- 1Neurology & Stroke, Australian Institute for Musculoskeletal Science, Melbourne Medical School, Sunshine Hospital, The University of Melbourne, Parkville, VIC, Australia
- 2School of Psychology and Public Health, College of Science, Health and Engineering, La Trobe University, Melbourne, Parkville, VIC, Australia
- 3Department of Medicine and Dean's Office, Rajarata University of Sri Lanka, Anuradhapura, Sri Lanka
- 4Department of Neurology, Australian Institute for Musculoskeletal Science, Level Three, Western Centre for Health Research and Education, Sunshine Hospital, Western Health & University Melbourne, St Albans, VIC, Australia
- 5Faculty of Social and Political Sciences, Tbilisi State University, Tbilisi, Georgia
Corona virus disease (COVID-19), caused by the severe acute respiratory syndrome coronavirus2 (SARS-CoV-2) is recognized as a global pandemic by WHO 2020 with 5,934 936 infections, 367,166 deaths and affecting over 200 countries as of 30th May 2020. Acute Ischemic Stroke (AIS) in brain is also emerging as an important neurovascular/neurological complication of COVID-19, associated with extreme immune responses leading to dysregulated coagulation system and generalized thrombo-embolic status and increased risk of AIS especially among usually less vulnerable younger adults in this cohort. Thus, in early June 2020, we aimed to review the clinical data on all published cases of COVID-19 and concomitant AIS, with a view to understanding the pertinent clinical, laboratory and imaging features. The neutrophil-lymphocyte ratio (NLR) at time of hospital admission for COVID infection correlates positively with the duration of time before onset of clinical features of AIS. Higher NLR, C-Reactive protein, serum ferritin, D-dimer and fibrinogen levels are associated with poor prognosis of AIS in COVID-19 with 75% of patients dying or being severely disabled at present. Currently it is too early to comment on the long-term outcomes for survivors.
Key Findings
• Acute ischemic stroke is an important, but an under recognized complication of SARS-CoV2 infection, that leaves most recovered patients with significant disabilities as of present stage July 2020 of the pandemic.
• Hypercoagulation markers such as D-dimer are substantially elevated among all patients early in the disease progression.
• Neutrophil to lymphocyte ratio, C-Reactive protein, and Serum Ferritin levels appear to be prognostic markers.
• Patients with higher admission neutrophil-lymphocyte ratios demonstrate a shorter interval between infective symptoms of COVID-19 and the clinical manifestation of Acute Ischemic Stroke.
• Large vessel occlusion is the main etiologic subtype, with only a minority of patients receiving standard of care treatment.
• Seventy five percent of the patients with COVID-19 and Acute Ischemic Stroke died or are still severely disabled.
• The COVID-19 pandemic has created a unique opportunity to advance the whole field of neurorehabilitation based on a better biological and scientific underpinning of precision neurorehabilitation protocols.
Introduction
In December 2019, a novel corona virus associated with a series of acute, atypical respiratory diseases was first detected in Wuhan China. Since then the virus, now known as SARS-CoV2 (Severe Acute Respiratory Syndrome coronavirus two), has spread to over 200 countries and is now recognized as a major world pandemic (1). As of May 30th 2020, the mortality rate of COVID-19 was reported with the number of confirmed deaths with recorded cases worldwide. Since the pathogenesis of SARS-CoV2 first began to emerge, numerous other clinical system manifestations have been identified.
Neurological manifestations of SARS-CoV 2 infection were first reported in a series of patients in Wuhan, China by Zhou et al. (2). Acute ischemic stroke (AIS) was diagnosed in 5% of the cases (2). However, a much lower rate of only 0.9% imaging confirmed AIS i.e., 32/3,556 total patients case number with COVID-19 was reported in New York USA (3). Subsequent retrospective reports from Europe have also confirmed AIS as a common neurovascular complications of SARS-CoV2 (4, 5). Interestingly Oxley et al. noted an increased occurrence of younger SARS CoV2 virus-infected patients with no significant traditional risk factors for AIS, presenting with large vessel occlusion (6). Putative mechanisms suggested as inducing AIS in association with SARS CoV2 have included systemic inflammation, inflammatory cytokine storm, hyper-coagulability, and imbalances in the classical and alternative Renin Angiotensin System (RAS) in relation to SARS-CoV-2 spike glycoprotein-ACE2 binding related molecular mechanisms (3, 7–19). The RAS system comprises both a plasma-based RAS regulating cardiovascular system and tissue-based RAS regulating long term changes via a complex hormonal system, endocrine, paracrine, and autocrine in action. Thus, the RAS controls renal, adrenal and cardiovascular systems with important implications on blood pressure control as well as fluid/electrolyte control which are critically important to maintain life being very susceptible to damage by SARS-CoV 2. The inflammatory pathway is core to the various clinical manifestations of SARS-CoV2 infection. Also referred to as the “cytokine storm,” it triggers an upsurge of various inflammatory cytokines such as IL-2, IL-7, IL-10 (20, 21), induces a state of lymphocytopenia (22–24) and also activates a spike of acute phase reactants such as CRP and ferritin (25, 26).
Various parameters have been proposed to predict prognosis and outcomes among patients with COVID, including the neutrophil to lymphocyte ratio (NLR) (27–30). A metanalysis of six studies involving 1,141 patients has demonstrated that an elevated NLR is associated with severe disease manifestation (28). The same meta-analysis has also revealed that along with ESR and IL-6, CRP was correlated with increased severity among patients with SARS-CoV2 infection (28). The role of ferritin as a predictor of mortality among confirmed SARS-CoV2, has also been confirmed in another metanalysis of 10 studies involving more than 1,400 subjects (31). Furthermore, elevated D-dimer and hyperfibrinogenemia, which are both biomarkers of inflammation and hypercoagulable state, have also been shown to predict the severity of the said infection (31, 32). Interestingly, similar biomarkers predict outcomes in stroke (33–39). In particular, it is known that patients who show elevated NLR, ferritin, CRP, D-dimer and fibrinogen have a higher risk for stroke and equate to potentially poorer clinical outcomes (33–39).
To date, despite the theoretical association of inflammatory and procoagulable states linking stroke and SARS-CoV2 infection, there is limited published literature on the actual co-occurrence of both. There is also limited information on the biological markers which may be associated with poor neurological outcomes. Thus, this study aims to describe the clinical characteristics of patients with acute ischemic stroke and concomitant SARS-CoV2 infection. By further analysis of available laboratory data, this will look at the trend of inflammatory biomarkers such as NLR, CRP, serum ferritin, fibrinogen and D-dimer and hospital discharge outcomes.
Currently, there is limited information about the clinical characteristics and specific neurorehabilitation issues of AIS patients with SARS-CoV 2 infection (40–43). However, it is expected that the surge in patient numbers, on-going issues with personal protective equipment (PPE) shortages, and associated health care workers anxiety and stress about the potential of getting infected with COVID-19 (and actual infection of health care workers and mandatory self isolation for 14 days even if these members are demonstrating minimum or no symptoms) will create a significant challenge to traditional neurorehabilitation practices and pathways, at least during the pandemic, possibly for a long time to come. Thus, these circumstances argue a strong case for converting the catastrophe [Complex rearrangement of hospital facilities as part of the preparation for the pandemic has also occasioned significant problems and added resource problems for health care systems across the world (44–50) into an opportunity for revamping of rehabilitation protocols]. Currently evidence is emerging for further expansion of telemedicine type paradigms, with incorporation of tablet based remote monitoring technology (Melbourne Rapid Field visual fields, wearable devices and artificial intelligence) suggesting as the way forward in neurorehabilitation of AIS in COVID19 pandemic era, at least for the foreseeable future (43, 51–53).
Thus, this systematic review aims to identify and collate the clinical and laboratory features, acute and long term treatment, and outcomes of all published reports on patients with concomitant diagnosis of confirmed SARS-CoV 2 infection and acute ischemic stroke and with a special emphasis on clinical and laboratory features.
Purpose
The present study was conducted to provide a systematic review of AIS and COVID-19 with respect to definition, prevalence, pathophysiology, clinical characteristics, acute, subacute features, prognostic markers outcomes.
Participants
Information regarding ischemic stroke patients with confirmed SARS-CoV2 infection and radiologically or clinically Confirmed AIS included in published studies from November 2019 to May 30th 2020 using the search strategy detailed below will be considered here.
Types of Studies
All types of studies including qualitative, systematic reviews, meta-analyses, case reports and case series, were included.
Search Methods
Published articles in English and on human subjects that were published from November 2019 until 30th May 2020 were the inclusion criteria for the search. The following search strategy was adopted:
1. In the first step MEDLINE, Cochrane and CINAHL databases were searched, followed by title and abstract search.
2. In the second step, the keywords were used when searching on Ovid MEDLINE, Cochrane, PubMed, CINAHL, and EMBASE databases.
3. In the third step, a manual search was carried out to ensure no study was inadvertently left out.
The keywords used to conduct the search were: Stroke, thrombosis, coronavirus, neurological complication, neurorehabilitation, COVID19, SARS-COV2.
Data Extraction
The Arksey and O'Malley methodological framework was employed in this review (54).
The bibliographies of individual studies were further hand-searched. Articles were screened by two independent investigators.
4. In the fourth step the secondary analysis was carried out as follows.
Clinical and laboratory data of every patient was extracted. Demographics and details of their respective laboratory details were also investigated. In particular, the following routine laboratory values were of interest to the researchers: NLR, CRP, ferritin, fibrinogen, and D-dimer. Individual patient outcomes were also accounted for and classified as good [with modified Rankin Scores (mRS) of 0, 1, 2, and 3 and poor mRS of 4, 5, 6]. Patients with no available laboratory data and outcomes were excluded in the quantitative analysis.
Search Results
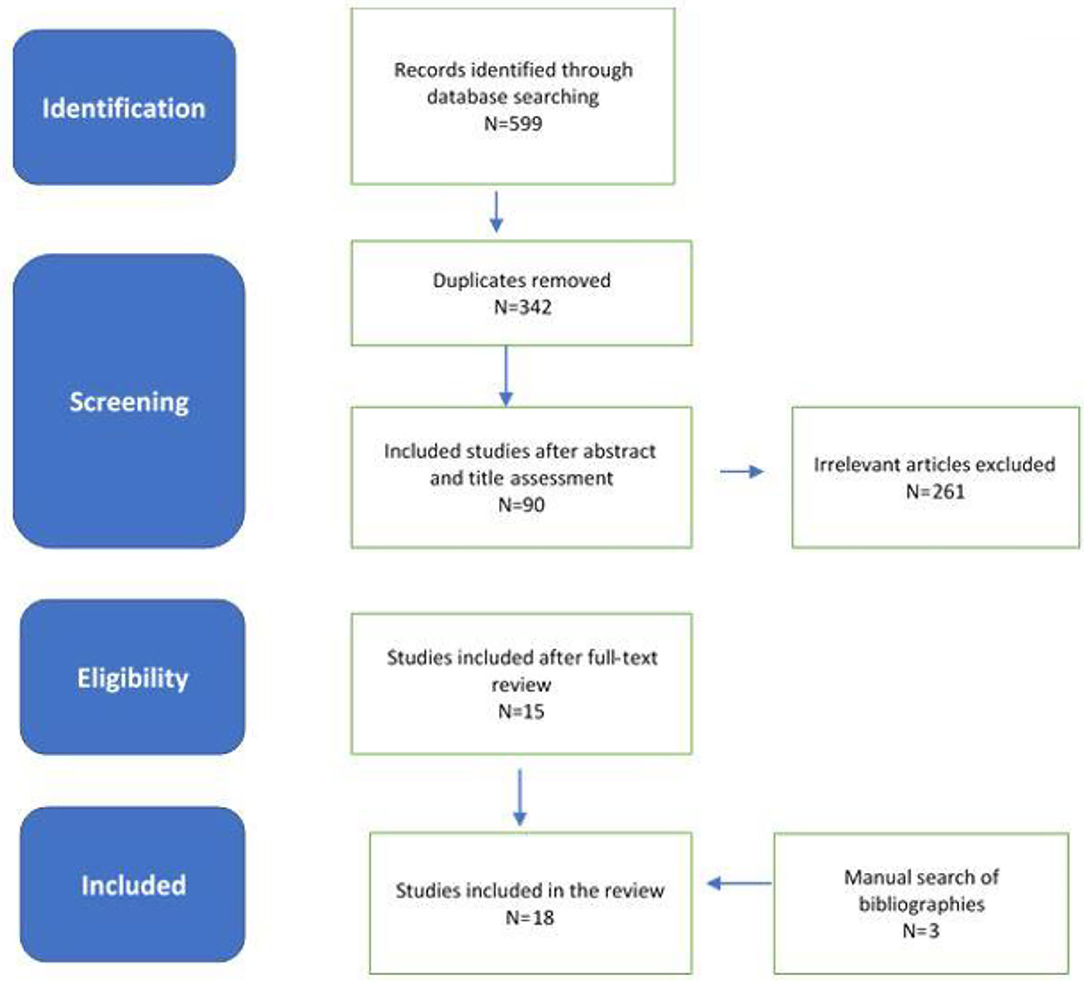
Extensive database search yielded 595 citations, and four studies were added by manual searching. A total of 257 duplicates were excluded resulting in 342 citations. These titles and abstracts were further screened yielding 90 final publications of relevance to consideration of stroke and SARS-COV2 infection, during the second screening process. One publication was non-existent despite being cited by multiple authors in their publications. Further evaluation of the full texts of the 89 studies by two independent neurologists (TW and CS) excluded 74 citations with 15 studies. Three further studies were added from hand-held search by TW and CS with 18 publications that were deemed to be included in this systematic review by all authors.
Year and Country of Study
The studies published from 2019 to 2020, Included literature were originated from North America, Europe, and Asia.
Study Population
This study included all patients with SARS-COV2 infection and a concomitant diagnosis of acute ischemic stroke and/or acute/subacute outcomes where available.
An electronic search performed on May 10 to 30th, 2020 using the identified keywords yielded 342 citations after removal of duplicates. This was further assessed at the title and abstract level which resulted in 90 articles. After full assessment of the full text of each, 18 were deemed relevant to the study, in addition to the three articles which were added from hand-held research. Figure 1 summarizes the search process.
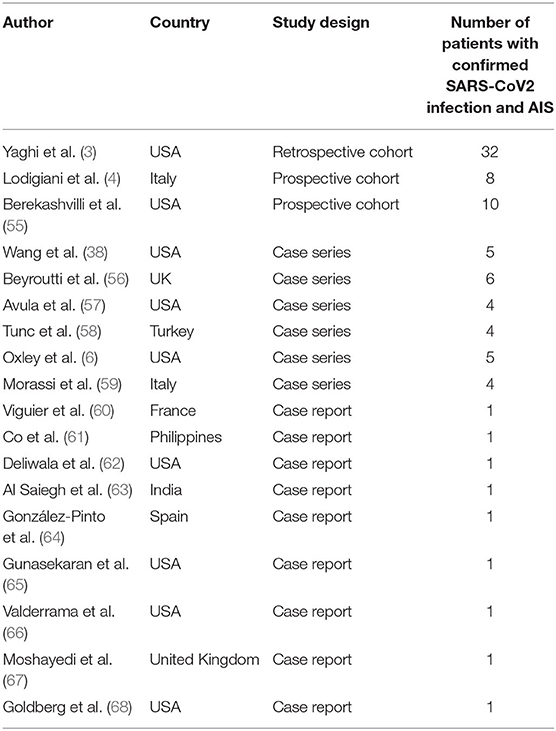
There were 18 articles included in the study consisting of 87 patients from USA, Italy, Turkey, France, Philippines, and United Kingdom. Most of the studies were case reports and case series while three of the included studies were retrospective and prospective cohorts. Table 1 outlines the characteristics of the individual studies.
Clinical characteristics of patients are described in Table 2.
The majority of the patients were within the 50–70 age group while almost one-third of the patients were <50 years old. The most common comorbidity was hypertension followed by diabetes, dyslipidemia and less frequently, atrial fibrillation. Mean hematologic parameters are also described. Neurovascular imaging either with magnetic resonance angiography (MRA) or computer tomographic angiography (CTA) was available for 35 patients, of whom the majority presented with anterior circulation, large vessel occlusion. Treatment regimens were also described for the majority of the patients and among whom a significant number received systemic anticoagulation, intravenous thrombolysis and mechanical thrombectomy. Of the 87 patients described, 72 outcomes are available, with almost 75% resulting in poor neurological outcomes of Modified Rankin score (mRS) 4 and above.
Inflammatory and coagulation markers of individual patients were also analyzed. Neurological outcomes were classified as either good (mRS 3 and below) or poor (mRS 4 and above). Respective inflammatory parameters such as neutrophil to lymphocyte ratio, C-reactive protein and serum ferritin were analyzed for each group. The same was performed for coagulation markers such as D-dimer and fibrinogen. Patients with good neurological outcomes had lower mean NLR, CRP and serum ferritin (4.39 ± 1.44, 53.09 ± 92.70 mg/L, 449 ± 482.3 ug/L, respectively), compared to patients with mRS 4 and above (7.51 ± 5.84, 88.69 ± 70.45 mg/L, 1,086 ± 1,220 ug/L, respectively). Similar trends were observed in terms of coagulation markers, with D-dimer and fibrinogen showing levels of 2,509 ± 4,093 ug/L and 4.70 ± 1.70 g/L, respectively, for patients with mRS 3 and below, while values for patients with poor neurological outcomes were 7,223 ± 6,781 ug/L for D-dimer and 6.086 ± 2.69 g/L for fibrinogen respectively. Summary of the said values are plotted in Figures 2A–E.
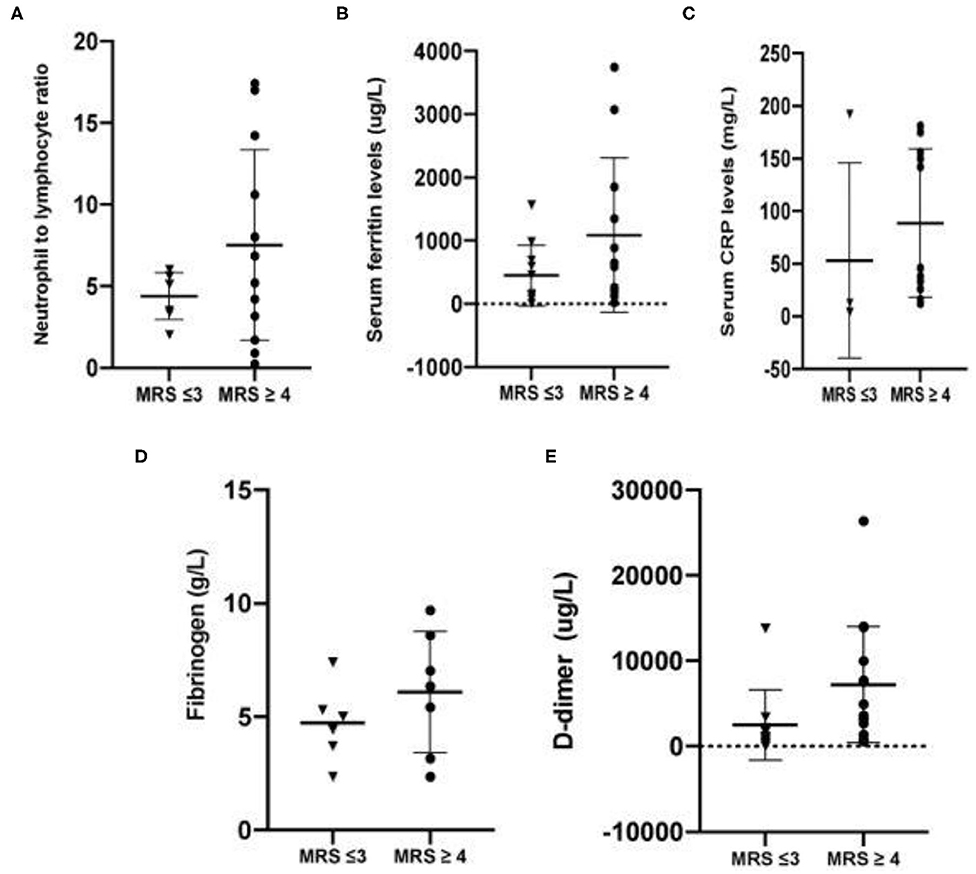
Figure 2. (A–C) Mean inflammatory markers among patients with stroke and confirmed SARA-CoV2 infections. (D–E) Mean coagulation markers among patients with stroke and confirmed SARS-CoV2 infection.
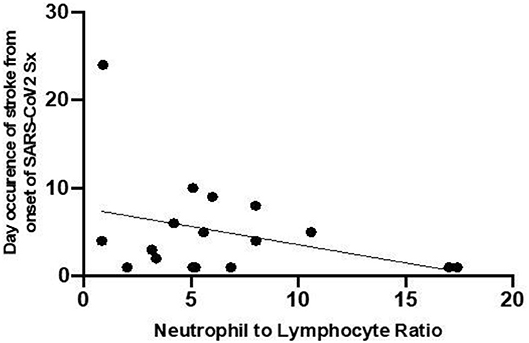
The relationship between the NLR on admission and the time interval from onset of SARS-CoV2 symptoms to the appearance onset of symptoms of stroke was established. As shown in Figure 3, patients who have higher NLR at the onset have a shorter time interval between infective symptoms and the occurrence of the ischemic event.
Discussion
To date, there is no comprehensive review describing the potential role of inflammatory and coagulation biomarkers in determining the clinical outcomes of patients with SARS-CoV2 infection and concomitant acute ischemic stroke. The data presented will also supplement currently limited information on the occurrence of neurovascular events among patients with SARS-CoV2 infection.
To date a number of theoretical models have been proposed to account for the occurrence of neurovascular events among SARS-CoV2 patients. Most build on the idea of the SARS-CoV2 virus infection inducing inflammation and associated immunological release of cytokines from blood and endothelial cells and the concurrent activation of platelets resulting in micro thrombosis (69). The depletion of the cardioprotective and neuroprotective ACE-2 receptors throughout the body and on microglia in the brain, as a result of the receptors being the preferential cellular target of the virus invasion, has also been proposed as another neuropathologic mechanism irrespective of age (8). However, the hypercoagulable state of SARS-COV2 infection as the sole basis of this mechanism is debateable given that vascular workups for cryptogenic stroke have not been detailed in most of the case studies. Furthermore, the increase in “burden of disease” especially in the elderly is likely to be further exacerbated by the expected age-related depletion in ACE-2 receptors resulting in the predominance of the end-organ damaging effects of increasing the ACE-1/Angiotensin II ratio (70–72).
To date, the majority of AIS lesion sites in the patients described in the literature, are related to large vessel occlusion. However, it remains unclear whether this is due to a mechanism related to thrombosis or embolism or the lack of brain imaging. Unfortunately, there are no studies to date, which fully report autopsy findings of the deaths recorded among the stroke patients with SARS-CoV infection. In a different, though recent, study describing the autopsy results of 12 SARS-CoV2 patients in a German center, the majority of cases showed massive venous thromboembolism with no arterial thrombosis being reported (73). Mechanisms which may contribute to intracranial arterial thrombosis include the cytokine-induced initiation of thrombin formation that triggers the activation of platelets that subsequently result in the development of micro and macrothrombi (74–87). This is worsened by the free conversion of fibrinogen to fibrin and inflammation-induced depletion of physiological anticoagulants such as antithrombin III, tissue factor pathway inhibitor, and the protein C system (74–88). In terms of treatment, while 30 cases were reported to have large vessel occlusion, only 20 mechanical thrombectomies were performed. A comprehensive stroke center in Barcelona, Spain reported an 18 and 23% drop in the number of strokes codes and mechanical thrombectomies during the start of the pandemic, respectively, albeit without any changes in reperfusion and clinical outcomes (89) The World Stroke Organization recognizes the said difficulties and emphasizes the utility of telemedicine as well as best practice sharing to further optimize and streamline stroke processes (90, 91).
While not depictive of the true epidemiologic picture, it is clear that patients with AIS and SARS-CoV2 infection have poor neurologic outcomes of either death or severe disability. Aggarwal et al. (92) concluded in a point analysis of four studies that patients with a previous history of stroke have a 2.5-fold increase in the odds of severe COVID infection but did not show any significant association with mortality (92). A retrospective cohort study of ischemic stroke reports a mortality rate close to 50% (3) while a prospective study involving 10 AIS patients resulted in four deaths (55) Clearly, more prospective studies involving a larger number of individual patients is necessary to ascertain the true mortality rate in this population.
In this study, there is a trend that patients with good outcomes have lower NLR, CRP, and serum ferritin compared to patients who died or remained critically ill. NLR has been shown to have a good predictive value in assessing patients who are likely to have severe SARS-CoV2 infection (30, 93–96). In particular, it has been proposed that patients who are older and have NLR values of more than 3 are likely to require intensive care (27). Yan et al. also predicted that high NLR values on admission is associated with greater odds of complications related to COVID-infection (97). On the other hand, it is known that high NLR is used as a poor prognosticating factor for patients with cerebral ischemia, intracerebral hemorrhage and post-stroke complications (98–107). The dual consequence of COVID-related lymphopenia along with migration of the neutrophils to the ischemic tissue may contribute to the significant increase in the NLR levels in patients with stroke and concomitant SARS-CoV2 infection (107).
Another hyperinflammatory biomarker which has been shown to stratify outcomes in patients with SARS-CoV2 infection is CRP. Aside from predicting severity and mortality, it has prognosticating value in determining which patients will eventually require mechanical ventilation (108–110). Published literature noted that elevated CRP is associated with poor outcomes in patients with neurovascular conditions (111, 112). There is also evidence to suggest that CRP is not just a “marker” but a “maker” of the atherogenesis (110). It has been demonstrated in experimental studies that exogenous CRP promotes atherogenesis by promoting the expression of adhesion molecules and cell mediators along with the decrease of arterial vasodilators (113–115). A meta-analysis of nine studies also provides evidence on the dose-dependent relationship of CRP and increased risk of venous thromboembolism (112). Whether the elevation of CRP is the causative etiology or the sequelae of a multifactorial process linking SARS-CoV2-infection to inflammation, atherogenesis or embolism needs further exploration.
Hyperferritinemia, which implies a heightened state of immunologic reactivity has also been associated with increased mortality in recent publications related to the SARS-CoV2 infection (116). It signals the activation of the macrophages and the reticuloendothelial system resulting in end-organ damage (117). Patients with SARS-CoV2 treated for pneumonia with Toculizumab had a marked decrease in the inflammatory markers such as CRP and ferritin, along with significant clinical improvement post-infusion (118). In patients with acute stroke, this iron storage protein can potentially worsen the iron-dependent oxidative stress in the ischemic penumbra which can lead to further neurologic decline (119). This is further validated in a study which shows a direct correlation between serum ferritin and markers of neural and blood-brain barrier disruption such as glutamate, interleukin-6, matrix metalloproteinase-9 and cellular fibronectin among patients receiving thrombolysis (39). The complementary inflammatory sequelae of SARS-CoV2 infection and ischemic stroke is the likely culprit of hyperferritinemia in SARS-CoV2 related strokes.
SARS-CoV2-related coagulopathy is responsible for various thrombotic events linked to mortality. Described as a fibrinolytic “shut-down,” SARS-CoV2 infection promotes a pro and hypercoagulable states resulting in disseminated (intravascular coagulation (DIC), microthrombi and other venous and arterial thrombotic phenomena (4, 120–122). D-dimer and fibrinogen are both recognized as important biomarkers of the severity of coagulopathy in patients with SARS-CoV 2 infection (123, 124). Olive et al. in a retrospective analysis of 21 patients with SARS-CoV infection concludes that D-dimer was associated with increased risk of pulmonary embolism (125). A similar observation was made in a larger study that suggests that D-dimer levels above 1 μg/mL may help in stratifying patients with poor prognosis at the onset (26). Fibrinogen increase was also observed among patients with severe SARS-CoV2 related pneumonia compared to mild presentation (126). The disproportionate increase of these biomarkers, especially at the early stages, warrant screening of thromboembolic events and initiation of thromboprophylaxis (124). The trend in these coagulation biomarkers are similarly observed in non-COVID related strokes. In the ARISTOTLE trial, patients with AF and increased D-dimer values had higher incidence of stroke, systemic embolism and all-cause mortality (127). Choi and colleagues also propose that D-dimer can be used as a biomarker for recurrence among patients with previous AF and non-AF related strokes (128). The EUROSTROKE study likewise confirms the utility of fibrinogen in predicting patients who are at risk for stroke (36). The said risk is equated to various clinical risks such as smoking, DM, MI, and HDL cholesterol (36). In this study, we have provided a scaffold on the potential trend between outcomes and coagulation parameters for SARS-CoV2 related strokes. While the most accepted mechanism behind this phenomenon is sepsis-induced disruption of the coagulation system, Iba et al. propose that more complex procoagulant responses resulting in a distinct interaction between the host's immunologic and the coagulation systems (124).
This study also highlights the occurrence of the ischemic event days to weeks after the onset of SARS-CoV2 symptoms. More importantly, we have established an inverse relationship between the inflammatory biomarker, NLR on admission and the duration between the stroke and the onset of SARS-CoV2 symptoms. This is likely related to the inflammatory burden which triggers a pro-coagulable cascade. Furthermore, Amiral et al. relate this to the alloimmune hypothesis, which has been demonstrated in rodents (129). The development of auto-antibodies to other ACE-2 receptors such as on the microglia in the brain after the onset of viral infection presumably resulted in the exponential increase in the cytokine storm and significant tissue destruction which may be linked to the delayed onset of the vascular event after the viral prodrome (129).
Lastly as the COVID-19 pandemic is distressing national health systems worldwide, a tsunami wave of neurorehabilitation needs and challenges regarding the long-term effects of the pandemic must be expected to begin to unfold soon. Thus, we believe that with strong humanity and collaboration across disciplines, this is the time to convert this situation into an opportunity that with vision, creativity, innovation, and use of smart technology can be harnessed with the aim of surviving this global health crisis (43, 130).
Conclusion
Stroke is an important neurovascular complication of SARS-CoV2 infection. The aetiopathogenesis of cerebral ischemia is related to the overactivation of immune and hypercoagulable mechanisms. This is supported by the disproportionate increase of biomarkers such as NLR, CRP, serum ferritin, D-dimer and fibrinogen among patients who died or were critically ill. An elevated NLR on admission also implies an increased burden of inflammation at the onset of SARS-CoV infection which may result in early manifestation of cerebral ischemic events.
Author Contributions
TW and LK conceived of the presented idea. TW, SC, and LK developed the theory. TW and CS performed the literature search. TW wrote the manuscript with support from CS, LK, and SC. All four authors approved the final manuscript.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. WHO. Coronavirus(COVID-19). Available online at: https://www.who.int/emergencies/diseases/novel-coronavirus-2019 (accessed May 30, 2020).
2. Zhou Y, Li W, Wang D, Mao L, Jin H, Li Y, et al. Clinical time course of COVID-19, its neurological manifestation and some thoughts on its management. Stroke Vasc Neurol. (2020) 5:177–9. doi: 10.1136/svn-2020-000398
3. Yaghi S, Ishida K, Torres J, Mac Grory B, Raz E, Humbert K, et al. SARS2-CoV-2 and stroke in a New York healthcare system. Stroke. (2020) 51:2002–11. doi: 10.1161/STROKEAHA.120.030335
4. Lodigiani C, Iapichino G, Carenzo L, Cecconi M, Ferrazzi P, Sebastian T, et al. Venous and arterial thromboembolic complications in COVID-19 patients admitted to an academic hospital in Milan, Italy. Thromb Res. (2020) 191:9–14. doi: 10.1016/j.thromres.2020.04.024
5. Caso V, Federico A. No lockdown for neurological diseases during COVID19 pandemic infection. Neurol Sci. (2020) 41:999–1001. doi: 10.1007/s10072-020-04389-3
6. Oxley TJ, Mocco J, Majidi S, Kellner CP, Shoirah H, Singh IP, et al. Large-vessel stroke as a presenting feature of Covid-19 in the young. N Engl J Med. (2020) 382:e60. doi: 10.1056/NEJMc2009787
7. Spiezia L, Boscolo A, Poletto F, Cerruti L, Tiberio I, Campello E, et al. COVID-19-related severe hypercoagulability in patients admitted to intensive care unit for acute respiratory failure. Thromb Haemost. (2020) 120:998–1000. doi: 10.1055/s-0040-1710018
8. Hess DC, Eldahshan W, Rutkowski E. COVID-19-related stroke. Transl Stroke Res. (2020) 11:322–5. doi: 10.1007/s12975-020-00818-9
9. Giacomelli E, Dorigo W, Fargion A, Calugi G, Cianchi G, Pratesi C. Acute thrombosis of an aortic prosthetic graft in a patient with severe COVID-19-related pneumonia. Ann Vasc Surg. (2020) 66:8–10. doi: 10.1016/j.avsg.2020.04.040
10. Griffin DO, Jensen A, Khan M, Chin J, Chin K, Parnell R, et al. Arterial thromboembolic complications in COVID-19 in low risk patients despite prophylaxis. Br J Haematol. (2020) 190:e11–e13. doi: 10.1111/bjh.16792
11. Benhamou D, Keita H, Bouthors AS, Group Cw. Coagulation changes and thromboembolic risk in COVID-19 pregnant patients. Anaesth Crit Care Pain Med. (2020) 39:351–3. doi: 10.1016/j.accpm.2020.05.003
12. Schultz K, Wolf JM. Digital ischemia in COVID-19 patients: case report. J Hand Surg Am. (2020) 45:518–22. doi: 10.1016/j.jhsa.2020.04.024
13. Viecca M, Radovanovic D, Forleo GB, Santus P. Enhanced platelet inhibition treatment improves hypoxemia in patients with severe Covid-19 and hypercoagulability. A case control, proof of concept study. Pharmacol Res. (2020) 158104950. doi: 10.1016/j.phrs.2020.104950
14. Wright FL, Vogler TO, Moore EE, Moore HB, Wohlauer MV, Urban S, et al. Fibrinolysis shutdown correlates to thromboembolic events in severe COVID-19 infection. J Am Coll Surg. (2020) 231:203–4. doi: 10.1016/j.jamcollsurg.2020.05.007
15. Terpos E, Ntanasis-Stathopoulos I, Elalamy I, Kastritis E, Sergentanis TN, Politou M, et al. Hematological findings and complications of COVID-19. Am J Hematol. (2020) 95:834–47. doi: 10.1002/ajh.25829
16. Panigada M, Bottino N, Tagliabue P, Grasselli G, Novembrino C, Chantarangkul V, et al. Hypercoagulability of COVID-19 patients in intensive care unit. A report of thromboelastography findings and other parameters of hemostasis. J Thromb Haemost. (2020) 18:1738–42. doi: 10.1111/jth.14850
17. Griffin DO, Jensen A, Khan M, Chin J, Chin K, Saad J, et al. Pulmonary embolism and increased levels of d-dimer in patients with coronavirus disease. Emerg Infect Dis. (2020) 26:1941–3. doi: 10.3201/eid2608.201477
18. Thachil J, Agarwal S. Understanding the COVID-19 coagulopathy spectrum. Anaesthesia. (2020). doi: 10.1111/anae.15141
19. Bian J, Zhao R, Zhai S, Li Z. Anti-RAS drugs and SARS-CoV-2 infection. Acta Pharm Sin B. (2020) 10:1251–2. doi: 10.1016/j.apsb.2020.04.013
20. Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. The Lancet. (2020) 395:497–506. doi: 10.1016/S0140-6736(20)30183-5
21. Conti P, Ronconi G, Caraffa A, Gallenga CE, Ross R, Frydas I, et al. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): anti-inflammatory strategies. J Biol Regul Homeost Agents. (2020) 34:327–31. doi: 10.23812/CONTI-E
22. Edition) NHCotPsRoCTDaTPf-nTST. The Diagnosis and Treatment Plan for 2019-nCoV. (2020). Available online at: http://www.nhc.gov.cn/yzygj/s7653p/202003/46c9294a7dfe4cef80dc7f5912eb1989.shtml (accessed May 30, 2020).
23. Kim ES, Chin BS, Kang CK, Kim NJ, Kang YM, Choi JP, et al. Clinical course and outcomes of patients with severe acute respiratory syndrome coronavirus 2 infection: a preliminary report of the first 28 patients from the Korean cohort study on COVID-19. J Korean Med Sci. (2020) 35:e142. doi: 10.3346/jkms.2020.35.e142
24. Yang X, Yu Y, Xu J, Shu H, Xia Ja, Liu H, et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med. (2020) 8:475–81. doi: 10.1016/S2213-2600(20)30079-5
25. Tan C, Huang Y, Shi F, Tan K, Ma Q, Chen Y, et al. C-reactive protein correlates with computed tomographic findings and predicts severe COVID-19 early. J Med Virol. (2020) 92:856–62. doi: 10.1002/jmv.25871
26. Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet. (2020) 395:1054–62. doi: 10.1016/S0140-6736(20)30566-3
27. Ciccullo A, Borghetti A, Zileri Dal Verme L, Tosoni A, Lombardi F, Garcovich M, et al. Neutrophil-to-lymphocyte ratio and clinical outcome in COVID-19: a report from the Italian front line. Int J Antimicrob Agents. (2020) 56:106017. doi: 10.1016/j.ijantimicag.2020.106017
28. Feng X, Li S, Sun Q, Zhu J, Chen B, Xiong M, et al. Immune-Inflammatory parameters in COVID-19 cases: a systematic review and meta-analysis. Front Med. (2020) 7:301. doi: 10.3389/fmed.2020.00301
29. Heneka MT, Golenbock D, Latz E, Morgan D, Brown R. Immediate and long-term consequences of COVID-19 infections for the development of neurological disease. Alzheimers Res Ther. (2020) 12:69. doi: 10.1186/s13195-020-00640-3
30. Liu J, Li S, Liu J, Liang B, Wang X, Wang H, et al. Longitudinal characteristics of lymphocyte responses and cytokine profiles in the peripheral blood of SARS-CoV-2 infected patients. EBioMedicine. (2020) 55:102763. doi: 10.1016/j.ebiom.2020.102763
31. Huang I, Pranata R, Lim MA, Oehadian A, Alisjahbana B. C-reactive protein, procalcitonin, D-dimer, and ferritin in severe coronavirus disease-2019: a meta-analysis. Therap Adv Respir Dis. (2020) 14:1753466620937175. doi: 10.1177/1753466620937175
32. Frazer JS, Tyrynis Everden AJ. Emerging patterns of hypercoagulability associated with critical COVID-19: A review. Trends Anaesth Criti Care. (2020). doi: 10.1016/j.tacc.2020.07.004
33. Switońska M, Słomka A, Korbal P, Piekuś-Słomka N, Sinkiewicz W, Sokal P, et al. Association of neutrophil-to-lymphocyte ratio and lymphocyte-to-monocyte ratio with treatment modalities of acute ischaemic stroke: a pilot study. Medicina. (2019) 55:342. doi: 10.3390/medicina55070342
34. Lim HH, Jeong IH, An GD, Woo KS, Kim KH, Kim JM, et al. Early prediction of severity in acute ischemic stroke and transient ischemic attack using platelet parameters and neutrophil-to-lymphocyte ratio. J Clin Lab Anal. (2019) 33:e22714. doi: 10.1002/jcla.22714
35. Fang Y-N, Tong M-S, Sung P-H, Chen Y-L, Chen C-H, Tsai N-W, et al. Higher neutrophil counts and neutrophil-to-lymphocyte ratio predict prognostic outcomes in patients after non-atrial fibrillation-caused ischemic stroke. Biomedical journal. (2017) 40:154–62. doi: 10.1016/j.bj.2017.03.002
36. Bots ML, Elwood PC, Salonen JT, Freire de Concalves A, Sivenius J, Di Carlo A, et al. Level of fibrinogen and risk of fatal and non-fatal stroke. EUROSTROKE: a collaborative study among research centres in Europe. J Epidemiol Community Health. (2002) 56(Suppl 1):i14–8. doi: 10.1136/jech.56.suppl_1.i14
37. Bustamante A, Simats A, Vilar-Bergua A, García-Berrocoso T, Montaner J. Blood/brain biomarkers of inflammation after stroke and their association with outcome: from C-reactive protein to damage-associated molecular patterns. Neurotherapeutics. (2016) 13:671–84. doi: 10.1007/s13311-016-0470-2
38. Wang A, Mandigo G, Yim P, Meyers P, Lavine S. Stroke and mechanical thrombectomy in patients with COVID-19: technical observations and patient characteristics. J Neurointerventional Surgery. (2020) 12:648–53. doi: 10.1136/neurintsurg-2020-016220
39. Millán M, Sobrino T, Arenillas JF, Rodríguez-Yáñez M, García M, Nombela F, et al. Biological signatures of brain damage associated with high serum ferritin levels in patients with acute ischemic stroke and thrombolytic treatment. Dis Markers. (2008) 25:181–8. doi: 10.1155/2008/380356
40. Carda S, Invernizzi M, Bavikatte G, Bensmail D, Bianchi F, Deltombe T, et al. The role of physical and rehabilitation medicine in the COVID-19 pandemic: the clinician's view. Ann Phys Rehabil Med. (2020). doi: 10.1016/j.rehab.2020.04.001
41. Leocani L, Diserens K, Moccia M, Caltagirone C. Disability through COVID-19 pandemic: neurorehabilitation cannot wait. Eur J Neurol. (2020). doi: 10.1111/ene.14320
42. Carda S, Invernizzi M, Bavikatte G, Bensmail D, Bianchi F, Deltombe T, et al. COVID-19 pandemic. What should PRM specialists do? A clinician's perspective. Eur J Phys Rehabil Med. (2020). doi: 10.23736/S1973-9087.20.06317-0
43. Bartolo M, Intiso D, Lentino C, Sandrini G, Paolucci S, Zampolini M, et al. Urgent measures for the containment of the coronavirus (Covid-19) epidemic in the neurorehabilitation/rehabilitation departments in the phase of maximum expansion of the epidemic. Front Neurol. (2020) 11:423. doi: 10.3389/fneur.2020.00423
44. Kampf G, Scheithauer S, Lemmen S, Saliou P, Suchomel M. COVID-19-associated shortage of alcohol-based hand rubs, face masks, medical gloves and gowns - proposal for a risk-adapted approach to ensure patient and healthcare worker safety. J Hosp Infect. (2020) 105:424–7. doi: 10.1016/j.jhin.2020.04.041
45. Rowan NJ, Laffey JG. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from coronavirus disease (COVID19) pandemic - case study from the Republic of Ireland. Sci Total Environ. (2020) 725:138532. doi: 10.1016/j.scitotenv.2020.138532
46. Dyer O. Covid-19: cases rise in Russia as health workers pay the price for PPE shortage. BMJ. (2020) 369:m1975. doi: 10.1136/bmj.m1975
47. Dyer C. Covid-19: doctors are warned not to go public about PPE shortages. BMJ. (2020) 369:m1592. doi: 10.1136/bmj.m1592
48. Garzotto F, Ceresola E, Panagiotakopoulou S, Spina G, Menotto F, Benozzi M, et al. COVID-19: ensuring our medical equipment can meet the challenge. Expert Rev Med Devices. (2020) 17:483–9. doi: 10.1080/17434440.2020.1772757
49. Ripp J, Peccoralo L, Charney D. Attending to the emotional well-being of the health care workforce in a New York City health system during the COVID-19 pandemic. Acad Med. (2020) 95:1136–9. doi: 10.1097/ACM.0000000000003414
50. Albott CS, Wozniak JR, McGlinch BP, Wall MH, Gold BS, Vinogradov S. Battle buddies: rapid deployment of a psychological resilience intervention for healthcare workers during the COVID-19 pandemic. Anesth Analg. (2020). doi: 10.1213/ANE.0000000000004912
51. Wijesundera C, Vingrys AJ, Wijeratne T, Crewther SG. Acquired visual deficits independent of lesion site in acute stroke. Front Neurol. (2020) 11:705. doi: 10.3389/fneur.2020.00705
52. Wijesundera C, Vingrys AJ, Kong YXG, Wijeratne T, Crewther SG. Visual function and visuo-motor coordination in acute stroke. Investig Ophthalmol Visual Sci. (2019) 60:1046.
53. Wijesundera CN, Crewther S, Vingrys A, Wijeratne T. Abstract WP187: significant recovery from acute visual deficits to three to six months post ischaemic stroke. Stroke. (2019) 50:AWP187-AWP. doi: 10.1161/str.50.suppl_1.WP187
54. Arksey H, O'Malley L. Scoping studies: towards a methodological framework. Int J Soc Res Methodol. (2005) 8:19–32. doi: 10.1080/1364557032000119616
55. Berekashvili K, Dmytriw AA, Vulkanov V, Agarwal S, Khaneja A, Turkel-Parella D, et al. Etiologic Subtypes of Ischemic Stroke in SARS-COV-2 Virus Patients. (2020). doi: 10.1101/2020.05.03.20077206
56. Beyrouti R, Adams ME, Benjamin L, et al. Characteristics of ischaemic stroke associated with COVID-19. J Neurol Neurosurg Psychiatry. (2020) 91:889–91. doi: 10.1136/jnnp-2020-323586
57. Avula A, Nalleballe K, Narula N, et al. COVID-19 presenting as stroke. Brain Behav Immun. (2020) 87:115–9. doi: 10.1016/j.bbi.2020.04.077
58. Tunç A, Ünlübaş Y Alemdar M Akyüz E. Coexistence of COVID-19 and acute ischemic stroke report of four cases. J Clin Neurosci. (2020) 77:227–9. doi: 10.1016/j.jocn.2020.05.018
59. Morassi M, Bagatto D, Cobelli M, et al. Stroke in patients with SARS-CoV-2 infection: case series. J Neurol. (2020) 267:2185–92. doi: 10.1007/s00415-020-09885-2
60. Viguier A, Delamarre L, Duplantier J, Olivot JM, Bonneville F. Acute ischemic stroke complicating common carotid artery thrombosis during a severe COVID-19 infection. J Neuroradiol. (2020) 47:393–4. doi: 10.1016/j.neurad.2020.04.003
61. Co COC, Yu JRT, Laxamana LC, David-Ona DIA. Intravenous thrombolysis for stroke in a COVID-19 positive Filipino patient, a case report. J Clin Neurosci. (2020) 77:234–6. doi: 10.1016/j.jocn.2020.05.006
62. Deliwala S, Abdulhamid S, Abusalih MF, Al-Qasmi MM, Bachuwa G. Encephalopathy as the sentinel sign of a cortical stroke in a patient infected with coronavirus disease-19 (COVID-19). Cureus. (2020) 12:e8121. doi: 10.7759/cureus.8121
63. Al Saiegh F, Ghosh R, Leibold A, et al. Status of SARS-CoV-2 in cerebrospinal fluid of patients with COVID-19 and stroke. J Neurol Neurosurg Psychiatry. (2020) 91:846–8. doi: 10.1136/jnnp-2020-323522
64. González-Pinto T, Luna-Rodríguez A, Moreno-Estébanez A, Agirre-Beitia G, Rodríguez-Antigüedad A, Ruiz-Lopez M. Emergency room neurology in times of COVID-19: malignant ischaemic stroke and SARS-CoV-2 infection. Eur J Neurol. (2020). doi: 10.1111/ene.14286
65. Gunasekaran K, Amoah K, Rajasurya V, Buscher MG. Stroke in a young COVID-19 patient. QJM. (2020) 113:573–4. doi: 10.1093/qjmed/hcaa177
66. Valderrama E, Humbert K, Lord A, Frontera J, Yaghi S. Severe acute respiratory syndrome coronavirus 2 infection and ischemic stroke. Stroke. (2020) 51. doi: 10.1161/strokeaha.120.030153
67. Moshayedi P, Ryan TE, Mejia LLP, Nour M, Liebeskind DS. Triage of acute ischemic stroke in confirmed COVID-19: large vessel occlusion associated with coronavirus infection. Front Neurol. (2020) 11:353. doi: 10.3389/fneur.2020.00353
68. Goldberg M, Goldberg M, Cerejo R, Tayal A. Cerebrovascular disease in COVID-19. Am J Neuroradiol. (2020) 41:1170–2. doi: 10.3174/ajnr.a6588
69. Bikdeli B, Madhavan MV, Jimenez D, Chuich T, Dreyfus I, Driggin E, et al. COVID-19 and thrombotic or thromboembolic disease: implications for prevention, antithrombotic therapy, and follow-up. J Am Coll Cardiol. (2020) 75:2950–73. doi: 10.1016/j.jacc.2020.04.031
70. Magrone T, Magrone M, Jirillo E. Focus on receptors for coronaviruses with special reference to angiotensin-converting enzyme 2 as a potential drug target - a perspective. Endocr Metab Immune Disord Drug Targets. (2020) 20:807–11. doi: 10.2174/1871530320666200427112902
71. Lopes RD, Macedo AVS, de Barros ESPGM, Moll-Bernardes RJ, Feldman A, D'Andrea Saba Arruda G, et al. Continuing versus suspending angiotensin-converting enzyme inhibitors and angiotensin receptor blockers: impact on adverse outcomes in hospitalized patients with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Am Heart J. (2020) 226:49–59. doi: 10.1016/j.ahj.2020.05.002
72. Larson AS, Savastano L, Kadirvel R, Kallmes DF, Hassan AE, Brinjikji W. COVID-19 and the Cerebro-cardiovascular systems: what do we know so far? J Am Heart Assoc. (2020) 9:e016793. doi: 10.1161/JAHA.120.016793
73. Wichmann D, Sperhake JP, Lutgehetmann M, Steurer S, Edler C, Heinemann A, et al. Autopsy findings and venous thromboembolism in patients with COVID-19. Ann Intern Med. (2020) 173:268–77. doi: 10.7326/M20-2003
74. Mehta P, McAuley DF, Brown M, Sanchez E, Tattersall RS, Manson JJ, et al. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. (2020) 395:1033–4. doi: 10.1016/S0140-6736(20)30628-0
75. Lin JH, Chen YC, Lu CL, Hsu YN, Wang WJ. Application of plasma exchange in association with higher dose CVVH in cytokine storm complicating COVID-19. J Formos Med Assoc. (2020) 119:1116–8. doi: 10.1016/j.jfma.2020.04.023
76. Zhou G, Chen S, Chen Z. Advances in COVID-19: the virus, the pathogenesis, and evidence-based control and therapeutic strategies. Front Med. (2020) 14:117–25. doi: 10.1007/s11684-020-0773-x
77. Zhu Z, Cai T, Fan L, Lou K, Hua X, Huang Z, et al. Clinical value of immune-inflammatory parameters to assess the severity of coronavirus disease (2019). Int J Infect Dis. (2020) 95:332–9. doi: 10.1016/j.ijid.2020.04.041
78. Jose RJ, Manuel A. COVID-19 cytokine storm: the interplay between inflammation and coagulation. Lancet Respir Med. (2020) 8:e46–7. doi: 10.1016/S2213-2600(20)30216-2
79. Tufan A, Avanoglu Guler A, Matucci-Cerinic M. COVID-19, immune system response, hyperinflammation and repurposing antirheumatic drugs. Turk J Med Sci. (2020) 50:620–32. doi: 10.3906/sag-2004-168
80. Yao Z, Zheng Z, Wu K, Junhua Z. Immune environment modulation in pneumonia patients caused by coronavirus: SARS-CoV, MERS-CoV and SARS-CoV-2. Aging. (2020) 12:7639–51. doi: 10.18632/aging.103101
81. Azkur AK, Akdis M, Azkur D, Sokolowska M, van de Veen W, Bruggen MC, et al. Immune response to SARS-CoV-2 and mechanisms of immunopathological changes in COVID-19. Allergy. (2020) 75:1564–81. doi: 10.1111/all.14364
82. Alijotas-Reig J, Esteve-Valverde E, Belizna C, Selva-O'Callaghan A, Pardos-Gea J, Quintana A, et al. Immunomodulatory therapy for the management of severe COVID-19. Beyond the anti-viral therapy: a comprehensive review. Autoimmun Rev. (2020) 19:102569. doi: 10.1016/j.autrev.2020.102569
83. Zheng Y, Li R, Liu S. Immunoregulation with mTOR inhibitors to prevent COVID-19 severity:a novel intervention strategy beyond vaccines and specific antiviral medicines. J Med Virol. (2020). doi: 10.20944/preprints202004.0060.v1
84. Serrano-Castro PJ, Estivill-Torrus G, Cabezudo-Garcia P, Reyes-Bueno JA, Ciano Petersen N, Aguilar-Castillo MJ, et al. Impact of SARS-CoV-2 infection on neurodegenerative and neuropsychiatric diseases: a delayed pandemic? Neurologia. (2020) 35:245–51. doi: 10.1016/j.nrleng.2020.04.002
85. Chau VQ, Oliveros E, Mahmood K, Singhvi A, Lala A, Moss N, et al. The imperfect cytokine storm: severe COVID-19 with ARDS in patient on durable LVAD support. JACC Case Rep. (2020) 2:1315–20. doi: 10.1016/j.jaccas.2020.04.001
86. Benhadou F, Del Marmol V. Improvement of SARS-CoV2 symptoms following guselkumab injection in a psoriatic patient. J Eur Acad Dermatol Venereol. (2020) 34:e363–4. doi: 10.1111/jdv.16590
87. Vaninov N. In the eye of the COVID-19 cytokine storm. Nat Rev Immunol. (2020) 20:277. doi: 10.1038/s41577-020-0305-6
88. Kowalewski M, Fina D, Slomka A, Raffa GM, Martucci G, Lo Coco V, et al. COVID-19 and ECMO: the interplay between coagulation and inflammation-a narrative review. Crit Care. (2020) 24:205. doi: 10.1186/s13054-020-02925-3
89. Rudilosso S, Laredo C, Vera V, Vargas M, Renu A, Llull L, et al. Acute stroke care is at risk in the Era of COVID-19: experience at a comprehensive stroke center in barcelona. Stroke. (2020) 51:1991–5. doi: 10.1161/STROKEAHA.120.030329
90. Markus HS, Brainin M. COVID-19 and stroke-A global world stroke organization perspective. Int J Stroke. (2020) 15:361–4. doi: 10.1177/1747493020923472
91. July J, Pranata R. Impact of the coronavirus disease pandemic on the number of strokes and mechanical thrombectomies: a systematic review and meta-analysis: COVID-19 and stroke care. J Stroke Cerebrovasc Dis. (2020) 29:105185. doi: 10.1016/j.jstrokecerebrovasdis.2020.105185
92. Aggarwal G, Lippi G, Michael Henry B. Cerebrovascular disease is associated with an increased disease severity in patients with coronavirus disease 2019 (COVID-19): a pooled analysis of published literature. Int J Stroke. (2020) 15:385–9. doi: 10.1177/1747493020921664
93. Liu Y, Du X, Chen J, Jin Y, Peng L, Wang HHX, et al. Neutrophil-to-lymphocyte ratio as an independent risk factor for mortality in hospitalized patients with COVID-19. J Infect. (2020) 81:e6–12. doi: 10.1016/j.jinf.2020.04.002
94. Xia X, Wen M, Zhan S, He J, Chen W. [An increased neutrophil/lymphocyte ratio is an early warning signal of severe COVID-19]. Nan Fang Yi Ke Da Xue Xue Bao. (2020) 40:333–6. doi: 10.12122/j.issn.1673-4254.2020.03.06
95. Liu Y-P, Li G-M, He J, Liu Y, Li M, Zhang R, et al. Combined use of the neutrophil-to-lymphocyte ratio and CRP to predict 7-day disease severity in 84 hospitalized patients with COVID-19 pneumonia: a retrospective cohort study. Ann Transl Med. (2020) 8:635. doi: 10.21037/atm-20-2372
96. Wang C, Deng R, Gou L, Fu Z, Zhang X, Shao F, et al. Preliminary study to identify severe from moderate cases of COVID-19 using combined hematology parameters. Ann Transl Med. (2020) 8:593. doi: 10.21037/atm-20-3391
97. Yan X, Li F, Wang X, Yan J, Zhu F, Tang S, et al. Neutrophil to lymphocyte ratio as prognostic and predictive factor in patients with coronavirus disease 2019: A retrospective cross-sectional study. J Med Virol. (2020). doi: 10.1002/jmv.26061
98. Celikbilek A, Ismailogullari S, Zararsiz G. Neutrophil to lymphocyte ratio predicts poor prognosis in ischemic cerebrovascular disease. J Clin Lab Anal. (2014) 28:27–31. doi: 10.1002/jcla.21639
99. Qun S, Tang Y, Sun J, Liu Z, Wu J, Zhang J, et al. Neutrophil-to-lymphocyte ratio predicts 3-month outcome of acute ischemic stroke. Neurotoxicity Research. (2017) 31:444–52. doi: 10.1007/s12640-017-9707-z
100. Zhao L, Dai Q, Chen X, Li S, Shi R, Yu S, et al. Neutrophil-to-lymphocyte ratio predicts length of stay and acute hospital cost in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. (2016) 25:739–44. doi: 10.1016/j.jstrokecerebrovasdis.2015.11.012
101. Kocaturk O, Besli F, Gungoren F, Kocaturk M, Tanriverdi Z. The relationship among neutrophil to lymphocyte ratio, stroke territory, and 3-month mortality in patients with acute ischemic stroke. Neurol Sci. (2019) 40:139–46. doi: 10.1007/s10072-018-3604-y
102. Guo Z, Yu S, Xiao L, Chen X, Ye R, Zheng P, et al. Dynamic change of neutrophil to lymphocyte ratio and hemorrhagic transformation after thrombolysis in stroke. J Neuroinflamm. (2016) 13:199. doi: 10.1186/s12974-016-0680-x
103. Qin J, Li Z, Gong G, Li H, Chen L, Song B, et al. Early increased neutrophil-to-lymphocyte ratio is associated with poor 3-month outcomes in spontaneous intracerebral hemorrhage. PLoS ONE. (2019) 14:e0211833. doi: 10.1371/journal.pone.0211833
104. Tao C, Hu X, Wang J, Ma J, Li H, You C. Admission neutrophil count and neutrophil to lymphocyte ratio predict 90-day outcome in intracerebral hemorrhage. Biomark Med. (2017) 11:33–42. doi: 10.2217/bmm-2016-0187
105. Lattanzi S, Brigo F, Trinka E, Cagnetti C, Di Napoli M, Silvestrini M. Neutrophil-to-lymphocyte ratio in acute cerebral hemorrhage: a system review. Transl Stroke Res. (2019) 10:137–45. doi: 10.1007/s12975-018-0649-4
106. Lattanzi S, Cagnetti C, Provinciali L, Silvestrini M. Neutrophil-to-lymphocyte ratio and neurological deterioration following acute cerebral hemorrhage. Oncotarget. (2017) 8:57489–94. doi: 10.18632/oncotarget.15423
107. Kolaczkowska E, Kubes P. Neutrophil recruitment and function in health and inflammation. Nat Rev Immunol. (2013) 13:159–75. doi: 10.1038/nri3399
108. Shang W, Dong J, Ren Y, Tian M, Li W, Hu J, et al. The value of clinical parameters in predicting the severity of COVID-19. J Med Virol. (2020). doi: 10.1002/jmv.26031
109. Zhang Z-L, Hou Y-L, Li D-T, Li F-Z. Laboratory findings of COVID-19: a systematic review and meta-analysis. Scand J Clin Lab Invest. (2020) 23:1–7. doi: 10.1080/00365513.2020.1768587
110. Herold T, Jurinovic V, Arnreich C, Lipworth BJ, Hellmuth JC, Bergwelt-Baildon MV, et al. Elevated levels of IL-6 and CRP predict the need for mechanical ventilation in COVID-19. J Allergy Clin Immunol. (2020) 146:128–36.e4. doi: 10.1016/j.jaci.2020.05.008
111. Nilsson J. CRP— marker or maker of cardiovascular disease? Arteriosclero Thrombo Vasc Biol. (2005) 25:1527–8. doi: 10.1161/01.ATV.0000174796.81443.3f
112. Kunutsor SK, Seidu S, Blom AW, Khunti K, Laukkanen JA. Serum C-reactive protein increases the risk of venous thromboembolism: a prospective study and meta-analysis of published prospective evidence. Eur J Epidemiol. (2017) 32:657–67. doi: 10.1007/s10654-017-0277-4
113. Pasceri V, Cheng JS, Willerson JT, Yeh ET. Modulation of C-reactive protein-mediated monocyte chemoattractant protein-1 induction in human endothelial cells by anti-atherosclerosis drugs. Circulation. (2001) 103:2531–4. doi: 10.1161/01.CIR.103.21.2531
114. Pasceri V, Willerson JT, Yeh ETH. Direct proinflammatory effect of c-reactive protein on human endothelial cells. Circulation. (2000) 102:2165–8. doi: 10.1161/01.CIR.102.18.2165
115. Venugopal SK, Devaraj S, Jialal I. C-reactive protein decreases prostacyclin release from human aortic endothelial cells. Circulation. (2003) 108:1676–8. doi: 10.1161/01.CIR.0000094736.10595.A1
116. Ruscitti P, Berardicurti O, Di Benedetto P, Cipriani P, Iagnocco A, Shoenfeld Y, et al. Severe COVID-19, another piece in the puzzle of the hyperferritinemic syndrome. An immunomodulatory perspective to alleviate the storm. Front Immunol. (2020) 11:1130. doi: 10.3389/fimmu.2020.01130
117. Kernan KF, Carcillo JA. Hyperferritinemia and inflammation. Int Immunol. (2017) 29:401–9. doi: 10.1093/intimm/dxx031
118. Toniati P, Piva S, Cattalini M, Garrafa E, Regola F, Castelli F, et al. Tocilizumab for the treatment of severe COVID-19 pneumonia with hyperinflammatory syndrome and acute respiratory failure: a single center study of 100 patients in Brescia, Italy. Autoimmun Rev. (2020) 19:102568. doi: 10.1016/j.autrev.2020.102568
119. Carbonell T, Rama R. Iron, oxidative stress and early neurological deterioration in ischemic stroke. Curr Med Chem. (2007) 14:857–74. doi: 10.2174/092986707780363014
120. Dolhnikoff M, Duarte-Neto AN, de Almeida Monteiro RA, da Silva LFF, de Oliveira EP, Saldiva PHN, et al. Pathological evidence of pulmonary thrombotic phenomena in severe COVID-19. J Thrombo Haemosta. (2020) 18:1517–9. doi: 10.1111/jth.14844
121. Zátroch I, Smudla A, Babik B, Tánczos K, Kóbori L, Szabó Z, et al. Procoagulatio, hypercoagulatio és fibrinolysis shut down” kimutatása ClotPro® viszkoelasztikus tesztek segítségével COVID−19-betegekben. Orvosi Hetilap. (2020) 161:899–907. doi: 10.1556/650.2020.31870
122. Tveita A, Hestenes S, Sporastøyl ER, Pettersen SA, Neple BL, Myrstad M, et al. Pulmonary embolism in cases of COVID-19. Tidsskrift Den Norske Legeforen. (2020) 190:58–9. doi: 10.1016/j.thromres.2020.04.011
123. Tang N, Li D, Wang X, Sun Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J Thromb Haemost. (2020) 18:844–7. doi: 10.1111/jth.14768
124. Iba T, Levy JH, Levi M, Connors JM, Thachil J. Coagulopathy of coronavirus disease 2019. Crit Care Med. (2020) 48:1358–64. doi: 10.1097/CCM.0000000000004458
125. Garcia-Olivé I, Sintes H, Radua J, Abad Capa J, Rosell A. D-dimer in patients infected with COVID-19 and suspected pulmonary embolism. Respir Med. (2020) 169:106023. doi: 10.1016/j.rmed.2020.106023
126. Zou Y, Guo H, Zhang Y, Zhang Z, Liu Y, Wang J, et al. Analysis of coagulation parameters in patients with COVID-19 in Shanghai, China. Biosci Trends. (2020). doi: 10.5582/bst.2020.03086
127. Christersson C, Wallentin L, Andersson U, Alexander JH, Ansell J, De Caterina R, et al. D-dimer and risk of thromboembolic and bleeding events in patients with atrial fibrillation–observations from the ARISTOTLE trial. J Thromb Haemost. (2014) 12:1401–12. doi: 10.1111/jth.12638
128. Choi K-H, Seo W-K, Park M-S, Kim J-T, Chung J-W, Bang OY, et al. Baseline D-dimer levels as a risk assessment biomarker for recurrent stroke in patients with combined atrial fibrillation and atherosclerosis. J Clin Med. (2019) 8:1457. doi: 10.3390/jcm8091457
129. Amiral J, Vissac AM, Seghatchian J. Covid-19, induced activation of hemostasis, and immune reactions: Can an auto-immune reaction contribute to the delayed severe complications observed in some patients? Transfus Apher Sci. (2020) 59:102804. doi: 10.1016/j.transci.2020.102804
Keywords: acute ischemic stroke, COVID-19, neurorehabilitation, white blood cells, neutrophil lymphocyte ratio, hyper coagulopathy, D-dimer, ferritin
Citation: Wijeratne T, Sales C, Karimi L and Crewther SG (2020) Acute Ischemic Stroke in COVID-19: A Case-Based Systematic Review. Front. Neurol. 11:1031. doi: 10.3389/fneur.2020.01031
Received: 17 June 2020; Accepted: 06 August 2020;
Published: 25 September 2020.
Edited by:
Giorgio Sandrini, University of Pavia, ItalyReviewed by:
Giovanni Morone, Santa Lucia Foundation (IRCCS), ItalyMarina Zettin, Centro Puzzle, Italy
Copyright © 2020 Wijeratne, Sales, Karimi and Crewther. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Tissa Wijeratne, twijeratne@gmail.com
 Tissa Wijeratne
Tissa Wijeratne Carmela Sales
Carmela Sales Leila Karimi
Leila Karimi Sheila Gillard Crewther
Sheila Gillard Crewther