1. Introduction
One of the main challenges for the agriculture in this 21st century is to be capable to feed the increasing world population in a sustainable way, because natural resources are becoming even more scarce [
1]. Crop protection measures can prevent yield losses due to pests [
2]. Herbicides have been the most used method to control weeds since their development, at the end of the Second World War because they are effective and economical [
3,
4].
Herbicides cause stress in crops and can make them more susceptible to other pests [
5]. Other problems derived from the overuse of herbicides are environmental pollution, toxicity for nontarget organisms, and the development of herbicide-resistant weed biotypes [
6]. In the latest 10 years, integrated weed management (IWM) strategies have been promoted worldwide [
7,
8] to control weeds. They consist of a combination of methods: cultural, mechanical, physical, biological, biotechnological, and chemical. In Europe, IWM has been promoted through the European Union Directive 2009/128/EC [
8].
The society is demanding new solutions for weed control and “greener” weed management products. The use of natural products as bioherbicides could be one alternative to reduce the stress that synthetic herbicides promote in crops and all their negative impacts aforementioned. Bioherbicides could be incorporated in IPM programs as an innovative weed control method. They are less persistent than synthetic herbicides and are potentially more environmentally friendly and safe [
9] and also, they have different modes of action, which can prevent the development of herbicide-resistant weed biotypes [
10].
Bailey [
11] defined bioherbicides as products of natural origin for weed control. The EPA (USA Environmental Protection Agency), considers three categories of biopesticides: (1) biochemical pesticides, which include naturally occurring substances that control pests; (2) microbial pesticides or biocontrol agents, which are microorganisms that control pests; and (3) plant-incorporated protectants, or PIPs, which are pesticide substances produced by plants that contain added genetic material) [
10]. In recent years, the search for natural substances that can act as bioherbicides has been very extensive.
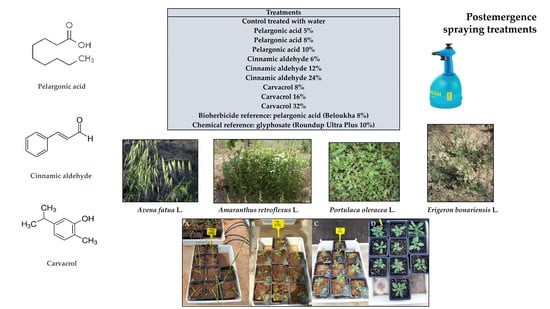
The weeds selected for this study were
Amaranthus retroflexus L.,
Avena fatua L.,
Portulaca oleracea L., and
Erigeron bonariensis L. because of their importance in many crops worldwide and their difficult management.
A. fatua is a very important weed mainly in cereals and also in other crops around the world [
12], and this weed is on the fourth position in resistance to herbicides worldwide, having developed resistance to nine different modes of action [
13].
A. retroflexus is a serious and aggressive weed in summer crops, with cosmopolite distribution [
14]. It has developed resistance to five modes of action and is on the eight position worldwide in resistance to herbicides [
13].
E. bonariensis, which can be found both in summer or winter crops, especially with no-tillage practices [
15], is on the ninth position in resistance to herbicides worldwide, with resistance to four modes of action.
P. oleracea, which is a summer weed difficult to control in Mediterranean crops [
16], has developed resistance only to two modes of action [
13].
A. fatua and
E. bonariensis have developed resistance to glyphosate, which is the herbicide most commonly used around the world [
13,
17].
There are several examples of natural products that have been tested as potential bioherbicides to control
A. fatua,
A. retroflexus,
E. bonariensis, and
P. oleracea, mainly essential oils (EOs) [
14,
18,
19,
20,
21,
22,
23,
24,
25,
26], or extracts from plants with different solvents [
27,
28,
29], or their isolated compounds [
30,
31]. Most studies have been carried out only in in vitro conditions. Of the weeds considered,
A. retroflexus has been the most tested. In vitro studies with EOs from
Artemisia vulgaris,
Mentha spicata,
Ocimum basilicum,
Salvia officinalis, and
Thymbra spicata from Turkey demonstrated high phytotoxic effects on seed germination and seedling growth of
A. retroflexus, with stronger effects with higher doses [
18]. EOs from
Tanacetum species growing in Turkey, rich in oxygenated monoterpenes, inhibited completely
A. retroflexus germination in in vitro assays [
19]. In addition, EOs from
Nepeta meyeri, with high content in oxygenated monoterpenes controlled completely
A. retroflexus germination [
20]. The phytotoxic potential of 12 EOs was studied in vitro against
A. retroflexus and
A. fatua, and the most phytotoxic EOs were those constituted mainly by oxygenated monoterpenes [
21]. Other EOs which showed strong herbicidal potential against
A. retroflexus seed germination and seedling growth were
Rosmarinus officinalis,
Satureja hortensis, and
Laurus nobilis [
14], and a nanoemulsion of
S. hortensis EO was tested against
A. retroflexus in greenhouse conditions killing the weed at 4000 µL/mL dose [
22].
P. oleracea germination was completely inhibited by
Eucalyptus camaldulensis EO in in vitro conditions [
23]. The application of leaf extracts (obtained using water, methanol, and ethanol as solvents) of cultivated
Cynara cardunculus in in vitro bioassays inhibited seed germination and germination time in
A. retroflexus and
P. oleracea [
27].
Different natural compounds have demonstrated herbicidal potential against the germination and seedling growth of
A. fatua, such as EOs from
Artemisia herba-alba [
24] and
Eucalyptus citriodora EOs [
25] and extracts from
Sapindus mukorossi, which inhibited
A. fatua and
A. retroflexus growth in vitro and in pots [
28] or from
Iris sibirica rhizomes [
29].
EOs from
Thymbra capitata,
Mentha piperita,
Eucalyptus camaldulensis, and
Santolina chamaecyparissus were tested in vivo against
E. bonariensis.
T. capitata EO, with high content in carvacrol, was the most effective to control
E. bonariensis, showing an excellent potential to develop bioherbicide formulations [
26].
Some studies carried out in recent years relate the herbicidal activity of plant extracts or EOs to their composition in monoterpenes, and these substances are postulated as the future of natural herbicide components [
32,
33,
34,
35]. For example, eugenol, a monoterpene that can be found in many EOs as the major compound, like in
Syzygium aromaticum EO, has shown strong phytotoxic potential against
A. retroflexus [
30] and
A. fatua [
31]. In
A. fatua, eugenol inhibited its seedling growth, affecting more the roots than the coleoptiles. In addition, sesquiterpenes, secondary metabolites in plants, present in some EOs, have demonstrated strong herbicidal activity [
36,
37].
The natural products studied on this work for their potential as bioherbicides were pelargonic acid, trans-cinnamaldehyde and carvacrol. Pelargonic acid (PA) (CH
3(CH
2)
7CO
2H, n-nonanoic acid), which is present as esters in the EO of
Pelargonium spp., is a saturated fatty acid with nine carbons in its structure [
28,
29,
30,
31,
32,
33,
34,
35,
36,
37,
38,
39,
40]. PA and its salts are used like active ingredients in bioherbicide formulations for garden and professional uses worldwide. They are applied as burndown herbicides, which in a short time, attack cell membranes, causing cell leakage, followed by breakdown of membrane acyl lipids [
41], and finally causing visible effects of desiccation of green areas of the weeds [
38]. All the symptoms caused by PA on weeds involve extreme phytotoxicity for the plants and their cells, which rapidly begin to oxidize, causing necrotic lesions on aerial parts of plants [
42,
43].
Herbicidal fatty acids have been used for a long time in weed management, and some of them are used as natural herbicides. Still, the high dosage and the high cost are some of the drawbacks of its practical application in the current agriculture. In 2015, the bioherbicide Beloukha
® was authorized as plant protection product to be marketed in Europe [
44]. It is derived from oleic acid from different origin. Actually, it is authorized also for markets in USA and Canada. This work aims to find an optimal formulation of PA capable to be effective at reduced doses compared to the existing products in the market.
Trans-cinnamaldehyde (CA) (C
9H
8O) is one of the major components of two different cinnamon species (
Cinnamomum zeylanicum and
Cinnamomum cassia) and their EOs [
45,
46,
47,
48]. This compound has shown strong antioxidant properties and is responsible for various observed biological activities of cinnamon like bactericidal, fungicidal, or acaricidal [
49,
50,
51,
52]. The antimicrobial activity of CA is well known, however, its potential as bioherbicide has been less studied. Despite that, recent research demonstrated the herbicidal activity of CA against
Echinochloa crus-galli by reducing the fresh weight and growth of this important weed [
53]. To our knowledge, the mode of action of CA on weeds has not been elucidated.
The third natural compound evaluated was carvacrol (CV), a phenolic monoterpene frequently present on EOs obtained from many species belonging to Lamiaceae family like
Thymus spp.,
Thymbra spp., and
Origanum spp. [
34]. CV presents antimicrobial properties that make it helpful for controlling diseases in crop protection [
54,
55,
56,
57,
58]. In relation to its mode of action, CV exhibited membrane-disrupting activity that was dependent on long exposure at high concentration [
33]. Postemergence exposure of plants to high concentrations of CV causes severe phytotoxicity. One of the effects associated with the mode of action of CV is the reduction of weed growth [
22,
41,
54].
This work is a collaboration between the Universitat Politècnica de València (UPV) and the company Seipasa S.A., which develops and commercializes biopesticides, with the purpose to manage agricultural ecosystems in a more sustainable way. The objective of the present study was to evaluate the herbicidal potential of the natural compounds pelargonic acid, trans-cinnamaldehyde, and carvacrol against important cosmopolite weeds (Amaranthus retroflexus L., Portulaca oleracea L., Erigeron bonariensis L., and Avena fatua L.) as an alternative to synthetic herbicides to reduce the abiotic stress that they cause on crops. Effective compounds were formulated as emulsifiable concentrates (ECs) by Seipasa S.A., and evaluated for their postemergence herbicidal activity in greenhouse conditions in the UPV (Spain).