The Immediate Effects of a Dynamic Orthosis on Gait Patterns in Children With Unilateral Spastic Cerebral Palsy: A Kinematic Analysis
- 1Faculdade de Motricidade Humana, Universidade de Lisboa, Lisbon, Portugal
- 2Escola Superior de Saúde do Alcoitão, Lisbon, Portugal
- 3Faculdade de Motricidade Humana, CIPER, Universidade de Lisboa, Lisbon, Portugal
- 4Universidade Europeia, Lisbon, Portugal
- 5Department of Biomechanics, University of Nebraska, Omaha, NE, United States
This study analyzes the immediate effects of wearing a Therasuit on sagittal plane lower limb angular displacements during gait in children with unilateral spastic cerebral palsy (US-CP). Seven participants (median age = 7.00 years; ranging from 5.83 to 9.00 years) with US-CP, levels I and II of the Gross Motor Function Classification System, were assessed with kinematic gait analysis in three different conditions: (A) Baseline; (B) Therasuit without elastics and (C) Therasuit with elastics. Significant improvements were observed at the hip joint of both lower limbs during most of the gait cycle in participants wearing a Therasuit, including a decrease in the flexion pattern at the initial contact and swing phase in both lower limbs, and an increase in the extension pattern in the paretic lower limb during the stance phase. At the knee joint in the paretic lower limb, significant differences were found between the baseline and Therasuit with elastics conditions on the knee angle at initial contact, and between baseline and both Therasuit conditions on the flexion angle at swing phase. However, the inter-individual variability in kinematic patterns at the knee joint was high. At the ankle joint, decreased plantar flexion at initial contact and increased dorsiflexion during stance and swing phases were observed at the Therasuit with elastics condition, helping to correct the equinus-foot in the paretic lower limb during the whole gait cycle. The Z-values showed large effect sizes particularly for most of the angular hip variables in both lower limbs and for the angular ankle variables in the paretic lower limb. The Therasuit seems to have some positive immediate effects on gait kinematics in children with spastic unilateral cerebral palsy by providing a more functional and safer gait pattern. Future investigations with larger samples are recommended to further support these findings.
Introduction
Cerebral Palsy (CP) is a group of pediatric disorders presenting movement and posture symptoms caused by disturbances occurring during the development of the fetal or infant brain. Children with unilateral (or hemiplegic) spastic CP (US-CP) have multiple physical impairments, including muscle weakness, sensory loss, and spasticity, in the upper and lower limbs of one side of the body (1), typically on the opposite side of the brain injury (2). Asymmetry between the paretic and the non-paretic sides is common (3), particularly a decrease in muscle volume on the paretic side (4, 5) and lower limb length discrepency (6). US-CP is the most common syndrome in children born at term and is second in frequency only to spastic diplegia among preterm infants (7–9).
Although “hemiplegia” refers to disorders affecting only one side of the body, hemiplegic children often also have motor impairments on the non-paretic side, particularly in more severe types of hemiplegia, which are typically characterized by altered gait patterns in both lower limbs (10). Compensatory movement patterns are adopted which often result in functional difficulty and musculoskeletal dysfunction. Physical therapy aims to maximize functional independence and minimize secondary complications (11).
New technologies have been introduced in rehabilitation programs to promote and/or enhance the engagement of children with CP in a variety of physical activities and tasks. For instance, dynamic orthoses (12) address different CP-related problems: balance control (11); limb symmetry, walking speed and cadence (13); trunk control (14); motor function in all categories of Gross Motor Function Measure (15); and self-care (16). Furthermore, since the 1990s, different types of therapeutic suits have been used in children with CP (16–18), such as Theratogs (TTs) and Therasuit® (TS). TTs are custom manufactured lycra garments covering the trunk and limbs that exert a compressive force on the body (16–19). The TS is a soft dynamic proprioceptive orthotic (including vest, shorts, knee pads, and specially adapted shoes) that aligns the body by placing pressure on specific areas through a system of interconnected elastic cords (13). The TS was created from a prototype developed for Russian astronauts to counter the effects of long-term weightlessness on the body while in space (13, 16, 20). The elastic cords are systematically adjusted based on the individual needs and functional limitations of the child (16). The TS Method was designed to be used in intensive rehabilitation programs, including vigorous strengthening and stretching exercises, and training of specific motor activities (13). However, despite the popularity of short intensive TS therapy interventions, a recent systematic review and meta-analysis showed they have small effects on the functional skills of children and adolescents with CP (21). The authors raised concern about the lack of evidence for the beneficial effects of this dynamic orthosis, and suggested that specific immediate effects of TS therapy on postural control and gait should be analyzed. The aim of this study is to determine the immediate effects of wearing TS on joint angle displacements in both lower limbs during gait in children with US-CP. The results from this analysis may provide evidence to support the applicability of TS in physical therapy interventions, more specifically on gait training programs.
Materials and Methods
Participants
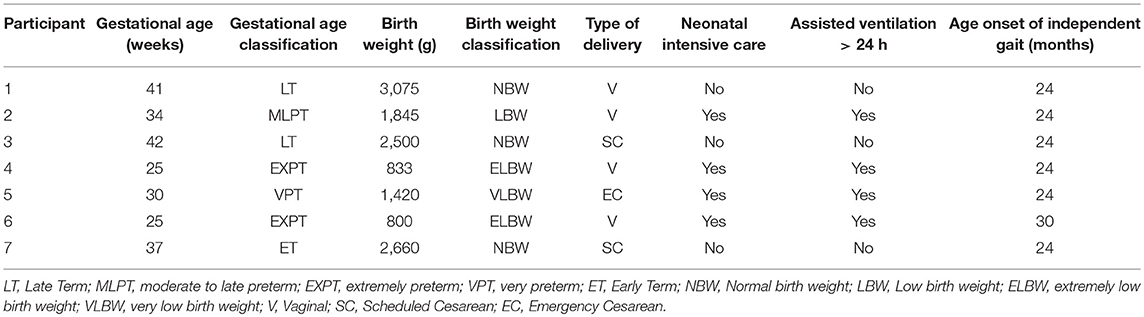
Seven participants (3 females and 4 males, mean age = 7.17 ± 1.01 yrs; ranging from 5 to 9 years) with a medical diagnosis of Unilateral Spastic Cerebral Palsy, level I and II of the Gross Motor Function Classification System (GMFCS), were evaluated using a quasi-experimental study design with one pre-test condition (baseline) and two post-test conditions (Therasuit without elastics and Therasuit with elastics). Participants 1, 2, and 3 had a medical diagnosis of left hemiparesis, and participants 4, 5, 6, and 7 had a medical diagnosis of right hemiparesis.
Inclusion criteria were: (a) unilateral spastic cerebral palsy; (b) ability to walk independently (level I or II according to GMFCS); (c) age range from 5 to 10 years old; (d) cognitive level and emotional state facilitating understanding and cooperation of the participant; (e) no prior experience with this type of dynamic orthosis (TS) before this study. Exclusion Criteria were: (a) another medical diagnosis, types and sub-types of spastic CP; (b) congenital heart disease and cardiorespiratory problems; (c) presence of structural deformities at the lower limbs and trunk, or instability in the ankle joint, which could compromise the child's safety and performance of the motor task; (d) epilepsy; (e) treatment with botulinum toxin in the calf muscles within the previous 6 months; (f) surgical intervention (e.g. tendon lengthening in lower limb) within the previous 12 months; (g) muscle tone scored ≥2, according to Modified Ashworth Scale; (h) severe affective or psychiatric impairments; (i) serious vision or hearing problems. The previous clinical history of the participants is presented on Table 1.
The Ethical Committee of the Rehabilitation Medicine Center in Alcoitão (CMRA), Portugal (PT) ensured and approved the conformity procedures regarding scientific research involving human beings. Parental consents and children assents were obtained. A convenience sample was used. Children that were attending the CMRA and who met the inclusion criteria were invited to participate.
Procedure
Data Collection
The protocol included two phases of data collection: (1) parental interview and clinical examination; (2) gait analyses in three different conditions: baseline (before TS), TS without elastics (TSWE) and complete TS, with elastics (TS). The option of testing two TS conditions was necessary because for gait analysis some markers that were placed on the skin in baseline condition required placement on the orthosis in the TS condition. In order to avoid a possible systematic bias due to placement of the markers on the orthosis the TSWE was included. However, the condition TSWE could not be considered a baseline condition because, even without elastics the TS may influence the gait patterns of the children since its fabric is not as soft and flexible as other orthosis (e.g., the TTs lycra).
Parental interview and clinical examination
The parental interview aimed to collect data regarding pre- and peri-natal history and developmental milestones (Table 1). The diagnosis of US-CP was confirmed by a pediatric neurologist and magnetic resonance exams. Data about orthopedic surgeries and pharmacological treatments of spasticity were also collected. All children had been submitted to at least one application of botulinum toxin (BTX), type A, in the paretic lower limb, with only one child (participant 1) undergoing a muscle stretching in the paretic lower limb (15 months prior to the study).
To understand the health conditions associated with CP and the impact on the functional activity, the clinical examination was performed by two-experienced pediatric physical therapists a week before gait analysis.
A bilateral goniometric assessment of passive range of movement (ROM) was performed for both lower limbs by the same trained physical therapist (22, 23) (see Supplementary Table 1), and the spasticity score of the paretic LL (PLL) was determined according to the Modified Ashworth's Scale (MAS) (24), (see Supplementary Table 2). All the participants showed no structural deformities or instability in the ankle joint of the PLL (dorsiflexion/plantarflexion range from 15° to −15°), which could compromise the child's safety and performance of the motor task and muscle tone scored <2. The length of the lower limbs was clinically assessed (distance from anterior superior iliac spine to medial malleolus).
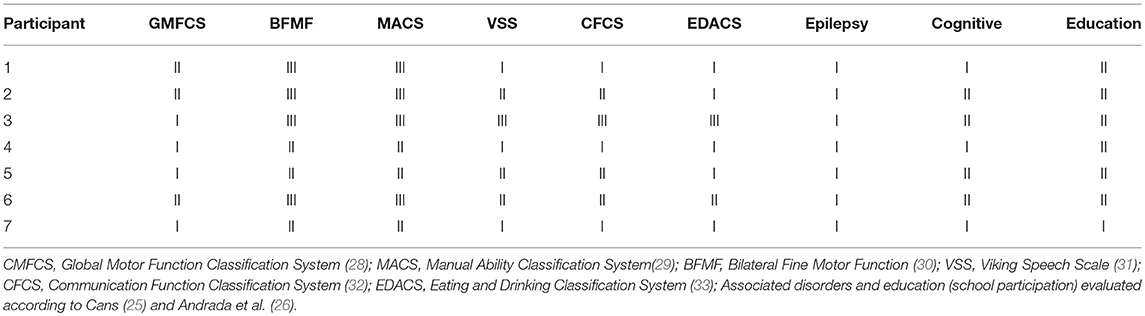
The functional profiles (Table 2) were based on validated tools to measure and classify the functional severity, according to the recommendation of Surveillance of Cerebral Palsy in Europe (SCPE) (25, 26) and the International Classification of Functioning, Disability and Health–Children & Youth (CIF-CY) conceptual model (27).
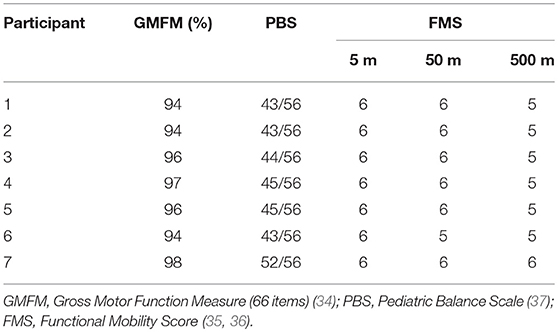
The gross motor function, balance assessments and functional mobility (Table 3), were performed using the Gross Motor Function Measure (GMFM-66 items) (34). Functional Mobility Score (FMS) (35, 36), and the Pediatric Balance Scale (PBS) (37) Additionally, a video gait analysis was done, to assess the gait patterns of each participant in the sagittal plane, based in the classification proposed by Winters, Gage and Hicks (see Supplementary Table 3) (38). ROM and MAS scores and the observational gait analysis were used to support the decision-making process regarding the placement of the elastic cords in each of the seven children (see Supplementary Table 4).
Gait analysis protocol
A week after the clinical examination, 3D gait analysis following a standardized protocol was conducted in three different conditions: (A) baseline (BL); (B) while wearing TS without elastics (TSWE); and (C) while wearing the complete TS (TS). The assessments were performed in 1 day by two experienced pediatric physical therapists, one of them with TS certification. The order of the conditions was the same for all children (i.e., baseline, TSWE, TS), increasing gradually the complexity of the orthosis, in order to shorten the total data collection time and to avoid possible stress or fatigue related with dressing and undressing the TS. Data from clinical examination (ROM and MAS) and observational gait analysis were used to guide the placing of the elastic cords of the TS according to each participant's specific needs. The participant's assent was obtained after an initial explanation prior to the data collection procedures.
Two video-digital cameras (Basler piA1000-48gc GigE) and six infrared cameras (VICON T10) sampled at 100 Hz in conjunction with four force-plates (AMTI OR6-7-2000) and four analog AMTI amplifiers were used to measure 3D motion of the lower limbs (LLs).
Anthropometric measures (height, body weight, tibial length, distance between the femoral condyles or diameter of the knee, distance between the malleoli or diameter of the ankle, distance between the anterior iliac spines and thickness of the pelvis) were collected in the two conditions. Height and weight were collected with shoes on in both conditions.
After collecting the anthropometric measures, in the BL condition sixteen retro-reflective markers were fixed with double-sided adhesive tape directly on the skin in anatomical landmarks of the pelvis and lower extremities identified by palpation. The placement of the markers in these anatomical landmarks allows the tracking of each functional segment's movement trajectory. In the TSWE and TS conditions, the markers were placed on the dynamic orthosis in positions that best reproduced the original anatomical references used in the BL condition (see Supplementary Figure 1). In some cases (e.g., pelvis) each marker was moved laterally by an equal distance along the ASIS-ASIS axis. The true inter-ASIS distance was then manually measured and entered in Vicon, following the Plug-in Gait model recommendations. In the thigh and leg segments, rigid clusters were also used according to the Lowerbody Vincon Model (Plug-in Gait) (39, 40). To avoid marker displacement between the BL and both TS conditions, and to reduce the influence of the footwear on the gait pattern, all participants used the same model of commercially available sneakers during the test protocol (41). The markers were placed in anatomical landmarks equivalent to those reported in the literature (42). For the hallux and heel, the markers were placed on the shoe in positions that best reproduced the reported barefoot anatomical references (42).
After placing the markers, a reference position was captured to determine the overall position and orientation of the markers within the body segments (43). To this end, the participants were instructed to remain in an orthostatic position with their feet aligned in the center of the force platform. The reference position was determined after collecting the marker data for 5 s in each condition. Next, the participants were instructed to walk up and down at a comfortable walking speed until they were asked to stop, in order to perform a total of 10 trials (about 20 m). Kinematic variables were recorded until at least five successful trials (range: 5–8) for each lower limb in each condition. Rest breaks of 20 min were allowed between conditions.
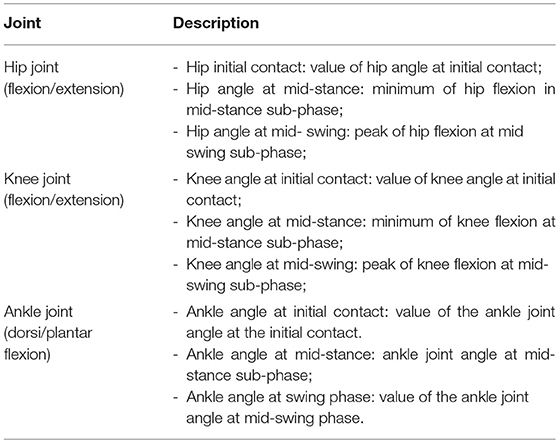
Different outcome variables regarding the kinematic measures of lower limbs in the sagittal plane were analyzed in this study (Table 4).
Data Analysis
Acquisition and processing of the biomechanical data were performed with the Vicon® system by using the software Nexus version 1.8.5. We analyzed the kinematic data with the Plug-in-Gait model (44), which is widely used in clinical gait analysis (45). The Polygon version 3.5.2. software was used for the analysis. All data used in this analysis was previously archived in folders and exported to Excel files. Each file stored task and evaluation data corresponding to one participant.
A descriptive exploratory analysis was carried out to identify aspects or behavior patterns characterizing the variables under study. The Wilcoxon test for paired samples, with Bonferroni correction, was performed to compare the mean ranks of each variable in the BL, TSWE and TS conditions. SPSS 24 was used for the statistical analyses with a p < 0.05 as the level of statistical significance. However, the p value cannot provide full information about the practical significance of the results or about whether or not the result is replicable. In studies with small samples the p-value could not reflect if the underlying difference is real. We therefore used the Z-scores to calculate correlation coefficients (46) and to quantify the effect sizes of the TS therapy. Values were considered small if r ≥ 0.10; moderate, if r ≥ 30 and large if r ≥ 0.50 (47–49).
Results
Hip Joint
The hip joint sagittal plane angular displacements in the seven children during a gait cycle, as well as the group average and SD values in the three conditions, for the PLL and NPLL, are presented in Figure 1. All participants showed a tendency to have lower hip flexion pattern in the TS conditions, in both lower limbs during the gait cycle.
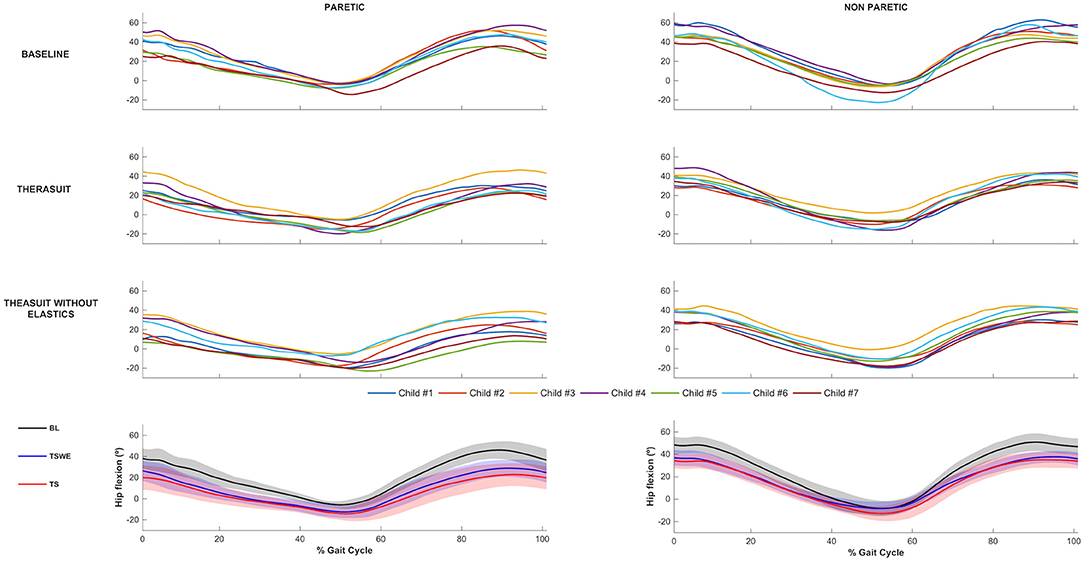
Figure 1. Hip joint flexion-extension. Top 3 rows represent the angular displacements in the seven children during a gait cycle, in the three conditions, for the PLL and NPLL. Bottom row represents the group average and SD values (shaded areas) in the three conditions, for the PLL and NPLL. Negative values represent hip extension.
Table 5 presents the comparison for the hip joint kinematic variables between conditions during the different stages of gait cycle.
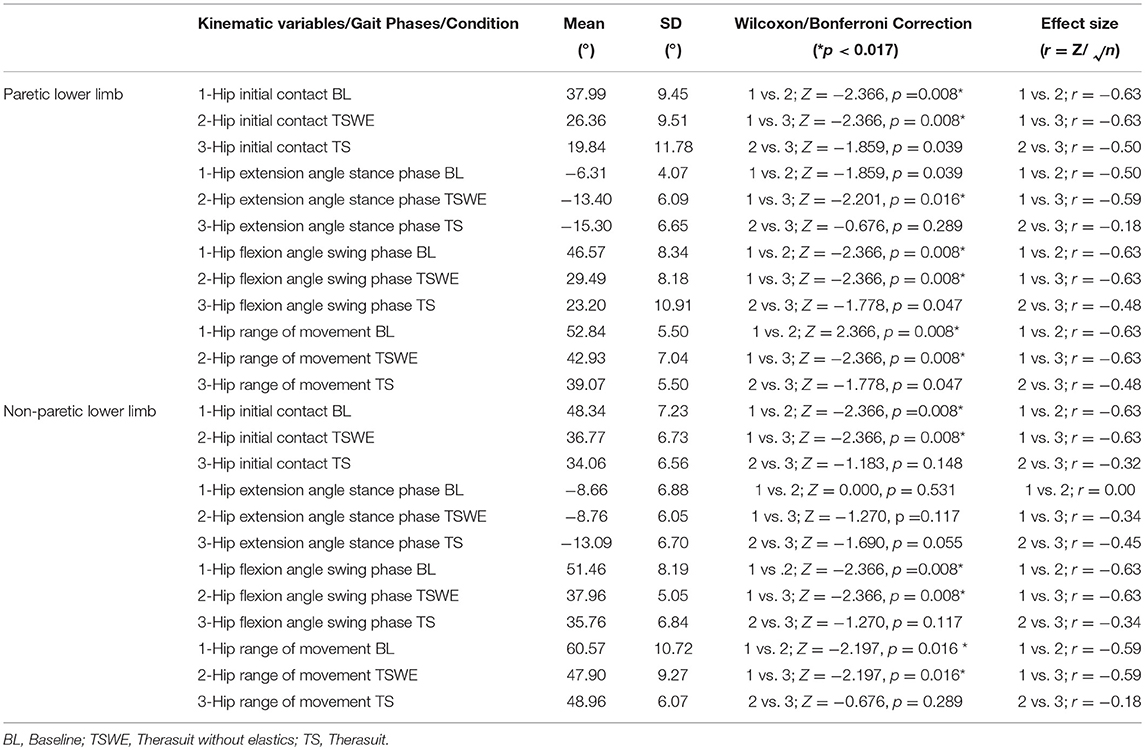
Table 5. Comparison for the hip joint kinematic variables between conditions, during the different stages of gait cycle, in both lower limbs (Wilcoxon test with Bonferroni correction and effect size).
Statistically significant differences between conditions were observed on the PLL at the different gait stages, namely: (i) hip angle at initial contact between BL and both TS conditions (p = 0.008, r = 0.63), (ii) hip extension angle at mid-stance between BL and TS (p = 0.016, r = 0.59); iii) hip flexion angle at mid-swing between BL and both TS conditions (p = 0.008, r = 0.63).
For the NPLL, differences were found at the following stages: (i) hip angle at initial contact between BL and both TS conditions (p = 0.008, r = 0.63) and; (ii) hip flexion angle at mid-swing between BL and both TS conditions (p = 0.008, r = 0.63).
Regarding ROM, significant differences were found between BL and both TS conditions at the hip of the PLL (p = 0.008, r = 0.63) and of the NPLL (p = 0.016, r = 0.59).
Despite the statistically non-significant differences, there were also large effect sizes in the PLL at: (i) initial contact between the two TS conditions (p = 0.039, r = 0.50); (ii) stance phase between BL and TSWE (p = 0.039, r = 0.50).
Knee Joint
The knee joint sagittal plane angular displacements in all participants during a gait cycle and also the group average and SD values in the three conditions, for the PLL and NPLL, are presented in Figure 2. In contrast to the hip joint, the seven participants showed high heterogeneity or inter-individual variability in knee joint motor patterns in the BL condition, which is possibly related to the lower limb discrepancy between the PLL and the NPLL exhibited by the children.
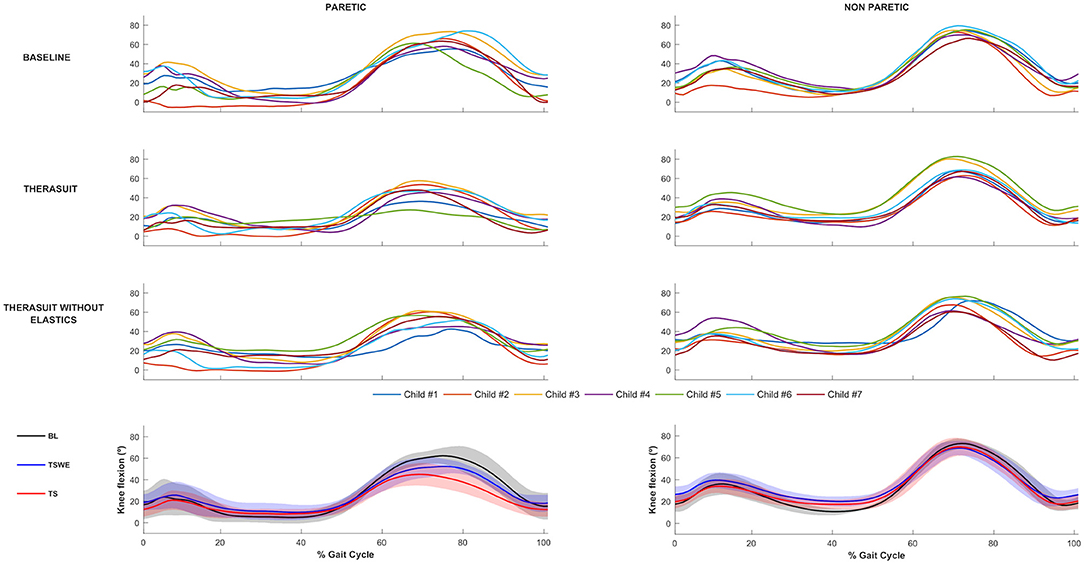
Figure 2. Knee joint flexion-extension Top 3 rows represent the angular displacements in the seven children during a gait cycle, in the three conditions, for the PLL and NPLL. Bottom row represents the group average and SD values (shaded areas) in the three conditions, for the PLL and NPLL. Negative values represent knee extension.
Table 6 presents the comparison for the knee joint kinematic variables between conditions during the different stages of gait cycle.
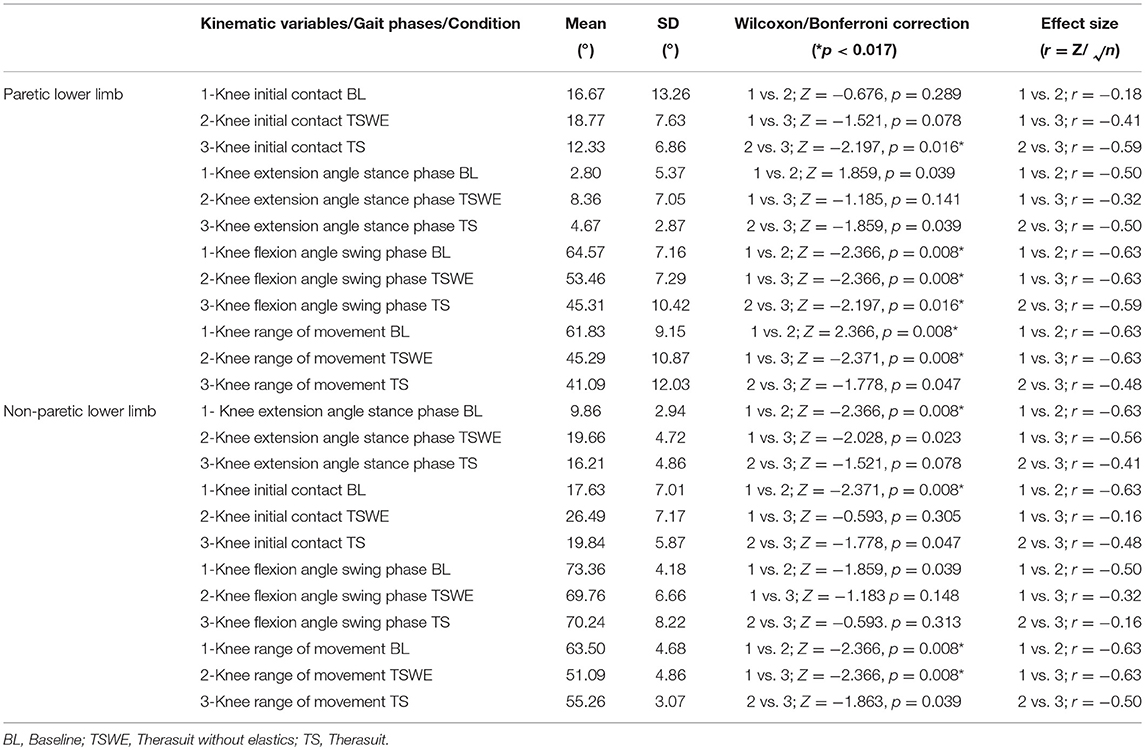
Table 6. Comparison for the knee joint kinematic variables between conditions, during the different stages of gait cycle, in both lower limbs (Wilcoxon test with Bonferroni correction and effect size).
Statistically significant differences for the PLL were found at the following stages of the gait cycle: (i) knee angle at initial contact between both TS conditions (p = 0.016, r = 0.59); (ii) knee flexion angle at mid-swing between BL and both TS conditions (p = 0.008, r = 0.63) and between both TS conditions (p = 0.016, r = 0.59).
For the NPLL differences were found at the following stages: (i) knee angle at initial contact between BL and TSWE (p = 0.008, r = 0.63), (ii) knee angle at mid-stance between BL and TSWE (p = 0.008, r = 0.63).
Regarding ROM, there were differences between BL and both TS conditions in the PLL (p = 0.008, r = 0.63) and in the NPLL (p = 0.008, r = 0.63).
Despite the lack of statistically significant differences, large effect sizes were observed at knee extension at mid-stance in both lower limbs. In the PLL large effects were observed between BL and TSWE (p = 0.039, r = 0.50), and between both TS conditions (p = 0.039, r = 0.50). In the NPLL, still at stance phase, large effect sizes were found between BL and TS (p = 0.023, r = 0.56). In swing phase, large effect sizes were noted between the BL and TSWE (p = 0.039, r = 0.50). Large effect sizes were also noted between the two TS conditions in the ROM of the NPLL (p = 0.039, r = 0.50).
Ankle Joint
The ankle joint sagittal plane angular displacements in all participants during a gait cycle, plus the group average and SD values in the three conditions, for the PLL and NPLL, are presented in Figure 3. Although all participants had similar kinematic patterns in the ankle joints, there were differences between the PLL and NPLL at different phases of the gait cycle.
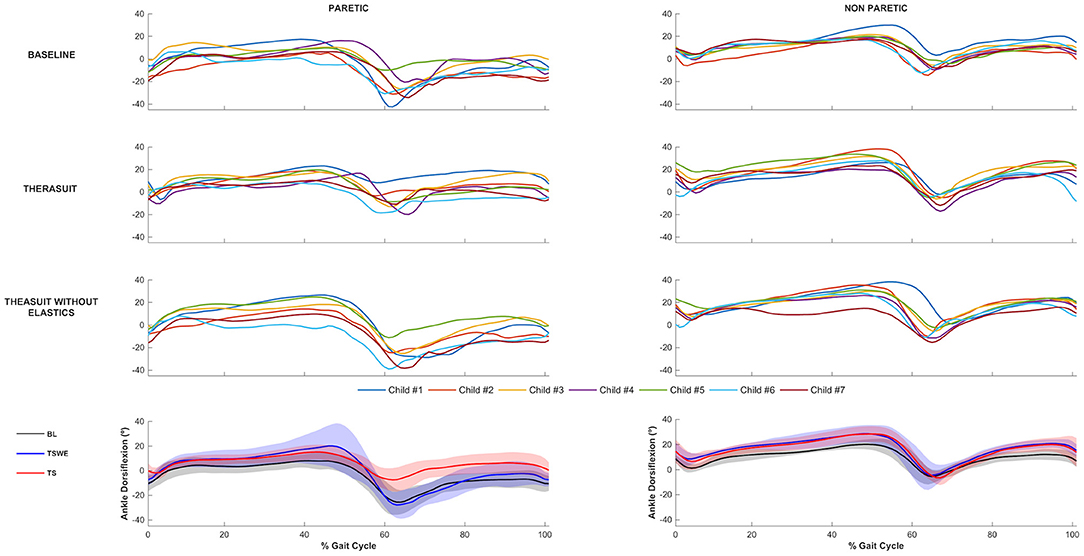
Figure 3. Ankle joint flexion-extension. Top 3 rows represent the angular displacements in the seven children during a gait cycle, in the three conditions, for the PLL and NPLL. Bottom row represents the group average and SD values (shaded areas) in the three conditions, for the PLL and NPLL. Negative values represent plantarflexion.
Table 7 presents the comparison for the ankle joint kinematic variables between conditions during the different stages of gait cycle.
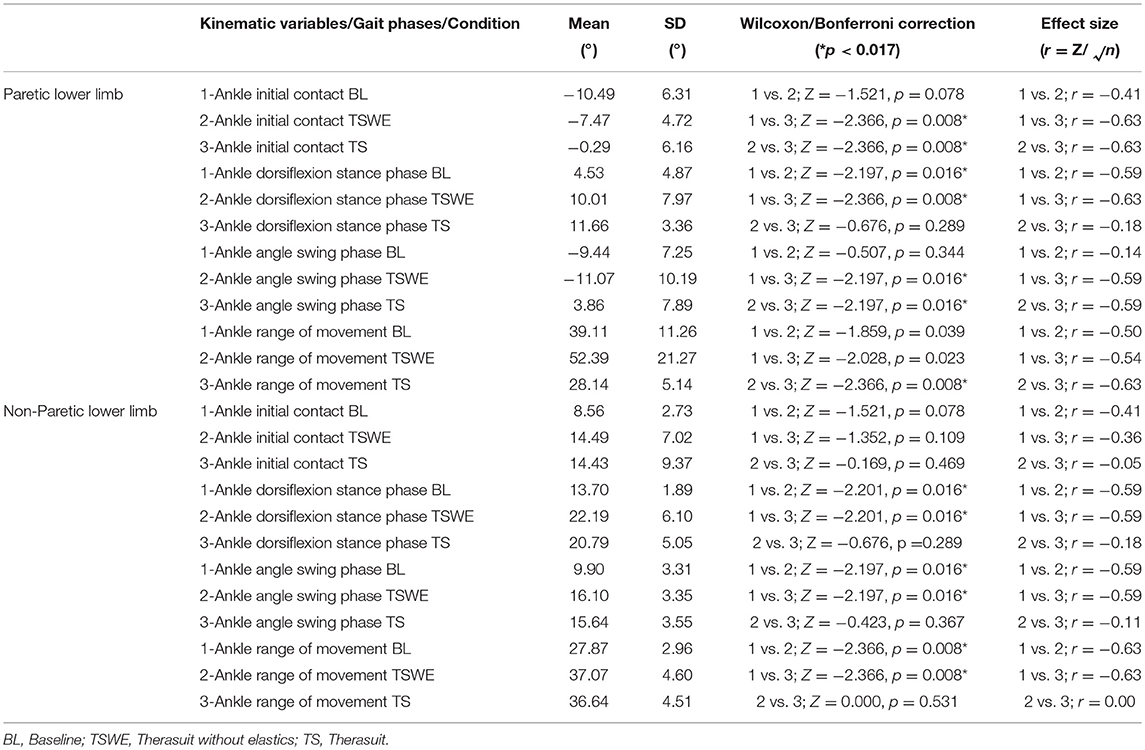
Table 7. Comparison for the ankle joint kinematic variables between conditions, during the different stages of gait cycle, in both lower limbs (Wilcoxon test with Bonferroni correction and effect size).
The results show significant differences on the PLL at different stages of gait cycle, namely: (i) ankle angle at initial contact between BL and TS (p = 0.008, r = 0.63) and between both TS conditions (p = 0.008, r = 0.63); (ii) dorsiflexion angle at stance phase between BL and both TS conditions (TSWE: p = 0.016, r = 0.59; TS: p = 0.008, r = 0.63); (iii) dorsiflexion angle at swing phase between BL and TS (p = 0.016, r = 0.59) and between both TS conditions (p = 0.016, r = 0.59).
Regarding the NPLL, there were significant differences only at the following stages of gait cycle: (i) dorsiflexion angle at stance phase between BL and both TS conditions (p = 0.016, r = 0.59); (ii) dorsiflexion angle at swing phase between BL and both TS conditions (p = 0.016, r = 0.59).
Concerning ankle ROM in the PLL, there were differences between both TS conditions (p = 0.008, r = 0.63). In the NPLL, there were differences in ankle ROM between BL and both TS conditions (p = 0.008, r = 0.63).
Despite the lack of statistically significant differences, there were large effect sizes at ROM of the PLL between BL and both TS conditions (TSWE: p = 0.039, r = 0.50; TS: p = 0.023, r = 0.54).
Discussion
This study assessed the immediate effects of wearing Therasuit on the angular displacements of the lower limbs during gait in children with unilateral spastic cerebral palsy (US-CP). Overall the TS showed some significant effects in the three analyzed joints. However, the positive results in the ankle are more reliable, since in this joint, markers were not moved between conditions, eliminating any possible systematic bias due to the placement of the markers between conditions. The discussion of the effects noted in the hip, knee and ankle joints is presented below.
The results showed that wearing a Therasuit (with or without elastics) has statistically significant effects and large effect sizes on most of the angular hip variables in both lower limbs, correcting the exaggerated flexion pattern during the whole gait cycle in all participants. Even though there were no statistically significant differences between both Therasuit conditions in this joint, the results were most notable on the TS condition than TSWE in the PLL, resulting in lower hip flexion pattern during whole gait cycle.
Children with US-CP often have an exaggerated hip flexion pattern during gait to compensate for motor control impairments in the PLL and for leg length discrepancy (i.e., longer NPLL) (50). Our results suggest that the Therasuit helps to reduce this greater hip flexion pattern during the gait cycle, and this is consistent with previous data showing an increase in hip extension at stance phase in children with bilateral spastic (diplegia) CP, wearing the Theratogs dynamic orthosis (51). In our study, the Therasuit promoted a greater hip extension pattern at initial contact in both lower limbs and at mid-stance sub-phase in the PLL and lower hip flexion pattern in both lower limbs, at mid-swing, resulting in a tendency to approach normative angular values for this joint (52). This effect is likely due to the decrease of the musculoskeletal constraints on the hip gait patterns in the PLL. According to Hussein et al. (53), the decrease in the typical spasticity flexion pattern allows agonist and antagonist muscles to work in better synchrony, leading to a smother movement.
The results in the knee joint were less clear than in the hip, probably also due to a greater heterogeneity between children. In the PLL, most relevant differences were found in swing phase, since the exaggerated flexion pattern exhibited in BL decreased toward normative values in the TS conditions, with significant differences and large effect sizes between the three conditions. Regarding the NPLL, there were significant differences on the knee angle at initial contact and stance phase, between BL and TSWE, with large effect sizes. Other large effect sizes (without statistically significant differences) were found on the knee extension angle at stance-phase between both TS conditions and on the knee flexion angle at swing phase between BL and TSWE conditions. One possible explanation for these results, may be related with the high inter-individual variability exhibited by the seven participants in the baseline condition, regarding the knee joint motor patterns in the PLL during the stance phase. This variability was especially visible at mid-stance, ranging from full extension angles similar to children with typical development (Child 4, Child 5, Child 6, and Child 7), to hyperextension (Child 2) and flexion patterns (Child 1 and Child 3), usually showed in gait patterns in children with US-CP. Considering the within participant changes, the TS seemed to help to correct the hyperextension pattern of Child 2 and had slightly positive effects on children with flexion pattern (Child 1 and Child 3).
Furthermore, all the participants have lower limbs length discrepancy and four of them (Child 1, Child 2, Child 6, and Child 7) have the PLL significantly shorter than the NPLL (54). This clinically significant (≥1.5 cm) lower limb length discrepancy (55) may have limited the effects of the Therasuit, more specifically, on the hip and knee joints angles in the NPLL at stance phase.
The fact that there were almost no significant differences between TSWE and TS conditions on the hip and knee joints might be related with the biomechanical constraints caused by the TS fabric.
Regarding the ankle joint, the results showed significant differences and large effect sizes on all kinematic variables in the PLL between BL and TS with elastics, during whole gait cycle. These differences with BL only occurred in stance phase for the TSWE condition. Comparing both TS conditions, the significant differences and large effect sizes were also noted, at initial contact and swing phases. Conversely, in the NPLL, there were significant differences and large effect sizes on the dorsiflexion angles, at stance and swing phases, between BL and both TS conditions.
The ankle joint showed an asymmetrical pattern. While in the baseline condition the ankle joint on the PLL is plantarflexed at initial contact and swing phases, the NPLL is characterized by a greater ankle dorsiflexion pattern, in these phases of the gait cycle. According to our findings, the TS with elastics successfully controlled the excessive ankle plantar flexion (equinus foot) in the PLL at swing phase, correctly pre-positioning the foot for an initial heel contact. This correction was observed in all the participants and led the ankle angles values at initial contact toward the normative values (52), with the exception of participant 1 (the only participant submitted to orthopedic surgery), who exhibited an ankle angle higher than the normative values. In turn, in the NPLL at initial contact in the BL condition, most participants showed a greater dorsiflexion than typically developing children (52), which was maintained in TS conditions. According to Allen et al. (55) and Cimolin et al. (52), the motor control changes observed in most participants in the NPLL, probably result from compensatory strategies used to overcome the structural and functional limitations of the PLL, thereby allowing greater stability over time.
Interestingly, the effects of the Therasuit were also observed on the distal ankle joint. These findings contradict previous research with other dynamic orthoses (i.e., Theratogs) that cause improvements at a proximal level but not at a distal level (56). These conflicting results may be explained by the differences in the samples (participants with bilateral spastic CP at Rennie's study vs. unilateral spastic CP in the present study) or by differences in the orthoses (Theratogs vs. Therasuit). In contrast to Therathogs, the Therasuit allows the correction of the ankle joint by adjusting the elastic cords attached to the shoes, thus augmenting the possibilities to improve distal control.
In summary, our results suggest that wearing a Therasuit could allow a greater extension pattern in the initial contact and stance phases on the PLL (hip and knee joints), as well as a greater dorsiflexion at the ankle joint during the whole gait cycle. These changes promote better dynamic control in the sagittal plane, caused by the reduction of the biomechanical constrains in that limb. These adaptations consequently lead to more functional gait patterns in our sample (seven 6- to 9-year-old children, with US-CP and with minimal degree of spasticity on the PLL).
Clinical Implications
The (re)habilitation of gait disorders in children with CP is one of the main objectives of physical therapeutic intervention, because the gait has a critical role in the autonomy and quality of life of a child and family (30). The implementation of therapeutic innovations, including Therasuit, into the physical therapist's clinical practice to facilitate more efficient gait patterns in children with US-CP, should be based on a clear understanding of the biomechanical factors underlying the motor and proprioceptive impairments of this subtype of CP. It is also important to note that the inherent complexity of the Therasuit orthosis requires experienced physical therapists for placing the elastic bands in a way that restrains compensatory patterns while simultaneously promoting new and more efficient gait patterns.
This study has important clinical implications since it emphasizes on one hand the presence of differentiated kinematic patterns between the paretic and non-paretic lower limbs (52) and, on other hand, it reinforces the presence of significant compensatory motor strategies in the NPLL that result from neuromuscular factors, biomechanical constraints and primary growth disturbances (e.g., limb length discrepancy) of the PLL (55).
In order to implement an evidence-based practice, the best research evidence should be available. Thus, from a clinical perspective, the identification and precise quantification of gait patterns in both lower limbs (paretic and non-paretic) of children with unilateral spastic CP is a central issue for development of effective and specific (re)habilitation programs. Despite the limitations presented next, the results of the present study may constitute a first step to guide physical therapists in the selection of appropriate dynamic orthoses to promote more functional gait patterns in this particular CP subtype (unilateral spastic).
Methodological Considerations and Limitations
To our knowledge, this is the first study to investigate the immediate effects of Therasuit on the gait pattern of children with CP. Most studies have addressed the effects of Therasuit interventions (21) and therefore have various confounding variables (e.g., different activities performed during therapy and different intensities of training), making the interpretation of the results challenging and the specific effects of Therasuit difficult to determine. The clinical type and severity level of children with CP influence the functional prognosis and the aims of (re)habilitation programs. For this reason, a previous systematic review has suggested the use of homogeneous samples for studying the effects of Therasuit in the gait pattern of children with CP (21). In this study, we used a homogeneous sample comprising children with similar medical diagnoses (US-CP). Moreover, two experienced pediatric physical therapists performed a detailed physical examination, to ensure similar functional profiles between the participants. Finally, the same two experienced physical therapists were responsible for placing the elastic bands of the TS, in order to ensure the best correction of the musculoskeletal constraints, according to individual needs of each participant.
Nevertheless, this study has some limitations, namely: (1) a convenience sample was used, although the authors had no previous therapeutic relationship with the children until the beginning of the study; (2) the small sample size does not allow the generalization of the results, which should always be made with caution in these type of population; (3) only the sagittal plane was analyzed, hindering the calculus of an index of overall gait pathology, such as the Gait Deviation Index (57) or Gait Profile Score (58), which could help to better understand the clinical significance of the results; (4) the inherent problems associated to the kinematic analysis using markers, namely soft tissue artifact, particularly when not applied directly on the skin in both TS conditions. The placement of some markers on the TS, requires an estimation of the position of anatomical landmarks, which may have produced some error in the results, even being performed by an experienced professional. Due to this, and since we cannot determine the error magnitudes associated with the placement of the markers on the soft tissue, we believe that results regarding the hip and knee joints should be interpreted with extra caution.
Recommendations for Future Research
Comparative studies with children with unilateral spastic CP are difficult to perform because cortical compromise may generate different patterns of gait. It therefore remains challenging to develop a universal gait classification system for standardizing participant samples and data comparisons between studies (59). The wide variety of interventions used in children with US-CP likely results from the current knowledge gap in what causes the gait pattern changes in these children. Indeed, many of these interventions are insufficiently supported by scientific evidence. Thus, we suggest that future research with more representative samples (i.e., bigger and larger age range samples) should address the immediate and long-term effects of the Therasuit dynamic orthosis on spatial, temporal and angular variables in the three planes (sagittal, frontal, and transverse), not only in this particular CP subtype, but also in other types of CP, such as the dyskinetic type, and in particular, the coreathetotid subtype. Coreathetotid children are characterized by low muscular tone, involuntary movements and loss of postural stability. For these reasons, it would be interesting to understand if the TS could promote adaptive changes in the gait patterns of children with a clinical diagnosis totally different from children with spastic CP. It would also be important to analyze the immediate effects of Therasuit on orthostatic postural control (and to calculate the symmetry index) to determine its potential in promoting a more symmetric postural alignment, a fundamental condition for all functional motor tasks. Finally, studies addressing the immediate effects of Therasuit on gait kinematic parameters in children with US-CP using other methodologies, such as muscle recruitment data, extracted from electromyography, could allow a better identification of the neuromuscular behavior, thereby providing better information and guidance for the placements of the Therasuit elastic cords.
Conclusion
The results in this study should be analyzed with caution due to the limitations previously mentioned (e.g., small convenience sample with minimal spasticity degree). Despite this, our results seem to suggest that wearing a Therasuit promotes: (1) positive kinematic changes on gait pattern in the paretic lower limb; (2) decrease hip flexion angles at initial contact in both lower limbs; (3) increase of the extension pattern at the hip joint during stance phase in the paretic lower limb, and a decrease of the flexion pattern during swing phase in both lower limbs; (4) a decrease of the equinus-foot pattern at the ankle joint in the paretic lower limb, during whole gait cycle, particularly on TS condition (with elastics). As this is the first study addressing the immediate effects of Therasuit on the gait pattern of children with hemiplegic CP, these findings may have important clinical implications.
Although our research only focused on the immediate effects of wearing Therasuit, the results suggest that the TS may be included by physical therapists in gait training programs for children with CP as an alternative dynamic orthosis, which may drive the child to use a new motor program, resulting in a safer and more functional gait pattern. Thus, our findings add to the growing body of evidence supporting the efficacy of Therasuit therapy for treating gait disorders in children with unilateral spastic CP.
Data Availability
The raw data supporting the conclusions of this manuscript will be made available by the authors, without undue reservation, to any qualified researcher.
Author Contributions
EM, RC, and RO conceived and designed the study. EM, JP, RC, and RO performed the experiments. EM, JV, and AD analyzed the data. EM, RC, RO, JP, AD, and JV wrote the paper.
Funding
This research was funded by the Foundation for Science and Technology (Portugal) PTDC/DTP-DES/6776/2014. JV is funded by NIH P20GM109090.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fped.2019.00042/full#supplementary-material
References
1. Boyd RN, Morris ME, Graham HK. Management of upper limb dysfunction in children with cerebral palsy: a systematic review. Eur J Neurol. (2001) 8 (Suppl. 5):150–66. doi: 10.1046/j.1468-1331.2001.00048.x
2. Charles J, Gordon AM. A critical review of constraint-induced movement therapy and forced use in children with hemiplegia. Neural Plast (2005) 12:245–61. doi: 10.1155/NP.2005.245
3. Novacheck TF, Gage JR. Orthopedic management of spasticity in cerebral palsy. Childs Nerv Syst. (2007) 23:1015–31. doi: 10.1007/s00381-007-0378-6
4. Lampe R, Grassl S, Mitternacht J, Gerdesmeyer L, Gradinger R. MRT-measurements of muscle volumes of the lower extremities of youths with spastic hemiplegia caused by cerebral palsy. Brain Dev. (2006) 28:500–6. doi: 10.1016/j.braindev.2006.02.009
5. Malaiya R, McNee AE, Fry NR, Eve LC, Gough M, Shortland AP. The morphology of the medial gastrocnemius in typically developing children and children with spastic hemiplegic cerebral palsy. J Electromyogr Kinesiol. (2007) 17:657–63. doi: 10.1016/j.jelekin.2007.02.009
6. Riad J, Finnbogason T, Brostrom E. Leg length discrepancy in spastic hemiplegic cerebral palsy: a magnetic resonance imaging study. J Pediatr Orthop. (2010) 30:846–50. doi: 10.1097/BPO.0b013e3181fc35dd
7. Himmelmann K, Hagberg G, Beckung E, Hagberg B, Uvebrant P. The changing panorama of cerebral palsy in Sweden. IX. Prevalence and origin in the birth-year period 1995-1998. Acta Paediatr. (2005) 94: 287–94. doi: 10.1111/j.1651-2227.2005.tb03071.x
8. Odding E, Roebroeck ME, Stam HJ. The epidemiology of cerebral palsy: incidence, impairments and risk factors. Disabil Rehabil. (2006) 28:183–91. doi: 10.1080/09638280500158422
9. Soleimani F, Vameghi R, Biglarian A. Antenatal and intrapartum risk factors for cerebral palsy in term and near-term newborns. Arch Iran Med. (2013) 16:213–6.
11. Morris C, Bowers R, Ross K, Stevens P, Phillips D. Orthotic management of cerebral palsy: recommendations from a consensus conference. Neurorehabilitation (2011) 28:37–46. doi: 10.3233/NRE-2011-0630
12. Novak I, McIntyre S, Morgan C, Campbell L, Dark L, Morton N, et al. A systematic review of interventions for children with cerebral palsy: state of the evidence. Dev Med Child Neurol. (2013) 55:885–910. doi: 10.1111/dmcn.12246
13. Bailes AF, Greve K, Schmitt LC. Changes in two children with cerebral palsy after intensive suit therapy: a case report. Pediatr Phys Ther. (2010) 22:76–85. doi: 10.1097/PEP.0b013e3181cbf224
14. Neves E, Krueger E, Pol S, Oliveira M, Szinke A, Rosário M. Benefícios da Terapia Neuromotora Intensiva (TNMI) para o Controle do Tronco de Crianças com Paralisia Cerebral. Rev Neuroci. (2013) 21:549–55. doi: 10.4181/RNC.2013.21.876.7p
15. Datorre ECS. Intensive Therapy Combined With Strengthening Exercises Using the Thera Suit in a Child With CP. A Case Report. (2005) Available online at : http://www.suittherapy.com/pdf%20research/Int.%20Therapy%20%20Research%20Datore.pdf (Accessed Feb 05, 2017).
16. Semenova KA. Basis for a method of dynamic proprioceptive correction in the restorative treatment of patients with residual-stage infantile cerebral palsy. Neurosci Behav Physiol. (1997) 27:639–43. doi: 10.1007/BF02461920
17. Blair E, Ballantyne J, Horsman S, Chauvel P. A study of a dynamic proximal stability splint in the management of children with cerebral palsy. Dev Med Child Neurol. (1995) 37:544–54. doi: 10.1111/j.1469-8749.1995.tb12041.x
18. Hylton N, Allen C. The development and use of SPIO Lycra compression bracing in children with neuromotor deficits. Pediatr Rehabil. (1997) 1:109–16. doi: 10.3109/17518429709025853
19. Attard J, Rithalia S. A review of the use of Lycra pressure orthoses for children with cerebral palsy. Int J Ther Rehabilit. (2004) 11:120–6. doi: 10.12968/ijtr.2004.11.3.13384
20. Scheeren EM, Mascarenhas LPG, Chiarello CR, Costin ACMS, Oliveira L, Neves EB. Description of the Pediasuit ProtocolTM. Fisioter Movimento (2012) 25:473–80. doi: 10.1590/S0103-51502012000300002
21. Martins E, Cordovil R, Oliveira R, Letras S, Lourenco S, Pereira I, et al. Efficacy of suit therapy on functioning in children and adolescents with cerebral palsy: a systematic review and meta-analysis. Dev Med Child Neurol (2016) 58:348–60. doi: 10.1111/dmcn.12988
22. Mutlu A, Livanelioglu A, Gunel MK. Reliability of goniometric measurements in children with spastic cerebral palsy. Med Sci Monit. (2007) 13:CR323–9.
23. Herrero P, Carrera P, Garcia E, Gomez-Trullen EM, Olivan-Blazquez B. Reliability of goniometric measurements in children with cerebral palsy: a comparative analysis of universal goniometer and electronic inclinometer. A pilot study. BMC Musculoskelet Disord (2011) 12:155. doi: 10.1186/1471-2474-12-155
24. Bohannon RW, Smith MB. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther. (1987) 67:206–7. doi: 10.1093/ptj/67.2.206
25. Cans C. Surveillance of cerebral palsy in Europe: a collaboration of cerebral palsy surveys and registers. Surveillance of Cerebral Palsy in Europe (SCPE). Dev Med Child Neurol (2000) 42:816–24. doi: 10.1111/j.1469-8749.2000.tb00695.x
26. Andrada MG, Calado E, Gouveia R, Virella D, Folha T. Programa de Vigilância da Paralisia Cerebral aos 5 anos de idade. Federação das Associações Portuguesas de Paralisia Cerebral (2009). Available online at: http://www.fappc.pt/wp-content/uploads/2017/04/Relat%C3%B3rio-PVNPC5A-dos-nascidos-em-2001-a-2007.pdf
27. World Health Organization (2007). International Classification of Functioning, Disability, and Health: Children & Youth Version: ICF-CY. Geneva: World Health Organization.
28. Palisano R, Rosenbaum P, Walter S, Russell D, Wood E, Galuppi B. Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol. (1997) 39:214–23. doi: 10.1111/j.1469-8749.1997.tb07414.x
29. Eliasson AC, Krumlinde-Sundholm L, Rosblad B, Beckung E, Arner M, Ohrvall AM, et al. The Manual Ability Classification System (MACS) for children with cerebral palsy: scale development and evidence of validity and reliability. Dev Med Child Neurol. (2006) 48:549–54. doi: 10.1017/S0012162206001162
30. Beckung E, Hagberg G, Uldall P, Cans C Surveillance of Cerebral Palsy in Europe. Probability of walking in children with cerebral palsy in Europe. Pediatrics (2008) 121:e187–92. doi: 10.1542/peds.2007-0068
31. Pennington L, Virella D, Mjoen T, da Graca Andrada M, Murray J, Colver A, et al. Development of The Viking Speech Scale to classify the speech of children with cerebral palsy. Res Dev Disabil. (2013) 34:3202–10. doi: 10.1016/j.ridd.2013.06.035
32. Hidecker MJ, Paneth N, Rosenbaum PL, Kent RD, Lillie J, Eulenberg JB, et al. Developing and validating the Communication Function Classification System for individuals with cerebral palsy. Dev Med Child Neurol. (2011) 53:704–10. doi: 10.1111/j.1469-8749.2011.03996.x
33. Sellers D, Mandy A, Pennington L, Hankins M, Morris C. Development and reliability of a system to classify the eating and drinking ability of people with cerebral palsy. Dev Med Child Neurol. (2014) 56:245–51. doi: 10.1111/dmcn.12352
34. Russell DJ, Avery LM, Rosenbaum PL, Raina PS, Walter SD, Palisano RJ. Improved scaling of the gross motor function measure for children with cerebral palsy: evidence of reliability and validity. Phys Ther (2000) 80:873–85. doi: 10.1093/ptj/80.9.873
35. Williams EN, Carroll SG, Reddihough DS, Phillips BA, Galea MP. Investigation of the timed ‘up & go’ test in children. Dev Med Child Neurol (2005) 47:518–24. doi: 10.1017/S0012162205001027
36. Harvey AR, Morris ME, Graham HK, Wolfe R, Baker R. Reliability of the functional mobility scale for children with cerebral palsy. Phys Occup Ther Pediatr. (2010) 30:139–49. doi: 10.3109/01942630903454930
37. Franjoine MR, Gunther JS, Taylor MJ. Pediatric balance scale: a modified version of the berg balance scale for the school-age child with mild to moderate motor impairment. Pediatr Phys Ther. (2003) 15:114–28. doi: 10.1097/01.PEP.0000068117.48023.18
38. Winters TF Jr, Gage JR, Hicks R. Gait patterns in spastic hemiplegia in children and young adults. J Bone Joint Surg Am. (1987) 69:437–41. doi: 10.2106/00004623-198769030-00016
39. Desloovere K, Molenaers G, Feys H, Huenaerts C, Callewaert B, Van de Walle P. Do dynamic and static clinical measurements correlate with gait analysis parameters in children with cerebral palsy? Gait Posture (2006) 24:302–313. doi: 10.1016/j.gaitpost.2005.10.008
40. Stebbins J, Harrington M, Thompson N, Zavatsky A, Theologis T. Gait compensations caused by foot deformity in cerebral palsy. Gait Posture (2010) 32:226–30. doi: 10.1016/j.gaitpost.2010.05.006
41. Wolf S, Simon J, Patikas D, Schuster W, Armbrust P, Doderlein L. Foot motion in children shoes: a comparison of barefoot walking with shod walking in conventional and flexible shoes. Gait Posture (2008) 27:51–9. doi: 10.1016/j.gaitpost.2007.01.005
42. Hollander K, Riebe D, Campe S, Braumann KM, Zech A. Effects of footwear on treadmill running biomechanics in preadolescent children. Gait Posture (2014) 40:381–5. doi: 10.1016/j.gaitpost.2014.05.006
43. Wu G, Siegler S, Allard P, Kirtley C, Leardini A, Rosenbaum D, et al. ISB recommendation on definitions of joint coordinate system of various joints for the reporting of human joint motion–part I: ankle, hip, and spine. Int Soc Biomech J Biomech. (2002) 35:543–8. doi: 10.1016/S0021-9290(01)00222-6
44. Kadaba MP, Ramakrishnan HK, Wootten ME. Measurement of lower extremity kinematics during level walking. J Orthop Res. (1990) 8:383–92. doi: 10.1002/jor.1100080310
45. Nieuwenhuys A, Papageorgiou E, Pataky T, De Laet T, Molenaers G, Desloovere K. Literature review and comparison of two statistical methods to evaluate the effect of botulinum toxin treatment on gait in children with cerebral palsy. PLoS ONE (2016) 11:e0152697. doi: 10.1371/journal.pone.0152697
46. Fritz CO, Morris PE, Richler JJ. Effect size estimates: current use, calculations, and interpretation. J Exp Psychol Gen. (2012) 141:2–18. doi: 10.1037/a0024338
47. Cohen J. (ed.). Statistical Power Analysis for the Behavioral Sciences, 2nd Edition. Hillsdale, NJ: Lawrence Erbaum (1988).
48. Cohen J. Statistical power analysis. Curr Direct Psychol Sci. (1992) 1:98–101. doi: 10.1111/1467-8721.ep10768783
49. Tomczak M, Tomczak E. The need to report effect size estimates revisited. An overview of some recommended measures of effect size TRENDS Sport Sci. (2014) 1:19–25.
50. Pasparakis D, Darras N, Intzoglou K, Tziomarki M. Gait patterns in hemiplegic children with cerebral palsy: the uninvolved side. EEXOT (2013) 64:40–3.
51. Flanagan A, Krzak J, Peer M, Johnson P, Urban M. Evaluation of short-term intensive orthotic garment use in children who have cerebral palsy. Pediatr Phys Ther. (2009) 21:201–4. doi: 10.1097/PEP.0b013e3181a347ab
52. Cimolin V, Galli M, Tenore N, Albertini G, Crivellini M. Gait strategy of uninvolved limb in children with spastic hemiplegia. Eura Medicophys. (2007) 43:303–10.
53. Hussein ZA, El-Wahab MSA, El-Shennawy SAW. Kinematic gait analysis of upper and lower limbs joints in hemiplegic children. Int J Med Health Sci. (2013) 7:38–42.
54. Kiapour A, Abdelgawad AA, Goel VK, Souccar A, Terai T, Ebraheim NA. Relationship between limb length discrepancy and load distribution across the sacroiliac joint–a finite element study. J Orthop Res. (2012) 30:1577–80. doi: 10.1002/jor.22119
55. Allen PE, Jenkinson A, Stephens MM, O'Brien T. Abnormalities in the uninvolved lower limb in children with spastic hemiplegia: the effect of actual and functional leg-length discrepancy. J Pediatr Orthop. (2000) 20:88–92. doi: 10.1097/01241398-200001000-00019
56. Rennie DJ, Attfield SF, Morton RE, Polak FJ, Nicholson J. An evaluation of lycra garments in the lower limb using 3-D gait analysis and functional assessment (PEDI). Gait Posture (2000) 12:1–6. doi: 10.1016/S0966-6362(00)00066-7
57. Schwartz MH, Rozumalski A. The Gait Deviation Index: a new comprehensive index of gait pathology. Gait Posture (2008) 28:351–7. doi: 10.1016/j.gaitpost.2008.05.001
58. Baker R, McGinley JL, Schwartz MH, Beynon S, Rozumalski A, Graham HK, et al. The gait profile score and movement analysis profile. Gait Posture (2009) 30:265–9. doi: 10.1016/j.gaitpost.2009.05.020
Keywords: cerebral palsy, hemiparetic, suit therapy, kinematic gait analysis, dynamic orthosis, physical therapy
Citation: Martins E, Cordovil R, Oliveira R, Pinho J, Diniz A and Vaz JR (2019) The Immediate Effects of a Dynamic Orthosis on Gait Patterns in Children With Unilateral Spastic Cerebral Palsy: A Kinematic Analysis. Front. Pediatr. 7:42. doi: 10.3389/fped.2019.00042
Received: 26 October 2018; Accepted: 01 February 2019;
Published: 21 February 2019.
Edited by:
Tomoki Arichi, King's College London, United KingdomReviewed by:
Dinesh Upadhya, Manipal Academy of Higher Education, IndiaSilvia Romano, Sapienza University of Rome, Italy
Naomi Rachel Winfield, Milton Keynes Hospital, United Kingdom
Copyright © 2019 Martins, Cordovil, Oliveira, Pinho, Diniz and Vaz. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rita Cordovil, ritacordovil@fmh.ulisboa.pt
 Elisabete Martins
Elisabete Martins Rita Cordovil
Rita Cordovil Raul Oliveira
Raul Oliveira Joana Pinho
Joana Pinho Ana Diniz
Ana Diniz Joao R. Vaz
Joao R. Vaz