Are Copper Nanoparticles Toxic to All Plants? A Case Study on Onion (Allium cepa L.)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Location
2.2. Soil Preparation and Organic Matter
2.3. Irrigation System
2.4. Plant Material and Transplantation of Seedlings
2.5. Fertilizer Application and Harvest
2.6. Synthesis of Copper Oxide Nanoparticles
2.7. Characterization of CuO Nanoparticles
2.8. Experimental Design
- NPK+ without foliar spray (Control).
- NPK+ copper sulfate foliar spray at rate of 20 ppm L−1 (CuSO4).
- NPK+ EDTA chelated copper foliar spray at rate of 20 ppm L−1 (Cu-EDTA).
- NPK+ copper oxide nanoparticles (CuO-NPs) mixed with irrigation water at the first spray, then foliar spray at a rate of 10 ppm L−1.
2.9. Yield and Morphological Parameters
- Plant height (cm), using a measuring tape.
- Number of leaves per plant.
- Fresh weight of leaves (g), using a sensitive balance.
- Number of obtained bulbs per treatment.
- Neck diameter (cm), using a caliper.
- Bulb diameter (cm), using a caliper.
- Bulbing ratio (the diameter of the neck divided by the bulb diameter).
- Bulb weight (g), using a sensitive balance.
- Fresh weight (leaves and bulbs), using a sensitive balance.
- Yield (ton ha−1).
2.10. Chemical Analysis
2.11. Data Analysis
3. Results
3.1. Soil Properties after Planting
3.2. Vegetative Growth
3.3. Yield
3.4. Nitrogen, Phosphorus, and Potassium Contents of Onion Bulbs
3.5. Trace Elements Content of Onion Bulbs
3.6. Phytochemicals Content of Onion Bulbs
3.7. Vitamin Contents of Onion Bulbs
3.8. Amino Acid Contents of Onion Bulbs
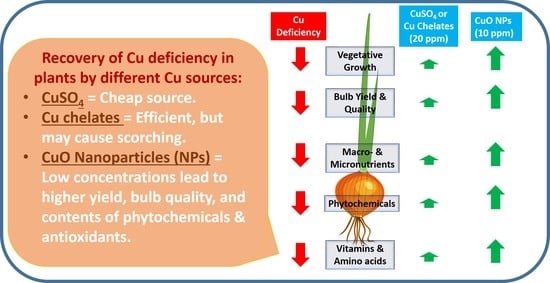
4. Discussion
4.1. Importance of Copper to Plants
4.2. Cu Deficiency Negatively Affects Bulb Quality Parameters of Onion
4.3. CuO NPs Form Is the Most Efficient Treatment in Increasing Onion Yields
4.4. CuO NPs Enhances the Content of Micro- and Macro-Elements in Onion Tissues
4.5. Cu Enhances Vitamin Contents in Onion Bulbs
4.6. Cu Enhances the Accumulation of Amino Acids in Onion Bulbs
4.7. Is There a Clear Limit between the Stimulating and Inhibiting/Toxic Concentrations of Cu NPs in Plants?
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- FAO-United Nations Statistics Division. Statistics Division of Food and Agriculture Organization of the United Nations; FAO-United Nations Statistics Division: Rome, Italy, 2018. [Google Scholar]
- Teshika, J.D.; Zakariyyah, A.M.; Zaynab, T.; Zengin, G.; Rengasamy, K.R.; Pandian, S.K.; Fawzi, M.M. Traditional and modern uses of onion bulb (Allium cepa L.): A systematic review. Crit. Rev. Food Sci. Nutr. 2019, 59, S39–S70. [Google Scholar] [CrossRef] [PubMed]
- Upadhyay, R.K. Nutraceutical, pharmaceutical and therapeutic uses of Allium cepa: A review. Int. J. Green Pharm. 2016, 10, 46–64. [Google Scholar]
- Marrelli, M.; Amodeo, V.; Statti, G.; Conforti, F. Biological properties and bioactive components of Allium cepa L.: Focus on potential benefits in the treatment of obesity and related comorbidities. Molecules 2019, 24, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khokhar, K.M. Mineral nutrient management for onion bulb crops—A review. J. Hortic. Sci. Biotechnol. 2019, 94, 703–717. [Google Scholar] [CrossRef]
- Broadley, M.; Brown, P.; Cakmak, I.; Rengel, Z.; Zhao, F. Function of Nutrients: Micronutrients. In Marschner’s Mineral Nutrition of Higher Plants; Elsevier: Amsterdam, The Netherlands, 2012; pp. 191–248. [Google Scholar]
- Rahimi, A.; Bussler, W. Physiologische Voraussetzungen für die Bildung der Kupfermangelsymptome. Z. Pflanz. Bodenkd. 1973, 136, 25–32. [Google Scholar] [CrossRef]
- Gupta, U.C. Copper in Crop and Plant Nutrition. In Handbook of Copper Compounds and Applications; Marcel Dekker, Inc.: New York, NY, USA, 1997; pp. 203–229. [Google Scholar]
- Fageria, N.K. The Use of Nutrients in Crop Plants; CRC press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Purvis, E.; Carolus, R. Nutrient Deficiencies in Vegetable Crops. In Hunger Signs in Crops; MacKay & Company, Inc.: New York, USA, 1964; pp. 245–286. [Google Scholar]
- Kasana, R.C.; Panwar, N.R.; Kaul, R.K.; Kumar, P. Biosynthesis and effects of copper nanoparticles on plants. Environ. Chem. Lett. 2017, 15, 233–240. [Google Scholar] [CrossRef]
- Shah, V.; Belozerova, I. Influence of metal nanoparticles on the soil microbial community and germination of lettuce seeds. Water Air Soil Pollut. 2009, 197, 143–148. [Google Scholar] [CrossRef]
- Nair, P.M.G.; Chung, I.M. Impact of copper oxide nanoparticles exposure on Arabidopsis thaliana growth, root system development, root lignificaion, and molecular level changes. Environ. Sci. Pollut. Res. 2014, 21, 12709–12722. [Google Scholar] [CrossRef]
- Fouda, K.F. Response of onion yield and Its chemical content to NPK fertilization and foliar application of some micronutrients. Egypt. J. Soil Sci. 2016, 56, 549–561. [Google Scholar] [CrossRef]
- Wang, Y.; Deng, C.; Cota-Ruiz, K.; Peralta-Videa, J.R.; Sun, Y.; Rawat, S.; Tan, W.; Reyes, A.; Hernandez-Viezcas, J.A.; Niu, G. Improvement of nutrient elements and allicin content in green onion (Allium fistulosum) plants exposed to CuO nanoparticles. Sci. Total Environ. 2020, 725, 138387. [Google Scholar] [CrossRef]
- Richards, L.A. Diagnosis and Improvement of Saline and Alkali Soils; Government Printing Office (Superintendent of Documents): Washington, DC, USA, 1954. [Google Scholar]
- Jackson, M. Soil Chemical Analysis Prentice; Hall of India Private Limited: New Delhi, India, 1967; p. 498. [Google Scholar]
- Keller, J.; Karmeli, D. Trickle Irrigation Design; Rain Bird Sprinkler Manufacturing Corporation: Glendora, CA, USA, 1975. [Google Scholar]
- Zhang, Q.; Zhang, K.; Xu, D.; Yang, G.; Huang, H.; Nie, F.; Liu, C.; Yang, S. CuO nanostructures: Synthesis, characterization, growth mechanisms, fundamental properties, and applications. Prog. Mater. Sci. 2014, 60, 208–337. [Google Scholar] [CrossRef]
- The Association of Official Analytical Chemists. Official Methods of Analysis: Changes in Official Methods of Analysis Made at the Annual Meeting, Supplement; Association of Official Analytical Chemists: Washington, DC, USA, 1990; Volume 15. [Google Scholar]
- Katoch, R. Analytical Techniques in Biochemistry and Molecular Biology; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- de Brouwer, V.; Storozhenko, S.; Stove, C.P.; van Daele, J.; van der Straeten, D.; Lambert, W.E. Ultra-performance liquid chromatography–tandem mass spectrometry (UPLC–MS/MS) for the sensitive determination of folates in rice. J. Chromatogr. B 2010, 878, 509–513. [Google Scholar] [CrossRef] [PubMed]
- Nazmul, H.; Akhtaruzzaman, M.; Zakir, S. Estimation of Vitamins B-Complex (B 2, B 3, B 5 and B 6) of Some Leafy Vegetables Indigenous to Bangladesh by HPLC Method. J. Anal. Sci. Methods Instrum. 2013, 3, 24–29. [Google Scholar]
- Wayne, W. A reliable methodology for quantitative extraction of fruit and vegetable physiological amino acids and their subsequent analysis with commonly available HPLC systems. Food Nutr. Sci. 2012, 3, 863–871. [Google Scholar]
- Sardella, R.; Lisanti, A.; Marinozzi, M.; Ianni, F.; Natalini, B.; Blanch, G.P.; del Castillo, M.L.R. Combined monodimensional chromatographic approaches to monitor the presence of D-amino acids in cheese. Food Control 2013, 34, 478–487. [Google Scholar] [CrossRef] [Green Version]
- Andresen, E.; Peiter, E.; Küpper, H. Trace metal metabolism in plants. J. Exp. Bot. 2018, 69, 909–954. [Google Scholar] [CrossRef]
- Pilon, M.; Abdel-Ghany, S.E.; Cohu, C.M.; Gogolin, K.A.; Ye, H. Copper cofactor delivery in plant cells. Curr. Opin. Plant Biol. 2006, 9, 256–263. [Google Scholar] [CrossRef] [PubMed]
- Yamasaki, H.; Pilon, M.; Shikanai, T. How do plants respond to copper deficiency? Plant Signal. Behav. 2008, 3, 231–232. [Google Scholar] [CrossRef] [Green Version]
- Masunaga, T.; Fong, J.D.M. Strategies for Increasing Micronutrient Availability in Soil for Plant Uptake. In Plant Micronutrient Use Efficiency; Elsevier: Amsterdam, The Netherlands, 2018; pp. 195–208. [Google Scholar]
- Mahmoud, A.W.M.; Abdeldaym, E.A.; Abdelaziz, S.M.; El-Sawy, M.B.; Mottaleb, S.A. Synergetic Effects of Zinc, Boron, Silicon, and Zeolite Nanoparticles on Confer Tolerance in Potato Plants Subjected to Salinity. Agronomy 2020, 10, 19. [Google Scholar] [CrossRef] [Green Version]
- Shambhavi, S.; Kumar, R.; Kumar, R.; Singh, M. Micronutrients Deficiency in Vegetable Crops and Their Management. Diseases of Fruits and Vegetable Crops: Recent Management Approaches; Apple Academic Press: Palm Bay, FL, USA, 2020; p. 382. [Google Scholar]
- Locascio, S. The relationship of copper availability and soil pH. Solutions 1978, 30–42. [Google Scholar]
- Mengel, K.; Kirkby, E.A.; Kosegarten, H.; Appel, T. Fertilizer Application. In Principles of Plant Nutrition; Springer: New York, NY, USA, 2001; pp. 337–396. [Google Scholar]
- Alloway, B.; Tills, A.R. Copper deficiency in world crops. Outlook Agric. 1984, 13, 32–42. [Google Scholar] [CrossRef]
- Price, G. Australian Soil Fertility Manual; CSIRO Publishing: Clayton South, VIC, Australia, 2006. [Google Scholar]
- Printz, B.; Guerriero, G.; Sergeant, K.; Audinot, J.-N.; Guignard, C.; Renaut, J.; Lutts, S.; Hausman, J.-F. Combining-omics to unravel the impact of copper nutrition on alfalfa (Medicago sativa) stem metabolism. Plant Cell Physiol. 2016, 57, 407–422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darwish, E.; Mottaleb, S.A.; Omara, M.; Safwat, G. Effect of salt stress on root plasticity and expression of ion transporter genes in tomato plants. Int. J. Bot. Res. 2016, 6, 13–26. [Google Scholar]
- Mottaleb, S.A.; Darwish, E.; Mostafa, M.; Safwat, G. Phenotyping Root System Architecture of Cotton (Gossypium barbadense L.) Grown Under Salinity. Agriculture (Pol’nohospodárstvo) 2017, 63, 142–150. [Google Scholar] [CrossRef] [Green Version]
- Shahbaz, M.; Ravet, K.; Peers, G.; Pilon, M. Prioritization of copper for the use in photosynthetic electron transport in developing leaves of hybrid poplar. Front. Plant. Sci. 2015, 6, 407. [Google Scholar] [CrossRef] [Green Version]
- Morales, F.; Pavlovič, A.; Abadía, A.; Abadía, J. Photosynthesis in Poor Nutrient Soils, in Compacted Soils, and Under Drought. In The Leaf: A Platform for Performing Photosynthesis; Springer: New York, NY, USA, 2018; pp. 371–399. [Google Scholar]
- Gupta, U.C.; Macleod, L. Response to copper and optimum levels in wheat, barley and oats under greenhouse and field conditions. Can. J. Soil Sci. 1970, 50, 373–378. [Google Scholar] [CrossRef]
- Brennan, R. Effectiveness of some copper compounds applied as foliar sprays in alleviating copper deficiency of wheat grown on copper-deficient soils of Western Australia. Aust. J. Exp. Agric. 1990, 30, 687–691. [Google Scholar] [CrossRef]
- Fleming, G.A.; Delaney, J. Copper and nitrogen in the nutrition of wheat on cutaway peat. Ir. J. Agric. Res. 1961, 1, 81–82. [Google Scholar]
- Brown, J.C. Calcium Movement in Barley and Wheat as Affected by Copper and Phosphorus 1. Agron. J. 1965, 57, 617–621. [Google Scholar] [CrossRef]
- Mengel, K.; Barker, A.; Pilbeam, D. Handbook of Plant Nutrition; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Cheng, T.; Allen, H.E. Prediction of uptake of copper from solution by lettuce (Lactuca sativa Romance). Environ. Toxicol. Chem. Int. J. 2001, 20, 2544–2551. [Google Scholar] [CrossRef]
- Ginocchio, R.; Rodríguez, P.H.; Badilla-Ohlbaum, R.; Allen, H.E.; Lagos, G.E. Effect of soil copper content and pH on copper uptake of selected vegetables grown under controlled conditions. Environ. Toxicol. Chem. Int. J. 2002, 21, 1736–1744. [Google Scholar] [CrossRef]
- Badilla-Ohlbaum, R.; Ginocchio, R.; Rodríguez, P.H.; Céspedes, A.; González, S.; Allen, H.E.; Lagos, G.E. Relationship between soil copper content and copper content of selected crop plants in central Chile. Environ. Toxicol. Chem. Int. J. 2001, 20, 2749–2757. [Google Scholar] [CrossRef]
- Kwiatkowska, C.A.; Finglas, P.M.; Faulks, R.M. The vitamin content of retail vegetables in the UK. J. Hum. Nutr. Diet. 1989, 2, 159–172. [Google Scholar] [CrossRef]
- Boubakri, H.; Gargouri, M.; Mliki, A.; Brini, F.; Chong, J.; Jbara, M. Vitamins for enhancing plant resistance. Planta 2016, 244, 529–543. [Google Scholar] [CrossRef] [PubMed]
- Bahram-Parvar, M.; Lim, L.T. Fresh-cut onion: A review on processing, health benefits, and shelf-life. Compr. Rev. Food Sci. Food Saf. 2018, 17, 290–308. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mozafar, A. Plant. Vitamins; CRC press: Boca Raton, FL, USA, 2018. [Google Scholar]
- Roje, S. Vitamin B biosynthesis in plants. Phytochemistry 2007, 68, 1904–1921. [Google Scholar] [CrossRef]
- Wan, P.; Moat, S.; Anstey, A. Pellagra: A review with emphasis on photosensitivity. Br. J. Dermatol. 2011, 164, 1188–1200. [Google Scholar] [CrossRef]
- Possingham, J. The effect of mineral nutrition on the content of free amino acids and amides in tomato plants IA comparison of the effects of deficiencies of copper, zinc, manganese, iron, and molybdenum. Aust. J. Biol. Sci. 1956, 9, 539–551. [Google Scholar] [CrossRef]
- Burkhead, J.L.; Gogolin Reynolds, K.A.; Abdel-Ghany, S.E.; Cohu, C.M.; Pilon, M. Copper homeostasis. New Phytol. 2009, 182, 799–816. [Google Scholar] [CrossRef]
- Pourkhaloee, A.; Haghighi, M.; Saharkhiz, M.J.; Jouzi, H.; Doroodmand, M.M. Carbon nanotubes can promote seed germination via seed coat penetration. Seed Technol. 2011, 33, 155–169. [Google Scholar]
- Siddiqui, M.H.; Al-Whaibi, M.H.; Faisal, M.; Al Sahli, A.A. Nano-silicon dioxide mitigates the adverse effects of salt stress on Cucurbita pepo L. Environ. Toxicol. Chem. 2014, 33, 2429–2437. [Google Scholar] [CrossRef] [PubMed]
- López-Vargas, E.R.; Ortega-Ortíz, H.; Cadenas-Pliego, G.; de Alba Romenus, K.; Cabrera de la Fuente, M.; Benavides-Mendoza, A.; Juárez-Maldonado, A. Foliar application of copper nanoparticles increases the fruit quality and the content of bioactive compounds in tomatoes. Appl. Sci. 2018, 8, 1020. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Hernández, H.; González-Morales, S.; Benavides-Mendoza, A.; Ortega-Ortiz, H.; Cadenas-Pliego, G.; Juárez-Maldonado, A. Effects of chitosan–PVA and Cu nanoparticles on the growth and antioxidant capacity of tomato under saline stress. Molecules 2018, 23, 178. [Google Scholar] [CrossRef] [Green Version]
- van Nguyen, D.; Nguyen, H.M.; Le, N.T.; Nguyen, K.H.; Nguyen, H.T.; Le, H.M.; Nguyen, A.T.; Dinh, N.T.T.; Hoang, S.A.; van Ha, C. Copper nanoparticle application enhances plant growth and grain yield in maize under drought stress conditions. J. Plant. Growth Regul. 2021, 2021, 1–12. [Google Scholar]
- Mittal, D.; Kaur, G.; Singh, P.; Yadav, K.; Ali, S. Nanoparticle-based sustainable agriculture and food science: Recent advances and future outlook. Front. Nanotechnol. 2020, 2020, 2. [Google Scholar]
- Paramo, L.A.; Feregrino-Pérez, A.A.; Guevara, R.; Mendoza, S.; Esquivel, K. Nanoparticles in Agroindustry: Applications, Toxicity, Challenges, and Trends. Nanomaterials 2020, 10, 1654. [Google Scholar] [CrossRef] [PubMed]
- Rivero-Montejo, S.d.J.; Vargas-Hernandez, M.; Torres-Pacheco, I. Nanoparticles as Novel Elicitors to Improve Bioactive Compounds in Plants. Agriculture 2021, 11, 134. [Google Scholar] [CrossRef]
- Zhao, L.; Hu, J.; Huang, Y.; Wang, H.; Adeleye, A.; Ortiz, C.; Keller, A.A. 1H NMR and GC–MS based metabolomics reveal nano-Cu altered cucumber (Cucumis sativus) fruit nutritional supply. Plant. Physiol. Biochem. 2017, 110, 138–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, O.S.; Pant, N.C.; Laishram, L.; Tewari, M.; Dhoundiyal, R.; Joshi, K.; Pandey, C. Effect of CuO nanoparticles on polyphenols content and antioxidant activity in Ashwagandha (Withania somnifera L. Dunal). J. Pharmacogn. Phytochem. 2018, 7, 3433–3439. [Google Scholar]
- Abbasifar, A.; Shahrabadi, F.; ValizadehKaji, B. Effects of green synthesized zinc and copper nano-fertilizers on the morphological and biochemical attributes of basil plant. J. Plant. Nutr. 2020, 43, 1104–1118. [Google Scholar] [CrossRef]
- Ahmad, M.A.; Javed, R.; Adeel, M.; Rizwan, M.; Ao, Q.; Yang, Y. Engineered ZnO and CuO nanoparticles ameliorate morphological and biochemical response in tissue culture regenerants of candyleaf (Stevia rebaudiana). Molecules 2020, 25, 1356. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Cao, W.; Rui, Y. Interactions between nanoparticles and plants: Phytotoxicity and defense mechanisms. J. Plant. Interact. 2017, 12, 158–169. [Google Scholar] [CrossRef]
- Rai, M.; Ingle, A.P.; Pandit, R.; Paralikar, P.; Shende, S.; Gupta, I.; Biswas, J.K.; da Silva, S.S. Copper and copper nanoparticles: Role in management of insect-pests and pathogenic microbes. Nanotechnol. Rev. 2018, 7, 303–315. [Google Scholar] [CrossRef] [Green Version]
- Shang, Y.; Hasan, M.; Ahammed, G.J.; Li, M.; Yin, H.; Zhou, J. Applications of nanotechnology in plant growth and crop protection: A review. Molecules 2019, 24, 2558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajput, V.; Minkina, T.; Suskova, S.; Mandzhieva, S.; Tsitsuashvili, V.; Chapligin, V.; Fedorenko, A. Effects of copper na-noparticles (CuO NPs) on crop plants: A mini review. BioNanoScience 2018, 8, 36–42. [Google Scholar] [CrossRef]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, S. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Arya, S.K.; Bhardwaj, N.; Khatri, M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts 2021, 23, 213–239. [Google Scholar] [CrossRef]
- Rasool, A.; Shah, W.H.; Tahir, I.; Rehman, R.U. Application of Nanoparticles in Crop Production and Protection. In Plant Nanobionics; Springer: New York, NY, USA, 2019; pp. 235–253. [Google Scholar]
- Singh, Z.; Singh, I. CTAB surfactant assisted and high pH nano-formulations of CuO nanoparticles pose greater cytotoxic and genotoxic effects. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
| Physical Properties | Value | Chemical Properties | Value | ||
|---|---|---|---|---|---|
| Particle Size Distribution (%) | Electrical conductivity (EC ds/m) | 1.70 | |||
| Coarse sand (2000-200µ) | 79.20 | pH (1:2.5) soil:water suspension | 7.98 | ||
| Fine sand (200-20 µ) | 13.80 | Soluble cations (meq/L) | |||
| Silt (20-2 µ) | 4.75 | Ca2+ | 5.30 | ||
| Clay (< 2 µ) | 2.25 | Mg2+ | 4.32 | ||
| Bulk density (g/cm3) | 1.56 | K+ | 2.39 | ||
| Total porosity (%) | 54.70 | Na+ | 5.40 | ||
| Pore Size Distribution as % of Total Porosity | Soluble Anions (meq/L) | ||||
| Macro (drainable)-pores (>28.8 µ) | 80.84 | CO3 2- | Nil | ||
| Micropores (<28.8 µ) | 19.16 | HCO3- | 1.65 | ||
| Water holding capacity | 21.56 | Cl- | 3.65 | ||
| Field capacity | 8.68 | SO42- | 12.11 | ||
| Wilting percentage | 4.15 | Total carbonate (%) | 0.20 | ||
| Available moisture | 4.53 | Organic matter (%) | 0.26 | ||
| Hydraulic conductivity (cm/h) | 6.30 | Micronutrients (ppm) | |||
| Cu | Zn | Mn | Fe | ||
| 12.05 | 15.63 | 14.61 | 42.26 | ||
| Property | Value |
|---|---|
| Moisture content (%) | 25 |
| PH (1:5) | 7.5 |
| EC (1:5 extract) dsm−1 | 3.1 |
| Organic C (%) | 33.11 |
| Organic matter (%) | 70 |
| Total N (%) | 1.82 |
| Total K (%) | 1.25 |
| C/N ratio | 15:1 |
| Total P (%) | 1.29 |
| Fe (ppm) | 97.5 |
| Mn (ppm) | 18.40 |
| Cu (ppm) | 5.25 |
| Zn (ppm) | 14.33 |
| Total content of bacteria (CFU g−1) | 2.5 × 107 |
| Phosphate dissolving bacteria (CFU g−1) | 2.5 × 106 |
| Weed seeds | 0 |
| Physical Properties | Value | Chemical Properties | Value | ||
|---|---|---|---|---|---|
| Particle Size Distribution (%) | Electrical conductivity (EC ds/m) | 1.64 | |||
| Coarse sand (2000–200µ) | 81.30 | pH (1:2.5) soil:water suspension | 7.80 | ||
| Fine sand (200–20 µ) | 12.40 | Soluble cations (meq/L) | |||
| Silt (20–2 µ) | 4.30 | Ca2+ | 5.13 | ||
| Clay (<2 µ) | 2.00 | Mg2+ | 4.22 | ||
| Bulk density (g/cm3) | 1.60 | K+ | 1.93 | ||
| Total porosity (%) | 53.78 | Na+ | 5.24 | ||
| Pore Size Distribution as % of Total Porosity | Soluble Anions (meq/L) | ||||
| Macro (drainable)-pores (>28.8 µ) | 82.54 | CO3 2- | Nil | ||
| Micropores (<28.8 µ) | 17.46 | HCO3- | 1.60 | ||
| Water holding capacity | 13.07 | Cl- | 3.44 | ||
| Field capacity | 8.46 | SO42- | 11.46 | ||
| Wilting percentage | 4.61 | Total carbonates (%) | 0.19 | ||
| Available moisture | 3.85 | Organic matter (%) | 0.22 | ||
| Hydraulic conductivity (cm/h) | 6.12 | Micronutrients (ppm) | |||
| Cu | Zn | Mn | Fe | ||
| 17.65 | 12.71 | 9.58 | 37.11 | ||
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||||
|---|---|---|---|---|---|---|---|---|
| Plant Height (cm) | No. of Leaves/Plant | Fresh Weight of Leaves (g) | No. of Bulbs | Plant Height (cm) | No. of Leaves/Plant | Fresh Weight of Leaves (g) | No. of Bulbs | |
| Control | 50.2 ± 0.32 b | 9.20 ± 0.25 a | 30.2 ± 0.12 b | 103 ± 2.5 c | 51.5 ± 1.5 b | 9.59 ± 1.3 a | 32.4 ± 1.2 b | 107 ± 2.1 c |
| CuSO4 | 52.1 ± 0.46 a | 9.60 ± 0.13 a | 32.5 ± 0.22 a | 124 ± 3.3 b | 52.8 ± 0.9 b | 9.73 ± 0.8 a | 33.6 ± 1.5 b | 125 ± 1.5 b |
| Cu-EDTA | 53.0 ± 1.02 a | 9.85 ± 0.57 a | 33.7 ± 0.15 a | 125 ± 2.4 b | 53.5 ± 0.7 b | 9.80 ± 1.1 a | 33.9 ± 1.1 b | 126 ± 2.1 b |
| Cu-NPs | 55.2 ± 1.32 a | 10.20 ± 0.41 a | 38.9 ± 0.36 a | 130 ± 1.9 a | 55.7 ± 0.6 a | 10.15 ± 2.3 a | 37.8 ± 1.6 a | 132 ± 1.3 a |
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Bulb Diameter (cm) | Neck Diameter (cm) | Bulbing Ratio | Bulb Weight (g) | Total Yield (ton/ha) | Bulb Diameter (cm) | Neck Diameter (cm) | Bulbing Ratio | Bulb Weight (g) | Total Yield (ton/ha) | |
| Control | 5.70 ± 0.04 b | 1.56 ± 0.02 b | 0.27 ± 0.0001 c | 85.3 ± 4 c | 18.6 ± 0.3 c | 5.93 ± 0.03 b | 1.58 ± 0.03 c | 0.27 ± 0.0001 b | 87.9 ± 7 c | 19.0 ± 0.4 c |
| CuSO4 | 5.86 ± 0.06 b | 1.63 ± 0.04 b | 0.28 ± 0.0001 b | 90.5 ± 6 c | 20.2 ± 0.9 c | 5.91 ± 0.04 b | 1.85 ± 0.02 b | 0.31 ± 0.0002 a | 96.4 ± 11 c | 26.4 ± 1.6 b |
| Cu-EDTA | 5.96 ± 0.04 b | 1.74 ± 0.05 b | 0.29 ± 0.0001 b | 130.2 ± 8 b | 25.2 ± 0.2 b | 6.03 ± 0.02 a | 1.86 ± 0.01 b | 0.31 ± 0.0001 a | 135.5 ± 7 b | 30.0 ± 0.9 b |
| Cu-NPs | 6.55 ± 0.07 a | 2.04 ± 0.02 a | 0.31 ± 0.0001 a | 156.1 ± 10 a | 32.0 ± 0.3 a | 6.93 ± 0.03 a | 2.19 ± 0.06 a | 0.32 ± 0.0002 a | 158.7 ± 9 a | 33.6 ± 0.5 a |
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||
|---|---|---|---|---|---|---|
| N % | P % | K % | N % | P % | K % | |
| Control | 1.70 ± 0.02 c | 0.14 ± 0.005 c | 1.35 ± 0.01 c | 1.79 ± 0.05 b | 0.15 ± 0.003 c | 1.25 ± 0.02 c |
| CuSO4 | 1.77 ± 0.01 b | 0.18 ± 0.002 b | 1.42 ± 0.03 b | 1.90 ± 0.02 b | 0.19 ± 0.001 b | 1.32 ± 0.01 b |
| Cu-EDTA | 1.75 ± 0.02 b | 0.21 ± 0.004 a | 1.47 ± 0.02 b | 2.08 ± 0.01 a | 0.22 ± 0.001 b | 1.39 ± 0.02 b |
| Cu-NPs | 1.92 ± 0.03 a | 0.22 ± 0.006 a | 1.61 ± 0.01 a | 2.30 ± 0.03 a | 0.25 ± 0.002 a | 1.46 ± 0.01 a |
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||||
|---|---|---|---|---|---|---|---|---|
| Fe (ppm) | Cu (ppm) | Zn (ppm) | Mn (ppm) | Fe (ppm) | Cu (ppm) | Zn (ppm) | Mn (ppm) | |
| Control | 143.7 ± 2 c | 7.50 ± 0.12 b | 22.9 ± 0.1 c | 19.9 ± 0.2 b | 137.6 ± 2 c | 7.98 ± 0.21 c | 23.9 ± 0.3 b | 20.4 ± 0.5 b |
| CuSO4 | 150.7 ± 1 b | 7.92 ± 0.25 b | 24.3 ± 0.2 b | 21.7 ± 0.1 a | 154.8 ± 3 b | 8.60 ± 0.25 b | 25.3 ± 0.1 a | 22.8 ± 0.2 a |
| Cu-EDTA | 157.1 ± 2 a | 8.56 ± 0.32 a | 24.9 ± 0.3 b | 23.0 ± 0.1 a | 166.3 ± 3 a | 8.80 ± 015 b | 26.0 ± 0.3 a | 23.1 ± 0.2 a |
| Cu-NPs | 159.6 ± 3 a | 8.90 ± 0.28 a | 25.9 ± 0.2 a | 23.4 ± 0.2 a | 169.1 ± 4 a | 9.39 ± 0.19 a | 26.8 ± 0.5 a | 23.8 ± 0.4 a |
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| TSS (%) | Carbohydrates (mg/g) | Protein (mg/g) | Dietary fibers (mg/g) | Water (mg/g) | TSS (%) | Carbohydrates (mg/g) | Protein (mg/g) | Dietary fibers (mg/g) | Water (mg/g) | |
| Control | 7.02 ± 0.1 c | 92.0 ± 0.2 c | 9.0 ± 0.2 b | 16.0 ± 0.2 b | 873.5 ± 3 a | 6.03 ± 0.3 b | 93.3 ± 0.4 b | 8.9 ± 0.02 c | 15.3 ± 0.3 c | 853.5 ± 9 a |
| CuSO4 | 7.23 ± 0.1 b | 93.5 ± 0.1 b | 10.3 ± 0.1 a | 16.4 ± 0.0 a | 862.3 ± 5 a | 6.50 ± 0.2 a b | 95.8 ± 0.9 b | 11.3 ± 0.00 b | 16.1 ± 0.2 b | 834.3 ± 5 b |
| Cu-EDTA | 7.63 ± 0.4 b | 94.3 ± 0.3 b | 10.5 ± 0.3 a | 16.8 ± 0.1 a | 857.8 ± 6 a | 6.67 ± 0.3 a | 101.3 ± 0.5 a | 11.5 ± 0.00 a | 16.5 ± 0.3 b | 816.6 ± 3 b |
| Cu-NPs | 8.23 ± 0.1 a | 95.3 ± 0.1 a | 11.1 ± 0.6 a | 17.1 ± 0.1 a | 820.0 ± 3 b | 6.75 ± 0.4 a | 102.8 ± 0.7 a | 11.6 ± 0.01 a | 17.0 ± 0.1 a | 800.0 ± 2 c |
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| B1 (mg) | B2 (mg) | B3 (mg) | B5 (mg) | B6 (mg) | B9 (µg) | C (mg) | B1 (mg) | B2 (mg) | B3 (mg) | B5 (mg) | B6 (mg) | B9 (µg) | C (mg) | |
| Control | 0.039(2) c | 0.023(1) c | 0.113(1) c | 0.129(2) a | 0.14 ± 0.02 a | 20 ± 0.1 a | 7.0 ± 0.2 a | 0.043(1) a | 0.030(1) a | 0.111(2) b | 0.124(6) a | 0.12 ± 0.01 b | 17 ± 0.2 b | 6.7 ± 0.2 b |
| CuSO4 | 0.041(1) b | 0.025(2) b | 0.115(3) b | 0.123(4) c | 0.11 ± 0.01 b | 19 ± 0.2 b | 7.2 ± 0.4 a | 0.040(1) b | 0.024(2) b | 0.110(2) b | 0.120(5) b | 0.16 ± 0.01 a | 15 ± 0.1 b | 7.9 ± 0.1 a |
| Cu-EDTA | 0.043(3) b | 0.026(2) b | 0.117(2) b | 0.125(3) b | 0.12 ± 0.01 b | 18 ± 0.3 b | 7.4 ± 0.2 a | 0.044(2) a | 0.020(4) c | 0.118(4) a | 0.125(6) a | 0.11 ± 0.02 b | 18 ± 0.2 a | 7.6 ± 0.3 a |
| Cu-NPs | 0.048(2) a | 0.029(1) a | 0.120(1) a | 0.120(2) c | 0.14 ± 0.03 a | 22 ± 0.3 a | 7.8 ± 0.6 a | 0.045(1) a | 0.031(7) a | 0.117(1) a | 0.121(4) b | 0.15 ± 0.01 a | 20 ± 0.5 a | 7.9 ± 0.2 a |
| Season 1 (2018–2019) | Season 2 (2019–2020) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Methionine (µg/mL) | Phenylalanine (µg/mL) | Leucine (µg/mL) | Tryptophan (µg/mL) | Cysteine( µg/mL) | Methionine (µg/mL) | Phenylalanine (µg/mL) | Leucine (µg/mL) | Tryptophan (µg/mL) | Cysteine (µg/mL) | |
| Control | 40.1 ± 0.3 c | 3.1 ± 0.2 c | 31.2 ± 0.3 c | 25.4 ± 0.6 b | 48.6 ± 0.1 c | 39.9 ± 0.2 c | 3.2 ± 0.7 d | 33.2 ± 0.3 b | 29.7 ± 0.5 a | 46.7 ± 0.5 c |
| CuSO4 | 44.3 ± 0.5 b | 11.2 ± 0.4 c | 33.4 ± 0.2 c | 20.1 ± 0.5 c | 50.0 ± 0.2 b | 42.8 ± 0.1 b | 11.2 ± 0.2 c | 34.5 ± 0.4 b | 22.4 ± 0.2 c | 49.2 ± 0.4 b |
| Cu-EDTA | 43.1 ± 0.9 b | 13.3 ± 0.1 b | 36.1 ± 0.6 b | 24.2 ± 0.2 b | 50.0 ± 0.5 b | 45.1 ± 0.6 a | 13.6 ± 0.4 b | 33.9 ± 0.7 b | 26.4 ± 03 b | 49.3 ± 0.2 b |
| Cu-NPs | 48.2 ± 0.6 a | 15.9 ± 0.6 a | 40.1 ± 0.8 a | 28.7 ± 0.4 a | 55.3 ± 0.3 a | 46.9 ± 0.5 a | 16.1 ± 0.2 a | 41.2 ± 0.2 a | 29.1 ± 0.4 a | 56.0 ± 0.3 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mottaleb, S.A.; Hassan, A.Z.A.; El-Bahbohy, R.; Mahmoud, A.W.M. Are Copper Nanoparticles Toxic to All Plants? A Case Study on Onion (Allium cepa L.). Agronomy 2021, 11, 1006. https://doi.org/10.3390/agronomy11051006
Mottaleb SA, Hassan AZA, El-Bahbohy R, Mahmoud AWM. Are Copper Nanoparticles Toxic to All Plants? A Case Study on Onion (Allium cepa L.). Agronomy. 2021; 11(5):1006. https://doi.org/10.3390/agronomy11051006
Chicago/Turabian StyleMottaleb, Shady Abdel, Ahmed Z. A. Hassan, Reham El-Bahbohy, and Abdel Wahab M. Mahmoud. 2021. "Are Copper Nanoparticles Toxic to All Plants? A Case Study on Onion (Allium cepa L.)" Agronomy 11, no. 5: 1006. https://doi.org/10.3390/agronomy11051006