- 1Cardiovascular Therapeutics Unit, Department of Biochemistry and Pharmacology, University of Melbourne, Parkville, VIC, Australia
- 2Australian Venom Research Unit, Department of Biochemistry and Pharmacology, University of Melbourne, Parkville, VIC, Australia
Human envenoming by Australian brown snakes (Pseudonaja spp.) may result in potentially life-threatening hypotension and subsequent cardiovascular collapse. There have been relatively few studies of the cardiovascular and sympathetic effects of Pseudonaja spp. venoms. In this study, we have examined the effects of venom from five brown snake species—P. affinis, aspidorhyncha, inframacula, nuchalis, and textilis—on cardiac inotropic and chronotropic responses, vascular tone, and sympathetic nerve-induced vascular contractions in rat isolated tissues. The role of phospholipases A2 (PLA2s) in venom-induced effects was assessed with the sPLA2 inhibitor varespladib. In rat isolated left and right atria, there were no physiologically relevant effects of Pseudonaja venoms (0.1–30 µg/ml) on left atrial force of contraction (inotropy) or right atrial rate (chronotropy). In contrast, in isolated small mesenteric arteries precontracted with a thromboxane mimetic, each of the five brown snake venoms (at 30 µg/ml) caused marked vasorelaxation (−60 to –90% of contractile tone). Pretreatment with varespladib (1 µM) significantly inhibited the vasorelaxation caused by P. aspidorhyncha, P. nuchalis, and P. textilis venoms. Electrically induced sympathetic nerve-mediated contractions of mesenteric arteries were significantly attenuated by only P. textilis, and P. affinis venoms (30 µg/ml) and these sympatholytic effects were inhibited by varespladib (1 µM). Based on their inhibition with the sPLA2 inhibitor varespladib, we conclude that PLA2 toxins in P. aspidorhyncha, P. nuchalis, and P. textilis venoms are involved in brown snake venom-induced vasorelaxation and the sympatholytic effects of P. affinis, and P. textilis venoms. Our study supports the promising potential role of varespladib as an initial (pre-referral) and/or adjunct (in combination with antivenom) therapeutic agent for brown snake envenoming.
1 Introduction
Venom is a functional trait, used by venomous species of snake to subdue prey animals or deter potential predators (Jackson and Fry, 2016). The venom itself is often described as a “cocktail” (Casewell et al., 2013) because it contains numerous toxin species, each of which may interact with a different component of the target organism’s physiology. Venom achieves its goal of subjugation or deterrence by virtue of these toxins interfering with the normal functioning of various regulatory (homeostatic) networks. These networks operate in a state of “criticality” (marginal stability—Daniels et al., 2018) and thus their subversion may lead to a catastrophic failure of homeostasis, either locally (i.e., in the vicinity of the bite site) or systemically. On the other hand, organisms are buffered systems and the effects of interference with a single gene product are often minimal at the level of the integrated phenotype (Noble, 2011). The complexity of the venom cocktail, therefore, is in service of the overall function of the trait–venoms typically attack multiple regulatory networks simultaneously and may attack each network on multiple fronts. This is also highlighted by the complexity of clinical envenoming syndromes in human bite victims (Gutiérrez et al., 2017).
According to the World Health Organisation, an estimated 2 million people are envenomed following a snakebite every year and approximately 100,000 of these cases are fatal (The Lancet, 2017). The majority of these cases occur in developing countries in sub-Saharan Africa, South Asia and South East Asia (Gutiérrez et al., 2017; Longbottom et al., 2018). Despite Australia’s reputation for deadly snakes, snakebite envenoming is far less frequent on that continent. However, deaths do occur there every year because of snakebite and brown snakes (genus Pseudonaja) are the most “medically significant” snake taxa, being responsible for 41% of all deaths from snakebite envenoming between 2005 and 2015 (Johnston et al., 2017).
Patients who are envenomed by Pseudonaja spp. may exhibit a range of clinical symptoms, including venom-induced consumption coagulopathy (VICC), hypotension or cardiovascular collapse. Despite the presence of both pre- and post-synaptic neurotoxins in the venom, paralysis is only a rare consequence of brown snake envenoming, a fact known as the “brown snake paradox” (Barber et al., 2012). VICC is the most common severe symptom observed in cases of human envenoming by Pseudonaja spp., though hypotension and subsequent cardiovascular collapse are also potentially life-threatening (Allen et al., 2012). Unlike VICC, which is caused by the prothrombinase toxin pseutarin C interfering with the coagulation cascade (Rao and Kini, 2002), the mechanisms underlying the induction of hypotension and collapse following snakebite remain contentious, although pseutarin C has also been implicated in these pathologies (Chaisakul et al., 2015). Hypotensive effects of snake venoms have also been attributed to the release of dilator autacoids (Chaisakul et al., 2014), blockade of L-type voltage-gated Ca2+ channels (Yagami et al., 2013), presence of bradykinin-potentiating (Ferreira et al., 1970; Ianzer et al., 2004; Camargo et al., 2012) and natriuretic (Fry et al., 2005; St Pierre et al., 2005) peptides, or as a side effect of disseminated intravascular coagulation (Tibballs et al., 1992).
The venoms of Pseudonaja spp. are notably diverse in toxin content and highly variable–intra-as well as interspecific variations have been documented (Flight et al., 2006; Jackson et al., 2016; Reeks et al., 2016). Diversity exists both in terms of the variety of toxin classes present within a given venom, as well as within toxin classes, such as post-synaptically neurotoxic “3-finger toxins” (3FTx), which may exist in more than 40 variants within a single venom (Jackson et al., 2013). As well as the prothrombinase homolog pseutarin C (Rao and Kini, 2002), the venoms of Pseudonaja spp. contain phospholipases A2 (PLA2), including the presynaptic neurotoxin textilotoxin (Su et al., 1983); long chain and short chain 3FTx including the post-synaptic neurotoxins pseudonajatoxin a (Barnett et al., 1980) and b (Tyler et al., 1987); serine protease inhibitors including textilinin-1 and -2 (Masci et al., 2000); cysteine-rich secretory peptides; and vasoactive C-type natriuretic peptides (Judge et al., 2002; Birrell et al., 2006; Viala et al., 2015; Jackson et al., 2016; Reeks et al., 2016).
PLA2 toxins exhibit diverse activities, including neurotoxicity (Su et al., 1983; Lambeau et al., 1989); myotoxicity (Harris and MacDonell, 1981; Dixon and Harris, 1996); procoagulant (Judge et al., 2002); synergistic/auxillary interactions with other toxins (Bougis et al., 1987; Mukherjee, 2010; Mora-Obando et al., 2014; Laustsen, 2016); and others, including both enzymatic and non-enzymatic activities (Kini, 2003). Inhibition of PLA2 toxins, therefore, may have considerable therapeutic benefit for patients suffering from snakebite envenoming, including those envenomed by Australian brown snakes. Lewin et al. (2016) found that varespladib–a secretory phospholipase A2 (sPLA2) inhibitor–inhibited the phospholipase activities of 28 snake venoms. Further, rats treated with varespladib before or after envenoming had higher survival rates. Its pro-drug methyl-varespladib, when administered in conjunction with antivenom, also rescued mice administered otherwise lethal doses of Oxyuranus scutellatus venom (Lewin et al., 2018). These studies have revealed the potential of varespladib as an adjunct therapy, to be administered along with antivenom and/or additional small molecule therapeutics, for snakebite envenoming.
In the present study, we examined the cardiac, vascular, and sympathetic effects induced by five Pseudonaja spp. venoms in rat isolated atria and small mesenteric arteries. Where the brown snake venoms caused significant effects, the role of sPLA2s in mediating these responses was assessed by treatment with varespladib in vitro. Further, the role of nitric oxide, prostaglandins or CGRP in venom-induced vascular relaxation was investigated.
2 Materials and Methods
2.1 Materials
Venoms from five of the nine species comprising the genus Pseudonaja were examined in this study: P. affinis (dugite), P. aspidorhyncha (strap-snouted brown snake), P. inframacula (Peninsula brown snake), P. textilis (common or eastern brown snake) and P. nuchalis (northern brown snake). Venoms of the first four brown snake species were collected from South Australian specimens, whereas P. nuchalis venom was from a Northern Territory specimen. Lyophilised venoms were obtained from Venom Supplies Pty. Ltd. (Tanunda, South Australia) and diluted in phosphate-buffered saline (PBS; Oxoid Ltd., Hampshire, England) to a w/v ratio of approximately 10 mg/ml. Actual protein concentrations for each venom batch were determined by a Bradford protein assay, and subsequently all venom concentrations were expressed as µg/ml of protein concentration.
Drugs and suppliers were as follows: acetylcholine bromide (Sigma-Aldrich, St. Louis, MO, United States); benextramine tetrahydrochloride (Sigma); calcitonin gene-related peptide (human) 8–37 (CGRP8–37; Synpeptide Co. Ltd., Shanghai, China); 9,11-dideoxy-9α,11α-methanoepoxy-prosta-5Z,13E-dien-1-oic acid (U46619; Cayman Chemical, Ann Arbor, MI, United States); indomethacin (Sigma); (-)-isoprenaline (+)-bitartrate salt (Sigma); Nω-nitro-L-arginine methyl ester hydrochloride (L-NAME; Sigma); noradrenaline bitartrate (Sigma); prazosin hydrochloride (Sigma); tetrodotoxin (Sigma); and varespladib (LY315920; Tocris Bioscience, Bristol, United Kingdom). All drug dilutions were in Milli-Q grade water (15 MΩ.cm), purified using the Elix® Essential Water Purification System (Merck Millipore, Darmstadt, Germany), except for varespladib and indomethacin, which were dissolved in 100% dimethyl sulfoxide (DMSO) or 0.01 M sodium bicarbonate, respectively.
2.2 Tissue Collection
Experiments were approved by the University of Melbourne Animal Ethics Committee and performed in accordance with the Australian code for the care and use of animals for scientific purposes (National Health and Medical Research Council, Canberra, Australia, 2013). Male Sprague-Dawley rats (250–350 g) were placed in a secure box and deeply anaesthetised by inhalation of isoflurane 5% (Baxter Healthcare Pty Ltd., New South Wales, Australia) in 95% O2 and killed by decapitation. Tissues were isolated, placed in a Silastic-coated petri dish and immersed in cold physiological salt solution (PSS) of the following composition (in mM): NaCl 119, KCl 4.69, MgSO4 1.17, KH2PO4 1.18, glucose 11 (5.5 for the myography preparations), NaHCO3 25, EDTA 0.026 and CaCl2 2.5; with pH 7.4.
2.3 Left and Right Atria
The left atrium and the spontaneously beating right atrium were separated from each other and pinned using two stainless steel hooks. The atria were then mounted in warm (37°C) PSS-filled 15 ml organ baths aerated with carbogen (95% O2, 5% CO2) for the duration of the experiment. Each atrium was attached to a Grass FT03C force transducer (Grass Instrument Co., Quincy, MA, United States) connected to a 6-channel amplifier (Octal Bridge Amp, ADInstruments, Sydney, Australia). Data were acquired by LabChart Acquisition Software (v7.0; ADInstruments). The atria were then stretched to 0.5 g and equilibrated for 10 min before another re-stretch to 0.5 g. The left atrium was electrically stimulated at 1 Hz, 0.25 ms at 150% threshold voltage via two punctate electrodes connected to a Grass S88 stimulator, while the right atrium was spontaneously beating. Tissues were allowed a 30 min equilibration period before their viability was tested with the β-adrenoceptor agonist isoprenaline (right atrium: 0.01 µM, left atrium: 0.1 µM). Tissues were washed with warm PSS and treated with one of P. textilis, P. affinis, P. inframacula or P. nuchalis venom in half-log10 cumulative increments (venom protein concentration 0.1–30 µg/ml). P. aspidorhyncha venom was not tested in the atrial bioassays.
2.4 Mesenteric Arteries
Second or third order arteries (i.d., 200–400 µm; 2 mm length segments) were dissected from the mesenteric vasculature and mounted in wire myograph chambers (Model 610M, Danish Myo Technology, Aarhus, Denmark). Vessels were submerged in 6 ml of PSS at 37°C and aerated with carbogen (95% O2, 5% CO2) for the duration of the experiments. To ensure that all mesenteric arteries were subjected to the same experimental conditions and optimal force development, each underwent a normalisation process by passive stretch as described by Angus and Wright (2000). Thirty min after the normalisation process, the PSS was replaced with 6 ml of potassium physiological salt solution (KPSS, with an equimolar substitution of KCl for NaCl−K+ 124 mM) to obtain each vessel’s depolarising maximum contraction response. These KPSS maximal responses were used as a reference point. To check that the endothelium layer remained intact, arteries were contracted with noradrenaline (3 µM) and tested for relaxation with acetylcholine (1 µM); only vessels with more than 50% relaxation were used in this study.
2.4.1 Vascular Relaxant Effects of Venoms
To examine whether Pseudonaja spp. venoms induce vasorelaxation, arteries were pre-contracted with the thromboxane A2 mimetic U46619 (100 nM) to approximately 80% of their KPSS-induced maximal contraction and a single concentration of a venom (30 µg/ml) was added. In sPLA2 inhibition studies, arteries were incubated with 1 µM of varespladib (Lewin et al., 2016) for 30 min before exposure to U46619. At the plateau contraction to U46619, a venom (30 µg/ml) was added. The protocol of using only a single concentration of each venom was chosen due to rapid desensitization in vascular relaxation responses observed with various Australian elapid snake venoms (Chaisakul et al., 2012; Chaisakul et al., 2013; Chaisakul et al., 2014). In a separate set of experiments, P. textilis venom (30 µg/ml) was tested after pretreatment (30 min; literature supporting each chosen antagonist concentration shown) with one of 1) the CGRP antagonist CGRP8–37 (3 µM) (Wisskirchen et al., 1998); 2) the cyclooxygenase inhibitor indomethacin (3 µM) (Kassab et al., 2017); or 3) the nitric oxide synthase inhibitor L-NAME (100 µM) (Angus et al., 2017).
2.4.2 Effects of Venoms on Sympathetic Nerve-Mediated Vascular Contractions
Arteries in myograph chambers were stimulated via platinum electrodes connected to a low-output-resistance stimulator (Grass S88). After the viability procedure described above, a test stimulation (3 s train duration every min, 25 Hz, 0.25 ms, 30 V dial setting) was conducted (Figure 1). Tissues were then incubated with 100 nM of prazosin for 5 min (to protect α1-adrenoceptors), followed by 30 µM of benextramine (an irreversible α1- and α2-adrenoceptor antagonist) for another 5 min to enhance the sympathetic-mediated responses of small resistance arteries via the blockade of presynaptic α2-adrenoceptors (Angus et al., 1988). Arteries were then washed with warm PSS every 5 min for 30 min, then exposed to 10 µM noradrenaline to ascertain that α1-adrenoceptor-mediated contractions were present; the noradrenaline was then washed out. Only arteries with a nerve stimulation contractile response of at least 50% KPSS were used. After 10 min, arteries were continuously stimulated until plateau, then a brown snake venom (30 µg/ml) was added, and nerve stimulation continued for 60 min.
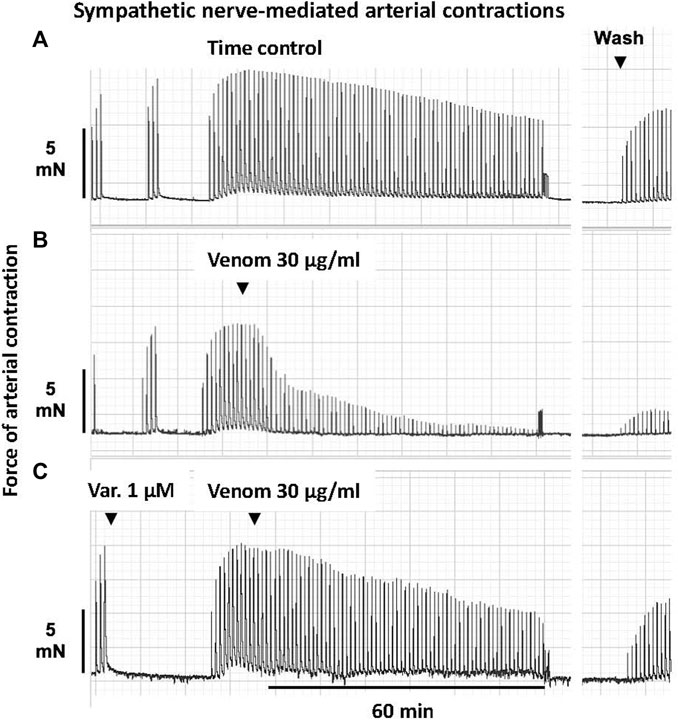
FIGURE 1. Representative computer traces of the sympatholytic effects of Pseudonaja affinis venom in the absence or presence of the sPLA2 inhibitor varespladib in rat isolated electrically stimulated small mesenteric arteries. (A) Time control–no venom or inhibitor treatment. (B) The addition of P. affinis venom (30 µg/ml) markedly inhibited nerve-mediated contractions (C) Pre-incubation with varespladib 1 µM (Var.) attenuated the sympatholytic effects of P. affinis venom. Wash, after 30 min of repeated washout of the bath solution, nerve stimulation responses were retested; very little recovery of contractile responses was observed after venom treatment alone (B).
In sPLA2 inhibition studies, arteries were incubated with varespladib (1 µM) for 30 min and then continuously stimulated. Following a plateau in the contractile response, 30 µg/ml of a brown snake venom was added. Nerve stimulation responses were recorded for a further 60 min. An example of this protocol is shown in Figure 1. Tetrodotoxin (0.3 µM) was used to ascertain that vascular smooth muscle was not directly stimulated.
2.5 Data and Statistical Analyses
All data are presented as the mean ± standard error of the mean (SEM) of n experiments (each tissue from a separate rat). Responses in left or right atria are expressed as the change (Δ) from resting baseline in contractile force (g) or in atrial rate (beats/min), respectively, within each tissue. Contractile responses in arteries are expressed as the percentage of the maximum reference contraction to KPSS (%KPSS), or as a percentage of the U46619-induced precontractile baseline tone, within each artery. For sympathetic nerve stimulation experiments, contractions in arteries are expressed as a percentage of the initial twitch height within each tissue.
Data were plotted and analysed using Prism 8 (GraphPad Software, La Jolla, CA, United States). For comparison of values between >2 treatment groups, one-way ANOVA with Dunnett’s or Tukey’s post hoc test, as appropriate, for multiple pairwise comparisons was performed. Responses in two groups were compared using a two-tailed Student’s unpaired t test, while paired responses within a group were compared with a Student’s paired t test. Within treatment group, atrial responses to increasing concentrations of venom were compared using repeated measures one-way ANOVA with Greenhouse-Geisser correction for correlation. In arteries, the effects of sympathetic nerve stimulation over time and between venom/vehicle treatments were compared using repeated measures two-way ANOVA with Greenhouse-Geisser correction for correlation and Dunnett’s post hoc test for multiple comparisons. Statistical significance was taken as p < 0.05.
3 Results
3.1 Cardiac Effects of Brown Snake Venoms
There were no significant differences between the baseline levels of contractility (left atria) or spontaneous atrial rate (right atria) between tissues allocated to the four venom treatment groups (p > 0.05, one-way ANOVA with Tukey’s post hoc test; data not shown). In rat isolated left atria, treatment with P. inframacula, P. nuchalis or P. textilis venoms elicited small increases in contractility at 10–30 µg/ml (+0.06–0.1 g, n = 5, 6; p < 0.05, repeated measures ANOVA; Figure 2A). To put this in context, these small positive inotropic responses were <10% of the pooled left atrial maximum contractile response to isoprenaline 0.1 µM of 1.02 ± 0.07 g (n = 21).
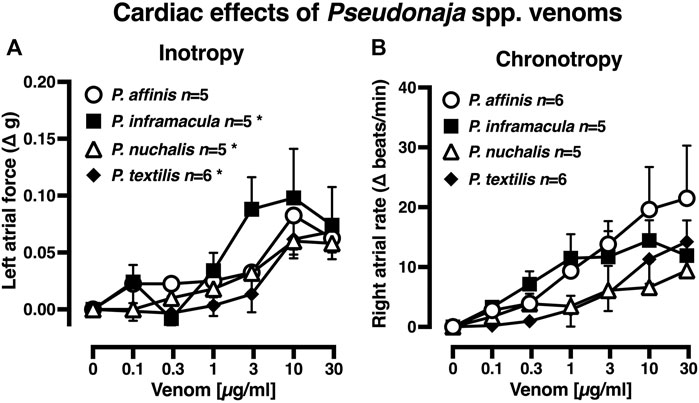
FIGURE 2. Effects of Pseudonaja spp. venoms (0.1–30 µg/ml) on (A) force of contraction of rat isolated left atria (responses are shown as change (Δ) in force (g) from baseline); and (B) rate of spontaneously beating isolated right atria (responses are Δ in atrial rate (beats/min) from baseline). Data are mean ± 1 SEM from n tissues from separate rats. *p < 0.05, repeated measures one-way ANOVA within treatment group over venom concentration range.
In isolated right atria, none of the four Pseudonaja venoms (0.1–30 µg/ml) had any significant effect on rate (Figure 2B; p > 0.05, repeated measures ANOVA). The pooled right atrial maximum chronotropic response to isoprenaline 0.01 µM was an increase from baseline of 139.0 ± 11.4 beats/min (n = 22). The venom of P. aspidorhyncha was not tested in atria due to the lack of physiologically significant effects of the other four Pseudonaja spp. venoms.
3.2 Vascular Effects of Brown Snake Venoms
The values of artery internal diameter (µm), contraction to depolarising PSS (KPSS; mN) and initial contraction (mN) when pre-contracted with U46619 or electrically stimulation were all consistent in arteries across the treatment groups (p > 0.05, one-way ANOVA; data not shown).
3.2.1 Vasorelaxant Effects of Brown Snake Venoms
In the time control (no venom) group, the pre-contractile U46619 tone remained stable (Figure 3A; n = 5, p > 0.05, two-tailed paired Student’s t test). The five brown snake venoms (30 µg/ml) all elicited marked vasorelaxation of −60–−92% of pre-contractile tone (Figure 3A, n = 5–12; p < 0.0001 compared with time control group, one-way ANOVA with Dunnett’s post hoc test).
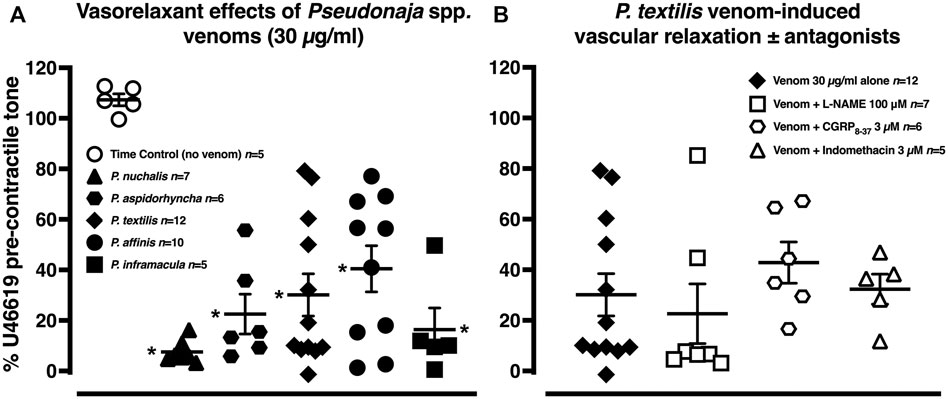
FIGURE 3. (A) Vascular relaxant effects of Pseudonaja spp. venoms (each 30 µg/ml) in rat isolated mesenteric arteries pre-contracted with U46619 (0.1 µM). (B) Vasorelaxation effects of Pseudonaja textilis venom (30 µg/ml) in the absence or presence of antagonists of nitric oxide synthase (L-NAME 100 µM), calcitonin gene-related peptide (CGRP8–37 3 µM) or cyclooxygenase (indomethacin 3 µM). Data are values of individual experiments expressed as a % of the pre-contractile U46619 tone. Horizontal bars are mean and vertical bars ± 1 SEM of n arteries from separate rats. *p < 0.05 vs time control (no venom), one-way ANOVA with Dunnett’s post hoc test.
The vasorelaxant effects of P. textilis venom (30 µg/ml) were explored further in separate experiments. There was no attenuation of these effects with antagonism of calcitonin gene-related peptide (CGRP) receptor, cyclooxygenase or nitric oxide synthase (Figure 3B, n = 5–7; p > 0.05, one-way ANOVA with Dunnett’s post hoc test), suggesting little role for CGRP, dilator prostaglandins or nitric oxide in the venom-induced vasorelaxation.
After pre-treatment with the sPLA2 inhibitor varespladib (1 µM), vascular relaxant effects of P. nuchalis, P. aspidorhyncha and P. textilis venoms were significantly attenuated (Figure 4). In the presence of varespladib, the vasorelaxation effects of P. nuchalis were −48 ± 9% of pre-contractile tone (n = 9), significantly less than the −92 ± 2% relaxation seen in the venom only group (n = 7, Figure 4A; p = 0.0007, unpaired Student’s t test). Varespladib also significantly attenuated the vasorelaxant effects of P. textilis venom (−21 ± 8%, n = 6, vs. −70 ± 8% venom alone, n = 12, Figure 4B; p = 0.0016) and P. aspidorhyncha venom (−37 ± 11% vs −78 ± 8% venom alone, n = 6 each, Figure 4C; p = 0.012, unpaired Student’s t test). Varespladib did not affect relaxation responses to P. affinis or P. inframacula venoms (p > 0.05; Figures 4D,E). Treatment with vehicle (0.1% DMSO, n = 6) or varespladib alone (n = 5) did not affect contractile responses in rat isolated mesenteric arteries (p > 0.05, compared with time control group, one-way ANOVA with Dunnett’s post hoc test; Figure 4F).
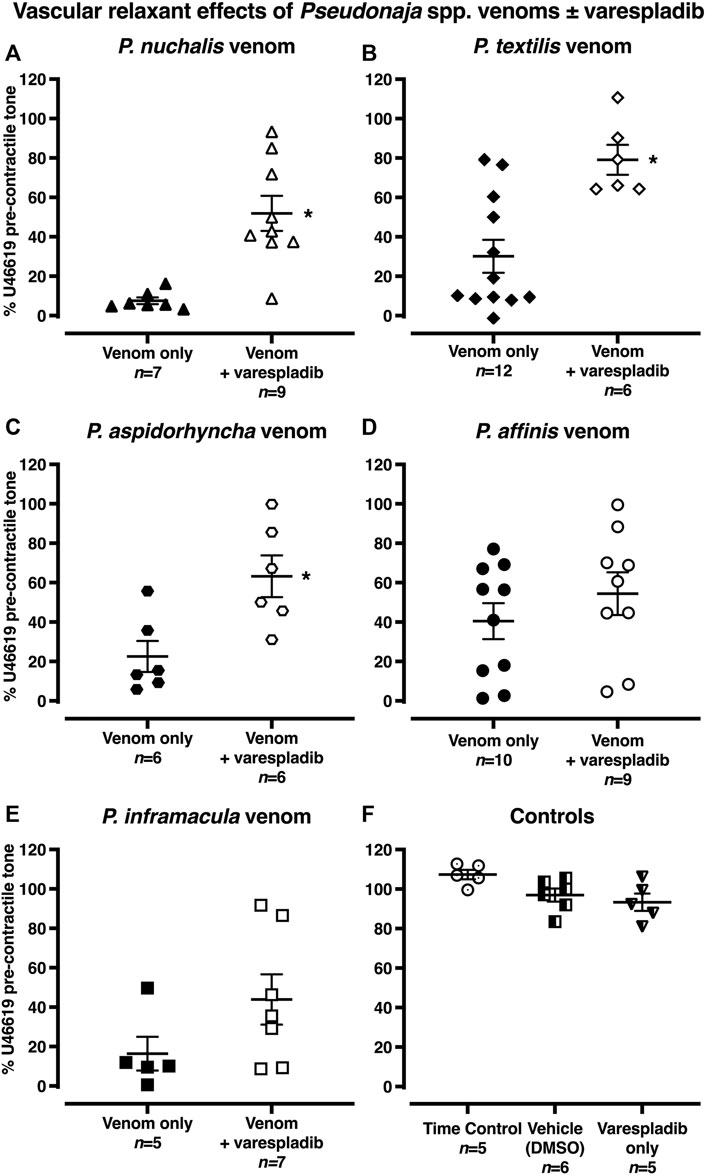
FIGURE 4. (A–E) Vascular relaxant effects of Pseudonaja spp. venoms (30 µg/ml) in the absence or presence of varespladib (1 µM) in rat isolated small mesenteric arteries. Responses are expressed as % of U46619 pre-contractile tone and individual values are shown. (F) Controls: time control-arteries did not receive any treatment; vehicle (DMSO)-arteries were only treated with DMSO (0.1% in bath); or varespladib only-arteries were treated only with varespladib (1 µM in 0.1% DMSO in bath). Horizontal bars are mean and vertical bars ± 1 SEM of n arteries from separate rats. *p < 0.05 vs the respective venom only group, Student’s unpaired t test.
3.2.2 Sympatholytic Effects of Pseudonaja spp. Venoms in Mesenteric Arteries
In the time control group, 20 min after the electrically stimulated contraction had reached a plateau, there was a decrease of −19 ± 3% of the initial twitch height (i.e., to 81 ± 3% of initial contraction, n = 5, Figures 1, 5A; p < 0.0001, repeated measures two-way ANOVA with Dunnett’s post hoc test). Of the five Pseudonaja spp. venoms (each 30 µg/ml), only P. affinis and P. textilis caused significant inhibition of nerve-mediated contractions (Figure 5A). P. affinis venom induced sympatholytic effects of −65 ± 5% of initial twitch height (n = 6, p < 0.0001 vs time control). Likewise, arteries treated with P. textilis venom were inhibited by −65 ± 7% (n = 6, p < 0.0001 vs time control). The administration of the other three venoms caused similar decreases in electrically induced contractions of −38 ± 3% (P. aspidorhyncha, n = 6), −37 ± 9% (P. nuchalis, n = 6) and −39 ± 7% (P. inframacula, n = 8), however these values were not significantly different to the time control group (p = 0.24, p = 0.28 and p = 0.15, respectively; one-way ANOVA with Dunnett’s post hoc test; Figure 5A).
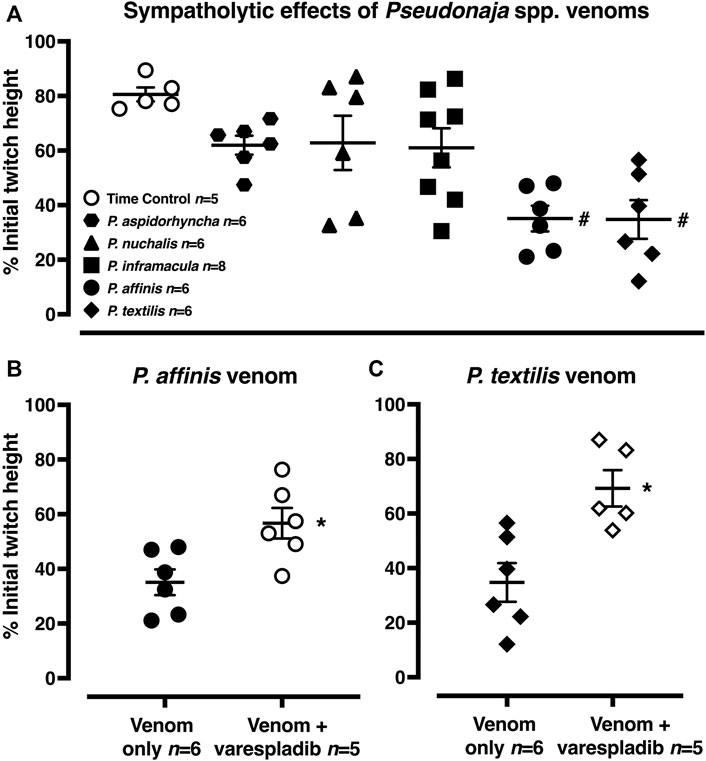
FIGURE 5. (A) Sympatholytic effects of five Pseudonaja spp. venoms (30 µg/ml each) 20 min after administration in rat isolated electrically stimulated small mesenteric arteries. (B) The sympatholytic effects of P. affinis and (C) P. textilis venoms (30 µg/ml) in the absence (venom only) or presence of varespladib (1 µM). Responses are expressed as % of the initial electrically induced contractile twitch height (at 0 min) and individual values are shown. Horizontal bars represent the mean and vertical bars ± 1 SEM of n arteries from separate rats. #p < 0.05 vs time control, one-way ANOVA with Dunnett’s post hoc test. *p < 0.05 vs. respective venom only groups, unpaired Student’s t test.
The effects of the sPLA2 inhibitor varespladib were tested against the significant sympatholytic effects of both P. affinis (Figures 1, 5B) and P. textilis (Figure 5C) venoms. Sympathetic nerve-mediated contractions in arteries that were exposed to varespladib and P. affinis venom were significantly greater than in the venom only group (−43 ± 6% vs. −65 ± 5%, respectively, n = 6 each, Figure 5B; p = 0.014). Varespladib treatment also caused a significant attenuation of the sympatholytic effects of P. textilis venom, as the contractile responses of arteries incubated with the sPLA2 inhibitor increased to −31 ± 7% of the initial twitch height (n = 5), compared to those treated with venom only (−65 ± 7%, n = 6, Figure 5C; p = 0.007, unpaired Student’s t test).
4 Discussion
The results of the present study highlight the variability of brown snake venoms, both in terms of their baseline toxicity and their relative inhibition with the sPLA2 inhibitor varespladib. They also contribute additional evidence in support of the potential utilisation of varespladib as an adjunct therapy for snakebite envenoming. Perhaps their most important contribution is to the investigation of the cardiovascular effects of these venoms, which have largely been neglected, despite cardiac arrest being the most common cause of death following snakebite envenoming in Australia (Allen et al., 2012; Johnston et al., 2017). Few studies have concentrated on deconvoluting the causes of cardiovascular collapse following brown snake envenoming, with the notable exceptions of in vitro (Chaisakul et al., 2015) and in vivo (Chaisakul et al., 2013) studies of the effects of P. textilis venom. Interestingly, in the present study we found little (P. inframacula, P. nuchalis and P. textilis) to no (P. affinis) positive inotropic effects, and no effects on chronotropy, induced by the four brown snake venoms tested. These results corroborate those of the in vivo study of P. textilis venom (Chaisakul et al., 2013), as well as one conducted by the same team with isolated sPLA2s from Oxyuranus scutellatus (taipan) venom (Chaisakul et al., 2014). Collectively, these results suggest that venom-induced cardiovascular collapse following envenoming by Australian snakes does not result from direct cardiac toxicity but is more likely to be mediated by effects in the vasculature.
Chaisakul et al. (2013) examined the pharmacology of whole P. textilis venom and found that it caused vasorelaxation. Our results agree with this finding for P. textilis and demonstrate that vasorelaxant activity is a marked effect of the venoms of four additional species of Pseudonaja (i.e., P. affinis, aspidorhyncha, inframacula and nuchalis), suggesting it is a widespread property of brown snake venoms. Inhibition with varespladib significantly attenuated the vasorelaxant effects of three of the venoms (P. nuchalis, textilis and aspidorhyncha), thus our results indicate that sPLA2s of these species may functionally antagonise vasocontraction (vascular tone) in mammalian arteries. However, varespladib (1 µM) pretreatment did not significantly inhibit vasorelaxant effects of P. affinis or P. inframacula venoms, suggesting that these effects may be mediated by a combination of toxins which are present in differing ratios in each of the venoms. One of the limitations of our study was the protocol of using only a single venom concentration, rather than the construction of a concentration-response curve for each Pseudonaja venom. However, it has been previously reported for both P. textilis and Oxyuranus scutellatus (Papuan taipan) venoms that rapid desensitization occurs with repeated exposure of isolated arteries in vitro (Chaisakul et al., 2012; Chaisakul et al., 2013; Chaisakul et al., 2014). The use of only a single venom concentration per tissue was therefore considered the most rigorous pharmacological approach.
Although our study did not examine the cardiovascular effects of isolated toxin fractions, the sPLA2-mediated vasorelaxant activity of Oxyuranus scutellatus (coastal taipan) venom has previously been investigated in a similar bioassay (Chaisakul et al., 2014). Oxyuranus is the sister genus to Pseudonaja and the venoms of both genera contain closely related toxins, a number of which are unique to the Oxyuranus/Pseudonaja clade (Jackson et al., 2016).
A fraction containing pseutarin C, the procoagulant toxin in P. textilis venom, was also found to induce a significant fall in mean arterial pressure without altering cardiac function (Chaisakul et al., 2015). In vitro experiments from the same study demonstrated that pseutarin C did not possess any vasorelaxant activity, further reinforcing the role of sPLA2s in the induction of vasorelaxation in mesenteric arteries. Taken together, these results indicate that multiple components–in this case, a prothrombin activator and sPLA2s (perhaps in addition to C-type natriuretic peptides also present in these venoms; St Pierre et al., 2006)–may contribute to the overall cardiovascular symptoms of snake envenomation. Notably, synergistic (potentiating) effects between PLA2s and other venom components have been described previously in a number of snake venoms, including those of the cobras Naja mossambica and Hemachatus haemachatus (Louw and Visser, 1978), the pit viper Bothrops asper (Mora-Obando et al., 2014), and the taipan O. scutellatus (Herrera et al., 2016).
Another popular hypothesis about snake venom-induced vasorelaxation invokes the release of dilator autacoids, particularly histamine (Feldberg and Kellaway, 1937; Teixeira et al., 2003) and bradykinin (Ferreira et al., 1970; Xu et al., 2015), as the proximal cause. Although this has largely been investigated using the venoms of viperid snakes (family Viperidae), a study using crude O. scutellatus venom showed that pre-treating rats with the cyclooxygenase inhibitor indomethacin, but not atropine (muscarinic receptor antagonist), mepyramine (histamine H1 receptor antagonist) or the nitric oxide synthase inhibitor L-NAME, could prevent vasodilation and sudden collapse (Chaisakul et al., 2012). PLA2 enzymes cause the release of lysophospholipids and fatty acids via hydrolysing glycerophospholipids (Kini, 2003). Chaisakul et al. (2014) showed that sPLA2-mediated hypotensive effects of O. scutellatus venom were inhibited in rats administered with indomethacin prior to venom administration, concluding significant release of dilator autacoids. An earlier study of the PLA2 fraction from V. russelli venom showed that PLA2 relaxed rat isolated aortic rings in an endothelium-independent manner, ruling out a role for the release of endothelial autacoids such as histamine and bradykinin (Huang and Lee, 1985). They found that inhibition of soluble guanylate cyclase with the early antagonist methylene blue partly attenuated the PLA2-induced aortic relaxation, suggesting a role for cyclic GMP in this response. In rat isolated mesenteric arteries, the relaxation caused by Papuan taipan venom was shown by Chaisakul et al. (2012) to involve, in part, protein kinase A and large-conductance Ca2+-activated K+ channels. In our study, pretreatment with indomethacin, L-NAME or the CGRP receptor antagonist CGRP8-37 did not abrogate the vasorelaxant effects of whole P. textilis venom in isolated mesenteric arteries, suggesting that the relaxation was not due to local release of dilator prostanoids or nitric oxide, or sensory nerve-released CGRP; the possible involvement of cyclic GMP formation, protein kinase A or large-conductance Ca2+-activated K+ channels was not tested and warrant further experimentation.
Although studies investigating the structure and activity of the main components of P. textilis venom–particularly pseutarin C and the presynaptically neurotoxic sPLA2 textilotoxin–have been conducted for more than 50 years, the remaining members of the genus Pseudonaja have received far less attention. PLA2s appear to be an almost ubiquitous component of Australian elapid snake venoms (Jackson et al., 2016) and have been detected in every Pseudonaja venom system investigated to-date (see e.g., Jackson et al., 2013; Jackson et al., 2016; Reeks et al., 2016). PLA2s have been identified in P. nuchalis and P. aspidorhyncha venoms as “homologues of textilotoxin PLA2 subunits” (Reeks et al., 2016); and in P. affinis venom as a “toxic PLA2 variant” (Judge et al., 2002). The latter study assayed the activity of purified PLA2s from P. affinis venom and found they induced contraction in rat tracheae–an activity like the venoms of O. microlepidotus and O. scutellatus, which was hypothesised by the authors to result from the downstream release of leukotrienes.
In the present study, only P. affinis and P. textilis venoms markedly inhibited sympathetic nerve-mediated vascular contractions of isolated mesenteric arteries. These effects were significantly inhibited by pretreatment with varespladib, indicating that PLA2 toxins play an important role in inducing this sympatholysis. As no studies have been performed which assess the sympatholytic effects of isolated textilotoxin homologues, their involvement in the inhibition of sympathetic transmission is unknown. Furthermore, neither P. nuchalis nor P. aspidorhyncha venoms, which contain textilotoxin isoforms (Reeks et al., 2016), induced significant sympatholytic effects in our assays. These data raise several questions: whether only certain isoforms of textilotoxin can cause these effects in rat mesenteric arteries; if particular concentrations must be achieved (i.e., whether the absence of the effect for some venoms is a consequence of the relative abundance of this toxin type); if synergy with post-synaptic neurotoxins (i.e., 3FTx) is required; or indeed if this presynaptic neurotoxin is involved at all. As varespladib significantly attenuated the sympatholytic effects of P. affinis and P. textilis venoms, it is reasonable to infer that some forms of sPLA2 act at synaptic terminals in vascular smooth muscle. Alternatively, they may act post-synaptically at the α1-adrenoceptor, which would explain the lack of effects on sympathetic nerve stimulation-induced inotropy (mediated by β1-adrenoceptors) in rat left atria–in contrast to the effects in blood vessels–in this study. Whether sPLA2s affect the function of α1-adrenoceptors in vascular smooth muscle is not known, however, PLA2 has been shown to desensitize α2-adrenoceptors in platelets (Rao and White, 1989), and in isolated membrane preparations (Cohen et al., 1985). This latter effect on α2-adrenoceptors is not relevant to the current study as these receptors were irreversibly blocked in the mesenteric arteries used in the sympathetic nerve stimulation experiments. The precise reason for the differential effects in atria and arteries is therefore impossible to ascertain based on our data but represents an interesting question for further investigation.
Previous studies indicate that presynaptic neurotoxins in snake venoms can deplete acetylcholine release from motor neurons in skeletal muscle via the destruction of synaptic terminals (Chang et al., 1973; Su and Chang, 1984; Cavalcante et al., 2017) and vesicular membranes (Montecucco et al., 2009). To complicate the matter further, whilst a proteomic study of P. affinis venom did not detect any textilotoxin homologues (Judge et al., 2002), multiple studies (Judge et al., 2002; Jackson et al., 2016) have detected an abundance of proteins in the 12–14 kDa range–the expected size of venom sPLA2s following decomplexation–in P. affinis venom. Thus, it is possible that the sympatholytic effects induced by this venom are caused by unknown sPLA2s. More detailed investigation into the composition of Pseudonaja venoms is therefore warranted to ascertain which PLA2 toxins are responsible for the sympatholytic effects observed in the present study.
5 Conclusion
Whilst all five brown snake venoms examined in the present study induced vasorelaxant effects, only two venoms–P. textilis and P. affinis–induced marked sympatholytic effects. The activity of the venoms (and their relative inhibition with varespladib) thus varied amongst species at the level of gross activity, as well as more subtly in terms of the potency of each activity. These findings corroborate earlier studies (e.g., Flight et al., 2006; Jackson et al., 2016; Reeks et al., 2016) that have uncovered considerable variability (at both inter- and intraspecific levels) in the composition and activity of brown snake venoms. Thus, our data contribute to the ongoing investigation of these biologically fascinating and medically significant venoms. However, it would be premature at this stage to conjecture about the evolutionary origins (i.e., the function sensu stricto), or possible clinical relevance of the variability we have reported here. Based on their inhibition with the sPLA2 inhibitor varespladib, we infer that PLA2 toxins in P. aspidorhyncha, P. nuchalis and P. textilis venoms are involved in brown snake venom-induced vasorelaxation and the sympatholytic effects of P. affinis and P. textilis venoms. Observed vasodilatory effects were likely endothelium-independent, although determination of the exact mechanism of action was outside the scope of this study. Given that little to no direct cardiac effects were observed in our assays, the cause of cardiovascular collapse following brown snake envenoming is likely due to a fall in mean arterial pressure unrelated to cardiac function. Further proteomic and pharmacological studies remain necessary to resolve the mechanistic details of this dangerous venom-induced pathology. Finally, our results are promising regarding the potential role of varespladib as an initial (pre-referral) and/or adjunct (in combination with antivenom) therapeutic agent for brown snake envenoming.
Data Availability Statement
The raw data supporting the conclusion of this article will be made available by the authors, without undue reservation.
Ethics Statement
The animal study was reviewed and approved by The Animal Ethics Committee of the University of Melbourne.
Author Contributions
NV performed all experiments, analysed the data, prepared figures and contributed to the writing of the manuscript; TJ contributed to the writing of the manuscript; CW designed the experiments, analysed the data, prepared figures and contributed to the writing of the manuscript.
Funding
This research received funding from the Struan Sutherland Trust.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
The authors thank Nathan Dunstan of Venom Supplies Pty Ltd. for the provision of venoms.
References
Allen, G. E., Brown, S. G., Buckley, N. A., O'Leary, M. A., Page, C. B., Currie, B. J., et al. (2012). Clinical Effects and Antivenom Dosing in Brown Snake (Pseudonaja spp.) Envenoming--Australian Snakebite Project (ASP-14). PLoS One 7, e53188. doi:10.1371/journal.pone.0053188
Angus, J. A., Broughton, A., and Mulvany, M. J. (1988). Role of Alpha-Adrenoceptors in Constrictor Responses of Rat, Guinea-Pig and Rabbit Small Arteries to Neural Activation. J. Physiol. 403, 495–510. doi:10.1113/jphysiol.1988.sp017260
Angus, J. A., Hughes, R. J. A., and Wright, C. E. (2017). Distortion of KB Estimates of Endothelin-1 ETA and ETB Receptor Antagonists in Pulmonary Arteries: Possible Role of an Endothelin-1 Clearance Mechanism. Pharmacol. Res. Perspect. 5. doi:10.1002/prp2.374
Angus, J. A., and Wright, C. E. (2000). Techniques to Study the Pharmacodynamics of Isolated Large and Small Blood Vessels. J. Pharmacol. Toxicol. Methods 44, 395–407. doi:10.1016/s1056-8719(00)00121-0
Barber, C. M., Isbister, G. K., and Hodgson, W. C. (2012). Solving the ‘Brown Snake Paradox’: In Vitro Characterisation of Australasian Snake Presynaptic Neurotoxin Activity. Toxicol. Lett. 210, 318–323. doi:10.1016/j.toxlet.2012.02.001
Barnett, D., Howden, M. E., and Spence, I. (1980). A Neurotoxin of Novel Structural Type from the Venom of the Australian Common Brown Snake. Naturwissenschaften 67, 405–406. doi:10.1007/BF00405486
Birrell, G. W., Earl, S., Masci, P. P., de Jersey, J., Wallis, T. P., Gorman, J. J., et al. (2006). Molecular Diversity in Venom from the Australian Brown Snake, Pseudonaja Textilis. Mol. Cel Proteomics 5, 379–389. doi:10.1074/mcp.M500270-MCP200
Bougis, P. E., Marchot, P., and Rochat, H. (1987). In Vivo synergy of Cardiotoxin and Phospholipase A2 from the Elapid Snake Naja Mossambica Mossambica. Toxicon 25, 427–431. doi:10.1016/0041-0101(87)90076-6
Camargo, A. C., Ianzer, D., Guerreiro, J. R., and Serrano, S. M. (2012). Bradykinin-potentiating Peptides: beyond Captopril. Toxicon 59, 516–523. doi:10.1016/j.toxicon.2011.07.013
Casewell, N. R., Wüster, W., Vonk, F. J., Harrison, R. A., and Fry, B. G. (2013). Complex Cocktails: the Evolutionary novelty of Venoms. Trends Ecol. Evol. 28, 219–229. doi:10.1016/j.tree.2012.10.020
Cavalcante, W. L. G., Noronha-Matos, J. B., Timóteo, M. A., Fontes, M. R. M., Gallacci, M., and Correia-de-Sá, P. (2017). Neuromuscular Paralysis by the Basic Phospholipase A2 Subunit of Crotoxin from Crotalus durissus Terrificus Snake Venom Needs its Acid Chaperone to Concurrently Inhibit Acetylcholine Release and Produce Muscle Blockage. Toxicol. Appl. Pharmacol. 334, 8–17. doi:10.1016/j.taap.2017.08.021
Chaisakul, J., Isbister, G. K., Konstantakopoulos, N., Tare, M., Parkington, H. C., and Hodgson, W. C. (2012). In Vivo and In Vitro Cardiovascular Effects of Papuan Taipan (Oxyuranus Scutellatus) Venom: Exploring “Sudden Collapse”. Toxicol. Lett. 213, 243–248. doi:10.1016/j.toxlet.2012.06.015
Chaisakul, J., Isbister, G. K., Kuruppu, S., Konstantakopoulos, N., and Hodgson, W. C. (2013). An Examination of Cardiovascular Collapse Induced by Eastern Brown Snake (Pseudonaja textilis) Venom. Toxicol. Lett. 221, 205–211. doi:10.1016/j.toxlet.2013.06.235
Chaisakul, J., Isbister, G. K., O'Leary, M. A., Parkington, H. C., Smith, A. I., Hodgson, W. C., et al. (2015). Prothrombin Activator-like Toxin Appears to Mediate Cardiovascular Collapse Following Envenoming by Pseudonaja textilis. Toxicon 102, 48–54. doi:10.1016/j.toxicon.2015.05.001
Chaisakul, J., Isbister, G. K., Tare, M., Parkington, H. C., and Hodgson, W. C. (2014). Hypotensive and Vascular Relaxant Effects of Phospholipase A2 Toxins from Papuan Taipan (Oxyuranus scutellatus) Venom. Eur. J. Pharmacol. 723, 227–233. doi:10.1016/j.ejphar.2013.11.028
Chang, C. C., Chen, T. F., and Lee, C. Y. (1973). Studies of the Presynaptic Effect of β-bungarotoxin on Neuromuscular Transmission. J. Pharmacol. Exp. Ther. 184, 339–345.
Cohen, R. M., McLellan, C., Dauphin, M., and Hirata, F. (1985). Glutaraldehyde Pretreatment Blocks Phospholipase A2 Modulation of Adrenergic Receptors. Life Sci. 36, 25–32. doi:10.1016/0024-3205(85)90282-6
Daniels, B. C., Kim, H., Moore, D., Zhou, S., Smith, H. B., Karas, B., et al. (2018). Criticality Distinguishes the Ensemble of Biological Regulatory Networks. Phys. Rev. Lett. 121, 138102. doi:10.1103/PhysRevLett.121.138102
Dixon, R. W., and Harris, J. B. (1996). Myotoxic Activity of the Toxic Phospholipase, Notexin, from the Venom of the Australian Tiger Snake. J. Neuropathol. Exp. Neurol. 55, 1230–1237. doi:10.1097/00005072-199612000-00006
Feldberg, W., and Kellaway, C. H. (1937). Liberation of Histamine from the Perfused Lung by Snake Venoms. J. Physiol. 90, 257–279. doi:10.1113/jphysiol.1937.sp003511
Ferreira, S. H., Bartelt, D. C., and Greene, L. J. (1970). Isolation of Bradykinin-Potentiating Peptides from Bothrops jararaca Venom. Biochemistry 9, 2583–2593. doi:10.1021/bi00815a005
Flight, S., Mirtschin, P., and Masci, P. P. (2006). Comparison of Active Venom Components between Eastern Brown Snakes Collected from South Australia and Queensland. Ecotoxicology 15, 133–141. doi:10.1007/s10646-005-0047-z
Fry, B. G., Wickramaratana, J. C., Lemme, S., Beuve, A., Garbers, D., Hodgson, W. C., et al. (2005). Novel Natriuretic Peptides from the Venom of the Inland Taipan (Oxyuranus microlepidotus): Isolation, Chemical and Biological Characterisation. Biochem. Biophys. Res. Commun. 327, 1011–1015. doi:10.1016/j.bbrc.2004.11.171
Gutiérrez, J. M., Calvete, J. J., Habib, A. G., Harrison, R. A., Williams, D. J., and Warrell, D. A. (2017). Snakebite Envenoming. Nat. Rev. Dis. Primers 3, 17079. doi:10.1038/nrdp.2017.79
Harris, J. B., and MacDonell, C. A. (1981). Phospholipase A2 Activity of Notexin and its Role in Muscle Damage. Toxicon 19, 419–430. doi:10.1016/0041-0101(81)90046-5
Herrera, M., de Cássia de O Collaço, R., Villalta, M., Segura, Á., Vargas, M., Wright, C. E., et al. (2016). Neutralization of the Neuromuscular Inhibition of Venom and Taipoxin from the Taipan (Oxyuranus Scutellatus) by F(ab')2 and Whole IgG Antivenoms. Toxicol. Lett. 241, 175–183. doi:10.1016/j.toxlet.2015.11.020
Huang, H. C., and Lee, C. Y. (1985). Relaxant Effect of Phospholipase A2 from Vipera Russelli Snake Venom on Rat Aorta. Eur. J. Pharmacol. 118, 139–146. doi:10.1016/0014-2999(85)90672-7
Ianzer, D., Konno, K., Marques-Porto, R., Vieira Portaro, F. C., Stöcklin, R., Martins de Camargo, A. C., et al. (2004). Identification of Five New Bradykinin Potentiating Peptides (BPPs) from Bothrops jararaca Crude Venom by Using Electrospray Ionization Tandem Mass Spectrometry after a Two-step Liquid Chromatography. Peptides 25, 1085–1092. doi:10.1016/j.peptides.2004.04.006
Jackson, T., and Fry, B. (2016). A Tricky Trait: Applying the Fruits of the “Function Debate” in the Philosophy of Biology to the “Venom Debate” in the Science of Toxinology. Toxins 8, 263. doi:10.3390/toxins8090263
Jackson, T. N., Koludarov, I., Ali, S. A., Dobson, J., Zdenek, C. N., Dashevsky, D., et al. (2016). Rapid Radiations and the Race to Redundancy: An Investigation of the Evolution of Australian Elapid Snake Venoms. Toxins 8, 309. doi:10.3390/toxins8110309
Jackson, T. N., Sunagar, K., Undheim, E. A., Koludarov, I., Chan, A. H., Sanders, K., et al. (2013). Venom Down under: Dynamic Evolution of Australian Elapid Snake Toxins. Toxins 5, 2621–2655. doi:10.3390/toxins5122621
Johnston, C. I., Ryan, N. M., Page, C. B., Buckley, N. A., Brown, S. G., O'Leary, M. A., et al. (2017). The Australian Snakebite Project, 2005-2015 (ASP-20). Med. J. Aust. 207, 119–125. doi:10.5694/mja17.00094
Judge, R. K., Henry, P. J., d’Aprile, A. C., Lynch, D., Jelinek, G. A., Wilce, M. C., et al. (2002). Identification of PLA(2) and Alpha-Neurotoxin Proteins in the Venom of Pseudonaja Affinis (Dugite). Toxicol. Appl. Pharmacol. 181, 184–191. doi:10.1006/taap.2002.9416
Kassab, S. E., Khedr, M. A., Ali, H. I., and Abdalla, M. M. (2017). Discovery of New Indomethacin-Based Analogs with Potentially Selective Cyclooxygenase-2 Inhibition and Observed Diminishing to PGE2 Activities. Eur. J. Med. Chem. 141, 306–321. doi:10.1016/j.ejmech.2017.09.056
Kini, R. M. (2003). Excitement Ahead: Structure, Function and Mechanism of Snake Venom Phospholipase A2 Enzymes. Toxicon 42, 827–840. doi:10.1016/j.toxicon.2003.11.002
Lambeau, G., Barhanin, J., Schweitz, H., Qar, J., and Lazdunski, M. (1989). Identification and Properties of Very High Affinity Brain Membrane-Binding Sites for a Neurotoxic Phospholipase from the Taipan Venom. J. Biol. Chem. 264, 11503–11510. doi:10.1016/s0021-9258(18)60492-2
Laustsen, A. H. (2016). Toxin Synergism in Snake Venoms. Toxin Rev. 35, 165–170. doi:10.1080/15569543.2016.1220397
Lewin, M., Samuel, S., Merkel, J., and Bickler, P. (2016). Varespladib (LY315920) Appears to Be a Potent, Broad-Spectrum, Inhibitor of Snake Venom Phospholipase A2 and a Possible Pre-referral Treatment for Envenomation. Toxins 8, 248. doi:10.3390/toxins8090248
Lewin, M. R., Gutiérrez, J. M., Samuel, S. P., Herrera, M., Bryan-Quirós, W., Lomonte, B., et al. (2018). Delayed Oral LY333013 Rescues Mice from Highly Neurotoxic, Lethal Doses of Papuan Taipan (Oxyuranus Scutellatus) Venom. Toxins 10, 380. doi:10.3390/toxins10100380
Longbottom, J., Shearer, F. M., Devine, M., Alcoba, G., Chappuis, F., Weiss, D. J., et al. (2018). Vulnerability to Snakebite Envenoming: A Global Mapping of Hotspots. Lancet 392, 673–684. doi:10.1016/S0140-6736(18)31224-8
Louw, A. I., and Visser, L. (1978). The Synergism of Cardiotoxin and Phospholipase A2 in Hemolysis. Biochim. Biophys. Acta 512, 163–171. doi:10.1016/0005-2736(78)90227-4
Masci, P. P., Whitaker, A. N., Sparrow, L. G., de Jersey, J., Winzor, D. J., Watters, D. J., et al. (2000). Textilinins from Pseudonaja textilis. Characterization of Two Plasmin Inhibitors that Reduce Bleeding in an Animal Model. Blood Coagul. Fibrinolysis 11, 385–393. doi:10.1097/00001721-200006000-00011
Montecucco, C., Rossetto, O., Caccin, P., Rigoni, M., Carli, L., Morbiato, L., et al. (2009). Different Mechanisms of Inhibition of Nerve Terminals by Botulinum and Snake Presynaptic Neurotoxins. Toxicon 54, 561–564. doi:10.1016/j.toxicon.2008.12.012
Mora-Obando, D., Fernández, J., Montecucco, C., Gutiérrez, J. M., and Lomonte, B. (2014). Synergism between Basic Asp49 and Lys49 Phospholipase A2 Myotoxins of Viperid Snake Venom In Vitro and In Vivo. PLoS One 9, e109846. doi:10.1371/journal.pone.0109846
Mukherjee, A. K. (2010). Non-Covalent Interaction of Phospholipase A(2) (PLA(2)) and Kaouthiotoxin (KTX) from Venom of Naja Kaouthia Exhibits Marked Synergism to Potentiate Their Cytotoxicity on Target Cells. J. Venom Res. 1, 37–42.
Noble, D. (2011). Differential and Integral Views of Genetics in Computational Systems Biology. Interf. Focus 1, 7–15. doi:10.1098/rsfs.2010.0444
Rao, G. H., and White, J. G. (1989). Influence of Phospholipase A2 on Human Blood Platelet Alpha Adrenergic Receptor Function. Thromb. Res. 53, 427–434. doi:10.1016/0049-3848(89)90197-7
Rao, V. S., and Kini, R. M. (2002). Pseutarin C, a Prothrombin Activator from Pseudonaja textilis Venom: Its Structural and Functional Similarity to Mammalian Coagulation Factor Xa-Va Complex. Thromb. Haemost. 88, 611–619. doi:10.1055/s-0037-1613264
Reeks, T., Lavergne, V., Sunagar, K., Jones, A., Undheim, E., Dunstan, N., et al. (2016). Deep Venomics of the Pseudonaja Genus Reveals Inter- and Intra-specific Variation. J. Proteomics 133, 20–32. doi:10.1016/j.jprot.2015.11.019
St Pierre, L., Flight, S., Masci, P. P., Hanchard, K. J., Lewis, R. J., Alewood, P. F., et al. (2006). Cloning and Characterisation of Natriuretic Peptides from the Venom Glands of Australian Elapids. Biochimie 88, 1923–1931. doi:10.1016/j.biochi.2006.06.014
St Pierre, L., Woods, R., Earl, S., Masci, P. P., and Lavin, M. F. (2005). Identification and Analysis of Venom Gland-specific Genes from the Coastal Taipan (Oxyuranus scutellatus) and Related Species. Cell Mol Life Sci 62, 2679–2693. doi:10.1007/s00018-005-5384-9
Su, M. J., and Chang, C. C. (1984). Presynaptic Effects of Snake Venom Toxins Which Have Phospholipase A2 Activity (Beta-bungarotoxin, Taipoxin, Crotoxin). Toxicon 22, 631–640. doi:10.1016/0041-0101(84)90003-5
Su, M. J., Coulter, A. R., Sutherland, S. K., and Chang, C. C. (1983). The Presynaptic Neuromuscular Blocking Effect and Phospholipase A2 Activity of Textilotoxin, a Potent Toxin Isolated from the Venom of the Australian Brown Snake, Pseudonaja textilis. Toxicon 21, 143–151. doi:10.1016/0041-0101(83)90057-0
Teixeira, C. F., Landucci, E. C., Antunes, E., Chacur, M., and Cury, Y. (2003). Inflammatory Effects of Snake Venom Myotoxic Phospholipases A2. Toxicon 42, 947–962. doi:10.1016/j.toxicon.2003.11.006
The Lancet (2017). Snake-Bite Envenoming: A Priority Neglected Tropical Disease. Lancet 390, 2. doi:10.1016/S0140-6736(17)31751-8
Tibballs, J., Sutherland, S. K., Rivera, R. A., and Masci, P. P. (1992). The Cardiovascular and Haematological Effects of Purified Prothrombin Activator from the Common Brown Snake (Pseudonaja textilis) and Their Antagonism with Heparin. Anaesth. Intensive Care 20, 28–32. doi:10.1177/0310057X9202000105
Tyler, M. I., Spence, I., Barnett, D., and Howden, M. E. (1987). Pseudonajatoxin B: Unusual Amino Acid Sequence of a Lethal Neurotoxin from the Venom of the Australian Common Brown Snake, Pseudonaja textilis. Eur. J. Biochem. 166, 139–143. doi:10.1111/j.1432-1033.1987.tb13493.x
Viala, V. L., Hildebrand, D., Trusch, M., Fucase, T. M., Sciani, J. M., Pimenta, D. C., et al. (2015). Venomics of the Australian Eastern Brown Snake (Pseudonaja textilis): Detection of New Venom Proteins and Splicing Variants. Toxicon 107, 252–265. doi:10.1016/j.toxicon.2015.06.005
Wisskirchen, F. M., Burt, R. P., and Marshall, I. (1998). Pharmacological Characterization of CGRP Receptors Mediating Relaxation of the Rat Pulmonary Artery and Inhibition of Twitch Responses of the Rat Vas Deferens. Br. J. Pharmacol. 123, 1673–1683. doi:10.1038/sj.bjp.0701783
Xu, X., Li, B., Zhu, S., and Rong, R. (2015). Hypotensive Peptides from Snake Venoms: Structure, Function and Mechanism. Curr. Top. Med. Chem. 15, 658–669. doi:10.2174/1568026615666150217113835
Keywords: Australian brown snake venoms, brown snake paradox, Pseudonaja brown snake, phospholipase A2, sympatholyic effects, vascular relaxation, cardiac effects, varespladib
Citation: Vuong NT, Jackson TNW and Wright CE (2021) Role of Phospholipases A2 in Vascular Relaxation and Sympatholytic Effects of Five Australian Brown Snake, Pseudonaja spp., Venoms in Rat Isolated Tissues. Front. Pharmacol. 12:754304. doi: 10.3389/fphar.2021.754304
Received: 06 August 2021; Accepted: 11 October 2021;
Published: 21 October 2021.
Edited by:
Ali H. Eid, Qatar University, QatarReviewed by:
Cho Yeow Koh, National University of Singapore, SingaporeMasashi Tawa, Osaka Medical and Pharmaceutical University, Japan
Rafael Borges, São Paulo State University, Brazil
Copyright © 2021 Vuong, Jackson and Wright. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Christine E. Wright, cewright@unimelb.edu.au
 Nhi Thuc Vuong1
Nhi Thuc Vuong1 Timothy N. W. Jackson
Timothy N. W. Jackson Christine E. Wright
Christine E. Wright