- 1Center of Physiology and Pharmacology, Medical University of Vienna, Vienna, Austria
- 21st Department of Pathology and Experimental Cancer Research, Semmelweis University, Budapest, Hungary
A fundamental subdivision of nociceptive sensory neurons is named after their unique sensitivity to capsaicin, the pungent ingredient in hot chili peppers: these are the capsaicin-sensitive afferents. The initial excitation by capsaicin of these neurons manifested as burning pain sensation is followed by a lasting refractory state, traditionally referred to as “capsaicin desensitization,” during which the previously excited neurons are unresponsive not only to capsaicin but a variety of unrelated stimuli including noxious heat. The long sought-after capsaicin receptor, now known as TRPV1 (transient receptor potential cation channel, subfamily V member 1), was cloned more than two decades ago. The substantial reduction of the inflammatory phenotype of Trpv1 knockout mice has spurred extensive efforts in the pharmaceutical industry to develop small molecule TRPV1 antagonists. However, adverse effects, most importantly hyperthermia and burn injuries, have so far prevented any compounds from progressing beyond Phase 2. There is increasing evidence that these limitations can be at least partially overcome by approaches outside of the mainstream pharmaceutical development, providing novel therapeutic options through TRPV1. Although ablation of the whole TRPV1-expressing nerve population by high dose capsaicin, or more selectively by intersectional genetics, has allowed researchers to investigate the functions of capsaicin-sensitive afferents in health and disease, several “mysteries” remain unsolved to date, including the molecular underpinnings of “capsaicin desensitization,” and the exact role these nerves play in thermoregulation and heat sensation. This review tries to shed some light on these capsaicin mechanisms.
Capsaicin as a Tool Before TRPV1 Was Discovered
Natural products like capsaicin afford a unique tool to dissect important physiological pathways. The recognition that consuming the fruits of capsicum plants evokes a characteristic “hot” burning sensation in the human tongue and oral mucosa is probably as old as the domestication and cultivation of these plants (going back to 8,000 years in South America) (Perry et al., 2007). And here is the first great “mystery” of capsaicin-sensitive afferents: how come that the very same pungent sensation that repels herbivores (like deer) from eating the capsicum pods is found pleasurable by so many humans? Recently, experts tried to solve this puzzle in the journal Temperature with such fascinating explanations like the cooling effect of spicy food (capsaicin as “natural air-conditioner”), the food-preserving, anti-microbial action of capsicum (capsaicin as “refrigerator”), or simply the “masochism” of chili-lovers (Szallasi, 2016). Nonetheless, the human fondness of, or aversion to hot pepper is probably far more complex than these models imply (Byrnes and Hayes, 2013).
From Capsicum, capsaicin was first isolated in 1846 (Thresh, 1846), and its chemical structure determined in 1919 (Nelson, 1919). In 1912, Wilbur Scoville invented his human tongue-based scale to measure the “hotness” of pepper extracts (Gmyrek, 2013; Sweat et al., 2016). Ever since, capsaicin has remained a reference agonist in sensory pharmacology (Szolcsányi, 1984; O’Neill et al., 2012). The early capsaicin findings were detailed elsewhere (Szolcsányi, 1984, 2004) and only the most important milestones are listed here.
The effect of capsaicin on thermoregulation was first noted 150 years ago when hot pepper extract applied to the stomach of dogs produced a fall in rectal temperature (Högyes, 1878). With regard to pain and inflammation, Nicholas (Miklós) Jancsó made the astute observation that capsaicin evoked strong and persistent “desensitization” after exposure to the rat cornea, skin, and airways (Jancsó and Jancsó, 1949; Jancsó et al., 1967).
Capsaicin research gathered speed in the 1970s. Electrical nerve stimulation was shown to cause neurogenic inflammation, and this could be ablated by prior high dose capsaicin exposure. Similarly, responsiveness to mustard oil or cigarette smoke (both turned out to activate TRPA1) or the sodium channel modulator veratridine was reduced. Since these substances only partially cross-desensitize, this indicated a prolonged silencing of the capsaicin-sensitive neurons, and an overlap with the respective receptor populations (Szolcsányi et al., 1975). In contrast, mechanical sensitivity was largely untouched by high concentrations of capsaicin. In the 1980s, researchers acquired important new tools to study capsaicin-sensitive afferents, including ruthenium red as the first (though non-selective) capsaicin antagonist (Maggi et al., 1988), capsazepine as the first synthetic and somewhat selective capsaicin antagonist (Urban and Dray, 1991), and resiniferatoxin, an ultrapotent capsaicin analog with a unique spectrum of actions (Szallasi and Blumberg, 1989).
Much of our early knowledge of capsaicin-sensitive pathways came from the desensitization experiments. It cannot be exphasized enough that the literature uses the term “capsaicin desensitization” loosely, in an ill-defined manner. By “desensitization”, some investigators mean a fully reversible capsaicin-induced refractory state, whereas others use it more broadly to include irreversible changes due to neuronal death (Szallasi and Blumberg, 1999).
It is not clear whether the reversible and irreversible refractoriness following capsaicin (or resiniferatoxin) administration reflect quantitative or qualitative differences. For example, in the human neurogenic bladder, topical resiniferatoxin induces a long lasting (up to several months), but fully reversible, increase in the cystoscopic volume at which the voiding urge is activated (Cruz et al., 1997), without causing any noticeable changes in the bladder biopsies at the light or electron microscopic level (Silva et al., 2001). By contrast, resiniferatoxin applied to the bodies of capsaicin-sensitive neurons causes irreversible changes by selectively ablating these cells via Ca2+ influx-mediated cytotoxicity (Karai L. et al., 2004).
Using resiniferatoxin as a “molecular scalpel” to achieve permanent analgesia has a clear therapeutic potential (Iadarola and Gonnella, 2013). Indeed, intrathecal resiniferatoxin is already undergoing clinical trials in severe osteoarthritic pain (www.clinicaltrials.gov, NCT 04044742 Sorrento Therapeutics Inc., 2020), and in cancer patients with chronic intractable pain (www.clinicaltrials.gov, NCT 00804154, 2020). Furthermore, a site-specific (intraarticular) trans-capsaicin (CNTX-4975) injection has shown promise in osteoarthritis knee pain (Stevens et al., 2019); the results of two on-going phase 3 trials are expected soon.
Calcium is clearly a key player in capsaicin actions (Wood et al., 1988; Bevan and Szolcsányi, 1990). Calcium overload also underlies both desensitization and neurotoxicity by resiniferatoxin (Olah et al., 2001). Of note, capsaicin and resiniferatoxin differ in the kinetics of the Ca2+ influx that they evoke: the current is fast and rapidly normalizing for capsaicin, whereas it is sustained and long-lasting for resiniferatoxin (Szallasi et al., 1999). This difference is so striking that it even led to the proposal (later discredited by the cloning of the vanilloid receptor, TRPV1) of distinct vanilloid receptors mediating capsaicin (C-type) and resiniferatoxin (R-type) actions, respectively (Bíró et al., 1998). One may argue that there is a fine and ill-defined line that separates reversible desensitization from irreversible toxicity. This may involve the route of application (peripheral nerve terminal versus cell body), the kinetics of Ca2+ influx, the phosphorylation state of the receptor protein, as well as other, as yet unidentified, mechanisms.
In rat models of chronic neuropathic pain, there appears to be a genetic reprogramming in injured nociceptive neurons in which pain-promoting mechanisms are up-regulated: it was referred to as messenger plasticity (Hökfelt et al., 1994). Interestingly, resiniferatoxin administration was found to change the phenotype of sensory neurons the opposite way, by down-regulating the expression of substances know to promote pain (e.g., substance P), and by up-regulating endogenous pain-countering compounds, like galanin (Szallasi, 1996). Importantly, resiniferatoxin also blocked the neuronal synthesis of its own receptor. These resiniferatoxin-induced neurochemical changes, collectively referred to as “vanilloid-induced messenger plasticity,” were fully reversible, and their recovery coincided with the return of pain sensitivity (Szallasi and Blumberg, 1999).
Early experiments already showed that acute systemic exposure to capsaicin reduced body temperature (hypothermia), and, after ablation of the sensitive neurons, it rendered animals unable of behavior saving themselves from overheating (hyperthermia) (Jancsó-Gábor et al., 1970). These observations could have served as an early warning of the thermoregulatory side-effects of TRPV1 antagonists that, somehow, had to be rediscovered later. It should be mentioned here that the undesirable effects on the body temperature have been minimized in the second generation TRPV1 antagonists (Gomtsyan et al., 2015), but whether this also reduced potential therapeutic uses in parallel is not yet known. Site and mechanism of the body temperature regulation fall beyond the scope of this review and are discussed elsewhere (Garami et al., 2018).
In the 1970s, based on the fairly strict structure-activity-requirements for capsaicin-like activity, Szolcsányi and coworkers postulated the existence of a specific capsaicin receptor (Szolcsányi and Jancsó-Gábor, 1975). In 1990, specific binding of resiniferatoxin provided the first biochemical proof for the existence of this receptor, called the vanilloid receptor VR1 (Szallasi and Blumberg, 1990), and [3H]resiniferatoxin autoradiography was used to visualize the expression of this receptor in several species, including man (Szallasi, 1995). In patch-clamped Xenopus oocytes, a capsaicin-induced current was observed following the injection of RNA extracted from rat sensory neurons (Szallasi, unpublished observations). With this, the hunt for the capsaicin receptor was on.
The Discovery of TRPV1
In 1997, the laboratory of David Julius was the first to identify the rat capsaicin (vanilloid) receptor via an expression cloning strategy that took advantage of the Ca2+ conductance (Caterina et al., 1997). The human isoform showed largely similar properties (Hayes et al., 2000). The availability of a plasmid led to rapid characterisation of the properties of the receptor protein, including pharmacological and biophysical properties. Importantly, the capsaicin receptor turned out to be a transient receptor potential (TRP) channel. Within the TRP superfamily, the capsaicin receptor as TRPV1 is the founding member of the now populous TRPV (vanilloid) subdivision, TRPV1 to TRPV6 (Clapham, 2003).
Regarding the “transient nature” of TRP channels, it is really a misnomer explained by the history of this naming convention. In 1969, a drosophila eye mutant labeled trp responded to lasting light stimulation with a transient depolarizing after-potential instead of the normal prolonged response (Cosens and Manning, 1969). The respective wild-type trp gene was isolated and could rescue this phenotype (Montell et al., 1985). So, the wild-type channel in fact causes a persistent (and not transient) current; nevertheless, this ion channel family now bears the name “transient”. This is in contrast to many ion channels, which fully adapt when exposed to constant stimulation, and is important to continuously code pain for a persistent stimulus.
Subsequently, the Julius laboratory generated and characterized the Trpv1 knockout mouse, which misses exon 13 that codes mainly for the pore loop and transmembrane domain 6. These animals looked normal, but lacked responses to capsaicin, showed normal responses to noxious mechanical stimuli, and expressed minimal inflammatory thermal hyperalgesia (Caterina et al., 2000). This phenotype was consistent with that of the capsaicin-desensitized rodents and rendered TRPV1 immediately as an attractive pharmacological target. In addition, which could have been an early warning of the burn injury in patients on TRPV1 antagonists, there was a reduced pain-related behavior to noxious heat, in particular at higher temperatures (Caterina et al., 2000). A separately generated Trpv1 knockout mouse led to similar results (Davis et al., 2000).
Moreover, the crystal structure of the TRPV1 protein was largely solved by cryo-electron microscopy (Liao et al., 2013), including an open conformation (Cao et al., 2013) and the transmembrane part in a chemically more native lipid nanodisc environment (Gao et al., 2016).
TRPV1 as a Sensor
TRPV1 is multimodally-gated channel, activated in concert by both physical and chemical stimuli. Importantly, from a “native state”, the channel’s sensitivity can be substantially increased by chemical modification, e.g., phosphorylation (Numazaki et al., 2002; Szallasi et al., 2007; Utreras et al., 2013; Nagy et al., 2014). Consequently, the adjustable working range of the responsiveness is surprisingly broad. Furthermore, different modes of activation can act in an additive or supraadditive fashion, and thus subthreshold stimuli acting together may reach the activation threshold.
TRPV1 is activated by heat (Caterina et al., 1997) with a threshold of just above 40 °C (Zhang et al., 2018): this is not far from the human heat pain threshold of about 40 °C (Yarnitsky et al., 1995; Rolke et al., 2006). The ion channel pore domain is responsible for the sensitivity to heat (Zhang et al., 2018). TRPV1 is also activated by acidic pH (Tominaga et al., 1998) with good concentration-dependent coding below pH 6. The biophysics of this activation along with the amino acid residues involved were detailed elsewhere (Jordt et al., 2000; Ryu et al., 2007; Aneiros et al., 2011). The proton-activation of TRPV1 is complicated by the proton-induced inhibition, which applies to most currents, including proton-activated ones (Fischer et al., 2003; Lee and Zheng, 2015).
Natural pharmacological agonists of TRPV1 include both pungent plant products (e.g., capsaicin, piperine and resiniferatoxin) and painful animal venoms and toxins (from spiders, scorpions, centipedes, snakes, and jelly fish, etc.) (Geron et al., 2017; Chu et al., 2020). In addition to capsaicin, chili peppers contain other less pungent compounds like capsiate, summarized under the term “capsinoids”. Of note, pungent compounds occur in various plant-derived spices like red and black pepper, mustard, horse radish, and wasabi. Although these spices taste similarly, their active ingredients act on different molecular targets, primarily TRPV1 and TRPA1.
The existence of endogenous TRPV1 activators (so-called “endovanilloids”) with physiological or pathophysiological relevance remains putative. Although several endogenous lipids, e.g., anandamide (Zygmunt et al., 1999) and other acylethanolamines (Brito et al., 2014), were reported to activate TRPV1 in vitro, this activation was observed at such high concentrations that are unlikely to occur in vivo. This is also true for lipid oxidation products (Hwang et al., 2000).
Voltage-dependent gating of TRP ion channels was first shown for TRPM4 (Launay et al., 2002). Voltage also seems to be a cooperative factor in the gating of TRPV1 (Voets et al., 2004). Osmotic activation of TRPV1 was also reported, but this has not been reproduced by others (Nishihara et al., 2011). TRPV1 is regulated by phospholipids, but the details are a controversial issue which was critically reviewed elsewhere (Rohacs, 2015).
Inflammatory sensitisation of TRPV1 is an important mechanism of on-going pain (Fischer et al., 2010; Malek et al., 2015). A fast and large degree of sensitisation can be achieved by phosphorylation of the TRPV1 protein, more so via protein kinase C than protein kinase A (Premkumar and Ahern, 2000; Vellani et al., 2001; Bhave et al., 2002; Mandadi et al., 2006; Fischer and Reeh, 2007; Wang et al., 2015). Furthermore, substances generated under inflammatory conditions (e.g., nerve growth factor) may either regulate TRPV1 expression (Amaya et al., 2004; Chu et al., 2011) and or act more directly (Zhang et al., 2005).
The substantial variation in the exact thermal activation threshold of TRPV1 is due to its phosphorylation state (Sugiura et al., 2002; Huang et al., 2006; Li et al., 2014). Conformational changes (sensitisation) by phosphorylation bring the channel closer to its activation state, thereby lowering the activation threshold for agonists. This concept applies not only to chemical agonists but to all modes of activation, including temperature and voltage. Protons and/or capsaicin can act with temperature in a supraadditive fashion (Jordt et al., 2000; Kichko and Reeh, 2004), and this also applies to voltage (Voets et al., 2004). The extent of channel activation by a specific agonist-binding site depends on the agonist, with a partial agonist showing less shift and acting antagonistic to a full agonist. Indeed, this expected phenomenon was demonstrated for iodo-resiniferatoxin (Shimizu et al., 2005). Less expected was the different number of agonist-bound sites for activation: using concatemers with inactivated capsaicin or proton binding sites, it was shown that one capsaicin is sufficient to activate TRPV1, but the same response needs four protons, indicating an agonist-site dependent shift toward activation (Hazan et al., 2015).
Since increased TRPV1 sensitivity might primarily occur as part of local pathology (e.g., local inflammation) (Malek et al., 2015), in contrast to addressing TRPV1 directly (Moran and Szallasi, 2018), selectively targeting sensitisation without affecting the native channel has been investigated as a novel therapeutic approach (Btesh et al., 2013; Fischer et al., 2013; Hanack et al., 2015; Sondermann et al., 2019). It is hoped that side effects that plagued the use of per os TRPV1 antagonists can be avoided by this approach.
Continuous or frequently repeated TRPV1 stimulation leads to receptor tachyphylaxis that should be clearly distinguished from capsaicin-induced defunctionalisation of the whole sensory neuron. Tachyphylaxis depends on Ca2+ influx, dephosphorylation (Docherty et al., 1996; Mohapatra and Nau, 2005), and association with protein complexes (Por et al., 2013); combined, these effects lead to decreased TRPV1 presence in the plasma membrane. When the Ca2+ influx is terminated, the channel is recycled to the plasma membrane from the intracellular depots by shuttle molecules like synaptotagmin 1 (Tian et al., 2019). Of note, a fraction of internalized TRPV1 gets degraded (Sanz-Salvador et al., 2012). Similar to cooperative activation, desensitization by different modalities is also at least partially convergent; for example, desensitization by heat also renders TRPV1 less sensitive to capsaicin (Sánchez-Moreno et al., 2018).
TRPV1 expression is found through the animal kingdom. Species-related differences in TRPV1 function allow a molecular dissection of ion channel biophysics. The Bactrian camel (Camelus ferus) and the thirteen-lined ground squirrel (Ictidomys tridecemlineatus) both show reduced sensitivity to heat, resisting activation of TRPV1 until 46°C (Laursen et al., 2016). Similarly, chicken (Gallus gallus) TRPV1 has an increased activation threshold of around 46°C, but is additionally insensitive to capsaicin (Jordt and Julius, 2002). At the other end of the thermal scale, there are species that have developed a high sensitivity to thermal stimuli such as the axolotl (Ambystoma mexicanum) or zebrafish (Danio rerio) that have TRPV1 activation thresholds of ∼31°C (Hori and Saitoh, 2020) and ∼33 °C (Gau et al., 2013), respectively. The platypus (Ornithorhynchus anatinus) exhibits a lack of heat induced desensitization of TRPV1 in the context of normal heat activation thresholds (Luo et al., 2019), rendering it more susceptible than more evolved species to heat induced damage. Here, the platypus TRPV1 helped to dissect the heat-induced desensitization in mouse TRPV1, where an interaction between the C- and N-termini leads to the rearrangement of the outer pore domain (Luo et al., 2019). In contrast to other modes of activation like heat, capsaicin sensitivity is more evolutionary conserved, notable exceptions being the avian TRPV1 (Jordt and Julius, 2002) and the tree shrew (Tupaia belangeri chinensis), the latter with an EC50 of 1.9 mM (Han et al., 2018). Medicinal leech (Hirudo medicinalis), clawed frog (Xenopus tropicalis), and rabbit (Oryctolagus cuniculus) TRPV1 all exhibit reduced capsaicin sensitivity, with EC50 values of 100, 85, and 15 μM, respectively (Gavva et al., 2004; Ohkita et al., 2012; Summers et al., 2014). Taken together, these observations imply that the acquisition of capsaicin sensitivity was an early event in evolution, and that birds lost their capsaicin sensitivity.
TRPV1 exerts its primary function in the plasma membrane as noxious signal integrator, although a large fraction is always in intracellular compartments with rapid cycling. Although the presence of TRPV1 in the endoplasmic reticulum is well-established (Turner et al., 2003; Gallego-Sandín et al., 2009), the functional relevance of this observation is unclear since the sensitivity of TRPV1 to capsaicin in the endoplasmic reticulum is about ∼100-fold lower than in the plasma membrane. Moreover, Ca2+ depletion of the endoplasmic reticulum poses substantial cellular stress which can lead to cell death, and this can be induced by higher capsaicin concentrations than required for TRPV1 activation at the plasma membrane (Karai L. J. et al., 2004).
In the mouse, two splice variants of TRPV1 (missing some exons) have been reported. These variants are not sensitive to capsaicin, but they interact with the full-length channel and act inhibitory in a concentration-dependent manner (Vos et al., 2006; Eilers et al., 2007). The physiological function of these negative variants remains unknown. Alternative splicing of TRPV1 in the trigeminal ganglia allows vampire bats (Desmodus rotundus) to detect infrared radiation; the respective short TRPV1 isoform has an activation threshold of about 30 °C (Gracheva et al., 2011), similar to how pit vipers and pythons use TRPA1 in their infrared sensing organs (Gracheva et al., 2010). Similar splicing occurs in cattle (Bos taurus), coast moles (Scapanus orarius), dogs (Canis lupus), and all members of the Laurasiatheria clade (a large group of placental mammals), highlighting a mechanism for physiological tuning of thermosensory nerve fibers (Gracheva et al., 2011).
The role of TRPV1 in body weight regulation also remains a mystery. One study reported that Trpv1 knockout mice remain lean on high-fat diet (Motter and Ahern, 2008), another study found no difference between the body weight of wild-type and Trpv1 knockout animals (Marshall et al., 2013), and a third study described a mouse which is lean and hyperactive when young, and lazy and fat when becomes old (Wanner et al., 2011).
The TRPV1-Positive Neuron Population
TRPV1 Expression
TRPV1 is expressed in a subpopulation of sensory afferents, primarily of the small to medium diameter (Szallasi, 2016). These are mainly slow-conducting, unmyelinated C-fibers, and a subpopulation of thin myelinated A-fibers (Mitchell et al., 2010, 2014; Blivis et al., 2017). The cell bodies of the neurons that give rise to these afferents are located in sensory (e.g., dorsal root and trigeminal) ganglia. These neurons transmit sensory information from the periphery to the dorsal horn of the spinal cord.
The fraction of TRPV1-expressing neurons in sensory ganglia depends on the species, as well as on the location. Expression data are mainly based on protein, mRNA, or reporters. In a study with direct comparison, TRPV1 was expressed in 37% of mouse and 47% of rat dorsal root ganglion (DRG) neurons (Orozco et al., 2001). There are systematic differences between laboratory mouse strains (Ono et al., 2015). A similar TRPV1 expression was found in trigeminal and visceral afferents (Helliwell et al., 1998). Interestingly, TRPV1 mRNA was found in 47% of rat DRG neurons with TRPA1 expression in a subpopulation of these and a mutually exclusive TRPM8 expression (Kobayashi et al., 2005). This was somewhat unexpected since TRPV1 is a heat sensor whereas TRPA1 (at least in some studies) and TRPM8 are both cold-responsive.
If TRPV1 is co-expressed with TRPA1, one has to assume that it has functional consequences. Indeed, functional interaction between these two channels was reported (Staruschenko et al., 2010), differential for neuronal subpopulations (Patil et al., 2020), and the properties of an enforced heteroconcatamer has been described (Fischer et al., 2014).
TRPV1 reporter mice allow a sensitive analysis of TRPV1 expression without dependency on antibody specificity. Reporting expression via LacZ, PLAP, or Cre-Lox systems all showed a robust TRPV1 presence in peripheral sensory neurons with good correlation to functional responses, but the percentage responding was not quantified (Wang et al., 2017). The extent of brain and extraneuronal TRPV1 expression is a controversial issue beyond the scope of this review (capsaicin-sensitive afferents), therefore the reader is referred to the literature (Mezey et al., 2000; Kauer and Gibson, 2009; Cavanaugh et al., 2011; Fernandes et al., 2012; Bevan et al., 2014; Martins et al., 2014).
TRPV1 is a useful marker of nociceptive neurons, but it is unclear what is common in these neurons beside their TRPV1 expression. Yes, TRPV1 activation can cause pain without any doubt, but there are neurons outside the TRPV1-positive populations which can transmit pain, and, conversely, there is no proof that every TRPV1-expressing neuron is nociceptive. Indeed, afferents expressing TRPV1 seem to serve distinct functions in different organs. For example, TRPV1-positive afferents in the pancreas has been implicated in the pathomechanism of diabetes; in the gastrointestinal system they were linked to thermoregulation; in the respiratory system their activation causes cough; and in the urinary bladder they alre involved in the micturition reflex (Moran et al., 2011). Another reflex pathway, the Bezold-Jarisch reflex (also known as the pulmonary chemoreflex) is also initiated by capsaicin-sensitive afferents (Harron and Kobinger, 1984). Even in the skin, TRPV1 contributes to multiple distinct sensations, as e.g., warmth and heat. The dual, sensory-afferent nature of these fibers further complicates the picture.
There is a partial overlap between TRPV1 expression and other commonly used markers in sensory pharmacology. Most, but not all, TRPV1-positive neurons are peptidergic, expressing calcitonin gene-related peptide (CGRP) and substance P (SP) (Holzer, 1988; Szallasi and Blumberg, 1999). In the mouse, peptidergic and isolectin B4-binding populations cover most sensory neurons and are mutually exclusive. However, despite clear differences in their spinal projection (Silverman and Kruger, 1990), ascribing a function to these two neurochemically distinct populations is less trivial. Therefore, these are often combined with TRPV1, which intersects differently with these populations in the mouse and rat (Dirajlal et al., 2003; Price and Flores, 2007).
With the advent of single-cell RNA sequencing, the sensory neuron populations can be clustered with high precision. Two studies analyzed mouse DRG neuron populations (Usoskin et al., 2015; Li et al., 2016) and a further study used murine trigeminal ganglia (Nguyen et al., 2017), but a synthesis of these is not trivial. Based on RNA profile, these studies described 10–17 subpopulations. In one study, TRPV1 was expressed in 6 of the 11 clusters and comprised 44% of all DRG neurons (Usoskin et al., 2015). In the second study, TRPV1 was detected in 8 of the 17 clusters, comprising 30% of all DRG neurons (Li et al., 2016). And in the third study, TRPV1 was found in 7 of the 13 clusters, comprising 50% of all trigeminal neurons (Nguyen et al., 2017). Human sensory neuron TRPV1 mRNA data have been compared to mouse (Ray et al., 2018). These data are from adult animals. In embryonic stage and in newborns, capsaicin-sensitive neurons are more widely distributed (Hjerling-Leffler et al., 2007).
Using RNA sequencing data, one may develop hypotheses about the TRPV1 responsiveness of populations which completely, partially or do not overlap with TRPV1 expression. For example, for MrgprA3 or TRPM8 there is little overlap with TRPV1, therefore at best minor changes are to be expected in response to TRPV1 activation after MrgprA3 lineage ablation. This is in line with experimental observations of an unchanged response to capsaicin (Han et al., 2013; Pogorzala et al., 2013). In contrast, NaV1.8 is largely overlapping, allowing to expect an absence of capsaicin response after NaV1.8 lineage ablation, which fits the experimental observation (Abrahamsen et al., 2008). Potential heterogeneity of the TRPV1-sensitive population can be further addressed by an intersectional approach, which eliminates a subfraction of these neurons. Ablation of CGRPα-expressing neurons reduced the time-spent licking after capsaicin injection to half, indicating that both the remaining as well as the ablated population contribute to pain-related behavior (McCoy and Zylka, 2014). Deletion of MrgprD eliminates primarily the non-peptidergic subfraction of neurons, comprising a small subset of the TRPV1-expressing neurons. Mice with MrgprD lineage ablation have been generated, but responsiveness to capsaicin has not been reported. This or a similar approach will allow in the future addressing, whether TRPV1-expressing neurons contain subpopulations, which equip the organism with distinct sensitivities.
Finally, RNA sequencing results allow an investigation of coexpressed proteins, whether they control TRPV1 expression, membrane presence and trafficking, and thereby facilitate or repress TRPV1 function.
Activation of TRPV1 in Sensory Neurons
Activation by capsaicin of TRPV1 causes a burning pain sensation in human skin and mucosa, the intensity and duration of which can be controlled over a wide range. The discovery that TRPV1 can also be activated by noxious heat provided a mechanistic explanation for the “burning” nature of capsaicin-evoked pain. Psychophysical experimentation with capsaicin in the human oral cavity was extensively used to study desensitization and change of temperature perception (Smutzer and Devassy, 2016). In fact, Szolcsányi and coworkers used the human tongue to establish structure-activity relations for capsaicin analogs (Szolcsányi and Jancsó-Gábor, 1975). The capsaicin threshold on the tongue is 0.15–1.0 μM (Rozin et al., 1981; Sizer and Harris, 1985).
Although the use of the human tongue as experimental model went out of favor, injection of capsaicin into the skin, first described in 1987, is still a broadly used human pain model (Simone et al., 1987). The concentration-dependence of intensity and duration of capsaicin-evoked pain is well-established (Simone et al., 1989). A frequent rating, a small volume of 50 μl and a low concentration of 3.2 μM capsaicin, which is close to the threshold of the originally described model, allows a reliable pain model that lasts about one-minute (Schwarz et al., 2017). A topical capsaicin cream can also be used as a pain model, for example to test analgesic actions in humans (Lee et al., 2019).
Intraplantar capsaicin injection is an established animal pain model, with observing pain-related aversive behavior as a readout. This model was extensively used to establish target occupation and on-target analgesia by TRPV1 antagonists (Li et al., 1999; Laird et al., 2001; Massaad et al., 2004; Gavva et al., 2005). Alternatively, TRPV1 activation can also be scrutinized using the eye-wiping test (Lee et al., 2001; Bates et al., 2010).
Capsaicin-induced pain is prevented by the TRPV1 antagonists, capsazepine and BCTC. The advent of capsazepine facilitated TRPV1 research, but given inhibition of voltage-gated calcium channels (Docherty et al., 1997) and activation of TRPA1 (Kistner et al., 2016), it cannot be considered state of the art. BCTC is a potent inhibitor of TRPV1 (Valenzano et al., 2003), but turned out also to inhibit TRPM8 (Behrendt et al., 2004). This is a more acceptable flaw for use in expression systems and also for sensory fibers, as there is little overlap in expression of TRPV1 and TRPM8, as discussed above. The use of these early compounds is supplanted by potent and highly selective TRPV1 antagonists (Aghazadeh Tabrizi et al., 2017; Moran and Szallasi, 2018).
The human capsaicin-induced pain model was used to demonstrate a local axon reflex flare, primary hyperalgesia and secondary hyperalgesia (Magerl et al., 1998; Serra et al., 1998). The cellular time course of tachyphylaxis is well reflected in responses to repetitive stimulation (Witting et al., 2000). The model appears suitable to test analgesics (Wang et al., 2008).
The response of human fibers to injection of capsaicin recorded by microneurography indicated the existence of two functional populations, a mechanosensitive and a mechano-insensitive (Schmelz et al., 2000; Serra et al., 2004), with the mechanosensitive population further differentiated into subpopulations in primates (Wooten et al., 2014). However, it is unclear how these functional subpopulations map into the neuronal subpopulations defined by RNA sequencing.
Intravenous capsaicin in humans exceeding 0.5 μg/kg caused a burning sensation in chest, face, rectum and extremities (Winning et al., 1986). The majority of orally consumed capsaicin is resorbed; the pharmacokinetic and further metabolism has been investigated (Chaiyasit et al., 2009). In oral consumption, the interaction with other gustatory and olfactory stimuli is interesting. The four primary taste qualities attenuated the effects of capsaicin, but capsaicin reduced only sweet, sour and bitter but not salty taste (Lawless and Stevens, 1984; Stevens and Lawless, 1986).
Ablation of the TRPV1-Sensitive Population
TRPV1 antagonists only block TRPV1, leaving the respective neurons functional and allowing these to be activated by a number of additional pain targets. By contrast, TRPV1 agonists like capsaicin desensitize the whole neuron, rendering it “silent” (Figure 1). This explains why the capsaicin desensitization experiments overestimated the analgesic potential of TRPV1 antagonists. Furthermore, as mentioned above, capsaicin “desensitization” can be reversible and permanent, with an ill-defined line between the two.
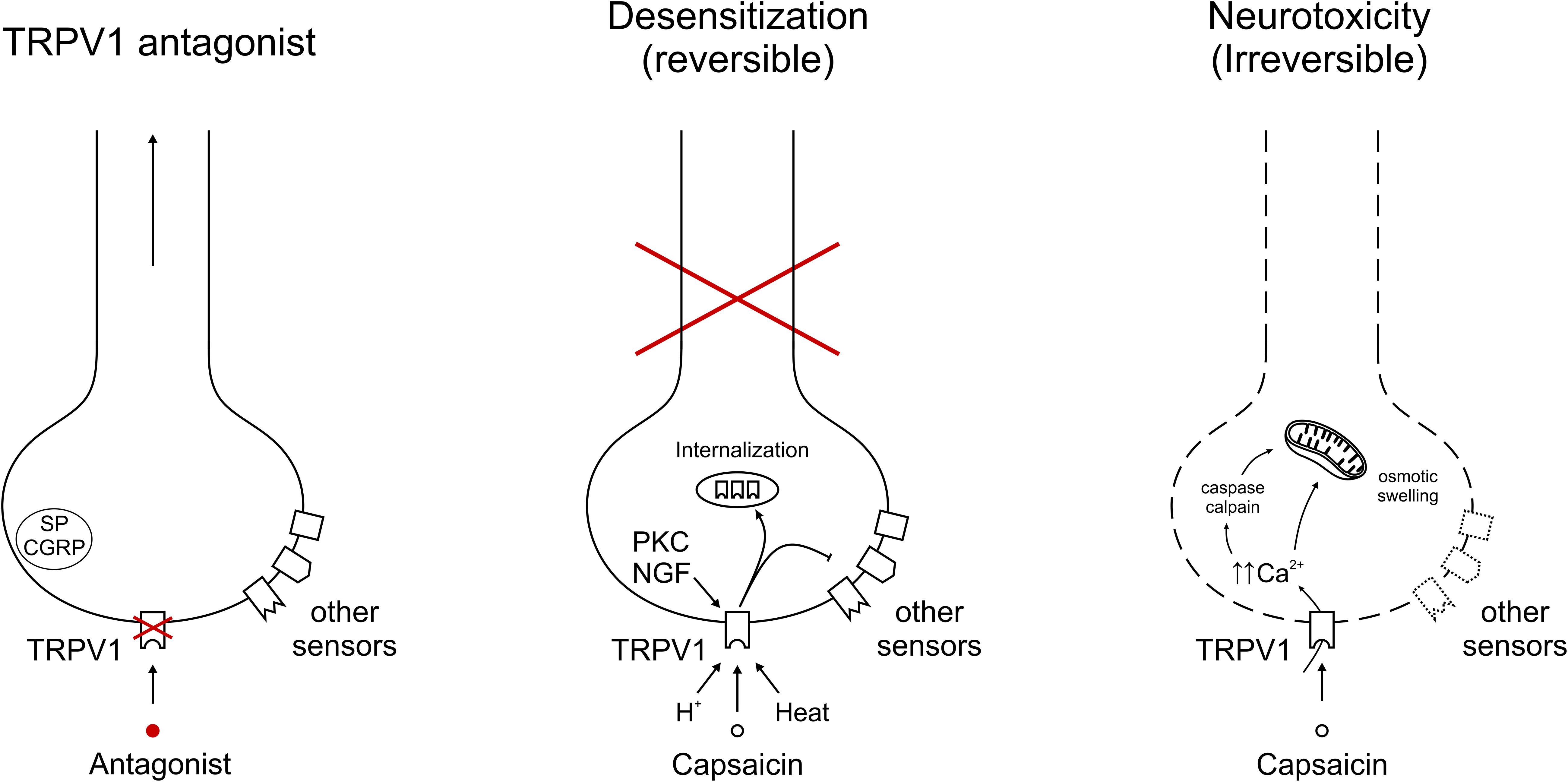
Figure 1. Representative depiction of TRPV1 modulation on afferent nerve endings. (Left) Specific TRPV1 antagonism blocks TRPV1-mediated signals, but allows transduction of the neuron through other sensors. Neuropeptides Substance P (SP) and calcitonin gene-related peptide (CGRP) indicate the potential to contribute to neurogenic inflammation. (Middle) Sustained and/or repeated TRPV1 gating leads to internalization of the receptor into intracellular stores, from which it is recycled or broken down. With capsaicin, heat or protons, exemplary TRPV1 agonists are depicted, in addition acute sensitization by PKC and longer-term expression regulation by NGF. The inhibitory arrow to other sensors indicates cross-desensitization of other sensors leading to a reversible inactivation of this neuron. (Right) TRPV1 activation leading to unsustainable intracellular calcium concentrations gives rise to irreversible damage. Mechanisms include cellular stress, e.g., through calcium, caspase and calpain activation.
There are multiple ways to achieve “capsaicin desensitization.” The traditional approach is injection of a TRPV1 agonist, in most studies capsaicin, with incrementally increasing doses (Jancsó and Jancsó-Gábor, 1959). The dose escalation can finally reach concentrations manyfold of the LD50 of naive animals. This is necessary because the therapeutic window of capsaicin is narrow, and using single doses allows only partial desensitization before respiratory depression by capsaicin kills the experimental animal (Palecek et al., 1989). Despite pulmonary dose-limiting side effects, systemic capsaicin administration leads to a critical drop of total peripheral resistance (Donnerer and Lembeck, 1982), which might be at least partially caused by the potent vasodilator CGRP released into the systemic circulation (Tang et al., 1997). The full capsaicin dose that is required for complete desensitization is usually given over a period of 5 days. In rats, desensitization can last up to 2–3 months (Jancsó et al., 1967). This is in keeping with the human experience using the topically administered high concentration capsaicin patch, Qutenza. Of note, resiniferatoxin has a much broader therapeutic window, and full desensitization can be achieved by means of a single s.c. injection (Szallasi and Blumberg, 1999).
In embryonic and in early neonatal stage, TRPV1 expression extends to a larger fraction of neurons (Hjerling-Leffler et al., 2007), therefore the fraction of capsaicin-ablated neurons is larger compared to adults (Ritter and Dinh, 1992), and there are even differences between prenatal and neonatal treatment (Perfumi and Sparapassi, 1999). The irreversible pharmacological ablation of a sensory neuronal subpopulation is still a unique and “mysterious” feature of capsaicin and its analogs.
The mechanisms underlying neurotoxicity by capsaicin remain unclear, since the so far identified mechanisms that are dependent on Ca2+ influx and activation of calpain and caspase are not particularly TRPV1-specific. Moreover, activation of other ion channels with high calcium conductance does not yield similar results. Adding to the confusion, neonatal capsaicin administration leads to widespread neuronal loss in the rat brain, interpreted by the investigators as hypoxic damage caused by the capsaicin-evoked respiratory arrest (Ritter and Dinh, 1992).
Ablation of the TRPV1-lineage is also possible by expressing the diphtheria toxin receptor as a cell death switch under the TRPV1 promoter. Injecting the diphtheria toxin in such animals allows ablation of the TRPV1-expressing neurons (Baral et al., 2018).
A further approach to investigate the TRPV1 lineage is a functional silencing, which has been described for the charged and membrane impermeable sodium channel blocker, QX314. Coadministration with a TRPV1 agonist allows selective uptake of QX314 through the ion channel pore of the TRPV1 channel (Brenneis et al., 2013). The treated animals showed deficits in heat and mechanical pressure, but not in pinprick and touch detection. They also showed reduced inflammatory hyperalgesia. However, QX314 also permeates the ion channel pore of TRPA1 (Stueber et al., 2016) which this limits specificity.
So, what are the main differences between Trpv1 knockout animals and those with “silenced” TRPV1 lineage? Trpv1 knockouts show only minor defects in physiological heat sensation (Caterina et al., 2000). In contrast, ablation of the whole TRPV1 lineage by diphtheria toxin eliminated withdrawal from a 55°C hot plate within the test cutoff values (Mishra et al., 2011). Profound thermal hypoalgesia was also noted in rats following resiniferatoxin administration (Xu et al., 1997; Bölcskei et al., 2010). There was also a strong effect on the thermal preference, with both extreme hot and cold temperatures largely ignored by these animals (Pogorzala et al., 2013). In humans, desensitization by capsaicin elevated the heat detection threshold and reduced suprathreshold pain (Rosenberger et al., 2020). The difference between eliminating the TRPV1 lineage and only TRPV1 suggested the presence of further heat sensing ion channels in neurons expressing TRPV1. Based on the observations, one may argue that TRPV1 plays only a minor role in physiological heat sensation of rodents, and the additional channels involved in mice were unexpected (Vandewauw et al., 2018). In contrast, TRPV1 inhibition in human subjects clearly elevated the heat thresholds, therefore TRPV1 acts a first line of defense against acute non-damaging heat (Arendt-Nielsen et al., 2016; Manitpisitkul et al., 2018). Interestingly, modality specific TRPV1 antagonist NEO6860, which does not block heat activation in vitro, did also not alter human heat thresholds (Brown et al., 2017). The behavioral response to “inflammatory soup” (that contains ATP, prostaglandins, bradykinin, histamine, and serotonin) was markedly reduced after TRPV1 lineage-ablation; however, this misses a direct comparison to the Trpv1 knockout as a relevant fraction of inflammatory sensitisation converges on TRPV1 (Caterina et al., 2000). Pain-related behavior induced by ATP was also reduced after TRPV1 lineage-ablation, indicating that the involved ATP receptors substantially overlap with TRPV1. The resting body temperature was not different after TRPV1-lineage ablation, but the counterregulation to thermal stress as well as the induction of fever by interleukin-1β was reduced. The TRPV1 lineage was also required for ongoing inflammatory pain induced by carrageenan, but TRPV1 inhibition could not antagonize this as resiniferatoxin pretreatment (Okun et al., 2011). Comparison of RNA sequencing of TRPV1-expressing neurons and the complementary population shows the relative abundance of many established pain targets (Goswami et al., 2014).
Topical capsaicin desensitization has a clear therapeutic potential to relieve pain, but has also mysterious inconsistencies. In fact, over-the-counter capsaicin creams are broadly available for muscle and arthritic pain, though their analgesic value is only marginally better than placebo (Szallasi and Blumberg, 1999). To increase clinical efficacy, site-specific capsaicin injections and dermal patches have been developed. Qutenza is a dermal patch (capsaicin, 8% w/w), indicated for neuropathic pain (Blair, 2018). It provides a variable, but clinically meaningful improvement in neuropathic pain patients for about 3 months (Martini et al., 2012; Wagner et al., 2013; Maihöfner and Heskamp, 2014).
For decades, sensitivity to capsaicin was recognized as a functional signature of primary sensory neurons. Indeed, for concentrations of up to 1 μM there is little evidence for non-specific, TRPV1-independent capsaicin actions. At 10 μM and above, however, capsaicin loses its selectivity for TRPV1 and starts interacting with various enzymes, changing membrane fluidity, and blocking other receptors (Holzer, 1991; Szallasi and Blumberg, 1999; Braga Ferreira et al., 2020). Indeed, cell death evoked by capsaicin 100 μM is well documented in Trpv1 knockout mice (Yang et al., 2014). When the clinical experience with high concentration capsaicin preparations is puzzling, the possibility that excessive capsaicin concentrations may also have acted on targets other than TRPV1 should be considered.
First, there is the high capsaicin concentration in the Qutenza patch. Acute desensitization can be achieved by low concentration (0.02–0.15% are common) capsaicin cremes (Derry and Moore, 2012), but a 1% dermal patch is less efficacious than the 8% patch to achieve a reversible “cutaneous nerve terminal axotomy”. The variable delivery through the skin and the systemic redistribution might only partially explain why Qutenza contains a concentration which is orders of magnitude (by about 4 × 106) higher than the EC50 of capsaicin at human TRPV1. Data on transdermal delivery indicate that within one hour about 1% of the capsaicin contained in the Qutenza patch is delivered (Wohlrab et al., 2015). A potential explanation for the required high concentration might be a need to reach intracellular TRPV1 (Zhao and Tsang, 2017), about 100-fold higher capsaicin concentrations compared to the plasma membrane were required for a half-maximal effect (Gallego-Sandín et al., 2009).
Second, in Qutenza-treated patients, the density of TRPV1-positive dermal fibers was reduced with corresponding reduction in the detection of touch and pinprick stimuli, but not heat sensation, which is at odds with expectations based on animal data obtained with TRPV1 ablation (Kennedy et al., 2010).
Third, the duration of clinical pain relief clearly exceeds the recovery of skin sensitivity, which is largely normalized after 21 days (Lo Vecchio et al., 2018).
Fourth, the Qutenza effect differs between patients and healthy volunteers. The induced pain in healthy volunteers shows high variability, including subjects classified as non-responders, of which many had barely any capsaicin patch-induced pain (Gustorff et al., 2013; Papagianni et al., 2018). The capsaicin patch may affect Aδ-fiber function in humans, and the extent was correlated with pain reduction by the capsaicin patch (Papagianni et al., 2018). Expression of TRPV1 in Aδ-fibers during disease states, but not under physiological conditions might serve as explanation.
Finally, one has to keep in mind that for Qutenza to be clinically effective, the treated patient must have functional capsaicin-sensitive nerves, which may not be the case for some individuals. For example, patients with advanced diabetes may lose most of their TRPV1+ afferents as implied by a murine model of diabetic peripheral neuropathy (Pabbidi et al., 2008).
Concluding Thoughts: The Mysteries of Capsaicin-Sensitive Afferents
Capsaicin research has a long and rich history. In dogs, Hõgyes reported the hypothermic action of intragastric pepper extract in 1878 as the first indication of the role that TRPV1-positive afferents play in thermoregulation (Högyes, 1878). Yet, almost 150 years later, the exact mechanism by which TRPV1 regulates body temperature remains a mystery. Early studies with microinjection of capsaicin into brain nuclei pointed to the existence of a capsaicin-responsive thermoregulation center in the preoptic area (Jancsó-Gábor et al., 1970). By contrast, results obtained with peripherally restricted TRPV1 antagonists implicated a peripheral target (Tamayo et al., 2008). Other studies (Szolcsányi, 2015) argued that capsaicin “tricks” animals into feeling hot, and these animals try to cool down by seeking out cold surfaces. Indeed, capsaicin-treated rodents have red ears due to vasodilation, and they spread out in their cages. Moreover, dogs pant and humans sweat in response to capsaicin. However, rats desensitized to capsaicin suffer heat stroke when moved to a heat chamber, implying that capsaicin may impair thermoregulation instead of simply causing hypothermia (Jancsó-Gábor et al., 1970).
TRPV1 agonists and antagonists induce opposite changes in body temperature, hypo- and hyperthermia, respectively. Based on these observations, it was proposed that TRPV1 has an endogenous tone (Gavva, 2008). This model located TRPV1+ afferents responsible for body temperature changes in the gastrointestinal tract. Yet, ablation by resiniferatoxin of the abdominal TRPV1-expressing afferents did not eliminate the capsaicin-sensitive thermoregulatory response (Szolcsányi, 2015). Adding to the confusion, global Trpv1 knockout mice have normal body temperature (Szelényi et al., 2004). Though one may argue that these animals may have developed alternative thermoregulatory pathways to compensate for the missing TRPV1.
Studies with TRPV1 antagonists further clouded the picture. It turned out that some antagonists caused a febrile reaction in human volunteers whereas others did not change body temperature perceptibly or, paradoxically, they even lowered body temperature [reviewed in Garami et al. (2020)]. Although rational drug design resulted in “temperature neutral” TRPV1 antagonists (substances that did not block proton activation did not cause hyperthermia either), some of these compounds proved “rat-specific” as they still caused hyperthermia in dogs and primates (Lehto et al., 2008). Nevertheless, with NEO6860 there is a TRPV1 antagonist which does not invoke hyperthermia in humans (Brown et al., 2017). As an interesting twist, the undesirable hyperthermia by TRPV1 antagonists in pain patients might even be useful in patients with surgically-induced hypothermia (Schmidt, 2017).
Although TRPV1 is clearly a heat-activated channel with an activation threshold about 40°C (Caterina et al., 1997), the role of TRPV1 in physiological heat sensation remains a mystery. It was suggested that TRPV1 is really a warmth receptor (Yarmolinsky et al., 2016), though the burn injuries in patients taking TRPV1 antagonists argue otherwise. Species-related differences should also be taken into consideration. For example, camel TRPV1 is not heat-sensitive (Laursen et al., 2016). Rats have heat-sensitive TRPV1 channels, but mole rats living in burrows underground do not.
But maybe the biggest, and probably most important, mystery of all is the discrepancy between the therapeutic potential of TRPV1 antagonists in preclinical models and clinical studies. In experimental animals, TRPV1 antagonists blocked inflammatory, neuropathic, and cancer pain (Szallasi et al., 2007; Moran et al., 2011; Moran and Szallasi, 2018). However, in patients with migraine and osteoarthritis, TRPV1 inhibition disappointed as therapeutic approach (Mayorga et al., 2017; Arsenault et al., 2018; Vécsei et al., 2019). Likewise, TRPV1 antagonists inhibited evoked cough in experimental animals, but did not show any antitussive activity in chronic cough patients (Khalid et al., 2014; Belvisi et al., 2017).
The explanation of the discrepant preclinical and clinical TRPV1 antagonist efficacies remains to be elucidated. Maybe TRPV1 plays a much less important role in human diseases than in their animal models. Or maybe one size simply does not fit all: one ought to select the patient population that could benefit from the TRPV1 antagonist therapy (targeted approach).
In conclusion, capsaicin has been an invaluable tool in sensory pharmacology to dissect a fundamental population of sensory afferents, and to explore their functions in health and disease. The capsaicin receptor was identified as TRPV1, and a number of selective and potent small molecule antagonists developed. Despite the tremendous progress in our understanding of TRPV1 mechanisms, several “mysteries” remain, ranging from the molecular mechanisms of capsaicin desensitization through the exact role of TRPV1 in thermoregulation and heat sensation to the therapeutic value of TRPV1 antagonists.
Author Contributions
All authors listed have made a substantial, direct and intellectual contribution to the work, and approved it for publication.
Funding
MJMF was supported by FWF grant P 32534-8.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
Abrahamsen, B., Zhao, J., Asante, C. O., Cendan, C. M., Marsh, S., Martinez-Barbera, J. P., et al. (2008). The cell and molecular basis of mechanical, cold, and inflammatory pain. Science 321, 702–705. doi: 10.1126/science.1156916
Aghazadeh Tabrizi, M., Baraldi, P. G., Baraldi, S., Gessi, S., Merighi, S., and Borea, P. A. (2017). Medicinal chemistry, pharmacology, and clinical implications of TRPV1 receptor antagonists. Med. Res. Rev. 37, 936–983. doi: 10.1002/med.21427
Amaya, F., Shimosato, G., Nagano, M., Ueda, M., Hashimoto, S., Tanaka, Y., et al. (2004). NGF and GDNF differentially regulate TRPV1 expression that contributes to development of inflammatory thermal hyperalgesia. Eur. J. Neurosci. 20, 2303–2310. doi: 10.1111/j.1460-9568.2004.03701.x
Aneiros, E., Cao, L., Papakosta, M., Stevens, E. B., Phillips, S., and Grimm, C. (2011). The biophysical and molecular basis of TRPV1 proton gating. EMBO J. 30, 994–1002. doi: 10.1038/emboj.2011.19
Arendt-Nielsen, L., Harris, S., Whiteside, G. T., Hummel, M., Knappenberger, T., O’Keefe, S., et al. (2016). A randomized, double-blind, positive-controlled, 3-way cross-over human experimental pain study of a TRPV1 antagonist (V116517) in healthy volunteers and comparison with preclinical profile. Pain 157, 2057–2067. doi: 10.1097/j.pain.0000000000000610
Arsenault, P., Chiche, D., Brown, W., Miller, J., Treister, R., Leff, R., et al. (2018). NEO6860, modality-selective TRPV1 antagonist: a randomized, controlled, proof-of-concept trial in patients with osteoarthritis knee pain. Pain Rep. 3:e696. doi: 10.1097/PR9.0000000000000696
Baral, P., Umans, B. D., Li, L., Wallrapp, A., Bist, M., Kirschbaum, T., et al. (2018). Nociceptor sensory neurons suppress neutrophil and γδ T cell responses in bacterial lung infections and lethal pneumonia. Nat. Med. 24, 417–426. doi: 10.1038/nm.4501
Bates, B., Mitchell, K., Keller, J. M., Chan, C.-C., Swaim, W. D., Yaskovich, R., et al. (2010). Prolonged analgesic response of cornea to topical resiniferatoxin, a potent TRPV1 agonist. Pain 149, 522–528. doi: 10.1016/j.pain.2010.03.024
Behrendt, H.-J., Germann, T., Gillen, C., Hatt, H., and Jostock, R. (2004). Characterization of the mouse cold-menthol receptor TRPM8 and vanilloid receptor type-1 VR1 using a fluorometric imaging plate reader (FLIPR) assay. Br. J. Pharmacol. 141, 737–745. doi: 10.1038/sj.bjp.0705652
Belvisi, M. G., Birrell, M. A., Wortley, M. A., Maher, S. A., Satia, I., Badri, H., et al. (2017). XEN-D0501, a novel transient receptor potential Vanilloid 1 Antagonist, does not reduce cough in patients with refractory cough. Am. J. Respir. Crit. Care Med. 196, 1255–1263. doi: 10.1164/rccm.201704-0769OC
Bevan, S., Quallo, T., and Andersson, D. A. (2014). “TRPV1,” in Mammalian Transient Receptor Potential (TRP) Cation Channels. Handbook of Experimental Pharmacology, eds B. Nilius, and V. Flockerzi (Berlin: Springer), Vol. 222, 207–245. doi: 10.1007/978-3-642-54215-2_9
Bevan, S., and Szolcsányi, J. (1990). Sensory neuron-specific actions of capsaicin: mechanisms and applications. Trends Pharmacol. Sci. 11, 330–333. doi: 10.1016/0165-6147(90)90237-3
Bhave, G., Zhu, W., Wang, H., Brasier, D. J., Oxford, G. S., and Gereau, R. W. (2002). cAMP-dependent protein kinase regulates desensitization of the capsaicin receptor (VR1) by direct phosphorylation. Neuron 35, 721–731. doi: 10.1016/s0896-6273(02)00802-4
Bíró, T., Maurer, M., Modarres, S., Lewin, N. E., Brodie, C., Acs, G., et al. (1998). Characterization of functional vanilloid receptors expressed by mast cells. Blood 91, 1332–1340. doi: 10.1182/blood.v91.4.1332
Blair, H. A. (2018). Capsaicin 8% dermal patch: a review in peripheral neuropathic pain. Drugs 78, 1489–1500. doi: 10.1007/s40265-018-0982-7
Blivis, D., Haspel, G., Mannes, P. Z., O’Donovan, M. J., and Iadarola, M. J. (2017). Identification of a novel spinal nociceptive-motor gate control for Aδ pain stimuli in rats. eLife 6:e23584. doi: 10.7554/eLife.23584
Bölcskei, K., Tékus, V., Dézsi, L., Szolcsányi, J., and Petho, G. (2010). Antinociceptive desensitizing actions of TRPV1 receptor agonists capsaicin, resiniferatoxin and N-oleoyldopamine as measured by determination of the noxious heat and cold thresholds in the rat. Eur. J. Pain 14, 480–486. doi: 10.1016/j.ejpain.2009.08.005
Braga Ferreira, L. G., Faria, J. V., Dos Santos, J. P. S., and Faria, R. X. (2020). Capsaicin: TRPV1-independent mechanisms and novel therapeutic possibilities. Eur. J. Pharmacol. 887:173356. doi: 10.1016/j.ejphar.2020.173356
Brenneis, C., Kistner, K., Puopolo, M., Segal, D., Roberson, D., Sisignano, M., et al. (2013). Phenotyping the function of TRPV1-expressing sensory neurons by targeted axonal silencing. J. Neurosci. 33, 315–326. doi: 10.1523/JNEUROSCI.2804-12.2013
Brito, R., Sheth, S., Mukherjea, D., Rybak, L. P., and Ramkumar, V. (2014). TRPV1: a potential drug target for treating various diseases. Cells 3, 517–545. doi: 10.3390/cells3020517
Brown, W., Leff, R. L., Griffin, A., Hossack, S., Aubray, R., Walker, P., et al. (2017). Safety, pharmacokinetics, and pharmacodynamics study in healthy subjects of oral NEO6860, a modality selective transient receptor potential vanilloid subtype 1 antagonist. J. Pain 18, 726–738. doi: 10.1016/j.jpain.2017.01.009
Btesh, J., Fischer, M. J. M., Stott, K., and McNaughton, P. A. (2013). Mapping the binding site of TRPV1 on AKAP79: implications for inflammatory hyperalgesia. J. Neurosci. 33, 9184–9193. doi: 10.1523/JNEUROSCI.4991-12.2013
Byrnes, N. K., and Hayes, J. E. (2013). Personality factors predict spicy food liking and intake. Food Qual. Prefer. 28, 213–221. doi: 10.1016/j.foodqual.2012.09.008
Cao, E., Liao, M., Cheng, Y., and Julius, D. (2013). TRPV1 structures in distinct conformations reveal activation mechanisms. Nature 504, 113–118. doi: 10.1038/nature12823
Caterina, M. J., Leffler, A., Malmberg, A. B., Martin, W. J., Trafton, J., Petersen-Zeitz, K. R., et al. (2000). Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science 288, 306–313. doi: 10.1126/science.288.5464.306
Caterina, M. J., Schumacher, M. A., Tominaga, M., Rosen, T. A., Levine, J. D., and Julius, D. (1997). The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature 389, 816–824. doi: 10.1038/39807
Cavanaugh, D. J., Chesler, A. T., Jackson, A. C., Sigal, Y. M., Yamanaka, H., Grant, R., et al. (2011). Trpv1 reporter mice reveal highly restricted brain distribution and functional expression in arteriolar smooth muscle cells. J. Neurosci. 31, 5067–5077. doi: 10.1523/JNEUROSCI.6451-10.2011
Chaiyasit, K., Khovidhunkit, W., and Wittayalertpanya, S. (2009). Pharmacokinetic and the effect of capsaicin in Capsicum frutescens on decreasing plasma glucose level. J. Med. Assoc. Thai. 92, 108–113.
Chu, C., Zavala, K., Fahimi, A., Lee, J., Xue, Q., Eilers, H., et al. (2011). Transcription factors Sp1 and Sp4 regulate TRPV1 gene expression in rat sensory neurons. Mol. Pain 7:44. doi: 10.1186/1744-8069-7-44
Chu, Y., Qiu, P., and Yu, R. (2020). Centipede venom peptides acting on ion channels. Toxins 12:230. doi: 10.3390/toxins12040230
Clapham, D. E. (2003). TRP channels as cellular sensors. Nature 426, 517–524. doi: 10.1038/nature02196
Cosens, D. J., and Manning, A. (1969). Abnormal electroretinogram from a Drosophila mutant. Nature 224, 285–287. doi: 10.1038/224285a0
Cruz, F., Guimaräes, M., Silva, C., and Reis, M. (1997). Suppression of bladder hyperreflexia by intravesical resiniferatoxin. Lancet 350, 640–641. doi: 10.1016/S0140-6736(05)63330-2
Davis, J. B., Gray, J., Gunthorpe, M. J., Hatcher, J. P., Davey, P. T., Overend, P., et al. (2000). Vanilloid receptor-1 is essential for inflammatory thermal hyperalgesia. Nature 405, 183–187. doi: 10.1038/35012076
Derry, S., and Moore, R. A. (2012). Topical capsaicin (low concentration) for chronic neuropathic pain in adults. Cochrane Datab. Syst. Rev. 2012:CD010111. doi: 10.1002/14651858.CD010111
Dirajlal, S., Pauers, L. E., and Stucky, C. L. (2003). Differential response properties of IB(4)-positive and -negative unmyelinated sensory neurons to protons and capsaicin. J. Neurophysiol. 89, 513–524. doi: 10.1152/jn.00371.2002
Docherty, R. J., Yeats, J. C., Bevan, S., and Boddeke, H. W. (1996). Inhibition of calcineurin inhibits the desensitization of capsaicin-evoked currents in cultured dorsal root ganglion neurones from adult rats. Pflugers Arch. 431, 828–837. doi: 10.1007/s004240050074
Docherty, R. J., Yeats, J. C., and Piper, A. S. (1997). Capsazepine block of voltage-activated calcium channels in adult rat dorsal root ganglion neurones in culture. Br. J. Pharmacol. 121, 1461–1467. doi: 10.1038/sj.bjp.0701272
Donnerer, J., and Lembeck, F. (1982). Analysis of the effects of intravenously injected capsaicin in the rat. Naunyn Schmiedebergs Arch. Pharmacol. 320, 54–57. doi: 10.1007/bf00499072
Eilers, H., Lee, S.-Y., Hau, C. W., Logvinova, A., and Schumacher, M. A. (2007). The rat vanilloid receptor splice variant VR.5’sv blocks TRPV1 activation. Neuroreport 18, 969–973. doi: 10.1097/WNR.0b013e328165d1a2
Fernandes, E. S., Fernandes, M. A., and Keeble, J. E. (2012). The functions of TRPA1 and TRPV1: moving away from sensory nerves. Br. J. Pharmacol. 166, 510–521. doi: 10.1111/j.1476-5381.2012.01851.x
Fischer, M. J. M., Balasuriya, D., Jeggle, P., Goetze, T. A., McNaughton, P. A., Reeh, P. W., et al. (2014). Direct evidence for functional TRPV1/TRPA1 heteromers. Pflugers Arch. 466, 2229–2241. doi: 10.1007/s00424-014-1497-z
Fischer, M. J. M., Btesh, J., and McNaughton, P. A. (2013). Disrupting sensitization of transient receptor potential vanilloid subtype 1 inhibits inflammatory hyperalgesia. J. Neurosci. 33, 7407–7414. doi: 10.1523/JNEUROSCI.3721-12.2013
Fischer, M. J. M., Mak, S. W. Y., and McNaughton, P. A. (2010). Sensitisation of nociceptors - what are ion channels doing? Open Pain J. 3, 82–96. doi: 10.2174/1876386301003010082
Fischer, M. J. M., and Reeh, P. W. (2007). Sensitization to heat through G-protein-coupled receptor pathways in the isolated sciatic mouse nerve. Eur. J. Neurosci. 25, 3570–3575. doi: 10.1111/j.1460-9568.2007.05582.x
Fischer, M. J. M., Reeh, P. W., and Sauer, S. K. (2003). Proton-induced calcitonin gene-related peptide release from rat sciatic nerve axons, in vitro, involving TRPV1. Eur. J. Neurosci. 18, 803–810. doi: 10.1046/j.1460-9568.2003.02811.x
Gallego-Sandín, S., Rodríguez-García, A., Alonso, M. T., and García-Sancho, J. (2009). The endoplasmic reticulum of dorsal root ganglion neurons contains functional TRPV1 channels. J. Biol. Chem. 284, 32591–32601. doi: 10.1074/jbc.M109.019687
Gao, Y., Cao, E., Julius, D., and Cheng, Y. (2016). TRPV1 structures in nanodiscs reveal mechanisms of ligand and lipid action. Nature 534, 347–351. doi: 10.1038/nature17964
Garami, A., Pakai, E., McDonald, H. A., Reilly, R. M., Gomtsyan, A., Corrigan, J. J., et al. (2018). TRPV1 antagonists that cause hypothermia, instead of hyperthermia, in rodents: compounds’ pharmacological profiles, in vivo targets, thermoeffectors recruited and implications for drug development. Acta Physiol. 223:e13038. doi: 10.1111/apha.13038
Garami, A., Shimansky, Y. P., Rumbus, Z., Vizin, R. C. L., Farkas, N., Hegyi, J., et al. (2020). Hyperthermia induced by transient receptor potential vanilloid-1 (TRPV1) antagonists in human clinical trials: insights from mathematical modeling and meta-analysis. Pharmacol. Ther. 208:107474. doi: 10.1016/j.pharmthera.2020.107474
Gau, P., Poon, J., Ufret-Vincenty, C., Snelson, C. D., Gordon, S. E., Raible, D. W., et al. (2013). The zebrafish ortholog of TRPV1 is required for heat-induced locomotion. J. Neurosci. 33, 5249–5260. doi: 10.1523/JNEUROSCI.5403-12.2013
Gavva, N. R. (2008). Body-temperature maintenance as the predominant function of the vanilloid receptor TRPV1. Trends Pharmacol. Sci. 29, 550–557. doi: 10.1016/j.tips.2008.08.003
Gavva, N. R., Klionsky, L., Qu, Y., Shi, L., Tamir, R., Edenson, S., et al. (2004). Molecular determinants of vanilloid sensitivity in TRPV1. J. Biol. Chem. 279, 20283–20295. doi: 10.1074/jbc.M312577200
Gavva, N. R., Tamir, R., Qu, Y., Klionsky, L., Zhang, T. J., Immke, D., et al. (2005). AMG 9810 [(E)-3-(4-t-butylphenyl)-N-(2,3-dihydrobenzo[b][1,4] dioxin-6-yl)acrylamide], a novel vanilloid receptor 1 (TRPV1) antagonist with antihyperalgesic properties. J. Pharmacol. Exp. Ther. 313, 474–484. doi: 10.1124/jpet.104.079855
Geron, M., Hazan, A., and Priel, A. (2017). Animal toxins providing insights into TRPV1 activation mechanism. Toxins 9:326. doi: 10.3390/toxins9100326
Gomtsyan, A., McDonald, H. A., Schmidt, R. G., Daanen, J. F., Voight, E. A., Segreti, J. A., et al. (2015). TRPV1 ligands with hyperthermic, hypothermic and no temperature effects in rats. Temperature 2, 297–301. doi: 10.1080/23328940.2015.1046013
Goswami, S. C., Mishra, S. K., Maric, D., Kaszas, K., Gonnella, G. L., Clokie, S. J., et al. (2014). Molecular signatures of mouse TRPV1-lineage neurons revealed by RNA-Seq transcriptome analysis. J. Pain 15, 1338–1359. doi: 10.1016/j.jpain.2014.09.010
Gracheva, E. O., Cordero-Morales, J. F., González-Carcacía, J. A., Ingolia, N. T., Manno, C., Aranguren, C. I., et al. (2011). Ganglion-specific splicing of TRPV1 underlies infrared sensation in vampire bats. Nature 476, 88–91. doi: 10.1038/nature10245
Gracheva, E. O., Ingolia, N. T., Kelly, Y. M., Cordero-Morales, J. F., Hollopeter, G., Chesler, A. T., et al. (2010). Molecular basis of infrared detection by snakes. Nature 464, 1006–1011. doi: 10.1038/nature08943
Gustorff, B., Poole, C., Kloimstein, H., Hacker, N., and Likar, R. (2013). Treatment of neuropathic pain with the capsaicin 8% patch: quantitative sensory testing (QST) in a prospective observational study identifies potential predictors of response to capsaicin 8% patch treatment. Scand. J. Pain 4, 138–145. doi: 10.1016/j.sjpain.2013.04.001
Han, L., Ma, C., Liu, Q., Weng, H.-J., Cui, Y., Tang, Z., et al. (2013). A subpopulation of nociceptors specifically linked to itch. Nat. Neurosci. 16, 174–182. doi: 10.1038/nn.3289
Han, Y., Li, B., Yin, T.-T., Xu, C., Ombati, R., Luo, L., et al. (2018). Molecular mechanism of the tree shrew’s insensitivity to spiciness. PLoS Biol. 16:e2004921. doi: 10.1371/journal.pbio.2004921
Hanack, C., Moroni, M., Lima, W. C., Wende, H., Kirchner, M., Adelfinger, L., et al. (2015). GABA blocks pathological but not acute TRPV1 pain signals. Cell 160, 759–770. doi: 10.1016/j.cell.2015.01.022
Harron, D. W., and Kobinger, W. (1984). Facilitation of the Bezold-Jarisch reflex by central stimulation of alpha 2 adrenoceptors in dogs. Naunyn Schmiedebergs Arch. Pharmacol. 325, 193–197. doi: 10.1007/bf00495942
Hayes, P., Meadows, H. J., Gunthorpe, M. J., Harries, M. H., Duckworth, D. M., Cairns, W., et al. (2000). Cloning and functional expression of a human orthologue of rat vanilloid receptor-1. Pain 88, 205–215. doi: 10.1016/s0304-3959(00)00353-5
Hazan, A., Kumar, R., Matzner, H., and Priel, A. (2015). The pain receptor TRPV1 displays agonist-dependent activation stoichiometry. Sci. Rep. 5:12278. doi: 10.1038/srep12278
Helliwell, R. J., McLatchie, L. M., Clarke, M., Winter, J., Bevan, S., and McIntyre, P. (1998). Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci. Lett. 250, 177–180. doi: 10.1016/s0304-3940(98)00475-3
Hjerling-Leffler, J., AlQatari, M., Ernfors, P., and Koltzenburg, M. (2007). Emergence of functional sensory subtypes as defined by transient receptor potential channel expression. J. Neurosci. 27, 2435–2443. doi: 10.1523/JNEUROSCI.5614-06.2007
Högyes, E. (1878). Beiträge zur physiologischen wirkung der bestandteile des Capsicum annuum. Arch. Exp. Pathol. Pharmakol. 9, 117–130.
Hökfelt, T., Zhang, X., and Wiesenfeld-Hallin, Z. (1994). Messenger plasticity in primary sensory neurons following axotomy and its functional implications. Trends Neurosci. 17, 22–30. doi: 10.1016/0166-2236(94)90031-0
Holzer, P. (1988). Local effector functions of capsaicin-sensitive sensory nerve endings: involvement of tachykinins, calcitonin gene-related peptide and other neuropeptides. Neuroscience 24, 739–768. doi: 10.1016/0306-4522(88)90064-4
Holzer, P. (1991). Capsaicin: cellular targets, mechanisms of action, and selectivity for thin sensory neurons. Pharmacol. Rev. 43, 143–201.
Hori, S., and Saitoh, O. (2020). Unique high sensitivity to heat of axolotl TRPV1 revealed by the heterologous expression system. Biochem. Biophys. Res. Commun. 521, 914–920. doi: 10.1016/j.bbrc.2019.10.203
Huang, J., Zhang, X., and McNaughton, P. A. (2006). Inflammatory pain: the cellular basis of heat hyperalgesia. Curr. Neuropharmacol. 4, 197–206. doi: 10.2174/157015906778019554
Hwang, S. W., Cho, H., Kwak, J., Lee, S. Y., Kang, C. J., Jung, J., et al. (2000). Direct activation of capsaicin receptors by products of lipoxygenases: endogenous capsaicin-like substances. Proc. Natl. Acad. Sci. U.S.A. 97, 6155–6160. doi: 10.1073/pnas.97.11.6155
Iadarola, M. J., and Gonnella, G. L. (2013). Resiniferatoxin for pain treatment: an interventional approach to personalized pain medicine. Open Pain J. 6, 95–107. doi: 10.2174/1876386301306010095
Jancsó, N., and Jancsó, A. (1949). Desensitization of sensory nerve endings. Kísérletes Orvostudom. 2(Suppl. 15), 179–226.
Jancsó, N., and Jancsó-Gábor, A. (1959). Dauerausschaltung der chemischen schmerzempfindlichkeit durch capsaicin. Naunyn Schmiedebergs Arch. 236, 142–145. doi: 10.1007/BF00259094
Jancsó, N., Jancsó-Gábor, A., and Szolcsányi, J. (1967). Direct evidence for neurogenic inflammation and its prevention by denervation and by pretreatment with capsaicin. Br. J. Pharmacol. Chemother. 31, 138–151. doi: 10.1111/j.1476-5381.1967.tb01984.x
Jancsó-Gábor, A., Szolcsányi, J., and Jancsó, N. (1970). Irreversible impairment of thermoregulation induced by capsaicin and similar pungent substances in rats and guinea-pigs. J. Physiol. 206, 495–507. doi: 10.1113/jphysiol.1970.sp009027
Jordt, S.-E., and Julius, D. (2002). Molecular basis for species-specific sensitivity to “hot” chili peppers. Cell 108, 421–430. doi: 10.1016/s0092-8674(02)00637-2
Jordt, S. E., Tominaga, M., and Julius, D. (2000). Acid potentiation of the capsaicin receptor determined by a key extracellular site. Proc. Natl. Acad. Sci. U.S.A. 97, 8134–8139. doi: 10.1073/pnas.100129497
Karai, L., Brown, D. C., Mannes, A. J., Connelly, S. T., Brown, J., Gandal, M., et al. (2004). Deletion of vanilloid receptor 1-expressing primary afferent neurons for pain control. J. Clin. Invest. 113, 1344–1352. doi: 10.1172/JCI20449
Karai, L. J., Russell, J. T., Iadarola, M. J., and Oláh, Z. (2004). Vanilloid receptor 1 regulates multiple calcium compartments and contributes to Ca2+-induced Ca2+ release in sensory neurons. J. Biol. Chem. 279, 16377–16387. doi: 10.1074/jbc.M310891200
Kauer, J. A., and Gibson, H. E. (2009). Hot flash: TRPV channels in the brain. Trends Neurosci. 32, 215–224. doi: 10.1016/j.tins.2008.12.006
Kennedy, W. R., Vanhove, G. F., Lu, S.-P., Tobias, J., Bley, K. R., Walk, D., et al. (2010). A randomized, controlled, open-label study of the long-term effects of NGX-4010, a high-concentration capsaicin patch, on epidermal nerve fiber density and sensory function in healthy volunteers. J. Pain 11, 579–587. doi: 10.1016/j.jpain.2009.09.019
Khalid, S., Murdoch, R., Newlands, A., Smart, K., Kelsall, A., Holt, K., et al. (2014). Transient receptor potential vanilloid 1 (TRPV1) antagonism in patients with refractory chronic cough: a double-blind randomized controlled trial. J. Allergy Clin. Immunol. 134, 56–62. doi: 10.1016/j.jaci.2014.01.038
Kichko, T. I., and Reeh, P. W. (2004). Why cooling is beneficial: non-linear temperature-dependency of stimulated iCGRP release from isolated rat skin. Pain 110, 215–219. doi: 10.1016/j.pain.2004.03.033
Kistner, K., Siklosi, N., Babes, A., Khalil, M., Selescu, T., Zimmermann, K., et al. (2016). Systemic desensitization through TRPA1 channels by capsazepine and mustard oil - a novel strategy against inflammation and pain. Sci. Rep. 6:28621. doi: 10.1038/srep28621
Kobayashi, K., Fukuoka, T., Obata, K., Yamanaka, H., Dai, Y., Tokunaga, A., et al. (2005). Distinct expression of TRPM8, TRPA1, and TRPV1 mRNAs in rat primary afferent neurons with adelta/c-fibers and colocalization with trk receptors. J. Comp. Neurol. 493, 596–606. doi: 10.1002/cne.20794
Laird, J. M., Roza, C., De Felipe, C., Hunt, S. P., and Cervero, F. (2001). Role of central and peripheral tachykinin NK1 receptors in capsaicin-induced pain and hyperalgesia in mice. Pain 90, 97–103. doi: 10.1016/s0304-3959(00)00394-8
Launay, P., Fleig, A., Perraud, A. L., Scharenberg, A. M., Penner, R., and Kinet, J. P. (2002). TRPM4 is a Ca2+-activated nonselective cation channel mediating cell membrane depolarization. Cell 109, 397–407. doi: 10.1016/s0092-8674(02)00719-5
Laursen, W. J., Schneider, E. R., Merriman, D. K., Bagriantsev, S. N., and Gracheva, E. O. (2016). Low-cost functional plasticity of TRPV1 supports heat tolerance in squirrels and camels. Proc. Natl. Acad. Sci. U.S.A. 113, 11342–11347. doi: 10.1073/pnas.1604269113
Lawless, H., and Stevens, D. A. (1984). Effects of oral chemical irritation on taste. Physiol. Behav. 32, 995–998. doi: 10.1016/0031-9384(84)90291-9
Lee, B. H., and Zheng, J. (2015). Proton block of proton-activated TRPV1 current. J. Gen. Physiol. 146, 147–159. doi: 10.1085/jgp.201511386
Lee, J., Lee, J., Kim, J., Kim, S. Y., Chun, M. W., Cho, H., et al. (2001). N-(3-Acyloxy-2-benzylpropyl)-N’-(4-hydroxy-3-methoxybenzyl) thiourea derivatives as potent vanilloid receptor agonists and analgesics. Bioorg. Med. Chem. 9, 19–32. doi: 10.1016/s0968-0896(00)00216-9
Lee, M. C., Bond, S., Wheeler, D., Scholtes, I., Armstrong, G., McNaughton, P., et al. (2019). A randomised, double blind, placebo-controlled crossover trial of the influence of the HCN channel blocker ivabradine in a healthy volunteer pain model: an enriched population trial. Pain 160, 2554–2565. doi: 10.1097/j.pain.0000000000001638
Lehto, S. G., Tamir, R., Deng, H., Klionsky, L., Kuang, R., Le, A., et al. (2008). Antihyperalgesic effects of (R,E)-N-(2-hydroxy-2,3-dihydro-1H-inden-4-yl)-3-(2-(piperidin-1-yl)-4-(trifluoromethyl)phenyl)-acrylamide (AMG8562), a novel transient receptor potential vanilloid type 1 modulator that does not cause hyperthermia in rats. J. Pharmacol. Exp. Ther. 326, 218–229. doi: 10.1124/jpet.107.132233
Li, C.-L., Li, K.-C., Wu, D., Chen, Y., Luo, H., Zhao, J.-R., et al. (2016). Somatosensory neuron types identified by high-coverage single-cell RNA-sequencing and functional heterogeneity. Cell Res. 26, 83–102. doi: 10.1038/cr.2015.149
Li, J., Daughters, R. S., Bullis, C., Bengiamin, R., Stucky, M. W., Brennan, J., et al. (1999). The cannabinoid receptor agonist WIN 55,212-2 mesylate blocks the development of hyperalgesia produced by capsaicin in rats. Pain 81, 25–33. doi: 10.1016/s0304-3959(98)00263-2
Li, L., Hasan, R., and Zhang, X. (2014). The basal thermal sensitivity of the TRPV1 ion channel is determined by PKCβII. J. Neurosci. 34, 8246–8258. doi: 10.1523/JNEUROSCI.0278-14.2014
Liao, M., Cao, E., Julius, D., and Cheng, Y. (2013). Structure of the TRPV1 ion channel determined by electron cryo-microscopy. Nature 504, 107–112. doi: 10.1038/nature12822
Lo Vecchio, S., Andersen, H. H., and Arendt-Nielsen, L. (2018). The time course of brief and prolonged topical 8% capsaicin-induced desensitization in healthy volunteers evaluated by quantitative sensory testing and vasomotor imaging. Exp. Brain Res. 236, 2231–2244. doi: 10.1007/s00221-018-5299-y
Luo, L., Wang, Y., Li, B., Xu, L., Kamau, P. M., Zheng, J., et al. (2019). Molecular basis for heat desensitization of TRPV1 ion channels. Nat. Commun. 10, 1–12. doi: 10.1038/s41467-019-09965-6
Magerl, W., Wilk, S. H., and Treede, R. D. (1998). Secondary hyperalgesia and perceptual wind-up following intradermal injection of capsaicin in humans. Pain 74, 257–268. doi: 10.1016/s0304-3959(97)00177-2
Maggi, C. A., Patacchini, R., Santicioli, P., Giuliani, S., Geppetti, P., and Meli, A. (1988). Protective action of ruthenium red toward capsaicin desensitization of sensory fibers. Neurosci. Lett. 88, 201–205. doi: 10.1016/0304-3940(88)90126-7
Maihöfner, C. G., and Heskamp, M.-L. S. (2014). Treatment of peripheral neuropathic pain by topical capsaicin: impact of pre-existing pain in the QUEPP-study. Eur. J. Pain 18, 671–679. doi: 10.1002/j.1532-2149.2013.00415.x
Malek, N., Pajak, A., Kolosowska, N., Kucharczyk, M., and Starowicz, K. (2015). The importance of TRPV1-sensitisation factors for the development of neuropathic pain. Mol. Cell. Neurosci. 65, 1–10. doi: 10.1016/j.mcn.2015.02.001
Mandadi, S., Tominaga, T., Numazaki, M., Murayama, N., Saito, N., Armati, P. J., et al. (2006). Increased sensitivity of desensitized TRPV1 by PMA occurs through PKCepsilon-mediated phosphorylation at S800. Pain 123, 106–116. doi: 10.1016/j.pain.2006.02.016
Manitpisitkul, P., Flores, C. M., Moyer, J. A., Romano, G., Shalayda, K., Tatikola, K., et al. (2018). A multiple-dose double-blind randomized study to evaluate the safety, pharmacokinetics, pharmacodynamics and analgesic efficacy of the TRPV1 antagonist JNJ-39439335 (mavatrep). Scand. J. Pain 18, 151–164. doi: 10.1515/sjpain-2017-0184
Marshall, N. J., Liang, L., Bodkin, J., Dessapt-Baradez, C., Nandi, M., Collot-Teixeira, S., et al. (2013). A role for TRPV1 in influencing the onset of cardiovascular disease in obesity. Hypertension 61, 246–252. doi: 10.1161/HYPERTENSIONAHA.112.201434
Martini, C., Yassen, A., Olofsen, E., Passier, P., Stoker, M., and Dahan, A. (2012). Pharmacodynamic analysis of the analgesic effect of capsaicin 8% patch (QutenzaTM) in diabetic neuropathic pain patients: detection of distinct response groups. J. Pain Res. 5, 51–59. doi: 10.2147/JPR.S30406
Martins, D., Tavares, I., and Morgado, C. (2014). “Hotheaded”: the role OF TRPV1 in brain functions. Neuropharmacology 85, 151–157. doi: 10.1016/j.neuropharm.2014.05.034
Massaad, C. A., Safieh-Garabedian, B., Poole, S., Atweh, S. F., Jabbur, S. J., and Saadé, N. E. (2004). Involvement of substance P, CGRP and histamine in the hyperalgesia and cytokine upregulation induced by intraplantar injection of capsaicin in rats. J. Neuroimmunol. 153, 171–182. doi: 10.1016/j.jneuroim.2004.05.007
Mayorga, A. J., Flores, C. M., Trudeau, J. J., Moyer, J. A., Shalayda, K., Dale, M., et al. (2017). A randomized study to evaluate the analgesic efficacy of a single dose of the TRPV1 antagonist mavatrep in patients with osteoarthritis. Scand. J. Pain 17, 134–143. doi: 10.1016/j.sjpain.2017.07.021
McCoy, E. S., and Zylka, M. J. (2014). Enhanced behavioral responses to cold stimuli following CGRPα sensory neuron ablation are dependent on TRPM8. Mol. Pain 10:69. doi: 10.1186/1744-8069-10-69
Mezey, E., Tóth, Z. E., Cortright, D. N., Arzubi, M. K., Krause, J. E., Elde, R., et al. (2000). Distribution of mRNA for vanilloid receptor subtype 1 (VR1), and VR1-like immunoreactivity, in the central nervous system of the rat and human. Proc. Natl. Acad. Sci. U.S.A. 97, 3655–3660. doi: 10.1073/pnas.060496197
Mishra, S. K., Tisel, S. M., Orestes, P., Bhangoo, S. K., and Hoon, M. A. (2011). TRPV1-lineage neurons are required for thermal sensation. EMBO J. 30, 582–593. doi: 10.1038/emboj.2010.325
Mitchell, K., Bates, B. D., Keller, J. M., Lopez, M., Scholl, L., Navarro, J., et al. (2010). Ablation of rat TRPV1-expressing Adelta/C-fibers with resiniferatoxin: analysis of withdrawal behaviors, recovery of function and molecular correlates. Mol. Pain 6:94. doi: 10.1186/1744-8069-6-94
Mitchell, K., Lebovitz, E. E., Keller, J. M., Mannes, A. J., Nemenov, M. I., and Iadarola, M. J. (2014). Nociception and inflammatory hyperalgesia evaluated in rodents using infrared laser stimulation after Trpv1 gene knockout or resiniferatoxin lesion. Pain 155, 733–745. doi: 10.1016/j.pain.2014.01.007
Mohapatra, D. P., and Nau, C. (2005). Regulation of Ca2+-dependent desensitization in the vanilloid receptor TRPV1 by calcineurin and cAMP-dependent protein kinase. J. Biol. Chem. 280, 13424–13432. doi: 10.1074/jbc.M410917200
Montell, C., Jones, K., Hafen, E., and Rubin, G. (1985). Rescue of the Drosophila phototransduction mutation trp by germline transformation. Science 230, 1040–1043. doi: 10.1126/science.3933112
Moran, M. M., McAlexander, M. A., Bíró, T., and Szallasi, A. (2011). Transient receptor potential channels as therapeutic targets. Nat. Rev. Drug Discov. 10, 601–620. doi: 10.1038/nrd3456
Moran, M. M., and Szallasi, A. (2018). Targeting nociceptive transient receptor potential channels to treat chronic pain: current state of the field. Br. J. Pharmacol. 175, 2185–2203. doi: 10.1111/bph.14044
Motter, A. L., and Ahern, G. P. (2008). TRPV1-null mice are protected from diet-induced obesity. FEBS Lett. 582, 2257–2262. doi: 10.1016/j.febslet.2008.05.021
Nagy, I., Friston, D., Valente, J. S., Torres Perez, J. V., and Andreou, A. P. (2014). Pharmacology of the capsaicin receptor, transient receptor potential vanilloid type-1 ion channel. Prog. Drug Res. 68, 39–76. doi: 10.1007/978-3-0348-0828-6_2
NCT00804154 (2020). Resiniferatoxin to Treat Severe Pain Associated With Advanced Cancer. ClinicalTrials.gov. Available online at: https://clinicaltrials.gov/ct2/show/NCT00804154 (accessed November 2, 2020).
NCT04044742 Sorrento Therapeutics Inc (2020). A Phase 3 Placebo-controlled Study to Evaluate the Efficacy and Safety of Intra-Articular Administration of Resiniferatoxin Versus Placebo for the Treatment of Moderate to Severe Pain Due to Osteoarthritis of the Knee. Clinicaltrials.gov. Available online at: https://clinicaltrials.gov/ct2/show/NCT04044742 (accessed October 29, 2020).
Nelson, E. K. (1919). The constitution of capsaicin, the pungent principle of capsicum. J. Am. Chem. Soc. 41, 1115–1121. doi: 10.1021/ja02228a011
Nguyen, M. Q., Wu, Y., Bonilla, L. S., von Buchholtz, L. J., and Ryba, N. J. P. (2017). Diversity amongst trigeminal neurons revealed by high throughput single cell sequencing. PLoS One 12:e0185543. doi: 10.1371/journal.pone.0185543
Nishihara, E., Hiyama, T. Y., and Noda, M. (2011). Osmosensitivity of transient receptor potential vanilloid 1 is synergistically enhanced by distinct activating stimuli such as temperature and protons. PLoS One 6:e22246. doi: 10.1371/journal.pone.0022246
Numazaki, M., Tominaga, T., Toyooka, H., and Tominaga, M. (2002). Direct phosphorylation of capsaicin receptor VR1 by protein kinase Cepsilon and identification of two target serine residues. J. Biol. Chem. 277, 13375–13378. doi: 10.1074/jbc.C200104200
Ohkita, M., Saito, S., Imagawa, T., Takahashi, K., Tominaga, M., and Ohta, T. (2012). Molecular cloning and functional characterization of Xenopus tropicalis frog transient receptor potential vanilloid 1 reveal its functional evolution for heat, acid, and capsaicin sensitivities in terrestrial vertebrates. J. Biol. Chem. 287, 2388–2397. doi: 10.1074/jbc.M111.305698
Okun, A., DeFelice, M., Eyde, N., Ren, J., Mercado, R., King, T., et al. (2011). Transient inflammation-induced ongoing pain is driven by TRPV1 sensitive afferents. Mol. Pain 7:4. doi: 10.1186/1744-8069-7-4
Olah, Z., Szabo, T., Karai, L., Hough, C., Fields, R. D., Caudle, R. M., et al. (2001). Ligand-induced dynamic membrane changes and cell deletion conferred by vanilloid receptor 1. J. Biol. Chem. 276, 11021–11030. doi: 10.1074/jbc.M008392200
O’Neill, J., Brock, C., Olesen, A. E., Andresen, T., Nilsson, M., and Dickenson, A. H. (2012). Unravelling the mystery of capsaicin: a tool to understand and treat pain. Pharmacol. Rev. 64, 939–971. doi: 10.1124/pr.112.006163
Ono, K., Ye, Y., Viet, C. T., Dang, D., and Schmidt, B. L. (2015). TRPV1 expression level in isolectin B4-positive neurons contributes to mouse strain difference in cutaneous thermal nociceptive sensitivity. J. Neurophysiol. 113, 3345–3355. doi: 10.1152/jn.00973.2014
Orozco, O. E., Walus, L., Sah, D. W., Pepinsky, R. B., and Sanicola, M. (2001). GFRalpha3 is expressed predominantly in nociceptive sensory neurons. Eur. J. Neurosci. 13, 2177–2182. doi: 10.1046/j.0953-816x.2001.01596.x
Pabbidi, R. M., Yu, S.-Q., Peng, S., Khardori, R., Pauza, M. E., and Premkumar, L. S. (2008). Influence of TRPV1 on diabetes-induced alterations in thermal pain sensitivity. Mol. Pain 4:9. doi: 10.1186/1744-8069-4-9
Palecek, F., Sant’Ambrogio, G., Sant’Ambrogio, F. B., and Mathew, O. P. (1989). Reflex responses to capsaicin: intravenous, aerosol, and intratracheal administration. J. Appl. Physiol. 67, 1428–1437. doi: 10.1152/jappl.1989.67.4.1428
Papagianni, A., Siedler, G., Sommer, C., and Üçeyler, N. (2018). Capsaicin 8% patch reversibly reduces A-delta fiber evoked potential amplitudes. Pain Rep. 3:e644. doi: 10.1097/PR9.0000000000000644
Patil, M. J., Salas, M., Bialuhin, S., Boyd, J. T., Jeske, N. A., and Akopian, A. N. (2020). Sensitization of small-diameter sensory neurons is controlled by TRPV1 and TRPA1 association. FASEB J. 34, 287–302. doi: 10.1096/fj.201902026R
Perfumi, M., and Sparapassi, L. (1999). Rat offspring treated prenatally with capsaicin do not show some of the irreversible effects induced by neonatal treatment with neurotoxin. Pharmacol. Toxicol. 84, 66–71. doi: 10.1111/j.1600-0773.1999.tb00876.x
Perry, L., Dickau, R., Zarrillo, S., Holst, I., Pearsall, D. M., Piperno, D. R., et al. (2007). Starch fossils and the domestication and dispersal of chili peppers (Capsicum spp. L.) in the Americas. Science 315, 986–988. doi: 10.1126/science.1136914
Pogorzala, L. A., Mishra, S. K., and Hoon, M. A. (2013). The cellular code for mammalian thermosensation. J. Neurosci. 33, 5533–5541. doi: 10.1523/JNEUROSCI.5788-12.2013
Por, E. D., Gomez, R., Akopian, A. N., and Jeske, N. A. (2013). Phosphorylation regulates TRPV1 association with β-arrestin-2. Biochem. J. 451, 101–109. doi: 10.1042/BJ20121637
Premkumar, L. S., and Ahern, G. P. (2000). Induction of vanilloid receptor channel activity by protein kinase C. Nature 408, 985–990. doi: 10.1038/35050121
Price, T. J., and Flores, C. M. (2007). Critical evaluation of the colocalization between calcitonin gene-related peptide, substance P, transient receptor potential vanilloid subfamily type 1 immunoreactivities and isolectin B4 binding in primary afferent neurons of the rat and mouse. J. Pain 8, 263–272. doi: 10.1016/j.jpain.2006.09.005
Ray, P., Torck, A., Quigley, L., Wangzhou, A., Neiman, M., Rao, C., et al. (2018). Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 159, 1325–1345. doi: 10.1097/j.pain.0000000000001217
Ritter, S., and Dinh, T. T. (1992). Age-related changes in capsaicin-induced degeneration in rat brain. J. Comp. Neurol. 318, 103–116. doi: 10.1002/cne.903180108
Rohacs, T. (2015). Phosphoinositide regulation of TRPV1 revisited. Pflugers Arch. 467, 1851–1869. doi: 10.1007/s00424-015-1695-3
Rolke, R., Baron, R., Maier, C., Tölle, T. R., Treede, R.-D., Beyer, A., et al. (2006). Quantitative sensory testing in the german research network on neuropathic pain (DFNS): standardized protocol and reference values. Pain 123, 231–243. doi: 10.1016/j.pain.2006.01.041
Rosenberger, D. C., Binzen, U., Treede, R.-D., and Greffrath, W. (2020). The capsaicin receptor TRPV1 is the first line defense protecting from acute non damaging heat: a translational approach. J. Transl. Med. 18:28. doi: 10.1186/s12967-019-02200-2
Rozin, P., Mark, M., and Schiller, D. (1981). The role of desensitization to capsaicin in chili pepper ingestion and preference. Chem. Sens. 6, 23–31. doi: 10.1093/chemse/6.1.23
Ryu, S., Liu, B., Yao, J., Fu, Q., and Qin, F. (2007). Uncoupling proton activation of vanilloid receptor TRPV1. J. Neurosci. 27, 12797–12807. doi: 10.1523/JNEUROSCI.2324-07.2007
Sánchez-Moreno, A., Guevara-Hernández, E., Contreras-Cervera, R., Rangel-Yescas, G., Ladrón-de-Guevara, E., Rosenbaum, T., et al. (2018). Irreversible temperature gating in trpv1 sheds light on channel activation. eLife 7:e36372. doi: 10.7554/eLife.36372
Sanz-Salvador, L., Andrés-Borderia, A., Ferrer-Montiel, A., and Planells-Cases, R. (2012). Agonist- and Ca2+-dependent desensitization of TRPV1 channel targets the receptor to lysosomes for degradation. J. Biol. Chem. 287, 19462–19471. doi: 10.1074/jbc.M111.289751
Schmelz, M., Schmid, R., Handwerker, H. O., and Torebjörk, H. E. (2000). Encoding of burning pain from capsaicin-treated human skin in two categories of unmyelinated nerve fibres. Brain 123(Pt 3), 560–571. doi: 10.1093/brain/123.3.560
Schmidt, W. K. (2017). Turning up the heat on surgical cold. Temperature 4, 97–100. doi: 10.1080/23328940.2017.1319453
Schwarz, M. G., Namer, B., Reeh, P. W., and Fischer, M. J. M. (2017). TRPA1 and TRPV1 antagonists do not inhibit human acidosis-induced pain. J. Pain 18, 526–534. doi: 10.1016/j.jpain.2016.12.011
Serra, J., Campero, M., Bostock, H., and Ochoa, J. (2004). Two types of C nociceptors in human skin and their behavior in areas of capsaicin-induced secondary hyperalgesia. J. Neurophysiol. 91, 2770–2781. doi: 10.1152/jn.00565.2003
Serra, J., Campero, M., and Ochoa, J. (1998). Flare and hyperalgesia after intradermal capsaicin injection in human skin. J. Neurophysiol. 80, 2801–2810. doi: 10.1152/jn.1998.80.6.2801
Shimizu, I., Iida, T., Horiuchi, N., and Caterina, M. J. (2005). 5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro. J. Pharmacol. Exp. Ther. 314, 1378–1385. doi: 10.1124/jpet.105.084277
Silva, C., Avelino, A., Souto-Moura, C., and Cruz, F. (2001). A light- and electron-microscopic histopathological study of human bladder mucosa after intravesical resiniferatoxin application. BJU Int. 88, 355–360. doi: 10.1046/j.1464-410x.2001.02339.x
Silverman, J. D., and Kruger, L. (1990). Selective neuronal glycoconjugate expression in sensory and autonomic ganglia: relation of lectin reactivity to peptide and enzyme markers. J. Neurocytol. 19, 789–801. doi: 10.1007/bf01188046
Simone, D. A., Baumann, T. K., and LaMotte, R. H. (1989). Dose-dependent pain and mechanical hyperalgesia in humans after intradermal injection of capsaicin. Pain 38, 99–107. doi: 10.1016/0304-3959(89)90079-1
Simone, D. A., Ngeow, J. Y., Putterman, G. J., and LaMotte, R. H. (1987). Hyperalgesia to heat after intradermal injection of capsaicin. Brain Res. 418, 201–203. doi: 10.1016/0006-8993(87)90982-6
Sizer, F., and Harris, N. (1985). The influence of common food additives and temperature on threshold perception of capsaicin. Chem. Sens. 10, 279–286. doi: 10.1093/chemse/10.3.279
Smutzer, G., and Devassy, R. K. (2016). Integrating TRPV1 receptor function with capsaicin psychophysics. Adv. Pharmacol. Sci. 2016:1512457. doi: 10.1155/2016/1512457
Sondermann, J. R., Barry, A. M., Jahn, O., Michel, N., Abdelaziz, R., Kügler, S., et al. (2019). Vti1b promotes TRPV1 sensitization during inflammatory pain. Pain 160, 508–527. doi: 10.1097/j.pain.0000000000001418
Staruschenko, A., Jeske, N. A., and Akopian, A. N. (2010). Contribution of TRPV1-TRPA1 interaction to the single channel properties of the TRPA1 channel. J. Biol. Chem. 285, 15167–15177. doi: 10.1074/jbc.M110.106153
Stevens, D. A., and Lawless, H. T. (1986). Putting out the fire: effects of tastants on oral chemical irritation. Percept. Psychophys. 39, 346–350. doi: 10.3758/bf03203002
Stevens, R. M., Ervin, J., Nezzer, J., Nieves, Y., Guedes, K., Burges, R., et al. (2019). Randomized, double-blind, placebo-controlled trial of intraarticular trans-capsaicin for pain associated with osteoarthritis of the knee. Arthrit. Rheumatol. 71, 1524–1533. doi: 10.1002/art.40894
Stueber, T., Eberhardt, M. J., Hadamitzky, C., Jangra, A., Schenk, S., Dick, F., et al. (2016). Quaternary lidocaine derivative QX-314 activates and permeates human TRPV1 and TRPA1 to produce inhibition of sodium channels and cytotoxicity. Anesthesiology 124, 1153–1165. doi: 10.1097/ALN.0000000000001050
Sugiura, T., Tominaga, M., Katsuya, H., and Mizumura, K. (2002). Bradykinin lowers the threshold temperature for heat activation of vanilloid receptor 1. J. Neurophysiol. 88, 544–548. doi: 10.1152/jn.2002.88.1.544
Summers, T., Holec, S., and Burrell, B. D. (2014). Physiological and behavioral evidence of a capsaicin-sensitive TRPV-like channel in the medicinal leech. J. Exp. Biol. 217, 4167–4173. doi: 10.1242/jeb.110049
Sweat, K. G., Broatch, J., Borror, C., Hagan, K., and Cahill, T. M. (2016). Variability in capsaicinoid content and Scoville heat ratings of commercially grown Jalapeño, Habanero and bhut jolokia peppers. Food Chem. 210, 606–612. doi: 10.1016/j.foodchem.2016.04.135
Szallasi, A. (1995). Autoradiographic visualization and pharmacological characterization of vanilloid (capsaicin) receptors in several species, including man. Acta Physiol. Scand. Suppl. 629, 1–68.
Szallasi, A. (1996). Vanilloid-sensitive neurons: a fundamental subdivision of the peripheral nervous system. J. Peripher. Nerv. Syst. 1, 6–18.
Szallasi, A. (2016). Some like it hot (ever more so in the tropics): a puzzle with no solution. Temperature 3, 54–55. doi: 10.1080/23328940.2016.1139964
Szallasi, A., and Blumberg, P. M. (1989). Resiniferatoxin, a phorbol-related diterpene, acts as an ultrapotent analog of capsaicin, the irritant constituent in red pepper. Neuroscience 30, 515–520. doi: 10.1016/0306-4522(89)90269-8
Szallasi, A., and Blumberg, P. M. (1990). Specific binding of resiniferatoxin, an ultrapotent capsaicin analog, by dorsal root ganglion membranes. Brain Res. 524, 106–111. doi: 10.1016/0006-8993(90)90498-z
Szallasi, A., and Blumberg, P. M. (1999). Vanilloid (Capsaicin) receptors and mechanisms. Pharmacol. Rev. 51, 159–212.
Szallasi, A., Blumberg, P. M., Annicelli, L. L., Krause, J. E., and Cortright, D. N. (1999). The cloned rat vanilloid receptor VR1 mediates both R-type binding and C-type calcium response in dorsal root ganglion neurons. Mol. Pharmacol. 56, 581–587. doi: 10.1124/mol.56.3.581
Szallasi, A., Cortright, D. N., Blum, C. A., and Eid, S. R. (2007). The vanilloid receptor TRPV1: 10 years from channel cloning to antagonist proof-of-concept. Nat. Rev. Drug Discov. 6, 357–372. doi: 10.1038/nrd2280
Szelényi, Z., Hummel, Z., Szolcsányi, J., and Davis, J. B. (2004). Daily body temperature rhythm and heat tolerance in TRPV1 knockout and capsaicin pretreated mice. Eur. J. Neurosci. 19, 1421–1424. doi: 10.1111/j.1460-9568.2004.03221.x
Szolcsányi, J. (1984). “Capsaicin sensitive chemoprotective neural system with dual sensory-afferent function,” in Antidromic Vasodilatation and Neurogenic Inflammation, eds L. A. Chalk, J. Szolcsányi, and F. Lembeck (Budapest: Akadémiai Kiadó), 27–56.
Szolcsányi, J. (2004). Forty years in capsaicin research for sensory pharmacology and physiology. Neuropeptides 38, 377–384. doi: 10.1016/j.npep.2004.07.005
Szolcsányi, J. (2015). Effect of capsaicin on thermoregulation: an update with new aspects. Temperature 2, 277–296. doi: 10.1080/23328940.2015.1048928
Szolcsányi, J., and Jancsó-Gábor, A. (1975). Sensory effects of capsaicin congeners I. Relationship between chemical structure and pain-producing potency of pungent agents. Arzneimittelforschung 25, 1877–1881.
Szolcsányi, J., Jancśo-Gábor, A., and Joo, F. (1975). Functional and fine structural characteristics of the sensory neuron blocking effect of capsaicin. Naunyn Schmiedebergs Arch. Pharmacol. 287, 157–169. doi: 10.1007/bf00510447
Tamayo, N., Liao, H., Stec, M. M., Wang, X., Chakrabarti, P., Retz, D., et al. (2008). Design and synthesis of peripherally restricted transient receptor potential vanilloid 1 (TRPV1) antagonists. J. Med. Chem. 51, 2744–2757. doi: 10.1021/jm7014638
Tang, Y. H., Lu, R., Li, Y. J., Deng, H. W., and Liu, G. Z. (1997). Protection by capsaicin against attenuated endothelium-dependent vasorelaxation due to lysophosphatidylcholine. Naunyn Schmiedebergs Arch. Pharmacol. 356, 364–367. doi: 10.1007/pl00005063
Tian, Q., Hu, J., Xie, C., Mei, K., Pham, C., Mo, X., et al. (2019). Recovery from tachyphylaxis of TRPV1 coincides with recycling to the surface membrane. Proc. Natl. Acad. Sci. U.S.A. 116, 5170–5175. doi: 10.1073/pnas.1819635116
Tominaga, M., Caterina, M. J., Malmberg, A. B., Rosen, T. A., Gilbert, H., Skinner, K., et al. (1998). The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron 21, 531–543. doi: 10.1016/s0896-6273(00)80564-4
Turner, H., Fleig, A., Stokes, A., Kinet, J.-P., and Penner, R. (2003). Discrimination of intracellular calcium store subcompartments using TRPV1 (transient receptor potential channel, vanilloid subfamily member 1) release channel activity. Biochem. J. 371, 341–350. doi: 10.1042/BJ20021381
Urban, L., and Dray, A. (1991). Capsazepine, a novel capsaicin antagonist, selectively antagonises the effects of capsaicin in the mouse spinal cord in vitro. Neurosci. Lett. 134, 9–11. doi: 10.1016/0304-3940(91)90496-G
Usoskin, D., Furlan, A., Islam, S., Abdo, H., Lönnerberg, P., Lou, D., et al. (2015). Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat. Neurosci. 18, 145–153. doi: 10.1038/nn.3881
Utreras, E., Prochazkova, M., Terse, A., Gross, J., Keller, J., Iadarola, M. J., et al. (2013). TGF-β1 sensitizes TRPV1 through Cdk5 signaling in odontoblast-like cells. Mol. Pain 9:24. doi: 10.1186/1744-8069-9-24
Valenzano, K. J., Grant, E. R., Wu, G., Hachicha, M., Schmid, L., Tafesse, L., et al. (2003). N-(4-tertiarybutylphenyl)-4-(3-chloropyridin-2-yl)tetrahydropyrazine -1(2H)-carbox-amide (BCTC), a novel, orally effective vanilloid receptor 1 antagonist with analgesic properties: I. in vitro characterization and pharmacokinetic properties. J. Pharmacol. Exp. Ther. 306, 377–386. doi: 10.1124/jpet.102.045674
Vandewauw, I., De Clercq, K., Mulier, M., Held, K., Pinto, S., Van Ranst, N., et al. (2018). A TRP channel trio mediates acute noxious heat sensing. Nature 555, 662–666. doi: 10.1038/nature26137
Vécsei, L., Lukács, M., Tajti, J., Fülöp, F., Toldi, J., and Edvinsson, L. (2019). The therapeutic impact of new migraine discoveries. Curr. Med. Chem. 26, 6261–6281. doi: 10.2174/0929867325666180530114534
Vellani, V., Mapplebeck, S., Moriondo, A., Davis, J. B., and McNaughton, P. A. (2001). Protein kinase C activation potentiates gating of the vanilloid receptor VR1 by capsaicin, protons, heat and anandamide. J. Physiol. 534, 813–825. doi: 10.1111/j.1469-7793.2001.00813.x
Voets, T., Droogmans, G., Wissenbach, U., Janssens, A., Flockerzi, V., and Nilius, B. (2004). The principle of temperature-dependent gating in cold- and heat-sensitive TRP channels. Nature 430, 748–754. doi: 10.1038/nature02732
Vos, M. H., Neelands, T. R., McDonald, H. A., Choi, W., Kroeger, P. E., Puttfarcken, P. S., et al. (2006). TRPV1b overexpression negatively regulates TRPV1 responsiveness to capsaicin, heat and low pH in HEK293 cells. J. Neurochem. 99, 1088–1102. doi: 10.1111/j.1471-4159.2006.04145.x
Wagner, T., Poole, C., and Roth-Daniek, A. (2013). The capsaicin 8% patch for neuropathic pain in clinical practice: a retrospective analysis. Pain Med. 14, 1202–1211. doi: 10.1111/pme.12143
Wang, H., Bolognese, J., Calder, N., Baxendale, J., Kehler, A., Cummings, C., et al. (2008). Effect of morphine and pregabalin compared with diphenhydramine hydrochloride and placebo on hyperalgesia and allodynia induced by intradermal capsaicin in healthy male subjects. J. Pain 9, 1088–1095. doi: 10.1016/j.jpain.2008.05.013
Wang, S., Joseph, J., Ro, J. Y., and Chung, M.-K. (2015). Modality-specific mechanisms of protein kinase C-induced hypersensitivity of TRPV1: S800 is a polymodal sensitization site. Pain 156, 931–941. doi: 10.1097/j.pain.0000000000000134
Wang, S., Wang, S., Asgar, J., Joseph, J., Ro, J. Y., Wei, F., et al. (2017). Ca2+ and calpain mediate capsaicin-induced ablation of axonal terminals expressing transient receptor potential vanilloid 1. J. Biol. Chem. 292, 8291–8303. doi: 10.1074/jbc.M117.778290
Wanner, S. P., Garami, A., and Romanovsky, A. A. (2011). Hyperactive when young, hypoactive and overweight when aged: connecting the dots in the story about locomotor activity, body mass, and aging in Trpv1 knockout mice. Aging 3, 450–454. doi: 10.18632/aging.100306
Winning, A. J., Hamilton, R. D., Shea, S. A., and Guz, A. (1986). Respiratory and cardiovascular effects of central and peripheral intravenous injections of capsaicin in man: evidence for pulmonary chemosensitivity. Clin. Sci. 71, 519–526. doi: 10.1042/cs0710519
Witting, N., Svensson, P., Arendt-Nielsen, L., and Jensen, T. S. (2000). Repetitive intradermal capsaicin: differential effect on pain and areas of allodynia and punctate hyperalgesia. Somatosens. Mot. Res. 17, 5–12. doi: 10.1080/08990220070256
Wohlrab, J., Neubert, R. H. H., Heskamp, M.-L., and Michael, J. (2015). Cutaneous drug delivery of capsaicin after in vitro administration of the 8% capsaicin dermal patch system. Skin Pharmacol. Physiol. 28, 65–74. doi: 10.1159/000362740
Wood, J. N., Winter, J., James, I. F., Rang, H. P., Yeats, J., and Bevan, S. (1988). Capsaicin-induced ion fluxes in dorsal root ganglion cells in culture. J. Neurosci. 8, 3208–3220. doi: 10.1523/JNEUROSCI.08-09-03208.1988
Wooten, M., Weng, H.-J., Hartke, T. V., Borzan, J., Klein, A. H., Turnquist, B., et al. (2014). Three functionally distinct classes of C-fibre nociceptors in primates. Nat. Commun. 5:4122. doi: 10.1038/ncomms5122
Xu, X. J., Farkas-Szallasi, T., Lundberg, J. M., Hökfelt, T., Wiesenfeld-Hallin, Z., and Szallasi, A. (1997). Effects of the capsaicin analogue resiniferatoxin on spinal nociceptive mechanisms in the rat: behavioral, electrophysiological and in situ hybridization studies. Brain Res. 752, 52–60. doi: 10.1016/s0006-8993(96)01444-8
Yang, R., Xiong, Z., Liu, C., and Liu, L. (2014). Inhibitory effects of capsaicin on voltage-gated potassium channels by TRPV1-independent pathway. Cell Mol. Neurobiol. 34, 565–576. doi: 10.1007/s10571-014-0041-1
Yarmolinsky, D. A., Peng, Y., Pogorzala, L. A., Rutlin, M., Hoon, M. A., and Zuker, C. S. (2016). Coding and plasticity in the mammalian thermosensory system. Neuron 92, 1079–1092. doi: 10.1016/j.neuron.2016.10.021
Yarnitsky, D., Sprecher, E., Zaslansky, R., and Hemli, J. A. (1995). Heat pain thresholds: normative data and repeatability. Pain 60, 329–332. doi: 10.1016/0304-3959(94)00132-x
Zhang, F., Jara-Oseguera, A., Chang, T.-H., Bae, C., Hanson, S. M., and Swartz, K. J. (2018). Heat activation is intrinsic to the pore domain of TRPV1. Proc. Natl. Acad. Sci. U.S.A. 115, E317–E324. doi: 10.1073/pnas.1717192115
Zhang, X., Huang, J., and McNaughton, P. A. (2005). NGF rapidly increases membrane expression of TRPV1 heat-gated ion channels. EMBO J. 24, 4211–4223. doi: 10.1038/sj.emboj.7600893
Zhao, R., and Tsang, S. Y. (2017). Versatile roles of intracellularly located TRPV1 channel. J. Cell. Physiol. 232, 1957–1965. doi: 10.1002/jcp.25704
Keywords: capsaicin, TRPV1 receptor, ion channel, pain, sensory neuron, inflammation, thermoregulation
Citation: Fischer MJM, Ciotu CI and Szallasi A (2020) The Mysteries of Capsaicin-Sensitive Afferents. Front. Physiol. 11:554195. doi: 10.3389/fphys.2020.554195
Received: 21 April 2020; Accepted: 13 November 2020;
Published: 16 December 2020.
Edited by:
Istvan Nagy, Imperial College London, United KingdomReviewed by:
Peter M. Blumberg, Other, Frederick, United StatesLivio Luongo, University of Campania Luigi Vanvitelli, Italy
Michael Iadarola, National Institutes of Health Clinical Center (NIH), United States
Copyright © 2020 Fischer, Ciotu and Szallasi. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Arpad Szallasi, szallasiarpad@gmail.com
 Michael J. M. Fischer
Michael J. M. Fischer Cosmin I. Ciotu1
Cosmin I. Ciotu1 Arpad Szallasi
Arpad Szallasi