- 1Department of Health Sciences, Université du Québec à Rimouski, Lévis, QC, Canada
- 2Population Health and Optimal Health Practices, CHU de Québec - Université Laval Research Center, Québec, QC, Canada
- 3Department of Medical and Surgical Sciences, University of Bologna, Bologna, Italy
- 4Institut national de Santé Publique du Québec, Québec, QC, Canada
- 5Faculty of Medicine, Université Laval, Québec, QC, Canada
- 6Faculty of Pharmacy, Université Laval, Québec, QC, Canada
- 7Quebec Excellence Centre on Aging, VITAM Research Centre on Sustainable Health, Québec, QC, Canada
Background: Schizophrenia is a severe psychiatric disorder associated with multiple psychiatric and non-psychiatric comorbidities. As adults with schizophrenia age, they may use many medications, i.e., have polypharmacy. While psychiatric polypharmacy is well documented, little is known about trends and patterns of global polypharmacy. This study aimed to draw a portrait of polypharmacy among older adults with schizophrenia from 2000 to 2016.
Methods: This population-based cohort study was conducted using the data of the Quebec Integrated Chronic Disease Surveillance System of the National Institute of Public Health of Quebec to characterize recent trends and patterns of medication use according to age and sex. We identified all Quebec residents over 65 years with an ICD-9 or ICD-10 diagnosis of schizophrenia between 2000 and 2016. We calculated the total number of medications used by every individual each year and the age-standardized proportion of individuals with polypharmacy, as defined by the usage of 5+, 10+, 15+, and 20+ different medications yearly. We identified the clinical and socio-demographic factors associated with polypharmacy using robust Poisson regression models considering the correlation of the responses between subjects and analyzed trends in the prevalence of different degrees of polypharmacy.
Results: From 2000 to 2016, the median number of medications consumed yearly rose from 8 in 2000 to 11 in 2016. The age-standardized proportion of people exposed to different degrees of polypharmacy also increased from 2000 to 2016: 5+ drugs: 76.6%–89.3%; 10+ drugs: 36.9%–62.2%; 15+: 13.3%–34.4%; 20+: 3.9%–14.4%. Non-antipsychotic drugs essentially drove the rise in polypharmacy since the number of antipsychotics remained stable (mean number of antipsychotics consumed: 1.51 in 2000 vs. 1.67 in 2016). In the multivariate regression, one of the main clinically significant factor associated with polypharmacy was the number of comorbidities (e.g., Polypharmacy-10+: RR[2 VS. 0–1] = 1.4; 99% IC:1.3–1.4, RR[3–4] = 1.7 (1.7–1.8); RR[5+] = 2.1 (2.1–2.2); Polypharmacy-15+: RR[2 VS 0–1] = 1.6; 99% IC:1.5–1.7, RR[3–4] = 2.5 (2.3–2.7); RR[5+] = 4.1 (3.8–4.5).
Conclusion: There was a noticeable increase in polypharmacy exposure among older adults with schizophrenia in recent years, mainly driven by non-antipsychotic medications. This raises concerns about the growing risks for adverse effects and drug-drug interactions in this vulnerable population.
Introduction
Schizophrenia is a severe disease characterized by hallucinations, delusions, disorganized speech, and abnormal thinking, which significantly impact the ability of patients to function in their daily lives and quality of life (Marder and Cannon, 2019). It is among the top 10 global causes of disability (Fleischhacker et al., 2014; Charlson et al., 2018), with an estimated worldwide prevalence that can reach up to 1% (Saha et al., 2005). Schizophrenia patients are more often sedentary, higher cigarette smokers and drug users (Fleischhacker et al., 2014). They have frequent physical comorbidities such as cardiovascular diseases (Fleischhacker et al., 2014), obesity (Mamakou et al., 2018), type two diabetes (Fleischhacker et al., 2014), metabolic syndrome (Jeon and Kim, 2017), and dementia (Stroup et al., 2021). Mental comorbidities such as depression (Remington et al., 2017), alcohol or substance abuse (Buchanan et al., 2010), and insomnia (Stummer et al., 2018) are also common in these patients. It is also hypothesized that the aging process is accelerated in schizophrenia patients (Nguyen et al., 2018).
On the other hand, patients with schizophrenia are underdiagnosed with physical conditions and, when the diagnosis arrives, these conditions are often at an advanced stage, requiring more intensive treatment and more medications (Fleischhacker et al., 2014). In the general older population, multimorbidity (Gontijo Guerra et al., 2019), is often associated with polypharmacy [i.e., taking multiple medications (Sirois et al., 2019a)]. Polypharmacy is a genuine concern in older individuals due to the higher risk for adverse drug events, drug-drug interactions, adherence problems, and potentially inappropriate prescriptions (Kojima et al., 2020; Lin, 2020). In recent years, polypharmacy has been studied in different populations of older individuals with chronic conditions, such as heart failure (Campeau Calfat et al., 2022), chronic obstructive pulmonary disease (COPD) (Sirois et al., 2019b), or diabetes (Oktora et al., 2021). These studies have shown an increase in polypharmacy in the last decades. Nevertheless, polypharmacy has not been studied in older patients with schizophrenia, despite this concern similarly exists for these patients because of their elevated risk of multimorbidity (Buchanan et al., 2010; Fleischhacker et al., 2014; Jeon and Kim, 2017; Remington et al., 2017; Mamakou et al., 2018; Nguyen et al., 2018; Stummer et al., 2018; Stroup et al., 2021).
The cornerstone of schizophrenia treatment is antipsychotic medications (Marder and Cannon, 2019). Antipsychotic drugs usually must be taken lifelong (Remington et al., 2017; Marder and Cannon, 2019). Antipsychotic polypharmacy (Jeon and Kim, 2017), namely the use of more than one antipsychotic at the same time, is frequent in clinical practice either to achieve reasonable control of psychosis or to treat specific symptoms such as insomnia (Stummer et al., 2018) or other side effects (Baandrup, 2020). In a recent study on hospitalized patients with schizophrenia-spectrum disorders, 28.1% of patients took four or more psychotropic drugs before hospitalization, with a mean number of 2.8 medications. Still, the number of non-psychotropic drugs was not mentioned (Gaudiano et al., 2018). We can hypothesize that global polypharmacy is significant, especially in older patients with schizophrenia, given multimorbidity, as age is a predictor of polypharmacy in psychiatric patients (Viola et al., 2004; Paudel et al., 2020).
Even if global polypharmacy may be frequent in older patients with schizophrenia, studies on this topic have focused only on the psychiatric polypharmacy (Zink et al., 2010), with the main emphasis on the antipsychotic combination therapy (Gaudiano et al., 2018; Baandrup, 2020; Lin, 2020). Considering the potential burden that polypharmacy may impose on these patients, it is important to quantify the problem and to identify factors associated with polypharmacy that may help identify those at higher risk of adverse consequences of polypharmacy. To the best of our knowledge, no study has investigated the trends and patterns of global polypharmacy in older adults with schizophrenia.
The objectives of this study were thus to draw a portrait of polypharmacy among Quebec older adults with schizophrenia from 2000 to 2016 and to identify factors associated with different degrees of polypharmacy.
Materials and methods
Data source and population
We performed a population-based observational study of annual cohorts (one cohort for each year under study) using the data of the Quebec Integrated Chronic Disease Surveillance System (QICDSS) of the National Institute of Public Health of Quebec (Institut National de Santé Publique du Québec−INSPQ) (Blais et al., 2014). The QICDSS database is composed of five different sources of medico-administrative data: information on the insurance plan of its members (i.e., starting and end date of eligibility), on hospitalizations (i.e., primary and secondary diagnostic codes according to the ninth and tenth revisions of the International classification of diseases–ICD-9 and ICD-10, respectively), on physician visits (primary ICD-9 diagnostic codes), on reimbursed drugs (i.e., drug name, dispensing date, days’ supply, the specialty of the prescriber) and on deaths. More than 90% of the Quebec population aged 65 years and above is covered by the public drug plan, and their information is in the QICDSS (Blais et al., 2014). Older adults in long-term care and those with a private drug plan are not covered by the public drug plan and are thus excluded.
This study identified all Quebec residents over 65 with an ICD-9 or ICD-10 diagnostic inpatient or outpatient code for schizophrenia (ICD-9: 295.0 to 295.9; ICD-10: F20.0 to F21.9, F23.2, F25.0 to F25.9) between April 1st, 2000 and March 31st, 2017. We constructed 17 cohorts (one for each year under study) which included both incident and prevalent cases of schizophrenia.
Definition of polypharmacy and medication use
We assessed the number of different medications used by each individual in every fiscal year, with the fiscal year beginning on April 1st and ending on March 31st. We included all the patients covered by the public drug insurance plan and alive for the year under investigation to assess the total number of medications used that year. Medications were classified according to the American Hospital Formulary Service (AHFS) classification (Francke, 1963) and common drug denomination (chemical name of the medication).
There is no consensus on the definition of polypharmacy, with the most common definitions used in the literature having a threshold of 5 or 10 medications (Sirois et al., 2019a). Considering the population of older adults with multimorbidity, we decided to use different thresholds to define polypharmacy. Thus, in this study, polypharmacy was referred to as the presence of prescription claims for at least 5, 10, 15, or 20 different medications in a fiscal year. We, therefore, assessed different degrees of polypharmacy for the time frame of a fiscal year, for every fiscal year in the study period. In accounting for the sum of medications claimed in each fiscal year, we considered only medications reimbursed by the public drug plan. Thus, over-the-counter drugs or other non-reimbursed medications (e.g., z-drugs) were not included. Medications used as needed (“prn”) and those for acute illnesses (e.g., antibiotics), if reimbursed by the public drug plan, were included.
Socio-demographic and clinical characteristics
Socio-demographic characteristics of individuals in each cohort included age, sex, material and social deprivation index (in quintiles) and residence area [based on the Quebec census geographical areas: Montreal (> 1,000,000 inhabitants), other census metropolitan (100,000 to 1,000,000 inhabitants), agglomerations (10,000 to 100,000 inhabitants), and rural (< 10,000 inhabitants)]. Material and social deprivation indexes represent a proxy of the socioeconomic status of the individual (Pampalon et al., 2009). These indexes, which are ecological indexes based on the census dissemination area, are divided into quintiles, with the first quintile including the least deprived and the fifth quintile the most deprived individuals (Pampalon et al., 2009). We also calculated a global deprivation index combining social and material deprivations according to five classes (most deprived, deprived, mostly socially deprived, mostly materially deprived, least deprived), as explained in Supplementary Figure S1. We identified the annual number of hospitalizations and the number of physician visits recorded in the QIDSS for each individual and each year under study. ICD-9 and ICD-10 codes were used to identify comorbid conditions according to validated QICDSS algorithms for Alzheimer’s disease, asthma, chronic obstructive pulmonary disease (COPD), diabetes, hypertension, mood disorders, osteoporosis, stroke, mood disorders, and dementia (Blais et al., 2014) during 5 years (the current year and the four preceding years). We used the combined Charlson-Elixhauser comorbidity index to calculate a score of the burden of comorbidities of each patient (Simard et al., 2018).
Statistical analysis
We used descriptive statistics to describe the subjects included in each cohort. For each subject, we assessed the number of different drugs claimed in every fiscal year by using the drug’s common denomination (identifying the chemical entity). We calculated the proportion of individuals exposed to different degrees of polypharmacy and then estimated the age-standardized annual prevalence of polypharmacy with the reference population of Quebec in 2011. We further identified the clinical and socio-demographic factors associated with polypharmacy using robust Poisson regression analyses, modeling the number of individuals who claimed at least 5, 10, 15, or 20 different medications in a fiscal year, depending on the model and considering the correlation of the responses between subjects. Thus, we calculated unadjusted and adjusted prevalence ratios (PRs) and their 99% confidence intervals (CIs). We also tested the trends of change in the mean annual prevalence of polypharmacy with the same models. We performed all the analyses using SAS Enterprise Guide 7.1.
Results
Cohorts comprised 2,566 individuals in 2000 and up to 4,634 in 2016 (Table 1). Female patients were the large majority (about two-third of each cohort), as for those in the 66–75 age group (about 70%), with some changes in the age distribution depending on the cohort. Indeed, the proportion of older individuals (>85 years) slightly increased from 4.2% to 5.9% over time. Between 2000 and 2016, the proportion of individuals with mood disorders decreased by 26% (from 40.3% to 30.0%), but those with diabetes, hypertension and osteoporosis largely increased, with relative changes of 104%, 54%, and 124%, respectively. Indeed, as expected, with the aging of the individuals being part of the annual cohorts from 2000 to 2016, the population was composed of older individuals with more physical comorbidities in more recent years.
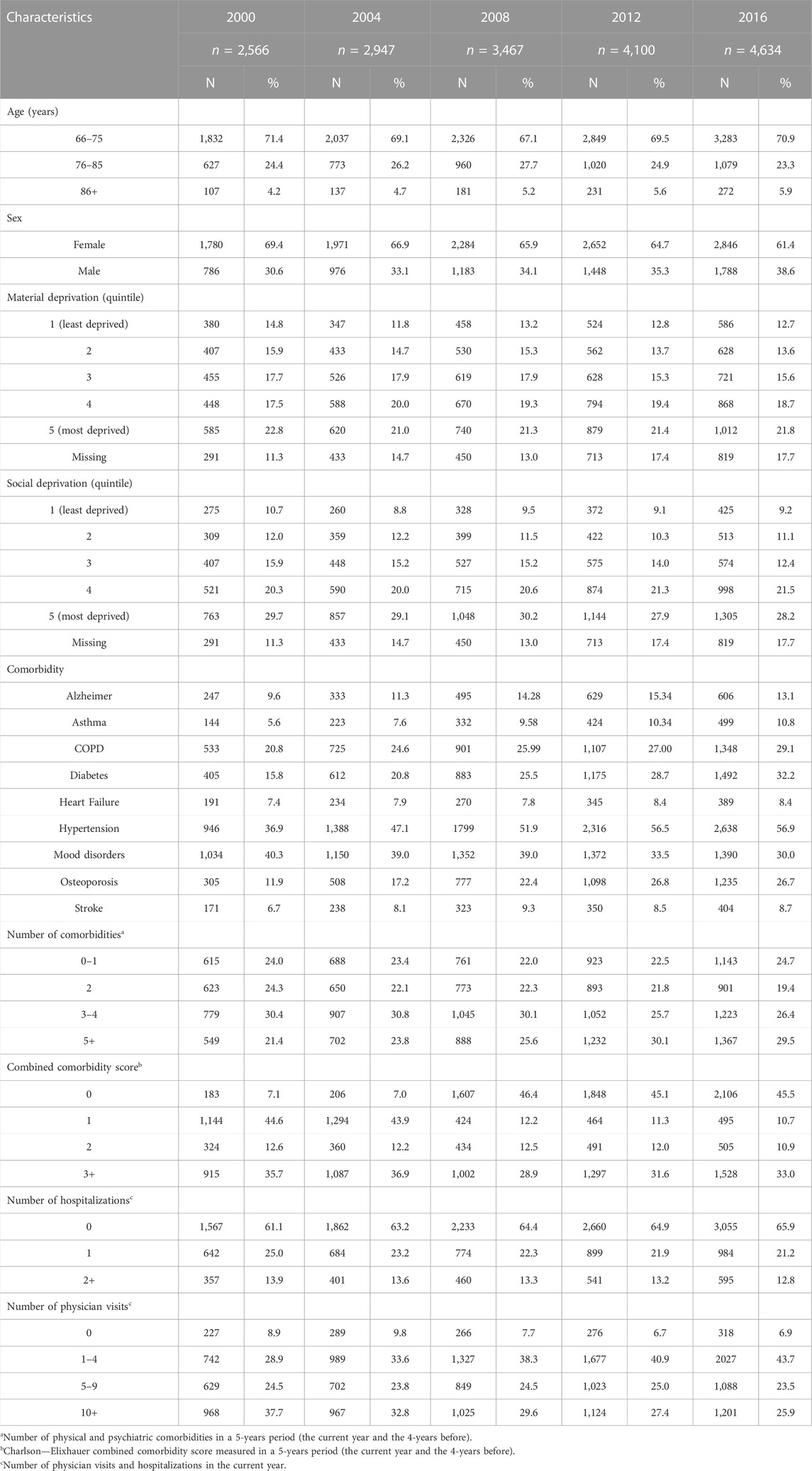
TABLE 1. Characteristics of the population studied from selected cohorts (2000; 2004; 2008; 2012 and 2016).
As reported in Figure 1, the number of different medications claimed increased over the 16 years, with the mean number of drugs claimed rising from 8.76 [standard deviation (SD) 5.29] in 2000 to 12.3 (SD 6.78) in 2016. Accordingly, the age-standardized prevalence of different degrees of polypharmacy also increased over time, with 36.9% of individuals being exposed to 10 drugs and above in 2000, increasing to 62.2% in 2016. Similarly, the prevalence of polypharmacy defined as 5 medications and above, as 15 medications and above, and as 20 medications and above went from 76.6%, 13.3%, and 3.9% in 2000 to 89.3%, 34.4%, and 14.4% in 2016, respectively (Figure 1). The trend analyses showed that the age-adjusted proportion of individuals exposed to different degrees of polypharmacy increased in the study period. Over the 17 years under investigation, the yearly mean increases of individuals exposed to varying degrees of polypharmacy were 0.8% (99% CI = 0.7%–0.9%) for 5 and more medications, 2.6% (99% CI = 2.4%–2.9%) for 10 medications and above, 4.5% (99% CI = 4.0%–4.9%) for 15 medications and above, and 5.2% (99% CI = 4.4%–5.9%) for 20 medications and above.
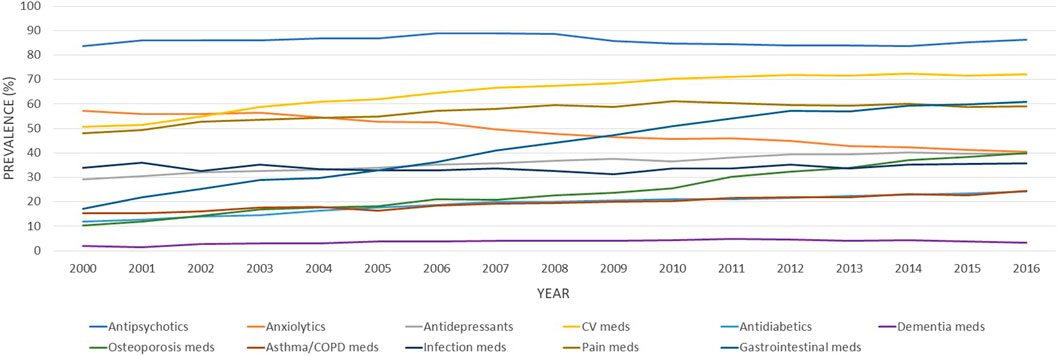
FIGURE 1. Age-standardized proportions of older adults with schizophrenia exposed to different degrees of polypharmacy (≥5, ≥10, ≥15, and ≥20 medications), between 2000 and 2016. Bars represent the age-adjusted prevalence of different degrees of polypharmacy and the line is the mean number of different medications claimed in the current fiscal year.
The rise in medication use was essentially driven by non-antipsychotic drugs, as presented in Table 2. The number of antipsychotics remained stable, with a mean number of antipsychotics consumed of 1.51 ± 0.75 in 2000 and 1.67 (±0.84) in 2016. When analyzing the prevalence of the main medication classes claimed, different patterns emerged. Some classes increased over the study period, such as cardiovascular medications, gastrointestinal medications (mainly driven by proton pump inhibitors–PPIs), and osteoporosis medications (Figure 2). Other classes, such as anxiolytics, showed an important decrease overtime. The more impacting diseases were cardiovascular and respiratory comorbidities, such as heart failure, stroke, asthma, and COPD.
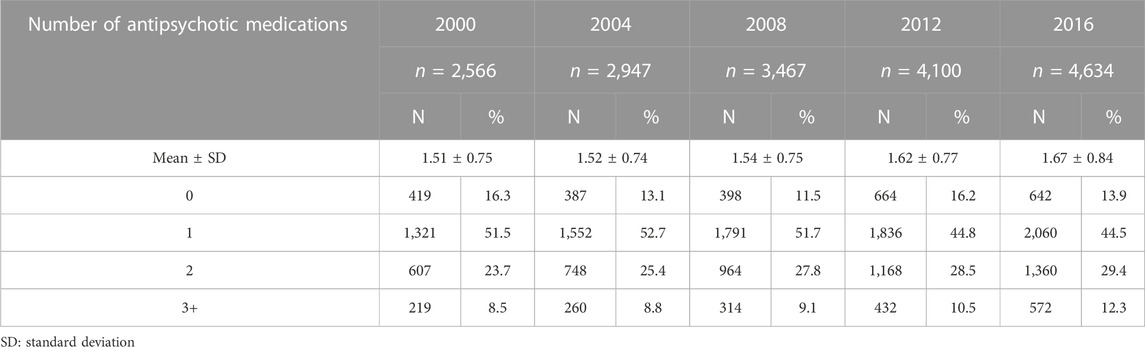
TABLE 2. Number of different antipsychotic medications claimed during one year-period by older people with schizophrenia from 2000 to 2016.
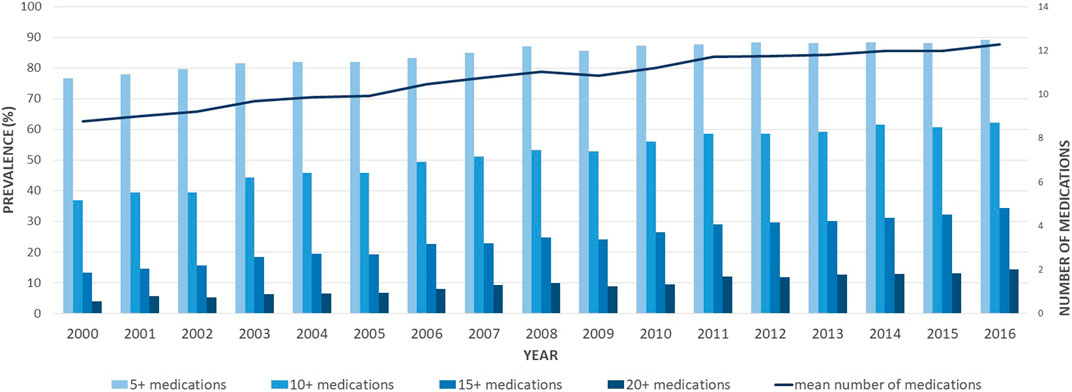
FIGURE 2. Age-standardized proportions of older adults with schizophrenia exposed to different classes of medications between 2000 and 2016. CV, cardiovascular; COPD, chronic obstructive pulmonary disease; Meds, medications.
In the multivariable robust Poisson regressions, women were more likely to be exposed to polypharmacy with adjusted prevalence ratios (PR) ranging from 1.05 for 5 to 1.22 for 20 medications and above (Table 3). Older individuals were slightly more likely to be exposed to lower degrees of polypharmacy (5+ and 10+ medications), while age was not a statistically significant factor for higher levels of polypharmacy (15+ and 20+). The only clinically significant factor statistically associated with polypharmacy was the number of comorbidities, with prevalence ratios increasing with the number of comorbidities and the degree of polypharmacy (see Table 3).
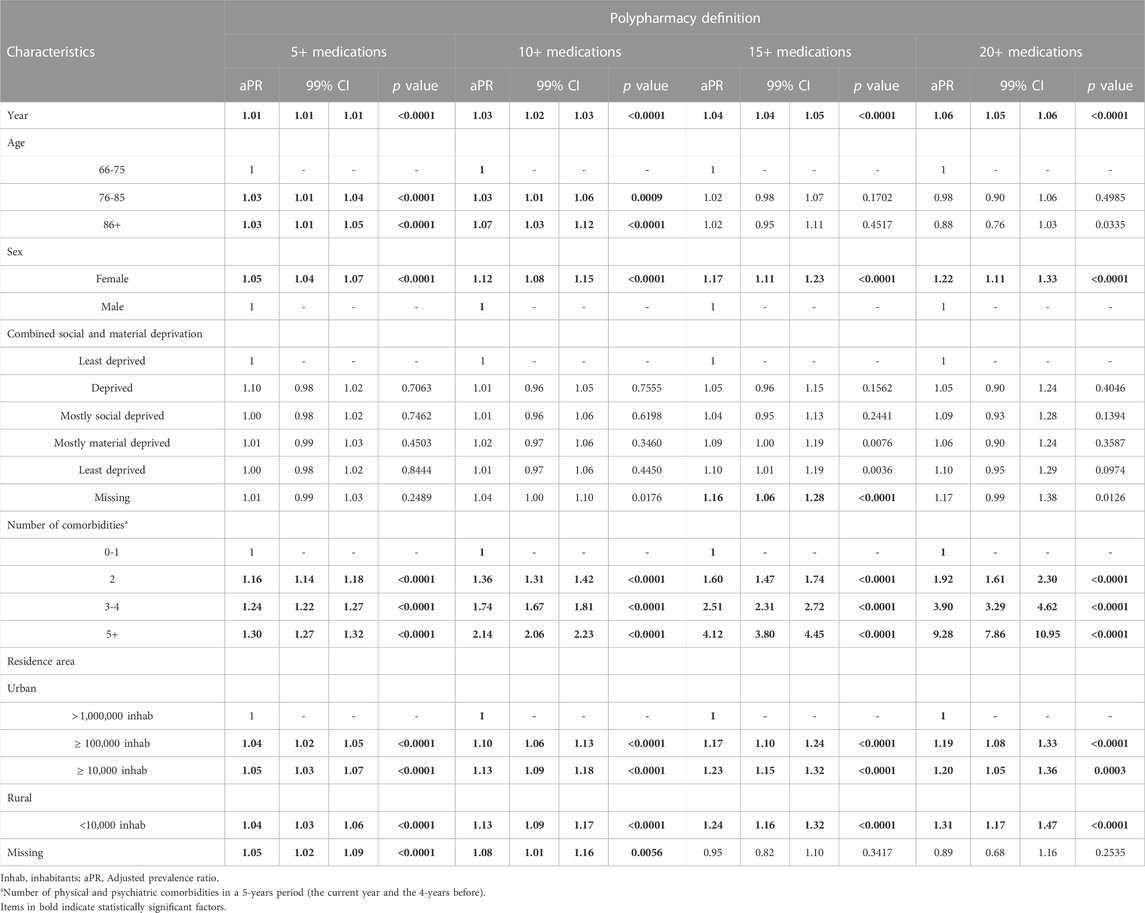
TABLE 3. Multivariable robust Poisson regressions of the factors associated with polypharmacy, defined as the claim of at least five, ten, fifteen, or twenty different medications in 1 year during the study period.
Discussion
The main result of this study is that polypharmacy has been increasing steadily over the last few years for older patients with schizophrenia. To the best of our knowledge, this study is the first one that has evaluated polypharmacy and not only psychiatric polypharmacy (e.g., the use of more than one psychotropic medication) in older individuals with schizophrenia. Some studies have evaluated psychotropic medication use and psychiatric polypharmacy in this population. In a study on schizophrenia-spectrum disorder patients, the authors found that, at hospitalization, 28.1% of patients received four or more psychotropic drugs with a mean number of 2.8 (Gaudiano et al., 2018). Those with four or more psychotropic drugs were older (43.0 vs. 38.6 years) and had more medical comorbidities, including metabolic conditions (Gaudiano et al., 2018). Even if the number of non-psychotropic drugs was not estimated in that study, we could hypothesize that global polypharmacy was significantly higher. A study analyzing prescriptions from office-based physicians in the United States to treat schizophrenia patients showed that 29% of them received at least two medications and 18% three or more (Dussias et al., 2010). Moreover, 58% of patients received one or more antipsychotic medications, and the others received a combination of antipsychotics and other psychiatric medications (20% antidepressants, 15% mood stabilizers, 7% anxiolytics, and 6% treatment for extrapyramidal symptoms) (Dussias et al., 2010). In another study evaluating central nervous system (CNS) medication prescriptions trends in patients with schizophrenia-related conditions between 2004 and 2012 (Heald et al., 2017), the authors found an increase in psychotropic polypharmacy over the study period. This rise corresponded to increased body mass index (BMI) and fasting blood glucose (Heald et al., 2017), conditions requiring additional pharmacological treatments. Despite the lack of studies on global pharmacology in older patients with schizophrenia, the cited studies evaluating psychotropic medications indicate that the pharmacological burden on these patients is significant. Psychiatric polypharmacy could indeed increase the burden of medication load leading to an increase in medications used for both mental and somatic conditions.
In the context of the lack of studies evaluating the global pharmacological burden affecting these patients, our study underlines the high prevalence of polypharmacy, with more than a third of patients having claimed at least 15 different medications in the last year under study. This study should be a starting point in the research on older patients with schizophrenia. Indeed, the long-term pharmacological treatment of these patients should be considered globally in a holistic point of view. The pharmacological treatment should thus be re-evaluated when the patient becomes older. Polypharmacy is a well-known risk factor for many adverse outcomes (Davies et al., 2020; Zaninotto et al., 2020; Franchi et al., 2021; Li et al., 2022). The high proportion of individuals exposed to this potential risk should raise concerns and stimulate new studies on this vulnerable population.
In our population, the rise in polypharmacy was mainly driven by non-antipsychotic medications, for which the use rested stable over time. Some classes, such as medications for osteoporosis or gastrointestinal and cardiovascular drugs, showed an increased use over time. These increases are due to the aging population during the study period and the presence of effective medications on the market (i.e., PPIs). Other classes, such as anxiolytics, showed a significant decrease over time, driven by the changes in clinical guidelines as reported also from a recent population-based study in Quebec (Gosselin et al., 2022). The most used medication classes were those for cardiovascular and respiratory comorbidities, such as heart failure, stroke, asthma, and COPD. Chronic somatic diseases are more frequent among schizophrenia patients than in the general older population. In a review including 25,692 schizophrenia patients, the prevalence of metabolic syndrome was estimated at 32.5%, increasing to 51.9% for patients treated with clozapine (Mitchell et al., 2013). Among older patients with schizophrenia, diabetes is highly prevalent (about 25% of patients), especially among women (Annamalai et al., 2017; Huo et al., 2021), with a 2 to 5-fold increased risk than in the general population (Annamalai et al., 2017; Mamakou et al., 2018). Similarly, these patients are at higher risk for hypertension (Meszaros et al., 2011; Mamakou et al., 2018), obesity (Allison et al., 2009; Annamalai et al., 2017), and dyslipidemia (Mamakou et al., 2018). The higher risk of schizophrenia patients for these comorbidities can be explained by the physiopathology of the disease itself and the utilization of psychotropic medications (Mitchell et al., 2013; Abosi et al., 2018; Mamakou et al., 2018). Antipsychotic medications are indeed associated with an important side effect burden, including metabolic side effects (Jeon and Kim, 2017). Antipsychotic side effects are common, and they may easily reach an intensity requiring another pharmacological treatment, such as benztropine for extrapyramidal side effects (Marder and Cannon, 2019), benzodiazepines, propranolol, or mirtazapine for akathisia, (Zink et al., 2010; Marder and Cannon, 2019), metformin or liraglutide for weight control (de Silva et al., 2016; Grigg et al., 2017), aripiprazole or hormone therapy for hyperprolactinemia (Myles et al., 2017), hormonal therapy for sexual dysfunctions (Grigg et al., 2017; Marder and Cannon, 2019), or laxatives for constipation (De Berardis et al., 2018).
We observed that the number of comorbidities increased over time and contributed to the burden of polypharmacy. This was confirmed by the multivariate regression models analyzing the factors associated with different degrees of polypharmacy. In those analyses, no matter the definition of polypharmacy used, multimorbidity was a statistically and clinically significant factor associated with polypharmacy. The American Psychiatric Association (APA) practice guidelines for managing patients with schizophrenia (Keepers et al., 2020) highlight the importance of addressing integrated medical care to prevent and treat comorbidities. They address weight management, smoke cessation, cardiovascular risk factors (metabolic syndrome, hypertension, dyslipidemia, or heart failure), and renal and liver function. Moreover, these guidelines recommend identifying optimal approaches to prevent and treat specific side effects of antipsychotic medications (i.e., weight gain, metabolic syndrome, cardiovascular toxicity) (Areas for Further Research in Individuals With Schizophrenia, 2021), with particular attention to older individuals for their higher risk for side effects of antipsychotic medications, and potential renal and hepatic impairment (Keepers et al., 2020). Nevertheless, the risks and benefits of exposure to many medications (e.g., polypharmacy) to treat comorbid conditions in older patients with schizophrenia are not well defined yet.
Polypharmacy is a real concern in all older individuals because it has been associated with negative health outcomes such as non-adherence (Franchi et al., 2021), drug-drug interactions (Davies et al., 2020), potentially inappropriate medications (Davies et al., 2020), falls (Zaninotto et al., 2020), hospitalizations (Davies et al., 2020), and mortality (Li et al., 2022), also in COVID-19 patients (Sirois et al., 2022). In this schizophrenia patients, the use of antipsychotic medications, which are necessary to control the symptoms of the disease, can increase the risk for physical comorbidities, especially cardiovascular and metabolic ones (Jeon and Kim, 2017). Older patients with schizophrenia represent a real challenge because of the high number of medications they receive for schizophrenia itself and the frequent comorbidities they are diagnosed with. Future studies should identify the effect of polypharmacy on the risk of negative health outcomes and mortality. They should also focus on which combinations of medications can provide the greatest benefits with the lowest risks for better integrated medical care, considering not only the control of schizophrenia and the management of its treatment with antipsychotics and psychiatric medications but also non-psychiatric comorbidities, their prevention and treatment.
This study highlighted how polypharmacy is frequent in older adults with schizophrenia, even when more restrictive thresholds as 15 or 20 medications and above are used to define it. These patients are indeed at elevated risk for drug-drug interactions, adverse drug effects interactions, and drug-disease interactions compared to their peers without schizophrenia because of the frequency of physical comorbidities and the already impacting burden of antipsychotic treatments.
We believe this study has the main strength of well highlighting the burden of global polypharmacy among older individuals with schizophrenia. Medico-administrative databases allowed us to access annual large cohorts of patients with schizophrenia throughout Québec, as well as all the reimbursed medications they claimed, the diagnoses they received, and their resource utilization. With this approach, we could observe the pharmacological burden of older patients with schizophrenia, putting the antipsychotic treatment in the context of the global treatment of the older individual. Moreover, we could analyze trends and patterns of different pharmacological classes over a period of almost 20 years, highlighting changes and practices.
The results of this study should, nevertheless, be considered in light of some limitations. First, because of the use of administrative databases, we could not clinically assess the diagnosis of schizophrenia or the presence of comorbidities. However, the algorithms used for the identification of such diagnoses are routinely used by the INSPQ for surveillance purposes and the QICDSS (Blais et al., 2014). We could also have overestimated polypharmacy. To measure polypharmacy, we used claims of prescribed medications during a 1-year time frame. This means that the medications considered could not have been used simultaneously, as happens when treatments are switched because of side effects or inefficacy. On the contrary, we could consider only prescribed medications reimbursed by the public drug plan. This could have thus led to an underestimation of polypharmacy since over-the-counter medications, such as anti-inflammatory drugs, or laxatives, have not been considered among the medications accounting for polypharmacy. Still, since the same operational definition of polypharmacy was used for every year of the study, the conclusion on the increasing burden of medications among older individuals with schizophrenia persist, with the same overestimation of individuals exposed to polypharmacy being homogenous over time. Finally, this study aimed to explore global polypharmacy in older patients with schizophrenia, its prevalence, trends, and patterns over time, and it was thus designed for these purposes only. Therefore, we did not assess the effects of polypharmacy, such as adverse events, hospitalizations or mortality in this population.
Conclusion
We found a noticeable increase in polypharmacy exposure in older adults with schizophrenia, with the proportion of subjects having claimed at least 5, 10, 15, and 20 medications increasing to about 90%, 60%, 35%, and 15% in 2016. This raises concerns about the growing risks of adverse effects and drug-drug interactions that could arise in these patients, especially considering the use of antipsychotic treatments.
The risks and benefits of polypharmacy in older patients with schizophrenia are not well defined yet. There is a need to better understand which combinations of medications provide the greatest benefits and lowest risks and consider the presence of non-psychiatric comorbidities and the concomitant use of psychiatric and non-psychiatric drugs.
Data availability statement
The data analyzed in this study is subject to the following licenses/restrictions: We do not have permission to share the data from the Quebec Integrated Chronic Diseases Surveillance System (QICDSS). Requests to access these datasets should be directed to the Quebec Information access commissioner—Commission d’accès à l’information du Québec.
Ethics statement
For this study, medico-administrative data were analyzed retrospectively. The study protocol was reviewed and approved by the Ethics Committee for sectoral research in population and first-line health of the CIUSSS de la Capitale-Nationale (#2021–2049, 2021–2049_SPPL) and the Ethics committee of the Université du Québec à Rimouski (CÉR-112-849). Written informed consent for participation was not required for this study in accordance with the national legislation and the institutional requirements.
Author contributions
CL and CS conceived the research protocol and the analyses. LR carried out the statistical analyses. AO, CL, CS, and VM interpreted the results and prepared the tables and figures. CL and IT took the lead in writing the manuscript. All authors provided critical feedback and contributed to the first draft discussion. All authors approved the submitted version of the manuscript.
Funding
This work was supported by the Réseau québécois de recherche sur les médicaments (RQRM) though the RQRM-Pharmacoépidémiologie 2020 grant (CL and CS as principal investigators). CS was the recipient of a Junior 2 salary award from the Fonds the recheche du Québec–Santé.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fphar.2023.1080073/full#supplementary-material
SUPPLEMENTAL FIGURE S1 | Graphical representation of the combined deprivation index. The combined deprivation index combines social and material deprivation into a grouped deprivation index. Green: most deprived; Yellow: deprived; Blue: mostly socially deprived Orange: mostly materially deprived; Purple: least deprived.
References
Abosi, O., Lopes, S., Schmitz, S., and Fiedorowicz, J. G. (2018). Cardiometabolic effects of psychotropic medications. Horm. Mol. Biol. Clin. Investig. 36 (1). doi:10.1515/hmbci-2017-0065
Allison, D. B., Newcomer, J. W., Dunn, A. L., Blumenthal, J. A., Fabricatore, A. N., Daumit, G. L., et al. (2009). Obesity among those with mental disorders: A national Institute of mental health meeting report. Am. J. Prev. Med. 36 (4), 341–350. doi:10.1016/j.amepre.2008.11.020
Annamalai, A., Kosir, U., and Tek, C. (2017). Prevalence of obesity and diabetes in patients with schizophrenia. World J. Diabetes 8 (8), 390–396. doi:10.4239/wjd.v8.i8.390
Areas for Further Research in Individuals With Schizophrenia (2021). The American psychiatric association practice guidelines for the treatment of patients with schizophrenia. Third Edition. Washington, DC: American Psychiatric Association.
Baandrup, L. (2020). Polypharmacy in schizophrenia. Basic Clin. Pharmacol. Toxicol. 126 (3), 183–192. doi:10.1111/bcpt.13384
Blais, C., Jean, S., Sirois, C., Rochette, L., Plante, C., Larocque, I., et al. (2014). Quebec integrated chronic disease surveillance system (QICDSS), an innovative approach. Chronic Dis. Inj. Can. 34 (4), 226–235. doi:10.24095/hpcdp.34.4.06
Buchanan, R. W., Kreyenbuhl, J., Kelly, D. L., Noel, J. M., Boggs, D. L., Fischer, B. A., et al. (2010). The 2009 schizophrenia PORT psychopharmacological treatment recommendations and summary statements. Schizophr. Bull. 36 (1), 71–93. doi:10.1093/schbul/sbp116
Campeau Calfat, A., Simard, M., Ouali, A., Blais, C., and Sirois, C. (2022). Polypharmacy among older individuals with heart failure: Trends between 2000 and 2017 in the province of Quebec, Canada. Ther. Adv. Cardiovasc Dis. 16, 17539447221113946. doi:10.1177/17539447221113946
Charlson, F. J., Ferrari, A. J., Santomauro, D. F., Diminic, S., Stockings, E., Scott, J. G., et al. (2018). Global epidemiology and burden of schizophrenia: Findings from the global burden of disease study 2016. Schizophr. Bull. 44 (6), 1195–1203. doi:10.1093/schbul/sby058
Davies, L. E., Spiers, G., Kingston, A., Todd, A., Adamson, J., and Hanratty, B. (2020). Adverse outcomes of polypharmacy in older people: Systematic review of reviews. J. Am. Med. Dir. Assoc. 21 (2), 181–187. doi:10.1016/j.jamda.2019.10.022
De Berardis, D., Rapini, G., Olivieri, L., Di Nicola, D., Tomasetti, C., Valchera, A., et al. (2018). Safety of antipsychotics for the treatment of schizophrenia: A focus on the adverse effects of clozapine. Ther. Adv. Drug Saf. 9 (5), 237–256. doi:10.1177/2042098618756261
de Silva, V. A., Suraweera, C., Ratnatunga, S. S., Dayabandara, M., Wanniarachchi, N., and Hanwella, R. (2016). Metformin in prevention and treatment of antipsychotic induced weight gain: A systematic review and meta-analysis. BMC Psychiatry 16 (1), 341. doi:10.1186/s12888-016-1049-5
Dussias, P., Kalali, A. H., and Citrome, L. (2010). Polypharmacy of schizophrenia. Psychiatry (Edgmont) 7 (8), 17–19.
Fleischhacker, W. W., Arango, C., Arteel, P., Barnes, T. R., Carpenter, W., Duckworth, K., et al. (2014). Schizophrenia-time to commit to policy change. Schizophr. Bull. 40, S165–S194. doi:10.1093/schbul/sbu006
Franchi, C., Ardoino, I., Ludergnani, M., Cukay, G., Merlino, L., and Nobili, A. (2021). Medication adherence in community-dwelling older people exposed to chronic polypharmacy. J. Epidemiol. Community Health 75 (9), 854–859. doi:10.1136/jech-2020-214238
Francke, D. E. (1963). Uses of AHFS classification system. Am. J. Hosp. Pharm. 20 (3), 119–120. doi:10.1093/ajhp/20.3.119
Gaudiano, B. A., Guzman Holst, C., Morena, A., Reeves, L. E., Sydnor, V. J., Epstein-Lubow, G., et al. (2018). Complex polypharmacy in patients with schizophrenia-spectrum disorders before a psychiatric hospitalization: Prescribing patterns and associated clinical features. J. Clin. Psychopharmacol. 38 (3), 180–187. doi:10.1097/JCP.0000000000000876
Gontijo Guerra, S., Berbiche, D., and Vasiliadis, H. M. (2019). Measuring multimorbidity in older adults: Comparing different data sources. BMC Geriatr. 19 (1), 166. doi:10.1186/s12877-019-1173-4
Gosselin, E., Simard, M., Lunghi, C., and Sirois, C. (2022). Trends in benzodiazepine and alternative hypnotic use in relation with multimorbidity among older adults in Quebec, Canada. Pharmacoepidemiol Drug Saf. 31 (3), 322–333. doi:10.1002/pds.5383
Grigg, J., Worsley, R., Thew, C., Gurvich, C., Thomas, N., and Kulkarni, J. (2017). Antipsychotic-induced hyperprolactinemia: Synthesis of world-wide guidelines and integrated recommendations for assessment, management and future research. Psychopharmacol. Berl. 234 (22), 3279–3297. doi:10.1007/s00213-017-4730-6
Heald, A., Livingston, M., Yung, A., and De Hert, M. A. (2017). Prescribing in schizophrenia and psychosis: Increasing polypharmacy over time. Hum. Psychopharmacol. 32 (2), e2579. doi:10.1002/hup.2579
Huo, L., Lu, X., Wu, F., Huang, X., Ning, Y., and Zhang, X. Y. (2021). Diabetes in late-life schizophrenia: Prevalence, factors, and association with clinical symptoms. J. Psychiatr. Res. 132, 44–49. doi:10.1016/j.jpsychires.2020.09.026
Jeon, S. W., and Kim, Y. K. (2017). Unresolved issues for utilization of atypical antipsychotics in schizophrenia: Antipsychotic polypharmacy and metabolic syndrome. Int. J. Mol. Sci. 18 (10), 2174. doi:10.3390/ijms18102174
Keepers, N., Servis, M., Young, A., Anzia, J., Buckley, P., Lyness, J., et al. (2020). The American psychiatric association practice guideline for the treatment of patients with schizophrenia. Am. J. Psychiatry 177, 868. doi:10.1176/appi.ajp.2020.177901
Kojima, T., Mizokami, F., and Akishita, M. (2020). Geriatric management of older patients with multimorbidity. Geriatr. Gerontol. Int. 20 (12), 1105–1111. doi:10.1111/ggi.14065
Li, Y., Zhang, X., Yang, L., Yang, Y., Qiao, G., Lu, C., et al. (2022). Association between polypharmacy and mortality in the older adults: A systematic review and meta-analysis. Archives Gerontology Geriatrics 100, 104630. doi:10.1016/j.archger.2022.104630
Lin, S. K. (2020). Antipsychotic polypharmacy: A dirty little secret or a fashion? Int. J. Neuropsychopharmacol. 23 (2), 125–131. doi:10.1093/ijnp/pyz068
Mamakou, V., Thanopoulou, A., Gonidakis, F., Tentolouris, N., and Kontaxakis, V. (2018). Schizophrenia and type 2 diabetes mellitus. Psychiatriki 29 (1), 64–73. doi:10.22365/jpsych.2018.291.64
Marder, S. R., and Cannon, T. D. (2019). Schizophrenia. N. Engl. J. Med. 381 (18), 1753–1761. doi:10.1056/NEJMra1808803
Meszaros, Z. S., Dimmock, J. A., Ploutz-Snyder, R., Chauhan, S. V. S., Abdul-Malak, Y., Middleton, F. A., et al. (2011). Accuracy of self-reported medical problems in patients with alcohol dependence and co-occurring schizophrenia or schizoaffective disorder. Schizophrenia Res. 132 (2), 190–193. doi:10.1016/j.schres.2011.07.033
Mitchell, A. J., Vancampfort, D., Sweers, K., van Winkel, R., Yu, W., and De Hert, M. (2013). Prevalence of metabolic syndrome and metabolic abnormalities in schizophrenia and related disorders-a systematic review and meta-analysis. Schizophr. Bull. 39 (2), 306–318. doi:10.1093/schbul/sbr148
Myles, N., Myles, H., Clark, S. R., Bird, R., and Siskind, D. (2017). Use of granulocyte-colony stimulating factor to prevent recurrent clozapine-induced neutropenia on drug rechallenge: A systematic review of the literature and clinical recommendations. Aust. N. Z. J. Psychiatry 51 (10), 980–989. doi:10.1177/0004867417720516
Nguyen, T. T., Eyler, L. T., and Jeste, D. V. (2018). Systemic biomarkers of accelerated aging in schizophrenia: A critical review and future directions. Schizophr. Bull. 44 (2), 398–408. doi:10.1093/schbul/sbx069
Oktora, M. P., Alfian, S. D., Bos, H. J., Schuiling-Veninga, C. C. M., Taxis, K., Hak, E., et al. (2021). Trends in polypharmacy and potentially inappropriate medication (PIM) in older and middle-aged people treated for diabetes. Br. J. Clin. Pharmacol. 87 (7), 2807–2817. doi:10.1111/bcp.14685
Pampalon, R., Hamel, D., and Gamache, P. (2009). A comparison of individual and area-based socio-economic data for monitoring social inequalities in health. Health Rep. 20 (4), 85–94.
Paudel, S., Vyas, C. M., and Stern, T. A. (2020). A prescription for deprescribing antipsychotics: Managing polypharmacy in schizophrenia. Prim. Care Companion CNS Disord. 22 (6), 20f02708. doi:10.4088/PCC.20f02708
Remington, G., Addington, D., Honer, W., Ismail, Z., Raedler, T., and Teehan, M. (2017). Guidelines for the pharmacotherapy of schizophrenia in adults. Can. J. Psychiatry 62 (9), 604–616. doi:10.1177/0706743717720448
Saha, S., Chant, D., Welham, J., and McGrath, J. (2005). A systematic review of the prevalence of schizophrenia. PLoS Med. 2 (5), e141. doi:10.1371/journal.pmed.0020141
Simard, M., Sirois, C., and Candas, B. (2018). Validation of the combined comorbidity index of Charlson and elixhauser to predict 30-day mortality across ICD-9 and ICD-10. Med. Care 56 (5), 441–447. doi:10.1097/MLR.0000000000000905
Sirois, C., Boiteau, V., Chiu, Y., Gilca, R., and Simard, M. (2022). Exploring the associations between polypharmacy and COVID-19-related hospitalisations and deaths: A population-based cohort study among older adults in Quebec, Canada. BMJ Open 12 (3), e060295. doi:10.1136/bmjopen-2021-060295
Sirois, C., Domingues, N. S., Laroche, M. L., Zongo, A., Lunghi, C., Guenette, L., et al. (2019). Polypharmacy definitions for multimorbid older adults need stronger foundations to Guide research, clinical practice and public health. Pharm. (Basel) 7 (3), 126. doi:10.3390/pharmacy7030126
Sirois, C., Ouali, A., and Simard, M. (2019). Polypharmacy among older individuals with COPD: Trends between 2000 and 2015 in Quebec, Canada. Copd 16 (3-4), 234–239. doi:10.1080/15412555.2019.1646716
Stroup, T. S., Olfson, M., Huang, C., Wall, M. M., Goldberg, T., Devanand, D. P., et al. (2021). Age-specific prevalence and incidence of dementia diagnoses among older US adults with schizophrenia. JAMA Psychiatry 78, 632–641. doi:10.1001/jamapsychiatry.2021.0042
Stummer, L., Markovic, M., and Maroney, M. E. (2018). Pharmacologic treatment options for insomnia in patients with schizophrenia. Med. (Basel) 5 (3), 88. doi:10.3390/medicines5030088
Viola, R., Csukonyi, K., Doro, P., Janka, Z., and Soos, G. (2004). Reasons for polypharmacy among psychiatric patients. Pharm. World Sci. 26 (3), 143–147. doi:10.1023/b:phar.0000026800.13888.b0
Zaninotto, P., Huang, Y. T., Di Gessa, G., Abell, J., Lassale, C., and Steptoe, A. (2020). Polypharmacy is a risk factor for hospital admission due to a fall: Evidence from the English longitudinal study of ageing. BMC Public Health 20 (1), 1804. doi:10.1186/s12889-020-09920-x
Keywords: polypharmacy, drug utilization, administrative databases, trends, older adults, elderly, schizophrenia
Citation: Lunghi C, Rochette L, Massamba V, Tardif I, Ouali A and Sirois C (2023) Psychiatric and non-psychiatric polypharmacy among older adults with schizophrenia: Trends from a population-based study between 2000 and 2016. Front. Pharmacol. 14:1080073. doi: 10.3389/fphar.2023.1080073
Received: 25 October 2022; Accepted: 20 January 2023;
Published: 07 February 2023.
Edited by:
Brian Godman, University of Strathclyde, United KingdomReviewed by:
Qingqing Xu, Shanghai Jiao Tong University, ChinaAngelo Barbato, Mario Negri Pharmacological Research Institute (IRCCS), Italy
Copyright © 2023 Lunghi, Rochette, Massamba, Tardif, Ouali and Sirois. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Carlotta Lunghi, carlotta_lunghi@uqar.ca, carlotta.lunghi@unibo.it
 Carlotta Lunghi
Carlotta Lunghi Louis Rochette4
Louis Rochette4 Isabelle Tardif
Isabelle Tardif Caroline Sirois
Caroline Sirois