Canine and feline papillomaviruses: an update
- 1Institute for Innovation, Capacity Building and Sustainability of Agri-food Production (Inov4Agro), Centre for Research and Technology of Agro-Environmental and Biological Sciences (CITAB), Vila Real, Portugal
- 2Molecular Oncology and Viral Pathology Group, Research Center of IPO Porto (CI-IPOP)/Health Research Network (RISE)@CI-IPOP, Portuguese Oncology Institute of Porto (IPO Porto)/Porto Comprehensive Cancer Center (Porto.CCC), Porto, Portugal
- 3Department of Zootechnics, School of Sciences and Technology, University of Évora, Évora, Portugal
- 4Comprehensive Health Research Center (CHRC), Évora, Portugal
- 5Faculty of Medicine, University of Porto, Porto, Portugal
- 6Abel Salazar Institute for the Biomedical Sciences, Porto, Portugal
- 7FP-I3ID, FP-ENAS, FP-BHS, University Fernando Pessoa, Porto, Portugal
- 8Faculty of Health Sciences, University Fernando Pessoa, Porto, Portugal
- 9Research Department, Portuguese League Against Cancer (NRNorte), Porto, Portugal
- 10Department of Veterinary Sciences, University of Trás-os-Montes and Alto Douro, Vila Real, Portugal
- 11Laboratory for Process Engineering, Environment, Biotechnology and Energy (LEPABE), Faculty of Engineering, University of Porto, Porto, Portugal
- 12Postgraduate Programme in Adult Health (PPGSAD), Department of Morphology, Federal University of Maranhão (UFMA), UFMA University Hospital (HUUFMA), São Luís, Brazil
Papillomaviruses are small viruses able to cause disease not only in mammalians, but also in birds and reptiles. In recent years, a rising number of papillomaviruses have been identified in dogs and cats, totaling 24 canine papillomavirus (CPV) and six feline papillomavirus (FcaPV). The canine and feline papillomaviruses (CPVs and FcaPVs, respectively) are responsible for multiple lesions in these domestic species but the potential pathological relevance of some recently identified types remains to be determined. CPVs are associated with oral papillomatosis, cutaneous papillomas and viral pigmented plaques, and have been rarely associated with the development of oral and cutaneous squamous cell carcinomas in their canine hosts. FcaPVs are associated with oral papillomas, viral plaques, and Bowenoid in situ carcinomas. The present review provides readers with the more recent advances on dog and cat papillomavirus research, bringing an update on this field to both veterinary practitioners and the virology community at large.
1. Introduction
Papillomaviruses are small, icosahedral, non-enveloped viruses with a capsid that involves the genome of a double-stranded circular DNA molecule. These viruses are able to cause multiple epithelial lesions not only in mammalians, but also in birds and reptiles (1). Their genome contains approximately 8,000 base pairs (bp) and includes five or six early (E) and two late (L) open reading frames (ORF) (2). Papillomaviruses are classified considering the L1 ORF sequence, with the papillomavirus of the same genus having more than 60% L1 ORF similarity and presenting similar host, location, and behavior. Different types of papillomaviruses have a similarity lower than 90% in the L1 ORF sequence (3). Additionally to this genome-based taxonomic classification, papillomaviruses may be also classified as those that cause hyperplastic papillomas (warts) or those that infect without causing clinical lesions (asymptomatic) (4). The papillomaviruses that cause warts may be transmitted between animals directly or via fomites, and mainly affect young adults. After infection, these papillomaviruses stimulate fast replication of epithelial cells, leading to epithelial hyperplasia and the development of a wart (5–7). In turn, warts stimulate an immune response inhibiting virus replication that results in the spontaneous resolution of the lesion (8). The majority of papillomaviruses do not cause a visible lesion, because they stimulate slow replication of epithelium (9, 10), and, usually, they are acquired during or soon after birth (11). Although there are few studies addressing the subclinical infection by papillomavirus in dogs and cats, a subclinical infection was already noticed in these species, suggesting that it is ubiquitous in companion animals, as observed in humans (12–16). Furthermore to the classifications presented above, as some of the papillomaviruses can be only detected in hyperplastic lesions and others may be detected in neoplastic lesions, the papillomavirues may also be classified as low-risk types (those asymptomatic or causing self-resolving warts) or high-risk types (those can cause neoplasia) (6).
Excepting some types of papillomaviruses from genus Deltapapillomavirus, papillomaviruses are highly species-specific (3, 6, 12). Bearing in mind their tissue specificity, papillomaviruses can be classed into those that affect cutaneous sites and those affecting mucosal sites. The life cycle of papillomavirus is coordinated with the division and maturation of stratified epithelium cells (4, 17). A papillomavirus infection initiates with the contact of the viral particles with the basal cells of the epithelium, after microtrauma (18). After infecting the keratinocytes of the basal layer, the viral genome multiples within the suprabasal epithelial layers. As the cells replicate, the daughter cells contain the papillomavirus DNA, allowing the persistence of the infection. The continuous replication of epithelial cells infected with the virus allows viral genome amplification leading to the formation of cells containing multiple viral copies (19).
Papillomaviruses induce a wide spectrum of lesions in animals, ranging from short-lived papillomas that regress spontaneously, to cancers. This behavior can be observed with those papillomaviruses infecting companion animals—the canine papillomaviruses (CPVs) and feline papillomaviruses (FcaPVs), with an increasing number of new viruses infecting dogs and cats identified over the years. There are many similarities between the diseases caused by papillomaviruses in humans and those caused by these viruses in animals like cattle, dogs, and cats (20). Considering the species and tissue specificity of papillomaviruses, their study in animals is essential to better understand viral biology and pathogenesis, and to search for new and more effective strategies to fight viral infection and its consequences (21, 22). Accordingly, this work aims to provide the readers with the more recent advances on dog and cat papillomavirus research, bringing an update on this field to both veterinary practitioners and scientific community.
2. Papillomaviruses in animals
Approximately 450 different types of human papillomavirus (HPV), classed into five genera (Alphapapillomavirus, Betapapillomavirus, Gammapapillomavirus, Mupapillomavirus, and Nupapillomavirus), were identified over the years (4). A lower number of papillomaviruses were identified in animals (23). However, the increasing interest and study of animal papillomaviruses in the last years, allowed the identification of more non-human papillomaviruses. Some of the best known papillomaviruses among animals affect mostly domestic species, since they are closer to humans and their lesions are more frequently detected by owners and reported (20). Ongoing studies in this field will continue to unveil more human and non-human papillomavirus and viral types.
3. Canine papillomaviruses (CPVs)
Presently, 24 types of CPVs have been identified in dogs, most of which associated with both mucosal and cutaneous lesions. Most of the types belong to the genus Chipapillomavirus (CPV 3, 4, 5, 8, 9, 10, 11, 12, 14, 15, 16, 18, 19, and 24), and the remaining types belong to the genus Lambdapapillomavirus (CPV 1 and 6) or the genus Taupapillomavirus (CPV 2, 7, 13, 17, 20, 21, 22, and 23) (Table 1). These papillomaviruses have tropism for different organs, with almost of all affecting the skin. Some of them, namely CPVs 1, 2, 3, 4, 6, 8, 13, 17, and 19, have tropism for more than one organ, affecting the skin and oral cavity. However, the tissue tropism and the possible pathogenicity of CPVs 20, 21, 22, and 23 remains to be determined (24–26) (Table 1). Over the years, CPVs have been classically associated with oral papilloma, cutaneous papilloma, inverted papilloma, and pigmented plaques in dogs. Rarely have these viruses been associated with the development of oral and cutaneous squamous cell carcinomas (SCCs) in this species, mostly under conditions of immune suppression The Lambdapapillomavirus are associated with oral and cutaneous papillomas. CPV1, in particular, is also associated with inverted papillomas and conjunctival epithelial hyperplasia. The papillomavirus belonging to the Chi genus are related to viral pigmented plaques and cutaneous squamous cell carcinomas, while the Taupapillomavirus are linked to cutaneous papillomas (24–26) (Table 1).
Cutaneous warts have been considered the second most frequent skin tumor in dogs under 1 year of age (27). These lesions may be induced by CPV1 or CPV2, or both types simultaneously (28, 29). Warts are frequently found on the feet and around the face and ears (30). Most canine cutaneous warts regress spontaneously, within 3 months, and do not cause discomfort. However, some of them may persist for 2 years before regressing (30). The progression of cutaneous warts into SCCs is extremely rare. Cutaneous warts in the anogenital region are rarely reported in dogs (31).
Oral papillomatosis in dogs presents as multiple exophytic smooth or cauliflower-like warts on the lips and mouth. Like cutaneous papillomatosis, oral papillomas are frequent in young animals (30). Most of oral warts are caused by CPV1 and regress spontaneously within 4–8 weeks (32, 33). In few cases, dogs can develop further warts that increase in size over a year and can spread from the oral cavity to the haired skin or progress to SCC (30). Few reports have suggested that the transmission between dogs is possible (8, 34).
Viral cutaneous plaques, also called pigmented plaques, are rarely reported in dogs. The development of these lesions has been associated with Chipapillomavirus types, and with immunosuppressive conditions and breed predisposition (35). The pigmented plaques are usually dark and multiple, and common on the ventral and medial aspects of the limbs (36). Extensive plaque can cause pruritus and pain, but in the majority of the cases the plaques do not impact the animals’ life and can regress spontaneously (35, 36). There are reports of HPV-associated canine cutaneous and oral SCC, but there is limited evidence suggesting the involvement of papillomaviruses in this kind of lesion (37). CPV types 2–17 and CPV type 19 have been found in cutaneous and oral SCC (Table 1). However, presence of the virus is insufficient to establish its etiological role and additional molecular evidence exists in some cases to support its causal involvement in SCC (38, 39). The CPV E5, E6, and E7 proteins share some characteristics with homologous oncoproteins from HPV 16 which are involved in malignancy (40) suggesting they may, at least partially, contribute for cell transformation in similar ways (1). Each protein has different contributions in different contexts: for instance, E5 is a major transforming protein in bovine delta PVs but seems to play a less central role in HPV (41). The particular role of each gene and each CPV genotype remains to be determined. From the data summarized in Table 1, it is clear that all genotypes possess the E6 and E7 genes, regardless of whether they were found in SCC or not. Conversely, the E5 is occasionally present in both groups. It would be interesting to systematically compare the genomes of CPV genotypes suggested to be involved in SCC with those involved only in benign lesions, to identify molecular determinants of malignant transformation.
4. Feline papillomaviruses (FcaPVs)
Feline papillomaviruses (FcaPVs) are thought to cause oral papilloma, cutaneous papilloma, viral plaques and Bowenoid in situ carcinomas (BISC). There is increasing evidence that FcaPVs may also be associated with the development of cutaneous squamous cell carcinomas (SCC). So far, six types of FcaPVs were described and associated with mucosal and cutaneous lesions (Table 2), as described for the dog. These viruses were grouped into sp. genera: Dyothetapapillomavirus, Lambdapapillomavirus, and Taupapillomaviru. FcaPV1 belongs to the genus Lambdapapillomavirus, has tropism for skin and oral cavity, and is associated with the development of cutaneous and oral papillomas. FcaPV2 is part of the genus Dyothetapapillomavirus, has tropism for the skin and is responsible for the development of viral plaques, BISC, cutaneous SCC and basal cell carcinoma. FcaPVs 3, 4, 5, and 6 belong to the genus Taupapillomavirus and have tropism for different organs. FcaPVs 3 and 5 have tropism for skin, and both are associated with the development of viral plaques and BISC. Moreover, FcaPV3 is also associated with the development of skin neoplasia and cutaneous SCC. FcaPV4 has tropism for the oral cavity and is related with the development of stomatitis and BISC. FcaPV6 was detected in the nasal planum and was associated with the development of cutaneous SCC. Additionally to these viruses, cats may also be affected by bovine papillomavirus (BPV)-14, a Deltapapillomavirus, that is responsible for the development of feline sarcoids, as observed in other species infected with Deltapapillomavirus BPVs (42, 43) (Table 2).
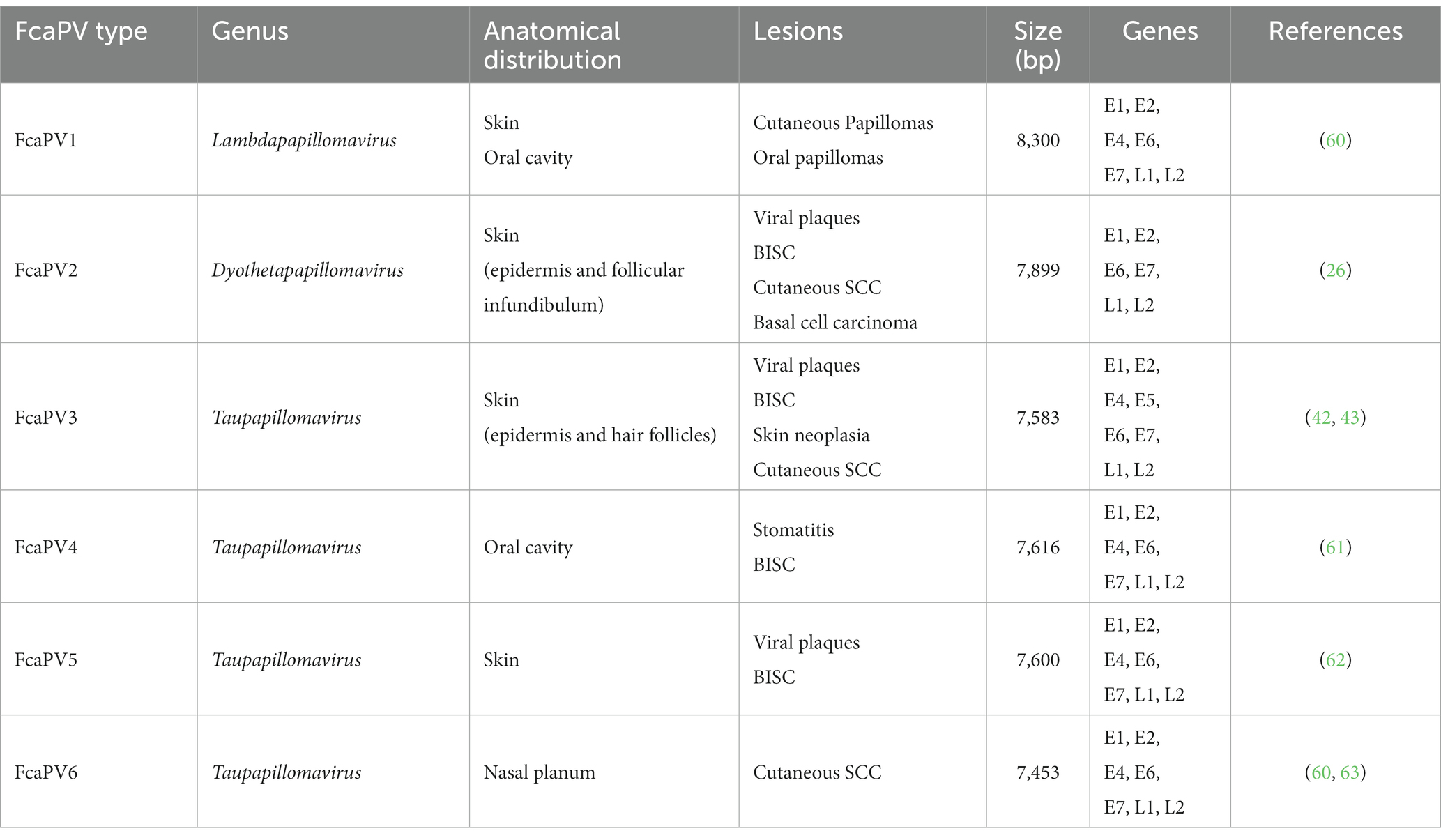
Table 2. FcaPV types and their associated lesions at multiple anatomic sites. Bowenoid in situ carcinomas (BISC).
Warts are less frequent in cats than in dogs. To date, only three cases of cutaneous warts were reported in cats, and they were small and solitary, two of them were found on the nasal planum and one on the eyelid, as previously reviewed (44). Cutaneous warts in the anogenital region were never reported in cats. Oral warts were rarely reported in cats and resolve spontaneously (45). Viral cutaneous plaques are rare in cats and affect mainly middle-aged or older animals. When compared with other breeds, Sphinx or Devon Rex cats develop plaques more frequently and at a younger age (46). These plaques present as multiple pigmented or non-pigmented lesions, that do not cause pain or pruritus, on the head, face, and neck (47).
There are strong evidence suggesting that papillomaviruses are part of the etiology of skin cancers in cats (45). FcaPV2, 3 and 6 have been associated with cutaneous SCC (Table 2). However, it is not clear whether SCC develops from viral plaques or directly from normal skin (21). As observed for canine PVs, all FcaPV genotypes contain the essential E6 and E7 genes, but only FcaPV3 has an E5 gene. Apart from some members of the Deltapapillomavirus genus, papillomaviruses have strong tropism for keratinizing epithelia and a recent study indicates that papillomaviruses do not frequently infect the lung, mammary gland, or the bladder of dogs and cats and, consequently, are unlikely to be determining factors for cancer development in those tissues (48).
5. Conclusion
To date, 24 types of CPVs and six types of FcaPV have been described, most often with tropism for the skin and oral cavity. The infection of cats by a bovine papillomavirus (BPV-14) was already reported and leads to sarcoids as in other animal species. Papillomaviruses are widely recognized as a cause of several oral and cutaneous lesions in both dogs and cats, with most of lesions are self-resolving. Papillomaviruses are also potentially related to malignant diseases in these species, especially in cats. Further research in this field will likely add new papillomavirus types to those already known and will add to our current knowledge of their epidemiology and pathologic features as well as support the development of new and more effective preventive and therapeutic approaches.
Author contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work and approved it for publication.
Funding
The present work was supported by European Investment Funds by FEDER/COMPETE/POCI-Operational Competitiveness and Internationalization Program, and National Funds by Portuguese Foundation for Science and Technology (FCT), under the projects PI86-CI-IPOP-66-2017 (CI-IPOP), UID/AGR/04033/2020 (CITAB), LA/P/0126/2020 (Inov4Agro), UIDB/00511/2020 (LEPABE), NORTE-01-0145-FEDER-000054 (Project 2SMART), and the PhD grant no. 2020.07675.BD to BM-F.
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
1. Cruz-Gregorio, A, Aranda-Rivera, AK, and Pedraza-Chaverri, J. Pathological similarities in the development of papillomavirus-associated cancer in humans, dogs, and cats. Animals. (2022) 12:2390. doi: 10.3390/ani12182390
2. Munday, JS, and Pasavento, P. Papillomaviridae and polyomaviridae In: NJ MacLachlan and E Dubovi, editors. Fenner’s veterinary virology. London, UK: Academic Press (2017). 229–43.
3. Bernard, HU, Burk, RD, Chen, Z, van Doorslaer, K, Hausen, H z, and de Villiers, EM. Classification of papillomaviruses (PVs) based on 189 PV types and proposal of taxonomic amendments. Virology. (2010) 401:70–9. doi: 10.1016/J.VIROL.2010.02.002
4. McBride, AA. Human papillomaviruses: diversity, infection and host interactions. Nat Rev Microbiol. (2021) 20:95–108. doi: 10.1038/s41579-021-00617-5
5. Altan, E, Seguin, MA, Leutenegger, CM, Phan, TG, Deng, X, and Delwart, E. Nasal virome of dogs with respiratory infection signs include novel taupapillomaviruses. Virus Genes. (2019) 55:191–7. doi: 10.1007/S11262-019-01634-6
6. Doorbar, J, Quint, W, Banks, L, Bravo, IG, Stoler, M, Broker, TR, et al. The biology and life-cycle of human papillomaviruses. Vaccine. (2012) 30:F55–70. doi: 10.1016/J.VACCINE.2012.06.083
7. Munday, JS, Knight, CG, and French, AF. Evaluation of feline oral squamous cell carcinomas for p16CDKN2A protein immunoreactivity and the presence of papillomaviral DNA. Res Vet Sci. (2011) 90:280–3. doi: 10.1016/J.RVSC.2010.06.014
8. Lane, HE, Weese, JS, and Stull, JW. Canine oral papillomavirus outbreak at a dog daycare facility. Can Vet J. (2017) 58:747–9.
9. Egawa, N, and Doorbar, J. The low-risk papillomaviruses. Virus Res. (2017) 231:119–27. doi: 10.1016/J.VIRUSRES.2016.12.017
10. Munday, JS, Thomson, NA, and Luff, JA. Papillomaviruses in dogs and cats. Vet J. (2017) 225:23–31. doi: 10.1016/J.TVJL.2017.04.018
11. Antonsson, A, Karanfilovska, S, Lindqvist, PG, and Hansson, BG. General acquisition of human papillomavirus infections of skin occurs in early infancy. J Clin Microbiol. (2003) 41:2509–14. doi: 10.1128/JCM.41.6.2509-2514.2003
12. Antonsson, A, and Hansson, BG. Healthy skin of many animal species harbors papillomaviruses which are closely related to their human counterparts. J Virol. (2002) 76:12537–42. doi: 10.1128/JVI.76.24.12537-12542.2002
13. Bogaert, L, Martens, A, Van Poucke, M, Ducatelle, R, De Cock, H, Dewulf, J, et al. High prevalence of bovine papillomaviral DNA in the normal skin of equine sarcoid-affected and healthy horses. Vet Microbiol. (2008) 129:58–68. doi: 10.1016/J.VETMIC.2007.11.008
14. Munday, JS, and Witham, AI. Frequent detection of papillomavirus DNA in clinically normal skin of cats infected and noninfected with feline immunodeficiency virus. Vet Dermatol. (2010) 21:307–10. doi: 10.1111/J.1365-3164.2009.00811.X
15. Lange, CE, and Favrot, C. Canine papillomaviruses. Vet Clin North Am Small Anim Pract. (2011) 41:1183–95. doi: 10.1016/J.CVSM.2011.08.003
16. Knight, CG, Dunowska, M, Munday, JS, Peters-Kennedy, J, and Rosa, BV. Comparison of the levels of Equus caballus papillomavirus type 2 (EcPV-2) DNA in equine squamous cell carcinomas and non-cancerous tissues using quantitative PCR. Vet Microbiol. (2013) 166:257–62. doi: 10.1016/J.VETMIC.2013.06.004
17. Graham, SV. The human papillomavirus replication cycle, and its links to cancer progression: a comprehensive review. Clin Sci. (2017) 131:2201–21. doi: 10.1042/CS20160786
18. Doorbar, J. The papillomavirus life cycle. J Clin Virol. (2005) 32:7–15. doi: 10.1016/J.JCV.2004.12.006
19. McBride, AA. The papillomavirus E2 proteins. Virology. (2013) 445:57–79. doi: 10.1016/J.VIROL.2013.06.006
20. Gil da Costa, RM, Peleteiro, MC, Pires, MA, and DiMaio, D. An update on canine, feline and bovine papillomaviruses. Transbound Emerg Dis. (2017) 64:1371–9. doi: 10.1111/tbed.12555
21. Munday, JS, Knight, CG, and Luff, JA. Papillomaviral skin diseases of humans, dogs, cats and horses: a comparative review. Part 1: papillomavirus biology and hyperplastic lesions. Vet J. (2022) 288:105897. doi: 10.1016/j.tvjl.2022.105897
22. Munday, JS, Knight, CG, and Luff, JA. Papillomaviral skin diseases of humans, dogs, cats and horses: a comparative review. Part 2: pre-neoplastic and neoplastic diseases. Vet J. (2022) 288:105898. doi: 10.1016/j.tvjl.2022.105898
23. Rector, A, and Van Ranst, M. Animal papillomaviruses. Virology. (2013) 445:213–23. doi: 10.1016/J.VIROL.2013.05.007
24. Isegawa, N, Ohta, M, Shirasawa, H, Tokita, H, Yamaura, A, and Simizu, B. Nucleotide-sequence of a canine oral papillomavirus containing a long noncoding region. Int J Oncol. (1995) 7:155–9. doi: 10.3892/IJO.7.1.155
25. Brandes, K, Fritsche, J, Mueller, N, Koerschgen, B, Dierig, B, Strebelow, G, et al. Detection of canine oral papillomavirus DNA in conjunctival epithelial hyperplastic lesions of three dogs. Vet Pathol. (2009) 46:34–8. doi: 10.1354/VP.46-1-34
26. Lange, CE, Tobler, K, Ackermann, M, Panakova, L, Thoday, KL, and Favrot, C. Three novel canine papillomaviruses support taxonomic clade formation. J Gen Virol. (2009) 90:2615–21. doi: 10.1099/VIR.0.014498-0/CITE/REFWORKS
27. Kim, D, Dobromylskyj, MJ, O’Neill, D, and Smith, KC. Skin masses in dogs under one year of age. J Small Anim Pract. (2022) 63:10–5. doi: 10.1111/JSAP.13418
28. Chang, CY, Chen, WT, Haga, T, Yamashita, N, Lee, CF, Tsuzuki, M, et al. The detection and association of canine papillomavirus with benign and malignant skin lesions in dogs. Viruses. (2020) 12:170. doi: 10.3390/V12020170
29. Orlandi, M, Mazzei, M, Vascellari, M, Melchiotti, E, Zanardello, C, Verin, R, et al. Localization and genotyping of canine papillomavirus in canine inverted papillomas. J Vet Diagn Invest. (2021) 33:1069–78. doi: 10.1177/10406387211035799
30. Gould, AP, Coyner, KS, Trimmer, AM, Tater, K, and Rishniw, M. Canine pedal papilloma identification and management: a retrospective series of 44 cases. Vet Dermatol. (2021) 32:509–e141. doi: 10.1111/VDE.12999
31. Lange, CE, Jennings, SH, Diallo, A, and Lyons, J. Canine papillomavirus types 1 and 2 in classical papillomas: high abundance, different morphological associations and frequent co-infections. Vet J. (2019) 250:1–5. doi: 10.1016/J.TVJL.2019.05.016
32. Oğuzoğlu, TÇ, Timurkan, MÖ, Koç, BT, and Alkan, F. Comparison of genetic characteristics of canine papillomaviruses in Turkey. Infect Genet Evol. (2017) 55:372–6. doi: 10.1016/J.MEEGID.2017.10.010
33. Sancak, A, Favrot, C, Geisseler, MD, Müller, M, and Lange, CE. Antibody titres against canine papillomavirus 1 peak around clinical regression in naturally occurring oral papillomatosis. Vet Dermatol. (2015) 26:57–e20. doi: 10.1111/VDE.12189
34. Yhee, JY, Kwon, BJ, Kim, JH, Yu, CH, Im, KS, Lee, SS, et al. Characterization of canine oral papillomavirus by histopathological and genetic analysis in Korea. J Vet Sci. (2010) 11:21–5. doi: 10.4142/JVS.2010.11.1.21
35. Tobler, K, Lange, C, Carlotti, DN, Ackermann, M, and Favrot, C. Detection of a novel papillomavirus in pigmented plaques of four pugs. Vet Dermatol. (2008) 19:21–5. doi: 10.1111/J.1365-3164.2007.00640.X
36. Gross, TL, Ihrke, PJ, Walder, EJ, and Affolter, V. Skin diseases of the dog and cat: clinical and histopathologic diagnosis. Oxford, UK: Blackwell Science Ltd. (2005).
37. Thaiwong, T, Sledge, DG, Wise, AG, Olstad, K, Maes, RK, and Kiupel, M. Malignant transformation of canine oral papillomavirus (CPV1)-associated papillomas in dogs: an emerging concern? Papillomavirus Res. (2018) 6:83–9. doi: 10.1016/J.PVR.2018.10.007
38. Alves, CDBT, Weber, MN, Guimarães, LLB, Cibulski, SP, da Silva, FRC, Daudt, C, et al. Canine papillomavirus type 16 associated to squamous cell carcinoma in a dog: virological and pathological findings. Braz J Microbiol. (2020) 51:2087–94. doi: 10.1007/S42770-020-00310-4
39. Luff, J, Mader, M, Rowland, P, Britton, M, Fass, J, and Yuan, H. Viral genome integration of canine papillomavirus 16. Papillomavirus Res. (2019) 7:88–96. doi: 10.1016/j.pvr.2019.02.002
40. Estêvão, D, Costa, NR, Gil da Costa, RM, and Medeiros, R. Hallmarks of HPV carcinogenesis: the role of E6, E7 and E5 oncoproteins in cellular malignancy. Biochim Biophys Acta Gene Regul Mech. (2019) 1862:153–62. doi: 10.1016/j.bbagrm.2019.01.001
41. Gil da Costa, RM, and Medeiros, R. Bovine papillomavirus: opening new trends for comparative pathology. Arch Virol. (2014) 159:191–8. doi: 10.1007/s00705-013-1801-9
42. Munday, JS, Thomson, NA, Henderson, G, Fairley, R, and Orbell, GM. Identification of Felis catus papillomavirus 3 in skin neoplasms from four cats. J Vet Diagn Invest. (2018) 30:324–8. doi: 10.1177/1040638717750852
43. Munday, JS, Dunowska, M, Hills, SF, and Laurie, RE. Genomic characterization of Felis catus papillomavirus-3: a novel papillomavirus detected in a feline Bowenoid in situ carcinoma. Vet Microbiol. (2013) 165:319–25. doi: 10.1016/J.VETMIC.2013.04.006
44. Munday, JS, Piripi, SA, Julian, A, and Martin, SJ. Long-term recurrent, yet nonprogressive, pedal viral papillomas in a dog. Vet Dermatol. (2020) 31:489–e128. doi: 10.1111/VDE.12888
45. Munday, JS, Fairley, RA, Mills, H, Kiupel, M, and Vaatstra, BL. Oral Papillomas associated with Felis catus papillomavirus type 1 in 2 domestic cats. Vet Pathol. (2015) 52:1187–90. doi: 10.1177/0300985814565133
46. Munday, JS, Benfell, MW, French, A, Orbell, GMB, and Thomson, N. Bowenoid in situ carcinomas in two Devon rex cats: evidence of unusually aggressive neoplasm behaviour in this breed and detection of papillomaviral gene expression in primary and metastatic lesions. Vet Dermatol. (2016) 27:215–e55. doi: 10.1111/vde.12319
47. Wilhelm, S, Degorce-Rubiales, F, Godson, D, and Favrot, C. Clinical, histological and immunohistochemical study of feline viral plaques and bowenoid in situ carcinomas. Vet Dermatol. (2006) 17:424–31. doi: 10.1111/J.1365-3164.2006.00547.X
48. Munday, JS, MacLachlan, CB, Perrott, MR, and Aberdein, D. Papillomavirus DNA is not amplifiable from bladder, lung, or mammary gland cancers in dogs or cats. Animals. (2019) 9:1–8. doi: 10.3390/ani9090668
49. Yuan, H, Ghim, S, Newsome, J, Apolinario, T, Olcese, V, Martin, M, et al. An epidermotropic canine papillomavirus with malignant potential contains an E5 gene and establishes a unique genus. Virology. (2007) 359:28–36. doi: 10.1016/J.VIROL.2006.08.029
50. Tobler, K, Favrot, C, Nespeca, G, and Ackermann, M. Detection of the prototype of a potential novel genus in the family Papillomaviridae in association with canine epidermodysplasia verruciformis. J Gen Virol. (2006) 87:3551–7. doi: 10.1099/VIR.0.82305-0
51. Luff, JA, Affolter, VK, Yeargan, B, and Moore, PF. Detection of six novel papillomavirus sequences within canine pigmented plaques. J Vet Diagn Invest. (2012) 24:576–80. doi: 10.1177/1040638712443360
52. Zhou, D, Luff, J, Usuda, Y, Affolter, V, Moore, P, Schlegel, R, et al. Complete genome sequence of canine papillomavirus type 11. Genome Announc. (2014) 2:529–43. doi: 10.1128/GENOMEA.00529-14
53. Zhou, D, Luff, J, Paul, S, Alkhilaiwi, F, Usuda, Y, Wang, N, et al. Complete genome sequence of canine papillomavirus virus type 12. Genome Announc. (2015) 3:e00294-15. doi: 10.1128/GENOMEA.00294-15
54. Lange, CE, Ackermann, M, Favrot, C, and Tobler, K. Entire genomic sequence of novel canine papillomavirus type 13. J Virol. (2012) 86:10226–7. doi: 10.1128/JVI.01553-12
55. Lange, CE, Tobler, K, Schraner, EM, Vetsch, E, Fischer, NM, Ackermann, M, et al. Complete canine papillomavirus life cycle in pigmented lesions. Vet Microbiol. (2013) 162:388–95. doi: 10.1016/J.VETMIC.2012.10.012
56. Bhatta, TR, Chamings, A, Vibin, J, and Alexandersen, S. Detection and characterisation of canine astrovirus, canine parvovirus and canine papillomavirus in puppies using next generation sequencing. Sci Rep. (2019) 9:1–10. doi: 10.1038/s41598-019-41045-z
57. Lange, CE, Diallo, A, Zewe, C, and Ferrer, L. Novel canine papillomavirus type 18 found in pigmented plaques. Papillomavirus Res. (2016) 2:159–63. doi: 10.1016/J.PVR.2016.08.001
58. Tisza, MJ, Yuan, H, Schlegel, R, and Buck, CB. Genomic sequence of canine papillomavirus 19. Genome Announc. (2016) 4:1380–96. doi: 10.1128/GENOMEA.01380-16
59. Munday, JS, Gedye, K, Knox, MA, Ravens, P, and Lin, X. Genomic characterisation of Canis Familiaris papillomavirus type 24, a novel papillomavirus associated with extensive pigmented plaque formation in a pug dog. Viruses. (2022) 14:2357. doi: 10.3390/V14112357
60. Munday, JS, and Thomson, NA. Papillomaviruses in domestic cats. Viruses. (2021) 13:1664. doi: 10.3390/V13081664
61. Vascellari, M, Mazzei, M, Zanardello, C, Melchiotti, E, Albanese, F, Forzan, M, et al. Felis catus papillomavirus types 1, 2, 3, 4, and 5 in feline Bowenoid in situ carcinoma: an in situ hybridization study. Vet Pathol. (2019) 56:818–25. doi: 10.1177/0300985819859874
62. Munday, JS, Dittmer, KE, Thomson, NA, Hills, SF, and Laurie, RE. Genomic characterisation of Felis catus papillomavirus type 5 with proposed classification within a new papillomavirus genus. Vet Microbiol. (2017) 207:50–5. doi: 10.1016/J.VETMIC.2017.05.032
Keywords: cancer, cat, dog, human, papillomavirus
Citation: Medeiros-Fonseca B, Faustino-Rocha AI, Medeiros R, Oliveira PA and Gil da Costa RM (2023) Canine and feline papillomaviruses: an update. Front. Vet. Sci. 10:1174673. doi: 10.3389/fvets.2023.1174673
Edited by:
Hui-Wen Chang, National Taiwan University, TaiwanReviewed by:
John Munday, Massey University, New ZealandCopyright © 2023 Medeiros-Fonseca, Faustino-Rocha, Medeiros, Oliveira and Gil da Costa. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Rui M. Gil da Costa, rmcosta@fe.up.pt
 Beatriz Medeiros-Fonseca
Beatriz Medeiros-Fonseca Ana I. Faustino-Rocha
Ana I. Faustino-Rocha Rui Medeiros
Rui Medeiros Paula A. Oliveira
Paula A. Oliveira Rui M. Gil da Costa
Rui M. Gil da Costa