- 1Women’s Hospital, School of Medicine, Zhejiang University, Hangzhou, China
- 2Device Evaluation Center Zhejiang Medical Products Administration, Hangzhou, China
- 3Center for Food and Drug Inspection of NMPA, Beijing, China
- 4Zhejiang Medical Products Administration, Hangzhou, China
Background: In this study, an investigation was conducted on clinical drug trials comprising pregnant women in China that provided data on the quantity, properties, source of funding, and geographical distribution regarding registration and post-marketing studies.
Methods: We conducted a cross-sectional descriptive study of clinical trials of pregnant women in China on 30 December 2021, and it was registered on the official Drug Clinical Trial Information Management Platform (ChiCTR) (http://www.chinadrugtrials.org.cn) established by the State Food and Drug Administration of China (Chinese FDA).
Results: This study encompassed 72 registered trials (0.46%, 72/15,539) for data analysis. Of these trials, 43.1% of trials were started between 2013 and 2016, and nearly half of the trials (48.6%) were completed. Industries were listed as the primary sponsor for 95.8% trials. Economically developed eastern China and northern China, accounting for 69.5% of the 72 registered trials, were the most frequently identified study locations. Regarding study designs of these trials, more than half of the trials (70.8%) were randomized, 61.1% were a parallel assignment, 33.3% were phase 3, and half of the trials (54.2%) were open label. In total, 23 trials met the requirements after excluding trials of cancer and/or of postmenopausal women, accounting for 0.15% of the 15,539 registered trials in the ChiCTR websites. Of the 72 clinical trials, 54 drugs for 18 indications were included. Of these indications, the highest proportion of the trials is osteoporosis (27.8%), followed by cancer (22.2%), assisted reproduction (13.9%), and other indications (13.9%).
Conclusion: This survey revealed a significant shortage of the development, evaluation, and safety trials of pregnancy-related drugs in China. Modifying or adding legislation and providing financial incentives may therefore encourage pharmaceutical companies to conduct additional clinical trials on pregnant women.
Introduction
Although women during pregnancy demonstrate concerns with regard to drug safety, numerous women still use prescription drugs throughout pregnancy due to disease or abnormal reactions that may occur in pregnancy (e.g., hypertensive disorders of pregnancy or gestational diabetes) (Anoshchenko et al., 2020). Approximately 40–80% of women receive at least one medication during pregnancy (Meyer et al., 2021). However, the vast majority of newly marketed medications have not been evaluated with respect to pregnant women (Ye et al., 2019; Carnovale et al., 2021). Although pregnant women are often excluded from clinical trials due to safety and ethical concerns (Saito et al., 2017; Kreuder et al., 2020; Van Der Straten et al., 2020), clinical trials constitute the most effective way to evaluate preventive and therapeutic strategies (Ranawaka et al., 2018), and they were widely regarded as comprising the most crucial evidential source of efficacy and safety (Ruff et al., 2014). However, the obstetric studies registered at http://clinicaltrials.gov from 2007 to 2012 accounted for less than 10% of the total number of registries (Stockmann et al., 2014). A systematic evaluation in 2016 reported that in all validly registered drug clinical trials, only 0.32% were for drugs during pregnancy and only 4.4% of these clinical trials of pregnancy drugs included preplanned pharmacokinetic (PK) studies (Scaffidi et al., 2017).
In September 2013, the State Food and Drug Administration of China (Chinese FDA) promulgated the Announcement of the Drug Clinical Trial Information Management Platform (https://www.nmpa.gov.cn/xxgk/ggtg/qtggtg/20130906120001263.html); this required the acquisition of a clinical trial approval from the Chinese FDA and to register and provide information disclosure in http://www.chinadrugtrials.org.cn/index.html to conduct a clinical trial.
The objective of the present study, then, was to investigate the current status and specific characteristics of clinical trials encompassing pregnant women in China and to provide valuable insights into the drafting relevance of countermeasure policies for the government and for the evaluation of drug clinical trials that include pregnant women.
Materials and Methods
Reporting Guideline
This study was a cross-sectional report in which we followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines (Zeng et al., 2015).
Search Strategy and Selection Criteria
Clinical trial data registered on the ChiCTR (http://www.chinadrugtrials.org.cn) websites were collected, and we employed the registry search function to search any of the following terms: “Obstetrics,” “Pregnancy,” “Pregnant,” “Fetus,” “Birth,” “Perinatal,” “Newborn,” “Postpartum,” “Prenatal,” “Maternal,” “Maternity,” “Mother,” or “Birth Outcome” between 1 September 2013 and 30 December 2021. This investigation also included clinical trials of traditional Chinese medicines, supplements, and vitamins.
Data Extraction
Information publicly accessible on the ChiCTR websites included sponsors and registered projects, such as funding organizations, types, and locations; basic information of clinical trials, such as titles, study sites, and date of first registration; and study designs, such as indications, name and category of drugs, study phase, design type, sample size, and primary and secondary endpoints. Two independent investigators searched ChiCTR websites using the same search terms and reviewed all of the retrieved studies individually and independently. If their opinions did not agree, a third reviewer was consulted, and the registered projects were re-reviewed for consensus.
We implemented a joint-phase nomenclature for phases 1/2 and phases 2/3 and classified them as phases 1 and 2, respectively, because of their small numbers (one project in phases 1/2, two projects in phases 2/3). The data from all of the aforementioned trial-related information were extracted and compiled into an Excel worksheet for subsequent checks and analysis.
Our exclusion criteria were 1) study participants ≤12 years of age; 2) contraceptive drug; 3) males; 4) where the pregnant women constituted an exclusion criterion in clinical experiments. In March 2016, the general office of the State Council of China promulgated the “Opinions on implementing the Consistency Evaluation for the Quality and Efficacy of Generic Drugs” (http://www.gov.cn/zhengce/content/2016-03/05/content_5049364.htm) and required clinicians to register clinical drug trials on ChiCTR (http://www.chinadrugtrials.org.cn) websites. The marketing authorization of a generic drug is based on the proof of BE trials (Viprey et al., 2020), which requires manufacturers to certify that their generic pharmaceuticals are bioequivalent to brand drugs (Zhong et al., 2018), and BE trials are often executed with healthy volunteers and avoided with respect to women during pregnancy (Government of Canada, 2018). Therefore, we removed the BE trials from our analyses to discern the impact of BE on our study. The inclusion and exclusion criteria in our clinical trial included the exclusion of pregnant women as an excluded object of this study, and the research subjects for the indications were not strictly screened, including postmenopausal women and cancer patients.
Data Analysis
Descriptive analyses were used, and primary sponsors were classified as the university, hospital, industry, or other sponsors. If different sites were analyzed in the same region, we were entered into the cumulative calculation for that region. Categorical data are reported as frequencies and percentages, and continuous variables as median and interquartile ranges. We assessed the differences between counts of categorical variables using the chi-squared test. Ordinary chi-squared analysis was applied for inspection when n ≥ 40 and T ≥ 5, whereas a calibrated chi-squared test was employed for inspection when n ≥ 40 and 1 ≤ T < 5. All of the analyses were executed using SPSS 20.0 software. p values < 0.05 were considered statistically significant.
Results
Screening and Included Trials
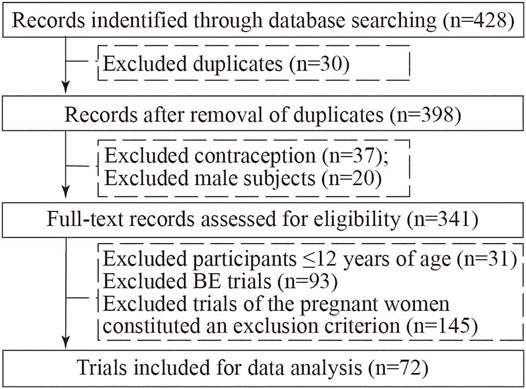
In our initial search, we found 428 clinical trials registered on ChiCTR websites on 30 December 2021, and after excluding duplicated trials, 398 trials remained; after carefully reviewing all the information, 37 contraception trials, 20 trials with male participants, 31 participants ≤12 years of age trials, 93 BE trials, and 145 trials where the pregnant women constituted an exclusion criterion in clinical experiments were excluded. Consequently, a total of 72 registered trials were ultimately evaluated (Figure 1).
General Characteristics of Included Trials
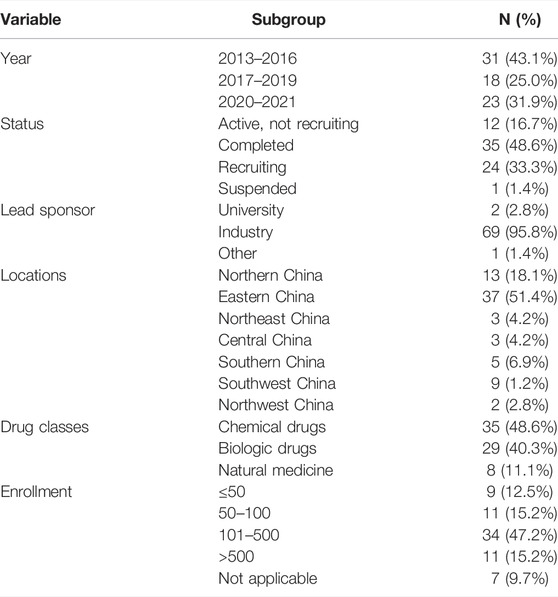
The characteristics of the included trials are shown in Table 1. Thirty-one trials (43.1%) were started between 2013 and 2016, eighteen (25.0%) between 2017 and 2019, and twenty-three trials (31.9%) since January 2020. Nearly half of the trials (48.6%) were completed, followed by those actively recruited (33.3%), and those active, but not recruited (16.7%). The proportion of industry trials was 95.8%, and the majority was accounted for by the lead sponsor. Economically developed eastern China (51.4%) and northern China (18.1%) were the most frequently identified study locations. Drug classes were chemical (48.6%), biologics (40.3%), and natural (11.1%). Samples sizes were relatively large, with 62.5% of trials enrolling 100 or more participants. The median number of participants per trial was 216 (70–369).
Study Designs of Included Trials
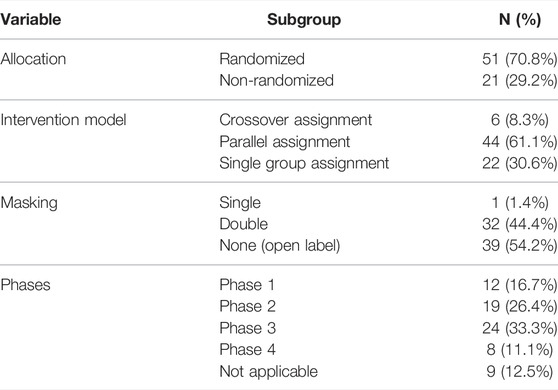
More than half of the trials (70.8%) were randomized. The intervention models were parallel assignment (61.1%), crossover assignment (8.3%), and single-group assignment (30.6%). Regarding masking, over half of the trials (54.2%) were open label, followed by double-blind studies (44.4%) and single-blind (1.4%). Trial phases were as follows: phase 1 (16.7%), phase 2 (26.4%), phase 3 (33.3%), phase 4 (11.1%), and not applicable (12.5%). (Detailed data are depicted in Table 2.)
Trial Characteristics and Study Design According to the Inclusion Criteria After Exclusion of Trials of Cancer and/or of Postmenopausal Women
We observed no significant differences between the two groups with respect to 1 year, drug classes, allocation, intervention model, masking, phases, and enrollment (p = 0.753, p = 0.698, p = 0.776, p = 1.000, p = 0.552, p = 0.936 and p = 0.661, respectively). Included pregnant women trials (41.3%) were principally initiated since January 2020, whereas excluded cancer and/or postmenopausal women trials (34.8%) primarily began during 2013–2016. (Detailed data are shown in Table 3.)
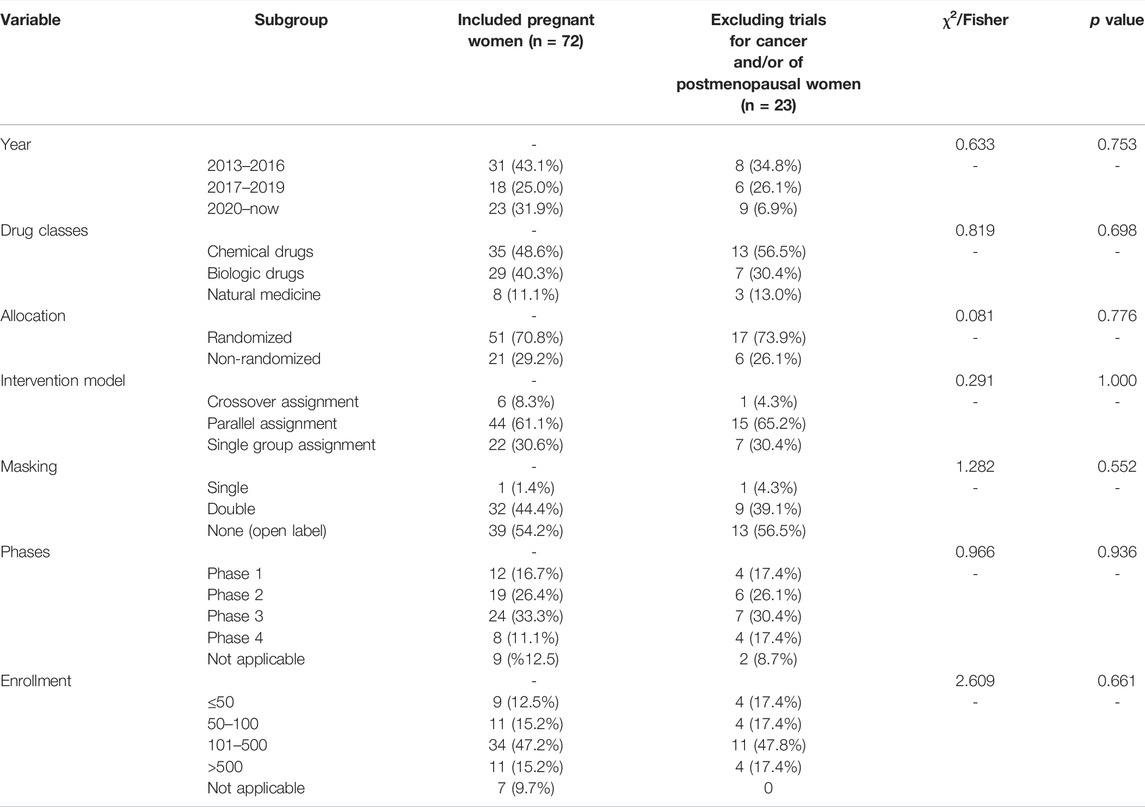
TABLE 3. Trial characteristics and study design according to the inclusion criteria after exclusion of trials of cancer and/or of postmenopausal women.
The characteristics of pregnant women trials according to the inclusion criteria and excluding trials for cancer and/or of postmenopausal women are given in Table 4. In total, 23 trials ultimately met our requirements, accounting for 0.15% of 15,539 registered trials in the ChiCTR websites, and 31.9% included 72 clinical trials.
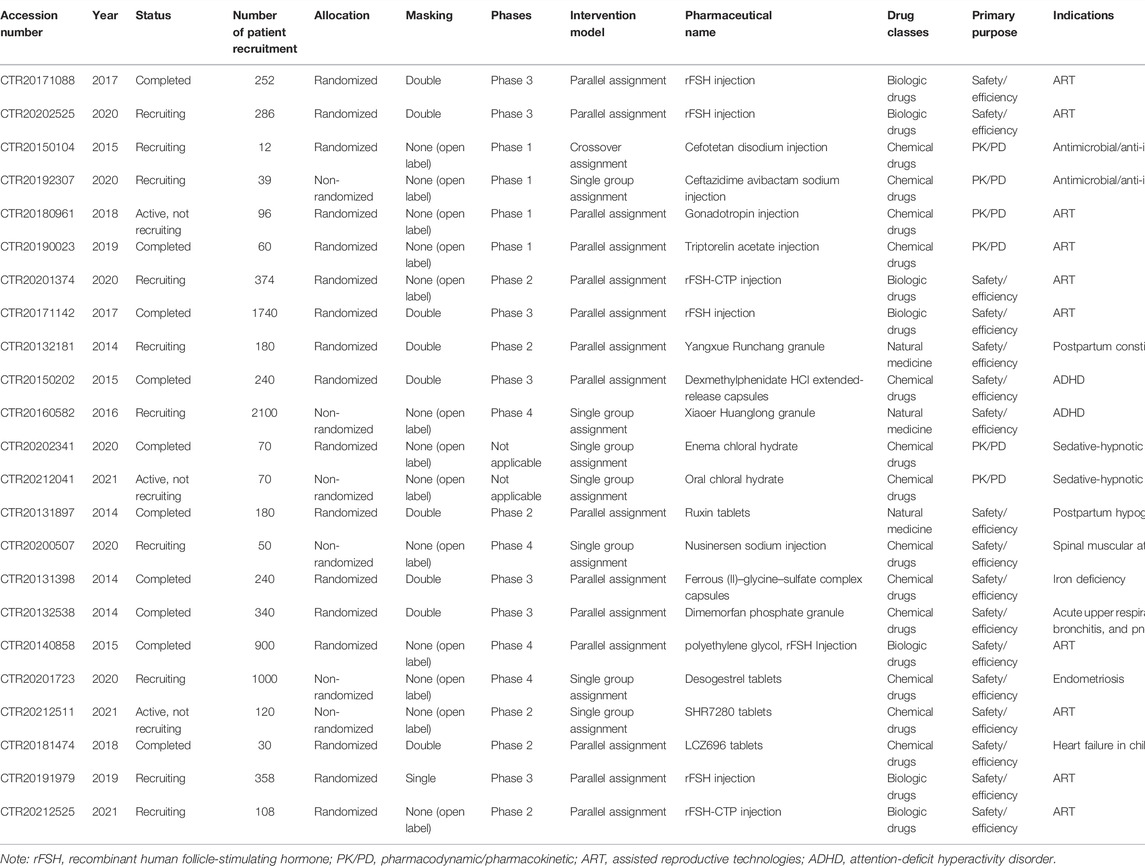
TABLE 4. Trials on pregnant women according to the inclusion criteria after exclusion of trials of cancer and/or of postmenopausal women.
Overview of Investigated Drugs
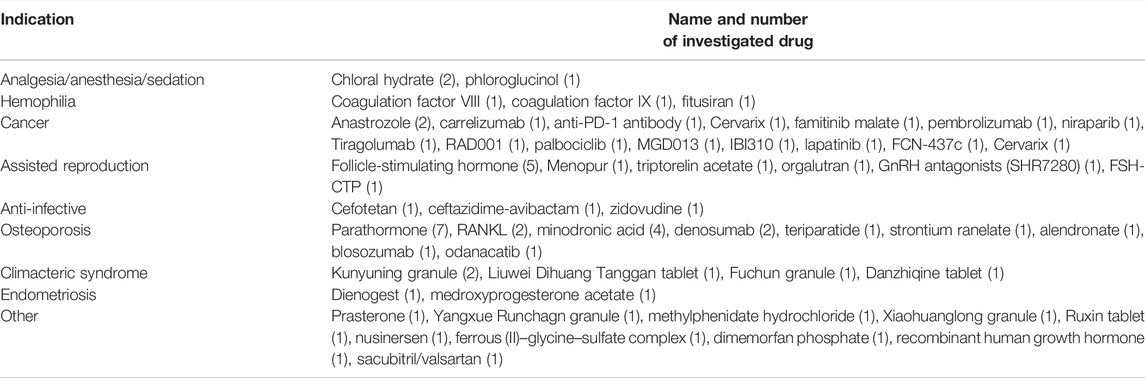
A summary of studied drugs is provided in Table 5. Of the 72 clinical trials, 54 drugs for 18 indications were included. Regarding indications, the highest proportion of the trials is for osteoporosis (27.8%), followed by cancer (22.2%), assisted reproduction (13.9%), and other indications (13.9%). Seven trials investigated the parathormone for the treatment of osteoporosis, and five trials investigated the follicle-stimulating hormone for assisted reproduction.
Discussion
Clinical trials are critical to clinical practice and decision-making (Mastroianni and Kahn, 2001). However, pregnant women were excluded from the majority of drug trials (Mofenson et al., 2019), yet other very relevant and/or more recent references of “drug utilization” studies could be used to show that pregnant women often (need to) use medication (Lupattelli et al., 2014; Ceulemans et al., 2022). As several investigators have determined, most trials have been funded by large pharmaceutical concerns that had better financial and organizational resources and more experts in conducting trials (Laterre and François, 2015). However, for ethical reasons, bias, and regarding potential harm to the fetus, large companies often excluded pregnant women from clinical trials (Fisk and Atun, 2008; Saito et al., 2017; Scaffidi et al., 2017; Kreuder et al., 2020; Van Der Straten et al., 2020), and these results were also similar to the present study. Reliable data on the quantity, location, source of funding, and therapeutic area of the trials on pregnancy-related drugs were provided. However, this demonstrated that there are very few clinical trials in which pregnant women were selected as research subjects. This finding is consistent with that reported in other countries, where the authors considered pregnant women as “drug orphans” (Kass et al., 2000; Mccormack and Best, 2014; Ayad and Costantine, 2015). Even when the number of pregnant women in a clinical trial is sufficient, the number of trials on pregnant women registered in China remains deficient compared to other regions of the world (e.g., North Africa/the Middle East, Europe, and North America) where trials of pregnancy-related drugs are actively pursued and implemented (Scaffidi et al., 2017). All of the clinical trials in the present study were approved by the Chinese FDA, with 95.8% funded by industry, and only 0.46% (72/15,539) of the clinical trials included pregnant women. After removing trials of cancer and/or on postmenopausal women, only 23 trials (0.15%, 23/15,539) included pregnant women. This result also indicated that studies on pregnant women might require government intervention, subsidies, incentives, and additional technologic advancements (Mastroianni and Kahn, 2001).
Neither the investigators nor the patients were particularly enthusiastic about participating in clinical trials. Our study found that since the establishment of the National Medical Products Administration (NMPA) registration platform in 2013, the total number of drug clinical trials registered on the platform and the CHiCTR platform was 44,505, which was only 1/5 the number of valid clinical trials on international registration platforms over the year. The results of our study also showed that the number of trials for new drugs and the assessments of efficacy and safety of the drugs for the treatment of common pregnancy conditions were insufficient and that there were no PK studies conducted on pregnant women. This was potentially due to the uncertainty of both the public and healthcare providers with regard to teratogenicity and other potential negative impacts of investigational drugs on fetal development, rather than on the mother (Kass et al., 2000; Ahmed et al., 2018). Collectively, our data revealed that the introduction of new drugs to the population of pregnant women would be difficult and complex. The medications most typically given to pregnant women would still be prescribed off label, and we acknowledge that data regarding the appropriate dosage, efficacy, and safety for pregnant women are still insufficient.
Randomized controlled, masked, and appropriate patient population trials are critical components of high-quality clinical trials (Zwierzyna et al., 2018). In our study, the percentage of randomized trials (70.8%) was lower than in previous studies 84.1% (Chen et al., 2020); 90.7% (Chen et al., 2019), which may be due to the robust effect of clinical trials that entailed cancer treatment or prevention/genetic diseases. After removing such trials, 51 trials (91.1%, 51/56) were ultimately randomized.
Strengths and Limitations
The essential advantage of this study was its surveillance of the clinical trial data of pregnant women registered on the clinical trial information platform in China using a systematic, unbiased approach. However, there were three significant limitations. First, since 6 September 2013, new clinical drug trials were required by the NMPA to be registered on the drug clinical trial registration and information disclosure platform. Therefore, previously registered trials may not have been included in our analysis. Second, although some pharmaceutical companies or clinical institutions have conducted clinical trials on pregnant women, they have not yet registered on this information platform. Third, we were also limited by the cross-sectional nature of our study, which restricted our analysis of the factors influencing results from pregnant women included in clinical trials.
Conclusion
Due to concerns regarding the fetus, it is common for pregnant women to show reluctance toward their inclusion in clinical trials. However, pregnant women and their spouses collectively agree that medical treatment should be administered for illnesses during pregnancy and that clinical trials of drugs during pregnancy are important and need to be performed. This practice paradoxically increases the risk to fetuses of untested or subtherapeutic drug regimens in clinical practice (Zhao et al., 2021). Based on our findings, we posit that the prospect for developing and evaluating drugs in pregnant women may not be favorable unless appropriate policies and measures are put in place. For example, studies on pregnant women might require government intervention, subsidies, incentives, and technological advancements (Mastroianni and Kahn, 2001). However, bioethicists, pharmacologists, regulators, and researchers elucidated on the need to include pregnant women in clinical trials to improve knowledge regarding the safety, dosage, and long-term effects drugs on pregnant women in the past few decades (Lyerly et al., 2008; White, 2015; Payne, 2019). Medical associations and regulatory agencies in various countries have been advocating for the removal of obstacles for pregnant women to be included in drug clinical research (Lyerly et al., 2008; Allesee and Gallagher, 2011; Payne, 2019). The U.S. FDA passed the “Pregnancy and Lactation Labeling Final Rule” (PLLR, Final Rule) in 2014. The rule requires an evaluation of the available information about a product’s use in pregnancy, which is expected to advance the development and implementation of clinical research on pregnant women (Gruber, 2015). Meanwhile, the results from this study should not be viewed as disappointing as there are still studies carried out on pregnant women at certain intervals by sponsors and clinical institutions in China. Nevertheless, greater attention needs to be given to the use of marketed drugs on pregnant women and to prioritize studies on drug PK in pregnant women.
Data Availability Statement
The original contributions presented in the study are included in the article/Supplementary Material, further inquiries can be directed to the corresponding author.
Author Contributions
YZ and GD planned and drafted the manuscript. HY and SW contributed to data quality control, analysis, and interpretation. QZ and ZZ provided administrative guidance and support with data interpretation. YZ led the overall planning and data interpretation. All authors reviewed and revised the manuscript.
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation (LGF21F020018).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Acknowledgments
We acknowledge LetPub (www.letpub.com) for its linguistic assistance during the preparation of this manuscript.
References
Ahmed, S. M., Nordeng, H., Sundby, J., Aragaw, Y. A., and De Boer, H. J. (2018). The Use of Medicinal Plants by Pregnant Women in Africa: A Systematic Review. J. Ethnopharmacol 224, 297–313. doi:10.1016/j.jep.2018.05.032
Allesee, L., and Gallagher, C. M. (2011). Pregnancy and Protection: The Ethics of Limiting a Pregnant Woman's Participation in Clinical Trials. J. Clin. Res. Bioeth. 2, 1000108. doi:10.4172/2155-9627.1000108
Anoshchenko, O., Prasad, B., Neradugomma, N. K., Wang, J., Mao, Q., and Unadkat, J. D. (2020). Gestational Age-dependent Abundance of Human Placental Transporters as Determined by Quantitative Targeted Proteomics. Drug Metab. Dispos 48, 735–741. doi:10.1124/dmd.120.000067
Ayad, M., and Costantine, M. M. (2015). Epidemiology of Medications Use in Pregnancy. Semin. Perinatol 39, 508–511. doi:10.1053/j.semperi.2015.08.002
Carnovale, C., Tombetti, E., Battini, V., Mazhar, F., Radice, S., Nivuori, M., et al. (2020). Inflammasome Targeted Therapy in Pregnancy: New Insights from an Analysis of Real-World Data from the FAERS Database and a Systematic Review. Front. Pharmacol. 11, 612259. doi:10.3389/fphar.2020.612259
Ceulemans, M., Foulon, V., Panchaud, A., Winterfeld, U., Pomar, L., Lambelet, V., et al. (2022). Self-Reported Medication Use Among Pregnant and Breastfeeding Women during the COVID-19 Pandemic: A Cross-Sectional Study in Five European Countries. Ijerph 19, 1389. doi:10.3390/ijerph19031389
Chen, L., Su, Y., Quan, L., Zhang, Y., and Du, L. (2019). Clinical Trials Focusing on Drug Control and Prevention of Ventilator-Associated Pneumonia: A Comprehensive Analysis of Trials Registered on ClinicalTrials.Gov. Front. Pharmacol. 9, 1574. doi:10.3389/fphar.2018.01574
Chen, L., Wang, M., Yang, Y., Shen, J., and Zhang, Y. (2020). Registered Interventional Clinical Trials for Old Populations with Infectious Diseases on ClinicalTrials.Gov: A Cross-Sectional Study. Front. Pharmacol. 11, 942. doi:10.3389/fphar.2020.00942
Fisk, N. M., and Atun, R. (2008). Market Failure and the Poverty of New Drugs in Maternal Health. Plos Med. 5, e22. doi:10.1371/journal.pmed.0050022
Government of Canada (2018). Guidance Document: Conduct and Analysis of Comparative Bioavailability Studies. Ottawa: Health Canada.
Gruber, M. F. (2015). The US FDA Pregnancy Lactation and Labeling Rule - Implications for Maternal Immunization. Vaccine 33, 6499–6500. doi:10.1016/j.vaccine.2015.05.107
Kass, N. E., Taylor, H. A., and Anderson, J. (2000). Treatment of Human Immunodeficiency Virus during Pregnancy: the Shift from an Exclusive Focus on Fetal protection to a More Balanced Approach. Am. J. Obstet. Gynecol. 182, 856–859. doi:10.1016/s0002-9378(00)70335-8
Kreuder, A. E., Bolaños-Rosales, A., Palmer, C., Thomas, A., Geiger, M. A., Lam, T., et al. (2020). Inspired by the Human Placenta: a Novel 3D Bioprinted Membrane System to Create Barrier Models. Sci. Rep. 10, 15606. doi:10.1038/s41598-020-72559-6
Laterre, P. F., and François, B. (2015). Strengths and Limitations of Industry vs. Academic Randomized Controlled Trials. Clin. Microbiol. Infect. 21, 906–909. doi:10.1016/j.cmi.2015.07.004
Lupattelli, A., Spigset, O., Twigg, M. J., Zagorodnikova, K., Mårdby, A. C., Moretti, M. E., et al. (2014). Medication Use in Pregnancy: a Cross-Sectional, Multinational Web-Based Study. BMJ Open 4, e004365. doi:10.1136/bmjopen-2013-004365
Lyerly, A. D., Little, M. O., and Faden, R. (2008). The Second Wave: Toward Responsible Inclusion of Pregnant Women in Research. Int. J. Fem Approaches Bioeth. 1, 5–22. doi:10.1353/ijf.0.0047
Mastroianni, A., and Kahn, J. (2001). Swinging on the Pendulum. Shifting Views of justice in Human Subjects Research. Hastings Cent. Rep. 31, 21–28. doi:10.2307/3527551
Mccormack, S. A., and Best, B. M. (2014). Obstetric Pharmacokinetic Dosing Studies Are Urgently Needed. Front. Pediatr. 2, 9. doi:10.3389/fped.2014.00009
Meyer, A., Fermaut, M., Drouin, J., Carbonnel, F., and Weill, A. (2021). Drug Use for Gastrointestinal Symptoms during Pregnancy: A French Nationwide Study 2010-2018. PloS one 16, e0245854. doi:10.1371/journal.pone.0245854
Mofenson, L. M., Pozniak, A. L., Wambui, J., Raizes, E., Ciaranello, A., Clayden, P., et al. (2019). Optimizing Responses to Drug Safety Signals in Pregnancy: the Example of Dolutegravir and Neural Tube Defects. J. Int. AIDS Soc. 22, e25352. doi:10.1002/jia2.25352
Payne, P. (2019). Including Pregnant Women in Clinical Research: Practical Guidance for Institutional Review Boards. Ethics Hum. Res. 41, 35–40. doi:10.1002/eahr.500036
Ranawaka, U. K., De Abrew, A., Wimalachandra, M., Samaranayake, N., and Goonaratna, C. (2018). Ten Years of Clinical Trial Registration in a Resource-Limited Setting: Experience of the Sri Lanka Clinical Trials Registry. J. Evid. Based Med. 11, 46–50. doi:10.1111/jebm.12284
Ruff, C. T., Giugliano, R. P., Braunwald, E., Hoffman, E. B., Deenadayalu, N., Ezekowitz, M. D., et al. (2014). Comparison of the Efficacy and Safety of New Oral Anticoagulants with Warfarin in Patients with Atrial Fibrillation: a Meta-Analysis of Randomised Trials. Lancet 383, 955–962. doi:10.1016/S0140-6736(13)62343-0
Saito, M., Gilder, M. E., Nosten, F., Mcgready, R., and Guérin, P. J. (2017). Systematic Literature Review and Meta-Analysis of the Efficacy of Artemisinin-Based and Quinine-Based Treatments for Uncomplicated Falciparum Malaria in Pregnancy: Methodological Challenges. Malar. J. 16, 488. doi:10.1186/s12936-017-2135-y
Scaffidi, J., Mol, B. W., and Keelan, J. A. (2017). The Pregnant Women as a Drug Orphan: a Global Survey of Registered Clinical Trials of Pharmacological Interventions in Pregnancy. BJOG 124, 132–140. doi:10.1111/1471-0528.14151
Stockmann, C., Sherwin, C. M., Koren, G., Campbell, S. C., Constance, J. E., Linakis, M., et al. (2014). Characteristics and Publication Patterns of Obstetric Studies Registered in ClinicalTrials.Gov. J. Clin. Pharmacol. 54, 432–437. doi:10.1002/jcph.212
Van Der Straten, A., Ryan, J. H., Reddy, K., Etima, J., Taulo, F., Mutero, P., et al. (2020). Influences on Willingness to Use Vaginal or Oral HIV PrEP during Pregnancy and Breastfeeding in Africa: the Multisite MAMMA Study. J. Int. AIDS Soc. 23, e25536. doi:10.1002/jia2.25536
Viprey, M., Xue, Y., Rousseau, A., Payet, C., Chapurlat, R., Caillet, P., et al. (2020). Adherence with Brand versus Generic Bisphosphonates Among Osteoporosis Patients: a New-User Cohort Study in the French National Healthcare Insurance Database. Sci. Rep. 10, 7446. doi:10.1038/s41598-020-64214-x
White, A. (2015). Accelerating the Paradigm Shift toward Inclusion of Pregnant Women in Drug Research: Ethical and Regulatory Considerations. Semin. Perinatol 39, 537–540. doi:10.1053/j.semperi.2015.08.008
Ye, X., Monchka, B. A., Righolt, C. H., and Mahmud, S. M. (2019). Maternal Use of Antibiotics and Cancer Incidence Risk in Offspring: A Population-Based Cohort Study in Manitoba, Canada. Cancer Med. 8, 5367–5372. doi:10.1002/cam4.2412
Zeng, X., Zhang, Y., Kwong, J. S., Zhang, C., Li, S., Sun, F., et al. (2015). The Methodological Quality Assessment Tools for Preclinical and Clinical Studies, Systematic Review and Meta-Analysis, and Clinical Practice Guideline: a Systematic Review. J. Evid. Based Med. 8, 2–10. doi:10.1111/jebm.12141
Zhao, Y., Zhang, L., and Geng, Y. (2021). Clinical Drug Trial Participation: Perspectives of Pregnant Women and Their Spouses. Patient Prefer Adherence 15, 2343–2352. doi:10.2147/PPA.S328969
Zhong, H., Chan, G., Hu, Y., Hu, H., and Ouyang, D. (2018). A Comprehensive Map of FDA-Approved Pharmaceutical Products. Pharmaceutics 10, 263. doi:10.3390/pharmaceutics10040263
Keywords: clinical trials, pregnant women, drug development, drug safety, registration and post-marketing studies
Citation: Zhao Y, Du G, Luan X, Yang H, Zhang Q, Zhang Z and Wang S (2022) Registered Clinical Trials Comprising Pregnant Women in China: A Cross-Sectional Study. Front. Pharmacol. 13:850080. doi: 10.3389/fphar.2022.850080
Received: 07 January 2022; Accepted: 10 March 2022;
Published: 05 April 2022.
Edited by:
Dalia M. Dawoud, National Institute for Health and Care Excellence, United KingdomReviewed by:
Ashwin Karanam, Pfizer, United StatesMichael Ceulemans, KU Leuven, Belgium
Laure Sillis, KU Leuven, Belgium, in collaboration with MC.
Copyright © 2022 Zhao, Du, Luan, Yang, Zhang, Zhang and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Yi Zhao, zhaoyi1124@zju.edu.cn
 Yi Zhao
Yi Zhao Guiping Du1
Guiping Du1