- 1Department of Obstetrics and Gynecology, Division of Gynecologic Oncology, Arthur G. James Cancer Hospital, The Ohio State University Wexner Medical Center, Columbus, OH, United States
- 2Department of Obstetrics and Gynecology, The Ohio State University Wexner Medical Center, Columbus, OH, United States
Purpose: We sought to evaluate the contribution of mismatch repair (MMR) status to traditional risk stratification algorithms used to predict nodal involvement and recurrence in a large single-institution cohort.
Methods: Endometrioid endometrial cancer (EC) cases from 2014-2020 were evaluated. MMR immunohistochemistry (IHC) was performed universally. Uterine factors assessed in the Mayo criteria were used to retrospectively classify patients as low or high risk for lymphatic spread. Patients were classified according to risk for recurrence using GOG 99 and PORTEC criteria. Associations were evaluated using chi-square and t-tests and contributing factors assessed using logistic regression models.
Results: 1,514 endometrioid EC were evaluated; 392 (25.9%) were MMR (MMR) deficient of which 80.4% of MMR defects were associated with epigenetic silencing of MLH1. Epigenetic MMR defects were significantly more likely to be high risk for lymph node (LN) metastasis based on Mayo criteria (74.9% vs 60.6%, p=<0.001) and with the presence of LN metastasis (20.3 vs 10.5%, p=0.003) compared to MMR proficient tumors. Tumors with epigenetic MMR defects were significantly more likely to be classified as high or high intermediate risk using GOG99 and PORTEC criteria. Furthermore, cases with epigenetic MMR defects classified as low or low intermediate risk were significantly more likely to recur (GOG99 p=0.013; PORTEC p=0.008) and independently associated with worse disease-free survival (DFS). MMR status was found to be independently associated with worse DFS (HR 1.90; 95% CI 1.34-2.70; p=0.003) but not overall survival.
Conclusion: While MMR deficient EC has been associated with poor prognostic features in prior reports; we demonstrate that only epigenetic MMR defects have poorer outcomes. Epigenetic MMR defect were independently associated with lymph node metastasis after controlling for risk criteria. Epigenetic MMR deficiency was found to be an independent predictor of recurrence beyond the factors considered in traditional risk stratification algorithms. Traditional uterine-based risk stratification algorithms may not fully reflect the risk for recurrence in MMR deficient tumors. Consideration should be given to implementing MMR status and MLH1 hypermethylation alongside traditional risk stratification algorithms. Performing MMR IHC on preoperative pathologic specimens may aid in risk stratification and patient counseling.
Introduction
Endometrial cancer (EC) is the most common gynecologic malignancy in the U.S. and more than 66,000 new cases will be diagnosed in 2023. MMR (MMR) deficiency is common in EC, occurring in 20-40% of cases (1, 2). Determination of MMR status in EC has several clinical implications. Loss of expression of MMR proteins may be associated with inherited germline defects in MMR genes (MLH1, MSH2, MSH6, PMS2). Approximately 3-5% of EC may be attributed to Lynch Syndrome (LS), a hereditary cancer predisposition caused by mutations in mismatch repair (MMR) genes. Women with LS have up to a 60% lifetime risk for developing EC as well as a significant risk for colorectal, ovarian, stomach, and other cancers (3–6). EC serves as an important ‘sentinel’ cancer and is the first cancer diagnosed in approximately 50% of women with LS (6, 7). While MMR deficiency in EC is common, the majority of cases can be explained by epigenetic silencing of the MLH1 promoter rather than germline defects (1, 2, 8, 9). While BRAFV600E mutations are frequently implicated in sporadic colorectal cancer (10), BRAF mutations are very rare in EC (0.1%) and testing is not recommended as part of universal screening for LS (11, 12).
Outside of genetic screening, MMR status is an important prognostic biomarker (13–15) and can be used to predict response to immunotherapy (16, 17). Currently, MMR testing is recommended by the National Comprehensive Cancer Network (NCCN) as a complement to morphologic assessment of EC and is used to separate EC into one of four molecular subgroups (POLE mutated, MMR-deficient/Microsatellite instability-high, copy number low, and copy number high) (18, 19). Our group, and others, have reported on the association between MMR deficiency and a number of poor prognostic indicators routinely used to guide the decision for adjuvant therapy in endometrioid EC. Epigenetic MMR defects have been associated with diagnosis at an older age, the presence of lymphovascular space invasion (LVSI), and higher-grade tumors, as well as diagnosis at a more advanced stage. EC with MLH1 hypermethylation has also been associated with larger tumor volumes increasing the risk for lymph node metastasis (9, 20). However, even with these poor prognostic features, data regarding outcomes in EC with MMR deficiency and epigenetic MMR defects have been inconsistent (9, 21). While many groups have reported on reduced recurrence free survival in EC with MMR defects others have reported that there is no effect or even an improvement in OS in these tumors (11, 22–33).
We sought to determine if MMR status might add to traditional risk stratification algorithms used to predict risk for lymph node metastasis and recurrence in a large, single-institution cohort.
Materials and methods
This was an institutional review board-approved retrospective review from the Ohio State University Comprehensive Cancer Center (OSUCCC) from June 1, 2014 to December 31, 2020. All patients who underwent surgery for an EC diagnosis at our institution were included. Clinical and demographic data were abstracted from medical records. Electronic health information exchange (HIE) was used to access medical records from outside institutions where available. A portion of this cohort was included in previous reports (20, 22).
Universal MMR IHC testing for protein expression of MLH1, PMS2, MSH2, and MSH6 was performed clinically on all EC specimens for LS screening as standard of care. Tumors with loss of expression of MLH1 or PMS2 on IHC underwent reflex MLH1 methylation testing using methylation-specific PCR to triage for genetics referral. MMR status of tumors was classified as MMR proficient (normal) if there was intact expression of MMR proteins. Patients’ tumors with loss of MLH1/PMS2 on IHC and methylation of the MLH1 promoter region were classified as having an epigenetic MMR defect. Tumors with abnormal IHC without MLH1 methylation were classified as MMR deficient due to a probable MMR mutation (probable Lynch syndrome or double somatic mutation).
The criteria established by Mariani et al. from the Mayo Clinic (i.e. tumor diameter, grade, and depth of invasion) were used to retrospectively classify patients as low or high risk for lymphatic spread (23). Patients were classified as low risk for lymph node metastasis if they were without evidence of extrauterine disease, with primary tumor diameter ≤2cm, FIGO grade 1 or 2 histology, and ≤50% myometrial invasion. Tumor grade and depth of myometrial invasion were abstracted from the final pathology report. Tumor size was based on hysterectomy gross tumor specimen measurements recorded by the evaluating pathologist. Tumor volume was calculated using the maximum tumor measurements for 3 lengths as previously described (20). Subjects were classified according to GOG99 and PORTEC risk criteria as previously reported (34–36). Briefly, patients were classified as high intermediate risk (HIR) by GOG 99 depending on age and the number of risk factors (grade 2 or 3 tumor, the presence of LVSI, and outer 1/3 myometrial invasion). Patients were classified as high intermediate risk by PORTEC if they had 2 of 3 clinicopathologic factors: age > 60 years, ≥50% myometrial invasion, and grade 3 histology.
Clinical-pathologic relationships were assessed using χ2, Fisher’s exact test, and t test. Where data were not normally distributed, the Wilcoxon signed-rank test was utilized. The Kaplan-Meier product limit was used to estimate survival. The log-rank test was used to test for differences in survival. Multivariable logistic regression models were developed, and odds ratios (ORs) were used to evaluate the risk factors associated with recurrence. Cox proportional hazard models were used to assess variables associated with disease-free (DFS) and overall survival (OS). All statistical analyses were performed using JMP® Pro, Version 15.2.0. SAS Institute Inc., Cary, NC, 2019.
Results
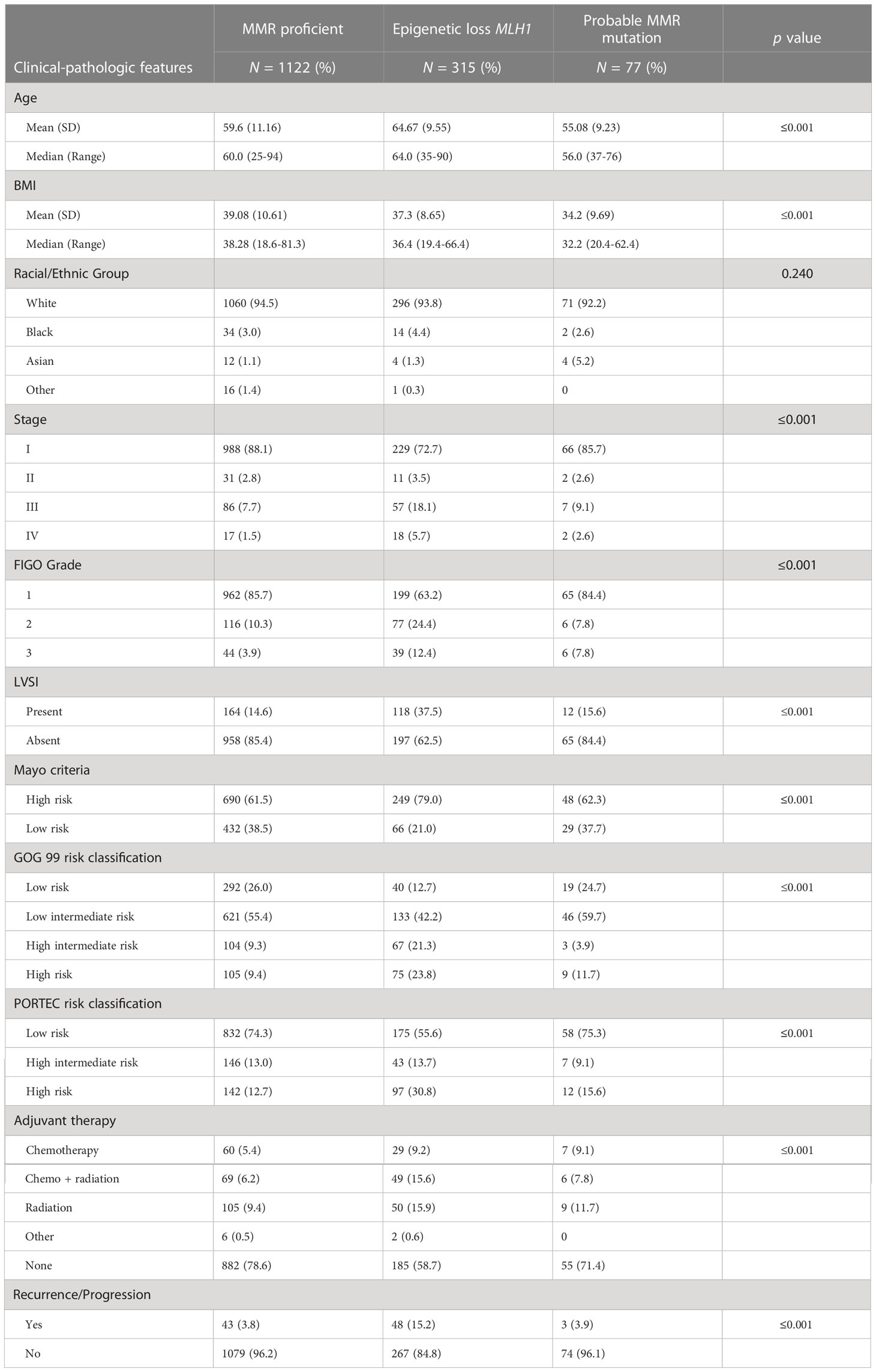
Data was collected for 1,718 ECs; for the purposes of this study analyses were limited to endometrioid histology EC (N=1,514). The median follow-up time was 2.5 years (range 20 days to 7.8 years). The clinical and pathologic features of the entire cohort, stratified by MMR status are presented in Table 1. Most patients were obese (82%), were stage I at diagnosis (83%), and had grade I tumors (81%). Three-hundred ninety-two (25.9%) patients’ tumors demonstrated MMR defect based on IHC. Eighty percent (315/392) of those were associated with MLH1 hypermethylation and classified as epigenetic MMR defects. Seventy-seven patients had MMR IHC loss of expression without MLH1 hypermethylation suggestive of MMR mutations. IHC staining for these cases revealed 15 with loss of MLH1/PMS2 without MLH1 hypermethylation, 9 with isolated loss of PMS2 without MLH1 hypermethylation, 25 with loss of MSH2/MSH6, and 28 with isolated loss of MSH6 staining. Of those 77 patients, germline testing results were available in 53 cases, 38 of whom (2.5% of the entire cohort) had confirmed LS. MSH6-related LS was diagnosed in 15 cases, PMS2-related LS in 14 cases, MSH2-related LS in 7 cases, and MLH1-related LS in 2 cases.
The only significant difference between patients with a probable MMR deficiency and those who were MMR proficient was age (median 56 vs 60 years, p=0.009) and BMI (median 32.2 vs 38.3, p=0.003). EC with probable MMR deficiency did not differ from MMR proficient EC in terms of stage, grade, LVSI, the receipt of adjuvant therapy, Mayo risk criteria, GOG 99, or PORTEC risk criteria. Comparatively, ECs with epigenetic loss of MLH1 were significantly more likely to be diagnosed at a more advanced stage (23.8%), with higher grade tumors (12.4%), with LVSI (37.5%), and to receive adjuvant therapy (41.2%) (Table 1).
MMR status and traditional risk stratification algorithms
Mayo criteria
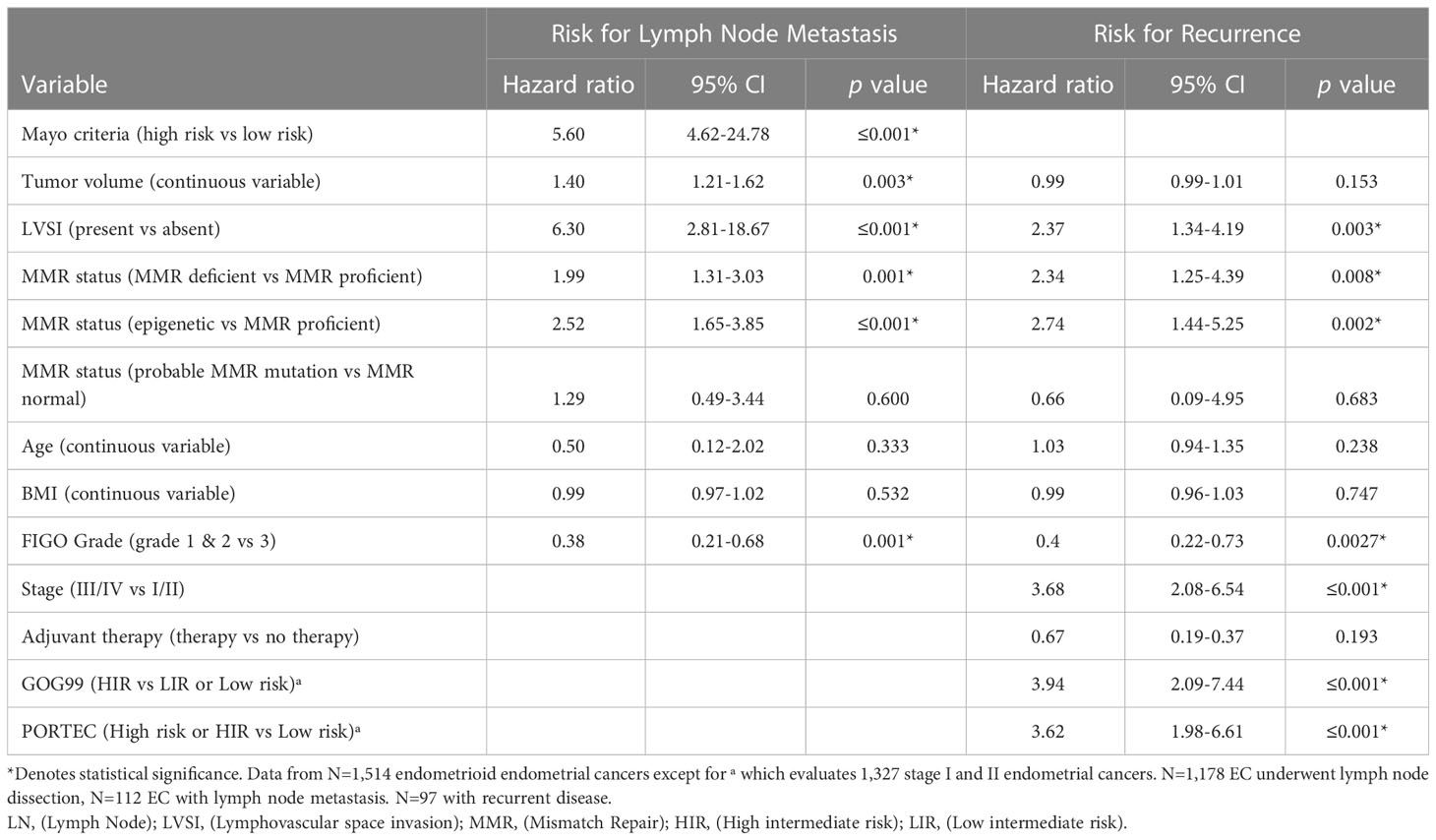
Given the reported increased risk of lymph node (LN) metastasis in EC with epigenetic loss of MLH1 we evaluated the contribution of MMR status to Mayo criteria. Mayo criteria published by Mariani et al. has been used to identify patients which may safely be excluded from routine lymphadenectomy due to low risk of LN metastasis (37). We evaluated 1,477 endometrioid EC without preoperative evidence of advanced disease (without evidence of metastatic disease or lymphadenopathy on preoperative imaging). The majority of EC in our cohort (77.8%) underwent LN assessment (sentinel lymph node biopsy or full lymphadenectomy) regardless of MAYO criteria risk. MMR deficient EC were significantly more likely to be deemed high-risk for lymph node metastasis by Mayo criteria (74.9% vs 60.6%, p=≤0.001) (Table 1). However, there was no significant difference in the characteristics that resulted in exclusion from the low-risk group (ie. Tumor size, myometrial invasion, grade) between MMR deficient and MMR proficient EC. In addition, in patients at high risk for lymphatic spread by Mayo criteria, ECs with epigenetic MMR defect were significantly more likely to have LN metastasis (20.3% vs 10.5%, p=0.003). There was no significant difference in the rate of LN metastasis between patients with probable MMR mutation and MMR proficient EC after selecting for those at high-risk by Mayo criteria (15.4% vs 10.5%, p=0.369). While Mayo criteria is not used routinely to omit lymph node assessment in our practice due to high utilization of sentinel lymphadenectomy, there was a significantly higher rate of lymphadenectomy in patients with MMR deficient EC compared to MMR proficient EC (83.7% vs 74.1%, p=≤0.001) reflecting the impact that intraoperative assessment of tumor volume may have in surgical decision making. Sixty-seven percent of patients at low risk by Mayo criteria underwent surgical lymph node assessment. There were 4 cases of lymph node metastases in patients deemed low risk by Mayo criteria; two of these occurred in patients with epigenetic MMR defect, and two in MMR proficient EC. The false negative rate of Mayo criteria in epigenetic MMR defects was 3.9% (compared to 0.7% in MMR proficient) (HR 5.44, 95% CI 0.78-37.8, p=0.105). There were two retroperitoneal recurrences that could be related to undiagnosed lymphatic spread in patients who did not undergo lymph node assessment; both patients were high-risk by Mayo criteria but did not undergo lymphatic dissection due to inadequate visualization and medical comorbidities. Both patients had MMR deficient tumors (one epigenetic loss and one with a probable MMR mutation). A nominal logistic regression model was used to evaluate the risk for lymph node metastasis. Epigenetic MMR defect was found to be an independent risk factor for lymph node metastasis (HR 2.52; 95% CI 1.65-3.85; p=≤0.001) after controlling for risk group by Mayo criteria, LVSI, and tumor volume (Table 2).
GOG99 and PORTEC risk classification
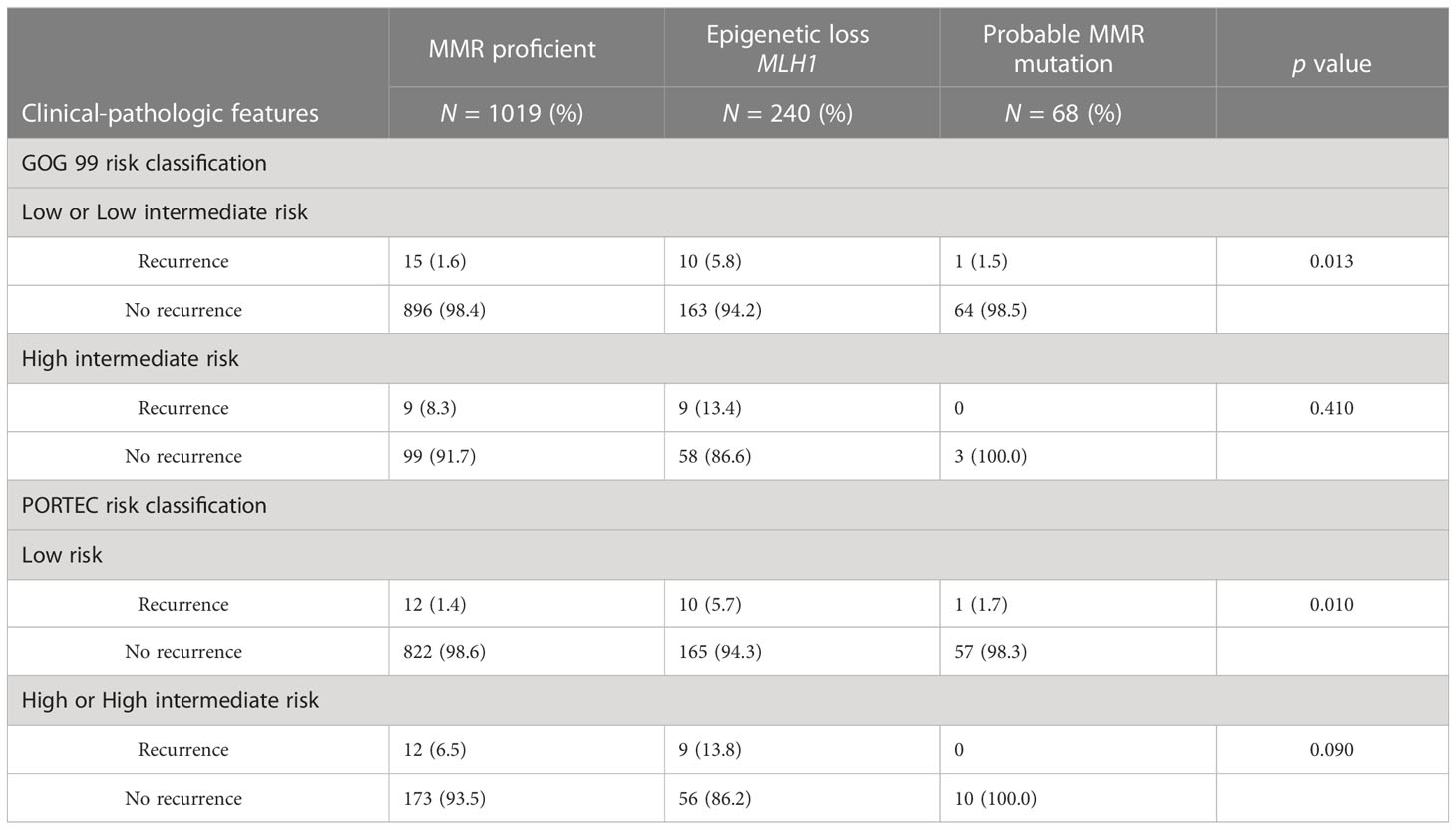
Adjuvant radiation has not been shown to improve survival in early-stage disease (34–36) and the role of adjuvant therapy in early-stage endometrial cancer remains uncertain. The GOG99 and PORTEC studies evaluated the role of adjuvant therapy in early-stage endometrial cancer (34–36) and identified patients that would benefit from adjuvant radiation to decrease the risk for pelvic recurrence. These trials arrived at different (but overlapping) criteria to determine high intermediate risk. We categorized the 1,327 early-stage endometrioid EC in our cohort according to the GOG99 and PORTEC criteria: Eighty seven percent were deemed low or low intermediate risk by GOG99 criteria, and 80.9% low risk by PORTEC criteria. MMR deficient ECs were significantly more likely to meet GOG99 HIR criteria (23.1% vs 10.3%, p=≤0.001) and high risk or HIR by PORTEC (23.4% vs 17.8%, p=0.004) (Table 1). A nominal logistic regression model was used to evaluate the risk of recurrence after controlling for risk classification. After controlling for GOG99 classification and PORTEC classification, MMR status as a dichotomous variable was found to be an independent risk factor for recurrence, (HR 2.34; 95% CI 1.25-4.39; p=0.008). When MMR status was evaluated as a trichotomous variable only epigenetic loss (rather than probable MMR mutation) remained independently associated with recurrence (HR 2.74; 95% CI 1.44-5.25; p=0.002) after controlling for GOG99 and PORTEC classification (Table 2). Epigenetic loss of MLH1 also demonstrated significant association with recurrence after correcting for receipt of any adjuvant therapy and type of adjuvant therapy. Indeed, EC with epigenetic MMR defect had a statistically and clinically meaningful increased rate of recurrence in early-stage EC compared to MMR proficient EC (7.9% vs 2.4%, p=0.005) (Table 3).
Survival
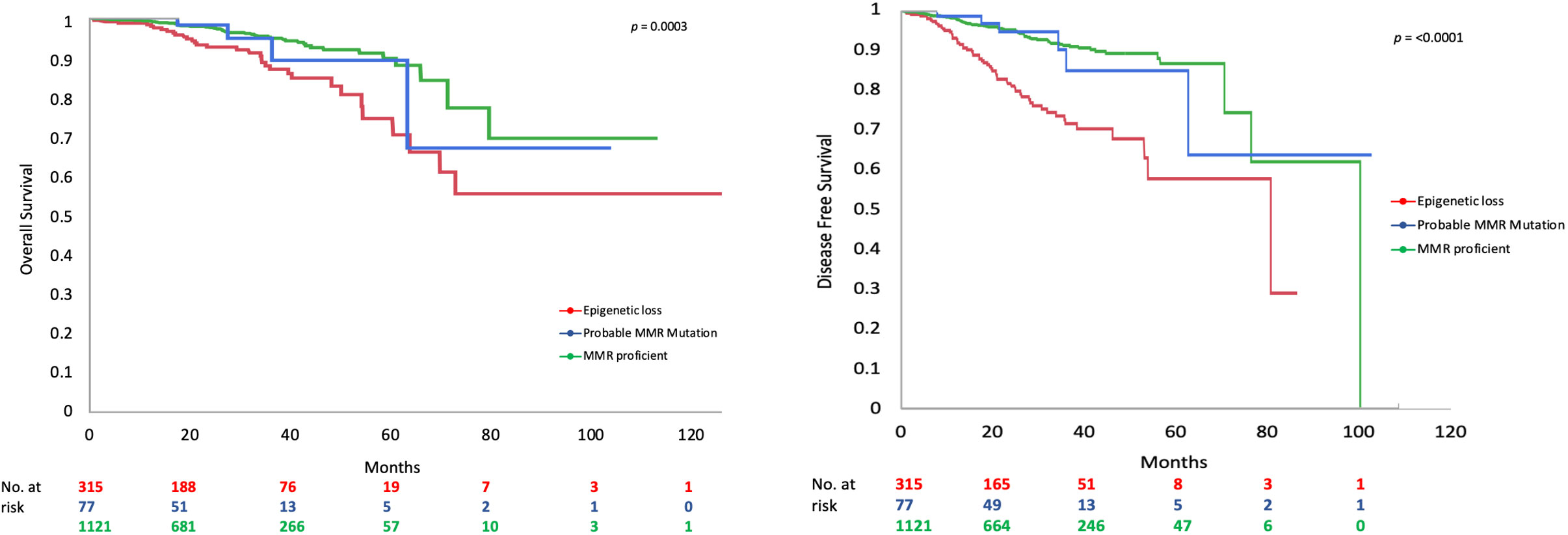
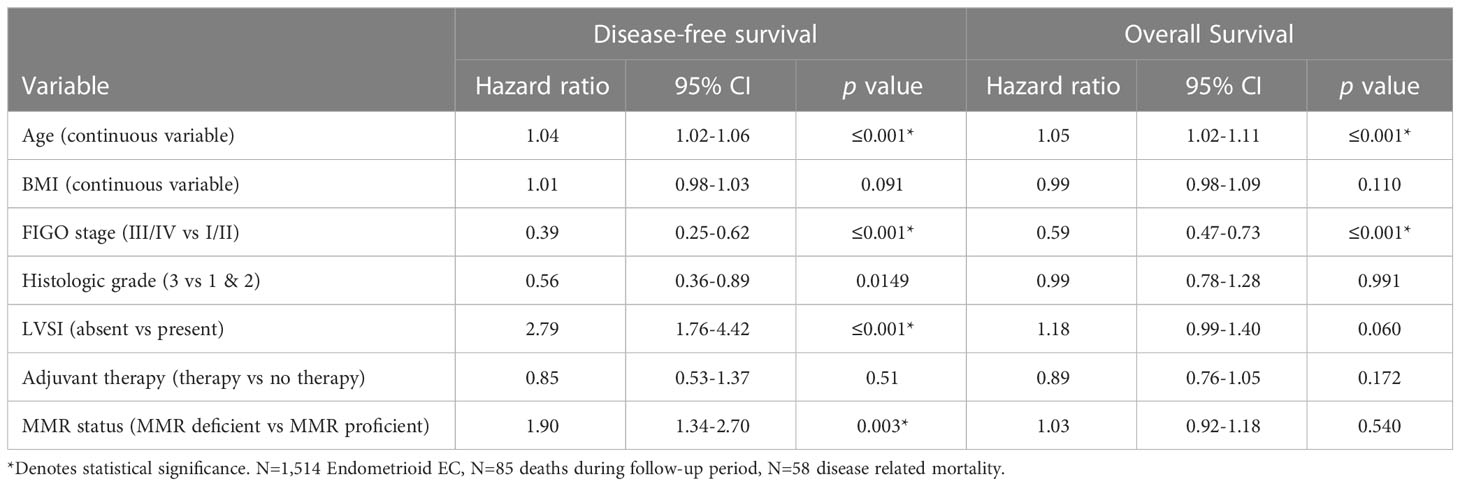
MMR deficient endometrioid EC had worse DFS (Figure 1) and OS than MMR proficient EC (OS data not shown). Only 65.0% of MMR deficient tumors were disease free at 5 years compared to 88.1% of MMR proficient tumors (p=≤0.001). This detrimental effect appears to be driven by the behavior of EC with epigenetic loss of MLH1. The 5-year DFS of EC with epigenetic loss was 57.5% compared to 85% in EC with probable MMR mutation and 88.1% in MMR proficient EC (p=≤0.001). The 5-year OS for EC with epigenetic loss of MLH1 was 74.6% compared to 89.1% for EC with probable MMR mutation and 90% for MMR proficient EC (p=0.003). Univariate analysis revealed that MMR status, age, BMI, stage, grade, and LVSI were significantly associated with survival. When the six factors significant in univariate analysis were included in multivariable analysis (along with adjuvant therapy) MMR status was found to be independently associated with DFS but not OS (HR 1.90; 95% CI 1.34-2.70; p=0.003) (Table 4).
In early-stage endometrioid EC, MMR deficiency was associated with significantly worse OS and DFS (Supplementary Figure S1). The effect of MMR deficiency on DFS was evaluated via Cox proportional hazard ratios; epigenetic loss of MLH1 was independently associated with worse DFS in early-stage EC after controlling for GOG99 and PORTEC risk classification (HR 2.75; 95% CI 1.69-4.48; p=≤0.001). Most striking, in patients at low and low-intermediate risk by GOG 99 criteria there was a significantly increased risk for recurrence and worse DFS (5-year DFS 80.8% vs 94.6% at 5 years, p=0.004). The effect of MMR deficiency on recurrence risk persisted when evaluating patients at low-risk for recurrence by PORTEC criteria (5-year DFS 70.8% vs 95.4%, p=≤0.001). Due to the relatively few patients with early-stage EC and probable MMR mutations who recurred (N=3), we are unable to comment on the effect of probable MMR mutation and recurrence risk.
Discussion
In this study, we confirm and expand on prior reports that epigenetic MMR deficiency in EC is associated with poor prognostic features (9, 24, 38, 39). However, for the first time we demonstrate that MMR deficiency was an independent predictor for lymph node metastasis and recurrence after controlling for these prognostic factors through traditional risk stratification algorithms.
In patients at high risk for LN metastasis by Mayo criteria, EC with epigenetic MMR defect was twice as likely to have LN metastasis (20% vs 10.5%). Tumor size is an established prognostic factor for lymph node involvement and thus has been integrated into risk stratification algorithms used to identify women in whom surgical lymphadenectomy can be safely omitted (23, 25). Indeed, the significantly different rates of lymphadenectomy between MMR deficient tumors (83.7%) and MMR proficient EC (74.1%) illustrates the effect tumor volume may have on surgical decision-making. Our group has previously reported on the association of epigenetic MMR defects with large tumor volume and lymph node metastasis (20) but in this study, we identify that epigenetic MMR defect is associated with lymph node metastasis independent of tumor volume. Recently, Diniz et al. advocated for the use of MMR status to triage patients who require lymphadenectomy (26); however, their analyses did not differentiate between epigenetic loss and those with probable MMR mutation. The association between epigenetic MMR deficits and LN positivity seen in our study suggests that the 22% (15/69) MMR deficient EC with positive LN in their study may largely be attributed to epigenetic MMR deficits.
Factors such as advanced age, higher grade, LVSI, and deeper myometrial invasion are utilized to predict patients at high risk for recurrence despite early stage disease. This study, and others (9, 13, 22, 27, 28), confirms that MMR deficient EC is associated negative prognostic indicators. However, we found that patients with probable MMR mutation did not differ from those with MMR proficient EC in terms of stage, grade, LVSI, and myometrial invasion. Rather, epigenetic MMR defects were the driver for the association of MMR defects with these negative prognostic factors. Given that these prognostic factors are accounted for through risk stratification algorithms (GOG99 and PORTEC) utilized to predict the risk for recurrence and guide adjuvant therapy in early-stage endometrioid EC, we sought to evaluate the role of MMR status after controlling for these factors. We found that there was not a significant difference between MMR proficient EC and EC with probable MMR mutations according to GOG99 and PORTEC risk classification. However, ECs with epigenetic MMR defects were significantly more likely to be classified as high or HIR by traditional risk stratification algorithms; 23.1% vs 10.3% (p=≤0.001) for GOG99 and 23.4% vs 17.8% (p=0.004) for PORTEC criteria. Epigenetic MMR defects were strongly associated with worse DFS in early-stage endometrioid EC independent of risk classification and the receipt of adjuvant therapy.
Finally, when we evaluated all endometrioid ECs, we found that MMR deficiency was independently associated with worse DFS but not OS after controlling for age, BMI, grade, LVSI, stage, and adjuvant therapy.
The association between MMR status and disease recurrence and survival has been extensively studied in a variety of malignancies. Although MMR deficiency has been associated with better prognosis in colorectal cancer its prognostic significance in EC is unclear (29). While many studies have reported worse DFS in patients with MMR deficiency (9, 20, 30, 39) other studies have reported improved outcomes (21, 31, 32, 38). A meta-analysis (33) that sought to evaluate the role of MMR status and clinical outcomes in EC highlights some possible reasons for these discrepancies. Many studies include very small sample sizes (the median sample size in the aforementioned meta-analysis was 112 subjects), heterogeneous patient populations including endometrioid and non-endometrioid histologies, and inconsistent methods for determining and classifying MMR status. Our study has several strengths that we feel empower the findings: (1) The large sample size of more than 1,500 ECs, (2) only endometrioid histology ECs were isolated to avoid histology as a confounding factor, (3) MMR status was classified using the expression of all 4 MMR proteins, and (4) universal MLH1 methylation testing was performed. Our study does have some important limitations to address including the relatively modest number of recurrences and the very limited number of recurrences in patients with a probable MMR mutation limits the ability to extrapolate the risk profile in this group. In addition, while the median follow-up period of 2.5 years is relatively short, data has shown that the majority of recurrences will occur within 2 years of initial diagnosis.
Conclusion
Traditional uterine-based risk stratification algorithms may not accurately reflect the risk for lymph node metastasis and recurrence in EC with epigenetic MMR defects. Our findings advocate for the use of molecular classification and MMR testing alongside traditional risk stratification algorithms based on uterine factors. Given the high concordance between MMR IHC status by preoperative biopsy compared to definitive surgical specimen (40–42) these findings also highlight the role of MMR testing on preoperative biopsy specimens to facilitate risk-stratification and patient-centered counseling.
Data availability statement
The raw data supporting the conclusions of this article will be made available by the authors, without undue reservation.
Ethics statement
The studies involving human participants were reviewed and approved by The Ohio State University Institutional Review Board. Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
Concept and design (CC, CR, PH). Acquisition, analysis, or interpretation of data (all authors). Drafting of the manuscript (CC, CR). Critical revision of the manuscript for important intellectual content (all authors). Statistical and data analysis (CR, CC). Study supervision (CC, PG, DC). All authors contributed to the article and approved the submitted version.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fonc.2023.1147657/full#supplementary-material
Supplementary Figure 1 | OS and DFS in early-stage EC by MMR status.
References
1. Backes FJ, Leon ME, Ivanov I, Suarez A, Frankel WL, Hampel H, et al. Prospective evaluation of DNA mismatch repair protein expression in primary endometrial cancer. Gynecologic Oncol (2009) 114(3):486–90. doi: 10.1016/j.ygyno.2009.05.026
2. Buchanan DD, Tan YY, Walsh MD, Clendenning M, Metcalf AM, Ferguson K, et al. Tumor mismatch repair immunohistochemistry and DNA MLH1 methylation testing of patients with endometrial cancer diagnosed at age younger than 60 years optimizes triage for population-level germline mismatch repair gene mutation testing. J Clin Oncol (2014) 32(2):90–100. doi: 10.1200/JCO.2013.51.2129
3. Lancaster JM, Powell CB, Chen LM, Richardson D, SGO Clinical Practice Committee, et al. Society of gynecologic oncology statement on risk assessment for inherited gynecologic cancer predispositions. Gynecologic Oncol (2015) 136(1):3–7. doi: 10.1016/j.ygyno.2014.09.009
4. Aarnio M, Sankila R, Pukkala E, Salovaara R, Aaltonen LA, Chapelle A, et al. Cancer risk in mutation carriers of DNA-mismatch-repair genes. Int J Cancer (1999) 81(2):214–8. doi: 10.1002/(SICI)1097-0215(19990412)81:2<214::AID-IJC8>3.0.CO;2-L
5. Hampel H, Stephens JA, Pukkala E, Sankila R, Aaltonen LA, Mecklin JP, et al. Cancer risk in hereditary nonpolyposis colorectal cancer syndrome: Later age of onset. Gastroenterology (2005) 129(2):415–21. doi: 10.1016/j.gastro.2005.05.011
6. Meyer LA, Broaddus RR, Lu KH. Endometrial cancer and lynch syndrome: clinical and pathologic considerations. Cancer Control (2009) 16(1):14–22. doi: 10.1177/107327480901600103
7. Lu KH, Dinh M, Kohlmann W, Watson P, Green J, Syngal S, et al. Gynecologic cancer as a "sentinel cancer" for women with hereditary nonpolyposis colorectal cancer syndrome. Obstet Gynecol (2005) 105(3):569–74. doi: 10.1097/01.AOG.0000154885.44002.ae
8. Levine DA, the Cancer Genome Atlas Research Network. Integrated genomic characterization of endometrial carcinoma. Nature (2013) 497(7447):67–73. doi: 10.1038/nature12113
9. McMeekin DS, Tritchler DL, Cohn DE, Mutch DG, Lankes HA, Geller MA, et al. Clinicopathologic significance of mismatch repair defects in endometrial cancer: An NRG Oncology/Gynecologic oncology group study. J Clin Oncol (2016) 34(25):3062–8. doi: 10.1200/JCO.2016.67.8722
10. Yan HHN, Lai JCW, Ho SL, Leung WK, Law WL, Lee JFY, et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut (2017) 66(9):1645–56. doi: 10.1136/gutjnl-2016-311849
11. Metcalf AM, Spurdle AB. Endometrial tumour BRAF mutations and MLH1 promoter methylation as predictors of germline mismatch repair gene mutation status: a literature review. Fam Cancer (2014) 13(1):1–12. doi: 10.1007/s10689-013-9671-6
12. Yan HHM, Lai JCW, Ho SL, Leung WK, Law WL, Lee JFY, et al. RNF43 germline and somatic mutation in serrated neoplasia pathway and its association with BRAF mutation. Gut (2017) 66(9):1645–56.
13. Loukovaara M, Pasanen A, Bützow R. Mismatch repair deficiency as a predictive and prognostic biomarker in molecularly classified endometrial carcinoma. Cancers (2021) 13(13). doi: 10.3390/cancers13133124
14. Backes FJ, Haag J, Cosgrove CM, Suarez A, Cohn DE, Goodfellow PJ, et al. Mismatch repair deficiency identifies patients with high-intermediate-risk (HIR) endometrioid endometrial cancer at the highest risk of recurrence: A prognostic biomarker. Cancer (2019) 125(3):398–405. doi: 10.1002/cncr.31901
15. Njoku K, Barr CE, Crosbie EJ. Current and emerging prognostic biomarkers in endometrial cancer. Front Oncol (2022) 12. doi: 10.3389/fonc.2022.890908
16. Le DT, Durham JN, Smith KN, Wang H, Bartlett BR, Aulakh LK, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science (2017) 357(6349):409–13. doi: 10.1126/science.aan6733
17. Green AK, Feinberg J, Makker V. A review of immune checkpoint blockade therapy in endometrial cancer. Am Soc Clin Oncol Educ Book (2020) 40):238–44. doi: 10.1200/EDBK_280503
18. Murali R, Delair DF, Bean SM, Abu-Rustum NR, Soslow RA. Evolving roles of histologic evaluation and Molecular/Genomic profiling in the management of endometrial cancer. J Natl Compr Canc Netw (2018) 16(2):201–9. doi: 10.6004/jnccn.2017.7066
19. National Comprehensive Cancer Network. Uterine NEoplasms. (2023) (Version 1.2023). Available at: https://www.nccn.org/professionals/physician_gls/pdf/uterine.pdf.
20. Cosgrove CM, Cohn DE, Hampel H, Frankel WL, Jones D, McElroy JP, et al. Epigenetic silencing of MLH1 in endometrial cancers is associated with larger tumor volume, increased rate of lymph node positivity and reduced recurrence-free survival. Gynecologic Oncol (2017) 146(3):588–95. doi: 10.1016/j.ygyno.2017.07.003
21. Fountzilas E, Kotoula V, Pentheroudakis G, Manousou K, Polychronidou G, Vrettou E, et al. Prognostic implications of mismatch repair deficiency in patients with nonmetastatic colorectal and endometrial cancer. ESMO Open (2019) 4(2):e000474. doi: 10.1136/esmoopen-2018-000474
22. Backes FJ, Haag J, Cosgrove CM, Suarez A, Cohn DE, Goodfellow PJ, et al. Mismatch repair deficiency identifies patients with high-intermediate–risk (HIR) endometrioid endometrial cancer at the highest risk of recurrence: A prognostic biomarker. Cancer (2019) 125(3):398–405. doi: 10.1002/cncr.31901
23. Mariani A, Dowdy SC, Cliby WA, Gostout BS, Jones MB, Wilson TO, et al. Prospective assessment of lymphatic dissemination in endometrial cancer: a paradigm shift in surgical staging. Gynecol Oncol (2008) 109(1):11–8. doi: 10.1016/j.ygyno.2008.01.023
24. Bilbao C, Lara PC, Ramirez R, Henriquez-Hernandez LA, Rodriguez F, Falcon O, et al. Microsatellite instability predicts clinical outcome in radiation-treated endometrioid endometrial cancer. Int J Radiat OncologyBiologyPhysics (2010) 76(1):9–13. doi: 10.1016/j.ijrobp.2009.09.035
25. Milam MR, Java J, Walker JL, Metzinger DS, Parker LP, Coleman RL, et al. Nodal metastasis risk in endometrioid endometrial cancer. Obstetrics Gynecology (2012) 119(2 Part 1):286–92. doi: 10.1097/AOG.0b013e318240de51
26. Diniz TP, Menezes JN, Goncalves BT, Faloppa CC, Mantoan H, Kumagai LY, et al. Can mismatch repair status be added to sentinel lymph node mapping algorithm in endometrioid endometrial cancer? Gynecologic Oncol (2023) 169:131–6. doi: 10.1016/j.ygyno.2022.12.010
27. Kim SR, Pina A, Albert A, McAlpine JN, Wolber R, Gilks B, et al. Mismatch repair deficiency and prognostic significance in patients with low-risk endometrioid endometrial cancers. Int J Gynecologic Cancer (2020) 30(6):783–8. doi: 10.1136/ijgc-2019-000910
28. Berg HF, Engerud H, Myrvold M, Lien HE, Hjelmeland ME, Halle MK, et al. Mismatch repair markers in preoperative and operative endometrial cancer samples; expression concordance and prognostic value. Br J Cancer (2022). doi: 10.1038/s41416-022-02063-3
29. Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol (2005) 23(3):609–18. doi: 10.1200/JCO.2005.01.086
30. Caduff RF, Johnston CM, Svoboda-Newman SM, Poy EL, Merajver SD, Frank TS, et al. Clinical and pathological significance of microsatellite instability in sporadic endometrial carcinoma. Am J Pathol (1996) 148(5):1671–8.
31. Maxwell GL, Rinsinger JI, Alvarez AA, Barrett JC, Berchuck A. Favorable survival associated with microsatellite instability in endometrioid endometrial cancers. Obstet Gynecol (2001) 97(3):417–22. doi: 10.1016/s0029-7844(00)01165-0
32. Kato M, Takano M, Miyamoto M, Sasaki N, Goto T, Tsuda H, et al. DNA Mismatch repair-related protein loss as a prognostic factor in endometrial cancers. J Gynecol Oncol (2015) 26(1):40–5. doi: 10.3802/jgo.2015.26.1.40
33. Diaz-Padilla I, Romero N, Amir E, Matias-Guiu X, Vilar E, Muggia F., et al. Mismatch repair status and clinical outcome in endometrial cancer: a systematic review and meta-analysis. Crit Rev Oncol Hematol (2013) 88(1):154–67. doi: 10.1016/j.critrevonc.2013.03.002
34. Keys HM, Roberts JA, Brunetto VL, Zaino RJ, Spirtos NM, Bloss JD, et al. A phase III trial of surgery with or without adjunctive external pelvic radiation therapy in intermediate risk endometrial adenocarcinoma: a gynecologic oncology group study. Gynecol Oncol (2004) 92(3):744–51. doi: 10.1016/j.ygyno.2003.11.048
35. Creutzberg CL, Putten WL, Koper PC, Lybeert ML, Jobsen JJ, Warlam-Rodenhuis CC, et al. Surgery and postoperative radiotherapy versus surgery alone for patients with stage-1 endometrial carcinoma: multicentre randomised trial. PORTEC study group. post operative radiation therapy in endometrial carcinoma. Lancet (2000) 355(9213):1404–11. doi: 10.1016/S0140-6736(00)02139-5
36. Nout RA, Smit VTHBM, Putter H, Jurgenliemk-Schulz IM, Jobsen JJ, Lutgens LCHW, et al. Vaginal brachytherapy versus pelvic external beam radiotherapy for patients with endometrial cancer of high-intermediate risk (PORTEC-2): an open-label, non-inferiority, randomised trial. Lancet (2010) 375(9717):816–23. doi: 10.1016/S0140-6736(09)62163-2
37. Mariani A, Webb MJ, Keeney GL, Haddock MG, Calori G, Podratz KC, et al. Low-risk corpus cancer: is lymphadenectomy or radiotherapy necessary? Am J Obstet Gynecol (2000) 182(6):1506–19. doi: 10.1067/mob.2000.107335
38. Black D, Soslow RA, Levine DA, Tornos C, Chen SC, Hummer AJ, et al. Clinicopathologic significance of defective DNA mismatch repair in endometrial carcinoma. J Clin Oncol (2006) 24(11):1745–53. doi: 10.1200/JCO.2005.04.1574
39. Cohn DE, Frankel WL, Resnick KE, Zanagnolo VL, Copeland LJ, Hampel H, et al. Improved survival with an intact DNA mismatch repair system in endometrial cancer. Obstetrics Gynecology (2006) 108(5):1208–15. doi: 10.1097/01.AOG.0000239097.42987.0c
40. Stelloo E, Nout RA, Naves LCLM, Ter Haar NT, Creutzberg CL, Smit VTHBM, et al. High concordance of molecular tumor alterations between pre-operative curettage and hysterectomy specimens in patients with endometrial carcinoma. Gynecologic Oncol (2014) 133(2):197–204. doi: 10.1016/j.ygyno.2014.02.012
41. Abdulfatah E, Wakeling E, Sakr S, Al-Abaidy K, Bandyopadhyay S, Morris R, et al. Molecular classification of endometrial carcinoma applied to endometrial biopsy specimens: Towards early personalized patient management. Gynecologic Oncol (2019) 154(3):467–74. doi: 10.1016/j.ygyno.2019.06.012
42. Chapel DB, Yamada SD, Cowan M, Lastra R. Immunohistochemistry for mismatch repair protein deficiency in endometrioid endometrial carcinoma yields equivalent results when performed on endometrial biopsy/curettage or hysterectomy specimens. Gynecologic Oncol (2018) 149(3):570–4. doi: 10.1016/j.ygyno.2018.04.005
Keywords: mismatch repair deficiency (MMR), epigenetic loss, Lynch syndrome, biomarker, risk stratification, endometrial cancer
Citation: Riedinger CJ, Brown M, Haight PJ, Backes FJ, Cohn DE, Goodfellow PJ and Cosgrove CM (2023) Epigenetic MMR defect identifies a risk group not accounted for through traditional risk stratification algorithms in endometrial cancer. Front. Oncol. 13:1147657. doi: 10.3389/fonc.2023.1147657
Received: 19 January 2023; Accepted: 10 March 2023;
Published: 06 April 2023.
Edited by:
Christina Therkildsen, Copenhagen University Hospital, DenmarkReviewed by:
Jon Ambæk Durhuus, Hvidovre Hospital, DenmarkStephanie M. McGregor, University of Wisconsin-Madison, United States
Copyright © 2023 Riedinger, Brown, Haight, Backes, Cohn, Goodfellow and Cosgrove. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Courtney J. Riedinger, courtney.riedinger@osumc.com
 Courtney J. Riedinger
Courtney J. Riedinger Morgan Brown
Morgan Brown Paulina J. Haight
Paulina J. Haight Floor J. Backes1
Floor J. Backes1 Paul J. Goodfellow
Paul J. Goodfellow Casey M. Cosgrove
Casey M. Cosgrove