- 1Department of Psychiatry, The University of Melbourne, Parkville, VIC, Australia
- 2Department of General Practice, The University of Melbourne, Parkville, VIC, Australia
- 3NorthWestern Mental Health, Melbourne, VIC, Australia
- 4Florey Institute of Neuroscience and Mental Health, The University of Melbourne, Parkville, VIC, Australia
- 5Centre for Human Psychopharmacology, Swinburne University of Technology, Hawthorne, VIC, Australia
Cross-sectional studies have demonstrated that the brain-derived neurotrophic factor (BDNF) Val66Met single-nucleotide polymorphism moderates the association between exposure to negative life events and depression outcomes. Yet, it is currently unclear whether this moderating effect is applicable to positive life events and if the moderating effect is stable over time. To address these gaps in the literature, we examined clinical and BDNF genotypic data from a 5-year prospective cohort of 310 primary care attendees. Primary care attendees were selected based on existence of depressive symptoms at screening. Depressive symptoms were assessed at baseline and annually for 5 years post-baseline using the Primary Care Evaluation of Mental Disorders Patient Health Questionnaire-9 (PHQ-9). Linear mixed models assessed differences in depressive symptom severity over the 5-year follow-up period by BDNF Val66Met and history of life events, both negative and positive. Analysis identified a novel three-way interaction between the BDNF Val66Met polymorphism, history of severe childhood abuse, and time. Post hoc analysis stratified by time showed a two-way interaction between Val66Met and severe childhood abuse at baseline that was not detectable at any other time point. An interaction between Val66Met and positive life events was not detected. Our longitudinal results suggest that the BDNF Val66Met polymorphism moderates the depressive symptom severity experienced by those with a history of severe childhood abuse but does so in a time-dependent manner. Our results further support the notion that gene–environment–depression interactions are dynamic and highlight the importance of longitudinal assessment of these interactions. Given these novel longitudinal findings; replication is required.
Introduction
The etiologies of depression are poorly understood, with the most recent etiological theories focusing on gene–environment interactions. Depressive phenotypes have long been associated with exposure to negative events, yet phenotypic variation is evident, with individuals exposed to the same environmental stress either not developing depressive symptoms or developing a range of symptom severity (1). This phenotypic variation is the basis of the diathesis–stress psychological framework (2), which suggests individuals possess “risk” factors (e.g., genetic variants), which make them more vulnerable to developing psychological symptoms when exposed to an adverse event. Historically, the diathesis–stress framework has guided research into gene–environment interactions. However, an emerging alternative framework is that of differential susceptibility. The differential susceptibility framework postulates that traditionally defined “risk” factors may better be defined as “phenotypic plasticity” factors in that individuals carrying these factors are more sensitive to both positive and negative environmental influences (3).
Environmental influences consistently linked to depression include adverse childhood events (4, 5) and current domestic abuse among women (6). However, environmental stress alone has been identified as insufficient to cause depression (7), emphasizing the gene–environment hypothesis. Current literature clearly identifies numerous genetic variants associated with mediating the differential response to negative life events in the development of depression (8). Among these genes is the brain-derived neurotrophic factor (BDNF), specifically the Met allele in the Val66Met single-nucleotide polymorphism located in the coding region of exon 2 (rs6265 – A66G) (8). BDNF is a pro-survival factor, involved in brain cell survival and proliferation (8) and has also been suggested to affect neuronal plasticity (9). Depressed individuals are shown to have decreased serum BDNF levels compared to controls (10–12) and carriers of the BDNF rs6265 Met allele show a reduction in BDNF activity (13).
Studies examining the interaction between BDNF genetic variation and stressful life events have reported conflicting results. A number of studies identified an association between the BDNF Met allele and greater depressive symptom severity in the context of childhood abuse or adult abuse (14). Other studies reported that BDNF moderates the response to some, but not all, forms of abuse (15, 16). While two large studies, one a cross-sectional study (17) and another a case–control study (18) reported no moderating effect of BDNF genetic variation on the association between stressful life events and depressive symptoms.
In light of these conflicting results, the role of BDNF genetic variation in moderating depressive symptoms in the context of stressful life events remains unclear. In addition, the role BDNF plays in moderating depressive symptom severity in the context of life events over time is not clear. As such, we examined whether the BDNF Val66Met polymorphism moderated the relationship between life events (negative and positive) and depressive symptom severity in a 5-year prospective cohort of 310 primary care attendees.
Materials and Methods
Participants
Participants were recruited from the Diagnosis, Management and Outcomes of Depression in Primary Care (diamond) study, an ongoing prospective cohort that commenced in 2005 (19). The diamond study aims to document the experiences, health outcomes, treatment, and service usage of primary care attendees identified as having clinically relevant depressed mood at screening; with patients recruited from 30 rural and metropolitan general practices randomly recruited in Victoria, Australia (19). Primary care patients were eligible for the diamond cohort if they were: (a) aged 18–75 years, (b) able to read English, (c) not terminally ill, (d) did not reside in a nursing home, and (e) scored 16 or higher on the Center for Epidemiologic Studies Depression Scale (CES-D). Participants were assessed annually using postal surveys as well as computer-assisted telephone interviews. In 2011 (cohort year 6), participants enrolled in the cohort were invited to provide a saliva sample for DNA extraction and genotyping. All procedures were conducted in accord with principles expressed in the Declaration of Helsinki and obtained approval from the University of Melbourne Human Research Ethics Committee (Ethics ID 1135247.1).
Depressive Symptom Measures
Longitudinal depressive symptoms were assessed at baseline and annually for 5 years thereafter using the self-administered Primary Care Evaluation of Mental Disorders Patient Health Questionnaire-9 (PHQ-9) (20). The PHQ-9 is based directly on the nine signs and symptoms of major depressive disorder as described in the Diagnostic and Statistical Manual of Mental Disorders-IV (DSM-IV) (21) and has been validated to screen and monitor depression severity in the primary care setting (22). The PHQ-9 asks respondents to rate their symptoms over the past 2 weeks and is scored on a scale of zero (“not at all”) to three (“nearly every day”) for each item with a range of 0–27 (20). Scores of 5, 10, 15, and 20 on the PHQ-9 represent cut-points for mild, moderate, moderately severe, and severe depression, respectively (20). DSM-IV criteria (23) for major depressive disorder was assessed using the Composite International Diagnostic Interview (CIDI) Auto version 2.1 (24) by a trained research assistant.
Childhood Abuse
At baseline exposure to physical and sexual childhood abuse was measured using the Child Maltreatment History Self-Report (CMHSR) (25). The CMHSR consists of 11 items, 7 pertaining to physical abuse and 4 to sexual abuse. The physical abuse questions have a four-point response option (never, rarely, sometimes, often), whereas the sexual abuse questions have a yes/no response option. Severe abuse was defined using the scoring algorithm previously described by Macmillan and associates (25).
Partner Abuse
History of partner abuse in adulthood was measured using a history of partner fear as a proxy marker. This was done because the available composite abuse scale (CAS) in our cohort has not been validated in males as well as to minimize the risk of under-reporting associated with the CAS and to reflect the importance on an individual’s perception in the experience of abuse (26).
Life Events
At baseline, participants completed a 13-item life events questionnaire adapted from the Life Experiences Scale and Life Events Questionnaire (27, 28). A full list of events measured is displayed in Table S1 in Supplementary Material. For each life event, participants were asked to indicate whether or not they experienced the life event in the past 12 months and if so, what impact the event had on them. There were six possible responses for each life event: (1) No, I have not experienced this event in the past 12 months; (2) Yes, and it has had an extremely negative impact on me; (3) Yes and it has had a slightly negative impact on me; (4) Yes but it has had no impact on me; (5) Yes and it has had a slightly positive impact on me; (6) Yes and it has had an extremely positive impact on me. In this study, presence of a positive event was defined as one or more events reported as slightly or extremely positive. Presence of a negative event was defined as one or more events reported as slightly or extremely negative.
Potential Confounding Variables
At baseline, potential confounding factors, including demographics, smoking status, quality of life (29), and self-rated health status (30) were assessed. Panic and other anxiety syndromes were also assessed using the anxiety module of the PHQ (22). Alcohol and drug abuse/dependence (i.e., cannabis, opioid, sedative, cocaine, amphetamine, hallucinogens, inhalants) was assessed using the CIDI Auto version 2.1 (24). At each assessment point, the use of antidepressants, anxiolytics, and antipsychotics, as well as self-reported visits in the past 12 months to a psychiatrist and/or psychologist were also assessed.
DNA Extraction and Genotyping
DNA extraction and genotyping details have previously been published (31). In brief, DNA was recovered from stabilized saliva samples and the rs6265 polymorphism was genotyped as part of a larger genotyping project with the Sequenom MassARRAY MALDI-TOF genotyping system (Sequenom Inc., San Diego, CA, USA). To detect for the presence of population stratification, 60 unlinked ancestry-informative markers (AIMs; Table S2 in Supplementary Material) representing the three HapMap phase III populations (Northern/Western European, Han Chinese, and Yoruba in Nigeria) were also genotyped (32).
Statistical Analysis
Chi-square analysis of BDNF genotype was used to detect departures from Hardy–Weinberg equilibrium. Due to the low number of Met/Met carriers (n = 9), individuals who carried the Met allele (Val/Met or Met/Met) were compared to homozygous Val carriers. To estimate the presence of population stratification, the 60 AIMs were used to assign each participant to the HapMap ancestral group (Northern/Western European, Han Chinese, and Yoruba in Nigeria) for which they carried the greatest proportion of that population’s AIMs.
Linear mixed models were used to determine trajectory differences in PHQ-9 depressive symptom severity over the 5-year follow-up period by genotype and abuse/life event type. The mixed models approach enables use of all repeated measurements, accounts for clustering of participants within primary care sites, and provides unbiased estimates in the presence of missing data. Prior to creation of interaction terms and modeling, genotypes, abuse, and life event variables as well as covariates were centered (33). Potential covariates were assessed for their association with each of the abuse/life event types and genotypes using chi-square, Fisher’s exact, or analysis of variance tests, depending on the variable structure. Covariates with p-values ≤0.05 were retained for adjusted analysis (Table S3 in Supplementary Material).
The unadjusted model included fixed effects of time, genotype, abuse/event, and a time × genotype, time × abuse/event, genotype × abuse/event, and time × genotype × abuse/event interaction terms. Random effects included individual, primary care site, intercept (PHQ baseline score), and slope (time). Unadjusted models that returned significant main effects (p < 0.05) were then adjusted for covariates to account for any bias from baseline characteristics. Adjusted models included relevant covariates as well as covariate × time, covariate × genotype, and covariate × abuse/event type interaction terms as previously recommended (34). Covariance models used for the random and repeated effects were unstructured and first-order autoregressive, respectively.
Adjusted models were then refined using a backward stepwise penalized likelihood model selection strategy by starting with the most complex model and removing the term with the largest p-value above 0.05 using unrestricted maximum likelihood estimations. If removal of a covariate term increased the Bayesian information criterion (BIC) compared to previous more complex model the covariate was retained. Missing data of longitudinal measurements were assumed missing at random. All analyses were performed using SPSS 21.0 (IBM, Armonk, NY, USA).
Results
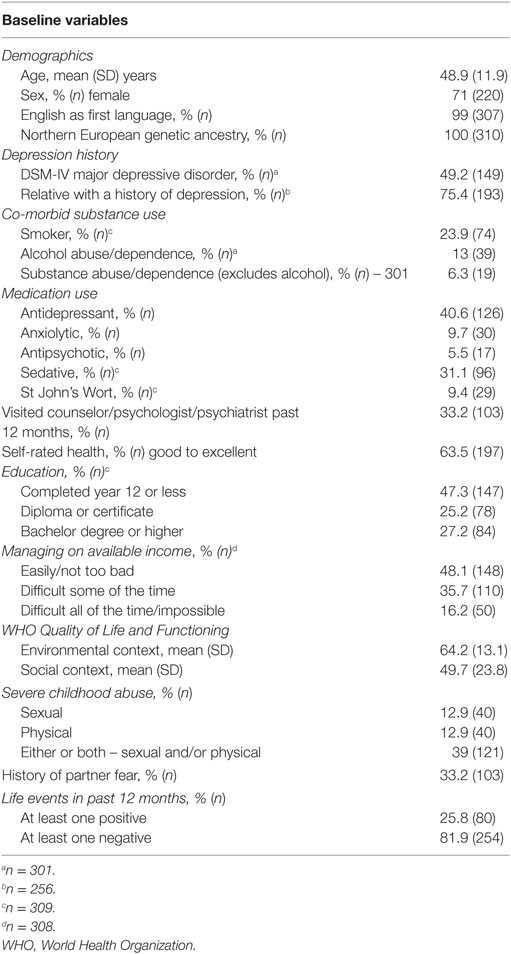
Sample Characteristics
A total of 789 participants were recruited into the diamond cohort of whom 498 were enrolled at the time of DNA collection (cohort year 6) and 344 (69%) consented and returned a DNA sample. Individuals missing genotype data (n = 22) or abuse data (n = 13) were excluded. A total sample of 310 participants was included in the analysis (Table 1). Genotype frequencies for BDNF rs6265 were 67% Val/Val, 33% Met carriers (30% Val/Met and 3% Met/Met) and were in Hardy–Weinberg equilibrium (p = 0.768). All participants were of Northern/Western European (CEU) ancestry based on 60 unlinked AIMs (Figure 1).
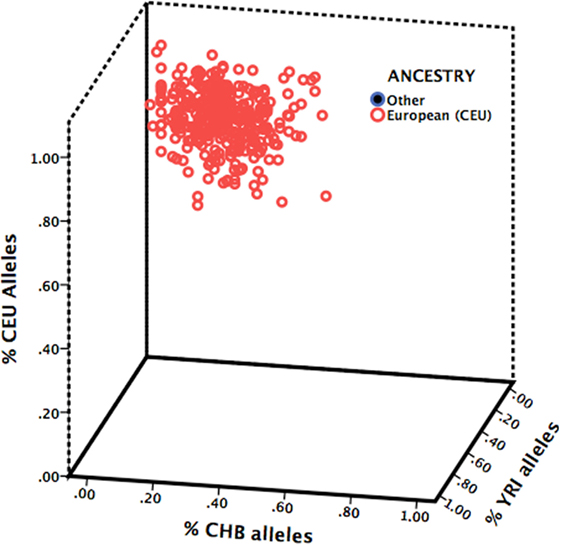
Figure 1. Ancestry estimation of study sample. Participants were assigned to the HapMap ancestral group (Northern/Western European, CEU; Han Chinese, CHB; or Yoruba in Nigeria, YRI) for which they carried the greatest proportion of that population’s ancestry-informative markers.
Unadjusted Findings
Unadjusted linear mixed models for the BDNF rs6265 genotype across the abuse and life event types are summarized in Table 2. A two-way interaction was not found between BDNF rs6265 and positive events (F1, 307 = 0.12, punadjusted = 0.728) or BDNF rs6265 and negative events (F1, 307 = 0.04, punadjusted = 0.844). However, a two-way interaction was detected between BDNF rs6265 and severe childhood abuse (F1, 307 = 4.6, punadjusted = 0.031) and a three-way interaction was observed between the BDNF rs6265 genotype, severe childhood abuse, and time (F1, 299 = 5.4, punadjusted = 0.021).
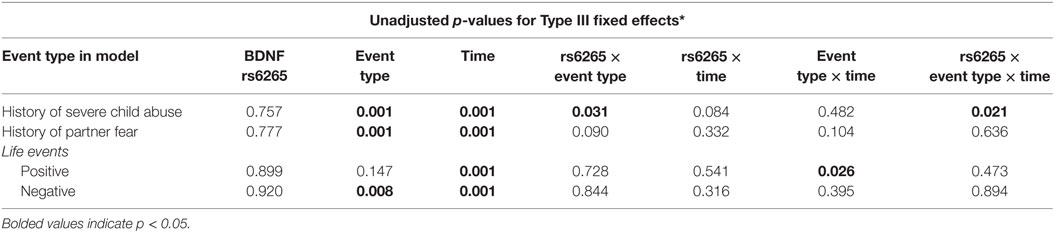
Table 2. General linear mixed model parameter p-values for main and interaction effects of BDNF rs6265 genotype, abusive experiences, and life events on 60-month depressive symptom trajectories (n = 310).
Covariate-Adjusted Findings
Only the three-way interaction survived adjustment for covariates (F1, 292 = 4.9, pcovariate adjusted = 0.027) (Table 3; Table S4 in Supplementary Material). Figure 2 shows Met allele carriers with a history of severe childhood abuse had a significantly greater reduction in depressive symptom severity over the 5-year follow-up period compared to their Val/Val carrying counterparts. Post hoc analysis stratified by time showed the two-way interaction between BDNF rs6265 and severe childhood abuse was present at baseline (F1, 297 = 5.4, punadjusted = 0.021) but no other time points (Figure 3). Importantly, further post hoc analyses suggested this time-dependent effect was not due to differential receipt of psychosocial (i.e., visits to counselor, psychologist, or psychiatrist) or pharmacological (i.e., antidepressant, anxiolytic, or antipsychotic) therapy over the 5-year study period (Figures 4 and 5).
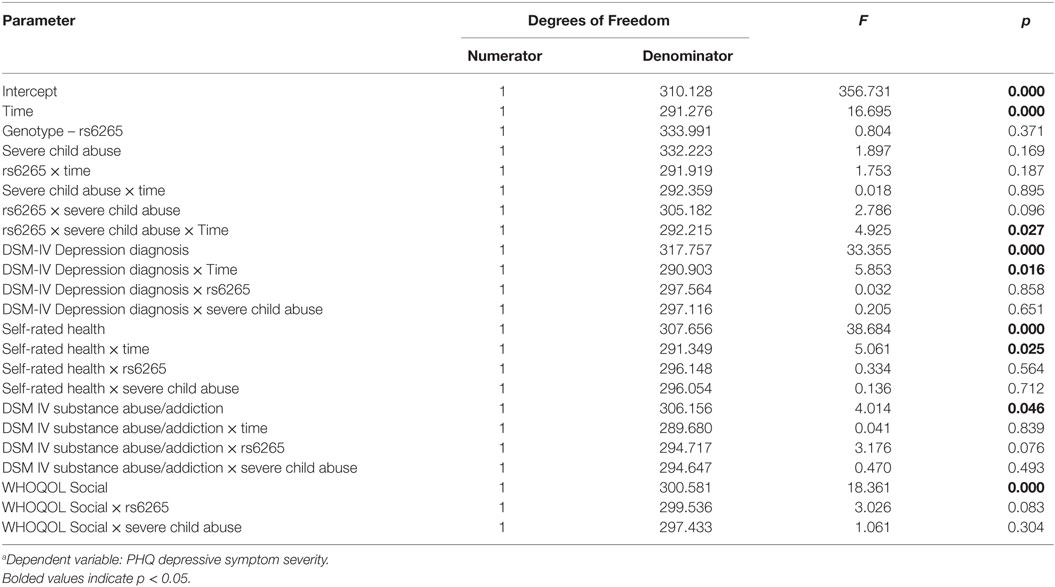
Table 3. Type III fixed effectsa for terms included in the final covariate-adjusted linear mixed model.
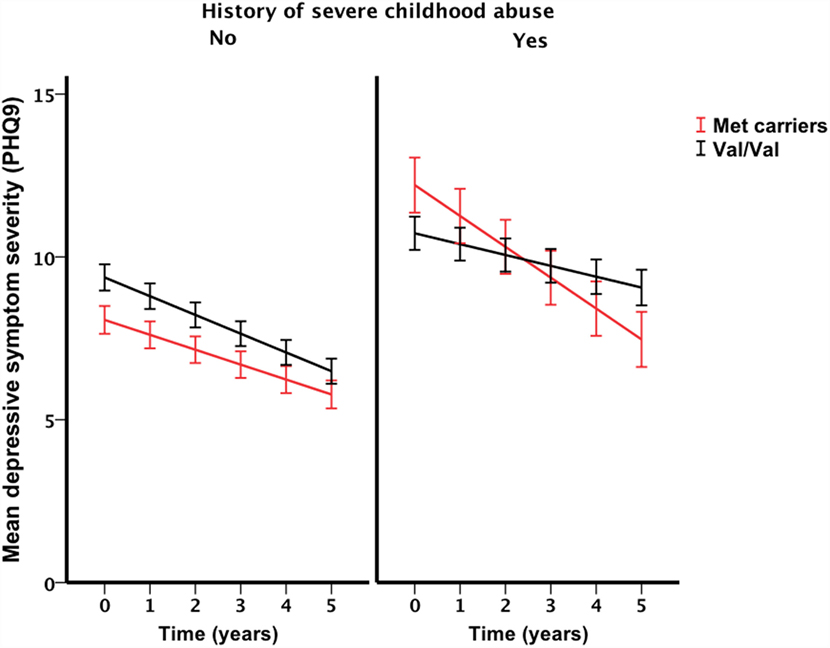
Figure 2. Interaction effect of severe childhood abuse and BDNF Val66Met genotype on 5-year depressive symptom trajectories. Baseline represented by time point 0, annual assessment thereafter. Points and associated SE bars represent predicted values based on the final covariate-adjusted model. PHQ-9 = Primary Care Evaluation of Mental Disorders Patient Health Questionnaire-9.
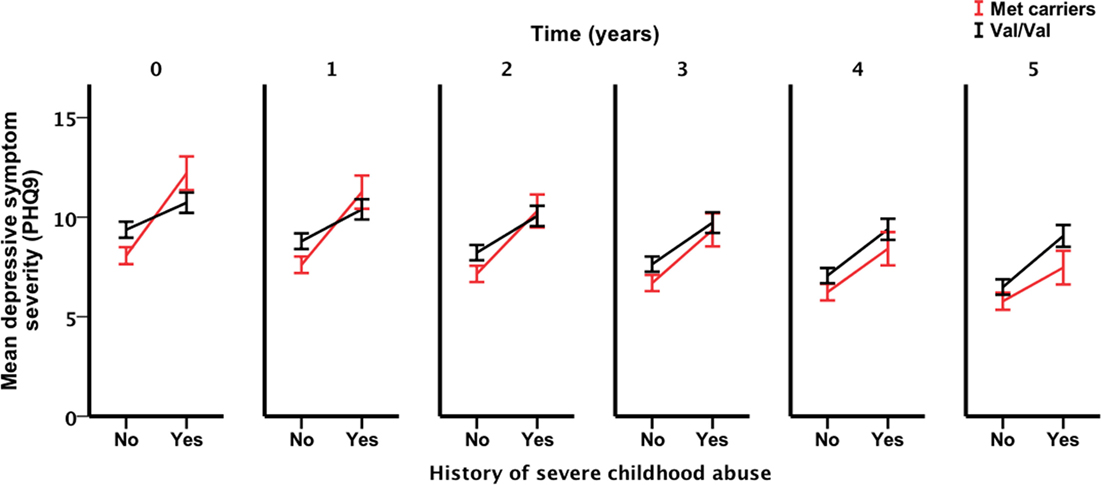
Figure 3. Interaction effect of severe child abuse and BDNF rs6265 genotype by time point. Time point zero represents baseline, measured annually from baseline. Points and associated SE bars represent predicted values based on the final covariate-adjusted model. PHQ-9 = Primary Care Evaluation of Mental Disorders Patient Health Questionnaire-9.
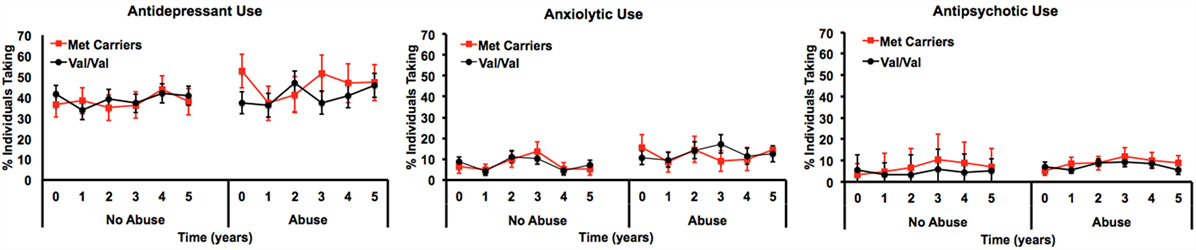
Figure 4. Longitudinal measurements of medication usage by percent of individuals reportedly taking the medication by genotype and history of severe child abuse.
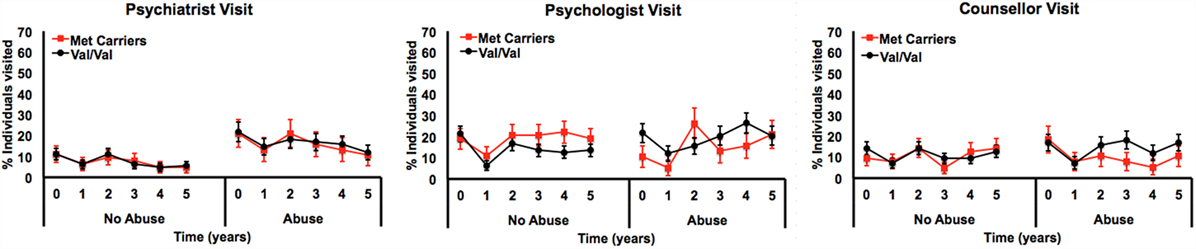
Figure 5. Longitudinal measurements of psychosocial treatment by percent of individuals reported as having seen a psychiatrist, psychologist, or counselor once or more in the preceding 12 months, by genotype and history of severe child abuse.
Discussion
We demonstrated a novel three-way interaction between the BDNF Val66Met polymorphism, a history of severe childhood abuse and time. This interaction withstood adjustment for key covariates and could not be attributed to differential receipt of psychosocial or pharmacological therapy. Previous studies have showed the BDNF Val66Met polymorphism moderates the relationship between history of childhood adversity and depression outcomes (15, 35, 36). Our longitudinal results not only support these previous findings but also suggest the moderating effect conferred by the BDNF Val66Met polymorphism may not be stable over time. In fact, we showed the presence of the interaction between BDNF rs6265 and severe childhood abuse was dependent on the time at which it was assessed; suggesting time as a key factor when testing gene–environment interactions.
The stability of gene–environment–depression interactions over time has only recently been described (37) but to our knowledge the current study is the first to demonstrate that these interactions may not be stable over time. Demonstrating the stability of a gene–environment interaction is critical to our conceptual and clinical understanding of depression and related outcomes. For example, at baseline, our analysis detected an interaction that is consistent with the differential susceptibility framework (3) in that Met allele carriers reported fewer depressive symptoms in the absence of severe childhood abuse and greater depressive symptoms in the presence of a history of severe childhood abuse, compared to Val/Val individuals who had similar depressive symptom severity regardless of their history of childhood abuse. However, over the course of 5 years, this interaction was gradually attenuated and support for the differential susceptibility framework was diminished. Furthermore, the presence of a time-dependent interaction may in part explain the sub-optimal reproducibility of BDNF by environment interactions in previous cross-sectional studies. Although other explanations for this interaction effect instability likely exist, we ruled out many of these explanations (e.g., therapy exposure) in our analyses. Nevertheless, further longitudinal examinations of BDNF by environment interactions are required to validate our findings.
Potential Mechanism(s)
The mechanism(s) by which the BDNF rs6265 polymorphism moderates the relationship between severe childhood abuse and depressive symptom severity in adulthood is unclear and is beyond the scope of this study. However, previous studies have shown BDNF gene expression and serum protein levels are decreased in depressed individuals compared to healthy controls (10–12) and are also decreased in individuals with the Met allele (13), albeit in healthy individuals Val/Val carriers had decreased BDNF levels (38). Nevertheless, there is modest evidence for a link between peripheral BDNF levels and depressive symptoms. Importantly, a number of studies have shown that serum BDNF levels increase with administration of antidepressant medication (39), which is negatively correlated with depressive symptom severity (10, 11). Additionally, Alder and Thakker-Varia (9) suggest that BDNF plays a role in neuronal plasticity, particularly emotional processing networks that are compromised in depression. However, further research into the effects of BDNF on neuroplasticity and neurodevelopment using post-mortem brain tissue and neuroimaging cohorts with childhood abuse exposure and depressive symptom data are needed before firm conclusions can be made on the moderating mechanisms by which BDNF genotypic variation acts.
Strengths and Limitations
This study has several notable strengths, including the longitudinal study design, comprehensive covariate adjustment, and centering of data. However, a number of limitations should be acknowledged. First, the assessment of environmental exposures and self-reporting of abuse is limited and subject to bias. Specifically, within the childhood abuse group, only those who had experienced severe childhood abuse either physical or sexual were included in analysis. Emotional abuse or neglect experienced during childhood has also been associated with depressive symptoms (40) yet were not considered in this study. Validity of self-reporting has also been questioned due to significant recall bias, often resulting in under-reporting of abuse (41) in both reporting stressful life events and recalling adverse childhood experiences (41, 42). Thus, our findings may represent a conservative estimate, which would dilute the interaction effect within the sample population and could contribute to negative results. Second, our measures of positive environmental exposure (e.g., positive life events) were sub-optimal and only capture adulthood experiences. Measures of “positive” exposures in childhood, such as maternal attachment or parental engagement, would be ideal but were not available. Third, BDNF serum levels were not measured in our study. Given that BDNF serum levels have been negatively correlated with depressive symptoms (39) and carriage of the Met allele (13), future study into BDNF genetic variation as a moderator of life events on depressive symptoms should include measurement of BDNF serum levels. Finally, rather than diagnosis of major depressive disorder, depressive symptom severity was the primary outcome measure limiting extrapolation and comparison to similar studies. However, the PHQ-9 scale consists of the nine diagnostic criteria outlined in DSM-IV and has been validated as a tool for assessing depressive symptom trajectories (20) and may translate better into the primary care setting.
Conclusion
We have identified a novel three-way interaction between BDNF genetic variation, a history of severe childhood abuse, and time that was associated with depressive symptom severity. This interaction highlights the dynamic nature of gene–environment–depression interactions and the importance of longitudinal assessment of these interactions. Discrepancies among previous study results could be associated with not just study design, but also time of analysis. Given this is the first longitudinal study of its kind; replication of results is needed.
Author Contributions
JG conceived and established the diamond cohort. CB, IE, and JG conceived the current study design and analysis. MP project managed the data collection. CW and CB conducted the analysis and wrote the first draft of the manuscript. All authors contributed to further drafts of the manuscript. All authors have read and approve the current version of the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
The reviewer CO declared a shared affiliation, though no collaboration or input in the work presented in this article, with the authors, to the handling Editor, who ensured that the process nevertheless met the standards of a fair and objective review.
Acknowledgments
The diamond study is funded by the National Health and Medical Research Council (IDs 299869, 454463, 566511, and 1002908) and the Victorian Centre for Excellence in Depression and Related Disorders, an initiative between beyondblue and the Victorian Government. The collection of DNA and genotyping was funded by the LEW Carty Chartable Fund (ID 7284). No funding body had a role in the study design; the collection, analysis, and interpretation of data; or the writing of the manuscript for publication. We acknowledge the 30 dedicated GPs, their patients, and practice staff for making this research possible. We thank the diamond project team, including associate investigators and researchers involved in the diamond study: Ms. Aves Middleton, Ms. Konstancja Densley, Professor Helen Herrman, Professor Christopher Dowrick, Dr. Gursharan Chana, and casual research staff.
Supplementary Material
The Supplementary Material for this article can be found online at http://journal.frontiersin.org/article/10.3389/fpsyt.2016.00151
References
1. Heim C, Binder EB. Current research trends in early life stress and depression: review of human studies on sensitive periods, gene-environment interactions, and epigenetics. Exp Neurol (2012) 233(1):102–11. doi:10.1016/j.expneurol.2011.10.032
2. Monroe SM, Simons AD. Diathesis-stress theories in the context of life stress research: implications for the depressive disorders. Psychol Bull (1991) 110(3):406. doi:10.1037/0033-2909.110.3.406
3. Belsky J, Jonassaint C, Pluess M, Stanton M, Brummett B, Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry (2009) 14(8):746–54. doi:10.1038/mp.2009.44
4. Arnow BA. Relationships between childhood maltreatment, adult health and psychiatric outcomes, and medical utilization. J Clin Psychiatry (2004) 65:10–5.
5. Chapman DP, Whitfield CL, Felitti VJ, Dube SR, Edwards VJ, Anda RF. Adverse childhood experiences and the risk of depressive disorders in adulthood. J Affect Disord (2004) 82(2):217–25. doi:10.1016/j.jad.2003.12.013
6. McCauley J, Kern DE, Kolodner K, Dill L, Schroeder AF, DeChant HK, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA (1997) 277(17):1362–8. doi:10.1001/jama.277.17.1362
7. Maniglio R. Child sexual abuse in the etiology of depression: a systematic review of reviews. Depress Anxiety (2010) 27(7):631–42. doi:10.1002/da.20687
8. Mandelli L, Serretti A. Gene environment interaction studies in depression and suicidal behavior: an update. Neurosci Biobehav Rev (2013) 37(10):2375–97. doi:10.1016/j.neubiorev.2013.07.011
9. Thakker-Varia S, Alder J. Neuropeptides in depression: role of VGF. Behav Brain Res (2009) 197(2):262–78. doi:10.1016/j.bbr.2008.10.006
10. Matrisciano F, Bonaccorso S, Ricciardi A, Scaccianoce S, Panaccione I, Wang L, et al. Changes in BDNF serum levels in patients with major depression disorder (MDD) after 6 months treatment with sertraline, escitalopram, or venlafaxine. J Psychiatr Res (2009) 43(3):247–54. doi:10.1016/j.jpsychires.2008.03.014
11. Yoshimura R, Mitoma M, Sugita A, Hori H, Okamoto T, Umene W, et al. Effects of paroxetine or milnacipran on serum brain-derived neurotrophic factor in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry (2007) 31(5):1034–7. doi:10.1016/j.pnpbp.2007.03.001
12. Piccinni A, Marazziti D, Catena M, Domenici L, Del Debbio A, Bianchi C, et al. Plasma and serum brain-derived neurotrophic factor (BDNF) in depressed patients during 1 year of antidepressant treatments. J Affect Disord (2008) 105(1):279–83. doi:10.1016/j.jad.2007.05.005
13. Egan MF, Kojima M, Callicott JH, Goldberg TE, Kolachana BS, Bertolino A, et al. The BDNF val66met polymorphism affects activity-dependent secretion of BDNF and human memory and hippocampal function. Cell (2003) 112(2):257–69. doi:10.1016/S0092-8674(03)00035-7
14. Bukh JD, Bock C, Vinberg M, Werge T, Gether U, Kessing LV. Interaction between genetic polymorphisms and stressful life events in first episode depression. J Affect Disord (2009) 119(1):107–15. doi:10.1016/j.jad.2009.02.023
15. Aguilera M, Arias B, Wichers M, Barrantes-Vidal N, Moya J, Villa H, et al. Early adversity and 5-HTT/BDNF genes: new evidence of gene-environment interactions on depressive symptoms in a general population. Psychol Med (2009) 39(9):1425–32. doi:10.1017/S0033291709005248
16. Brown GW, Craig TK, Harris TO, Herbert J, Hodgson K, Tansey KE, et al. Functional polymorphism in the brain-derived neurotrophic factor gene interacts with stressful life events but not childhood maltreatment in the etiology of depression. Depress Anxiety (2014) 31(4):326–34. doi:10.1002/da.22221
17. Elzinga BM, Molendijk ML, Voshaar RCO, Bus BA, Prickaerts J, Spinhoven P, et al. The impact of childhood abuse and recent stress on serum brain-derived neurotrophic factor and the moderating role of BDNF Val66Met. Psychopharmacology (2011) 214(1):319–28. doi:10.1007/s00213-010-1961-1
18. Lavebratt C, Åberg E, Sjöholm LK, Forsell Y. Variations in FKBP5 and BDNF genes are suggestively associated with depression in a Swedish population-based cohort. J Affect Disord (2010) 125(1):249–55. doi:10.1016/j.jad.2010.02.113
19. Gunn JM, Gilchrist GP, Chondros P, Ramp M, Hegarty KL, Blashki GA, et al. Who is identified when screening for depression is undertaken in general practice? Baseline findings from the Diagnosis, Management and Outcomes of Depression in Primary Care (diamond) longitudinal study. Med J Aust (2008) 188(12):S119.
20. Kroenke K, Spitzer RL, Williams JB. The PHQ-9 – validity of a brief depression severity measure. J Gen Intern Med (2001) 16(9):606–13. doi:10.1046/j.1525-1497.2001.016009606.x
21. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: DSM-IV-TR. 4th ed. Washington, DC: American Psychiatric Association (2000).
22. Spitzer RL, Kroenke K, Williams JB, Group PHQPCS. Validation and utility of a self-report version of PRIME-MD: the PHQ primary care study. JAMA (1999) 282(18):1737–44. doi:10.1001/jama.282.18.1737
23. American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders: Diagnostic Criteria from DSM-IV. Washington, DC: American Psychiatric Association (1994).
24. World Health Organization. Composite International Diagnostic Interview (CIDI), Version 2.1, 12-Months. Geneva: World Health Organization (1997).
25. MacMillan HL, Fleming JE, Trocmé N, Boyle MH, Wong M, Racine YA, et al. Prevalence of child physical and sexual abuse in the community: results from the Ontario Health Supplement. JAMA (1997) 278(2):131–5. doi:10.1001/jama.1997.03550020063039
26. Loxton D, Powers J, Fitzgerald D, Forder P, Anderson A, Taft A, et al. The community Composite Abuse Scale: reliability and validity of a measure of intimate partner violence in a community survey from the ALSWH. J Womens Health, Issues Care (2013) 2:4. doi:10.4172/2325-9795.1000115
27. Norbeck JS. Modification of life event questionnaires for use with female respondents. Res Nurs Health (1984) 7(1):61–71. doi:10.1002/nur.4770070110
28. Sarason IG, Johnson JH, Siegel JM. Assessing the impact of life changes: development of the Life Experiences Survey. J Consult Clin Psychol (1978) 46(5):932. doi:10.1037/0022-006X.46.5.932
29. Organization WH. The World Health Organization quality of life assessment (WHOQOL): development and general psychometric properties. Soc Sci Med (1998) 46(12):1569–85. doi:10.1016/S0277-9536(98)00009-4
30. Ware JE Jr, Kosinski M, Keller SD. A 12-item short-form health survey: construction of scales and preliminary tests of reliability and validity. Med Care (1996) 34(3):220–33. doi:10.1097/00005650-199603000-00003
31. Bousman CA, Potiriadis M, Everall IP, Gunn JM. Methylenetetrahydrofolate reductase (MTHFR) genetic variation and major depressive disorder prognosis: a five-year prospective cohort study of primary care attendees. Am J Med Genet B Neuropsychiatr Genet (2014) 165(1):68–76. doi:10.1002/ajmg.b.32209
32. Enoch M-A, Shen P-H, Xu K, Hodgkinson C, Goldman D. Using ancestry-informative markers to define populations and detect population stratification. J Psychopharmacol (2006) 20(4 Suppl):19–26. doi:10.1177/1359786806066041
33. Kraemer HC, Blasey CM. Centring in regression analyses: a strategy to prevent errors in statistical inference. Int J Methods Psychiatr Res (2004) 13(3):141–51. doi:10.1002/mpr.170
34. Keller MC. Gene × environment interaction studies have not properly controlled for potential confounders: the problem and the (simple) solution. Biol Psychiatry (2014) 75(1):18–24. doi:10.1016/j.biopsych.2013.09.006
35. Juhasz G, Dunham JS, McKie S, Thomas E, Downey D, Chase D, et al. The CREB1-BDNF-NTRK2 pathway in depression: multiple gene-cognition-environment interactions. Biol Psychiatry (2011) 69(8):762–71. doi:10.1016/j.biopsych.2010.11.019
36. Hosang GM, Shiles C, Tansey KE, McGuffin P, Uher R. Interaction between stress and the BDNF Val66Met polymorphism in depression: a systematic review and meta-analysis. BMC Med (2014) 12(1):7. doi:10.1186/1741-7015-12-7
37. Bousman CA, Gunn JM, Potiriadis M, Everall IP. Polygenic phenotypic plasticity moderates the effects of severe childhood abuse on depressive symptom severity in adulthood: a 5-year prospective cohort study. World J Biol Psychiatry (2016):1–20. doi:10.3109/15622975.2016.1153710
38. Lang UE, Hellweg R, Sander T, Gallinat J. The Met allele of the BDNF Val66Met polymorphism is associated with increased BDNF serum concentrations. Mol Psychiatry (2009) 14(2):120–2. doi:10.1038/mp.2008.80
39. Sen S, Duman R, Sanacora G. Serum brain-derived neurotrophic factor, depression, and antidepressant medications: meta-analyses and implications. Biol Psychiatry (2008) 64(6):527–32. doi:10.1016/j.biopsych.2008.05.005
40. Spertus IL, Yehuda R, Wong CM, Halligan S, Seremetis SV. Childhood emotional abuse and neglect as predictors of psychological and physical symptoms in women presenting to a primary care practice. Child Abuse Negl (2003) 27(11):1247–58. doi:10.1016/j.chiabu.2003.05.001
41. Hardt J, Rutter M. Validity of adult retrospective reports of adverse childhood experiences: review of the evidence. J Child Psychol Psychiatry (2004) 45(2):260–73. doi:10.1111/j.1469-7610.2004.00218.x
Keywords: depression, gene–environment, brain-derived neurotrophic factor, childhood adversity, stressful life events, longitudinal cohort
Citation: Webb C, Gunn JM, Potiriadis M, Everall IP and Bousman CA (2016) The Brain-Derived Neurotrophic Factor Val66Met Polymorphism Moderates the Effects of Childhood Abuse on Severity of Depressive Symptoms in a Time-Dependent Manner. Front. Psychiatry 7:151. doi: 10.3389/fpsyt.2016.00151
Received: 11 March 2016; Accepted: 17 August 2016;
Published: 29 August 2016
Edited by:
Ming D. Li, Seton Hall University, USAReviewed by:
Carlos M. Opazo, The University of Melbourne, AustraliaZhongli Yang, Zhejiang University, China
Copyright: © 2016 Webb, Gunn, Potiriadis, Everall and Bousman. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) or licensor are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Chad A. Bousman, cbousman@unimelb.edu.au
 Caitlin Webb1
Caitlin Webb1 Jane M. Gunn
Jane M. Gunn Chad A. Bousman
Chad A. Bousman