of the notion that the scope of the spectrum of premutation-related clinical and brain changes, especially those in infratentorial regions specifically affected in FXTAS, appear to extend beyond the classical syndromic forms of FXTAS, and across the categories of non-affected and non-syndromic carriers, comprising asymptomatic, asyndromic and FXTAS groups (19). In addition, in the FXTAS group we have demonstrated a highly significant relationship between supratentorial (periventricular) lesions and parkinsonism, and between both periventricular and supratentorial deep white matter lesions and the ICARS ataxia score. Furthermore, our data demonstrate that white matter lesions in infratentorial regions—the regional wmhs most characteristic of FXTAS—are significantly (negatively) correlated with a range of cognitive scores, namely processing speed, matrix reasoning and similarities, as well as Prorated IQ, in the total sample of carriers. These findings further support the relevance of white matter changes in different brain regions to the motor and cognitive deficits across the spectrum of premutation involvement.
Notably, when taken together with our previous study (19), the current findings imply a continuity in the clinical and white matter changes extending from non-affected older male carriers, to non-syndromic forms separate from FXTAS, and eventually to the full manifestation of FXTAS. Moreover, the finding of significant correlations between the wmhs in infratentorial regions and the motor and cognitive scores in the total sample of PM carriers suggests that the underlying primary pathology might be initially confined to this region. These findings corroborate previous studies using more sophisticated techniques to examine white matter microstructure in vivo by the sensitive MRI technique of DTI (15, 17). These authors speculated that the presymptomatic alterations in structural connectivity seen in several brain regions in non-affected premutation carriers may represent a subgroup at risk of developing clinical manifestations of FXTAS with increasing age. Moreover, the significant associations between reduced tract volume in the superior cerebellar peduncle and CGG repeat size in both unaffected premutation carriers and those with FXTAS (37) provides support for a continuum of white matter changes irrespective of the clinical category. It is therefore possible that this underlying pathology, if aggravated by aging and other possible genetic or non-genetic risk factors including stress-related cellular damage, could progress and expand beyond the “primary” infratentorial area to involve the supratentorial regions leading to the more severe clinical changes seen in FXTAS.
Although our data on the relationships between white matter lesions and cognitive scores were less clear, the significant correlations which survived correction for multiple comparisons in the combined PM sample: between infratentorial wmhs score and each of the non-verbal problem solving and processing speed scores, constitute major aspects of cognitive functioning known to be impaired in FXTAS (16). Our findings are generally concordant with the results of the first study of the relationship between white matter disease and cognitive functioning in a small sample of 13 older male PM carriers (8). In contrast, this study applied more advanced and sensitive MRI techniques than the conventional MRI imaging employed in the current study—DTI and magnetic resonance spectroscopy (MRS)—and showed that microstructural white matter abnormalities in the MCPs and the genu and splenium of the corpus callosus, were correlated with executive dysfunction and slowed processing speed (8). The most interesting result from that study revealed decreased levels of N-acetyl aspartate (NAA) in the MCP, which suggested the presence of axonal damage (8). Although direct comparison between findings from that study and our own results is not possible, given the differences in MRI techniques employed (wmh rating scale vs. DTI and MRS), and the utilization of different cognitive measures (Matrix Reasoning vs. Behavioral Dyscontrol Scale and COWAT), both studies' results suggest that the dominant lesion in the white matter of the cerebellar peduncles in PM carriers might disrupt fronto-cerebellar networks, which in turn could affect input from the contralateral cerebral cortex to the inferolateral cerebellum, thus leading to the non-motor impairments seen in affected PM carriers (38). Notably, both these studies have been consistent in showing the continuity of neural pathology from non-FXTAS to the full manifestation of FXTAS.
There are several limitations of our study that merit consideration. The small sample size combined with the inclusion of multiple measures in regression analysis may have accounted for a limited number of significant correlations between cognitive functioning and the hemispheric white matter lesions, and also for a number of significant interactions for FXTAS group that might be due to small sample size and considerable variability. Another limitation is the lack of a control group with normal FMR1 alleles with comparable age range to evaluate severity of pathological processes specifically related to the continuity of the changes in the FMR1 premutation. Future studies using longitudinal designs, with larger cohorts of PM carriers including those affected with FXTAS, asymptomatic carriers, those affected with non-syndromic neurological features and controls are necessary to confirm the postulated early clinical and neurodegenerative changes, which may lead to divergent clinical outcomes. Using conventional MRI with semiquantitative lesion scores may also be considered a limitation, since it does not permit more sensitive and tract-based analysis of white matter microstructure. However, this approach clearly served the purpose of our study, which aimed to establish whether white matter lesions in supratentorial, as well as infratentorial regions, contribute to motor and cognitive changes characteristic of the spectrum of premutation involvement. The validity of the wmh scores applied in this study was further attested by demonstration of the highly significant associations of all the wmhs scores used here with the levels of “toxic” FMR1 mRNA in a previous study (19). Finally, our study design did not include examination of the gray matter changes that have been reported by others in FXTAS (13, 14, 39, 40).
In summary, our results provide preliminary evidence for the relevance of the regional and total macrostructural white matter lesions to the neurological changes occurring in FMR1 premutation carriers, by finding linear relationships between infratentorial wmhs and measures of motor and specific cognitive impairments across all categories of clinical status. Further studies based on larger cohorts of premutation carriers and longitudinal modeling are needed to confirm our hypothesis that the neural and clinical changes may occur across the entire spectrum of PM carriers with continuity of the underlying neurodegenerative process. Notably, our results have suggested an involvement of the locations other than infratentorial region in this process; however, the specific factors that lead to progressive changes that involve the deep hemispheric and periventricular regions in the severity of motor impairments in the full manifestation of FXTAS remains to be determined. By using sensitive MRI techniques such as DTI and MRS, these studies will enable assessment of microstructural white matter changes and gray matter loss, and their association with motor and cognitive impairments reminiscent of FXTAS. This may reveal the earliest changes that precede the severe neurodegeneration associated with obvious clinical manifestations seen in FXTAS, and thereby lead to identification of targets for therapeutic intervention.
Data Availability
The datasets generated from the statistical analysis for this study are available on request to the corresponding author.
Ethics Statement
This study was approved by the La Trobe University Human Ethics Committee (No. 01/85). All participants gave informed consent for their involvement.
Author Contributions
DH wrote the first draft of the manuscript and provided intellectual input into the interpretation of the data. DL conceptualized and designed the study, provided intellectual input into the interpretation of the data, and co-wrote the first draft of the manuscript. NT contributed to MRI protocol and ratings and input on the interpretation of the findings. MB provided statistical analysis and input into interpretation of the data. EH conducted neuropsychological testing. DF and FT conducted genetic molecular FMR1 testings. EH, DF, and FT provided intellectual input into interpretation of data. ES assessed the subjects neurologically and contributed to drafting the final version of the manuscript.
Funding
This study was supported by the National Institute of Child Health and Human Development Grant, US, No. HD 36071, to DL and FT.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank all participants involved in this study and their families.
References
1. Leehey MA, Hall DA, Liu Y, Hagerman RJ. Clinical neurological phenotype of FXTAS. In: Tassone F, Hall DA, editors. FXTAS, FXPOI, and Other Premutation Disorders. Cham: Springer International Publishing (2016). p. 1–24.
2. Apartis E, Blancher A, Meissner WG, Guyant-Marechal L, Maltete D, De Broucker T, et al. FXTAS: new insights and the need for revised diagnostic criteria. Neurology. (2012) 79:1898–907. doi: 10.1212/WNL.0b013e318271f7ff
3. Chonchaiya W, Utari A, Pereira G, Tassone F, Hessl D, Hagerman RJ. Broad clinical involvement in a family affected by the fragile X premutation. J Dev Behav Pediatr. (2009) 30:544–51. doi: 10.1097/DBP.0b013e3181c35f25
4. Hall DA, Tassone F, Klepitskaya O, Leehey M. Fragile X–associated tremor ataxia syndrome in FMR1 gray zone allele carriers. Mov Disord. (2012) 27:296–300. doi: 10.1002/mds.24021
5. Liu Y, Winarni TI, Zhang L, Tassone F, Hagerman RJ. Fragile X-associated tremor/ataxia syndrome (FXTAS) in grey zone carriers. Clin Genet. (2013) 84:74–7. doi: 10.1111/cge.12026
6. Tassone F, Hagerman RJ, Taylor AK, Gane LW, Godfrey TE, Hagerman PJ. Elevated levels of FMR1 mRNA in carrier males: a new mechanism of involvement in the fragile-X syndrome. Am J Hum Genet. (2000) 66:6–15. doi: 10.1086/302720
7. Hagerman R, Hagerman P. Advances in clinical and molecular understanding of the FMR1 premutation and fragile X-associated tremor/ataxia syndrome. Lancet Neurol. (2013) 12:786–98. doi: 10.1016/S1474-4422(13)70125-X
8. Filley CM, Brown MS, Onderko K, Ray M, Bennett RE, Berry-Kravis E, et al. White matter disease and cognitive impairment in FMR1 premutation carriers. Neurology. (2015) 84:2146–52. doi: 10.1212/WNL.0000000000001612
9. Filley CM. Fragile X tremor ataxia syndrome and white matter dementia. Clin Neuropsychol. (2016) 30:901–12. doi: 10.1080/13854046.2016.1165805
10. Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS). Brain. (2006) 129 (Pt 1):243–55. doi: 10.1093/brain/awh683
11. Kalus S, King J, Lui E, Gaillard F. Fragile X-associated tremor/ataxia syndrome: an under-recognised cause of tremor and ataxia. J Clin Neurosci. (2016) 23:162–4. doi: 10.1016/j.jocn.2015.08.010
12. Loesch DZ, Kotschet K, Trost N, Greco CM, Kinsella G, Slater HR, et al. White matter changes in basis pontis in small expansion FMR1 allele carriers with parkinsonism. Am J Med Genet B Neuropsychiatr Genet. (2011) 156b:502–6. doi: 10.1002/ajmg.b.31189
13. Hashimoto R, Javan AK, Tassone F, Hagerman RJ, Rivera SM. A voxel-based morphometry study of grey matter loss in fragile X-associated tremor/ataxia syndrome. Brain. (2011) 134 (Pt 3):863–78. doi: 10.1093/brain/awq368
14. Wang JY, Hagerman RJ, Rivera SM. A multimodal imaging analysis of subcortical gray matter in fragile X premutation carriers. Mov Disord. (2013) 28:1278–84. doi: 10.1002/mds.25473
15. Battistella G, Niederhauser J, Fornari E, Hippolyte L, Gronchi Perrin A, Lesca G, et al. Brain structure in asymptomatic FMR1 premutation carriers at risk for fragile X-associated tremor/ataxia syndrome. Neurobiol Aging. (2013) 34:1700–7. doi: 10.1016/j.neurobiolaging.2012.12.001
16. Grigsby J, Cornish K, Hocking D, Kraan C, Olichney JM, Rivera SM, et al. The cognitive neuropsychological phenotype of carriers of the FMR1 premutation. J Neurodev Disord. (2014) 6:28. doi: 10.1186/1866-1955-6-28
17. Hashimoto R, Srivastava S, Tassone F, Hagerman RJ, Rivera SM. Diffusion tensor imaging in male premutation carriers of the fragile X mental retardation gene. Mov Disord. (2011) 26:1329–36. doi: 10.1002/mds.23646
18. Wang JY, Hessl DH, Hagerman RJ, Tassone F, Rivera SM. Age-dependent structural connectivity effects in fragile X premutation. Arch Neurol. (2012) 69:482–9. doi: 10.1001/archneurol.2011.2023
19. Loesch DZ, Trost N, Bui MQ, Hammersley E, Lay ST, Annesley SJ, et al. The spectrum of neurological and white matter changes and premutation status categories of older male carriers of the FMR1 alleles are linked to genetic (CGG and FMR1 mRNA) and cellular stress (AMPK) markers. Front Genet. (2018) 9:531. doi: 10.3389/fgene.2018.00531
20. Famula JL, McKenzie F, McLennan YA, Grigsby J, Tassone F, Hessl D, et al. Presence of middle cerebellar peduncle sign in FMR1 premutation carriers without tremor and ataxia. Front Neurol. (2018) 9:695. doi: 10.3389/fneur.2018.00695
21. Tassone F, Greco CM, Hunsaker MR, Seritan AL, Berman RF, Gane LW, et al. Neuropathological, clinical and molecular pathology in female fragile X premutation carriers with and without FXTAS. Genes Brain Behav. (2012) 11:577–85. doi: 10.1111/j.1601-183X.2012.00779.x
22. Wahlund LO, Barkhof F, Fazekas F, Bronge L, Augustin M, Sjogren M, et al. A new rating scale for age-related white matter changes applicable to MRI and CT. Stroke. (2001) 32:1318–22. doi: 10.1161/01.STR.32.6.1318
23. van Straaten EC, Harvey D, Scheltens P, Barkhof F, Petersen RC, Thal LJ, et al. Periventricular white matter hyperintensities increase the likelihood of progression from amnestic mild cognitive impairment to dementia. J Neurol. (2008) 255:1302–8. doi: 10.1007/s00415-008-0874-y
24. Brunberg JA, Jacquemont S, Hagerman RJ, Berry-Kravis EM, Grigsby J, Leehey MA, et al. Fragile X premutation carriers: characteristic MR imaging findings of adult male patients with progressive cerebellar and cognitive dysfunction. AJNR Am J Neuroradiol. (2002) 23:1757–66.
25. Rivera SM, Stebbins GT, Grigsby J. Radiological findings in FXTAS. In: Tassone F, Berry-Kravis EM, editors. The Fragile X-Associated Tremor Ataxia Syndrome (FXTAS). New York, NY: Springer (2010). p. 55–66.
26. Scheltens P, Barkhof F, Leys D, Pruvo JP, Nauta JJ, Vermersch P, et al. A semiquantative rating scale for the assessment of signal hyperintensities on magnetic resonance imaging. J Neurol Sci. (1993) 114:7–12. doi: 10.1016/0022-510X(93)90041-V
27. Trost N, Cook M, Hammersley E, Bui MQ, Brotchie P, Burgess T, et al. White matter changes in patients with Parkinson's disease carrying small CGG expansion FMR1 alleles: a pilot study. Neurodegener Dis. (2014) 14:67–76. doi: 10.1159/000356190
28. Grigsby J, Brega AG, Engle K, Leehey MA, Hagerman RJ, Tassone F, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. (2008) 22:48–60. doi: 10.1037/0894-4105.22.1.48
29. Wechsler D. WAIS-III: Administration and Scoring Manual. Orlando, FL: The Psychological Corporation (1997).
31. Trouillas P, Takayanagi T, Hallett M, Currier RD, Subramony SH, Wessel K, et al. International cooperative ataxia rating scale for pharmacological assessment of the cerebellar syndrome. The Ataxia Neuropharmacology Committee of the World Federation of Neurology. J Neurol Sci. (1997) 145:205–11. doi: 10.1016/S0022-510X(96)00231-6
32. Fahn S, Elton RL, Members of the UPDRS Development Committee. The unified Parkinson's disease rating scale. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent Developments in Parkinson's Disease. Florham Park, NJ: Macmillan Healthcare Information (1987). p. 153–64.
33. Fahn S, Tolosa E, Marin C. Clinical rating scale for tremor. In: Jankovic J, Tolosa E, editors. Parkinson's Disease and Movement Disorders. Baltimore, MD: Munich Urban and Schwarzenberg (1988). p. 225–34.
34. Khaniani MS, Kalitsis P, Burgess T, Slater HR. An improved diagnostic PCR assay for identification of cryptic heterozygosity for CGG triplet repeat alleles in the fragile X gene (FMR1). Mol Cytogenet. (2008) 1:5. doi: 10.1186/1755-8166-1-5
35. Loesch DZ, Godler DE, Khaniani M, Gould E, Gehling F, Dissanayake C, et al. Linking the FMR1 alleles with small CGG expansions with neurodevelopmental disorders: preliminary data suggest an involvement of epigenetic mechanisms. Am J Med Genet A. (2009) 149a:2306–10. doi: 10.1002/ajmg.a.32990
36. Tassone F, Hagerman RJ, Garcia-Arocena D, Khandjian EW, Greco CM, Hagerman PJ. Intranuclear inclusions in neural cells with premutation alleles in fragile X associated tremor/ataxia syndrome. J Med Genet. (2004) 41:e43. doi: 10.1136/jmg.2003.012518
37. Wang JY, Hessl D, Schneider A, Tassone F, Hagerman RJ, Rivera SM. Fragile X-associated tremor/ataxia syndrome: influence of the FMR1 gene on motor fiber tracts in males with normal and premutation alleles. JAMA Neurol. (2013) 70:1022–9. doi: 10.1001/jamaneurol.2013.2934
38. Stoodley CJ, Schmahmann JD. Evidence for topographic organization in the cerebellum of motor control versus cognitive and affective processing. Cortex. (2010) 46:831–44. doi: 10.1016/j.cortex.2009.11.008
39. Cohen S, Masyn K, Adams J, Hessl D, Rivera S, Tassone F, et al. Molecular and imaging correlates of the fragile X-associated tremor/ataxia syndrome. Neurology. (2006) 67:1426–31. doi: 10.1212/01.wnl.0000239837.57475.3a
Keywords: FMR1 premutation carriers, FXTAS, MRI, white matter hyperintensities, motor scores, cognitive impairment
Citation: Hocking DR, Loesch DZ, Trost N, Bui MQ, Hammersley E, Francis D, Tassone F and Storey E (2019) Total and Regional White Matter Lesions Are Correlated With Motor and Cognitive Impairments in Carriers of the FMR1 Premutation. Front. Neurol. 10:832. doi: 10.3389/fneur.2019.00832
Received: 21 March 2019; Accepted: 18 July 2019;
Published: 13 August 2019.
Edited by:
Gianfranco Spalletta, Fondazione Santa Lucia (IRCCS), ItalyReviewed by:
Ryuichiro Hashimoto, Showa University, JapanMichael Ryan Hunsaker, The University of Utah, United States
Copyright © 2019 Hocking, Loesch, Trost, Bui, Hammersley, Francis, Tassone and Storey. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Danuta Z. Loesch, d.loesch@latrobe.edu.au
†These authors share first authorship
 Darren R. Hocking
Darren R. Hocking Danuta Z. Loesch
Danuta Z. Loesch Nicholas Trost3
Nicholas Trost3 Flora Tassone
Flora Tassone