- 1Department of Methods of Brain Imaging and Functional Research of Nervous System, Nalecz Institute of Biocybernetics and Biomedical Engineering, Polish Academy of Sciences, Warsaw, Poland
- 2Department of Clinical Sciences, Umeå University, Umeå, Sweden
- 3Institute of Physiology, Faculty of Medicine, University of Lisbon, Lisbon, Portugal
- 4Department of Neurology, University Hospital Jena, Jena, Germany
- 5Jena Centre for Healthy Aging, University Hospital Jena, Jena, Germany
- 6Department of Neurology, Medical University of Warsaw, Warsaw, Poland
- 7Clinic for Neurology, Hannover Medical School, Hanover, Germany
- 8Akdeniz University Faculty of Medicine, Antalya, Turkey
It may seem useless to propose preventive measures for a disease without established pathogenesis and successful therapy, such as amyotrophic lateral sclerosis (ALS). However, we will show that ALS shares essential molecular mechanisms with aging and that established anti-aging strategies, such as healthy diet or individually adjusted exercise, may be successfully applied to ameliorate the condition of ALS patients. These strategies might be applied for prevention if persons at ALS risk could be identified early enough. Recent research advances indicate that this may happen soon.
Introduction
Amyotrophic lateral sclerosis (ALS) is a fatal, progressive neurodegenerative disease characterized by the loss of motoneuron function in the brain, brainstem, and spinal cord. Approximately, ALS incidence is 1–2.6 and prevalence is six per 100,000 person-years (Talbott et al., 2016), but an adult lifetime risk is estimated at 1 in 400 (Kiernan et al., 2011). The pathogenesis of ALS remains to be defined, so neither effective therapies nor preventive measures have yet been developed.
Despite the intensive research conducted in recent years, the only established risk factors for ALS remain advanced age, male gender, and certain genetic mutations (Ingre et al., 2015; Niccoli et al., 2017). Any human individual living in contemporary world is subjected to a variety of harmful environmental factors, which results in an “age-related cascade of neurodegeneration” (Drechsel et al., 2012). The effects of environmental pathogens accumulate with age (Pamphlett and Kum Jew, 2016; Bektas et al., 2018; Escobar et al., 2019; Ferrucci et al., 2020), which accelerates neurodegeneration cascade. Indeed, the age-related accumulation of heavy metals was observed in human spinal interneurons and motoneurons. Recent studies highlight several pathogenic mechanisms shared between the aging process and ALS, such as oxidative stress, metabolic deficiencies, protein aggregation, decline in mitochondrial and microglial function, and inflammation (Niccoli et al., 2017; Bektas et al., 2018; Elmore et al., 2018; Ising and Heneka, 2018). Therefore, ALS is widely considered an age-related disease (Logroscino et al., 2015; Marin et al., 2018; Pandya and Patani, 2019).
Below, we will present evidence that many anti-aging strategies may ameliorate condition of ALS patients (PALS) or decrease risk of the disease. Therefore, they could be applied also for ALS prevention. Verifying this assumption is at present impossible, since the population to which proposed procedures might be applied is indefinable. However, this situation may change in the near future due to ongoing research. In this respect, we should note the recent study of Kiernan et al. (2019), who hypothesize that adult-onset neurodegenerative conditions might have their roots in early developmental derangements and that unraveling the very early molecular events may be crucial in developing a better understanding of ALS. If this hypothesis was proven to be true, it should open the possibility to determine groups of the highest risk early enough to apply preventive strategies.
Anti-Aging Strategies Related to ALS
Dietary Recommendations
Literature data clearly demonstrate that dietary intervention can positively modulate the aging process and represent a prevention for many age-related diseases (Aiello et al., 2016).
The nutrients promoted as anti-aging foods (Chrysohoou and Stefanadis, 2014; Skrovankova et al., 2015) are rich in antioxidants and anti-inflammatory components, which may lower age-related risk of developing neurodegenerative diseases (Joseph et al., 2009; Yavari et al., 2015). Several phytochemicals normally present in foods, such as polyphenols, have anti-inflammatory effects on microglia (Joseph et al., 2009; Peña-Altamira et al., 2017; Fernandes et al., 2018). In particular, pomegranates contain high levels of antioxidant polyphenolic substances, as compared to other fruits and vegetables (Subash et al., 2014, 2015). It was also suggested that silibinin, a polyphenol isolated from milk thistle, exhibits neuroprotective activity by attenuating oxidative damage and astrocyte activation (Fernandes et al., 2018).
However, the studies on diet as a preventive or modifying factor for ALS are rare. A recently published big study based on the results of a multicenter American project ALS COSMOS (Nieves et al., 2016) is the first one where the associations between diet and patient’s function were evaluated in detail on the sample of 302 PALS. The results have shown that higher intakes of fiber, antioxidants, and carotenes from fruits and vegetables (“good” foods) were associated with better function, measured by ALS Functional Rating Scale Revised (ALSFRS-R) and Forced Vital Capacity scores. Few earlier studies also lend support to the use of healthy foods for prevention of this disease. A decreased ALS risk was observed in individuals whose diet was rich in fiber (Nelson et al., 2000) or vegetables and citrus fruits (Okamoto et al., 2009; Pupillo et al., 2018).
Foods to Avoid
The higher ALS risk was associated with increased dietary uptake of fat and glutamate (Nelson et al., 2000; Huisman et al., 2015). Also, several studies show an association between increased intake of protein from meat and aging-related diseases in elderly population [e.g., (Verburgh, 2015; Pupillo et al., 2018)]. Moreover, WHO recommendations for healthy diet1 postulate restriction of free sugars and trans-fats, including natural trans-fats found in meat and dairy foods from ruminant animals. Interestingly, much earlier in two papers of Patten et al. (Felmus et al., 1976; Pierce-Ruhland and Patten, 1981), the evidence that PALS drank more milk than control subjects was presented. This corresponds well to the results of Nieves et al. (2016) who indicated that “bad” foods, of which the primary component was milk, were negatively and significantly associated with ALSFRS-R score. Dairy product consumption was also found to be associated with greater risk of other age-related diseases (Grant, 1998; Hughes et al., 2017).
Micronutrients and Supplements
Micronutrients (vitamins and minerals) play a central part in metabolism and in the maintenance of tissue function. Their influence on health, cognition, and aging is increasingly supported by experimental studies (Thomas, 2006; Hoeft et al., 2012; González-Sarríase et al., 2013). Although the amount of vitamins that are required for the proper functions of our body is relatively small, deficiency of vitamins and minerals is harmful for health (Lee et al., 2015).
Many micronutrients, such as vitamins B, C, E, D, and A have been shown to be neuroprotective, mostly because of their antioxidant properties, which is important for both anti-aging and ALS prevention (Gasperi et al., 2019; Li et al., 2019).
In the above cited study of Nieves et al. (2016), positive and significant associations with ALS function were found for selected micronutrients such as vitamins B2, B3, B6, E, K, and selenium. Vitamin E supplementation has also been shown in several other studies to correlate with lower ALS rates (Wang et al., 2011) or with better patient’s function (Patel and Hamadeh, 2009; Ngo et al., 2017). In addition, folic acid, coenzyme Q10, and melatonin were indicated as supplements holding promise to alleviate the ALS symptoms (Jacob et al., 2002; Ferrante et al., 2005; Sofic et al., 2005; Kaufmann et al., 2009; Patel and Hamadeh, 2009).
Among micronutrients, special attention has been given to vitamin D, which is known to regulate pathogenic processes involved in aging (Nagpal et al., 2005; Buell and Dawson-Hughes, 2008; DeLuca et al., 2013; Berridge, 2017). The evidence presented in the review of Hayes (2010) strongly indicates the major preventive role of vitamin D in aging.
In PALS, vitamin D blood level was proposed as a reliable prognostic factor of the disease (Karam et al., 2013; Wang et al., 2017). A severe vitamin D deficiency accelerated by four times the rate of ALSFRS-R score decline and was associated with a marked shorter life expectancy (Camu et al., 2014). Karam et al. (2013) have shown that vitamin D supplementation at 2000 international units daily resulted in a smaller decline over a period of 9 months. However, several other studies [e.g., (Yang et al., 2016; Libonati et al., 2017; Trojsi et al., 2020)] did not find any influence of vitamin D on prognosis or progression of ALS.
The most credible explanation of diverse effects of vitamin supplementation seems to be hormesis, which means that the individual dose–response relationship for a substance required for normal physiological function and survival is U-shaped, so that low-dose and high-dose regions have negative effects, while doses in the middle are beneficial for health (Hayes, 2007; Tuohimaa, 2009; Fricker et al., 2018). This aspect is important and should be taken into account in planning future studies. Vitamin D has been shown to be toxic in very high doses (Marcinowska-Suchowierska et al., 2018), while its deficiency has been associated with increased risk of several diseases (Holick and Chen, 2008). Moreover, it should be noted that moderate levels of reactive oxygen species (ROS) are beneficial for health and longevity (Schieber and Navdeep, 2014; Yan, 2014; Pizzino et al., 2017). Thus, antioxidant vitamins might interfere with these benefits (Lee et al., 2015), so finding the proper therapeutical dose seems crucial. However, the difficulty is to determine the proper dose for a particular person (Shenkin, 2006).
Caloric Restriction and Gut Microbiota
Caloric restriction and fasting have long been recognized for their neuroprotective and life span-extending properties (Lanza and Nair, 2010; Lettieri-Barbato et al., 2018; Longo, 2018). Nutritional studies show that aging in animals (Calabrese et al., 2008; Kincaid and Bossy-Wetzel, 2013) and in humans (Wang et al., 2010; Chrysohoou and Stefanadis, 2014; Escobar et al., 2019) can be significantly slowed by dietary restriction.
However, in PALS, malnutrition and weight loss are commonly observed and usually associated with accelerated progression and shorter survival (Desport et al., 2000; Körner et al., 2013). Therefore, the nutritional studies in ALS focus mostly on maintenance of body weight and diets proposed for patients are usually calorie-rich (Rosenfeld and Ellis, 2008; Körner et al., 2013; Wills et al., 2014). Summing up, suggesting low-calorie diet for PALS is not appropriate.
Malnutrition in PALS may be related to diverse factors, such as difficulties in swallowing, disability that restricts access to food, or dysfunction of endogenous processes that regulate hunger, satiety, and appetite (Ngo et al., 2017, 2019). However, in about 50% of patients, the malnutrition is related to hypermetabolism, which is also linked to shorter survival (Muscaritoli et al., 2012; Ahmed et al., 2018). The causes of hypermetabolism in ALS remain undefined, although recently a new hypothesis emerged, concerning the involvement of impaired energy homeostasis in ALS pathophysiology (Ngo et al., 2015), which may be related to hypothalamic defects (Vercruysse et al., 2018).
In this respect, we should mention the recent study that revealed signs of leaky intestine and impaired microbiome in G93A-SOD1 mice (Wu et al., 2015), accompanied by an increased concentration of inflammatory cytokine IL-17A. The latter finding was also reported in a study in PALS (Fiala et al., 2010). When the intestinal microbial homeostasis was restored in these mice, gut integrity was improved and life span was prolonged (Zhang et al., 2017).
We may hypothesize that the observed discordance between presymptomatic increase in total daily energy intake and decrease in body mass index in PALS (Huisman et al., 2015; Ahmed et al., 2018) is due to the leaking intestine. If this hypothesis was proven to be true, the treatment of PALS with appropriate probiotics could restore their energy balance and allow for applying low-calorie or at least low-protein diet. Recent data confirm that dysbiosis of gut microbiota may contribute to ALS pathogenesis and progression and be a potential therapeutic target (De Marchi et al., 2018; Mazzini et al., 2018; Wright et al., 2018).
The crucial role of the gut microbiota in the host physiology and health status is being confirmed in the constantly increasing number of studies [e.g., (Kim and Jazwinski, 2018; Mangiola et al., 2018; Rothschild et al., 2018)]. The aging process deeply affects the structure of the human gut microbiota, as well as its homeostasis with the host’s immune system (Biagi et al., 2010; Dinan and Cryan, 2017; Choi et al., 2018). Age-related gut dysbiosis has also been shown to be linked to other age-related and neurodegenerative disorders (Fang, 2016; Scheperjans, 2016; Rowin et al., 2017). Therefore, supplementation with probiotics may provide novel approaches for both disease prevention and treatment (Yan and Polk, 2011; Duncan and Flint, 2014; Nagpal et al., 2018).
Exercise
Being physically active is a key factor in maintaining health across the life span. Regular moderate-intensity training reduces oxidative stress (Webb et al., 2017; Simioni et al., 2018), decreases inflammatory markers IL-6 and CRP levels in elderly (Monteiro-Junior et al., 2017), helps to preserve cardiovascular fitness and brain function (Hotta et al., 2017; Sayegh and Degani-Costa, 2017; Shibata et al., 2018), and protects individuals from the negative effects of stress on cell aging (Puterman et al., 2010; Rebelo-Marques et al., 2018). In skeletal muscle, it attenuates mitochondrial deficits, which improves muscle function (Nyberg et al., 2012; Joseph et al., 2016; Wyckelsma et al., 2017). On the other hand, strenuous exercises generate high levels of ROS known to cause oxidative stress, activate certain pathogenic pathways, and accelerate aging (Gomez-Cabrera et al., 2009; Sahl et al., 2017).
The physical activity induces the cellular adaptations in the brain, spinal cord, and skeletal muscles that could counteract the oxidative stress complication in ALS. Therefore, it is conceivable that exercise should be beneficial for PALS (Elbasiouny and Schuster, 2011; Kincaid and Bossy-Wetzel, 2013). In G93A-SOD1 mice, moderate but not strenuous exercise delayed the onset of motor deficit (Carreras et al., 2010) and spinal motoneuron death (Deforges et al., 2009). Also, several studies in patients indicated that regular low and moderate exercise can improve functional outcome in ALS (Dalbello-Haas et al., 2008; Patel and Hamadeh, 2009; Braga et al., 2018), whereas exercises of high intensity may be harmful (Patel and Hamadeh, 2009; Wang et al., 2017).
Telomeres
Telomeres are gene sequences present at chromosomal ends, which are responsible for maintaining genome integrity. Aging is accompanied by telomere shortening; thus, telomere length (TL) serves as a biomarker of chronological aging and an early predictor of onset of disease and increased mortality (Shay, 2018; Wang et al., 2018). For any given individual at any age, TL depends on the newborn (initial) value and the magnitude of telomere erosion from birth onward (Heidinger et al., 2012; Shalev et al., 2013).
The rate of telomere shortening may be modified by the psychosocial, environmental, and behavioral factors (Starkweather et al., 2014). All anti-aging strategies described above (such as exercise or eating foods rich in fiber and vitamins) are related to longer telomeres, whereas bad habits (such as eating processed meats and trans-fats, excessive alcohol consumption, or cigarette smoking) are related to shorter telomeres (Valdes et al., 2005; Paul, 2011; Pavanello et al., 2011). Therefore, healthy lifestyle including regular physical activity and ideal diet composition represents major preserving strategies (Seals et al., 2015).
The research on TL clearly indicates that its excessive decrease is associated with the susceptibility to age-related neurodegenerative diseases (Forero et al., 2016; Hou et al., 2019). In line with this, accelerated telomere shortening was observed in leukocytes from sporadic PALS (De Felice et al., 2014) and in ALS mice (Linkus et al., 2016). In contrast, in the recent study (Al Khleifat et al., 2019), ALS was associated with the longer telomeres. On the other hand, in the same study, the longer telomeres in patients correlated with increased survival, which would suggest that longer telomeres played a protective role. These controversies certainly call for further investigation.
Psychological Stress
It is known that psychological stress is a major factor influencing telomere erosion (Epel et al., 2004; O’Donovan et al., 2012; Shalev et al., 2013). It is also significantly associated with lower telomerase activity and higher oxidative stress (Lavretsky and Newhouse, 2012; Mathur et al., 2016). Given that individuals who are exposed to stress during their early years show a fastest erosion rate of TL (Price et al., 2013; Savolainen et al., 2014), early intervention and prevention strategies can potentially slow down aging processes. Healthy lifestyle and environment can help to buffer the deleterious effects of stress on telomere erosion (Puterman et al., 2010; Puterman and Epel, 2012; Schutte and Malouff, 2014).
Unfortunately, the studies investigating association of ALS with psychological stress are very rare. McDonald et al. (1994) stated that psychological status is strongly related to outcome in ALS. The study of Okamoto et al. (2009) found higher ALS risk in patients reporting increased susceptibility to stress and high or moderate level of stress.
Given that PALS are subjected to enormous amount of stress, the interventions targeting resilience to it should be included in ALS modifying and preventive strategies. It should also be mentioned here that neuroprotective mechanisms can be bolstered by intellectual and physical activities.
Therapeutic Measures Applied by Patients (ALS Reversals)
It happens very rarely that a person diagnosed with ALS stops progressing and regains significant motor function. Recently, Harrison et al. (2018) have published a study on 36 cases in whom ALS diagnosis and sustained improvement in functions were confirmed. The control group consisted of PALS without reversal, whose data were accessible from web databases. The authors reported the evident differences in the lifestyle between cases and controls. In particular, the consumption of certain supplements such as curcumin, vitamin D, or fish oil was greater for cases than for controls.
A few cases included in this study were identified from the internet2, where more stories of PALS claiming reversals of the disease can be found. Some of these patients have healed partially, others almost completely. Many live with the disease for 20 years or more. Most of them apply different types of detoxification (including amalgam dental filling removal). Virtually all changed their diet to consume little or no sugar, low carbohydrates and grains, and very high amounts of organic fruits and vegetables. Many of them have regular exercises, including yoga and tai-chi. Unfortunately, it is virtually impossible to confirm all their diagnoses and reversals due to the lack of authorized medical information, although the healing procedures they apply roughly correspond to those described in this review.
All those PALS changed their mental attitude to a positive one and removed or reduced most of their mental stress by applying diverse relaxation techniques, including meditations. However, most of them recall a moment of breakdown when they received the final diagnosis from a doctor and learned that there is practically nothing to be done. Only few patients were strong enough to change their attitude and decide to live with ALS instead of dying from ALS, looking for possible unconventional therapies to ameliorate their condition. The results of the study on ALS reversals (Harrison et al., 2018) prove that some of them were successful. Therefore, it is important that “A positive attitude, with an “expect-the-worst” but “prepare” and “hope-for-the-best” philosophy, is important for boosting patient morale during the management of progressive neurodegenerative diseases” (Jugdutt, 2018) should become the gold standard for providing the patient with the final diagnosis.
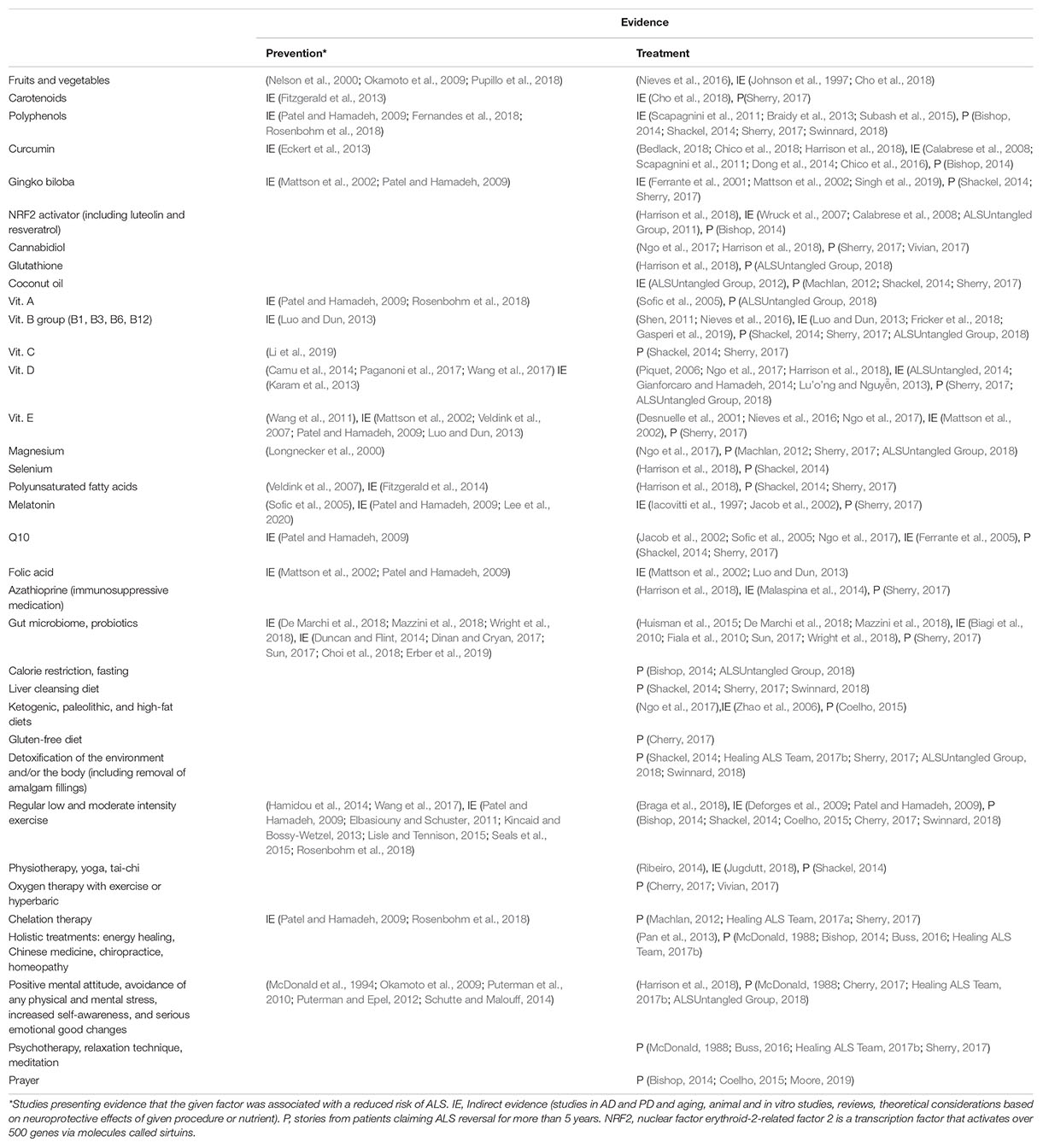
All the potential measures for ALS prevention and treatment are collected in Table 1, which contains references to the papers presenting clinical studies or meta-analyses with positive outcomes. Moreover, the references preceded by “IE” present indirect evidence justifying the neuroprotective action of a given procedure or nutraceutical, and those preceded by “P” contain links to PALS’ web pages.
Summary and Discussion
The anti-aging strategies have been shown in several studies to decrease ALS risk or ameliorate PALS condition. Although there are other studies that did not find such effects, the lack of an evidence is not the evidence of nonexistence. Therefore, in this mini-review, we have concentrated on the positive results. Especially encouraging are stories of patients with reversals, which should be the object of future research efforts focused on establishing the most effective lifestyle practices for maintaining function in ALS (Seals et al., 2015). Moreover, the doses optimal for maximum effects will have to be determined for personalized therapeutic and/or preventive interventions.
It has been shown that the early-life diet plays an essential role in the individual’s health status and longevity, as well as the later development of aging-related chronic diseases (Vaiserman, 2014). Also, the telomeres are the most susceptible to erosion in the first years of life (Price et al., 2013). Therefore, applying healthy lifestyle from the earliest stages of human development is very important. There is an urgent need for actions that would popularize such lifestyle among the wide public, which might prolong the health span, decreasing the risk of ALS and many other age-related diseases. This is an extremely difficult task, but not impossible. The recent study in a group of adolescents has proved that the special psychological intervention, presenting unhealthy dietary choices as incompatible with important values, can change youngsters’ dietary attitudes toward healthy food (Bryan et al., 2019).
Author Contributions
MP wrote the draft. MP, BK, HG, TP-M, participated in collection of the references. MdC supervised the project. All the authors participated in discussions leading to the final version of the manuscript and accepted this version for publication.
Conflict of Interest
The Reviewer DSF declared a past co-authorship with authors SP and JG to the handling Editor.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding
This manuscript was partly supported by grant ONWebDUALS No JPND/01/2015 funded by the Polish National Center of Research and Development in frames of EU Joint Program of Neurodegenerative Research.
Footnotes
- ^ https://www.who.int/news-room/fact-sheets/detail/healthy-diet
- ^ https://healingals.org/ and https://www.alswinners.com/
References
Ahmed, R. M., Dupuis, L., and Kiernan, M. C. (2018). Paradox of amyotrophic lateral sclerosis and energy metabolism. J. Neurol. Neurosurg. Amp Psychiatry 89:1013. doi: 10.1136/jnnp-2018-318428
Aiello, A., Accardi, G., Candore, G., Carruba, G., Davinelli, S., Passarino, G., et al. (2016). Nutrigerontology: a key for achieving successful ageing and longevity. Immunity Ageing 13:17. doi: 10.1186/s12979-016-0071-2
Al Khleifat, A., Iacoangeli, A., Shatunov, A., Fang, T., Sproviero, W., Jones, A. R., et al. (2019). Telomere length is greater in ALS than in controls: a whole genome sequencing study. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 229–234. doi: 10.1080/21678421.2019.1586951
ALSUntangled, T. (2014). ALSUntangled No. 24: vitamin D. Amyotroph. Lateral Scler. Frontotemporal Degener. 15, 318–320. doi: 10.3109/21678421.2014.888871
ALSUntangled Group (2011). ALSUntangled No. 10: luteolin and lutimax. Amyotroph. Lateral Scler. 12, 235–237. doi: 10.3109/17482968.2011.578872
ALSUntangled Group (2018). ALSUntangled No 41: “Eric is winning”. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 157–160. doi: 10.1080/21678421.2017.1350532
Bedlack, R. (2018). ALSUntangled 44: curcumin. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 623–629. doi: 10.1080/21678421.2018.1440738
Bektas, A., Schurman, S. H., Sen, R., and Ferrucci, L. (2018). Aging, inflammation and the environment. Exp. Gerontol. 105, 10–18. doi: 10.1016/j.exger.2017.12.015
Berridge, M. J. (2017). Vitamin D deficiency accelerates ageing and age-related diseases: a novel hypothesis. J. Physiol. 595, 6825–6836. doi: 10.1113/JP274887
Biagi, E., Nylund, L., Candela, M., Ostan, R., Bucci, L., Pini, E., et al. (2010). Through ageing, and beyond: gut microbiota and inflammatory status in seniors and centenarians. PLoS One 5:e10667. doi: 10.1371/journal.pone.0010667
Bishop, S. (2014). ALS Living – The Bishops’ Perspective. Available online at: https://alsliving.wordpress.com/ (accessed April 7, 2020).
Braga, A. C. M., Pinto, A., Pinto, S., and de Carvalho, M. (2018). The role of moderate aerobic exercise as determined by cardiopulmonary exercise testing in ALS. Neurol. Res. Int. 2018:8218697. doi: 10.1155/2018/8218697
Braidy, N., Selvaraju, S., Essa, M. M., Vaishnav, R., Al-Adawi, S., Al-Asmi, A., et al. (2013). Neuroprotective effects of a variety of pomegranate juice extracts against MPTP-induced cytotoxicity and oxidative stress in human primary neurons. Oxid. Med. Cell. Longev. 2013:685909. doi: 10.1155/2013/685909
Bryan, C. J., Yeager, D. S., and Hinojosa, C. P. (2019). A values-alignment intervention protects adolescents from the effects of food marketing. Nat. Hum. Behav. 3, 596–603. doi: 10.1038/s41562-019-0586-6
Buell, J. S., and Dawson-Hughes, B. (2008). Vitamin D and neurocognitive dysfunction: preventing “D”ecline? Mol. Aspects Med. 29, 415–422. doi: 10.1016/j.mam.2008.05.001
Calabrese, V., Cornelius, C., Mancuso, C., Pennisi, G., Calafato, S., Bellia, F., et al. (2008). Cellular stress response: a novel target for chemoprevention and nutritional neuroprotection in aging, neurodegenerative disorders and longevity. Neurochem. Res. 33, 2444–2471. doi: 10.1007/s11064-008-9775-9
Camu, W., Tremblier, B., Plassot, C., Alphandery, S., Salsac, C., Pageot, N., et al. (2014). Vitamin D confers protection to motoneurons and is a prognostic factor of amyotrophic lateral sclerosis. Neurobiol. Aging 35, 1198–1205. doi: 10.1016/j.neurobiolaging.2013.11.005
Carreras, I., Yuruker, S., Aytan, N., Hossain, L., Choi, J.-K., Jenkins, B. G., et al. (2010). Moderate exercise delays the motor performance decline in a transgenic model of ALS. Brain Res. 1313, 192–201. doi: 10.1016/j.brainres.2009.11.051
Cherry, K. (2017). ALS Winners – The Road to Recovery. Available online at: https://www.alswinners.com/ (accessed April 7, 2020).
Chico, L., Ienco, E. C., Bisordi, C., Gerfo, A. L., Petrozzi, L., Petrucci, A., et al. (2018). Amyotrophic lateral sclerosis and oxidative stress: a double-blind therapeutic trial after curcumin supplementation. CNS Neurol. Disord. Drug Targets 17, 767–779. doi: 10.2174/1871527317666180720162029
Chico, L., Ienco, E. C., Bisordi, C., Gerfo, A. L., Schirinzi, E., and Siciliano, G. (2016). Curcumin as an ROS scavenger in amyotrophic lateral sclerosis. React. Oxygen Spec. 2, 339–354.
Cho, K. S., Shin, M., Kim, S., and Lee, S. B. (2018). Recent advances in studies on the therapeutic potential of dietary carotenoids in neurodegenerative diseases. Oxid. Med. Cell. Longev. 2018:4120458. doi: 10.1155/2018/4120458
Choi, J., Hur, T.-Y., and Hong, Y. (2018). Influence of altered gut microbiota composition on aging and aging-related diseases. J. Lifestyle Med. 8, 1–7. doi: 10.15280/jlm.2018.8.1.1
Chrysohoou, C., and Stefanadis, C. (2014). Longevity and diet. Myth or pragmatism? Maturitas 76, 303–307. doi: 10.1016/j.maturitas.2013.09.014
Coelho, B. P. (2015). 18 Comments to “Report of ALS Improvement in Portugal: Bernardo Tells His Story”. Available online at: http://healingwithdrcraig.com/healing-methods/spiritual/report-of-als-improvement-in-portugal-bernardo-pinto-coelho-tells-his-story/#comment-586899 (accessed April 7, 2020).
Dalbello-Haas, V., Florence, J. M., and Krivickas, L. S. (2008). Therapeutic exercise for people with amyotrophic lateral sclerosis or motor neuron disease. Cochrane Database Syst. Rev. 2:Cd005229. doi: 10.1002/14651858.CD005229.pub2
De Felice, B., Annunziata, A., Fiorentino, G., Manfellotto, F., D’Alessandro, R., Marino, R., et al. (2014). Telomerase expression in amyotrophic lateral sclerosis (ALS) patients. J. Hum. Genet. 59, 555–561. doi: 10.1038/jhg.2014.72
De Marchi, F., Mazzini, L., Mogna, L., Amoruso, A., Pane, M., Aloisio, I., et al. (2018). Potential role of gut microbiota in Amyotrophic Lateral Sclerosis (ALS) pathogenesis (P1.318). Neurology 90:318.
Deforges, S., Branchu, J., Biondi, O., Grondard, C., Pariset, C., Lécolle, S., et al. (2009). Motoneuron survival is promoted by specific exercise in a mouse model of amyotrophic lateral sclerosis. J. Physiol. 587, 3561–3572. doi: 10.1113/jphysiol.2009.169748
DeLuca, G. C., Kimball, S. M., Kolasinski, J., Ramagopalan, S. V., and Ebers, G. C. (2013). Review: the role of vitamin D in nervous system health and disease. Neuropathol. Appl. Neurobiol. 39, 458–484. doi: 10.1111/nan.12020
Desnuelle, C., Dib, M., Garrel, C., and Favier, A. (2001). A double-blind, placebo-controlled randomized clinical trial of α-tocopherol (vitamin E) in the treatment of amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 2, 9–18. doi: 10.1080/146608201300079364
Desport, J. C., Preux, P. M., Truong, C. T., Courat, L., Vallat, J. M., and Couratier, P. (2000). Nutritional assessment and survival in ALS patients. Amyotroph. Lateral Scler. Other Motor Neuron Disord. 1, 91–96. doi: 10.1080/14660820050515386
Dinan, T. G., and Cryan, J. F. (2017). Gut instincts: microbiota as a key regulator of brain development, ageing and neurodegeneration. J. Physiol. 595, 489–503. doi: 10.1113/JP273106
Dong, H., Xu, L., Wu, L., Wang, X., Duan, W., Li, H., et al. (2014). Curcumin abolishes mutant TDP-43 induced excitability in a motoneuron-like cellular model of ALS. Neuroscience 272, 141–153. doi: 10.1016/j.neuroscience.2014.04.032
Drechsel, D. A., Estevez, A. G., Barbeito, L., and Beckman, J. S. (2012). Nitric oxide-mediated oxidative damage and the progressive demise of motor neurons in ALS. Neurotox. Res. 22, 251–264. doi: 10.1007/s12640-012-9322-y
Duncan, S. H., and Flint, H. J. (2014). Probiotics and prebiotics and health in ageing populations. Maturitas 75, 44–50. doi: 10.1016/j.maturitas.2013.02.004
Eckert, G. P., Schiborr, C., Hagl, S., Abdel-Kader, R., Müller, W. E., Rimbach, G., et al. (2013). Curcumin prevents mitochondrial dysfunction in the brain of the senescence-accelerated mouse-prone 8. Neurochem. Int. 62, 595–602. doi: 10.1016/j.neuint.2013.02.014
Elbasiouny, S. M., and Schuster, J. (2011). The effect of training on motoneuron survival in amyotrophic lateral sclerosis: which motoneuron type is saved? Front. Physiol. 2:18. doi: 10.3389/fphys.2011.00018
Elmore, M. R. P., Hohsfield, L. A., Kramár, E. A., Soreq, L., Lee, R. J., Pham, S. T., et al. (2018). Replacement of microglia in the aged brain reverses cognitive, synaptic, and neuronal deficits in mice. Aging Cell 17:e12832. doi: 10.1111/acel.12832
Epel, E. S., Blackburn, E. H., Lin, J., Dhabhar, F. S., Adler, N. E., Morrow, J. D., et al. (2004). Accelerated telomere shortening in response to life stress. Proc. Natl. Acad. Sci. U.S.A. 101, 17312–17315. doi: 10.1073/pnas.0407162101
Erber, A. C., Cetin, H., Berry, D., and Schernhammer, E. S. (2019). The role of gut microbiota, butyrate and proton pump inhibitors in amyotrophic lateral sclerosis: a systematic review. Int. J. Neurosci. doi: 10.1080/00207454.2019.1702549 [Epub ahead of print],
Escobar, K. A., Cole, N. H., Mermier, C. M., and VanDusseldorp, T. A. (2019). Autophagy and aging: maintaining the proteome through exercise and caloric restriction. Aging Cell 18:e12876. doi: 10.1111/acel.12876
Fang, X. (2016). Potential role of gut microbiota and tissue barriers in Parkinson’s disease and amyotrophic lateral sclerosis. Int. J. Neurosci. 126, 771–776. doi: 10.3109/00207454.2015.1096271
Felmus, M. T., Patten, B. M., and Swanke, L. (1976). Antecedent events in amyotrophic lateral sclerosis. Neurology 26, 167–172. doi: 10.1159/000110782
Fernandes, V., Sharma, D., Kalia, K., and Tiwari, V. (2018). Neuroprotective effects of silibinin: an in silico and in vitro study. Int. J. Neurosci. 128, 935–945. doi: 10.1080/00207454.2018.1443926
Ferrante, K. L., Shefner, J., Zhang, H., Betensky, R., O’Brien, M., Yu, H., et al. (2005). Tolerance of high-dose (3,000 mg/day) coenzyme Q10 in ALS. Neurology 65, 1834–1836. doi: 10.1212/01.wnl.0000187070.35365.d7
Ferrante, R. J., Klein, A. M., Dedeoglu, A., and Beal, M. F. (2001). Therapeutic efficacy of EGb761 (Gingko biloba extract) in a transgenic mouse model of amyotrophic lateral sclerosis. J. Mol. Neurosci. 17, 89–96. doi: 10.1385/jmn:17:1:89
Ferrucci, L., Gonzalez-Freire, M., Fabbri, E., Simonsick, E., Tanaka, T., Moore, Z., et al. (2020). Measuring biological aging in humans: a quest. Aging Cell 19:e13080. doi: 10.1111/acel.13080
Fiala, M., Chattopadhay, M., Cava, A. La, Tse, E., Liu, G. H., Lourenco, E., et al. (2010). IL-17A is increased in the serum and in spinal cord CD8 and mast cells of ALS patients. J. Neuroinflamm. 7:76. doi: 10.1186/1742-2094-7-76
Fitzgerald, K. C., O’Reilly, E. J., Falcone, G. J., McCullough, M. L., Park, Y., Kolonel, L. N., et al. (2014). Dietary omega-3 polyunsaturated fatty acid intake and risk for amyotrophic lateral sclerosis. JAMA Neurol. 71, 1102–1110. doi: 10.1001/jamaneurol.2014.1214
Fitzgerald, K. C., O’Reilly, E. J., Fondell, E., Falcone, G. J., McCullough, M. L., Park, Y., et al. (2013). Intakes of vitamin C and carotenoids and risk of amyotrophic lateral sclerosis: pooled results from 5 cohort studies. Ann. Neurol. 73, 236–245. doi: 10.1002/ana.23820
Forero, D. A., González-Giraldo, Y., López-Quintero, C., Castro-Vega, L. J., Barreto, G. E., and Perry, G. (2016). Meta-analysis of telomere length in Alzheimer’s disease. J. Gerontol. Ser. A 71, 1069–1073. doi: 10.1093/gerona/glw053
Fricker, R. A., Green, E. L., Jenkins, S. I., and Griffin, S. M. (2018). The influence of nicotinamide on health and disease in the central nervous system. Int. J. Tryptophan Res. 11, 1–11. doi: 10.1177/1178646918776658
Gasperi, V., Sibilano, M., Savini, I., and Catani, M. V. (2019). Niacin in the central nervous system: an update of biological aspects and clinical applications. Int. J. Mol. Sci. 20:E974. doi: 10.3390/ijms20040974
Gianforcaro, A., and Hamadeh, M. J. (2014). Vitamin D as a potential therapy in amyotrophic lateral sclerosis. CNS Neurosci. Ther. 20, 101–111. doi: 10.1111/cns.12204
Gomez-Cabrera, M. C., Vina, J., and Ji, L. L. (2009). Interplay of oxidants and antioxidants during exercise: implications for muscle health. Phys. Sportsmed. 37, 116–123. doi: 10.3810/psm.2009.12.1749
González-Sarríase, A., Larrosa, M., García-Conesa, M. T., Tomás-Barberán, F. A., and Espín, J. C. (2013). Nutraceuticals for older people: facts, fictions and gaps in knowledge. Maturitas 75, 313–334. doi: 10.1016/j.maturitas.2013.05.006
Grant, W. B. (1998). Milk and other dietary influences on coronary heart disease. Altern. Med. Rev. 3, 281–294.
Hamidou, B., Couratier, P., Besancon, C., Nicol, M., Preux, P. M., and Marin, B. (2014). Epidemiological evidence that physical activity is not a risk factor for ALS. Eur. J. Epidemiol. 29, 459–475. doi: 10.1007/s10654-014-9923-2
Harrison, D., Mehta, P., van Es, M. A., Stommel, E., Drory, V. E., Nefussy, B., et al. (2018). “ALS reversals”: demographics, disease characteristics, treatments, and co-morbidities. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 495–499. doi: 10.1080/21678421.2018.1457059
Hayes, D. P. (2007). Nutritional hormesis. Eur. J. Clin. Nutr. 61, 147–159. doi: 10.1038/sj.ejcn.1602507
Healing ALS Team (2017a). Cathy Cummins Lives a Full Life Despite ALS Diagnosis. Available online at: https://healingals.org/cathy-cummins-lives-a-full-life-despite-als-diagnosis/ (accessed April 7, 2020).
Healing ALS Team (2017b). Joyce Now 83, 29 Years After ALS Diagnosis. Available online at: https://healingals.org/2017/05/ (accessed April 7, 2020).
Heidinger, B. J., Blount, J. D., Boner, W., Griffiths, K., Metcalfe, N. B., and Monaghan, P. (2012). Telomere length in early life predicts lifespan. Proc. Natl. Acad. Sci. U.S.A. 109, 1743–1748. doi: 10.1073/pnas.1113306109
Hoeft, B., Weber, P., and Eggersdorfer, M. (2012). Micronutrients – a global perspective on intake, health benefits and economics. Int. J. Vitam. Nutr. Res. 82, 316–320. doi: 10.1024/0300-9831/a000125
Holick, M. F., and Chen, T. C. (2008). Vitamin D deficiency: a worldwide problem with health consequences. Am. J. Clin. Nutr. 87, 1080s–1086s. doi: 10.1093/ajcn/87.4.1080S
Hotta, K., Chen, B., Behnke, B. J., Ghosh, P., Stabley, J. N., Bramy, J. A., et al. (2017). Exercise training reverses age-induced diastolic dysfunction and restores coronary microvascular function. J. Physiol. 595, 3703–3719. doi: 10.1113/JP274172
Hou, Y., Dan, X., Babbar, M., Wei, Y., Hasselbalch, S. G., Croteau, D. L., et al. (2019). Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 15, 565–581. doi: 10.1038/s41582-019-0244-7
Hughes, K. C., Gao, X., Kim, I. Y., Wang, M., Weisskopf, M. G., Schwarzschild, M. A., et al. (2017). Intake of dairy foods and risk of Parkinson disease. Neurology 89, 46–52. doi: 10.1212/WNL.0000000000004057
Huisman, M. B., Seelen, M., van Doormaal, P. C., de Jong, S. W., de Vries, J. H., van der Kooi, A. J., et al. (2015). Effect of presymptomatic body mass index and consumption of fat and alcohol on amyotrophic lateral sclerosis. JAMA Neurol. 72, 1155–1162. doi: 10.1001/jamaneurol.2015.1584
Iacovitti, L., Stull, N. D., and Johnston, K. (1997). Melatonin rescues dopamine neurons from cell death in tissue culture models of oxidative stress. Brain Res. 768, 317–326. doi: 10.1016/s0006-8993(97)00668-9
Ingre, C., Roos, P. M., Piehl, F., Kamel, F., and Fang, F. (2015). Risk factors for amyotrophic lateral sclerosis. Clin. Epidemiol. 7, 181–193. doi: 10.2147/CLEP.S37505
Ising, C., and Heneka, M. T. (2018). Functional and structural damage of neurons by innate immune mechanisms during neurodegeneration. Cell Death Dis. 9:120. doi: 10.1038/s41419-017-0153-x
Jacob, S., Poeggeler, B., Weishaupt, J. H., Siren, A. L., Hardeland, R., Bahr, M., et al. (2002). Melatonin as a candidate compound for neuroprotection in amyotrophic lateral sclerosis (ALS): high tolerability of daily oral melatonin administration in ALS patients. J. Pineal Res. 33, 186–187. doi: 10.1034/j.1600-079x.2002.02943.x
Johnson, W. J., Phillips, M. C., and Rothblat, G. H. (1997). Lipoproteins and cellular cholesterol homeostasis. Subcell Biochem. 28, 235–276.
Joseph, A.-M., Adhihetty, P. J., and Leeuwenburgh, C. (2016). Beneficial effects of exercise on age-related mitochondrial dysfunction and oxidative stress in skeletal muscle. J. Physiol. 594, 5105–5123. doi: 10.1113/JP270659
Joseph, J., Cole, G., Head, E., and Ingram, D. (2009). Nutrition, brain aging, and neurodegeneration. J. Neurosci. 29:12795. doi: 10.1523/JNEUROSCI.3520-09.2009
Jugdutt, B. I. (2018). Can yoga and physiotherapy benefit patients in weight loss programs and with ALS? J. Yoga & Physio. 4, 555643.
Karam, C., Barrett, M. J., Imperato, T., MacGowan, D. J., and Scelsa, S. (2013). Vitamin D deficiency and its supplementation in patients with amyotrophic lateral sclerosis. J. Clin. Neurosci. 20, 1550–1553. doi: 10.1016/j.jocn.2013.01.011
Kaufmann, P., Thompson, J. L., Levy, G., Buchsbaum, R., Shefner, J., Krivickas, L. S., et al. (2009). Phase II trial of CoQ10 for ALS finds insufficient evidence to justify phase III. Ann. Neurol. 66, 235–244. doi: 10.1002/ana.21743
Kiernan, M. C., Vucic, S., Cheah, B. C., Turner, M. R., Eisen, A., Hardiman, O., et al. (2011). Amyotrophic lateral sclerosis. Lancet 377, 942–955. doi: 10.1016/s0140-6736(10)61156-7
Kiernan, M. C., Ziemann, U., and Eisen, A. (2019). Amyotrophic lateral sclerosis: origins trace to impaired balance between neural excitation and inhibition in the neonatal period. Muscle Nerve 60, 232–235. doi: 10.1002/mus.26617
Kim, S., and Jazwinski, S. M. (2018). The gut microbiota and healthy aging: a mini-review. Gerontology 64, 513–520. doi: 10.1159/000490615
Kincaid, B., and Bossy-Wetzel, E. (2013). Forever young: SIRT3 a shield against mitochondrial meltdown, aging, and neurodegeneration. Front. Aging Neurosci. 5:48. doi: 10.3389/fnagi.2013.00048
Körner, S., Hendricks, M., Kollewe, K., Zapf, A., Dengler, R., Silani, V., et al. (2013). Weight loss, dysphagia and supplement intake in patients with amyotrophic lateral sclerosis (ALS): impact on quality of life and therapeutic options. BMC Neurol. 13:84. doi: 10.1186/1471-2377-13-84
Lanza, I., and Nair, K. (2010). Mitochondrial function as a determinant of life span. Pflügers Arch. Eur. J. Physiol. 459, 277–289. doi: 10.1007/s00424-009-0724-5
Lavretsky, H., and Newhouse, P. A. (2012). Stress, inflammation, and aging. Am. J. Geriatr. Psychiatry 20, 729–733. doi: 10.1097/JGP.0b013e31826573cf
Lee, D., Hwang, W., Artan, M., Jeong, D.-E., and Lee, S.-J. (2015). Effects of nutritional components on aging. Aging Cell 14, 8–16. doi: 10.1111/acel.12277
Lee, J. H., Yoon, Y. M., Song, K.-H., Noh, H., and Lee, S. H. (2020). Melatonin suppresses senescence-derived mitochondrial dysfunction in mesenchymal stem cells via the HSPA1L–mitophagy pathway. Aging Cell 19:e13111. doi: 10.1111/acel.13111
Lettieri-Barbato, D., Cannata, S. M., Casagrande, V., Ciriolo, M. R., and Aquilano, K. (2018). Time-controlled fasting prevents aging-like mitochondrial changes induced by persistent dietary fat overload in skeletal muscle. PLoS One 13:e0195912. doi: 10.1371/journal.pone.0195912
Li, L., Li, Y., Fan, Z., Wang, X., Li, Z., Wen, J., et al. (2019). Ascorbic acid facilitates neural regeneration after sciatic nerve crush injury. Front. Cell. Neurosci. 13:108. doi: 10.3389/fncel.2019.00108
Libonati, L., Onesti, E., Gori, M. C., Ceccanti, M., Cambieri, C., Fabbri, A., et al. (2017). Vitamin D in amyotrophic lateral sclerosis. Funct. Neurol. 32, 35–40.
Linkus, B., Wiesner, D., Meßner, M., Karabatsiakis, A., Scheffold, A., Rudolph, K. L., et al. (2016). Telomere shortening leads to earlier age of onset in ALS mice. Aging 8, 382–393. doi: 10.18632/aging.100904
Lisle, S., and Tennison, M. (2015). Amyotrophic lateral sclerosis: the role of exercise. Curr. Sports Med. Rep. 14, 45–46. doi: 10.1249/JSR.0000000000000122
Logroscino, G., Tortelli, R., Rizzo, G., Marin, B., Preux, P. M., and Malaspina, A. (2015). Amyotrophic lateral sclerosis: an aging-related disease. Curr. Geriatr. Rep. 4, 142–153. doi: 10.1007/s13670-015-0127-8
Longnecker, M. P., Kamel, F., Umbach, D. M., Munsat, T. L., Shefner, J. M., Lansdell, L. W., et al. (2000). Dietary intake of calcium, magnesium and antioxidants in relation to risk of amyotrophic lateral sclerosis. Neuroepidemiology 19, 210–216. doi: 10.1159/000026258
Longo, D. V. (2018). Programmed longevity, youthspan, and juventology. Aging Cell 18:e12843. doi: 10.1111/acel.12843
Luo, J., and Dun, N. (2013). Should homocysteine be a therapeutic target for neurological disorders? Brain Disord Ther. 2:e107. doi: 10.4172/2168-975X.1000e107
Lu’o’ng, K. V. Q., and Nguyễn, L. T. H. (2013). Roles of vitamin D in amyotrophic lateral sclerosis: possible genetic and cellular signaling mechanisms. Mol. Brain 6:16. doi: 10.1186/1756-6606-6-16
Machlan, C. B. (2012). ALS Patient Two Year Update On Coconut Oil and Magnesium Chloride. Available online at: http://coconutketones.blogspot.com/2012/05/als-patient-two-year-update-on-coconut.html (accessed April 7, 2020).
Malaspina, A., Puentes, F., and Amor, S. (2014). Disease origin and progression in amyotrophic lateral sclerosis: an immunology perspective. Int. Immunol. 27, 117–129. doi: 10.1093/intimm/dxu099
Mangiola, F., Nicoletti, A., Gasbarrini, A., and Ponziani, F. R. (2018). Gut microbiota and aging. Eur. Rev. Med. Pharmacol. Sci. 22, 7404–7413. doi: 10.26355/eurrev_201811_16280
Marcinowska-Suchowierska, E., Kupisz-Urbanska, M., Lukaszkiewicz, J., Pludowski, P., and Jones, G. (2018). Vitamin D toxicity-A clinical perspective. Front. Endocrinol. (Lausanne) 9:550. doi: 10.3389/fendo.2018.00550
Marin, B., Fontana, A., Arcuti, S., Copetti, M., Boumediene, F., Couratier, P., et al. (2018). Age-specific ALS incidence: a dose-response meta-analysis. Eur. J. Epidemiol. 33, 621–634. doi: 10.1007/s10654-018-0392-x
Mathur, M. B., Epel, E., Kind, S., Desai, M., Parks, C. G., Sandler, D. P., et al. (2016). Perceived stress and telomere length: a systematic review, meta-analysis, and methodologic considerations for advancing the field. Brain Behav. Immunity 54, 158–169. doi: 10.1016/j.bbi.2016.02.002
Mattson, M. P., Chan, S. L., and Duan, W. (2002). Modification of brain aging and neurodegenerative disorders by genes, diet, and behavior. Physiol. Rev. 82, 637–672. doi: 10.1152/physrev.00004.2002
Mazzini, L., Mogna, L., Marchi, F. De, Amoruso, A., Pane, M., Aloisio, I., et al. (2018). Potential role of gut microbiota in ALS pathogenesis and possible novel therapeutic strategies. J. Clin. Gastroenterol. 2017, S68–S70. doi: 10.1097/mcg.0000000000001042
McDonald, E. (1988). Another Perspective of ALS. Holistic Medicine. Available online at: http://ahha.org/selfhelp-articles/another-perspective-of-als/ (accessed April 7, 2020).
McDonald, E. R., Wiedenfeld, S. A., Hillel, A., Carpenter, C. L., and Walter, R. A. (1994). Survival in amyotrophic lateral sclerosis. The role of psychological factors. Arch. Neurol. 51, 17–23. doi: 10.1001/archneur.1994.00540130027010
Monteiro-Junior, R. S., de Tarso Maciel-Pinheiro, P., da Matta Mello Portugal, E., da Silva Figueiredo, L. F., Terra, R., Carneiro, L. S. F., et al. (2017). Effect of exercise on inflammatory profile of older persons: systematic review and meta-analyses. J. Phys. Activity Health 15, 64–71. doi: 10.1123/jpah.2016-0735
Moore, W. (2019). Wendy Moore – Healed of ALS. Available online at: https://prayingmedic.com/category/als-healed/ (accessed April 7, 2020).
Muscaritoli, M., Kushta, I., Molfino, A., Inghilleri, M., Sabatelli, M., and Rossi Fanelli, F. (2012). Nutritional and metabolic support in patients with amyotrophic lateral sclerosis. Nutrition 28, 959–966. doi: 10.1016/j.nut.2012.01.011
Nagpal, R., Mainali, R., Ahmadi, S., Wang, S., Singh, R., Kavanagh, K., et al. (2018). Gut microbiome and aging: physiological and mechanistic insights. Nutr. Healthy Aging 4, 267–285. doi: 10.3233/NHA-170030
Nagpal, S., Na, S., and Rathnachalam, R. (2005). Noncalcemic actions of vitamin D receptor ligands. Endocr. Rev. 26, 662–687. doi: 10.1210/er.2004-0002
Nelson, L. M., Matkin, C., Longstreth, W. T. Jr., and McGuire, V. (2000). Population-based case-control study of amyotrophic lateral sclerosis in western Washington State. II. Diet. Am. J. Epidemiol. 151, 164–173. doi: 10.1093/oxfordjournals.aje.a010184
Ngo, S. T., Mi, J. D., Henderson, R. D., McCombe, P. A., and Steyn, F. J. (2017). Exploring targets and therapies for amyotrophic lateral sclerosis: current insights into dietary interventions. Degener. Neurol. Neuromuscul. Dis. 7, 95–108. doi: 10.2147/dnnd.s120607
Ngo, S. T., Steyn, F. J., Huang, L., Mantovani, S., Pfluger, C. M., Woodruff, T. M., et al. (2015). Altered expression of metabolic proteins and adipokines in patients with amyotrophic lateral sclerosis. J. Neurol. Sci. 357, 22–27. doi: 10.1016/j.jns.2015.06.053
Ngo, S. T., van Eijk, R. P. A., Chachay, V., van den Berg, L. H., McCombe, P. A., Henderson, R. D., et al. (2019). Loss of appetite is associated with a loss of weight and fat mass in patients with amyotrophic lateral sclerosis. Amyotroph. Lateral Scler. Frontotemporal Degener. 20, 497–505. doi: 10.1080/21678421.2019.1621346
Niccoli, T., Partridge, L., and Isaacs, A. M. (2017). Ageing as a risk factor for ALS/FTD. Hum. Mol. Genet. 26, R105–R113. doi: 10.1093/hmg/ddx247
Nieves, J. W., Gennings, C., Factor-Litvak, P., Hupf, J., Singleton, J., Sharf, V., et al. (2016). Association between dietary intake and function in amyotrophic lateral sclerosis. JAMA Neurol. 73, 1425–1432. doi: 10.1001/jamaneurol.2016.3401
Nyberg, M., Blackwell, J. R., Damsgaard, R., Jones, A. M., Hellsten, Y., and Mortensen, S. P. (2012). Lifelong physical activity prevents an age-related reduction in arterial and skeletal muscle nitric oxide bioavailability in humans. J. Physiol. 590, 5361–5370. doi: 10.1113/jphysiol.2012.239053
O’Donovan, A., Tomiyama, A. J., Lin, J., Puterman, E., Adler, N. E., Kemeny, M., et al. (2012). Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav. Immunity 26, 573–579. doi: 10.1016/j.bbi.2012.01.007
Okamoto, K., Kihira, T., Kondo, T., Kobashi, G., Washio, M., Sasaki, S., et al. (2009). Lifestyle factors and risk of amyotrophic lateral sclerosis: a case-control study in Japan. Ann. Epidemiol. 19, 359–364. doi: 10.1016/j.annepidem.2009.01.015
Paganoni, S., Macklin, E. A., Karam, C., Yu, H., Gonterman, F., Fetterman, K. A., et al. (2017). Vitamin D levels are associated with gross motor function in amyotrophic lateral sclerosis. Muscle Nerve 56, 726–731. doi: 10.1002/mus.25555
Pamphlett, R., and Kum Jew, S. (2016). Age-related uptake of heavy metals in human spinal interneurons. PLoS One 11:e0162260. doi: 10.1371/journal.pone.0162260
Pan, W., Chen, X., Bao, J., Bai, Y., Lu, H., Wang, Q., et al. (2013). The use of integrative therapies in patients with amyotrophic lateral sclerosis in Shanghai, China. Evid. Based Comp. Altern. Med. 2013:613596. doi: 10.1155/2013/613596
Pandya, V. A., and Patani, R. (2019). Decoding the relationship between ageing and amyotrophic lateral sclerosis: a cellular perspective. Brain 143, 1057–1072. doi: 10.1093/brain/awz360
Patel, B. P., and Hamadeh, M. J. (2009). Nutritional and exercise-based interventions in the treatment of amyotrophic lateral sclerosis. Clin. Nutr. 28, 604–617. doi: 10.1016/j.clnu.2009.06.002
Paul, L. (2011). Diet, nutrition and telomere length. J. Nutr. Biochem. 22, 895–901. doi: 10.1016/j.jnutbio.2010.12.001
Pavanello, S., Hoxha, M., Dioni, L., Bertazzi, P. A., Snenghi, R., Nalesso, A., et al. (2011). Shortened telomeres in individuals with abuse in alcohol consumption. Int. J. Cancer 129, 983–992. doi: 10.1002/ijc.25999
Peña-Altamira, E., Petralla, S., Massenzio, F., Virgili, M., Bolognesi, M. L., and Monti, B. (2017). Nutritional and pharmacological strategies to regulate microglial polarization in cognitive aging and Alzheimer’s disease. Front. Aging Neurosci. 9:175. doi: 10.3389/fnagi.2017.00175
Pierce-Ruhland, R., and Patten, B. M. (1981). Repeat study of antecedent events in motor neuron disease. Ann. Clin. Res. 13, 102–107.
Piquet, M. A. (2006). Nutritional approach for patients with amyotrophic lateral sclerosis. Rev. Neurol. (Paris) 162, 4S177–4S184.
Pizzino, G., Irrera, N., Cucinotta, M., Pallio, G., Mannino, F., Arcoraci, V., et al. (2017). Oxidative stress: harms and benefits for human health. Oxid. Med. Cell. Longev. 2017:13. doi: 10.1155/2017/8416763
Price, L. H., Kao, H. T., Burgers, D. E., Carpenter, L. L., and Tyrka, A. R. (2013). Telomeres and early-life stress: an overview. Biol. Psychiatry 73, 15–23. doi: 10.1016/j.biopsych.2012.06.025
Pupillo, E., Bianchi, E., Chiò, A., Casale, F., Zecca, C., Tortelli, R., et al. (2018). Amyotrophic lateral sclerosis and food intake. Amyotroph. Lateral Scler. Frontotemporal Degener. 19, 267–274. doi: 10.1080/21678421.2017.1418002
Puterman, E., and Epel, E. (2012). An intricate dance: life experience, multisystem resiliency, and rate of telomere decline throughout the lifespan. Soc. Pers. Psychol. Comp. 6, 807–825. doi: 10.1111/j.1751-9004.2012.00465.x
Puterman, E., Lin, J., Blackburn, E., O’Donovan, A., Adler, N., and Epel, E. (2010). The power of exercise: buffering the effect of chronic stress on telomere length. PLoS One 5:e10837. doi: 10.1371/journal.pone.0010837
Rebelo-Marques, A., Lages, A. De Sousa, Andrade, R., Ribeiro, C. F., Mota-Pinto, A., Carrilho, F., et al. (2018). Aging hallmarks: the benefits of physical exercise. Front. Endocrinol. (Lausanne) 9:258. doi: 10.3389/fendo.2018.00258
Ribeiro, S. (2014). Iyengar yoga therapy as an intervention for cramp management in individuals with amyotrophic lateral sclerosis: three case reports. J. Altern. Complement Med. 20, 322–326. doi: 10.1089/acm.2013.0340
Rosenbohm, A., Nagel, G., Peter, R. S., Brehme, T., Koenig, W., Dupuis, L., et al. (2018). Association of serum retinol-binding protein 4 concentration with risk for and prognosis of amyotrophic lateral sclerosis. JAMA Neurol. 75, 600–607. doi: 10.1001/jamaneurol.2017.5129
Rosenfeld, J., and Ellis, A. (2008). Nutrition and dietary supplements in motor neuron disease. Phys. Med. Rehabil. Clin. 19, 573–589. doi: 10.1016/j.pmr.2008.03.001
Rothschild, D., Weissbrod, O., Barkan, E., Kurilshikov, A., Korem, T., Zeevi, D., et al. (2018). Environment dominates over host genetics in shaping human gut microbiota. Nature 555:210. doi: 10.1038/nature25973
Rowin, J., Xia, Y., Jung, B., and Sun, J. (2017). Gut inflammation and dysbiosis in human motor neuron disease. Physiol. Rep. 5:e13443. doi: 10.14814/phy2.13443
Sahl, R. E., Andersen, P. R., Gronbaek, K., Morville, T. H., Rosenkilde, M., Rasmusen, H. K., et al. (2017). Repeated excessive exercise attenuates the anti-inflammatory effects of exercise in older men. Front. Physiol. 8:407. doi: 10.3389/fphys.2017.00407
Savolainen, K., Eriksson, J. G., Kananen, L., Kajantie, E., Pesonen, A. K., Heinonen, K., et al. (2014). Associations between early life stress, self-reported traumatic experiences across the lifespan and leukocyte telomere length in elderly adults. Biol. Psychol. 97, 35–42. doi: 10.1016/j.biopsycho.2014.02.002
Sayegh, A. L. C., and Degani-Costa, L. H. (2017). Effects of exercise training on endothelial and diastolic age-related dysfunctions: a new view of an old problem. J. Physiol. 595, 4591–4592. doi: 10.1113/jp274531
Scapagnini, G., Vasto, S., Abraham, N. G., Caruso, C., Zella, D., and Fabio, G. (2011). Modulation of Nrf2/ARE pathway by food polyphenols: a nutritional neuroprotective strategy for cognitive and neurodegenerative disorders. Mol. Neurobiol. 44, 192–201. doi: 10.1007/s12035-011-8181-5
Scheperjans, F. (2016). Can microbiota research change our understanding of neurodegenerative diseases? Neurodegener. Dis. Manag. 6, 81–85. doi: 10.2217/nmt-2015-0012
Schieber, M., and Navdeep, S. (2014). Chandel. ROS function in redox signaling and oxidative stress. Curr. Biol. 24, R453–R462. doi: 10.1016/j.cub.2014.03.034
Schutte, N. S., and Malouff, J. M. (2014). A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrinology 42, 45–48. doi: 10.1016/j.psyneuen.2013.12.017
Seals, D. R., Justice, J. N., and LaRocca, T. J. (2015). Physiological geroscience: targeting function to increase healthspan and achieve optimal longevity. J. Physiol. 594, 2001–2024. doi: 10.1113/jphysiol.2014.282665
Shackel, S. (2014). ALS - Amyotrophic Lateral Sclerosis and Motor Neuron Disease - MND. Available online at: http://www.shackel.org/home.html (accessed April 7, 2020).
Shalev, I., Entringer, S., Wadhwa, P. D., Wolkowitz, O. M., Puterman, E., Lin, J., et al. (2013). Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38, 1835–1842. doi: 10.1016/j.psyneuen.2013.03.010
Shay, J. W. (2018). Telomeres and aging. Curr. Opin. Cell Biol. 52, 1–7. doi: 10.1016/j.ceb.2017.12.001
Shen, L. (2011). Further support for vitamin D supplement in delaying the progression of ALS. Med. Hypotheses 77:698. doi: 10.1016/j.mehy.2011.07.057
Shenkin, A. (2006). Micronutrients in health and disease. Postgrad. Med. J. 82, 559–567. doi: 10.1136/pgmj.2006.047670
Sherry, S. (2017). Stephen Sherry ALS Survivor since 1999. Available online at: https://healingals.org/stephen-sherry-als-survivor-since-1999/ (accessed April 7, 2020).
Shibata, S., Fujimoto, N., Hastings Jeffrey, L., Carrick-Ranson, G., Bhella Paul, S., Hearon Christopher, M., et al. (2018). The effect of lifelong exercise frequency on arterial stiffness. J. Physiol. 596, 2783–2795. doi: 10.1113/JP275301
Simioni, C., Zauli, G., Martelli, A. M., Vitale, M., Sacchetti, G., Gonelli, A., et al. (2018). Oxidative stress: role of physical exercise and antioxidant nutraceuticals in adulthood and aging. Oncotarget 9, 17181–17198. doi: 10.18632/oncotarget.24729
Singh, S. K., Srivastav, S., Castellani, R. J., Plascencia-Villa, G., and Perry, G. (2019). Neuroprotective and antioxidant effect of ginkgo biloba extract against AD and other neurological disorders. Neurotherapeutics 16, 666–674. doi: 10.1007/s13311-019-00767-8
Skrovankova, S., Sumczynski, D., Mlcek, J., Jurikova, T., and Sochor, J. (2015). Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 16, 24673–24706. doi: 10.3390/ijms161024673
Sofic, E., Rimpapa, Z., Kundurovic, Z., Sapcanin, A., Tahirovic, I., Rustembegovic, A., et al. (2005). Antioxidant capacity of the neurohormone melatonin. J. Neural Trans. 112, 349–358.
Starkweather, A. R., Alhaeeri, A. A., Montpetit, A., Brumelle, J., Filler, K., Montpetit, M., et al. (2014). An integrative review of factors associated with telomere length and implications for biobehavioral research. Nurs. Res. 63, 36–50. doi: 10.1097/nnr.0000000000000009
Subash, S., Braidy, N., Essa, M. M., Zayana, A. B., Ragini, V., Al-Adawi, S., et al. (2015). Long-term (15 mo) dietary supplementation with pomegranates from Oman attenuates cognitive and behavioral deficits in a transgenic mice model of Alzheimer’s disease. Nutrition 31, 223–229. doi: 10.1016/j.nut.2014.06.004
Subash, S., Essa, M. M., Al-Asmi, A., Al-Adawi, S., Vaishnav, R., Braidy, N., et al. (2014). Pomegranate from oman alleviates the brain oxidative damage in transgenic mouse model of Alzheimer’s disease. J. Tradit. Complement Med. 4, 232–238. doi: 10.4103/2225-4110.139107
Sun, J. (2017). Commentary: target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. J. Neurol. Neuromed. 2, 13–15. doi: 10.29245/2572.942x/2017/6.1136
Swinnard, D. (2018). ALS Since 1997 Still Golfing in 2018. Available online at: https://healingals.org/derek-1997/ (accessed April 7, 2020).
Talbott, E. O., Malek, A. M., and Lacomis, D. (2016). The epidemiology of amyotrophic lateral sclerosis. Handb. Clin. Neurol. 138, 225–238. doi: 10.1016/B978-0-12-802973-2.00013-6
Thomas, D. R. (2006). Vitamins in aging, health, and longevity. Clin. Interv. Aging 1, 81–91. doi: 10.2147/ciia.2006.1.1.81
Trojsi, F., Siciliano, M., Passaniti, C., Bisecco, A., Russo, A., Lavorgna, L., et al. (2020). Vitamin D supplementation has no effects on progression of motor dysfunction in amyotrophic lateral sclerosis (ALS). Eur. J. Clin. Nutr. 74, 167–175. doi: 10.1038/s41430-019-0448-3
Vaiserman, A. M. (2014). Early-life nutritional programming of longevity. J. Dev. Orig. Health Dis. 5, 325–338. doi: 10.1017/s2040174414000294
Valdes, A. M., Andrew, T., Gardner, J. P., Kimura, M., Oelsner, E., Cherkas, L. F., et al. (2005). Obesity, cigarette smoking, and telomere length in women. Lancet 366, 662–664. doi: 10.1016/S0140-6736(05)66630-5
Veldink, J. H., Kalmijn, S., Groeneveld, G. J., Wunderink, W., Koster, A., de Vries, J. H., et al. (2007). Intake of polyunsaturated fatty acids and vitamin E reduces the risk of developing amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 78, 367–371. doi: 10.1136/jnnp.2005.083378
Verburgh, K. (2015). Nutrigerontology: why we need a new scientific discipline to develop diets and guidelines to reduce the risk of aging-related diseases. Aging Cell 14, 17–24. doi: 10.1111/acel.12284
Vercruysse, P., Vieau, D., Blum, D., Petersén, Å, and Dupuis, L. (2018). Hypothalamic alterations in neurodegenerative diseases and their relation to abnormal energy metabolism. Front. Mol. Neurosci. 11:2. doi: 10.3389/fnmol.2018.00002
Vivian, J. D. (2017). ALS Patient Cathy Jordan: ‘Pot Stopped My Disease’. Available online at: http://floridafoodandfarm.com/cannabis/als-patient-cathy-jordan-pot-stopped-my-disease/ (accessed April 7, 2020).
Wang, C., Maddick, M., Miwa, S., Jurk, D., Czapiewski, R., Saretzki, G., et al. (2010). Adult-onset, short-term dietary restriction reduces cell senescence in mice. Aging (Albany NY) 2, 555–566. doi: 10.18632/aging.100196
Wang, H., O’Reilly, ÉJ., Weisskopf, M. G., Logroscino, G., McCullough, M. L., Schatzkin, A., et al. (2011). Vitamin E intake and risk of amyotrophic lateral sclerosis: a pooled analysis of data from 5 prospective cohort studies. Am. J. Epidemiol. 173, 595–602. doi: 10.1093/aje/kwq416
Wang, M.-D., Little, J., Gomes, J., Cashman, N. R., and Krewski, D. (2017). Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology 61, 101–130. doi: 10.1016/j.neuro.2016.06.015
Wang, Q., Zhan, Y., Pedersen, N. L., Fang, F., and Hagg, S. (2018). Telomere length and all-cause mortality: a meta-analysis. Ageing Res. Rev. 48, 11–20. doi: 10.1016/j.arr.2018.09.002
Webb, R., Hughes, M. G., Thomas, A. W., and Morris, K. (2017). The ability of exercise-associated oxidative stress to trigger redox-sensitive signalling responses. Antioxidants(Basel) 6:63. doi: 10.3390/antiox6030063
Wills, A.-M., Hubbard, J., Macklin, E. A., Glass, J., Tandan, R., Simpson, E. P., et al. (2014). Hypercaloric enteral nutrition in patients with amyotrophic lateral sclerosis: a randomised, double-blind, placebo-controlled phase 2 trial. Lancet 383, 2065–2072. doi: 10.1016/S0140-6736(14)60222-1
Wright, M. L., Fournier, C., Houser, M. C., Tansey, M., Glass, J., and Hertzberg, V. S. (2018). Potential role of the gut microbiome in ALS: a systematic review. Biol. Res. Nurs. 20, 513–521. doi: 10.1177/1099800418784202
Wruck, C. J., Claussen, M., Fuhrmann, G., Römer, L., Schulz, A., Pufe, T., et al. (2007). Luteolin Protects rat PC 12 and C6 Cells Against MPP+ Induced Toxicity Via an ERK Dependent Keapl-Nrf2-ARE Pathway. Vienna: Springer.
Wu, S., Yi, J., Zhang, Y. G., Zhou, J., and Sun, J. (2015). Leaky intestine and impaired microbiome in an amyotrophic lateral sclerosis mouse model. Physiol. Rep. 3:e12356. doi: 10.14814/phy2.12356
Wyckelsma, V. L., Levinger, I., McKenna, M. J., Formosa, L. E., Ryan, M. T., Petersen, A. C., et al. (2017). Preservation of skeletal muscle mitochondrial content in older adults: relationship between mitochondria, fibre type and high-intensity exercise training. J. Physiol. 595, 3345–3359. doi: 10.1113/JP273950
Yan, F., and Polk, D. B. (2011). Probiotics and immune health. Curr. Opin. Gastroenterol. 27, 496–501. doi: 10.1097/MOG.0b013e32834baa4d
Yan, L.-J. (2014). Positive oxidative stress in aging and aging-related disease tolerance. Redox Biol. 2, 165–169. doi: 10.1016/j.redox.2014.01.002
Yang, J., Park, J. S., Oh, K. W., Oh, S. I., Park, H. M., and Kim, S. H. (2016). Vitamin D levels are not predictors of survival in a clinic population of patients with ALS. J. Neurol. Sci. 367, 83–88. doi: 10.1016/j.jns.2016.05.007
Yavari, A., Javadi, M., Mirmiran, P., and Bahadoran, Z. (2015). Exercise-induced oxidative stress and dietary antioxidants. Asian J. Sports Med. 6:e24898. doi: 10.5812/asjsm.24898
Zhang, Y. G., Wu, S., Yi, J., Xia, Y., Jin, D., Zhou, J., et al. (2017). Target intestinal microbiota to alleviate disease progression in amyotrophic lateral sclerosis. Clin. Ther. 39, 322–336. doi: 10.1016/j.clinthera.2016.12.014
Keywords: amyotrophic lateral sclerosis, aging, dietary habits, exercise, gut microbiome, psychological stress
Citation: Kuraszkiewicz B, Goszczyńska H, Podsiadły-Marczykowska T, Piotrkiewicz M, Andersen P, Gromicho M, Grosskreutz J, Kuźma-Kozakiewicz M, Petri S, Stubbendorf B, Szacka K, Uysal H and de Carvalho M (2020) Potential Preventive Strategies for Amyotrophic Lateral Sclerosis. Front. Neurosci. 14:428. doi: 10.3389/fnins.2020.00428
Received: 11 October 2019; Accepted: 07 April 2020;
Published: 26 May 2020.
Edited by:
Francesca Trojsi, University of Campania Luigi Vanvitelli, ItalyReviewed by:
Dongsheng Fan, Peking University Third Hospital, ChinaChristian Lunetta, University Hospital Policlinico G. Martino, Italy
Mauro Ceroni, Fondazione Casimiro Mondino National Neurological Institute (IRCCS), Italy
Copyright © 2020 Kuraszkiewicz, Goszczyńska, Podsiadły-Marczykowska, Piotrkiewicz, Andersen, Gromicho, Grosskreutz, Kuźma-Kozakiewicz, Petri, Stubbendorf, Szacka, Uysal and de Carvalho. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: M. Piotrkiewicz, masia@ibib.waw.pl
 B. Kuraszkiewicz
B. Kuraszkiewicz H. Goszczyńska
H. Goszczyńska T. Podsiadły-Marczykowska1
T. Podsiadły-Marczykowska1 M. Piotrkiewicz
M. Piotrkiewicz P. Andersen
P. Andersen M. Gromicho
M. Gromicho J. Grosskreutz
J. Grosskreutz M. Kuźma-Kozakiewicz
M. Kuźma-Kozakiewicz K. Szacka
K. Szacka M. de Carvalho
M. de Carvalho