1
Brain and Body Centre, University of Nottingham, Nottingham, UK
2
Montreal Neurological Institute, Montreal, QC, Canada
3
Institut Pasteur, Paris, France
Recent studies with magnetic resonance imaging suggest that age-related changes in white matter during male adolescence may indicate an increase in g ratio wherein the radial growth of an axon outpaces a corresponding increase in myelin thickness. We review the original Rushton (1951)
model where a g ratio of ∼0.6 represents an optimal relationship between the axon and fibre diameters vis-à-vis conduction velocity, and point out evidence indicating slightly higher g ratio in large-diameter fibres. We estimate that fibres with a diameter larger than 9.6 μm will have a relatively thinner myelin sheath, and brains with increasingly larger proportions of such large-diameter fibres will have progressively lower concentration of myelin. We conclude by pointing out possible implications of “suboptimal” g ratio for the emergence of “disconnection” disorders, such as schizophrenia, in late adolescence.
In vivo studies of the human brain have revealed the presence of striking sex differences in the volume and microstructure of white matter (WM). In adults, the overall volume of WM is higher in men than women; in most studies, this is true even after accounting for sex differences in brain size (e.g. Gur et al., 2002
). On the other hand, sex differences in the “density” (e.g. Good et al., 2001
; the term “density” or “concentration” refers to the probability of classifying a voxel as belonging to a specific type of tissue without making any inference about possible underlying neurobiological processes) and fractional anisotropy (e.g. Hsu et al., 2008
) of WM constituting various fibre tracts appear to vary across the tracts, being higher in women than men in some WM pathways and vice versa in others.
Many of these sex differences in WM emerge during adolescence. We (Perrin et al., 2009
) and others (De Bellis et al., 2001
; Giedd et al., 1999
; Lenroot et al., 2007
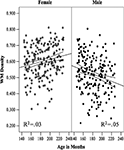
) have shown a steep increase in the WM volume during adolescence in boys but not girls. In our sample of 400 adolescents, relative lobar volumes of WM were very similar in boys and girls at the age of 12 years, after which they diverged to reach—by the age of 18 years—a sex difference varying between 9% in the frontal lobe and 25% in the occipital lobe (Perrin et al., 2009
; see Figure 1
).
Figure 1. The relationship between age and relative white matter (WM) volume in male and female adolescents in the (A) frontal, (B) parietal, (C) temporal and (D) occipital lobes. The lines represent the regression equation with 95% confidence intervals. Reprinted from Perrin et al. (2009)
.
The total volume of WM is determined by the number of axons, their calibre and the thickness of myelin sheath produced by the oligodendrocytes. Given the known elimination of axons in the early post-natal period (e.g. LaMantia and Rakic, 1990
, 1994
), age-related increases in the volume of WM during brain development in childhood and adolescence can be accounted for by increases in axonal calibre and/or thickness of the myelin sheath. Recent data from our laboratory provide indirect evidence in favour of age-related changes in axonal calibre during male adolescence. First, we have obtained values of magnetization-transfer ratio (MTR), which provides an indirect index of myelination (Kucharczyk et al., 1994
; Schmierer et al., 2004
). As can be seen in Figure 2
, MTR values in lobar WM were about the same in boys and girls at the age of 12 years after which they also diverged, increasing slightly in girls (in the frontal lobe) while decreasing in boys (in the parietal and occipital lobes); the sex by age interaction was significant in all lobes indicating the opposite effect of age on MTR in boys and girls throughout the brain (Perrin et al., 2009
). Second, we have examined WM density in the cortico-spinal tract (CST) at the level of the internal capsule and observed a similar divergence, namely age-related increases and decreases in WM density in girls and boys, respectively (Perrin et al., 2009
; Figure 3
).
Figure 2. The relationship between age and mean values of magnetization-transfer ratio (MTR) in white matter (WM) in male and female adolescents in the (A) frontal, (B) parietal, (C) temporal and (D) occipital lobes. The lines represent the regression equation with the 95% confidence intervals. Reprinted from Perrin et al. (2009)
.
Figure 3. The relationship between Age and white-matter (WM) density in the left cortico-spinal tract in female and male adolescents. The lines represent the regression equation with the 95% confidence intervals. Reprinted from Perrin et al. (2009)
.
Thus, in the same sample of typically developing adolescents, we have observed the following three phenomena: (1) WM volume increases steeply during adolescence in boys but not girls; (2) MTR decreases with age in boys while it does not change (or increases slightly) in girls; and (3) WM density in the CST decreases with age in adolescent boys but increases in girls. We have proposed that these phenomena may be best explained by a disproportionate growth of the axon compared with the growth of its myelin sheath and, as such, by changes in g ratio (axon diameter/fibre diameter; see below). We have speculated that a subtle shift in g ratio in WM of the male brain during adolescence would affect the proportion of WM tissue occupied by axons and myelin, thus explaining the emergence of sex differences in WM density and MTR described above (Herve et al., 2009
; Perrin et al., 2008
, 2009
). Here we will formalize this proposal by considering in more detail the relationship between fibre diameter and g ratio.
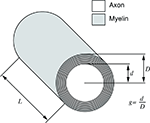
G ratio refers to the ratio between axon diameter d and fibre diameter D; fibre diameter is the sum of the axon diameter (or calibre) and the thickness of the myelin sheath (Figure 4
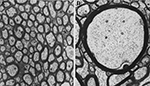
). Figure 5
illustrates the wide variations in axonal morphology vis-à-vis axon diameter and the thickness of myelin sheath. In the monkey corpus callosum (CC), unmyelinated axons, accounting for about 30% of all axons in the genu and <10% elsewhere in the CC, have a small diameter (∼0.1 μm), whereas the diameter of myelinated axons varies between 0.1 and 2.5 μm, with the largest axons (2.5 μm) found in the CC midbody (LaMantia and Rakic, 1990
; see also Aboitiz et al., 1992
for similar findings in the human CC). The largest axons in the human brain are found in the internal capsule and, most likely, correspond to the cortico-spinal neurons (Lassek, 1942
; Verhaart, 1950
); the size of these axons increases from birth (∼1 μm), through childhood (12 μm at 7 years of age) into adulthood (24 μm). These numbers correspond to the fibre diameter and, as such, reflect both the radial growth of the cortico-spinal axons and their increased myelination with age. We have suggested that changes in the WM density in the CST observed in our studies in relation to plasma levels of testosterone reflect changes in the diameter of these axons (Herve et al., 2009
).
Figure 4. Schematic representation of an axon and its myelin sheath illustrating g ratio.
Figure 5. Electron micrograms of axons in the monkey corpus callosum. Note wide variations in the axonal diameter and myelin thickness. (A) Asterisks indicate small unmyelinated axons. (B) A large myelinated axon in the midbody of the corpus callosum. Same magnification (8,500×) for both micrographs. Reprinted from LaMantia and Rakic (1990)
.
In general, axons of large diameter have a thick myelin sheath. It turns out that the g ratio is relatively stable at ∼0.6 (Rushton, 1951
). This observation, together with the small diameter of unmyelinated axons, suggests possible coupling between myelination and axon diameter (see below). But the range of g ratio varies considerably across axons of the same fibre tract (Berthold et al., 1983
), as a function of age (Berthold et al., 1983
; Jeronimo et al., 2008
), mouse strain (Little and Heath, 1994
) or disease (Friede and Beuche, 1985
). Importantly, g ratio appears to increase as a function of axon diameter, indicating a relatively thinner myelin sheath in large axons (Berthold et al., 1983
; Chatzopoulou et al., 2008
; Gillespie and Stein, 1983
). Even though most of the above morphometric studies of g ratio were carried out in peripheral nerves, the most recent and comprehensive study of g ratio was carried out in the optic nerve, which is a part of the central nervous system and whose myelination is generated by oligodendrocytes (Chatzopoulou et al., 2008
). As mentioned above, a subtle shift in the g ratio could change the proportion of tissue occupied by axons and myelin, respectively.
By assuming that geometrically similar fibres should possess similar membrane properties, Rushton (1951)
derived a formula showing that, for a constant g ratio, the conduction velocity v and fibre length l should be proportional to the fibre diameter D:
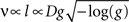
where g = d/D.
Rushton observed that this expression has a maximum for g = e−1/2 ≈ 0.6. A fibre with a g ratio of 0.6 will have the maximum conduction velocity for its length. Note that the theory applies to a generic (peripheral and central) myelinated axon. The optimal g-ratio was obtained from purely theoretical considerations, namely by maximising conduction velocity in the equation. In Figure 6
, we plot the variation of fibre length as a function of fibre diameter and axonal diameter. The continuous line shows the values of D and d that, according to Rushton, have the “optimal” g ratio of 0.6 thus maximising conduction velocity.
Figure 6. Relationship between axon diameter and fibre diameter (axon diameter + thickness of the myelin sheath) in fibres of different length. After Rushton (1951
), conduction velocity is maximum when the proportion of axon diameter to fibre diameter, i.e. g ratio, is close to 0.6 (solid line). If the axon diameter is taken to be proportional to the cross-section of the myelin sheath, the relationship between axon diameter and fibre diameter is given by the discontinuous curve. Note that g ratio expressed in the latter way is higher than 0.6 in large-diameter fibres, which would be more typical for longer (>13 cm) fibres. Grey levels: fibre length from 0 to 20 cm.
Rushton noticed, however, that (peripheral) fibres of large diameter have also a larger g ratio, that is, they have a relatively thinner myelin sheath. In a later work, Berthold et al. (1983
) presented an analysis of the variability and development of myelination in the peripheral nervous system of the cat. Again, they observed that axonal diameter plotted against fibre diameter produced a non-linear curve, where the larger fibres have also increasingly larger axons. A similar observation has been made recently by Chatzopoulou in the central nervous system, namely in the mouse optic nerve (Chatzopoulou et al., 2008
; Figure 7
).
Figure 7. G ratio (top), number of microtubules (middle) and number of neurofilaments (bottom) according to axonal diameter in the optic nerve of adult Tag-1+/− and Tag-1−/− mice (TAG-1 is a cell-adhesion molecule). Reprinted from Chatzopoulou et al. (2008)
.
Interestingly, when the cross-sectional area of the myelin sheath was plotted against axonal diameter, the relationship became linear, as if the volume of the myelin sheath depended on the surface of the axonal fibre (Berthold et al., 1983
). Based on this observation, here we model this relationship between axonal diameter and fibre diameter as
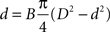
where B is a constant of proportionality. Solving the quadratic equation for d we obtain two roots, only one giving positive diameters:
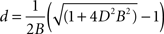
We estimated the value of Bertehold’s proportionality constant to be B = 0.1 μm−1, from the slope of the scatter plots in Figures 7d and 7e in Berthold et al. (1983
). The discontinuous curve in Figure 6
shows the values of D and d for which the axonal diameter is proportional to the myelin cross-section. This curve crosses the line showing the optimal g ratio when the fibres have a diameter of
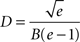
for which the axonal diameter is
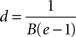
For our estimated value of B, this indicates that fibres with a diameter larger than 9.6 μm will have a relatively thinner myelin sheath; and brains with increasingly larger proportions of such large diameter fibres will have progressively lower concentration of myelin.
What determines axonal diameter? Axon consists mainly of neurofilaments (NF) and microtubules (MT), the former outnumbering the latter 5–10 times (Lee and Cleveland, 1996
). Neurofilaments support the cylindrical structure of an axon and, as such, protect the core from compressive stress, securing its unobstructed state (Kumar et al., 2002
). Axonal diameter is influenced both by the number of NF and their spacing (Hoffman et al., 1984
, 1987
; Kumar et al., 2002
). The former is regulated by NF synthesis (gene expression) and the amount of NF undergoing (slow) axonal transport (Hoffman et al., 1987
, 1984
); not surprisingly, the number of NF, but also that of MT, varies as a function of axon diameter (Chatzopoulou et al., 2008
; Figure 7
). The latter depends on the phosphorylation of NF sidearms (e.g. Garcia et al., 2003
; Kumar et al., 2002
). This process appears to be regulated by a protein synthesized by oligodendrocytes, namely myelin-associated glycoprotein; this “outside-in” signalling pathway provides a cellular mechanism for the coupling between myelination and axonal calibre (Yin et al., 1998
; Garcia et al., 2003
). Theoretically, sex differences in g ratio could emerge either through differences in the rate of synthesis and/or axonal transport of NF, or the rate of phosphorylation of the NF sidearms.
The initial work from our laboratory has suggested a disproportionate growth of axon and myelin sheath, respectively, during male adolescence (Perrin et al., 2008
). This phenomenon turned our attention to possible sex differences in g ratio. Both the experimental work reviewed above and the model predict lower concentration of myelin in WM regions occupied by large-diameter fibres; we have observed, indirectly, this phenomenon in the CST at the level of internal capsule (Herve et al., 2009
). It is likely that the male brain contains, overall, a larger proportion of large-diameter fibres. What might be the neurobiology underlying such a sex difference?
As pointed out above, axonal diameter depends primarily on the number of NF and their configuration. Although the number of MT also increases with the axonal diameter, directionality of this relationship is not clear. The link between testosterone and cellular processes relevant vis-à-vis cytoskeleton are rather tentative at present. Initial in vitro studies suggest that testosterone up-regulates α- and β-tubulin, the building blocks of MT (Butler et al., 2001
). Furthermore, neuropathy is one of the key symptoms of Kennedy’s disease (or X-linked spinal and bulbar muscular atrophy), a condition caused by an expanded polyglutamine (polyQ) stretch in the androgen receptor (Brooks and Fischbeck, 1995
; Hsiao et al., 1999
; La Spada et al., 1991
). This effect might be mediated by a PolyQ-AR induced inhibition of kinesin-mediated axonal transport (Morfini et al., 2006
) or a decrease in the expression of dynactin 1, a motor for retrograde axonal transport (Katsuno et al., 2006
); slow axonal transport is essential for moving elements of cytoskeleton (Barry et al., 2007
).
Is the rate of axonal transport different in small- and large-diameter fibres? This appears to be the case. Murayama et al. (2006)
used MRI to measured the rate of in vivo transport of Mn++ ions from the eye to the lateral geniculate nucleus of the monkey and found that a faster transport in the magno-cellular that parvocellular pathway. The Mn++ transport depends, at least in part, on kinesin-based processes underlying (active) axonal transport (Bearer et al., 2007
). In general, axonal cytoskeleton (i.e. NF and MT) and motor proteins are essential contributors to a large number of cellular processes, such as cell metabolism (e.g. transport of mitochondria and glycolytic enzymes) and neurotransmission (e.g. transport of synaptic-vesicle precursors), as well as growth and cell survival (e.g. retro-grade transport of growth factors). Thus, any sex differences in axonal transport may have a number of downstream effects.
Finally, the model presented above suggests that the g ratio will increase in large fibres beyond the optimal value of 0.6. We speculate that the presumed testosterone-induced changes in axonal diameter during male adolescence increase the probability of suboptimal g ratio in large-diameter fibres and, in turn, decrease conduction velocity in these fibres. Such a disturbance may interfere with the timing of inter-regional communication and contribute to the emergence of a “disconnection” syndrome hypothesized to underlie disorders such as schizophrenia (Friston and Frith, 1995
; Goldberg et al., 1997
).
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Tomáš Paus is supported by the Canadian Institutes of Health Research, the Royal Society (UK), and the National Institutes of Health (USA). The work on the maturation of white matter during adolescence benefited significantly from the many contributions made by Jennifer Perrin and Pierre-Yves Hervé.
Chatzopoulou, E., Miguez, A., Savvaki, M., Levasseur, G., Muzerelle, A., Muriel, M. P., Goureau, O., Watanabe, K., Goutebroze, L., Gaspar, P., Zalc, B., Karagogeos, D., and Thomas, J. L. (2008). Structural requirement of TAG-1 for retinal ganglion cell axons and myelin in the mouse optic nerve. J. Neurosci. 28, 7624–7636.
Garcia, M. L., Lobsiger, C. S., Shah, S. B., Deerinck, T. J., Crum, J., Young, D., Ward, C. M., Crawford, T. O., Gotow, T., Uchiyama, Y., Ellisman, M. H., Calcutt, N. A., and Cleveland, D. W. (2003). NF-M is an essential target for the myelin-directed “outside-in” signaling cascade that mediates radial axonal growth. J. Cell Biol. 163, 1011–1020.
Lenroot, R. K., Gogtay, N., Greenstein, D. K., Wells, E. M., Wallace, G. L., Clasen, L. S., Blumenthal, J. D., Lerch, J., Zijdenbos, A. P., Evans, A. C., Thompson, P. M., and Giedd, J. N. (2007). Sexual dimorphism of brain developmental trajectories during childhood and adolescence. Neuroimage 36, 1065–1073.