IgE-expressing long-lived plasma cells in persistent sensitization
- 1Department of Allergy, Center for Asthma Prevention and Lung Function Laboratory, Children's Hospital of Capital Institute of Pediatrics, Beijing, China
- 2Department of Pediatrics, Graduate School of Peking Union Medical College, Beijing, China
- 3Department of Pediatrics, The Second Xiangya Hospital, Central South University, Changsha, China
Persistent allergies affect the quality of life of patients and increase economic burdens. Many clinical observations indicate the presence of IgE+ long-lived plasma cells (LLPCs), which account for the persistent secretion of specific IgE; however, the characteristics of the IgE+ LLPCs have yet to be identified clearly. In this review, we summarized the generation of IgE+ PCs, discussed the prosurvival factors in the microenvironment, and reviewed the unique IgE-BCR signaling, which may bring insights into understanding the survival mechanisms of IgE+ LLPCs.
Introduction
Allergic diseases are a global health problem and a significant burden to society and patients. Over the past two decades, the prevalence of various allergic diseases has increased (1, 2). Allergic sensitization during childhood is a dynamic process. Some allergies can outgrow naturally, especially food allergies, such as cow milk allergy, egg allergy, and wheat allergy (3). According to a study by Bernard and colleagues, the remission rate of aeroallergen allergy within 2 years was 60% (4). However, some allergies (e.g., tree nuts, peanuts, fish, shellfish) are lifelong (3). People with persistent sensitization have an increased risk of developing allergic diseases, such as asthma and allergic rhinitis, thus impairing patient quality of life and incurring substantial economic costs (5, 6). Up to date, allergen immunotherapy (AIT) is the only way to cure allergies. AIT for dust-mite, pollen, milk, egg and peanut allergies has been used widely. However, the shortcomings, such as the long duration of therapy, potential adverse reactions, unclear long-term effects, and limited applications, make it challenging to meet clinical requirements (7). Thus, it is essential to understand the mechanism of persistent allergy and develop rational therapies to shorten the duration of allergy.
Long-lived plasma cells (LLPCs) are critical for maintaining adaptive humoral immunity after recovery from infection or vaccination. However, LLPCs also generate pathogenic antibodies, thus causing a variety of diseases or problems, such as systemic lupus erythematosus, allograft rejection, and persistent allergy (8, 9). IgE is the critical factor of type I hypersensitivity and mediates the degranulation of mast cells and basophils, leading to the rapid manifestation of symptoms after allergen exposure. The half-life of serum IgE is short [2–3 days in humans (10) and 12 h in mice (11)]. In contrast, allergen-specific IgE could maintain for an extended period with the absence or significantly attenuated exposure of allergen both in mice (12) and humans (13, 14), which suggests that IgE+ LLPCs may account for persistent IgE secretion (15). To clarify the potential role of IgE+ LLPCs in allergic conditions, we summarized the generation of IgE+ plasma cells (PCs), discussed the prosurvival factors in the microenvironment, and reviewed the IgE-BCR downstream pathways which uniquely regulate IgE+ PCs longevity.
Evidence of IgE+ LLPCs in mice and humans
Much evidence has been raised that IgE+ LLPCs were inducible in allergic mouse models. Mice injected intraperitoneally with a single dose of OVA with or without aluminum hydroxide (HA) had a persistent titer of OVA-specific IgE in serum and long-lived IgE-secreting cells in the bone marrow (BM) and spleen. Moreover, administration with X-irradiation, a lethal dose of x-rays sufficient to deplete B memory cells rather than LLPCs, only partially affected IgE levels and IgE+ secreting cell counts (12). Another study induced systemic sensitization in mice by intraperitoneal injection with OVA-HA on days 1, 14, and 21 and subsequently treated them with cyclophosphamide for 4 days (16). Researchers found that OVA-specific IgE-secreting cells survived in the BM and lesser in the spleen at day 100. Unlike short-lived plasma cells (SLPCs), LLPCs are refractory to cyclophosphamide, and these IgE-secreting cells were thought to be IgE+ LLPCs (16). However, intraperitoneal injection with allergens is not a natural route to allergen exposure. Asrat et al. (17) developed an allergic model by intranasal exposure to house dust mite (HDM) extract. In mice that were chronically exposed to HDM and left unmanipulated for additional periods, the serum IgE decreased initially but maintained constant after 14 weeks. Parallelly, IgE+ BMPCs were present in BM for at least 32 weeks and were not significantly affected by anti-CD20 antibodies administration. Because the lifespan of IgE and IgE+ SLPCs is short and the absence of allergen exposure and anti-CD20 antibodies administration block the de novo IgE+ PCs generation, the IgE+ PCs persistent in BM were LLPCs and contributed to long-term positive IgE (17). In addition to the aeroallergen allergy, the food allergy mouse model induced by peanut butter with cholera toxin intragastrically also demonstrated that cyclophosphamide-resistant IgE+ PCs could be induced and maintained in BM by intestinal allergic sensitization (18). Despite the differences between allergens, routes of allergen exposure, and the genetic background of mice, IgE+ LLPCs could be induced and mainly reside in BM.
In humans, many clinical observations indicate the presence of IgE+ LLPCs; however, direct evidence is lacking. For instance, with the mugwort pollen dropped, the allergen-specific T cells in patients with mugwort pollen allergy almost disappeared, but allergen-specific IgE persisted for several years (13). In cat-sensitization individuals, the level of specific IgE to cat did not significantly change after avoiding cat allergen for 20 months (14). More recently, Pitlick and Pongdee conducted a study in which patients received combining biologics targeting IgE+ IL-5/IL5R, IgE+ IL4/IL13, IgE+ IL-15+ IL4/IL13, and IL-15+ IL4/IL13 respectively. During the treatment, IgE levels in some patients cannot decrease to the normal range (19). IgE+ BMPCs could be found in allergic patients but not in nonallergic participants, and these IgE+ BMPCs secret allergen-specific IgE which could stimulate allergic reactions (17). However, it is difficult to identify the longevity of IgE+ BMPCs in atopic individuals. According to Zhang's study, IgE+ PCs were found in human nasal polyps and could secrete IgE constantly for 1-month ex vivo without stimulation (20). Moreover, a proportion of PCs (BCL2+ CD138+ PCs) could survive ex vivo for at least 32 days, a lifespan of LLPCs in the human intestine, indicating the existence of LLPCs in nasal polyps (20). Nevertheless, this study did not raise direct evidence that IgE+ PCs were LLPCs. LLPCs have several features which could distinguish them from SLPCs and B cells. LLPCs are long-lived, cyclophosphamide-resistant (16), and radioresistant (12). At the molecular level, LLPCs express anti-apoptosis proteins (such as MCL1 and BCL2), CD28, and the BCMA receptor (21, 22). These features could help to identify the long-lived population of IgE+ PCs in humans.
Generation of IgE+ PCs
The germinal center (GC)-dependent pathway
Typically, B cells migrate to the GC, a primary place for class switch recombination (CSR) and affinity maturation, after interacting with antigen-dependent T cells. GC B cells further differentiate into PCs under the interaction with follicular dendritic cells and T follicular (Tfh) cells (23, 24). Tfh cells are essential for affinity maturation and class switching of GC B cells. Different profiles of cytokines derived from Tfh cells induce B cells to switch into different isoforms of immunoglobulin (Ig) (24). For IgE-inducing in mice, Tfh cells that secret IL-4 are sufficient for IgE production, typically low-affinity IgE (25, 26). Tfh-derived IL-21 plays a negative role in IgE CSR, which could be attenuated by CD40 signaling (27). A recently identified IL-4+ IL-13+ Tfh13 population in mice and humans, a subtype of Tfh induced by various allergens, might play a crucial role in high-affinity IgE generation (26). IgE+ cells experiencing indirect isotype switching mainly from IgG1 (IgM → IgG1 → IgE) are more likely to produce high-affinity IgE. In contrast, low-affinity IgE derives from IgE+ cells undergoing direct class switching (IgM → IgE) (28–30). In humans, IgE can also switch from other Ig isotypes (28, 31).
He et al. analyzed Sγ1 remnants in switch regions of IgE+ GC B cells and IgE+ PCs in mice immunized with OVA + PEP1 or infected with N. brasiliensis. They found that Sγ1 remnants presented in a proportion of IgE+ PCs but not in IgE+ GC B cells, indicating that IgG1+ cells could be the precursors of IgE+ PCs besides IgE+ GC B cells (32). IgE+ GC B cells are transient in GC and are predisposed to differentiate into SLPCs (33). Therefore, IgE+ GC B cells are more likely to experience direct class switching and are the precursors of low-affinity IgE+ SLPCs. IgG1+ PCs are terminally differentiated, so they cannot further differentiate into IgE+ PCs (32). IgG1+ memory cells and IgG1+ GC cells may be the primary IgG1+ cells to switch to IgE+ PCs, which has been demonstrated by Talay (34, 35) and He (32). IgE+ SLPCs also could arise from IgE+ memory B cells in mice after the second challenge with N. brasiliensis (34, 35). These results are consistent with research based on humans. IgE+ memory B cells fitted with the GC-dependent and GC-independent pathways were detected in human peripheral blood samples by analyzing the replication history and SHM levels. In vitro, the two types of memory B cells could differentiate into IgE+ PCs cultured with anti-CD40 and IL-21 (36).
A small proportion of PCs migrate to BM and survive as LLPCs (37). IgE+ BMPCs are detectable in mice with N. brasiliensis infection, and most of them experience sequential class switching from IgG1 (32). Compared with irradiated mice receiving IgE− PCs, mice receiving IgE+ PCs purified from mice immunized with OVA + HA/Alum had higher levels of long-lasting serum IgE as well as increased expression of IgE transcripts in BM and spleen (32). This finding indicates that a proportion of IgE+ PCs could migrate to BM and might be the LLPCs. However, direct evidence is lacking (Figure 1).
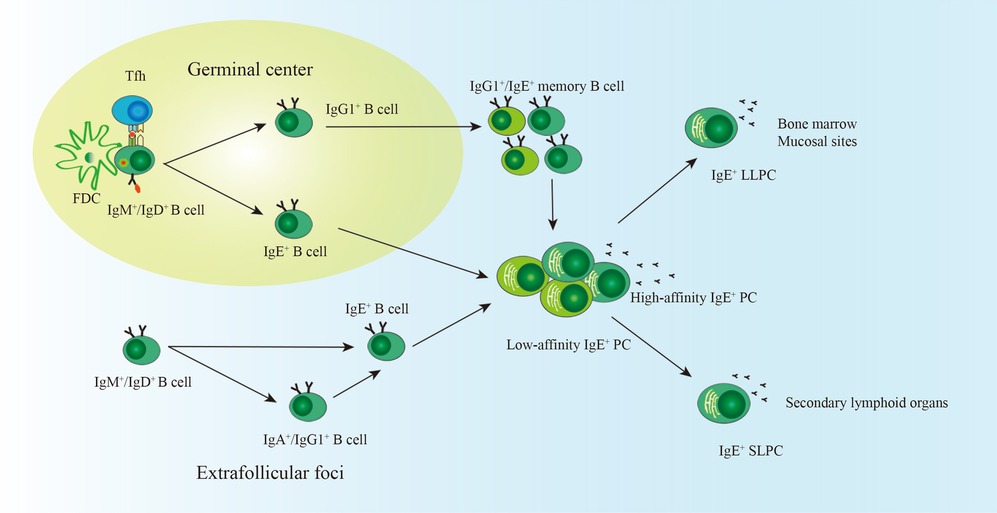
Figure 1. The generation of IgE+ LLPCs. With the help of FDC and Tfh cells in the germinal center, most naïve B cells experience somatic hypermutation and class switch recombination, including direct class switching (IgM/IgD → IgE) and indirect class switching (IgM/IgD → IgG1 → IgE). B cells experiencing direct class switching are more likely to differentiate into low-affinity IgE+ PCs. High-affinity IgE+ PCs may derive from B cells undergoing indirect class switching. IgE+ GC B cells, IgE+ memory B cells, and IgG1+ memory B cells are precursors of IgE+ PCs. A small proportion of IgE+ PCs migrate into the bone marrow and become LLPCs. SLPCs reside in secondary lymphoid organs. IgE+ PCs can also be generated in the extrafollicular foci and are more likely the SLPCs. FDC, follicular dendritic cell; Tfh, follicular helper T cell; PCs, plasma cells; LLPCs, long-lived plasma cells; SLPCs, short-lived plasma cells.
The GC-independent pathway
Recently, increasing evidence has shown that IgE+ PCs can be derived by the extrafollicular pathway (Figure 1). Kwon1 and colleagues showed that thymic B cells differentiated into IgE+ PCs in the thymus and produced natural IgE in mice (38). Another study showed that IgD+ B cells could differentiate into IgE+ PCs directly (IgD → IgE) or sequentially (IgD → IgA/IgG → IgE) in the nasal mucosa (39). The GC is critical for persistent humoral IgE responses because abnormal GC formation leads to a faster decrease in specific IgE in the peanut allergy mouse model (18). Thus, extrafollicular-derived IgE+ PCs are more likely to be SLPCs. GC is the primary place for CSR and affinity maturation. From this viewpoint, impaired GC formation may lead to the generation of low-affinity IgE, which competes with high-affinity IgE and prevents anaphylaxis (29, 40). However, a study by Jiménez-Saiz found that IgE in Bcl-6B cell knock-out mice with impaired GC formation could trigger anaphylaxis (18). Researchers did not further analyze the switch region in DNA fragments of IgE+ PCs and did not test the affinity of IgE derived from Bcl-6B cell knock-out mice.
Mechanisms for IgE+ PCs survival
IgE+ B cells and IgE+ PCs are poorly characterized because of their scarcity and low expression of membrane IgE. We reviewed the microenvironment of IgE+ LLPCs and the unique mechanisms mediated by IgE-BCR, which may bring insights to understand the survival mechanisms of IgE+ PCs.
The microenvironment for IgE+ PCs survival
LLPCs expressing the chemokine receptor CXCR4 will migrate to the BM, where these cells receive various survival signals. In contrast, SLPCs mostly die within 1 week in secondary lymphoid organs (17, 41). In mice induced chronically by HDM, IgE+ BMPCs express CXCR4 at a similar level with IgG1+ BMPCs (17). Moreover, IgE+ LLPCs induced in mice mainly reside in BM, suggesting that, like other LLPCs, BM is also the primary harbor of IgE+ LLPCs (12, 16–18). Additional reservoirs of IgE+ LLPCs besides BM were also reported, such as lungs and nasal polyps, implying that prosurvival factors in these mucosal sites contribute to the longevity of PCs (17, 20). The BM microenvironment is essential for PC survival and has been well-reviewed (23). Briefly, the adhesion molecules VAL-4 and LFA-1 are responsible for LLPC retention by interacting with ligands expressed by stromal cells, such as ICAM-1, ACAM-1, and fibronectin (42, 43). In the BM niche, stromal cells and hematopoietic cells (e.g., monocytes, eosinophils, basophils, BM dendritic cells, and megakaryocytes) could offer survival signals through cell-to-cell contact and cytokines, such as a proliferation-inducing ligand (APRIL), B-cell activating factor of the TNF family (BAFF), and interleukin-6 (IL-6) (37).
Identifying the prosurvival factors in the microenvironment other than BM is interesting. Many studies explored the cytology and cytokine profiles in nasal polyps and lungs. A higher level of eosinophils and IL-6 in eosinophilic nasal polyps and bronchoalveolar lavage fluid (BALF) of asthmatic patients was detected (44, 45). These two factors could promote the survival of LLPCs in the BM (43). Increased expression of other cytokines, such as IL-4, IL-13, IL-5, IL-8, et al., was also reported (45–47). IL-4 and IL-13 contribute to the class switching of IgE and promote memory B cells to differentiate into IgE+ PCs; however, they may not contribute to survival (48). IL-5 play a role in supporting the survival of BMPC (49). The expression of IL-5R was upregulated in PCs in nasal polyps from subjects with aspirin-exacerbated respiratory disease, and stimulation with IL-5 in vitro led to high expression of transcripts related to cell proliferation (50). Multiple myeloma (MM) cells are malignant PCs that share some common characteristics with LLPCs. IL-8 could protect MM cells from cell death induced by serum starvation (51). Collectively, some cytokines and cells that could support PC survival are also present in nasal polyps and lungs. However, the prosurvival role was identified in the BM or in vitro; whether these factors play the same effect in nasal polyps and lungs remains to be clarified.
The IgE-BCR signaling in IgE+ PCs
The serum level of IgE (50–200 ng/ml) is the lowest of all the Ig subclasses in non-atopic persons (52). In addition, IgE+ cells are rare, and IgE+ B cells are likely to differentiate into SLPCs and undergo apoptosis (53). Thus, the IgE production and IgE-secreting cells' lifespan are strictly regulated, or uncontrolled IgE response could cause allergic reactions. Unlike other PCs, IgE+ PCs have several unique features. Firstly, IgE+ PCs are scarce and short-lived in healthy conditions (33). Besides, IgE-BCR is paradoxically upregulated in IgE+ PCs (54). Moreover, IgE-BCR could activate autonomously without antigen engagement (55). Thus, IgE-BCR signaling may play an essential role in IgE+ PC regulation, and identifying the specific molecular mechanism helps discover the potential pathogenesis of the allergic disease.
IgE-BCR in humans and mice
Membrane IgE (mIgE) has two isoforms sharing the same mRNA precursor but alternative splicing sites. The long form of mIgE (mIgEL) has an additional extra-membrane proximal domain (EMPD) region between Cε4 and the transmembrane M1 domain. EMPD contains 52 amino acid residues and exists in some primate species, including humans (56). Other mammal animals, including mice, only express short mIgE (mIgES). In the human B cell line, the amount of mIgEL was much lower than mIgES on the cell surface due to EMPD. EMPD could act as an autonomous endoplasmic reticulum (ER) retention domain and might restrict the transport of mIgEL from ER to the cellular membrane (56). Moreover, modulation of the two isoforms exists. In human tonsil B cells cultured with anti-CD40 and IL-4, mIgEL was downregulated in IgE+ PCs along the differentiation pathway and compensated by mIgES (54). Humans have the propensity to develop allergic diseases but apparently not in mice, indicating a unique role of mIgEL isoform in allergy development.
Compared to wild-type mice, mice with IgE-BCR mutation show a reduction of IgE+ PC population after infection with N. brasiliensis, indicating a crucial role of IgE-BCR for PC accumulation (57). In vitro, spleen B cells isolated from naïve mice with IgE-BCR (mIgES) mutation and cultured with IL-4 and anti-CD40 antibodies show an impaired formation of IgE+ PCs (57). Another study developed murine spleen B cells that only expressed one isotype of BCRs and culture them with IL-4 on feeder cells expressing CD40l and BAFF. Among these isotypes (IgM/IgD/IgA/IgG/IgE-BCR), only IgE-BCR could induce B cells to differentiate into PCs and apoptosis (55). Thus, IgE-BCR could facilitate IgE+ PC differentiation and apoptosis in an antigen-independent way. In a human B cell line, stimulating mIgES of DG75 B cells with anti-IgE antibodies also leads to apoptosis (56). Studies on mIgEL are controversial. Haniuda et al. found that mIgEL could stimulate activation-induced cell apoptosis in vitro but is less efficient than mIgES (56). In contrast, one study developed transfected murine B cells that express immunoreceptors with or without EMPD. A higher proportion of B cells lacking EMPD underwent apoptosis, suggesting a role of EMPD in controlling apoptosis (58). However, the degree to which these findings in vitro apply to IgE+ PC cells in vivo is unclear.
The signaling pathways of IgE-BCR
Like other BCRs, IgE-BCR is non-covalently associated with CD79a and CD79b signaling devices. However, IgE-BCR could activate the downstream signaling autonomously (59). CD79a and CD79b contain an immunoreceptor tyrosine-based activation motif (ITAM). Phosphorylated ITAM recruits and activates Syk, which phosphorylates BLNK after activation. In addition, CD79a could recruit and activate BLNK by a non-ITAM tyrosine residue. BLNK controls calcium signaling pathways via activating PLC-γ2 (60). In contrast to IgM and IgD-BCR, IgE-BCR and IgG-BCR have a cytoplasmic Ig tail tyrosine (ITT) motif, enhancing ITAM-induced calcium signaling as well as Erk MAP kinase pathways (60–62). The IgG-BCR ITT motif could incorporate Grb2/Btk signaling module and amplify downstream signaling. However, the IgE-BCR ITT motif recruits Grb2 and Grb2-related adaptor proteins to enhance BCR signaling (62) (Figure 2).
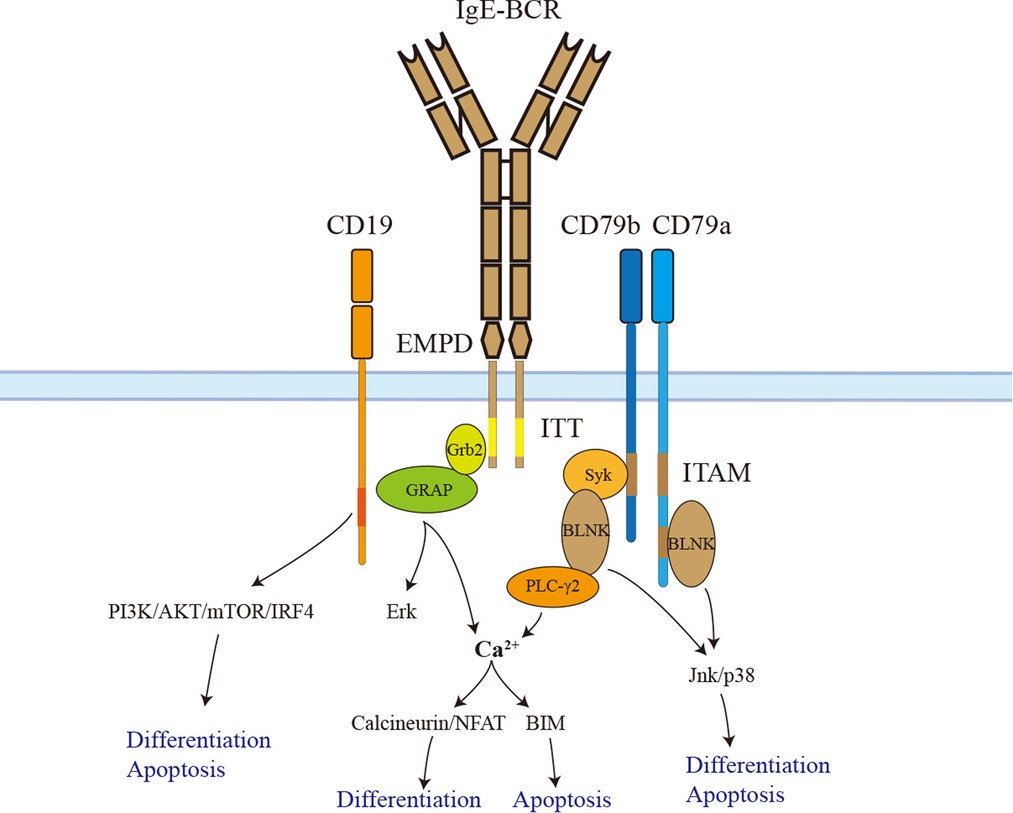
Figure 2. The signaling pathways of IgE-BCR. IgE-BCR is non-covalently associated with CD79a and CD79b, both of which have an ITAM motif in their cytoplasmic tail. Phosphorylated ITAM recruits and activates Syk, which phosphorylates BLNK after activation. In addition, CD79a could recruit and activate BLNK by a non-ITAM tyrosine residue. BLNK controls calcium signaling pathways via activating PLC-γ2. The downstream signaling calcineurin/NFAT promotes IgE+ PC differentiation. Sustained elevation of calcium contributes to BIM-dependent apoptosis. BLNK also facilitates IgE+ PC differentiation and apoptosis via Jnk/p38. In addition, the ITT motif recruits Grb2 and Grb2-related adaptor proteins to enhance BCR downstream signaling. The long isoform of IgE-BCR has an additional EMPD region that could interact and activate CD19. Activated CD19 triggers IgE+ PC differentiation and apoptosis via the PI3K-Akt-mTOR-IRF4 pathway. ITAM, immunoreceptor tyrosine-based activation motif; EMPD, extra-membrane proximal domain; ITT, immunoglobulin tail tyrosine; GRAP, Grb2-related adaptor protein.
Haniuda et al. (55) transduced GC-like B cells with mIgE or mIgG1 and examined the activation of the BCR signaling cascades without BCR stimulation. Compared to mIgG1, mIgE showed spontaneous activation of BCR signaling (BLNK-Jnk/p38) and CD19 downstream signaling (CD19-PI3K-Akt-IRF4). In vitro, the CD19 pathway was proven to play a crucial role in IgE+ SLPCs differentiation but not apoptosis. Further, researchers immunized mice with the TD Ag NP-CGG in alum to clarify the function of CD19 in vivo. CD19+/− mice had attenuated IgE+ PC differentiation but a long-lasting serum IgE titer. BLNK-Jnk/p38 rather than Erk pathway donated to IgE+ SLPCs differentiation and apoptosis. Immunized BLNK−/− mice also had a long-lasting serum IgE titer and generation of IgE+ LLPCs (55). Therefore, IgE-BCR triggers SLPCs generation and apoptosis via activating BLNK-Jnk/p38 and CD19-PI3K-Akt-IRF4 pathways (Figure 2). The PI3K-mTOR-IRF4 pathway also drives the differentiation of IgE+ and IgG1+ PCs. This result is consistent with studies that rapamycin could cause a reduction in serum IgE and IgG1 in mice induced by allergens (63–65). The calcium signaling pathway is another downstream of BCR. The basal intracellular calcium concentration in IgE+ PCs and IgE+ GC-like B cells elevates due to the autonomous activation of Syk/BLNK (55, 65). Enhancing the calcium signaling in IgE+ PCs caused mitochondrial apoptosis mediated by BIM (65). Calcineurin-NFAT is downstream of calcium signaling and may contribute to IgE+ PCs differentiation rather than apoptosis (65) (Figure 2).
Both ectodomain and cytoplastic tail contribute to IgE-BCR signaling. Chimeric BCR containing transmembrane cytoplastic tail of IgG1 and ectodomains of IgE could induce PC differentiation and apoptosis; however, the ectodomains of IgG1 and transmembrane cytoplastic tail of IgE could not, implying that the ectodomains of IgE-BCR contributed to downstream signaling spontaneous activation (55). Moreover, EMPD of IgE-BCR could interact and activate CD19, thus promoting PC differentiation. As mIgES isoform lacking EMPD could also activate downstream signaling of BCR without stimulation, there should be other alternative regions for spontaneous activation. The domains CH1-CH4 of IgE are sufficient to active Syk and BLNK in chimeric BCR without EMPD (55). More recently, the ITT motif of IgE-BCR has been demonstrated to promote IgE surface expression, accumulation of IgE+ PCs, and memory IgE responses in mice (60). As mentioned, the ITT motif could enhance calcium signaling and Erk MAP kinase pathways. Several studies found that Erk MAP kinase pathways did not play a significant role in IgE+ PCs differentiation and apoptosis (55, 65). ITT motif may contribute to IgE+ PCs maintenance through calcium signaling pathways. In addition, ITT promotes IgE surface expression, which may facilitate the activation of IgE-BCR (60).
The molecular mechanisms based on mouse models or mouse cell lines are associated with mIgES. To clarify the distinct functions of mIgEL and mIgES, Vanshylla et al. (56) generated mIgEL and mIgES expressing cells. They found that mIgEL-expressing cells had much weaker signaling of PI3K/Akt, MAPK, and calcium signaling pathways. Moreover, compared with full-length IgE-BCR, IgE-BCR with EMPD deletion significantly promotes murine B cell apoptosis (58). As discussed above, autonomous activation of mIgES signaling promotes SLPCs differentiation and apoptosis; impaired mIgES function contributes to LLPC generation and long-lasting serum IgE titer (55). Whether weaker signaling mediated by mIgEL could compete and attenuate the mIgES pro-apoptosis effect remained to be elucidated. If that is the case, the ratio mIgEL/mIgES on PCs surface may be implicated in the survival of PCs.
Discussion
Clinical observations indicate the responsibility of IgE+ LLPCs for persistent allergies, but the characteristics of IgE+ LLPCs are poorly studied, especially in humans. IgE+ LLPCs could be induced in the allergic mouse model, but only indirect evidence supports the presence of IgE+ LLPCs in allergic patients. IgE+ LLPCs mainly reside in BM and are lesser in the spleen and mucous. The prosurvival factors in the BM have been extensively studied, but a few focus on the mucosal microenvironment where IgE+ LLPCs exist. In non-atopy conditions, IgE+ B cells showed the propensity to differentiate into SLPCs due to the autonomous activation of IgE-BCR signaling. It is interesting to investigate the change of these signaling pathways in subjects with persistent allergies. In addition, mIgEL and mIgES deliver altered signaling, which may play distinct roles in IgE+ PCs survival. Applying mutant mice expressing mIgEL isoform helps to explore the unique role of mIgEL in allergic conditions.
Author contributions
SX and YJ drafted this manuscript. CL revised it. All authors contributed to the article and approved the submitted version.
Acknowledgments
We would like to thank the associate editor and the reviewers for their useful feedback that improved this paper.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher's note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Toit G, Tsakok T, Lack S, Lack G. Prevention of food allergy. J Allergy Clin Immunol. (2016) 137:998–1010. doi: 10.1016/j.jaci.2016.02.005
2. Jackson KD, Howie LJD, Akinbami LJ. Trends in allergic conditions among children: United States, 1997–2011. NCHS Data Brief. (2013) 121:1–8. PMID: 23742874
3. Oriel RC, Wang J. Diagnosis and management of food allergy. Immunol Allergy Clin North Am. (2021) 41:571–85. doi: 10.1016/j.iac.2021.07.012
4. Bernard A, Sardella A, Voisin C, Dumont X. Nasal epithelium injury by chlorination products and other stressors predicts persistent sensitization to aeroallergens in young schoolchildren. Environ Res. (2017) 158:145–52. doi: 10.1016/j.envres.2017.06.009
5. Fong WCG, Chan A, Zhang H, Holloway JW, Roberts G, Kurukulaaratchy R, et al. Childhood food allergy and food allergen sensitisation are associated with adult airways disease: a birth cohort study. Pediatr Allergy Immunol. (2021) 32:1764–72. doi: 10.1111/pai.13592
6. Warren CM, Jiang J, Gupta RS. Epidemiology and burden of food allergy. Curr Allergy Asthma Rep. (2021) 20:6. doi: 10.1007/s11882-020-0898-7
7. Nurmatov U, Dhami S, Arasi S, Pajno GB, Fernandez-Rivas M, Muraro A, et al. Allergen immunotherapy for IgE-mediated food allergy: a systematic review and meta- analysis. Allergy. (2017) 72:1133–47. doi: 10.1111/all.13124
8. Markmann C, Bhoj VG. On the road to eliminating long-lived plasma cells—“are we there yet?”. Immunol Rev. (2021) 303:154–67. doi: 10.1111/imr.13015
9. Pollok K, Mothes R, Ulbricht C, Liebheit A, Gerken JD, Uhlmann S, et al. The chronically inflamed central nervous system provides niches for long-lived plasma cells. Acta Neuropathol Commun. (2017) 5:88. doi: 10.1186/s40478-017-0487-8
10. Waldmann TA, Iio A, Ogawa M, McIntyre OR, Strober W. The metabolism of IgE. Studies in normal individuals and in a patient with IgE myeloma. J Immunol. (1976) 117:1139–44. doi: 10.4049/jimmunol.117.4.1139
11. Vieira P, Rajewsky K. The half-lives of serum immunoglobulins in adult mice. Eur J Immunol. (1988) 18:313–6. doi: 10.1002/eji.1830180221
12. Holt PG, Sedgwick JD, O'Leary C, Krska K, Leivers S. Long-lived IgE- and IgG-secreting cells in rodents manifesting persistent antibody responses. Cell Immunol. (1984) 89:281–9. doi: 10.1016/0008-8749(84)90330-7
13. Van Hemelen D, Hemmer W, Kmenta M, Berger UB, Kinaciyan T, Bohle B, et al. Dramatically decreased T cell responses but persistent IgE upon reduced pollen exposure. Immunobiology. (2019) 224:645–8. doi: 10.1016/j.imbio.2019.07.003
14. Erwin EA, Woodfolk JA, James HR, Satinover SM, Platts-Mills TA. Changes in cat specific IgE and IgG antibodies with decreased cat exposure. Ann Allergy Asthma Immunol. (2014) 112:545–550.e1. doi: 10.1016/j.anai.2014.03.007
15. Laffleur B, Debeaupuis O, Dalloul Z, Cogné M. B cell intrinsic mechanisms constraining IgE memory. Front Immunol. (2017) 8:1277. doi: 10.3389/fimmu.2017.01277
16. Luger EO, Fokuhl V, Wegmann M, Abram M, Tillack K, Achatz G, et al. Induction of long-lived allergen-specific plasma cells by mucosal allergen challenge. J Allergy Clin Immunol. (2009) 124:819–826.e4. doi: 10.1016/j.jaci.2009.06.047
17. Asrat S, Kaur N, Liu X, Ben LH, Kajimura D, Murphy AJ, et al. Chronic allergen exposure drives accumulation of long-lived IgE plasma cells in the bone marrow, giving rise to serological memory. Sci Immunol. (2020) 5:eaav8402. doi: 10.1126/sciimmunol.aav8402
18. Jiménez-Saiz R, Chu DK, Mandur TS, Walker TD, Gordon ME, Chaudhary R, et al. Lifelong memory responses perpetuate humoral TH2 immunity and anaphylaxis in food allergy. J Allergy Clin Immunol. (2017) 140:1604–15.e5. doi: 10.1016/j.jaci.2017.01.018
19. Pitlick MM, Pongdee T. Combining biologics targeting eosinophils (IL-5/IL-5R), IgE, and IL-4/IL-13 in allergic and inflammatory diseases. World Allergy Organ J. (2022) 15:100707. doi: 10.1016/j.waojou.2022.100707
20. Zhang YN, Song J, Zhai GT, Wang H, Luo RZ, Li JX, et al. Evidence for the presence of long-lived plasma cells in nasal polyps. Allergy Asthma Immunol Res. (2020) 12:274–91. doi: 10.4168/aair.2020.12.2.274
21. Robinson MJ, Webster RH, Tarlinton DM. How intrinsic and extrinsic regulators of plasma cell survival might intersect for durable humoral immunity. Immunol Rev. (2020) 296:87–103. doi: 10.1111/imr.12895
22. Liu X, Yao J, Zhao Y, Wang J, Qi H. Heterogeneous plasma cells and long-lived subsets in response to immunization, autoantigen and microbiota. Nat Immunol. (2022) 23:1564–76. doi: 10.1038/s41590-022-01345-5
23. Nguyen DC, Duan M, Ali M, Ley A, Sanz I, Lee FE. Plasma cell survival: the intrinsic drivers, migratory signals, and extrinsic regulators. Immunol Rev. (2021) 303:138–53. doi: 10.1111/imr.13013
24. Crotty S. T follicular helper cell biology: a decade of discovery and diseases. Immunity. (2019) 50:1132–48. doi: 10.1016/j.immuni.2019.04.011
25. Kobayashi T, Iijima K, Dent AL, Kita H. Follicular helper T cells mediate IgE antibody response to airborne allergens. J Allergy Clin Immunol. (2017) 139:300–13.e7. doi: 10.1016/j.jaci.2016.04.021
26. Gowthaman U, Chen JS, Zhang B, Flynn WF, Lu Y, Song W, et al. Identification of a T follicular helper cell subset that drives anaphylactic IgE. Science. (2019) 365:eaaw6433. doi: 10.1126/science.aaw6433
27. Yang Z, Wu CM, Targ S, Allen CDC. IL-21 is a broad negative regulator of IgE class switch recombination in mouse and human B cells. J Exp Med. (2020) 217:e20190472. doi: 10.1084/jem.20190472
28. Looney TJ, Lee JY, Roskin KM, Hoh RA, King J, Glanville J, et al. Human B-cell isotype switching origins of IgE. J Allergy Clin Immunol. (2016) 137:579–86.e7. doi: 10.1016/j.jaci.2015.07.014
29. Xiong H, Dolpady J, Wabl M, de Lafaille MAC, Lafaille JJ. Sequential class switching is required for the generation of high affinity IgE antibodies. J Exp Med. (2012) 209:353–64. doi: 10.1084/jem.20111941
30. Ramadani F, Upton N, Hobson P, Chan YC, Mzinza D, Bowen H, et al. Intrinsic properties of germinal center-derived B cells promote their enhanced class switching to IgE. Allergy. (2015) 70:1269–77. doi: 10.1111/all.12679
31. Hoh RA, Joshi SA, Lee J-Y, Martin BA, Varma S, Kwok S, et al. Origins and clonal convergence of gastrointestinal IgE+ B cells in human peanut allergy. Sci Immunol. (2020) 5:eaay4209. doi: 10.1126/sciimmunol.aay4209
32. He JS, Meyer-Hermann M, Xiangying D, Zuan LY, Jones LA, Ramakrishna L, et al. The distinctive germinal center phase of IgE+ B lymphocytes limits their contribution to the classical memory response. J Exp Med. (2013) 210:2755–71. doi: 10.1084/jem.20131539
33. Wade-Vallance AK, Allen CDC. Intrinsic and extrinsic regulation of IgE B cell responses. Curr Opin Immunol. (2021) 72:221–9. doi: 10.1016/j.coi.2021.06.005
34. Talay O, Yan D, Brightbill HD, Straney EEM, Zhou M, Ladi E, et al. IgE+ memory B cells and plasma cells generated through a germinal-center pathway. Nat Immunol. (2012) 13:396–404. doi: 10.1038/ni.2256
35. Talay O, Yan D, Brightbill HD, Straney EE, Zhou M, Ladi E, et al. IgE+ memory B cells and plasma cells generated through a geminal-center pathway. Nat Immunol. (2013) 14:1302–4. doi: 10.1038/ni.2770
36. Berkowska MA, Heeringa JJ, Hajdarbegovic E, van der Burg M, Thio HB, van Hagen PM, et al. Human IgE+ B cells are derived from T cell-dependent and T cell-independent pathways. J Allergy Clin Immunol. (2014) 134:688–697.e6. doi: 10.1016/j.jaci.2014.03.036
37. Lightman SM, Utley A, Lee KP. Survival of long-lived plasma cells (LLPC): piecing together the puzzle. Front Immunol. (2019) 10:965. doi: 10.3389/fimmu.2019.00965
38. Kwon DI, Park ES, Kim M, Choi YH, Lee MS, Joo SH, et al. Homeostatic serum IgE is secreted by plasma cells in the thymus and enhances mast cell survival. Nat Commun. (2022) 13:1418. doi: 10.1038/s41467-022-29032-x
39. Corrado A, Ramonell RP, Woodruff MC, Tipton C, Wise S, Levy J, et al. Extrafollicular IgD+ B cells generate IgE antibody secreting cells in the nasal mucosa. Mucosal Immunol. (2021) 14:1144–59. doi: 10.1038/s41385-021-00410-w
40. Victora GD, Nussenzweig MC. Germinal centers. Annu Rev Immunol. (2012) 30:429–57. doi: 10.1146/annurev-immunol-020711-075032
41. Tellier J, Kallies A. Finding a home for plasma cells—a niche to survive. Eur J Immunol. (2014) 44:2243–6. doi: 10.1002/eji.201444871
42. Nguyen DC, Garimalla S, Xiao H, Kyu S, Albizua I, Galipeau J, et al. Factors of the bone marrow microniche that support human plasma cell survival and immunoglobulin secretion. Nat Commun. (2018) 9:3698. doi: 10.1038/s41467-018-05853-7
43. Belnoue E, Tougne C, Rochat A-F, Lambert P-H, Pinschewer DD, Siegrist C-A. Homing and adhesion patterns determine the cellular composition of the bone marrow plasma cell niche. J Immunol. (2012) 188:1283–91. doi: 10.4049/jimmunol.1103169
44. Zhu Z, Wang W, Zhang X, Wang X, Zha Y, Chen Y, et al. Nasal fluid cytology and cytokine profiles of eosinophilic and non-eosinophilic chronic rhinosinusitis with nasal polyps. Rhinology. (2020) 58:314–22. doi: 10.4193/Rhin19.275
45. Lou Y, Ke Q, Cui H, Shang Y, Yang C. Correlation study of cytokine levels in alveolar lavage fluid with exhaled nitric oxide and lung function in children with bronchialasthma. Transl Pediatr. (2021) 10:2069–75. doi: 10.21037/tp-21-322
46. Biggs TC, Hayes SM, Harries PG, Allan RN, Walls AF, Pender SLF, et al. Immunological profiling of key inflammatory drivers of nasal polyp formation and growth in chronic rhinosinusitis. Rhinology. (2019) 57:336–42. doi: 10.4193/Rhin19.167
47. Carsuzaa F, Béquignon É, Dufour X, de Bonnecaze G, Lecron JC, Favot L. Cytokine signature and involvement in chronic rhinosinusitis with nasal polyps. Int J Mol Sci. (2022) 23:417. doi: 10.3390/ijms23010417
48. Turqueti-Neves A, Otte M, Schwartz C, Schmitt MER, Lindner C, Pabst O, et al. The extracellular domains of IgG1 and T cell-derived IL-4/IL-13 are critical for the polyclonal memory IgE response in vivo. Plos Biol. (2015) 13:e1002290. doi: 10.1371/journal.pbio.1002290
49. Cassese G, Arce S, Hauser AE, Lehnert K, Moewes B, Mostarac M, et al. Plasma cell survival is mediated by synergistic effects of cytokines and adhesion-dependent signals. J Immunol. (2003) 171:1684–90. doi: 10.4049/jimmunol.171.4.1684
50. Buchheit KM, Dwyer DF, Ordovas-Montanes J, Katz HR, Lewis E, Vukovic M, et al. IL-5Rα marks nasal polyp IgG4- and IgE-expressing cells in aspirin-exacerbated respiratory disease. J Allergy Clin Immunol. (2020) 145:1574–84. doi: 10.1016/j.jaci.2020.02.035
51. Herrero AB, García-Gómez A, Garayoa M, Corchete LA, Hernández JM, San Miguel J, et al. Effects of IL-8 up-regulation on cell survival and osteoclastogenesis in multiple myeloma. Am J Pathol. (2016) 186:2171–82. doi: 10.1016/j.ajpath.2016.04.003
52. Dullaers M, De Bruyne R, Ramadani F, Gould HJ, Gevaert P, Lambrecht BN. The who, where, and when of IgE in allergic airway disease. J Allergy Clin Immunol. (2012) 129:635–45. doi: 10.1016/j.jaci.2011.10.029
53. Yang Z, Sullivan BM, Allen CD. Fluorescent in vivo detection reveals that IgE(+) B cells are restrained by an intrinsic cell fate predisposition. Immunity. (2012) 36:857–72. doi: 10.1016/j.immuni.2012.02.009
54. Ramadani F, Bowen H, Upton N, Hobson PS, Chan YC, Chen JB, et al. Ontogeny of human IgE-expressing B cells and plasma cells. Allergy. (2017) 72:66–76. doi: 10.1111/all.12911
55. Haniuda K, Fukao S, Kodama T, Hasegawa H, Kitamura D. Autonomous membrane IgE signaling prevents IgE-memory formation. Nat Immunol. (2016) 17:1109–17. doi: 10.1038/ni.3508
56. Vanshylla K, Opazo F, Gronke K, Wienands J, Engels N. The extracellular membrane-proximal domain of membrane-bound IgE restricts B cell activation by limiting B cell antigen receptor surface expression. Eur J Immunol. (2018) 48:441–53. doi: 10.1002/eji.201747196
57. Schmitt MER, Lutz J, Haase P, Bösl MR, Wienands J, Engels N, et al. The B- cell antigen receptor of IgE-switched plasma cells regulates memory IgE responses. J Allergy Clin Immunol. (2020) 146:642–651.e5. doi: 10.1016/j.jaci.2020.02.015
58. Poggianella M, Bestagno M, Burrone OR. The extracellular membrane-proximal domain of human membrane IgE controls apoptotic signaling of the B cell receptor in the mature B cell line A20. J Immunol. (2006) 177:3597–605. doi: 10.4049/jimmunol.177.6.3597
59. Batista FD, Anand S, Presani G, Efremov DG, Burrone OR. The two membrane isoforms of human IgE assemble into functionally distinct B cell antigen receptors. J Exp Med. (1996) 184:2197–205. doi: 10.1084/jem.184.6.2197
60. Engels N, König LM, Schulze W, Radtke D, Vanshylla K, Lutz J, et al. The immunoglobulin tail tyrosine motif upgrades memory-type BCRs by incorporating a Grb2-btk signaling module. Nat Commun. (2014) 5:5456. doi: 10.1038/ncomms6456
61. Engels N, König LM, Heemann C, Lutz J, Tsubata T, Griep S, et al. Recruitment of the cytoplasmic adaptor Grb2 to surface IgG and IgE provides antigen receptor-intrinsic costimulation to class-switched B cells. Nat Immunol. (2009) 10:1018–25. doi: 10.1038/ni.1764
62. Vanshylla K, Bartsch C, Hitzing C, Krümpelmann L, Wienands J, Engels N. Grb2 and GRAP connect the B cell antigen receptor to Erk MAP kinase activation in human B cells. Sci Rep. (2018) 8:4244. doi: 10.1038/s41598-018-22544-x
63. Mushaben EM, Kramer EL, Brandt EB, Khurana Hershey GK, Le Cras TD. Rapamycin attenuates airway hyperreactivity, goblet cells, and IgE in experimental allergic asthma. J Immunol. (2011) 187:5756–63. doi: 10.4049/jimmunol.1102133
64. Yamaki K, Yoshino S. Preventive and therapeutic effects of rapamycin, a mammalian target of rapamycin inhibitor, on food allergy in mice. Allergy. (2012) 67:1259–70. doi: 10.1111/all.12000
Keywords: long-lived plasma cells, IgE, B cell receptor, microenviroment, class switch recombination, persistent sensitization
Citation: Xiong S, Jia Y and Liu C (2022) IgE-expressing long-lived plasma cells in persistent sensitization. Front. Pediatr. 10:979012. doi: 10.3389/fped.2022.979012
Received: 27 June 2022; Accepted: 17 November 2022;
Published: 5 December 2022.
Edited by:
Hai Qi, Tsinghua University, ChinaReviewed by:
Wataru Ise, Osaka University, JapanRonald van Ree, Amsterdam University Medical Center, Netherlands
© 2022 Xiong, Jia and Liu. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Shiqiu Xiong xsq20180224@163.com Chuanhe Liu liuchcip@126.com
Specialty Section: This article was submitted to Pediatric Immunology, a section of the journal Frontiers in Pediatrics
 Shiqiu Xiong
Shiqiu Xiong Yang Jia
Yang Jia Chuanhe Liu1,2*
Chuanhe Liu1,2*