- 1Operational Management Office, West China Second University Hospital, Sichuan University, Chengdu, China
- 2Key Laboratory of Birth Defects and Related Diseases of Women and Children (Sichuan University), Ministry of Education, Chengdu, China
- 3Emergency Department, West China Second University Hospital, Sichuan University, Chengdu, China
Interleukin-6 (IL-6) is a pleiotropic cytokine involved in immune regulation. It can activate janus kinase 2 (JAK2)-signal transducer and activator of transcription 3 (STAT3) signaling pathway. As one of the important signal transduction pathways in cells, JAK2/STAT3 signaling pathway plays a critical role in cell proliferation and differentiation by affecting the activation state of downstream effector molecules. The activation of JAK2/STAT3 signaling pathway is involved in tumorigenesis and development. It contributes to the formation of tumor inflammatory microenvironment and is closely related to the occurrence and development of many human tumors. This article focuses on the relationship between IL-6/JAK2/STAT3 signaling pathway and liver cancer, breast cancer, colorectal cancer, gastric cancer, lung cancer, pancreatic cancer and ovarian cancer, hoping to provide references for the research of cancer treatment targeting key molecules in IL-6/JAK2/STAT3 signaling pathway.
Introduction
With the participation of many cytokines, the tumor microenvironment (TME) thereby forms a local milieu which is conducive to tumor propagation (1). Cytokines such as interleukin-6 (IL-6) may have a significant impact on cancer progression through signal cascades. Among them, IL-6/janus kinase 2 (JAK2)/signal transducer and activator of transcription 3 (STAT3) signaling pathways may play a key role in the development of malignant tumors, participating in the entire process of invasion and metastasis. Excessive release of IL-6 in response to inflammatory stimulation is a potent activator of JAK/STAT signaling pathway. IL-6 may play a pro-inflammatory role by activating this pathway to promote the process of epithelial-mesenchymal transition (EMT) (2, 3). IL-6/JAK2/STAT3 signaling pathway is one of the important inflammatory signaling pathways found at present. It participates in many physiological and pathological processes such as immune regulation, angiogenesis and cell proliferation and differentiation. It is closely related to biological behaviors such as tumor occurrence, development, metastasis and invasion, and its abnormal expression has guiding significance for tumor prognosis (4, 5).
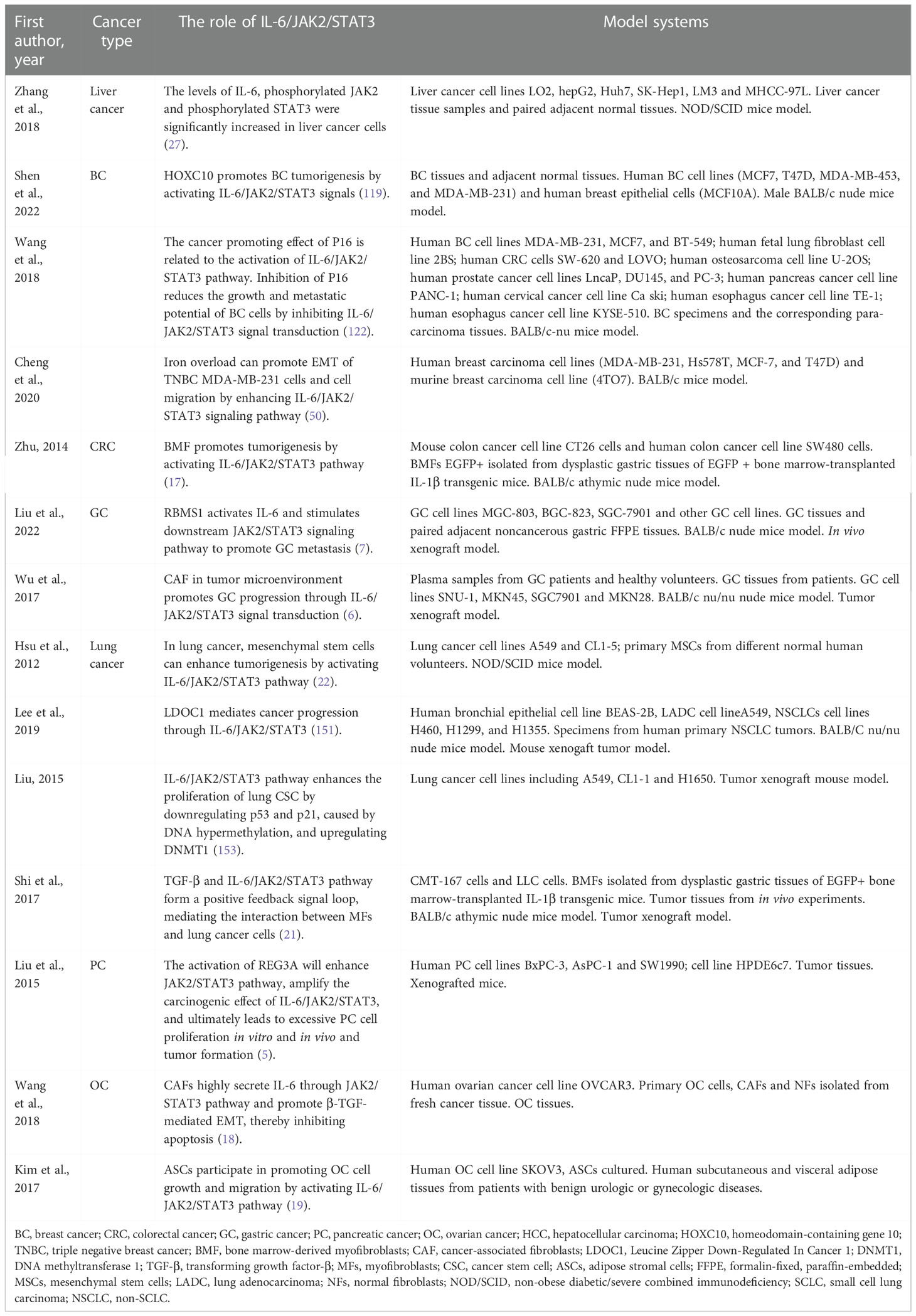
A large number of studies have shown that IL-6/JAK2/STAT3 signaling pathway is abnormally highly activated in a variety of cancers, such as gastric cancer (GC) (6, 7), breast cancer (BC) (8–10), liver cancer (11–13), colorectal cancer (CRC) (14, 15), colon cancer (16, 17), ovarian cancer (OC) (18, 19), lung cancer (20–22), pancreatic cancer (PC) (4, 5). It strongly inhibits anti-tumor immune response (23). In glioma tissues, syndecan-binding protein (SDCBP), which controls the proliferation and invasion of cancer cells, is positively correlated with IL-6 expression level, and IL-6 stimulation induces SDCBP expression at mRNA and protein levels in a dose- and time-dependent manner (24). Phosphorylation of STAT3 and JAK2 was significantly enhanced in glioma cells, and inhibition of IL-6/JAK2/STAT3 signaling pathway could significantly inhibit the proliferation of glioma cells and promote cell apoptosis (25, 26). IL-6, phosphorylated JAK2 and phosphorylated STAT3 protein levels were significantly increased in liver cancer cells. The treatment of liver cancer cells with JAK2 inhibitor and IL-6 neutralizing antibody enhanced the adriamycin-induced aging of cells, and also significantly inhibited the proliferation rate of the cells (27). IL-6/JAK2/STAT3 pathway is more active in CD44+CD24- BC cells than in other tumor types, and inhibition of JAK2 reduces their numbers and prevents xenograft growth (28). In CRC, CRC-derived mesenchymal stem cells (CC-MSCs) increase the migration and invasion of CRC cells through EMT in vitro, leading to the occurrence of CRC; and promote the growth and metastasis of CRC in vivo (29). The use of STAT3 inhibitors can weaken the CRC promoting effect of CC-MSCs (14). Total STAT3 and phosphorylated STAT3 in intestinal GC were increased compared with normal stomach (30). In lung cancer, mesenchymal stem cells can enhance tumorigenesis by activating IL-6/JAK2/STAT3 pathway (22). IL-6 and its downstream JAK2/STAT3 pathway have become the most important factors in the regulation of inflammation-related PC (31). Compared with normal ovaries and benign tumors, JAK2/STAT3 is activated in high-grade OC and is involved in cancer progression and EMT (32). Activation of IL-6/JAK2/STAT3 pathway is closely associated with EMT and stem cell-like features, ultimately leading to poor prognosis in patients with various cancers (33). Therefore, the inhibition and regulation of IL-6/JAK2/STAT3 signaling pathway is conducive to the prevention and treatment of tumors and the improvement of prognosis, and it is also one of the important targets for screening anti-tumor drugs (34). Targeting molecules in this pathway have significant effects on slowing down cancer progression (35–37). Thus, it is a very promising research object for cancer treatment. This paper reviewed the relationship between IL-6/JAK2/STAT3 signaling pathway and various cancers, providing a reference for cancer therapy targeting this pathway.
IL-6, JAK2 and STAT3
IL-6
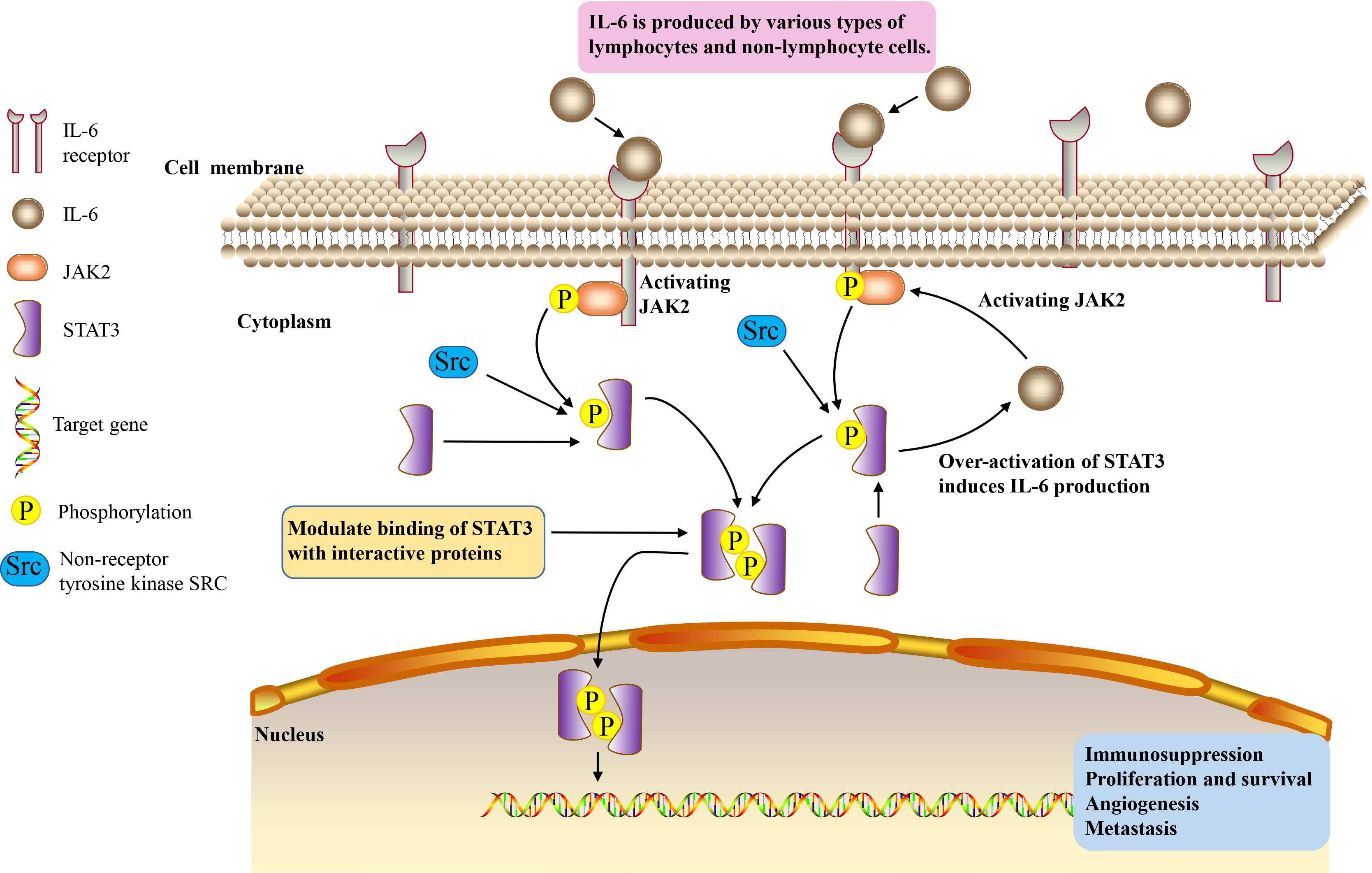
Interleukin is a kind of cytokines produced by and acting on many kinds of cells. It can interact with many types of cells, alter the immune system, and play a role in a variety of cancers (38). Interleukin can be divided into several families, with more than 40 subfamily members (38). Among them, IL-6 is a pleiotropic cytokine involved in immune regulation. It regulates almost all aspects of the innate immune system, including hematopoiesis and neutrophil accumulation at infection or trauma sites by controlling granulopoiesis (39–41).
The human gene for IL-6 was cloned and reported in 1986. It is mapped to 7p15–p21 chromosome, consisting of four introns and five exons (42). The IL-6 gene encodes the 212 amino acid length IL-6 precursor protein, including a 28-amino acid signal peptide and a 184-amino acid mature segment (42, 43). Its molecular masses vary from 21 kDa to 28 kDa, depending on the cellular source and post-translational modification including N-/O-glycosylation and phosphorylation (44). IL-6 is produced by various types of lymphocytes and non-lymphocyte cells, such as T and B lymphocytes, fibroblasts, monocytes, mesangial cells, endothelial cells, keratinocytes, and several tumor cells (45). IL-6 enacts a broad set of physiological functions traditionally related with immune cell regulation, host defense, proliferation and differentiation (46), and can directly stimulate the proliferation, survival, metastasis and invasion of tumor cells (47–49). It has a wide range of effects on immune system cells and non-immune system cells, usually showing hormone-like characteristics that affect homeostatic processes (41). IL-6 has context-dependent pro- and anti-inflammatory properties, and is regarded as a prominent target for clinical intervention (41). IL-6 levels were elevated in patients with chronic inflammation and a large number of hematopoietic malignancies and solid tumors. About 25 percent of adult cancers are caused by chronic inflammation (50). Associated with inflammation, IL-6 is involved in the progression of cancer. IL-6 exerts its biological effects by binding to its receptors, IL-6α receptors (glycoprotein 80, gp80) and IL-6β receptors (glycoprotein 130, gp130) (51). Homodimer composed of IL-6 and gp130 phosphorylates downstream janus tyrosine kinase (JAK), and then activates various downstream transcription factors (52). Tumor-associated macrophages increase tumor initiating ability and drug resistance of tumor stem cells by secreting IL-6 (53). IL-6 is involved in the progression of many tumors (54–58) and it is an important cytokine in tumor.
JAK2
Janus kinase (JAK) is a non-receptor tyrosine kinase with a molecular weight of 120-140 kDa (59). It can mediate the cascade activation of signal molecules after the binding of cytokines and receptors. JAK kinase family includes four members, namely JAK1, JAK2, JAK3 and TYK2 (60, 61). Among them, JAK1, JAK2 and TYK2 are expressed in any tissue and cell, which is also the basis for their extensive participation in various molecular signal transduction processes. JAK3 is generally expressed only in medullary and lymphoid tissues and is highly expressed in activated T cells, B cells and monocytes (62). In JAK family, JAK2 has become an important target for cancer therapy due to its role in cell growth and survival. Although most solid tumors do not have JAK2 mutations (63–65), more and more evidence shows that abnormal JAK2 signaling acts importantly in solid tumors (66) such as CRC (14, 67, 68), BC (69, 70), GC (71), lung cancer (72) and prostate cancer (73).
STAT3
Signal transducer and activator of transcription (STAT) protein family plays a key role in regulating cytokine dependent inflammation and immunity, consisting of seven members: STAT1, STAT2, STAT3, STAT4, STAT5A, STAT5B and STAT6 (16). Among them, STAT3 is a transcription factor that has been profoundly studied in cancer and inflammation.
STAT3 is a protein composed of 770 amino acids, characterized by the existence of 6 functional conservative domains (74). SRC homology 2 (SH2) is the most conservative STAT domain and plays a key role in signal transduction by binding to specific phosphotyrosine motifs (75). STAT3 is activated by many cytokines and growth factors (76), including cytokines utilizing the IL-6 signal-transducing receptor chain gp130 [such as IL-6 (77, 78), interleukin-11 (78–80), oncostatin M (81)] or homodimeric cytokine receptors [such as granulocyte colony-stimulating factor (G-CSF) (82)], as well as growth factors acting through protein tyrosine kinase receptors (such as epidermal growth factor (77, 83). Thereby, it is involved in carcinogenic signaling pathways and intracellular signal transduction pathways, including IL-11-STAT3 signaling (80), G-CSF-STAT3 pathway (84), NF-κB pathway (85). Once enter the nucleus, the STAT molecule binds to specific promoter DNA sequences, leading to transcription of genes regulating cell proliferation, differentiation, and apoptosis (86–88). Apoptosis related proteins B cell lymphoma-2 (Bcl-2) and Bcl-2 associated protein X (Bax) play an important role in regulating cell survival and are key transcription targets of STAT3 (89, 90). Over-activation of STAT3 can promote tumor growth either directly through tumor autonomic mechanisms or indirectly by modulating antitumor responses of tumor-associated stroma and immune system. Constitutively activated STAT3 in tumor cells not only eliminates anti-tumor immune response by continuously promoting IL-6 (91), IL-10 (91) or vascular endothelial growth factor (VEGF) (92) in the TME, but also transcribes and activates key oncogenes involved in immunosuppression such as programmed cell death-ligand 1 (PD-L1) (93), indoleamine 2, 3-dioxygenase 1 (IDO1) (94). The high expression of STAT3 thus enhances immune escape ability or establishes immune tolerance through a variety of mechanisms, and the inflammatory microenvironment further promotes tumor angiogenesis and the growth, invasion and metastasis of tumor cells (95). Whether in the initial stage of malignant transformation or during the progression of cancer, STAT3 plays a crucial role in selectively inducing and maintaining the carcinogenic inflammatory microenvironment (96).
IL-6/JAK2/STAT3 signaling pathway
IL-6/JAK2/STAT3 signaling pathway plays a crucial role in the development and progression of cancer. JAK2/STAT3 can induce systemic inflammatory response and is associated with the occurrence of tumor cachexia (97, 98). IL-6 binds to membrane receptors and then activates non-receptor tyrosine kinases, including JAK2. These phosphotyrosine residues act as docking sites for STAT3 protein recruitment, and STAT3 protein acts as a cellular mediator of IL-6 (4). Oncogene STAT3 responds to extracellular signals and JAK2 pathway after activation (99). Once tyrosine phosphorylate, the two STAT3 monomers form a dimer, transfer to the nucleus, and then bind to the STAT3 specific DNA response element of the target gene and induce gene transcription (100). Thus, IL-6 induces the activation of its downstream cascade JAK2/STAT3 pathway, contributing to tumorigenesis by regulating cell cycle progression, angiogenesis and tumor cell escape of the immune system (101–103). Over-activation of STAT3 in tumor cells also induces the production of IL-6, resulting in a positive feedback loop (104) (Figure 1). The activation of this signaling pathway plays an important role in cancer cachexia, and it is significantly related to the proliferation, invasion and migration of cancer cells (59, 105).
IL-6/JAK2/STAT3 signaling pathway plays a role in various cancers
A large number of studies have shown that IL-6/JAK2/STAT3 signaling pathway is abnormally highly activated in many types of cancer and strongly inhibits anti-tumor immune response (23). Activation of IL-6/JAK2/STAT3 pathway is closely associated with EMT and stem cell-like features, ultimately leading to poor outcomes in various human cancer patients (33). Through EMT, cancer cells can acquire more invasive characteristics (6). The motility and invasiveness enhanced by EMT are crucial in the metastasis initiation of cancer progression. The acquisition of mesenchymal phenotype also enhances the resistance to chemotherapy and poor prognosis (106, 107).
Liver cancer
The levels of IL-6, phosphorylated JAK2 and phosphorylated STAT3 were significantly increased in liver cancer cells (27). The treatment of liver cancer cells with JAK2 inhibitor and IL-6 neutralizing antibody enhanced the adriamycin-induced aging of cells, and also significantly inhibited the proliferation rate of the cells (27).
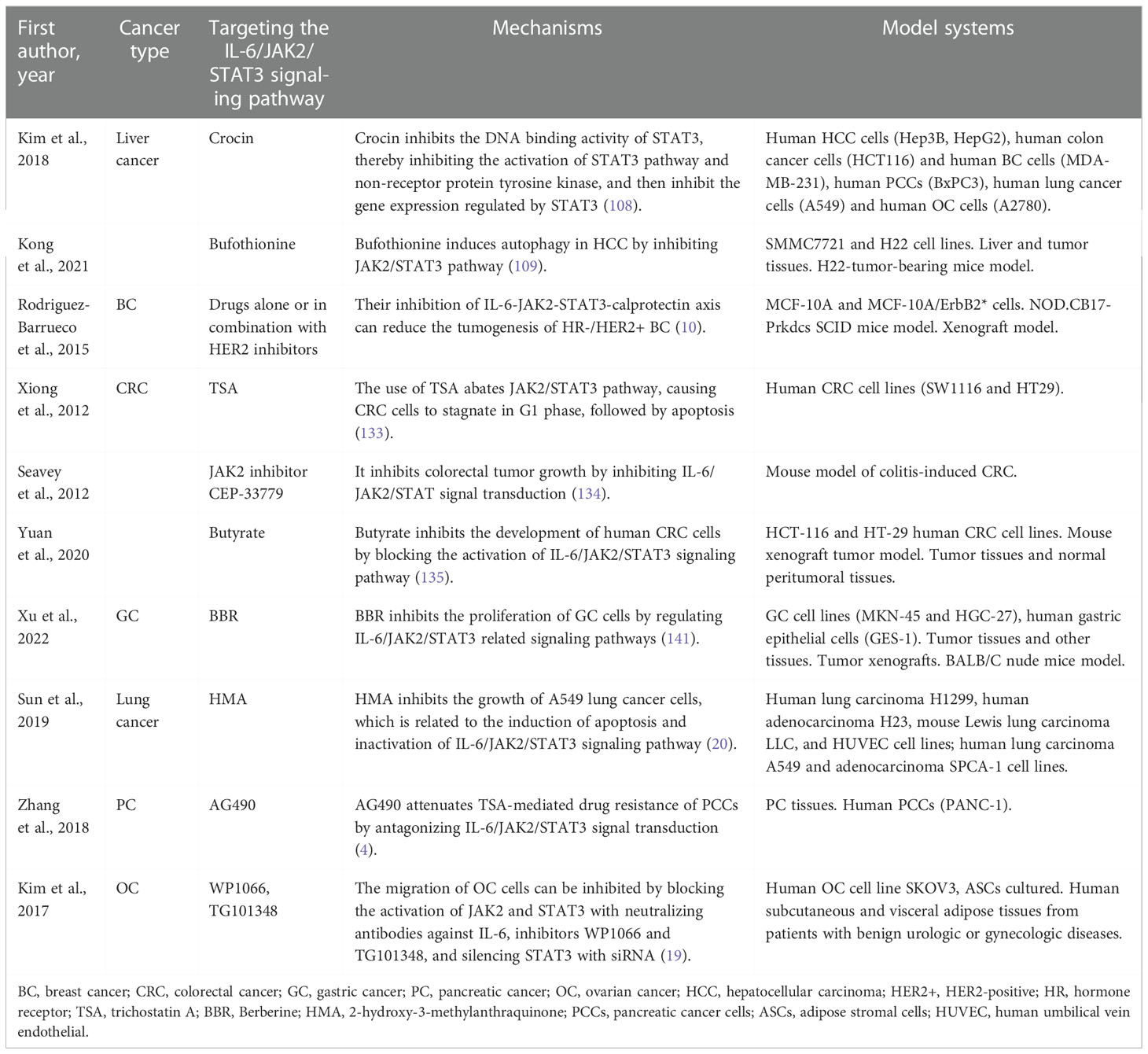
Saffron can promote the apoptosis of liver cancer cells. It is a major glycosyl carotenoid, with a variety of pharmacological effects, such as antioxidant, anti-atherosclerotic, antidepressant and anti-inflammatory activities. Saffron can inhibit the activation of STAT3 pathway and non-receptor protein tyrosine kinase by inhibiting the DNA binding activity of STAT3 in IL-6 stimulated liver cancer cells. Then, it inhibits gene expression regulated by STAT3, downregulates gene expression related to cell proliferation, survival, apoptosis and invasion, activates apoptotic protein-3 and apoptotic protein-9, and thus promotes the dependent apoptosis of liver cancer cells (108). In addition, bufothionine induces autophagy in HCC by inhibiting JAK2/STAT3 pathway (109). These indicate that regulating IL-6/JAK2/STAT3 signaling pathway may be a therapeutic strategy for liver cancer.
Breast cancer
JAK2/STAT3 pathway is necessary for the growth of human CD44+CD24- stem cell like BC cells. IL-6/JAK2/STAT3 pathway is more active in CD44+CD24- BC cells than in other tumor types, and inhibition of JAK2 reduces their numbers and prevents xenograft growth (28). IL6 is involved in the formation of mammary globules enriched with stem cell-like cancer cells (110) and progenitor cells (111). The activation of its downstream effector STAT3 is sufficient and necessary to maintain the undifferentiated status of mouse embryonic stem (ES) cells (112). Matsuda et al. (112) constructed a fusion protein STAT3ER, composed of the entire coding region of STAT3 and the ligand binding domain of the estrogen receptor. ES cells transfected with STAT3ER cultured in the presence of 4-hydroxytamoxifen (4HT) maintained an undifferentiated state (112).
Triple negative BC (TNBC) is an invasive BC subtype with no effective targeted therapy (113, 114). Iron overload may be related to the development of BC to a more malignant phenotype (115). It can promote EMT (the expression of mesenchymal markers N-cadherin, fibronectin and vimentin increased) and migration of MDA-MB-231 cells of TNBC by enhancing IL-6/JAK2/STAT3 signaling pathway (50). Abnormal activation of JAK2/STAT3 signal mediated by IL-6 is positively correlated with EMT and metastasis of human BC (116, 117). In a paracrine or autocrine inflammatory environment rich in IL-6, iron overload can lead to inducible IL-6 expression, thereby promoting the malignant transformation of BC cells.
HER2-positive (HER2+) breast adenocarcinoma is a heterogeneous group, in which the status of hormone receptor (HR) affects the treatment strategy and prognosis of patients. HR−/HER2+ cells secrete high levels of IL-6, which induces the activation of STAT3 and increases the production of calprotectin. The increase of calprotectin level activates the proliferation and resistance pathway. Inhibiting IL-6-JAK2-STAT3-calprotectin axis with drugs alone or in combination with HER2 inhibitors can reduce the tumorigenicity of HR–/HER2+ BC (10).
Homeodomain-containing gene 10 (HOXC10) is related to the progression of a variety of human malignant tumors (118). Its expression is increased in BC and is associated with poor prognosis (119). In vitro and in vivo experiments showed that HOXC10 promoted BC tumorigenesis by activating IL-6/JAK2/STAT3 signals (119). In addition, abnormal P16 expression is related to the metastatic potential of BC (120). The expression of P16 in invasive BC was significantly higher than that in non-invasive BC (120). In BC patients, P16 overexpression is closely associated with tumor invasion into accessory tissues (121). Inhibition of P16 reduced the growth and metastasis potential of BC cells by inhibiting IL-6/JAK2/STAT3 signals (122). It may be a potential therapeutic strategy for BC to affect the oncogenic effect of BC related genes through IL-6/JAK2/STAT3 signaling pathway.
Colorectal cancer
Colon cancer is composed of cancer cells and stromal cells, including endothelial cells, inflammatory cells, bone marrow-derived myeloid cells and myofibroblasts (MFs), such as bone marrow-derived myofibroblasts (BMF) (123, 124). These matrix components create a favorable microenvironment for tumor cell survival and tumor growth at the primary and metastatic site (125). BMF or BMF conditioned medium (BMF-CM) can induce colon cancer cells to form cancer stem cell-like spheres (17). Anti-IL-6 neutralizing antibody, JAK2 inhibitor and STAT3 gene knockout in mouse cancer cells reduced BMF and BMF-CM induced colon cancer cell spheroid formation (17). This indicates that BMF promotes tumorigenesis by activating IL-6/JAK2/STAT3 pathway (17).
CC-MSCs increase the migration and invasion of CRC cells through EMT in vitro, promote the occurrence of CRC, and enhance the growth and metastasis of CRC in vivo (14, 29). IL-6 is the highest expressed cytokine under CC-MSCs conditions. Under the stimulation of IL-6, the phosphorylation levels of JAK2 and STAT3 in CRC cells are increased, and the activation of STAT3 is dose-dependent (126). Activation of STAT3 in CRC cells can be promoted through the IL-6/JAK2/STAT3 signaling pathway. STAT3 inhibitors can attenuate the CRC-promoting effect of CC-MSCs (14).
Serum IL-6 level in CRC patients is significantly increased, and it is positively correlated with the mortality and prognosis of CRC (127, 128). IL-6 can act as a paracrine cytokine to promote the proliferation of CRC cells (129) and enhance EMT mediated CRC invasion and metastasis (130). In CRC mouse model, IL-6 promoted the occurrence of CRC, whereas the knockout of IL-6 or STAT3 gene inhibited CRC (131). STAT3 is constitutively active in CRC (132). Inhibition of JAK2/STAT3 pathway can induce cell cycle arrest and apoptosis of CRC cells (14). The use of histone deacetylase inhibitors trichostatin A (TSA) abated JAK2/STAT3 pathway, causing CRC cells to stagnate in G1 phase, followed by apoptosis (133). JAK2 inhibitor CEP-33779 inhibited colorectal tumor growth by inhibiting IL-6/JAK2/STAT3 signal transduction (134). Butyrate inhibits the development of human CRC cells by blocking the activation of IL-6/JAK2/STAT3 signaling pathway (135). Therefore, blocking IL-6/JAK2/STAT3 signal axis and its biological effects may be a treatment strategy of CRC.
Gastric cancer
High serum IL-6 level is an independent predictor of poor prognosis of GC and GC cells can secrete IL-6, promoting tumor growth, development and migration (136, 137). Jackson et al. (30) detected STAT3 in gastric antrum biopsy and proved that total STAT3 and phosphorylated STAT3 in intestinal GC were increased compared with normal stomach. Zhang et al. (138) also found that activated STAT3 was positive in early GC, poorly differentiated adenocarcinoma and metastatic lymph node tissue. Liu et al. (7) elucidated the potential molecular mechanism of RBMS1 promoting GC metastasis: RBMS1trans-activates IL-6 and stimulates JAK2/STAT3 pathway based on in vitro and in vivo experiments.
Cancer-associated fibroblasts (CAF) is an important regulator of tumor progression (139, 140). CAF isolated from GC produces large amounts of IL-6 (6). CAFs enhance the migration and EMT of GC cells by secreting IL-6, which activates JAK2/STAT3 pathway in GC cells. Deprivation of IL-6 with neutralizing antibodies or inhibition of JAK/STAT3 pathway with specific inhibitor AG490 can significantly attenuate these phenotypes in CAF-induced GC cells (6). In addition, inhibition of IL-6 expression in CAFs or JAK2/STAT3 pathway in GC cells can impair the peritoneal metastasis of tumor induced by CAFs in vivo (6). These suggest that CAF in tumor microenvironment promotes the progress of GC through IL-6/JAK2/STAT3 signal transduction, and IL-6 targeted therapy may become a complementary treatment for GC by acting on stromal fibroblasts (6). Apart from that, berberine (BBR) from Chinese herbal medicine inhibited the proliferation of GC cells by regulating IL-6/JAK2/STAT3 related signal pathway (141), indicating that IL-6/JAK2/STAT3 pathway is significantly important in the treatment of GC.
Lung cancer
IL-6 increases in serum and malignant pleural effusion of patients with lung adenocarcinoma (49, 142). Elevated serum IL-6 levels in patients with lung cancer predict adverse clinical outcomes (143). The level of serum IL-6 in patients with non-small cell lung cancer (NSCLC) decreases significantly after chemotherapy, which is related to reducing cancer recurrence and prolonging survival (144).
The signal changes of pro-inflammatory cytokine transforming growth factor-β (TGF-β) are closely related to various activities concerning cancer onset and migration (145, 146). The JAK/STAT3 signaling pathway in lung cancer cells is regulated by TGF-β (147). TGF-β can promote MFs proliferation (21). MFs will promote the development and progression of cancer (125, 148–150). TGF-β and IL-6/JAK2/STAT3 signal pathway form a positive feedback signal loop, mediating the interaction between MFs and lung cancer cells (21).
In lung cancer, mesenchymal stem cells can enhance tumorigenesis by activating IL-6/JAK2/STAT3 pathway (22). The downregulation of Leucine Zipper Down-Regulated In Cancer 1 (LDOC1) in cancer patients is associated with the low survival rate of lung cancer patients (151). LDOC1 deficiency leads to enhanced IL-6/JAK2/STAT3 loop, through which LDOC1 mediates cancer progression (151). DNA methyltransferase 1 (DNMT1) is related to human tumorigenesis (152). IL-6/JAK2/STAT3pathway enhances the occurrence of cancer and the proliferation of lung cancer stem cells (CSCs) by downregulating p53 and p21, which are cell cycle regulators caused by DNA hypermethylation, and upregulating DNMT1 (153). After blocking IL-6/JAK2/STAT3 pathway and inhibiting DNMT1, the proliferation of lung CSCs, the formation of spheres and the ability to initiate tumor growth decrease (153). These data suggest that targeting IL-6/JAK2/STAT3 signaling pathway and DNMT1 may become an important strategy for the treatment of lung cancer (153).
Sun et al. (20) evaluated the effect of 2-hydroxy-3-methylanthraquinone (HMA) on lung cancer cells in vitro, aiming to test the hypothesis that HMA may partially inhibit the growth, migration and/or invasion of lung cancer cells by downregulating IL-6-induced JAK2/STAT3 pathway. Their results showed that HMA had an effective inhibitory effect on the growth of highly invasive and metastatic A549 lung cancer cells, and significantly inhibited the growth and invasion of A549 lung cancer cells induced by IL-6, which was related to the induced apoptosis and inactivation of IL-6/JAK2/STAT3 signaling pathway (20). Culturing A549 or CL1-5 lung cancer cells with bone marrow mesenchymal stem cells (MSCs) can increase spheroid formation, drug resistance and overexpression of pluripotent markers by activating IL-6/JAK2/STAT3 pathway (22). Blocking the pathway attenuates the tumor-forming ability of A549 and CL1-5 cells (22). Therefore, targeted inhibition of this signal loop may become a new way for the prevention and treatment of lung cancer.
Pancreatic cancer
The pathogenesis of PC is complex. At present, it is believed that inflammatory reaction is closely related to its occurrence, development, metastasis and prognosis, which accelerates the process of the disease (154, 155). Various factors, including pancreatitis and injury, contribute to the development of PC (156). IL-6 is overexpressed in PC patients (157). Its serum level is directly associated with cachexia, advanced tumor and increased mortality in PC patients (5, 86, 158). The increase of IL-6 level is positively correlated with lymph node metastasis, tumor differentiation and vascular invasion of PC (4). IL-6 and its downstream pathways, especially the JAK2/STAT3 pathway, have become the most important factors in the regulation of inflammation-related PC (31).
Regenerating gene protein (REG) 3A plays a role as a tumor promoter in inflammation-related PC (159). After pancreatic inflammatory injury, the expression of REG3A was significantly increased (5). Overexpression of REG3A is associated with excessive proliferation, invasion, migration, distant metastasis and tumor invasiveness (160, 161). The activation of REG3A will enhance the JAK2/STAT3 pathway and form a positive feedback loop of REG3A-JAK2/STAT3, thereby amplifying the carcinogenic effect of IL-6/JAK2/STAT3, and ultimately leads to excessive PC cell proliferation in vitro and in vivo and tumor formation (5).
Androgen receptor (AR) is important for cell migration (86). The expression of AR in PC cells is higher than that in normal pancreatic cells (162). IL-6 enhanced the phosphorylation of STAT3 and mitogen-activated protein kinase (MAPK), thereby enhancing AR-mediated transcription in PC cell lines (86).
Drug resistance is the key reason why PC chemotherapy is in effective. PC cells are resistant to the histone deacetylase (HDAC) inhibitor TSA (163). The expression and phosphorylation of STAT3 were significantly upregulated in TSA-resistant cells compared with TSA non-resistant cells (4). In invasive malignant PC cell lines, a significant increase in IL-6 expression predicts more invasive cell types and poor clinical outcomes (4). Tyrphostin B42, also known as AG490, attenuates TSA-mediated drug resistance in PC cells (PCCS) by antagonizing IL-6/JAK2/STAT3 signal transduction (4). Therefore, targeted inhibition of IL-6/JAK2/STAT3 signaling over-activation may provide a strategy for treating TSA resistance (4). These findings suggest that blocking IL-6/JAK2/STAT3 signaling may inhibit the occurrence and development of PC and play a role in its treatment.
Ovarian cancer
Wang et al. (18) isolated primary OC cells, CAFs and normal fibroblasts (NFs) from fresh cancer tissues and found that CAFs were the main source of IL-6 in OC tissues. CAFs highly secrete IL-6 through JAK2/STAT3 pathway and promote β-TGF-mediated EMT, thereby inhibiting apoptosis (18).
Compared with normal ovaries and benign tumors, JAK2/STAT3 is activated in high-grade OC and is involved in cancer progression and EMT (32). Adipose stromal cells (ASCs) are involved in promoting the growth and migration of OC cells by activating the IL-6/JAK2/STAT3 pathway (19). CA125 is a useful predictor of advanced OC (164). It can bind JAK2 and activate STAT3, highlighting the importance of JAK2 in the pathogenesis of cancer expressing CA125 (165). Therefore, targeting JAK2 with multiple inhibitors may be an important therapeutic strategy to alleviate OC transmission (19) (Tables 1, 2 and Figure 2).
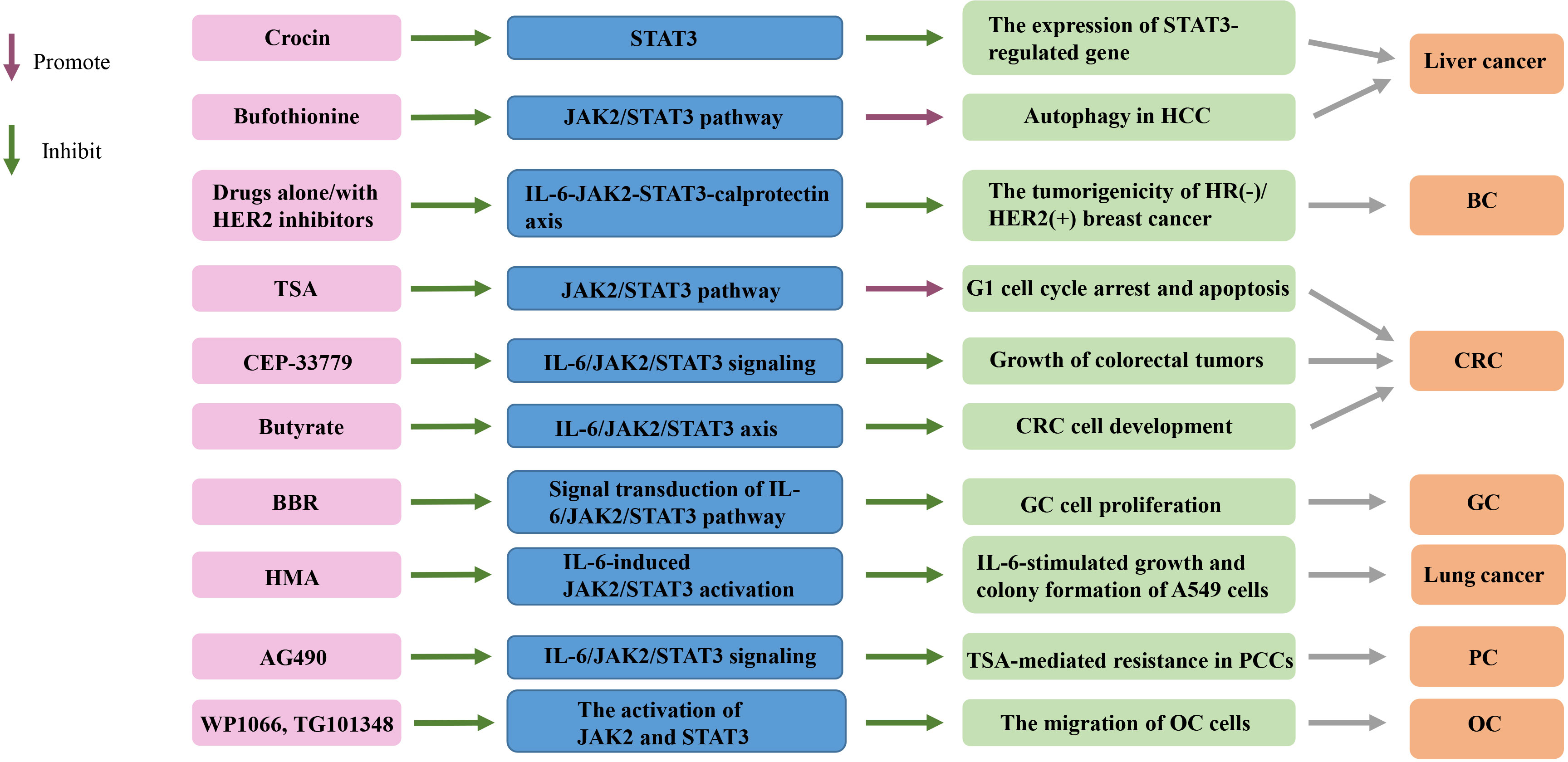
Figure 2 Targeting the IL-6/JAK2/STAT3 signaling pathway in cancers. TSA, trichostatin A; BBR, Berberine; HMA, 2-hydroxy-3-methylanthraquinone; HCC, hepatocellular carcinoma; HR, hormone receptor; HER2+, HER2-positive; CRC, colorectal cancer; GC, gastric cancer; PCCs, pancreatic cancer cells; OC, ovarian cancer; BC, breast cancer.
Conclusions and prospects
Intracellular signal transducers and activators of transcription play a key role in the process of information transmission. IL-6/JAK2/STAT3 signaling pathway deserves attention in the treatment of human cancer. More and more evidence shows that it plays an important role in the invasion and metastasis of many types of tumors. IL-6/JAK2/STAT3 pathway has considerable potential in inhibiting tumor growth and restoring anti-tumor immunity. In recent years, a large number of studies have shown that drug therapy targeting this pathway is effective for various cancers. The specific targeted intervention of related proteins and enzymes in this pathway can develop new ideas for cancer treatment. This signaling pathway can provide reference for tumor mechanism research and drug design, and become one of the directions of cancer treatment research.
Author contributions
BH drafted manuscript and prepared tables. XHL and XLL edited and revised manuscript. BH, XLL, and XHL approved final version of manuscript.
Funding
This study was supported by The National Natural Science Foundation of China: 82071353 (to XHL), The National Key Research and Development Program of China: 2017YFA 0104201 (to XHL), Key Research and Development Projects of Sichuan Province in China: 2021YFS0029 (to XHL).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Li L, Yu R, Cai T, Chen Z, Lan M, Zou T, et al. Effects of immune cells and cytokines on inflammation and immunosuppression in the tumor microenvironment. Int Immunopharmacol (2020) 88:106939. doi: 10.1016/j.intimp.2020.106939
2. Xiao J, Gong Y, Chen Y, Yu D, Wang X, Zhang X, et al. IL-6 promotes epithelial-to-mesenchymal transition of human peritoneal mesothelial cells possibly through the JAK2/STAT3 signaling pathway. Am J Physiol Renal Physiol (2017) 313(2):F310–f8. doi: 10.1152/ajprenal.00428.2016
3. Singh AK, Bhadauria AS, Kumar U, Raj V, Rai A, Kumar P, et al. Novel indole-fused benzo-oxazepines (IFBOs) inhibit invasion of hepatocellular carcinoma by targeting IL-6 mediated JAK2/STAT3 oncogenic signals. Sci Rep (2018) 8(1):5932. doi: 10.1038/s41598-018-24288-0
4. Zhang X, Lu H, Hong W, Liu L, Wang S, Zhou M, et al. Tyrphostin B42 attenuates trichostatin a-mediated resistance in pancreatic cancer cells by antagonizing IL-6/JAK2/STAT3 signaling. Oncol Rep (2018) 39(4):1892–900. doi: 10.3892/or.2018.6241
5. Liu X, Wang J, Wang H, Yin G, Liu Y, Lei X, et al. REG3A accelerates pancreatic cancer cell growth under IL-6-associated inflammatory condition: Involvement of a REG3A-JAK2/STAT3 positive feedback loop. Cancer Lett (2015) 362(1):45–60. doi: 10.1016/j.canlet.2015.03.014
6. Wu X, Tao P, Zhou Q, Li J, Yu Z, Wang X, et al. IL-6 secreted by cancer-associated fibroblasts promotes epithelial-mesenchymal transition and metastasis of gastric cancer via JAK2/STAT3 signaling pathway. Oncotarget (2017) 8(13):20741–50. doi: 10.18632/oncotarget.15119
7. Liu M, Li H, Zhang H, Zhou H, Jiao T, Feng M, et al. RBMS1 promotes gastric cancer metastasis through autocrine IL-6/JAK2/STAT3 signaling. Cell Death Dis (2022) 13(3):287. doi: 10.1038/s41419-022-04747-3
8. Banerjee K, Resat H. Constitutive activation of STAT3 in breast cancer cells: A review. Int J Cancer (2016) 138(11):2570–8. doi: 10.1002/ijc.29923
9. Li H, Yang P, Wang J, Zhang J, Ma Q, Jiang Y, et al. HLF regulates ferroptosis, development and chemoresistance of triple-negative breast cancer by activating tumor cell-macrophage crosstalk. J Hematol Oncol (2022) 15(1):2. doi: 10.1186/s13045-021-01223-x
10. Rodriguez-Barrueco R, Yu J, Saucedo-Cuevas LP, Olivan M, Llobet-Navas D, Putcha P, et al. Inhibition of the autocrine IL-6-JAK2-STAT3-calprotectin axis as targeted therapy for HR-/HER2+ breast cancers. Genes Dev (2015) 29(15):1631–48. doi: 10.1101/gad.262642.115
11. Singh AK, Bhadauria AS, Kumar U, Raj V, Maurya V, Kumar D, et al. Novel fused oxazepino-indoles (FOIs) attenuate liver carcinogenesis via IL-6/JAK2/STAT3 signaling blockade as evidenced through data-based mathematical modeling. Life Sci (2018) 201:161–72. doi: 10.1016/j.lfs.2018.02.029
12. Kang JH, Li MJ, Luan PP, Jiang DK, Chen YW, Xu X, et al. NLRC3 silencing accelerates the invasion of hepatocellular carcinoma cell via IL-6/JAK2/STAT3 pathway activation. Cell Biol Int (2020) 44(10):2053–64. doi: 10.1002/cbin.11414
13. Lin Y, Jian Z, Jin H, Wei X, Zou X, Guan R, et al. Long non-coding RNA DLGAP1-AS1 facilitates tumorigenesis and epithelial-mesenchymal transition in hepatocellular carcinoma via the feedback loop of miR-26a/b-5p/IL-6/JAK2/STAT3 and wnt/β-catenin pathway. Cell Death Dis (2020) 11(1):34. doi: 10.1038/s41419-019-2188-7
14. Zhang X, Hu F, Li G, Li G, Yang X, Liu L, et al. Human colorectal cancer-derived mesenchymal stem cells promote colorectal cancer progression through IL-6/JAK2/STAT3 signaling. Cell Death Dis (2018) 9(2):25. doi: 10.1038/s41419-017-0176-3
15. Raj V, Bhadauria AS, Singh AK, Kumar U, Rai A, Keshari AK, et al. Novel 1,3,4-thiadiazoles inhibit colorectal cancer via blockade of IL-6/COX-2 mediated JAK2/STAT3 signals as evidenced through data-based mathematical modeling. Cytokine (2019) 118:144–59. doi: 10.1016/j.cyto.2018.03.026
16. Fang X, Hong Y, Dai L, Qian Y, Zhu C, Wu B, et al. CRH promotes human colon cancer cell proliferation via IL-6/JAK2/STAT3 signaling pathway and VEGF-induced tumor angiogenesis. Mol Carcinogene (2017) 56(11):2434–45. doi: 10.1002/mc.22691
17. Zhu L, Cheng X, Ding Y, Shi J, Jin H, Wang H, et al. Bone marrow-derived myofibroblasts promote colon tumorigenesis through the IL-6/JAK2/STAT3 pathway. Cancer Lett (2014) 343(1):80–9. doi: 10.1016/j.canlet.2013.09.017
18. Wang L, Zhang F, Cui JY, Chen L, Chen YT, Liu BW. CAFs enhance paclitaxel resistance by inducing EMT through the IL−6/JAK2/STAT3 pathway. Oncol Rep (2018) 39(5):2081–90. doi: 10.3892/or.2018.6311
19. Kim B, Kim HS, Kim S, Haegeman G, Tsang BK, Dhanasekaran DN, et al. Adipose stromal cells from visceral and subcutaneous fat facilitate migration of ovarian cancer cells via IL-6/JAK2/STAT3 pathway. Cancer Res Treat (2017) 49(2):338–49. doi: 10.4143/crt.2016.175
20. Sun C, Yang J, Cheng HB, Shen WX, Jiang ZQ, Wu MJ, et al. 2-Hydroxy-3-methylanthraquinone inhibits lung carcinoma cells through modulation of IL-6-induced JAK2/STAT3 pathway. Phytomed: Int J phytother phytopharmacol (2019) 61:152848. doi: 10.1016/j.phymed.2019.152848
21. Shi J, Feng J, Xie J, Mei Z, Shi T, Wang S, et al. Targeted blockade of TGF-β and IL-6/JAK2/STAT3 pathways inhibits lung cancer growth promoted by bone marrow-derived myofibroblasts. Sci Rep (2017) 7(1):8660. doi: 10.1038/s41598-017-09020-8
22. Hsu HS, Lin JH, Hsu TW, Su K, Wang CW, Yang KY, et al. Mesenchymal stem cells enhance lung cancer initiation through activation of IL-6/JAK2/STAT3 pathway. Lung Cancer (Amsterdam Netherlands) (2012) 75(2):167–77. doi: 10.1016/j.lungcan.2011.07.001
23. Brooks AJ, Putoczki T. JAK-STAT signalling pathway in cancer. Cancers (2020) 12(7):1971. doi: 10.3390/cancers12071971
24. Cao F, Zhang Q, Chen W, Han C, He Y, Ran Q, et al. IL-6 increases SDCBP expression, cell proliferation, and cell invasion by activating JAK2/STAT3 in human glioma cells. Am J Trans Res (2017) 9(10):4617–26.
25. Stanzani E, Martínez-Soler F, Mateos TM, Vidal N, Villanueva A, Pujana MA, et al. Radioresistance of mesenchymal glioblastoma initiating cells correlates with patient outcome and is associated with activation of inflammatory program. Oncotarget (2017) 8(43):73640–53. doi: 10.18632/oncotarget.18363
26. Zhou J, Jiang Y, Zhao J, Zhang H, Fu J, Luo P, et al. Dp44mT, an iron chelator, suppresses growth and induces apoptosis via RORA-mediated NDRG2-IL6/JAK2/STAT3 signaling in glioma. Cell Oncol (Dordrecht) (2020) 43(3):461–75. doi: 10.1007/s13402-020-00502-y
27. Zhang K, Che S, Pan C, Su Z, Zheng S, Yang S, et al. The SHH/Gli axis regulates CD90-mediated liver cancer stem cell function by activating the IL6/JAK2 pathway. J Cell Mol Med (2018) 22(7):3679–90. doi: 10.1111/jcmm.13651
28. Marotta LL, Almendro V, Marusyk A, Shipitsin M, Schemme J, Walker SR, et al. The JAK2/STAT3 signaling pathway is required for growth of CD44+CD24- stem cell-like breast cancer cells in human tumors. J Clin Invest (2011) 121(7):2723–35. doi: 10.1172/JCI44745
29. Jin W. Role of JAK/STAT3 signaling in the regulation of metastasis, the transition of cancer stem cells, and chemoresistance of cancer by epithelial-mesenchymal transition. Cells (2020) 9(1):217. doi: 10.3390/cells9010217
30. Jackson CB, Judd LM, Menheniott TR, Kronborg I, Dow C, Yeomans ND, et al. Augmented gp130-mediated cytokine signalling accompanies human gastric cancer progression. J Pathol (2007) 213(2):140–51. doi: 10.1002/path.2218
31. Holmer R, Goumas FA, Waetzig GH, Rose-John S, Kalthoff H. Interleukin-6: a villain in the drama of pancreatic cancer development and progression. Hepatobil pancreatic Dis international: HBPD Int (2014) 13(4):371–80. doi: 10.1016/S1499-3872(14)60259-9
32. Colomiere M, Ward AC, Riley C, Trenerry MK, Cameron-Smith D, Findlay J, et al. Cross talk of signals between EGFR and IL-6R through JAK2/STAT3 mediate epithelial-mesenchymal transition in ovarian carcinomas. Br J Cancer (2009) 100(1):134–44. doi: 10.1038/sj.bjc.6604794
33. Xia XH, Xiao CJ, Shan H. Facilitation of liver cancer SMCC7721 cell aging by sirtuin 4 via inhibiting JAK2/STAT3 signal pathway. Eur Rev Med Pharmacol Sci (2017) 21(6):1248–53.
34. Zhao H, Guo Y, Li S, Han R, Ying J, Zhu H, et al. A novel anti-cancer agent icaritin suppresses hepatocellular carcinoma initiation and malignant growth through the IL-6/Jak2/Stat3 pathway. Oncotarget (2015) 6(31):31927–43. doi: 10.18632/oncotarget.5578
35. Lee H, Jeong AJ, Ye SK. Highlighted STAT3 as a potential drug target for cancer therapy. BMB Rep (2019) 52(7):415–23. doi: 10.5483/BMBRep.2019.52.7.152
36. Tabassum S, Abbasi R, Ahmad N, Farooqi AA. Targeting of JAK-STAT signaling in breast cancer: Therapeutic strategies to overcome drug resistance. Adv Exp Med Biol (2019) 1152:271–81. doi: 10.1007/978-3-030-20301-6_14
37. Xie Q, Yang Z, Huang X, Zhang Z, Li J, Ju J, et al. Ilamycin c induces apoptosis and inhibits migration and invasion in triple-negative breast cancer by suppressing IL-6/STAT3 pathway. J Hematol Oncol (2019) 12(1):60. doi: 10.1186/s13045-019-0744-3
38. Li J, Huang L, Zhao H, Yan Y, Lu J. The role of interleukins in colorectal cancer. Int J Biol Sci (2020) 16(13):2323–39. doi: 10.7150/ijbs.46651
39. Chou DB, Sworder B, Bouladoux N, Roy CN, Uchida AM, Grigg M, et al. Stromal-derived IL-6 alters the balance of myeloerythroid progenitors during toxoplasma gondii infection. J leukocyte Biol (2012) 92(1):123–31. doi: 10.1189/jlb.1011527
40. Liu F, Poursine-Laurent J, Wu HY, Link DC. Interleukin-6 and the granulocyte colony-stimulating factor receptor are major independent regulators of granulopoiesis in vivo but are not required for lineage commitment or terminal differentiation. Blood (1997) 90(7):2583–90. doi: 10.1182/blood.V90.7.2583
41. Hunter CA, Jones SA. IL-6 as a keystone cytokine in health and disease. Nat Immunol (2015) 16(5):448–57. doi: 10.1038/ni.3153
42. Hirano T, Yasukawa K, Harada H, Taga T, Watanabe Y, Matsuda T, et al. Complementary DNA for a novel human interleukin (BSF-2) that induces b lymphocytes to produce immunoglobulin. Nature (1986) 324(6092):73–6. doi: 10.1038/324073a0
43. Zilberstein A, Ruggieri R, Korn JH, Revel M. Structure and expression of cDNA and genes for human interferon-beta-2, a distinct species inducible by growth-stimulatory cytokines. EMBO J (1986) 5(10):2529–37. doi: 10.1002/j.1460-2075.1986.tb04531.x
44. Ataie-Kachoie P, Pourgholami MH, Richardson DR, Morris DL. Gene of the month: Interleukin 6 (IL-6). J Clin Pathol (2014) 67(11):932–7. doi: 10.1136/jclinpath-2014-202493
45. Ataie-Kachoie P, Pourgholami MH, Morris DL. Inhibition of the IL-6 signaling pathway: A strategy to combat chronic inflammatory diseases and cancer. Cytokine Growth factor Rev (2013) 24(2):163–73. doi: 10.1016/j.cytogfr.2012.09.001
46. Ridker PM, Rane M. Interleukin-6 signaling and anti-Interleukin-6 therapeutics in cardiovascular disease. Circ Res (2021) 128(11):1728–46. doi: 10.1161/CIRCRESAHA.121.319077
47. Wang H, Lathia JD, Wu Q, Wang J, Li Z, Heddleston JM, et al. Targeting interleukin 6 signaling suppresses glioma stem cell survival and tumor growth. Stem Cells (Dayton Ohio) (2009) 27(10):2393–404. doi: 10.1002/stem.188
48. Gao SP, Mark KG, Leslie K, Pao W, Motoi N, Gerald WL, et al. Mutations in the EGFR kinase domain mediate STAT3 activation via IL-6 production in human lung adenocarcinomas. J Clin Invest (2007) 117(12):3846–56. doi: 10.1172/JCI31871
49. Yeh HH, Lai WW, Chen HH, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene (2006) 25(31):4300–9. doi: 10.1038/sj.onc.1209464
50. Cheng M, Liu P, Xu LX. Iron promotes breast cancer cell migration via IL-6/JAK2/STAT3 signaling pathways in a paracrine or autocrine IL-6-rich inflammatory environment. J inorganic Biochem (2020) 210:111159. doi: 10.1016/j.jinorgbio.2020.111159
51. Murakami M, Hibi M, Nakagawa N, Nakagawa T, Yasukawa K, Yamanishi K, et al. IL-6-induced homodimerization of gp130 and associated activation of a tyrosine kinase. Sci (New York NY) (1993) 260(5115):1808–10. doi: 10.1126/science.8511589
52. Zhang C, Xin H, Zhang W, Yazaki PJ, Zhang Z, Le K, et al. CD5 binds to interleukin-6 and induces a feed-forward loop with the transcription factor STAT3 in b cells to promote cancer. Immunity (2016) 44(4):913–23. doi: 10.1016/j.immuni.2016.04.003
53. Jinushi M, Chiba S, Yoshiyama H, Masutomi K, Kinoshita I, Dosaka-Akita H, et al. Tumor-associated macrophages regulate tumorigenicity and anticancer drug responses of cancer stem/initiating cells. Proc Natl Acad Sci USA (2011) 108(30):12425–30. doi: 10.1073/pnas.1106645108
54. Su K, Zhao Q, Bian A, Wang C, Cai Y, Zhang Y. A novel positive feedback regulation between long noncoding RNA UICC and IL-6/STAT3 signaling promotes cervical cancer progression. Am J Cancer Res (2018) 8(7):1176–89.
55. Pencik J, Wiebringhaus R, Susani M, Culig Z, Kenner L. IL-6/STAT3/ARF: the guardians of senescence, cancer progression and metastasis in prostate cancer. Swiss Med weekly (2015) 145:w14215. doi: 10.4414/smw.2015.14215
56. Li R, Huang Y, Lin J. Distinct effects of general anesthetics on lung metastasis mediated by IL-6/JAK/STAT3 pathway in mouse models. Nat Commun (2020) 11(1):642. doi: 10.1038/s41467-019-14065-6
57. Abana CO, Bingham BS, Cho JH, Graves AJ, Koyama T, Pilarski RT, et al. IL-6 variant is associated with metastasis in breast cancer patients. PloS One (2017) 12(7):e0181725. doi: 10.1371/journal.pone.0181725
58. Ge J, Han T, Shan L, Na J, Li Y, Wang J. Long non-coding RNA THOR promotes ovarian cancer cells progression via IL-6/STAT3 pathway. J Ovarian Res (2020) 13(1):72. doi: 10.1186/s13048-020-00672-1
59. Johnson DE, O’Keefe RA, Grandis JR. Targeting the IL-6/JAK/STAT3 signalling axis in cancer. Nat Rev Clin Oncol (2018) 15(4):234–48. doi: 10.1038/nrclinonc.2018.8
60. Lin TE, HuangFu WC, Chao MW, Sung TY, Chang CD, Chen YY, et al. A novel selective JAK2 inhibitor identified using pharmacological interactions. Front Pharmacol (2018) 9:1379. doi: 10.3389/fphar.2018.01379
61. Menet CJ, Rompaey LV, Geney R. Advances in the discovery of selective JAK inhibitors. Prog med Chem (2013) 52:153–223. doi: 10.1016/B978-0-444-62652-3.00004-1
62. Coricello A, Mesiti F, Lupia A, Maruca A, Alcaro S. Inside perspective of the synthetic and computational toolbox of JAK inhibitors: Recent updates. Mol (Basel Switzerland) (2020) 25(15):3321. doi: 10.3390/molecules25153321
63. Lee JW, Soung YH, Kim SY, Nam SW, Park WS, Lee JY, et al. Absence of JAK2 V617F mutation in gastric cancers. Acta Oncol (Stockholm Sweden) (2006) 45(2):222–3. doi: 10.1080/02841860500341223
64. Zhao J, Moch H. Absence of JH2 domain mutation of the tyrosine kinase JAK2 in renal cell carcinomas. Acta Oncol (Stockholm Sweden) (2008) 47(3):474–6. doi: 10.1080/02841860701499390
65. Herreros-Villanueva M, Garcia-Girón C, Er TK. No evidence for JAK2 V617F mutation in colorectal cancer. Br J Biomed Sci (2010) 67(4):220–2. doi: 10.1080/09674845.2010.11978229
66. Harry BL, Eckhardt SG, Jimeno A. JAK2 inhibition for the treatment of hematologic and solid malignancies. Expert Opin investigational Drugs (2012) 21(5):637–55. doi: 10.1517/13543784.2012.677432
67. Park SY, Lee CJ, Choi JH, Kim JH, Kim JW, Kim JY, et al. The JAK2/STAT3/CCND2 axis promotes colorectal cancer stem cell persistence and radioresistance. J Exp Clin Cancer research: CR (2019) 38(1):399. doi: 10.1186/s13046-019-1405-7
68. Liu C, Pan Z, Chen Q, Chen Z, Liu W, Wu L, et al. Pharmacological targeting PTK6 inhibits the JAK2/STAT3 sustained stemness and reverses chemoresistance of colorectal cancer. J Exp Clin Cancer research: CR (2021) 40(1):297. doi: 10.1186/s13046-021-02059-6
69. Wang S, Liang K, Hu Q, Li P, Song J, Yang Y, et al. JAK2-binding long noncoding RNA promotes breast cancer brain metastasis. J Clin Invest (2017) 127(12):4498–515. doi: 10.1172/JCI91553
70. Liu Q, Ai B, Kong X, Wang X, Qi Y, Wang Z, et al. JAK2 expression is correlated with the molecular and clinical features of breast cancer as a favorable prognostic factor. Int Immunopharmacol (2021) 90:107186. doi: 10.1016/j.intimp.2020.107186
71. Ding L, Xu Y, Zhang W, Deng Y, Si M, Du Y, et al. MiR-375 frequently downregulated in gastric cancer inhibits cell proliferation by targeting JAK2. Cell Res (2010) 20(7):784–93. doi: 10.1038/cr.2010.79
72. Wang Y, Zhang Y, Wang Y, Shu X, Lu C, Shao S, et al. Using network pharmacology and molecular docking to explore the mechanism of shan ci gu (Cremastra appendiculata) against non-small cell lung cancer. Front Chem (2021) 9:682862. doi: 10.3389/fchem.2021.682862
73. Udhane V, Maranto C, Hoang DT, Gu L, Erickson A, Devi S, et al. Enzalutamide-induced feed-forward signaling loop promotes therapy-resistant prostate cancer growth providing an exploitable molecular target for Jak2 inhibitors. Mol Cancer Ther (2020) 19(1):231–46. doi: 10.1158/1535-7163.MCT-19-0508
74. Zou S, Tong Q, Liu B, Huang W, Tian Y, Fu X. Targeting STAT3 in cancer immunotherapy. Mol Cancer (2020) 19(1):145. doi: 10.1186/s12943-020-01258-7
75. Sgrignani J, Garofalo M, Matkovic M, Merulla J, Catapano CV, Cavalli A. Structural biology of STAT3 and its implications for anticancer therapies development. Int J Mol Sci (2018) 19(6):9289. doi: 10.3390/ijms19061591
76. Hillmer EJ, Zhang H, Li HS, Watowich SS. STAT3 signaling in immunity. Cytokine Growth factor Rev (2016) 31:1–15. doi: 10.1016/j.cytogfr.2016.05.001
77. Zhong Z, Wen Z, Darnell JE Jr. Stat3: a STAT family member activated by tyrosine phosphorylation in response to epidermal growth factor and interleukin-6. Sci (New York NY) (1994) 264(5155):95–8. doi: 10.1126/science.8140422
78. Heichler C, Scheibe K, Schmied A, Geppert CI, Schmid B, Wirtz S, et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut (2020) 69(7):1269–82. doi: 10.1136/gutjnl-2019-319200
79. Moon EJ, Mello SS, Li CG, Chi JT, Thakkar K, Kirkland JG, et al. The HIF target MAFF promotes tumor invasion and metastasis through IL11 and STAT3 signaling. Nat Commun (2021) 12(1):4308. doi: 10.1038/s41467-021-24631-6
80. Wang D, Zheng X, Fu B, Nian Z, Qian Y, Sun R, et al. Hepatectomy promotes recurrence of liver cancer by enhancing IL-11-STAT3 signaling. EBioMedicine (2019) 46:119–32. doi: 10.1016/j.ebiom.2019.07.058
81. Geethadevi A, Nair A, Parashar D, Ku Z, Xiong W, Deng H, et al. Oncostatin m receptor-targeted antibodies suppress STAT3 signaling and inhibit ovarian cancer growth. Cancer Res (2021) 81(20):5336–52. doi: 10.1158/0008-5472.CAN-21-0483
82. Tian SS, Lamb P, Seidel HM, Stein RB, Rosen J. Rapid activation of the STAT3 transcription factor by granulocyte colony-stimulating factor. Blood (1994) 84(6):1760–4. doi: 10.1182/blood.V84.6.1760.1760
83. Ruff-Jamison S, Zhong Z, Wen Z, Chen K, Darnell JE Jr., Cohen S. Epidermal growth factor and lipopolysaccharide activate Stat3 transcription factor in mouse liver. J Biol Chem (1994) 269(35):21933–5. doi: 10.1016/S0021-9258(17)31735-0
84. Nguyen-Jackson HT, Li HS, Zhang H, Ohashi E, Watowich SS. G-CSF-activated STAT3 enhances production of the chemokine MIP-2 in bone marrow neutrophils. J leukocyte Biol (2012) 92(6):1215–25. doi: 10.1189/jlb.0312126
85. Taniguchi K, Karin M. NF-κB, inflammation, immunity and cancer: Coming of age. Nat Rev Immunol (2018) 18(5):309–24. doi: 10.1038/nri.2017.142
86. Okitsu K, Kanda T, Imazeki F, Yonemitsu Y, Ray RB, Chang C, et al. Involvement of interleukin-6 and androgen receptor signaling in pancreatic cancer. Genes Cancer (2010) 1(8):859–67. doi: 10.1177/1947601910383417
87. Bharadwaj U, Marin-Muller C, Li M, Chen C, Yao Q. Mesothelin overexpression promotes autocrine IL-6/sIL-6R trans-signaling to stimulate pancreatic cancer cell proliferation. Carcinogenesis (2011) 32(7):1013–24. doi: 10.1093/carcin/bgr075
88. Thoennissen NH, Iwanski GB, Doan NB, Okamoto R, Lin P, Abbassi S, et al. Cucurbitacin b induces apoptosis by inhibition of the JAK/STAT pathway and potentiates antiproliferative effects of gemcitabine on pancreatic cancer cells. Cancer Res (2009) 69(14):5876–84. doi: 10.1158/0008-5472.CAN-09-0536
89. Gozgit JM, Bebernitz G, Patil P, Ye M, Parmentier J, Wu J, et al. Effects of the JAK2 inhibitor, AZ960, on Pim/BAD/BCL-xL survival signaling in the human JAK2 V617F cell line SET-2. J Biol Chem (2008) 283(47):32334–43. doi: 10.1074/jbc.M803813200
90. Bai J, Sui J, Demirjian A, Vollmer CM Jr., Marasco W, Callery MP. Predominant bcl-XL knockdown disables antiapoptotic mechanisms: Tumor necrosis factor-related apoptosis-inducing ligand-based triple chemotherapy overcomes chemoresistance in pancreatic cancer cells in vitro. Cancer Res (2005) 65(6):2344–52. doi: 10.1158/0008-5472.CAN-04-3502
91. Wang T, Niu G, Kortylewski M, Burdelya L, Shain K, Zhang S, et al. Regulation of the innate and adaptive immune responses by stat-3 signaling in tumor cells. Nat Med (2004) 10(1):48–54. doi: 10.1038/nm976
92. Wang YS, Chen C, Zhang SY, Li Y, Jin YH. (20S) ginsenoside Rh2 inhibits STAT3/VEGF signaling by targeting annexin A2. Int J Mol Sci (2021) 22(17):69–125. doi: 10.3390/ijms22179289
93. Song TL, Nairismägi ML, Laurensia Y, Lim JQ, Tan J, Li ZM, et al. Oncogenic activation of the STAT3 pathway drives PD-L1 expression in natural killer/T-cell lymphoma. Blood (2018) 132(11):1146–58. doi: 10.1182/blood-2018-01-829424
94. Xiang Z, Zhou Z, Song S, Li J, Ji J, Yan R, et al. Dexamethasone suppresses immune evasion by inducing GR/STAT3 mediated downregulation of PD-L1 and IDO1 pathways. Oncogene (2021) 40(31):5002–12. doi: 10.1038/s41388-021-01897-0
95. Wang Y, Shen Y, Wang S, Shen Q, Zhou X. The role of STAT3 in leading the crosstalk between human cancers and the immune system. Cancer Lett (2018) 415:117–28. doi: 10.1016/j.canlet.2017.12.003
96. Bollrath J, Phesse TJ, von Burstin VA, Putoczki T, Bennecke M, Bateman T, et al. gp130-mediated Stat3 activation in enterocytes regulates cell survival and cell-cycle progression during colitis-associated tumorigenesis. Cancer Cell (2009) 15(2):91–102. doi: 10.1016/j.ccr.2009.01.002
97. Zimmers TA, Fishel ML, Bonetto A. STAT3 in the systemic inflammation of cancer cachexia. Semin Cell Dev Biol (2016) 54:28–41. doi: 10.1016/j.semcdb.2016.02.009
98. Shen H, Guo M, Wang L, Cui X. MUC16 facilitates cervical cancer progression via JAK2/STAT3 phosphorylation-mediated cyclooxygenase-2 expression. Genes Genomics (2020) 42(2):127–33. doi: 10.1007/s13258-019-00885-9
99. Kim BH, Yi EH, Ye SK. Signal transducer and activator of transcription 3 as a therapeutic target for cancer and the tumor microenvironment. Arch pharmacal Res (2016) 39(8):1085–99. doi: 10.1007/s12272-016-0795-8
100. Bowman T, Garcia R, Turkson J, Jove R. STATs in oncogenesis. Oncogene (2000) 19(21):2474–88. doi: 10.1038/sj.onc.1203527
101. Pop VV, Seicean A, Lupan I, Samasca G, Burz CC. IL-6 roles - molecular pathway and clinical implication in pancreatic cancer - a systemic review. Immunol Lett (2017) 181:45–50. doi: 10.1016/j.imlet.2016.11.010
102. Miyamoto Y, Hosotani R, Doi R, Wada M, Ida J, Tsuji S, et al. Interleukin-6 inhibits radiation induced apoptosis in pancreatic cancer cells. Anticancer Res (2001) 21(4a):2449–56.
103. Fofaria NM, Srivastava SK. STAT3 induces anoikis resistance, promotes cell invasion and metastatic potential in pancreatic cancer cells. Carcinogenesis (2015) 36(1):142–50. doi: 10.1093/carcin/bgu233
104. Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, Daly L, et al. The IL-6/JAK/Stat3 feed-forward loop drives tumorigenesis and metastasis. Neoplasia (New York NY) (2013) 15(7):848–62. doi: 10.1593/neo.13706
105. Tsai KS, Yang SH, Lei YP, Tsai CC, Chen HW, Hsu CY, et al. Mesenchymal stem cells promote formation of colorectal tumors in mice. Gastroenterology (2011) 141(3):1046–56. doi: 10.1053/j.gastro.2011.05.045
106. Bae YK, Choi JE, Kang SH, Lee SJ. Epithelial-mesenchymal transition phenotype is associated with clinicopathological factors that indicate aggressive biological behavior and poor clinical outcomes in invasive breast cancer. J Breast Cancer (2015) 18(3):256–63. doi: 10.4048/jbc.2015.18.3.256
107. Zheng X, Carstens JL, Kim J, Scheible M, Kaye J, Sugimoto H, et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature (2015) 527(7579):525–30. doi: 10.1038/nature16064
108. Kim B, Park B. Saffron carotenoids inhibit STAT3 activation and promote apoptotic progression in IL-6-stimulated liver cancer cells. Oncol Rep (2018) 39(4):1883–91. doi: 10.3892/or.2018.6232
109. Kong WS, Shen FX, Xie RF, Zhou G, Feng YM, Zhou X. Bufothionine induces autophagy in H22 hepatoma-bearing mice by inhibiting JAK2/STAT3 pathway, a possible anti-cancer mechanism of cinobufacini. J ethnopharmacol (2021) 270:113848. doi: 10.1016/j.jep.2021.113848
110. Bromberg J, Wang TC. Inflammation and cancer: IL-6 and STAT3 complete the link. Cancer Cell (2009) 15(2):79–80. doi: 10.1016/j.ccr.2009.01.009
111. Sansone P, Storci G, Tavolari S, Guarnieri T, Giovannini C, Taffurelli M, et al. IL-6 triggers malignant features in mammospheres from human ductal breast carcinoma and normal mammary gland. J Clin Invest (2007) 117(12):3988–4002. doi: 10.1172/JCI32533
112. Matsuda T, Nakamura T, Nakao K, Arai T, Katsuki M, Heike T, et al. STAT3 activation is sufficient to maintain an undifferentiated state of mouse embryonic stem cells. EMBO J (1999) 18(15):4261–9. doi: 10.1093/emboj/18.15.4261
113. Bonotto M, Gerratana L, Poletto E, Driol P, Giangreco M, Russo S, et al. Measures of outcome in metastatic breast cancer: Insights from a real-world scenario. oncol (2014) 19(6):608–15. doi: 10.1634/theoncologist.2014-0002
114. den Brok WD, Speers CH, Gondara L, Baxter E, Tyldesley SK, Lohrisch CA. Survival with metastatic breast cancer based on initial presentation, de novo versus relapsed. Breast Cancer Res Treat (2017) 161(3):549–56. doi: 10.1007/s10549-016-4080-9
115. Rehman S, Husnain SM. A probable risk factor of female breast cancer: Study on benign and malignant breast tissue samples. Biol Trace element Res (2014) 157(1):24–9. doi: 10.1007/s12011-013-9865-7
116. Huang C, Yang G, Jiang T, Zhu G, Li H, Qiu Z. The effects and mechanisms of blockage of STAT3 signaling pathway on IL-6 inducing EMT in human pancreatic cancer cells in vitro. Neoplasma (2011) 58(5):396–405. doi: 10.4149/neo_2011_05_396
117. Looyenga BD, Hutchings D, Cherni I, Kingsley C, Weiss GJ, Mackeigan JP. STAT3 is activated by JAK2 independent of key oncogenic driver mutations in non-small cell lung carcinoma. PloS One (2012) 7(2):e30820. doi: 10.1371/journal.pone.0030820
118. Dai BW, Yang ZM, Deng P, Chen YR, He ZJ, Yang X, et al. HOXC10 promotes migration and invasion via the WNT-EMT signaling pathway in oral squamous cell carcinoma. J Cancer (2019) 10(19):4540–51. doi: 10.7150/jca.30645
119. Shen J, Wang M, Li F, Yan H, Zhou J. Homeodomain-containing gene 10 contributed to breast cancer malignant behaviors by activating interleukin-6/Janus kinase 2/Signal transducer and activator of transcription 3 pathway. Bioengineered (2022) 13(1):1335–45. doi: 10.1080/21655979.2021.2016088
120. Shan M, Zhang X, Liu X, Qin Y, Liu T, Liu Y, et al. P16 and p53 play distinct roles in different subtypes of breast cancer. PloS One (2013) 8(10):e76408. doi: 10.1371/journal.pone.0076408
121. Chae SW, Sohn JH, Kim DH, Choi YJ, Park YL, Kim K, et al. Overexpressions of cyclin B1, cdc2, p16 and p53 in human breast cancer: The clinicopathologic correlations and prognostic implications. Yonsei Med J (2011) 52(3):445–53. doi: 10.3349/ymj.2011.52.3.445
122. Wang L, Zhan X, Shen X, Li M, Yang J, Yu W, et al. P16 promotes the growth and mobility potential of breast cancer both in vitro and in vivo: the key role of the activation of IL-6/JAK2/STAT3 signaling. Mol Cell Biochem (2018) 446(1-2):137–48. doi: 10.1007/s11010-018-3281-4
123. Peddareddigari VG, Wang D, Dubois RN. The tumor microenvironment in colorectal carcinogenesis. Cancer Microenviron (2010) 3(1):149–66. doi: 10.1007/s12307-010-0038-3
124. Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell (2011) 144(5):646–74. doi: 10.1016/j.cell.2011.02.013
125. Martin M, Pujuguet P, Martin F. Role of stromal myofibroblasts infiltrating colon cancer in tumor invasion. Pathol Res Pract (1996) 192(7):712–7. doi: 10.1016/S0344-0338(96)80093-8
126. Tantawy MA, El-Sherbeeny NA, Helmi N, Alazragi R, Salem N, Elaidy SM. Synthetic antiprotozoal thiazolide drug induced apoptosis in colorectal cancer cells: Implications of IL-6/JAK2/STAT3 and p53/caspases-dependent signaling pathways based on molecular docking and in vitro study. Mol Cell Biochem (2020) 469(1-2):143–57. doi: 10.1007/s11010-020-03736-4
127. Knüpfer H, Preiss R. Serum interleukin-6 levels in colorectal cancer patients–a summary of published results. Int J colorectal Dis (2010) 25(2):135–40. doi: 10.1007/s00384-009-0818-8
128. Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, et al. Circulating levels of inflammatory cytokines and risk of colorectal adenomas. Cancer Res (2008) 68(1):323–8. doi: 10.1158/0008-5472.CAN-07-2924
129. Schneider MR, Hoeflich A, Fischer JR, Wolf E, Sordat B, Lahm H. Interleukin-6 stimulates clonogenic growth of primary and metastatic human colon carcinoma cells. Cancer Lett (2000) 151(1):31–8. doi: 10.1016/S0304-3835(99)00401-2
130. Rokavec M, Öner MG, Li H, Jackstadt R, Jiang L, Lodygin D, et al. IL-6R/STAT3/miR-34a feedback loop promotes EMT-mediated colorectal cancer invasion and metastasis. J Clin Invest (2014) 124(4):1853–67. doi: 10.1172/JCI73531
131. Grivennikov S, Karin E, Terzic J, Mucida D, Yu GY, Vallabhapurapu S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell (2009) 15(2):103–13. doi: 10.1016/j.ccr.2009.01.001
132. Rodriguez JA, Huerta-Yepez S, Law IK, Baay-Guzman GJ, Tirado-Rodriguez B, Hoffman JM, et al. Diminished expression of CRHR2 in human colon cancer promotes tumor growth and EMT via persistent IL-6/Stat3 signaling. Cell Mol Gastroenterol Hepatol (2015) 1(6):610–30. doi: 10.1016/j.jcmgh.2015.08.001
133. Xiong H, Du W, Zhang YJ, Hong J, Su WY, Tang JT, et al. A histone deacetylase inhibitor, suppresses JAK2/STAT3 signaling via inducing the promoter-associated histone acetylation of SOCS1 and SOCS3 in human colorectal cancer cells. Mol carcinogene (2012) 51(2):174–84. doi: 10.1002/mc.20777
134. Seavey MM, Lu LD, Stump KL, Wallace NH, Hockeimer W, O’Kane TM, et al. Therapeutic efficacy of CEP-33779, a novel selective JAK2 inhibitor, in a mouse model of colitis-induced colorectal cancer. Mol Cancer Ther (2012) 11(4):984–93. doi: 10.1158/1535-7163.MCT-11-0951
135. Yuan Y, Li B, Kuang Y, Ni S, Zhuge A, Yang J, et al. The fiber metabolite butyrate reduces gp130 by targeting TRAF5 in colorectal cancer cells. Cancer Cell Int (2020) 20:212. doi: 10.1186/s12935-020-01305-9
136. Sánchez-Zauco N, Torres J, Gómez A, Camorlinga-Ponce M, Muñoz-Pérez L, Herrera-Goepfert R, et al. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer (2017) 17(1):384. doi: 10.1186/s12885-017-3310-9
137. Li W, Zhang X, Wu F, Zhou Y, Bao Z, Li H, et al. Gastric cancer-derived mesenchymal stromal cells trigger M2 macrophage polarization that promotes metastasis and EMT in gastric cancer. Cell Death Dis (2019) 10(12):918. doi: 10.1038/s41419-019-2131-y
138. Zhang XM, Zhou C, Gu H, Yan L, Zhang GY. Correlation of RKIP, STAT3 and cyclin D1 expression in pathogenesis of gastric cancer. Int J Clin Exp Pathol (2014) 7(9):5902–8.
139. Kalluri R, Zeisberg M. Fibroblasts in cancer. Nat Rev Cancer (2006) 6(5):392–401. doi: 10.1038/nrc1877
140. Cirri P, Chiarugi P. Cancer associated fibroblasts: The dark side of the coin. Am J Cancer Res (2011) 1(4):482–97.
141. Xu M, Ren L, Fan J, Huang L, Zhou L, Li X, et al. Berberine inhibits gastric cancer development and progression by regulating the JAK2/STAT3 pathway and downregulating IL-6. Life Sci (2022) 290:120266. doi: 10.1016/j.lfs.2021.120266
142. Brooks GD, McLeod L, Alhayyani S, Miller A, Russell PA, Ferlin W, et al. IL6 trans-signaling promotes KRAS-driven lung carcinogenesis. Cancer Res (2016) 76(4):866–76. doi: 10.1158/0008-5472.CAN-15-2388
143. Pine SR, Mechanic LE, Enewold L, Chaturvedi AK, Katki HA, Zheng YL, et al. Increased levels of circulating interleukin 6, interleukin 8, c-reactive protein, and risk of lung cancer. J Natl Cancer Institute (2011) 103(14):1112–22. doi: 10.1093/jnci/djr216
144. Su C, Zhou C, Zhou S, Xu J. Serum cytokine levels in patients with advanced non-small cell lung cancer: Correlation with treatment response and survival. Med Oncol (Northwood London England) (2011) 28(4):1453–7. doi: 10.1007/s12032-010-9645-6
145. Karsdal MA, Hjorth P, Henriksen K, Kirkegaard T, Nielsen KL, Lou H, et al. Transforming growth factor-beta controls human osteoclastogenesis through the p38 MAPK and regulation of RANK expression. J Biol Chem (2003) 278(45):44975–87. doi: 10.1074/jbc.M303905200
146. Biswas S, Chytil A, Washington K, Romero-Gallo J, Gorska AE, Wirth PS, et al. Transforming growth factor beta receptor type II inactivation promotes the establishment and progression of colon cancer. Cancer Res (2004) 64(14):4687–92. doi: 10.1158/0008-5472.CAN-03-3255
147. Liu RY, Zeng Y, Lei Z, Wang L, Yang H, Liu Z, et al. JAK/STAT3 signaling is required for TGF-β-induced epithelial-mesenchymal transition in lung cancer cells. Int J Oncol (2014) 44(5):1643–51. doi: 10.3892/ijo.2014.2310
148. Havenith MG, Dingemans KP, Cleutjens JP, Wagenaar SS, Bosman FT. Basement membranes in bronchogenic squamous cell carcinoma: An immunohistochemical and ultrastructural study. Ultrastructural Pathol (1990) 14(1):51–63. doi: 10.3109/01913129009050874
149. Micke P, Ostman A. Tumour-stroma interaction: Cancer-associated fibroblasts as novel targets in anti-cancer therapy? Lung Cancer (Amsterdam Netherlands) (2004) 45 Suppl 2:S163–75. doi: 10.1016/j.lungcan.2004.07.977
150. Rønnov-Jessen L, Petersen OW, Bissell MJ. Cellular changes involved in conversion of normal to malignant breast: Importance of the stromal reaction. Physiol Rev (1996) 76(1):69–125. doi: 10.1152/physrev.1996.76.1.69
151. Lee CH, Yang JR, Chen CY, Tsai MH, Hung PF, Chen SJ, et al. Novel STAT3 inhibitor LDOC1 targets phospho-JAK2 for degradation by interacting with LNX1 and regulates the aggressiveness of lung cancer. Cancers (2019) 11(1):63. doi: 10.3390/cancers11010063
152. Morita R, Hirohashi Y, Suzuki H, Takahashi A, Tamura Y, Kanaseki T, et al. DNA Methyltransferase 1 is essential for initiation of the colon cancers. Exp Mol Pathol (2013) 94(2):322–9. doi: 10.1016/j.yexmp.2012.10.004
153. Liu CC, Lin JH, Hsu TW, Su K, Li AF, Hsu HS, et al. IL-6 enriched lung cancer stem-like cell population by inhibition of cell cycle regulators via DNMT1 upregulation. Int J Cancer (2015) 136(3):547–59. doi: 10.1002/ijc.29033
154. Wang Y, Yang G, You L, Yang J, Feng M, Qiu J, et al. Role of the microbiome in occurrence, development and treatment of pancreatic cancer. Mol Cancer (2019) 18(1):173. doi: 10.1186/s12943-019-1103-2
155. Stone ML, Beatty GL. Cellular determinants and therapeutic implications of inflammation in pancreatic cancer. Pharmacol Ther (2019) 201:202–13. doi: 10.1016/j.pharmthera.2019.05.012
156. Kolodecik T, Shugrue C, Ashat M, Thrower EC. Risk factors for pancreatic cancer: underlying mechanisms and potential targets. Front Physiol (2013) 4:415. doi: 10.3389/fphys.2013.00415
157. Noh KW, Pungpapong S, Wallace MB, Woodward TA, Raimondo M. Do cytokine concentrations in pancreatic juice predict the presence of pancreatic diseases? Clin Gastroenterol Hepatol (2006) 4(6):782–9. doi: 10.1016/j.cgh.2006.03.026
158. Feurino LW, Zhang Y, Bharadwaj U, Zhang R, Li F, Fisher WE, et al. IL-6 stimulates Th2 type cytokine secretion and upregulates VEGF and NRP-1 expression in pancreatic cancer cells. Cancer Biol Ther (2007) 6(7):1096–100. doi: 10.4161/cbt.6.7.4328
159. Wang J, Zhou H, Han Y, Liu X, Wang M, Wang X, et al. SOCS3 methylation in synergy with Reg3A overexpression promotes cell growth in pancreatic cancer. J Mol Med (Berlin Germany) (2014) 92(12):1257–69. doi: 10.1007/s00109-014-1184-8
160. Fukushima N, Koopmann J, Sato N, Prasad N, Carvalho R, Leach SD, et al. Gene expression alterations in the non-neoplastic parenchyma adjacent to infiltrating pancreatic ductal adenocarcinoma. Modern Pathol (2005) 18(6):779–87. doi: 10.1038/modpathol.3800337
161. Xie MJ, Motoo Y, Iovanna JL, Su SB, Ohtsubo K, Matsubara F, et al. Overexpression of pancreatitis-associated protein (PAP) in human pancreatic ductal adenocarcinoma. Digestive Dis Sci (2003) 48(3):459–64. doi: 10.1023/A:1022520212447
162. Corbishley TP, Iqbal MJ, Wilkinson ML, Williams R. Androgen receptor in human normal and malignant pancreatic tissue and cell lines. Cancer (1986) 57(10):1992–5. doi: 10.1002/1097-0142(19860515)57:10<1992::AID-CNCR2820571019>3.0.CO;2-0
163. Wang B, Zou Q, Sun M, Chen J, Wang T, Bai Y, et al. Reversion of trichostatin a resistance via inhibition of the wnt signaling pathway in human pancreatic cancer cells. Oncol Rep (2014) 32(5):2015–22. doi: 10.3892/or.2014.3476
164. Kim HS, Choi HY, Lee M, Suh DH, Kim K, No JH, et al. Systemic inflammatory response markers and CA-125 levels in ovarian clear cell carcinoma: A two center cohort study. Cancer Res Treat (2016) 48(1):250–8. doi: 10.4143/crt.2014.324
Keywords: IL-6/JAK2/STAT3 signaling pathway, liver cancer, breast cancer, colorectal cancer, gastric cancer, lung cancer, pancreatic cancer, ovarian cancer
Citation: Huang B, Lang X and Li X (2022) The role of IL-6/JAK2/STAT3 signaling pathway in cancers. Front. Oncol. 12:1023177. doi: 10.3389/fonc.2022.1023177
Received: 19 August 2022; Accepted: 06 December 2022;
Published: 16 December 2022.
Edited by:
Maria Felice Brizzi, University of Turin, ItalyReviewed by:
Yi-Jia Li, Beckman Research Institute, City of Hope, United StatesRavi Manoharan, University of Madras, India
Larissa Menezes dos Reis, State University of Campinas, Brazil
Copyright © 2022 Huang, Lang and Li. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiaoling Lang, 49137515@qq.com; Xihong Li, lixihonghxey@163.com
 Bei Huang
Bei Huang Xiaoling Lang
Xiaoling Lang Xihong Li
Xihong Li