- 1Immpheron, Inc., Lexington, KY, USA
- 2Department of Microbiology and Immunology, The Peter Doherty Institute for Infection and Immunity, University of Melbourne, Melbourne, VIC, Australia
- 3Department of Microbiology and Immunology, University of Kentucky, Lexington, KY, USA
- 4Immunology and Infectious Diseases Program, Division of BioMedical Sciences, Faculty of Medicine, Memorial University of Newfoundland, St. John’s, NL, Canada
In this review, we trace the concept and potential functional role of regulatory idiotypes in the immune response to human immunodeficiency virus type 1 (HIV-1), simian immunodeficiency virus, and hepatitis C virus (HCV). A major idiotype involved in these viral infections is recognized and defined by a murine monoclonal antibody (1F7). Antibodies expressing the idiotype defined by 1F7 are dominant in HIV-1 infection and are also found on many broadly neutralizing antibodies against HIV-1. This regulatory idiotypic axis offers opportunities for exploitation in vaccine development for HIV-1, HCV, and other chronic viral infections.
Anti-Idiotypic Antibodies and Immune Regulation
Oudin and Jerne are the founders of the Idiotype Concept (1, 2). They “discovered” that one antibody could recognize another antibody as an individual and unique member of the immune system. The uniqueness of an antibody lies in the variable region of heavy and light chains and has been named idiotype. Essential insight into the Idiotypic network has emerged from studies on the immune response of mice to phosphorylcholine (PC) [for review, see Ref. (3)]. Evidence that idiotype–anti-idiotype interactions may play a role in the regulation of the immune response came in part from the observation that auto-anti-idiotypic antibodies appear during an immune response to PC (4, 5). Similar evidence of auto-anti-idiotypic antibodies was later discovered in human immunodeficiency virus type 1 (HIV-1)-infected individuals who had antibodies against the 1F7 idiotype (6).
The utility of anti-idiotypic antibodies as immune regulatory molecules was initially demonstrated by Cosenza and Kohler who showed that an anti-idiotype antibody suppressed the response to PC (7, 8). Eichmann and Rajewski later suggested that anti-idiotype antibodies could be used as vaccines (9). In the following decades, anti-idiotypic antibodies have been investigated and probed as therapeutic antibodies in a variety of infectious diseases and malignancies. These anti-idiotypes were used successfully as surrogate antigens, termed Ab2beta, internal image or network antigen, thus replacing the original antigens used as therapeutic vaccines (10).
Anti-idiotypic antibodies were defined as Ab2alpha, Ab2beta, and Ab2gamma, reflecting their biological function as therapeutic antibodies. While Ab2alphas are non-internal images, which are non-biologically effective antibodies, Ab2gammas are non-internal images, which are biologically effective antibodies. Therefore, Ab2beta and Ab2gamma, being both biological effective as therapeutic antibodies, were integrated under the term network antigens (11). The term “idiotype” is used to refer to the entire collection of unique idiotopes within the variable region of a monoclonal antibody (mAb), whereas the term “idiotope” refers to a single determinant recognized within the variable region of an antibody.
Idiotypes expressed on anti-pathogen antibodies might also be utilized as disease markers (12). In the following, we will review the recent progress or lack thereof in the utilization of anti-idiotypes as therapeutic antibodies or disease markers. We will focus on HIV-1 and hepatitis C virus (HCV) infection as both HIV-1 and HCV can lead to cancer in late stages of infectious disease (13, 14).
Expression of the 1F7 Idiotype on Antibodies against Immune Deficiency-Causing Retroviruses
Several HIV infection-related anti-idiotype studies preceded 1F7 anti-idiotype research. These previous studies demonstrated that, in contrast to 1F7, some anti-idiotypes could be used as surrogate antigen to stimulate immune responses against HIV.
Morrow et al. demonstrated that anti-idiotypic antisera against a mAb specific for a p24 gag region epitope detected a common interspecies idiotype associated with anti-HIV response (15). Kang et al. produced an anti-idiotypic mAb (3C9) related to gp120-affinity purified human anti-gp120 antibodies directed against the conserved CD4 attachment site of gp120. It was subsequently shown that immunization with anti-idiotype 3C9 elicited broadly neutralizing antibodies (BnAbs) in naive, non-HIV-infected monkeys (16).
The murine monoclonal antibody defining the 1F7 idiotype, and named 1F7, was originally raised against pooled human antibodies against HIV-1, subcloned, and further selected by ELISA against specific anti-HIV-1 antibodies captured by HIV-1 proteins gp120 and p24 (17). Pooled human immunoglobulin from non-HIV-1-infected individuals (IVIG) was used as negative control in the selection process. Further investigation revealed that human monoclonal anti-gp120, and anti-p24 antibodies, as well as anti-HIV-1 human serum antibodies, share the common idiotype/clonotype 1F7 (18).
Subsequent research identified a region (FR3–CDR3) on three human mAb directed against gp120 or p24 that bound to the murine anti-1F7 mAb, allowing production of a peptide mimicking the idiotypic region. Design of the peptide was based on the molecular recognition theory. As such, regions of inverse hydropathy between the variable sequence of the anti-1F7 and the human mAb, which are assumed to be involved in the idiotype–anti-idiotype contacts, were selected for the peptide design (19). Human anti-HIV-1 serum antibodies from a variety of HIV-1-infected individuals bound to this peptide, indicating an auto-anti-idiotypic humoral immune response to the 1F7 idiotype (6). Further evidence of a biological role for the 1F7 idiotype in HIV-1 infection was provided by studies of cellular immunity. Culture of peripheral blood mononuclear cells (PBMC) from HIV-1-infected individuals with 1F7-induced apoptosis of CD4+ and CD8+ T cells and the 1F7 mAb selectively blocked cytotoxicity of CD8+ T cells from HIV-1-infected individuals (20, 21). The 1F7 idiotype is also expressed on antibodies against the envelope glycoprotein of simian immunodeficiency virus (SIV)- and chimeric simian-human immunodeficiency virus (SHIV) in infected rhesus monkeys (22, 23). As such, the impact of idiotypic regulation through targeting the 1F7 idiotype was explored in the SHIV-IIIB infection model (24, 25). Suppressing expression of the 1F7 idiotype through administration of the murine 1F7 mAb to macaques chronically infected with SHIV-IIIB increased the potency and breadth of neutralizing antibodies, with an increase in neutralization titer against HIV-IIIB and cross-neutralization of HIV-MN as tested by syncytium forming microassay (25). This was interpreted as a result of relieving clonal dominance of the 1F7 idiotype in the humoral response to SHIV, such that new antibodies could arise.
The murine 1F7 mAb has been designated as an Ab2delta antibody to distinguish it from Ab2alpha, Ab2beta, and Ab2gamma anti-idiotypes (26). Recently, we found that this anti-idiotypic clone also stained local SIV gp41-antibodies emerging in naive macaques after intravenous vaccination with SIVmac239 delta Nef and vaginal challenge with wild type SIV (27). Local antibody production in the genital tract correlated with the maturation of protection against high-dose pathogenic SIV vaginal challenge (28).
Expression of the 1F7 Idiotype on Anti-HCV Antibodies
Since 1F7 is a widely shared idiotype/clonotype, some cross-reactivity with antibodies directed to similar structures is not surprising. Parallel research revealed that the 1F7 idiotype is also expressed on antibodies against a number of different HCV proteins (29). Since approximately 20% of HCV-exposed individuals spontaneously clear HCV infection and chronic HCV infection can be cured through treatment, this finding enabled investigation of a possible relationship between expression levels of antibodies bearing the 1F7 idiotype and development or persistence of chronic infection (30). Direction of anti-pathogen immune responses along pathways with inherent limitations as to the potency of induced antibodies was previously suggested as a mechanism by which chronic pathogens may have evolved to subvert the immune response (29, 31, 32). For example, if the dominant antigens of the pathogen have some similarity to self antigens, then conserved aspects of immunological self-tolerance could limit the efficacy of responses against the pathogen (33, 34). In the context of idiotypic regulation of the immune system, we have also proposed that chronic pathogens may have evolved to stimulate immune responses along conserved idiotypic axes defining areas of high network connectivity (29, 30). Stimulating B cells with extensive internal connectivity would propagate high levels of idiotypic recognition with reciprocal stimulation of potentially irrelevant B cell clones and favor chronic oligoclonal activation over the focused selection of high-affinity antibodies with effective potency against the pathogen. Davtyan et al. compared levels of HCV core-specific antibodies expressing the 1F7 idiotype in HCV-exposed individuals who either cleared infection or progressed to chronic infection. Levels of 1F7 idiotype expression were significantly higher on anti-HCV core antibodies from HCV-exposed individuals who progressed to chronic infection than on the anti-HCV core antibodies of those who spontaneously cleared HCV infection (30). In addition, a hierarchy of 1F7 idiotype expression levels was discernable in total circulating IgG and IgM with chronic infection greater than spontaneous clearance greater than HCV non-exposed individuals (30). The concept that pathogens establishing chronic infection, despite strong humoral responses, have evolved to select antibodies along a common idiotypic axis of the immune network represented by the 1F7 idiotype may also relate to previous studies of a public idiotype termed 16/6. Elevated levels of the 16/6 idiotype occur in autoimmune disease and microbial infection, while injection of mice with antibodies bearing the 16/6 idiotype induces chronic immune activation and systemic lupus erythematosus (SLE)-like symptoms (35–38). In this case, there also appears to be a link between induction of antibodies along a common idiotypic axis demarcated by the 16/6 idiotype, immune activation, and autoreactivity. Based on these studies, the concept of pathogenic idiotypes arose, where idiotypes such as 16/6 identify antibodies that are directly autoreactive or have the capacity to disturb idiotypic regulation in ways that promote chronic activation and development of autoimmunity (39, 40). Identification of the distinct B1 B cell subset as the primary source of neonatal and natural antibodies with both a high frequency of autoreactivity and high connectivity apparently separated the B cell repertoire into subdivisions engaged in developmental regulation versus protection against pathogens. Although B1 B cells are distinguished by their developmental pathway, T cell independence and scarcity of somatic hypermutation or isotype switching, their use of the same set of germ-line antigen receptor genes as B2 B cells guarantees some level of idiotypic overlap between the two repertoires.
The fraction of CD5-expressing (B1) and CD5-negative B cells carrying the 1F7 idiotype was measured in uninfected controls and individuals with chronic HCV infection by flow cytometry. While there was expansion of 1F7-expressing B cells in both subsets in chronic HCV infection, it was clearly skewed toward the CD5-expressing subset with up to 30% of B1 B cells expressing the 1F7 Id in some HCV-infected individuals compared to <2% in uninfected controls (30). Despite this marked effect of HCV infection on expansion of B1 B cells expressing the 1F7 idiotype, the majority of circulating antibodies against HCV and HIV-1 that express the 1F7 idiotype have undergone secondary receptor modification and, therefore, appear to originate from the B2 B cell subset (17, 18, 22, 23, 29).
Anti-HIV-1 Antibody Breadth within the 1F7 Idiotype
Engagement of both the B1 and B2 subsets in response to an infection is unusual with respect to their perceived independent roles, different interactions with T cells, level of connectivity, and frequency of autoreactivity. Chronic infection allows for the investigation of the long-term influence that selective expression of a common idiotype has on evolution of antibodies against an infectious agent. Despite demonstrations of anti-HIV-1 antibodies against different proteins and across numerous HIV-1-infected donors carrying the 1F7 idiotype; until recently, little attention was paid to the breadth of these idiotypic antibodies (17, 18). An ideal starting point for assessing the breadth of 1F7-idiotypic antibodies is to screen anti-HIV-1 BnAbs for expression of the idiotype as the process of antibody evolution within a common idiotypic space is intricately illustrated in the development of BnAbs against HIV-1. Anti-HIV-1 BnAbs are a subset of antibodies capable of neutralizing wide arrays of HIV-1 isolates, both within and across viral subtypes (41). These antibodies have great potential as prophylactics against HIV-1, as upon passive transfer or gene delivery to macaques and humanized mice they provide protection from challenges with SHIV and HIV-1, respectively (42–46). Approximately 20% of HIV-1-infected individuals develop antibodies with broadly neutralizing activity against different HIV viruses (47). The HIV-1 BnAbs are unique in that the variable regions have undergone extensive levels of somatic hypermutation not replicated using currently available vaccine formulations (48). Germ-line precursors of the HIV-1 BnAb generally bind HIV-1 envelope glycoproteins poorly, if at all (49). As such, selection of such antibodies may initially require non-HIV-1 antigens (50). Fine-tuning the anti-HIV-1 specificity of these antibodies, as well as the generation of broad neutralization capacity, would likely require additional rounds of selection and somatic hypermutation. To assess the breadth of 1F7-idiotypic antibodies, Parsons et al. screened six well-characterized HIV-1 BnAbs (i.e., 2G12, b12, VRC01, 2F5, 4E10, and Z13e1) for expression of the 1F7 idiotype via ELISA, and demonstrated the presence of the 1F7 idiotype on all six antibodies (51). Given that 1F7-idiotypic antibodies are observed within a majority of HIV-1-infected individuals, but BnAbs are only detected in approximately 20% of infected individuals, this observation raised questions about how BnAbs develop from antibodies within the relatively common 1F7-idiotypic node.
Previous studies demonstrated that anti-HIV-1 antibody responses are subject to a form of original antigenic sin, known as repertoire freeze and deceptive imprinting (32, 52, 53). Establishment of antibodies against the contemporaneous autologous virus initiates this phenomenon during acute infection. Although the virus rapidly escapes to evade the abilities of these antibodies to suppress viral replication, B-cells carrying B-cell receptors (BCR) corresponding to these antibodies sufficiently recognize the escaped virus to be selected to undergo further rounds of somatic hypermutation (or at least keep responding to subsequent antigenic challenges). The continuous reselection of these early-established B-cell responses sustains the early-induced antibody response at the expense of novel B-cell responses more specific for the contemporaneous autologous viruses of chronic infection. Evidence of this phenomenon includes maintenance of skewed κ/λ ratios throughout infection, restricted and biased variable region gene usage throughout infection and persistence of antibody clones recognizing earlier viral variants, despite escape many months prior (54–57). Given the occurrence of repertoire freeze during HIV-1 infection, Parsons et al. hypothesized that BnAbs develop from 1F7-idiotypic antibodies established during early infection through multiple rounds of selection and somatic hypermutation (51). To assess this possibility, they screened serial plasma samples from six HIV-infected donors over time points ranging from acute through chronic infection to determine if 1F7-idiotypic antibodies arise during acute infection and are sustained throughout infection. Indeed, 1F7-idiotypic antibodies were detected in plasma samples collected within the first 3 months of HIV-1 infection, and these antibodies were expanded and sustained with longer duration of infection. This observation suggests that 1F7-idiotypic antibodies engaged during acute infection and sustained throughout infection in an idiotypic repertoire freeze are driven through repeated selection and somatic hypermutation to develop into BnAbs. This putative route to BnAb development is consistent with pathways hypothesized by others and has implications for designing BnAb-inducing vaccines.
Consistent with the hypothesis that BnAbs develop through repeated selection and somatic hypermutation of 1F7-idiotypic antibodies induced early in infection, Liao et al. recently demonstrated directly that antibodies capable of neutralizing a broad range of HIV-1 isolates develop as a result of repeated selection and mutation of antibodies by constantly evolving autologous HIV-1 (58). Haynes et al. hypothesized that BnAbs might be inducible through vaccination strategies that sequentially expose vaccine recipients to HIV-1 envelope antigens selected on the basis of binding to chronologically relevant antibody isolates, derived from HIV-1-infected donors that developed BnAbs (59). This vaccination strategy might benefit from inclusion of a prime and/or boost with F(ab)2 of the murine monoclonal anti-idiotypic antibody used to detect the 1F7 idiotype in in vitro assays. As anti-HIV-1 BnAbs appear to be selected from the 1F7-idiotypic repertoire, a prime with the anti-1F7 F(ab)2 should focus the B-cell response of later immunizations with relevant HIV-1 envelope antigens within the relevant B-cell repertoire. Similarly, boosts with anti-1F7 F(ab)2 might have the benefit of increasing the frequency of the relevant B-cell responses induced by the sequential vaccination strategy.
In addition to the ability of BnAb to prevent HIV-1 infection, non-neutralizing anti-HIV-1 antibodies have also recently gained attention as potentially protective moieties. Indeed, the ability of non-neutralizing antibodies to mediate antibody-dependent cellular cytotoxicity (ADCC) against HIV-1 was linked to the partial success of the RV144 Thai vaccine trial (60–62). Similarly, the ability of antibodies to mediate ADCC has been linked to protection against infection with pathogenic SIV challenge in macaques immunized with live-attenuated SIV (63). The ADCC competency of antibodies passively transferred via breast milk from HIV-1-infected mothers to their children was also associated with a lower likelihood of mother-to-child transmission (64). The breadth of these non-neutralizing ADCC competent antibodies, defined as the ability of such antibodies to trigger ADCC against target cells expressing HIV-1 envelope antigens from different viral subtypes, appears important to their protective capacity. In fact, a recent assessment of the breadth of ADCC antibodies from HIV-1-infected controllers and progressors demonstrated more breadth in controllers (65). Therefore, it is likely imperative to understand the origin of HIV-1 envelope binding antibodies with broad recognition profiles. In this regard, Parsons et al. recently assessed expression of the 1F7 idiotype on broadly reactive anti-HIV-1 antibodies from individuals infected with HIV-1 subtype B that cross-reacted with HIV-1 envelopes (i.e., gp140) from subtype B, A, and AE viral isolates (66). The 1F7 idiotype was detected on antibodies binding to each HIV-1 subtype envelope, suggesting broad recognition within the anti-HIV-1 1F7-idiotypic node. Similarly, when expression of the 1F7 idiotype was assessed on subtype B envelope (i.e., gp120)-binding anti-HIV-1 antibodies derived from individuals infected with non-subtype B viruses, the idiotype was observed on antibodies from 15/19 donors. Together with data regarding 1F7-idiotypic expression on anti-HIV-1 BnAbs, the expression profile of the 1F7 idiotype on anti-HIV-1-binding antibodies with broad recognition capabilities suggests that vaccines focusing anti-HIV-1 antibody responses within the 1F7-idiotypic repertoire can achieve a substantial breadth of HIV-1 recognition.
How best to exploit the immense breadth available within the 1F7-idiotype domain remains an open question. The potential breadth of this node may be dramatically narrowed by repertoire freezes induced by HIV-1 infection itself or by vaccination with single strains. Recently, Kohler discussed that the establishment of such repertoire freezes results in “back-boosts” upon exposure to antigenically related, yet distinct, antigens, as would be the case upon challenge with a different viral strain than that utilized for vaccination (67). He further proposed utilizing the full breadth of the 1F7-idiotypic repertoire by “forward-boosting” or priming the entire 1F7 repertoire prior to exposure to any HIV-1 antigens. Future research should evaluate the ability of such a “forward-boosting” protocol to establish broadly reactive anti-HIV-1 antibodies capable of mediating potent effector functions against HIV-1 or HIV-1 infected cells (see Figure 1 depicting the forward boosting strategy).
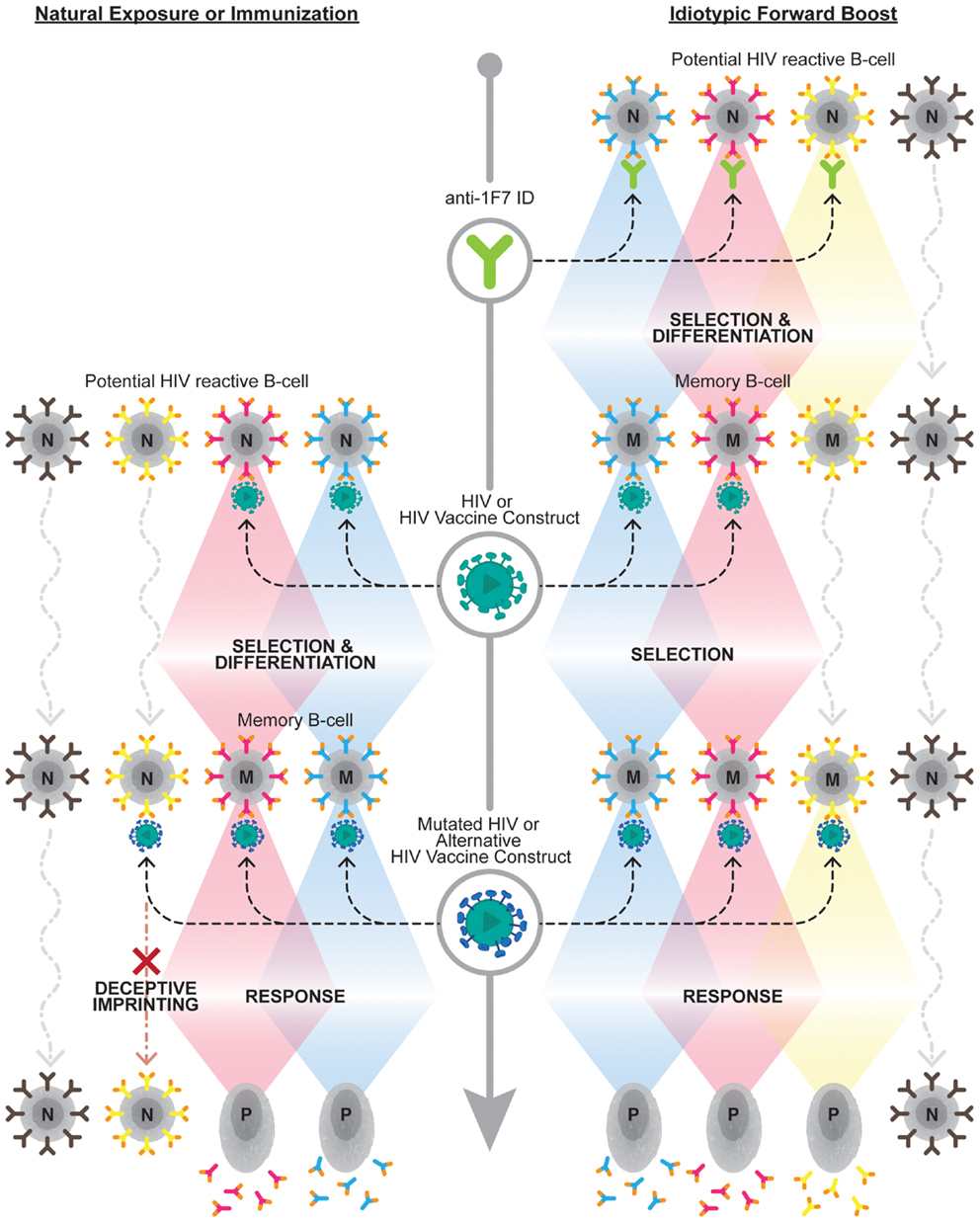
Figure 1. Overcoming deceptive imprinting through forward boosting. The left side of the diagram represents the B cell response to HIV-1 upon natural exposure to HIV-1 or immunization with an HIV-1 vaccine construct. This exposure results in a subset of naive B cell clones (N), which carry B cell receptors capable of binding HIV-1 antigens, being selected to undergo differentiation into memory B cells (M). This initial selection process induces a phenomenon known as deceptive imprinting. This phenomenon results in a reselection of previously selected B cell clones, at the expense of naive B cells carrying novel antigen-binding B cell receptor specificities, upon exposure to mutated HIV-1 or alternative HIV-1 vaccine constructs. The right side of the diagram depicts the forward-boosting strategy. This strategy predicts priming 1F7-idiotypic B cell clones (i.e., those expressing orange tipped B cell receptors), which exhibit broad HIV-1 reactivity, will establish a larger repertoire of B cell clones that can contribute to the anti-HIV-1 antibody response following future exposure or immunization. Upon future exposure to HIV-1 or an HIV-1 vaccine construct boosting of a subset of the memory B cells established from the initial anti-1F7-idiotype prime will be observed. Additional anti-1F7-idiotype primed clones should be boosted, and not impeded by deceptive imprinting, upon exposure to cognate antigens on mutated HIV-1 or alternative HIV-1 vaccine constructs. The bottom of the diagram, on both the left and right sides, demonstrates that selected B cell clones contribute to the antibody response upon becoming long-lived plasma cells (P).
Summary
The concept and significance of dominant idiotypes surfaced more than 40 years ago, and the practical value of internal image antibodies or network antigens as immunogens was realized shortly afterwards. Regulatory idiotypes, the levels of which might reflect immune trajectories toward autoimmunity or immune deficiency, were proposed in the mid 1980s and continue to be studied today in autoimmunity, cancer, and infectious disease. In this review, we described how antibodies against multiple antigens of retroviruses capable of causing immune deficiency syndromes, including HIV-1, SIV, and SHIV, and also antibodies against HCV share a common idiotype designated 1F7. Expression of this idiotype is associated with a form of clonal dominance that appears to restrict development of neutralizing antibodies against contemporaneous infecting strains of HIV-1 and imposes a trajectory toward chronic infection with HCV. However, BnAbs that develop against HIV-1 do bear the 1F7 idiotype, indicating that the same idiotypic axis accommodating clonal dominance and original antigenic sin also accommodates antibody evolution toward the highly mutated progeny capable of neutralizing diverse strains of HIV-1. Learning how to exploit this axis selectively to prime for the development of BnAbs in advance of viral exposure may be a key to more effective vaccination against chronic viruses, such as HIV-1, HCV, and others.
Author Contributions
Dr. SM describes her invention of mAb 1F7 and subsequent experimental findings. Prof. MG describes his findings about HCV related to 1F7 idiotype expression. Dr. MP describes findings of 1F7 expression on neutralizing anti-HIV antibodies. Dr. HK has written an additional paragraph on general anti-idiotypic regulation.
Conflict of Interest Statement
Matthew Parsons and Michael Grant serve on the scientific advisory board of Network Immunology Inc., a private company with an interest in developing vaccines against human immunoodeficiency virus. The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Jason Thean Kit Ooi for help preparing the figure.
Funding
MP is supported by a postdoctoral fellowship from the Canadian Institutes of Health Research (CIHR).
References
1. Jerne NK. Towards a network theory of the immune system. Ann Immunol (Paris) (1974) 125C(1–2):373–89.
3. Kohler H. The response to phosphorylcholine: dissecting an immune response. Transplant Rev (1975) 27:24–56.
4. Kluskens L, Kohler H. Regulation of immune response by autogenous antibody against receptor. Proc Natl Acad Sci U S A (1974) 71(12):5083–7. doi: 10.1073/pnas.71.12.5083
5. Cosenza H, Augustin A, Julius MH. Induction and characterization of “autologous” anti-isiotypic antibodies. Eur J Immunol (1977) 7(5):273–8. doi:10.1002/eji.1830070506
6. Wang QL, Wang HT, Blalock E, Müller S, Köhler H. Identification of an idiotypic peptide recognized by autoantibodies in human immunodeficiency virus-1-infected individuals. J Clin Invest (1995) 96(2):775–80. doi:10.1172/JCI118122
7. Cosenza H, Kohler H. Specific inhibition of plaque formation to phosphorylcholine by antibody against antibody. Science (1972) 176(4038):1027–9. doi:10.1126/science.176.4038.1027
8. Cosenza H, Kohler H. Specific suppression of the antibody response by antibodies to receptors. Proc Natl Acad Sci U S A (1972) 69(9):2701–5. doi:10.1073/pnas.69.9.2701
9. Eichmann K, Rajewsky K. Induction of T and B cell immunity by anti-idiotypic antibody. Eur J Immunol (1975) 5(10):661–6. doi:10.1002/eji.1830051002
10. Kohler H, McNamara M, Ward RE. Idiotypes as internal antigens. Prog Clin Biol Res (1985) 172B:343–53.
11. Köhler H, Kieber-Emmons T, Srinivasan S, Kaveri S, Morrow WJ, Müller S, et al. Revised immune network concepts. Clin Immunol Immunopathol (1989) 52(1):104–16. doi:10.1016/0090-1229(89)90197-9
12. Kohler H, Bhattacharya-Chatterjee M, Muller S, Foon KA. Idiotype manipulation in disease management. Adv Exp Med Biol (1995) 383:117–22. doi:10.1007/978-1-4615-1891-4_14
13. Omland LH, Jepsen P, Krarup H, Christensen PB, Weis N, Nielsen L, et al. Liver cancer and non-Hodgkin lymphoma in hepatitis C virus-infected patients: results from the DANVIR cohort study. Int J Cancer (2012) 130(10):2310–7. doi:10.1002/ijc.26283
14. Serraino D, De Paoli A, Zucchetto A, Pennazza S, Bruzzone S, Spina M, et al. The impact of Kaposi sarcoma and non-Hodgkin lymphoma on mortality of people with AIDS in the highly active antiretroviral therapies era. Cancer Epidemiol (2010) 34(3):257–61. doi:10.1016/j.canep.2010.03.011
15. Morrow WJ, Gaston I, Anderson T, Haigwood N, McGrath MS, Rosen J, et al. Anti-idiotypic antisera raised against monoclonal antibody specific for a p24 gag region epitope detects a common interspecies idiotype associated with anti-HIV responses. Viral Immunol (1990) 3(2):99–109. doi:10.1089/vim.1990.3.99
16. Kang CY, Nara P, Chamat S, Caralli V, Chen A, Nguyen ML, et al. Anti-idiotype monoclonal antibody elicits broadly neutralizing anti-gp120 antibodies in monkeys. Proc Natl Acad Sci U S A (1992) 89(7):2546–50. doi:10.1073/pnas.89.7.2546
17. Müller S, Wang HT, Kaveri SV, Chattopadhyay S, Köhler H. Generation and specificity of monoclonal anti-idiotypic antibodies against human HIV-specific antibodies. I. Cross-reacting idiotopes are expressed in subpopulations of HIV-infected individuals. J Immunol (1991) 147(3):933–41.
18. Wang H, Müller S, Zolla-Pazner S, Köhler H. Human monoclonal and polyclonal anti-human immunodeficiency virus-1 antibodies share a common clonotypic specificity. Eur J Immunol (1992) 22(7):1749–55. doi:10.1002/eji.1830220713
19. Blalock JE. Complementarity of peptides specified by ‘sense’ and ‘antisense’ strands of DNA. Trends Biotechnol (1990) 8(6):140–4. doi:10.1016/0167-7799(90)90159-U
20. Grant M, Smaill F, Muller S, Kohler H, Rosenthal K. The anti-idiotypic antibody 1F7 selectively inhibits cytotoxic T cells activated in HIV-1 infection. Immunol Cell Biol (2000) 78(1):20–7. doi:10.1046/j.1440-1711.2000.00879.x
21. Muller S, Brams P, Collins D, Kohler H, Dorigo O, Wong HT. Apoptosis of CD4+ and CD8+ cells from HIV-1 infected individuals: role of anti-idiotypic antibodies. Vaccin Res (1995) 4:229–38.
22. Grant MD, Whaley MD, Mayne A, Hoffmann GW, Ansari AA. Similar abnormalities of idiotype and immunoglobulin light chain expression and of cell-mediated cytotoxicity in HIV-infected humans and simian immunodeficiency virus (SIV)-infected rhesus macaques. Immunol Cell Biol (1996) 74(1):38–44. doi:10.1038/icb.1996.5
23. Muller S, Margolin DH, Min G. An HIV-1 infection-related idiotype/clonotype (1F7) is expressed on antibodies directed to envelope glycoprotein in simian immunodeficiency virus- and chimeric simian/human immunodeficiency virus-infected rhesus monkeys. Hybridoma (1997) 16(1):17–21. doi:10.1089/hyb.1997.16.17
24. Muller S, Margolin DH, Min G, Lou D, Nara P, Axthelm MK, et al. Stimulation of antiviral antibody response in SHIV-IIIB-infected macaques. Scand J Immunol (2001) 54(4):383–95. doi:10.1046/j.1365-3083.2001.00982.x
25. Müller S, Margolin DH, Nara PL, Alvord WG, Köhler H. Stimulation of HIV-1-neutralizing antibodies in simian HIV-IIIB-infected macaques. Proc Natl Acad Sci U S A (1998) 95(1):276–81. doi:10.1073/pnas.95.1.276
26. Hoffmann GW, Muller S, Kohler H. Towards an HIV vaccine based on immune network theory. Curr Trends Immunol (2012) 13:69–79.
27. Smith AJ, Wietgrefe SW, Shang L, Reilly CS, Southern PJ, Perkey KE, et al. Live simian immunodeficiency virus vaccine correlate of protection: immune complex-inhibitory Fc receptor interactions that reduce target cell availability. J Immunol (2014) 193(6):3126–33. doi:10.4049/jimmunol.1400822
28. Li Q, Zeng M, Duan L, Voss JE, Smith AJ, Pambuccian S, et al. Live simian immunodeficiency virus vaccine correlate of protection: local antibody production and concentration on the path of virus entry. J Immunol (2014) 193(6):3113–25. doi:10.4049/jimmunol.1400820
29. Grant MD. Antibody convergence along a common idiotypic axis in immunodeficiency virus and hepatitis C virus infections. J Med Virol (2002) 66(1):13–21. doi:10.1002/jmv.2105
30. Davtyan TK, Hovsepyan MP, Mkhitaryan LM, Hakobyan GS, Brazil A, Barrett L, et al. The 1F7 idiotype is selectively expressed on CD5+ B cells and elevated in chronic hepatitis C virus infection. Immunol Cell Biol (2009) 87(6):457–63. doi:10.1038/icb.2009.18
31. Kohler H. Structured therapy interruption (STI) and deceptive imprinting. Int Rev Immunol (2004) 23(5–6):455–63. doi:10.1080/08830180490432857
32. Kohler H, Muller S, Nara PL. Deceptive imprinting in the immune response against HIV-1. Immunol Today (1994) 15(10):475–8. doi:10.1016/0167-5699(94)90192-9
33. Cohen IR. The cognitive principle challenges clonal selection. Immunol Today (1992) 13(11):441–4. doi:10.1016/0167-5699(92)90071-E
34. Cohen IR. The cognitive paradigm and the immunological homunculus. Immunol Today (1992) 13(12):490–4. doi:10.1016/0167-5699(92)90024-2
35. Abu-Shakra M, Shoenfeld Y. Human anti-DNA idiotype (16/6 idiotype): pathogenic role in autoimmunity. Hum Antibodies Hybridomas (1990) 1(1):10–4.
36. el-Roeiy A, Gleicher N, Isenberg D, Kennedy RC, Shoenfeld Y. A common anti-DNA idiotype and other autoantibodies in sera of offspring of mothers with systemic lupus erythematosus. Clin Exp Immunol (1987) 68(3):528–34.
37. Mendlovic S, Brocke S, Shoenfeld Y, Ben-Bassat M, Meshorer A, Bakimer R, et al. Induction of a systemic lupus erythematosus-like disease in mice by a common human anti-DNA idiotype. Proc Natl Acad Sci U S A (1988) 85(7):2260–4. doi:10.1073/pnas.85.7.2260
38. Sela O, el-Roeiy A, Isenberg DA, Kennedy RC, Colaco CB, Pinkhas J, et al. A common anti-DNA idiotype in sera of patients with active pulmonary tuberculosis. Arthritis Rheum (1987) 30(1):50–6. doi:10.1002/art.1780300107
39. Shoenfeld Y, Teplizki HA, Mendlovic S, Blank M, Mozes E, Isenberg DA. The role of the human anti-DNA idiotype 16/6 in autoimmunity. Clin Immunol Immunopathol (1989) 51(3):313–25. doi:10.1016/0090-1229(89)90030-5
40. Shoenfeld Y. Idiotypic induction of autoimmunity: a new aspect of the idiotypic network. FASEB J (1994) 8(15):1296–301.
41. Walker LM, Phogat SK, Chan-Hui PY, Wagner D, Phung P, Goss JL, et al. Broad and potent neutralizing antibodies from an African donor reveal a new HIV-1 vaccine target. Science (2009) 326(5950):285–9. doi:10.1126/science.1178746
42. Balazs AB, Chen J, Hong CM, Rao DS, Yang L, Baltimore D. et al. Antibody-based protection against HIV infection by vectored immunoprophylaxis. Nature (2011) 481(7379):81–4. doi:10.1038/nature10660
43. Hessell AJ, Rakasz EG, Tehrani DM, Huber M, Weisgrau KL, Landucci G, et al. Broadly neutralizing monoclonal antibodies 2F5 and 4E10 directed against the human immunodeficiency virus type 1 gp41 membrane-proximal external region protect against mucosal challenge by simian-human immunodeficiency virus SHIVBa-L. J Virol (2010) 84(3):1302–13. doi:10.1128/JVI.01272-09
44. Luo XM, Lei MY, Feidi RA, West AP Jr, Balazs AB, Bjorkman PJ, et al. Dimeric 2G12 as a potent protection against HIV-1. PLoS Pathog (2010) 6(12):e1001225. doi:10.1371/journal.ppat.1001225
45. Saunders KO, Wang L, Joyce MG, Yang ZY, Balazs AB, Cheng C, et al. Broadly neutralizing human immunodeficiency virus type 1 antibody gene transfer protects non-human primates from mucosal simian-human immunodeficiency virus infection. J Virol (2015) 89(16):8334–45. doi:10.1128/JVI.00908-15
46. Veazey RS, Shattock RJ, Pope M, Kirijan JC, Jones J, Hu Q, et al. Prevention of virus transmission to macaque monkeys by a vaginally applied monoclonal antibody to HIV-1 gp120. Nat Med (2003) 9(3):343–6. doi:10.1038/nm833
47. Doria-Rose NA, Klein RM, Daniels MG, O’Dell S, Nason M, Lapedes A, et al. Breadth of human immunodeficiency virus-specific neutralizing activity in sera: clustering analysis and association with clinical variables. J Virol (2010) 84(3):1631–6. doi:10.1128/JVI.01482-09
48. Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: understanding nature’s pathways. Immunol Rev (2013) 254(1):225–44. doi:10.1111/imr.12075
49. Xiao X, Chen W, Feng Y, Zhu Z, Prabakaran P, Wang Y, et al. Germline-like predecessors of broadly neutralizing antibodies lack measurable binding to HIV-1 envelope glycoproteins: implications for evasion of immune responses and design of vaccine immunogens. Biochem Biophys Res Commun (2009) 390(3):404–9. doi:10.1016/j.bbrc.2009.09.029
50. Yang Z, Li J, Liu Q, Yuan T, Zhang Y, Chen LQ, et al. Identification of non-HIV immunogens that bind to germline b12 predecessors and prime for elicitation of cross-clade neutralizing HIV-1 antibodies. PLoS One (2015) 10(5):e0126428. doi:10.1371/journal.pone.0126428
51. Parsons MS, Rouleau D, Routy JP, LeBlanc R, Grant MD, Bernard NF. Selection of human anti-HIV broadly neutralizing antibodies occurs within the context of frozen 1F7-idiotypic repertoire. AIDS (2011) 25(10):1249–64. doi:10.1097/QAD.0b013e328347f9fa
52. Kohler H, Goudsmit J, Nara P. Clonal dominance: cause for a limited and failing immune response to HIV-1 infection and vaccination. J Acquir Immune Defic Syndr (1992) 5(11):1158–68.
53. Parsons MS, Muller S, Kohler H, Grant MD, Bernard NF. On the benefits of sin: can greater understanding of the 1F7-idiotypic repertoire freeze enhance HIV vaccine development? Hum Vaccin Immunother (2013) 9(7):1532–8. doi:10.4161/hv.24460
54. Li L, Wang XH, Banerjee S, Volsky B, Williams C, Virland D, et al. Different pattern of immunoglobulin gene usage by HIV-1 compared to non-HIV-1 antibodies derived from the same infected subject. PLoS One (2012) 7(6):e39534. doi:10.1371/journal.pone.0039534
55. Chung AW, Isitman G, Navis M, Kramski M, Center RJ, Kent SJ, et al. Immune escape from HIV-specific antibody-dependent cellular cytotoxicity (ADCC) pressure. Proc Natl Acad Sci U S A (2011) 108(18):7505–10. doi:10.1073/pnas.1016048108
56. Müller S, Wang H, Silverman GJ, Bramlet G, Haigwood N, Köhler H. B-cell abnormalities in AIDS: stable and clonally-restricted antibody response in HIV-1 infection. Scand J Immunol (1993) 38(4):327–34. doi:10.1111/j.1365-3083.1993.tb01734.x
57. Richman DD, Wrin T, Little SJ, Petropoulos CJ. Rapid evolution of the neutralizing antibody response to HIV type 1 infection. Proc Natl Acad Sci U S A (2003) 100(7):4144–9. doi:10.1073/pnas.0630530100
58. Liao HX, Lynch R, Zhou T, Gao F, Alam SM, Boyd SD, et al. Co-evolution of a broadly neutralizing HIV-1 antibody and founder virus. Nature (2013) 496(7446):469–76. doi:10.1038/nature12053
59. Bonsignori M, Alam SM, Liao HX, Verkoczy L, Tomaras GD, Haynes BF, et al. HIV-1 antibodies from infection and vaccination: insights for guiding vaccine design. Trends Microbiol (2012) 20(11):532–9. doi:10.1016/j.tim.2012.08.011
60. Haynes BF, Gilbert PB, McElrath MJ, Zolla-Pazner S, Tomaras GD, Alam SM, et al. Immune-correlates analysis of an HIV-1 vaccine efficacy trial. N Engl J Med (2012) 366(14):1275–86. doi:10.1056/NEJMoa1113425
61. Rerks-Ngarm S, Pitisuttithum P, Nitayaphan S, Kaewkungwal J, Chiu J, Paris R, et al. Vaccination with ALVAC and AIDSVAX to prevent HIV-1 infection in Thailand. N Engl J Med (2009) 361(23):2209–20. doi:10.1056/NEJMoa0908492
62. Tomaras GD, Ferrari G, Shen X, Alam SM, Liao HX, Pollara J, et al. Vaccine-induced plasma IgA specific for the C1 region of the HIV-1 envelope blocks binding and effector function of IgG. Proc Natl Acad Sci U S A (2013) 110(22):9019–24. doi:10.1073/pnas.1301456110
63. Alpert MD, Harvey JD, Lauer WA, Reeves RK, Piatak M Jr, Carville A, et al. ADCC develops over time during persistent infection with live-attenuated SIV and is associated with complete protection against SIV(mac)251 challenge. PLoS Pathog (2012) 8(8):e1002890. doi:10.1371/journal.ppat.1002890
64. Mabuka J, Nduati R, Odem-Davis K, Peterson D, Overbaugh J. HIV-specific antibodies capable of ADCC are common in breastmilk and are associated with reduced risk of transmission in women with high viral loads. PLoS Pathog (2012) 8(6):e1002739. doi:10.1371/journal.ppat.1002739
65. Madhavi V, Wren LH, Center RJ, Gonelli C, Winnall WR, Parsons MS, et al. Breadth of HIV-1 Env-specific antibody-dependent cellular cytotoxicity: relevance to global HIV vaccine design. AIDS (2014) 28(13):1859–70. doi:10.1097/QAD.0000000000000310
66. Parsons MS, Center RJ, Routy JP, Rouleau D, Leblanc R, Wainberg MA, et al. Short communication: antibody responses to human immunodeficiency virus envelope from infections with multiple subtypes utilize the 1F7-idiotypic repertoire. AIDS Res Hum Retroviruses (2013) 29(5):778–83. doi:10.1089/AID.2012.0094
Keywords: idiotype, 1F7, HIV, SIV, HCV
Citation: Muller S, Parsons MS, Kohler H and Grant M (2016) The Significance of a Common Idiotype (1F7) on Antibodies against Human Immune Deficiency Virus Type 1 and Hepatitis C Virus. Front. Oncol. 6:11. doi: 10.3389/fonc.2016.00011
Received: 09 September 2015; Accepted: 12 January 2016;
Published: 05 February 2016
Edited by:
Thomas Kieber-Emmons, University of Arkansas for Medical Sciences, USAReviewed by:
Jennifer Wu, Medical University of South Carolina, USAKyle K. Payne, The Wistar Institute, USA
Copyright: © 2016 Muller, Parsons, Kohler and Grant. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Sybille Muller, immpheron@aol.com
 Sybille Muller
Sybille Muller Matthew S. Parsons
Matthew S. Parsons Heinz Kohler
Heinz Kohler Michael Grant
Michael Grant