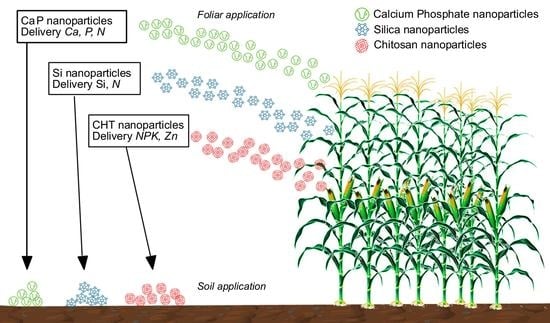
Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures
Abstract
:1. Introduction
2. Nanotechnologies: A Powerful Source of Innovation in Agriculture
3. Nanofertilizers
- Metal nanomaterials, metal-based materials commonly regarded as nanosilver, nanogold, quantum dots, and metal oxides [32];
- Ceramic nanomaterials, inorganic, heat-resistant, nonmetallic solids that can be made of both metallic and nonmetallic compounds [33];
- Polymeric nanomaterials, macromolecules composed of many repeating units organized in a chain-like molecular architecture exhibiting a multiplicity of compositions, structures, and properties [34].
- Macronutrient nanofertilizers: e.g., hydroxyapatite nanoparticles, calcium carbonate nanoparticles, and magnesium oxide nanoparticles;
- Micronutrient nanofertilizers: e.g., iron oxide nanoparticles, manganese oxide nanoparticles, zinc oxide and copper oxide nanoparticles;
- Nanomaterial-enhanced fertilizers (NEF): according to Liu and Lal [36] NEF are nanomaterials “loaded with plant nutrient(s), aimed at increasing plant-uptake efficiency of the nutrient(s) and/or reducing the adverse impacts of fertilizer application, but the nanomaterials themselves do not contain or supply the targeted nutrient(s).” Some examples of NEF are nanozeolites, silica nanoparticles, and nano chitosan (CHT);
- Plant growth stimulating nanomaterials: e.g., titanium oxide nanoparticles, cerium oxide nanoparticles, single-walled carbon nanotubes (SWCTNs), multiple walled carbon nanotubes (MWCTNs), graphene, and fullerenes. For these nanomaterials, stimulating action on plant growth has been demonstrated. However, in particular, as far as carbon compounds, studies are still ongoing [37].
4. Nanoscopic Calcium Phosphate Compounds
4.1. Hydroxyapatite Nanoparticles
4.1.1. Nano-Hydroxyapatite as Source of Phosphorus
4.1.2. Nano-Hydroxyapatite as Nitrogen Carrier
4.1.3. Nano-Hydroxyapatite as Micronutrients Carrier
| Material | Species | Treatment | Experimental Conditions | Results | Reference |
|---|---|---|---|---|---|
| nHAP, 16 nm | Glycine max | 21.8 mg L−1 as P | Perlite-peat moss (1:1), nutrient solution, greenhouse. | Increased growth rate (+32.6%), aerial biomass (+18.2%) and seed yield (+20.4%) than control. | [39] |
| nHAP, 94–163 nm | Solanum lycopersicum | 0, 2, 20, 200, 500, 1000, 2000 mg L−1 | Germination, hydroponics. | Stimulation of root elongation; no plant toxicity. | [51] |
| nHAP, primary size 22 nm | Triticum aestivum | 0–150 mg kg−1 P nHAP, bulk-HA, triple superphosphate (TSP) | Soil columns; glasshouse pot experiment; Andisol and Oxisol. | Increased shoot dry matter and P uptake than bulk-HA but less than the conventional P fertilizer. | [53] |
| nHAP (+), nHAP (0), nHAP (−), average size 25.7 nm | Helianthus annuus | 150 kg ha−1 nHAP (+); nHAP (0); nHAP (−); (iv) triple superphosphate (TSP); rock phosphate (RS) | Glasshouse pot experiment; P-deficient Ultisol (pH 4.2) and Vertisol (pH 8.2). | In Ultisol nHA (−) more effective in supplying than TSP; in Vertisol nHAP did not increase plant growth. | [54] |
| nHAP, rod shaped 59.5 × 10.6 nm | Adansonia digitata | Control (unfertilized); MAP; DAP; nHAP | Pot experiment; sandy soil. Foliar application of 20 mL of different P sources weekly. | Increased plant growth (plant height, leaf area, plant fractions dry matter) compared to other P sources. | [55] |
| Urea–nHAP nanohybrid, <100 nm | Camellia sinensis | 50% NPK 4 Splits; 50% NPK 2 Splits; 100% N (HA-urea nanohybrid) + 100% K MOP (2 Splits); 100% N (Urea-nHAP) + 100% K MOP (4 Splits); 50% N (Urea-nHAP) + 100% K MOP (4 Splits); 50% N (Urea-nHAP) + 100% K MOP (2 Splits); 100% conventional NPK fertilizer (4 Splits). | Field experiments in three different locations; Urea-nHAP nanohybrid provided as ground fertilizer. | Enhancement of NUE; increased quality parameters of tea leaves (e.g., total polyphenols and total amino acids). | [56] |
| nHAP with natural and synthetic humic substances (HA) | Zea mays | nHAP-natural HA; nHAP-synthetic HA; Superphosphate; nHA | Growth chamber; pot experiment. | Early growth, better salt stress tolerance and yield. | [57] |
| Material | Species | Treatment | Experimental Conditions | Results | Reference |
|---|---|---|---|---|---|
| Urea-Hydroxyapatite Montmorillonite (U-nHAP-MMT); Urea-Hydroxyapatite encapsulated wood chips (U-nHAP-wood) | Festuca arundinacea | Nutrients (g pot−1): 1.8 N, 1.2 P2O5, 1.2 K2O; 1.8 N, 1.2 P2O5, 1.2 K2O | Pot experiment; Ceylon tea soil. | Decreased N leaching; improvement NUE. | [58] |
| Nano Urea (nU); nHAP composite | Vigna radiata | Conventional U; chemically synthesized nU + nHAP; biologically synthesized nU + nHAP | Pot experiment. | Promoted seed germination; increased seedling growth. | [59] |
| Urea-Hydroxyapatite Montmorillonite nanohybrid composite (U-nHAP-MMT) | Festuca arundinacea | Conventional fertilizer: 120 kg ha−1 N; 40 kg ha−1 P2O5, 40 kg ha−1 K2O | Soil columns, pot experiment; Ceylon tea soil. | Slower N release; significant yield enhancement compared to control. | [60] |
| Urea-modified Hydroxyapatite nanohybrid composite (U-nHAP). | Oryza sativa | Granular urea compared to U-nHAP | Field experiment. | Slower N release relative to conventional urea. | [61] |
| nHAP and Urea-nHAP | Oryza sativa | 10 mg kg−1, 50 mg kg−1, and 100 mg kg−1. Control (untreated), Urea; conventional P salt; nHAP; Urea-nHAP | Germination; Petri dishes. Soil columns. | Enhancement germination; increased α-amylase activity and starch content. | [62] |
| Hydroxyapatite nanoparticles (nHAP, 40–60 nm), Urea doped hydroxyapatite nanoparticles (Ur@nHAP) | Oryza sativa | Water (Control); conventional Urea; Ca dihydrogen phosphate conventional P salt (Ca dihydrogen phosphate), nHAP, Ur@nHAP | Germination; Petri dishes. | Ur@nHAP more efficient than conventional N-P fertilizers. | [63] |
| Material | Species | Treatment | Experimental Conditions | Results | Reference |
|---|---|---|---|---|---|
| nHAP + micronutrients; rod shaped 20–25 nm × 86 nm | Asparagus officinalis | Micronutrients nano system: Alginate-nHAP loaded with Ag, Co, Cu, Fe, and Zn | Germination experiment; 90 d plant growth. | Slower micronutrients release; faster germination rate than control. | [64] |
| Hybrid nanofertilizer (HNF) nUrea + nHAP + micronutrient nanoparticles | Abelmoschus esculentus | nUrea-nHAP-nCu-nFe-nZn; 50 mg per week of nanohybrid; 5 g of conventional fertilizer | N/A | Enhancement of NUE and crop yield. Increase of Cu2+, Fe2+, and Zn2+ uptake in treated plants. | [65] |
4.2. Amorphous Calcium Phosphate Nanoparticles
| Material | Species | Treatment | Experimental Conditions | Results | Reference |
|---|---|---|---|---|---|
| U-nACP | Triticum durum | Control (no fertilizer); U-ACP, 15 kg N ha−1 sprayed suspension + 60 kg N ha−1 granular DAP; 150 kg N ha−1 granular DAP. | Pot trial, growth chamber. 1:1 clay-loam soil/sand mixture (12:12 h light/dark cycle). | Crop yield parameters and grain quality parameters unaltered in comparison to positive control plants. | [66] |
| nano U-NPK | Triticum durum | Control (only water); 150 kg N ha−1 granular DAP; nano U-NPK 15 kg N ha−1 sprayed aqueous suspension + 60 kg N ha−1 DAP. | Pot trial, growth chamber. 1:1 clay-loam soil/sand mixture (12:12 h light/dark cycle). | Same grain yield from conventional fertilization and nano U-NPK (−40% N). | [67] |
| U-nACP | Cucumis sativus | Control (N-starvation), U-nACP (1 mM U), U-nACP 0.5 (0.5 mM U), Urea (1 mM). | Hydroponics. 7 d full nutrient solution; 7 d N-starvation followed by treatments | U-ACP with a 50% reduced N content resulted in similar root and shoot biomass than conventional U. | [68] |
| U-nACP | Vitis vinifera cv. Tempranillo | Control, solution Urea 3 kg N ha−1, solution Urea 6 kg N ha−1, suspension U-nACP 0.4 kg N ha−1. | Field trial | U-ACP treated plants have similar levels of yeast assimilable N and aminoacids than other treatments. | [72] |
| U-nACP | Vitis vinifera cv. Pinot Gris | Control (unfertilized), N1 (granular NH4NO3 27%, 45 kg N ha−1), N2 (fertigation U-nACP 36 kg N ha−1), N3 (granular fertilization + foliar U-nACP, 36 kg N ha−1). | Pot trial, outdoor conditions, sand–peat–clay (50–35–15% by volume) | Despite the restrained dosage of N applied with the nanoparticles quanti-qualitative parameters were comparable to those of plants managed with conventional strategies. | [74] |
5. Silica Nanoparticles
Si Nanoparticles as Plant Nutrient Carrier
| (a) | |||||
| Material | Species | Treatment | Experimental Conditions | Results | Reference |
| Urea loaded zinc aluminosilicate (UZAS) mesoporous nanocomposite, 55.2 nm (pore size: 13 nm) | Oryza sativa | 0 mL UZAS (0 g N pot−1); 20 mL UZAS (0.45 g N pot−1); 40 mL UZAS (0.90 g N pot−1); 60 mL UZAS (1.35 g N pot−1); 0 mL UZAS (1.80 g N pot−1); 100 mL UZAS (2.25 g N pot−1) | Pot experiment. | Increased yield and higher nitrogen recovery efficiency compared to commercial urea. | [83] |
| Micro–nanostructured silica spheres (solid silica spheres (S-Si), hollow silica spheres (H-Si) and sea urchin-like micro–nanostructured hollow silica spheres (SUH-Si); particle diameters ~500 nm | Zea maysArachis hypogaea | Foliar application (50 mL) of: deionized water, NH4Cl solution (5 g L−1), S-Si–N (1.5 g L−1), HSi–N (1.5 g L−1) and SUH-Si–N (1.5 g L−1). 50 mL of deionized water simulated rainwater scouring. | Pot experiment in growth chamber (26 °C/21 °C; 14 h/10 h light–dark; light intensity 300 mol photons per m2 s−1). | Leaf adhesion of SUH-Si–N higher than S-Si–N and H-Si–N at 1.5 g L−1; SUH-Si–N enhanced root length, chlorophyll content and plant height in maize. | [84] |
| Amorphous silica nanoparticles (20–40 nm) | Zea mays | Silica nanoparticles and bulk Si 15 g L−1 | Pot experiment (sandy loam soil; pH 7.0 ± 0.5). In vitro cytotoxicity experiment. | Augmented Si accumulation and regulated expression of defense compounds. | [85] |
| Highly soluble silicate and amorphous nanosilica (<200 nm) | Oryza sativa | Nanosilica (Si = 106 g L−1) and soluble silicate (Si = 115 g L−1) at 0, 605, 1210, and 2420 g ha−1. | Pot experiment. | Increase of lignin content in leaves. Enhanced Si uptake. | [86] |
| Amorphous nanosilica (4.0 nm), silicic acid, stabilized sodium, potassium silicate, and potassium silicate | Sorghum bicolor | Foliar application of 0, 0.5, 1.0, and 1.5 g L−1 of Si. | Pot experiment. | nSi spraying at V4, V8, R1 increased plant growth; Si concentration enhanced photosynthesis. | [87] |
| (b) | |||||
| Material | Species | Treatment | Experimental Conditions | Results | Reference |
| Sodium silicate (Na2SiO3), micron silica (SiO2), silicic acid (H4SiO4), tetraethyl orthosilicate [Si(OC2H5)4], nanoSi from rice husk (50 nm; pore size 1.52 nm) | Zea mays | 10 mg L−1 Si from Na2SiO3, SiO2, H4SiO4, Si(OC2H5)4 and nanoSi | Germination trial in soil (pots). | Enhanced seed germination. | [88] |
| Amorphous nSiO2 (12 nm, SSA 200 m2 g−1) | Lycopersicum esculentum | nSiO2 0, 2, 4, 6, 8, 10 and 12 g L−1 | Germination trial in Petri dishes. | Enhanced % germination and seedling dry weight. | [89] |
| SiO2 microparticles, nSiO2 (25 nm, SSA 274 m2/g, pore size 1.46 nm) | Zea mays | micro SiO2 and nSiO2 1000 mg kg−1 | Germination trial in Petri dishes, cotton method and Soil method. | Enhanced root elongation. | [90] |
| Mesoporous silica nanoparticles MSN (20 nm, pore size 2.78 nm) | Triticum aestivumLupinus albus | MSN 0, 200, 500, 1000, 2000 mg L−1 | Germination trial in Petri dishes and seedling growth in hydroponics. | Enhancement of seed germination, increased plant biomass, total protein and chlorophyll content and photosynthesis (500–1000 mg kg−1). No oxidative stress or cell membrane damage even at 2000 mg kg−1. | [91] |
| Amorphous nSiO2 (5–50 nm; SSA 50–500 m2 g−1) | Lens culinaris | 0, 25, 50, 75, 100, 200, 300 μg mL−1 | Germination trial in Petri dishes. | Increased seed germination, seed vigour index and seedling biomass at lower concentrations. Dose-dependent decrease in mitotic index at higher concentrations. | [92] |
| Amorphous nSiO2 (4 nm, SSA 750 m2 g−1), monosilicic acid + PEG-400 (Si-acid), Na-K silicate stabilized + sorbitol (Sialkali); Si-K | Sugarcane | Si sources at 0, 0.25, 0.5, 0.75, 1.0 mmol L−1 | Pot trials. (i) foliar treatment (0.16 ml per plant) 18, 25, 32, 39 and 46 DAE; (ii) foliar treatment (1.92 ml plant−1) 101, 114, 128 and 144 DAE. | Si beneficial effects on plant growth more evident for Si soluble sources with stabilizers than Si-K and nSi. | [93] |
6. Chitosan Nanoparticles
6.1. Nutrient Delivery by Chitosan Nanoparticles
6.2. Hybrid Functionalization and Multiple Distribution by Chitosan Nanomaterials
| (a) | |||||
| Material | Species | Treatment | Experimental Conditions | Evidences | Reference |
| Zinc loaded chitosan nanoparticles (Zn-CNP), bulk Zn (ZnSO4) | Triticum durum | Foliar spray (i) Water; (ii) ZnSO4. (400 mg L−1 Zn); (iii) Zn-CNP (40 mg L−1 Zn). | Field trial. | Foliar application influences expression of genes implicated in Zn transport. Zn enrichment on grain storage proteins. | [109] |
| Zn loaded chitosan nanoparticles Zn-CNP1 (40 mg L−1 Zn) Zn-CNP2 (4 mg L−1 Zn) | Triticum durum | Leaf spray (i) Water; (ii) Urea; (iii) Urea + ZnSO4 (400 mg L−1 Zn); (iv) Urea + ZnSO4 (40 mg L−1 Zn); (v) Urea + Zn-CNP1; (vi) Urea + Zn-CNP2. | Field trials | Increased grain Zn content without affecting grain yield, protein content, spikelets per spike and kernel weight. | [110] |
| Zn loaded chitosan nanoparticles (200–300 nm) | Zea mays | Foliar spray Zn-chitosan NPs 0.01–0.16%. | Petri dishes; pot trial and field experiment | Strengthening of plant immunity by elevating antioxidant and defense enzymes; in field better control on Curvularia leaf spot disease; increased grain yield and enriched Zn content. | [111] |
| Zn loaded chitosan/TPP (Tripolyphosphate)–Zein coated nanoparticles | Gossypium hirsutum | (i) Control; foliar spray 50 mg L−1 of (ii) ZnSO4; (iii) Zn loaded chitosan;, (iv) Zn loaded chitosan/TPP–Zein coated 0.1%; (v) Zn loaded chitosan/TPP–Zein coated 0.2%; (vi) Zn loaded chitosan/TPP–Zein coated 0.3%. | Pot trial | Particles coated with Zein (0.1%) increased plant height, number of leaves and root length. | [112] |
| Chitosan-polymethacrylic acid-NPK nanoparticles | Proof of concept | 500 mg/mL of N, 60 mg/mL of P and 400 mg/mL of K in the most concentrated nanoformulation. | Laboratory conditions. | NP mean diameter (in the dry state) ≈ 78 ± 1.5 nm; ζ potential varies depending on the amount of nutrient encapsulated. | [113] |
| (b) | |||||
| Material | Species | Treatment | Experimental conditions | Evidences | Reference |
| Chitosan-polymethacrylic acid-NPK NPs | Triticum aestivum | 500 mg/mL of N, 60 mg/mL of P and 400 mg/mL of K in the most concentrated nanoformulation. | Pot filled with sandy soil, foliar distribution of nutrient solution, outdoor conditions. | Enhanced plant height, main spike weight, crop yield, and harvest index. | [114] |
| Cu and salicylic acid (SA) co-encapsulated chitosan | Zea mays | Nanofertilizer (0.01, 0.04, 0.08, 0.12, 0.16%, w/v), water (Ctrl), bulk chitosan (0.01%, w/v), SA (0.01%, w/v) and CuSO4 (0.01%) used to treat seeds and as foliar spray on adult plants. | Germination trial; pot trial. | Increased activities of antioxidant enzymes, and enhanced chlorophyll contents in leaves. | [116] |
| Chitosan-TPP-NPKS nanoparticles (220–530 nm) | Zea mays | (i) Control; (ii) conventional NPK; (iii) conventional NPKS; (iv) chitosan-TPP; (v) chitosan-TPP-NPKS. 0.125, 0.25, 0.5 and 1% chitosan. | Pot trial. | Increased plant height, number of leaves, and chlorophyll content. | [117] |
| nCuO–PEC [Chitosan/Alginate] (300 nm) | Fortunella margarita | (i) nCuO 10 ppm; (ii) nCuO 50 ppm; (iii) nCuO 100 ppm; (iv) nCuO–PEC 10 ppm; (v) nCuO–PEC 50 ppm; (vi) nCuO–PEC 100 ppm. | Petri dishes | Increased seed germination. | [118] |
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Springmann, M.; Clark, M.; Mason-D’Croz, D.; Wiebe, K.; Bodirsky, B.L.; Lassaletta, L.; de Vries, W.; Vermeulen, S.J.; Herrero, M.; Carlson, K.M.; et al. Options for keeping the food system within environmental limits. Nature 2018, 562, 519–525. [Google Scholar] [CrossRef] [PubMed]
- United Nations, Department of Economic and Social Affairs, Population Division. World Population Prospects 2019: Highlights; ST/ESA/SER.A/423; UNDESA: New York, NY, USA, 2019. [Google Scholar]
- EC. EU Agricultural Outlook for Markets and Income, 2019–2030; European Commission, DG Agriculture and Rural Development: Brussels, Belgium, 2019. [Google Scholar]
- Food and Agriculture Organization of the United Nations. How to Feed the World in 2050. 2009. Available online: www.fao.org/fileadmin/templates/wsfs/docs/expert_paper/How_to_Feed_the_World_in_2050.pdf (accessed on 24 May 2021).
- FAO. World Fertilizer Trends and Outlook to 2020: Summary Report; Food and Agriculture Organization of the United Nations: Rome, Italy, 2017. [Google Scholar]
- Drescher, A.; Glaser, R.; Richert, C.; Nippes, K.-R. Demand for Key Nutrients (NPK) in the Year 2050; Report for the European Commission Joint Research Centre: Brussels, Belgium, 2011; Available online: https://esdac.jrc.ec.europa.eu/projects/NPK/Documents/Freiburg_Demand_for_key_nutrients_in_2050_Drescher.pdf (accessed on 17 June 2021).
- Renner, A.; Cadillo-Benalcazar, J.J.; Benini, L.; Giampietro, M. Environmental pressure of the European agricultural system: Anticipating the biophysical consequences of internalization. Ecosyst. Serv. 2020, 46, 101195. [Google Scholar] [CrossRef]
- Baligar, V.C.; Fageria, V.K.; He, Z.L. Nutrient use efficiency in plants. Commun. Soil Sci. Plant Anal. 2001, 32, 921–950. [Google Scholar] [CrossRef]
- Smith, A.M.; Gilbertson, L.M. Rational ligand design to improve agrochemical delivery efficiency and advance agriculture sustainability. ACS Sustain. Chem. Eng. 2018, 6, 13599–13610. [Google Scholar] [CrossRef]
- Foley, J.A.; Ramankutty, N.; Brauman, K.A.; Cassidy, E.S.; Gerber, J.S.; Johnston, M.; Mueller, N.D.; O’Connell, C.; Ray, D.K.; West, P.C.; et al. Solutions for a cultivated planet. Nature 2011, 478, 337–342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urso, J.H.; Gilbertson, L.M. Atom conversion efficiency: A new sustainability metric applied to nitrogen and phosphorus use in agriculture. ACS Sustain. Chem. Eng. 2018, 6, 4453–4463. [Google Scholar] [CrossRef]
- Willett, W.; Rockström, J.; Loken, B.; Springmann, M.; Lang, T.; Vermeulen, S.; Garnett, T.; Tilman, D.; DeClerck, F.; Wood, A.; et al. Food in the Anthropocene: The EAT–Lancet Commission on healthy diets from sustainable food systems. Lancet 2019, 393, 447–492. [Google Scholar] [CrossRef]
- Lambert, H.; Gupte, J.; Fletcher, H.; Hammond, L.; Lowe, N.; Pelling, P.; Raina, N.; Shahid, T.; Shanks, S. COVID-19 as a global challenge: Towards an inclusive and sustainable future. Lancet Planet. Health 2020, 4, E312–E314. [Google Scholar] [CrossRef]
- European Commission. The European Green Deal, COM (2019) 640 Final. 11 December 2019. Available online: https://www.politico.eu/wp-content/uploads/2019/12/The-European-Green-Deal-Communication.pdf (accessed on 17 June 2021).
- F2F. Farm to Fork Strategy. 2020. Available online: https://ec.europa.eu/food/farm2fork_en (accessed on 30 November 2020).
- OECD-FAO Agricultural Outlook 2020–2029; OECD Publishing: Paris, France; FAO: Rome, Italy, 2020.
- Mulla, D.; Khosla, R. Historical evolution and recent advances in precision farming. In Soil Specific Farming: Precision Agriculture; Lal, R., Stewart, B.A., Eds.; Advances in Soil Science; Taylor and Francis Publisher: Boca Raton, FL, USA, 2015; pp. 1–35. [Google Scholar]
- FAO. Save and Grow. A Policymaker’s Guide to the Sustainable Intensification of Smallholder Crop Production; Food and Agriculture Organization of the United Nations: Rome, Italy, 2011. [Google Scholar]
- Rose, D.C.; Wheeler, R.; Winter, M.; Lobley, M.; Chivers, C.-A. Agriculture 4.0: Making it work for people, production, and the planet. Land Use Policy 2021, 100, 104933. [Google Scholar] [CrossRef]
- Gogos, A.; Knauer, K.; Bucheli, T.D. Nanomaterials in plant protection and fertilization: Current state, foreseen applications, and research priorities. J. Agric. Food Chem. 2012, 60, 9781–9792. [Google Scholar] [CrossRef] [PubMed]
- Manjunatha, S.B.; Biradar, D.P.; Aladakatti, Y.R. Nanotechnology and its applications in agriculture: A review. J. Farm Sci. 2016, 29, 1–13. [Google Scholar]
- Singh, H.; Sharma, A.; Bhardwaj, S.K.; Arya, S.K.; Bhardwaj, N.; Khatri, M. Recent advances in the applications of nano-agrochemicals for sustainable agricultural development. Environ. Sci. Process. Impacts 2021, 23, 213–239. [Google Scholar] [CrossRef]
- Lowry, G.V.; Avellan, A.; Gilbertson, L.M. Opportunities and challenges for nanotechnology in the agri-tech revolution. Nat. Nanotechnol. 2019, 14, 517–522. [Google Scholar] [CrossRef]
- Bandala, E.R.; Berli, M. Engineered nanomaterials (ENMs) and their role at the nexus of food, energy, and water. Mater. Sci. Technol. 2019, 2, 29–40. [Google Scholar] [CrossRef]
- Gomez, A.; Narayana, M.; Zhao, L.; Jia, X.; Bernal, R.A.; Lopez-Moreno, M.L.; Peralta-Videa, J.R. Effects of nano-enabled agricultural strategies on food quality: Current knowledge and future research needs. J. Hazard. Mater. 2021, 401, 123385. [Google Scholar] [CrossRef]
- Pulizzi, F. Nano in the future of crops. Nat. Nanotechnol. 2019, 14, 507. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raliya, R.; Saharan, V.; Dimkpa, C.; Biswas, P. Nanofertilizer for precision and sustainable agriculture: Current state and future perspectives. J. Agric. Food Chem. 2018, 26, 6487–6503. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Chhipa, H. Nanofertilizers and nanopesticides for agriculture. Environ. Chem. Lett. 2016, 15, 15–22. [Google Scholar] [CrossRef]
- Zuverza-Mena, N.; Martínez-Fernández, D.; Du, W.; Hernandez-Viezcas, J.A.; Bonilla-Bird, N.; López-Moreno, M.L.; Komárek, M.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Exposure of engineered nanomaterials to plants: Insights into the physiological and biochemical responses—A review. Plant Physiol. Biochem. 2017, 110, 236–264. [Google Scholar] [CrossRef] [Green Version]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nanofertilizers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Trotta, F.; Mele, A. Nanomaterials: Classification and Properties. In Nanosponges: Synthesis and Applications; Trotta, F., Mele, A., Eds.; Wiley-VCH: Weinheim, Germany, 2019; pp. 1–26. [Google Scholar]
- Singh, D.; Singh, S.; Sahu, J.; Srivastava, S.; Singh, M.R. Ceramic nanoparticles: Recompense, cellular uptake and toxicity concerns. Artif. Cells Nanomed. Biotechnol. 2016, 44, 401–409. [Google Scholar] [CrossRef]
- Moreno-Vega, A.I.; Gómez-Quintero, T.; Nuñez-Anita, R.E.; Acosta-Torres, L.S.; Castaño, V. Polymeric and ceramic nanoparticles in biomedical applications. J. Nanotechnol. 2012, 2012, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Marchiol, L.; Iafisco, M.; Fellet, G.; Adamiano, A. Nanotechnology support the next agricultural revolution: Perspectives to enhancement of nutrient use efficiency. Adv. Agron. 2020, 161, 27–116. [Google Scholar]
- Liu, R.; Lal, R. Potentials of engineered nanoparticles as fertilizers for increasing agronomic productions. Sci. Total Environ. 2015, 514, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Choi, K.S.; Kim, S.; Gwon, Y.; Kim, J. Graphene oxide-assisted promotion of plant growth and stability. Nanomaterials 2020, 10, 758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojeda-Barrios, D.L.; Morales, I.; Juárez-Maldonado, A.; Sandoval-Rangeld, A.; Fuentes-Lara, L.O.; Benavides-Mendoza, A. Importance of nanofertilizers in fruit nutrition. In Fruit Crops: Diagnosis and Management of Nutrient Constraints; Srivastava, A.K., Hu, C., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 497–507. [Google Scholar]
- Liu, R.; Lal, R. Synthetic apatite nanoparticles as a phosphorus fertilizer for soybean (Glycine max). Sci. Rep. 2014, 4, 5686. [Google Scholar] [CrossRef]
- González-Gómez, H.; Ramírez-Godina, F.; Ortega-Ortiz, H.; Benavides-Mendoza, A.; Robledo Torres, V.; Cabrera-De la Fuente, M. Use of chitosan-PVA hydrogels with copper nanoparticles to improve the growth of grafted watermelon. Molecules 2017, 22, 1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iavicoli, I.; Leso, V.; Beezhold, D.H.; Shvedova, A.A. Nanotechnology in agriculture: Opportunities, toxicological implications, and occupational risks. Toxicol. Appl. Pharmacol. 2017, 329, 96–111. [Google Scholar] [CrossRef] [PubMed]
- Dorozhkin, S.V.; Epple, M. Biological and medical significance of calcium phosphates. Angew. Chem. Int. Ed. 2002, 41, 3130–3146. [Google Scholar] [CrossRef]
- Epple, M. Review of Potential Health Risks Associated with Nanoscopic Calcium Phosphate. Acta Biomater. 2018, 77, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Maghsoodi, M.R.; Ghodszad, L.; Asgari Lajayer, B. Dilemma of hydroxyapatite nanoparticles as phosphorus fertilizer: Potentials, challenges and effects on plants. Environ. Technol. Innov. 2020, 19, 100869. [Google Scholar] [CrossRef]
- Ferraz, M.P.; Monteiro, F.J.; Manuel, C.M. Hydroxyapatite nanoparticles: A review of preparation methodologies. J. Appl. Biomater. Biomech. 2004, 2, 74–80. [Google Scholar] [PubMed]
- Rahavi, S.S.; Ghaderi, O.; Monshi, A.; Fathi, M.H. A comparative study on physicochemical properties of hydroxyapatite powders derived from natural and synthetic sources. Russ. J. Non-Ferr. Metals 2017, 58, 276–286. [Google Scholar] [CrossRef]
- Boutinguiza, M.; Pou, J.; Comesaña, R.; Lusquiños, F.; de Carlos, A.; León, B. Biological hydroxyapatite obtained from fish bones. Mater. Sci. Eng. C 2012, 32, 478–486. [Google Scholar] [CrossRef]
- Mohd Pu’ad, N.A.S.; Koshy, P.; Abdullah, H.Z.; Idris, M.I.; Lee, T.C. Syntheses of hydroxyapatite from natural sources. Heliyon 2019, 5, e01588. [Google Scholar] [CrossRef] [Green Version]
- Kirkby, E.A.; Johnston, A.E. Soil and fertilizer phosphorus in relation to crop nutrition. In The Ecophysiology of Plant-Phosphorus Interactions; White, P.J., Hammond, J.P., Eds.; Springer: Dordrecht, The Netherlands, 2008. [Google Scholar]
- Alewell, C.; Ringeval, B.; Ballabio, C.; Robinson, D.A.; Panagos, P.; Borrelli, P. Global phosphorus shortage will be aggravated by soil erosion. Nat. Commun. 2020, 11, 4546. [Google Scholar] [CrossRef]
- Marchiol, L.; Filippi, A.; Adamiano, A.; Iafisco, M.; Degli Esposti, L.; Mattiello, A.; Petrussa, E.; Braidot, E. Influence of hydroxyapatite nanoparticles on germination and plant metabolism of tomato (Solanum lycopersicum L.): Preliminary evidence. Agronomy 2019, 9, 161. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Yuan, L.; Zhang, J.; Li, H.; Bai, Z.; Chen, X.; Zhang, W.; Zhang, F. Phosphorus dynamics: From soil to plant. Plant Physiol. 2011, 156, 997–1005. [Google Scholar] [CrossRef] [Green Version]
- Montalvo, D.; McLaughlin, M.J.; Degryse, F. Efficacy of hydroxyapatite nanoparticles as phosphorus fertilizers in Andisols and Oxisols. Soil Sci. Soc. Am. J. 2015, 79, 551–558. [Google Scholar] [CrossRef]
- Xiong, L.; Wang, P.; Hunter, M.N.; Kopittke, P. Bioavailability and movement of phosphorus applied as hydroxyapatite nanoparticles (HA-NPs) in soils. Environ. Sci. Nano 2018, 5, 2888–2898. [Google Scholar] [CrossRef]
- Soliman, A.S.; Hassan, M.; Abou-Elella, F.; Hanafy Ahmed, A.H.; El-Feky, S.A. Effect of nano and molecular phosphorus fertilizers on growth and chemical composition of Baobab (Adansonia digitata L.). J. Plant Sci. 2016, 11, 52–60. [Google Scholar] [CrossRef] [Green Version]
- Raguraj, S.; Wijayathunga, W.M.S.; Gunaratne, G.P.; Amali, R.K.A.; Priyadarshana, G.; Sandaruwan, C.; Karunaratne, V.; Hettiarachchi, L.S.K.; Kottegoda, N. Urea–hydroxyapatite nanohybrid as an efficient nutrient source in Camellia sinensis (L.) Kuntze (tea). J. Plant Nutr. 2020, 43, 2383–2394. [Google Scholar] [CrossRef]
- Yoon, H.Y.; Lee, J.G.; Degli Esposti, L.; Iafisco, M.; Kim, P.J.; Shin, S.G.; Jeon, J.-R.; Adamiano, A. Synergistic release of crop nutrients and stimulants from hydroxyapatite nanoparticles functionalized with humic substances: Toward a multifunctional nanofertilizer. ACS Omega 2020, 5, 6598–6610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottegoda, N.; Munaweera, I.; Madusanka, N.; Karunaratne, V. A green slow-release fertilizer composition based on urea-modified hydroxyapatite nanoparticles encapsulated wood. Curr. Sci. 2011, 101, 73–78. [Google Scholar]
- Subbaiya, R.; Priyanka, M.; Masilamani Selvam, M. Formulation of green nano-fertilizer to enhance the plant growth through slow and sustained release of nitrogen. J. Pharm. Res. 2012, 5, 5178–5183. [Google Scholar]
- Gunaratne, G.P.; Kottegoda, N.; Madusanka, N.; Munaweera, I.; Sandaruwan, C.; Priyadarshana, W.M.G.I.; Siriwardhana, A.; Madhushanka, B.A.D.; Rathnayake, U.A.; Karunaratne, V. Two new plant nutrient nanocomposites based on urea coated hydroxyapatite: Efficacy and plant uptake. Indian J. Agric. Sci. 2016, 86, 494–499. [Google Scholar]
- Madusanka, N.; Sandaruwana, C.; Kottegodaa, N.; Sirisena, D.; Munaweera, I.; De Alwis, A.; Karunaratnea, V.; Amaratunga, G.A.J. Urea–hydroxyapatite-montmorillonite nanohybrid composites as slow release nitrogen compositions. Appl. Clay Sci. 2017, 150, 303–308. [Google Scholar] [CrossRef]
- Kottegoda, N.; Sandaruwan, C.; Priyadarshana, G.; Siriwardhana, A.; Rathnayake, U.A.; Berugoda Arachchige, D.M.; Kumarasinghe, A.R.; Dahanayake, D.; Karunaratne, V.; Amaratunga, G.A.J. Urea-hydroxyapatite nanohybrids for slow release of nitrogen. ACS Nano 2017, 11, 1214–1221. [Google Scholar] [CrossRef]
- Pradhan, S.; Durgam, M.; Mailapalli, D.R. Urea loaded hydroxyapatite nanocarrier for efficient delivery of plant nutrients in rice. Arch. Agron. Soil Sci. 2020, 67, 371–382. [Google Scholar] [CrossRef]
- Phan, K.S.; Nguyen, H.T.; Huong Le, T.T.; Thuy Vu, T.T.; Do, H.D.; Oanh Vuong, T.K.; Nguyen, H.N.; Tran, C.H.; Hang Ngo, T.T.; Ha, P.T. Fabrication and activity evaluation on Asparagus officinalis of hydroxyapatite based multimicronutrient nano systems. Adv. Nat. Sci. Nanosci. Nanotechnol. 2019, 10, 025011. [Google Scholar] [CrossRef]
- Tarafder, C.; Daizy, M.; Alam, M.M.; Ali, M.R.; Islam, M.J.; Islam, R.; Ahommed, S.; Saad Aly, M.A.; Hossain Khan, M.Z. Formulation of a hybrid nanofertilizer for slow and sustainable release of micronutrients. ACS Omega 2020, 5, 23960–23966. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-Rodríguez, G.B.; Dal Sasso, G.; Carmona, F.J.; Miguel-Rojas, C.; Pérez-de-Luque, A.; Masciocchi, N.; Guagliardi, A.; Delgado-López, J.M. Engineering Biomimetic Calcium Phosphate Nanoparticles: A Green Synthesis of Slow-Release Multinutrient (NPK) Nanofertilizers. ACS Appl. Bio Mater. 2020, 3, 1344–1353. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Miguel-Rojas, C.; Montanha, G.S.; Carmona, F.J.; Dal Sasso, G.; Sillero, J.C.; Skov Pedersen, J.; Masciocchi, N.; Guagliardi, A.; Pérez-de-Luque, A.; et al. Reducing Nitrogen Dosage in Triticum durum Plants with Urea-Doped Nanofertilizers. Nanomaterials 2020, 10, 1043. [Google Scholar] [CrossRef]
- Carmona, F.J.; Dal Sasso, G.; Ramírez-Rodríguez, G.B.; Youry, P.; Delgado-López, J.M.; Guagliardi, A.; Masciocchi, N. Urea-functionalized amorphous calcium phosphate nanofertilizers: Optimizing the synthetic strategy towards environmental sustainability and manufacturing costs. Sci. Rep. 2021, 11, 3419. [Google Scholar] [CrossRef]
- Marin, A.; Armengol, J.; Carbonell-Bejerano, P.; Escalona, J.M.; Gramaje, D.; Hernández-Montes, E.; Intrigliolo, D.S.; Martínez-Zapater, J.M.; Medrano, H.; Mirás-Avalos, J.M.; et al. Challenges of viticulture adaptation to global change: Tackling the issue from the roots. Aust. J. Grape Wine Res. 2021, 27, 8–25. [Google Scholar] [CrossRef]
- Battiston, E.A.; Salvatici, M.C.; Lavacchi, A.; Gatti, A.; Di Marco, S.; Mugnai, L. Functionalization of a nanostructured hydroxyapatite with Cu(II) compounds as a pesticide: In situ transmission electron microscopy and environmental scanning electron microscopy observations of treated Vitis vinifera L. leaves. Pest Manag. Sci. 2018, 74, 1903–1915. [Google Scholar] [CrossRef]
- Battiston, E.A.; Antonielli, L.; Di Marco, S.; Fontaine, F.; Mugnai, L. Innovative delivery of Cu(II) ions by a nanostructured hydroxyapatite: Potential application in planta to enhance the sustainable control of Plasmopara viticola. Phytopathology 2019, 109, 748–759. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, E.P.; Ramírez-Rodríguez, G.B.; Carmona, F.J.; Martínez-Vidaurre, J.M.; Masciocchi, N.; Guagliardi, A.; Garde-Cerdán, T.; Delgado-López, J.M. Towards a more sustainable viticulture: Foliar application of N-doped calcium phosphate nanoparticles on Tempranillo grapes. J. Sci. Food Agric. 2021, 101, 1307–1313. [Google Scholar] [CrossRef]
- Pérez-Álvarez, E.P.; Garde-Cerdán, T.; García-Escudero, E.; Martínez-Vidaurre, J.M. Effect of two doses of urea foliar application on leaves and grape nitrogen composition during two vintages. J. Sci. Food Agric. 2017, 97, 2524–2532. [Google Scholar] [CrossRef] [PubMed]
- Gaiotti, F.; Lucchetta, M.; Rodegher, G.; Lorenzoni, D.; Longo, E.; Boselli, E.; Cesco, S.; Belfiore, N.; Lovat, L.; Delgado-López, J.M.; et al. Urea-doped calcium phosphate nanoparticles as sustainable nitrogen nanofertilizers for viticulture: Implications on yield and quality of Pinot gris grapevines. Agronomy 2021, 11, 1026. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Ma, J.F.; Bélanger, R.R. Editorial: Role of silicon in plants. Front. Plant Sci. 2017, 8, 1858. [Google Scholar] [CrossRef] [Green Version]
- Hattori, T.; Inanaga, S.; Araki, H.; An, P.; Morita, S.; Luxová, M.; Lux, A. Application of silicon enhanced drought tolerance in Sorghum bicolor. Physiol. Plant. 2005, 123, 459–466. [Google Scholar] [CrossRef]
- Epstein, E. Silicon: Its manifold roles in plants. Ann. Appl. Biol. 2009, 155, 155–160. [Google Scholar] [CrossRef]
- Rains, D.W.; Epstein, E.; Zasoski, R.J.; Aslam, M. Active silicon uptake by wheat. Plant Soil 2006, 280, 223–228. [Google Scholar] [CrossRef]
- Yamaji, N.; Mitatni, N.; Ma, J.F. A Transporter regulating silicon distribution in rice shoots. Plant Cell 2008, 20, 1381–1389. [Google Scholar] [CrossRef] [Green Version]
- Deshmukh, R.K.; Vivancos, V.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Luyckx, M.; Hausman, J.-F.; Lutts, S.; Guerriero, G. Silicon and plants: Current knowledge and technological perspectives. Front. Plant Sci. 2017, 8, 411. [Google Scholar] [CrossRef] [Green Version]
- Schurt, D.A.; Rodrigues, F.Á.; Colodette, J.L.; Carré-Missio, V. Efeito do silício nas concentrações de lignina e de açúcaresem bainhas de folhas de arroz infectadas por Rhizoctonia solani. Bragantia 2013, 72, 360–366. [Google Scholar] [CrossRef] [Green Version]
- Naseem, F.; Zhi, Y.; Farrukh, M.A.; Hussain, F.; Yin, Z. Mesoporous ZnAl2Si10O24 nanofertilizers enable high yield of Oryza sativa L. Sci. Rep. 2020, 10, 10841. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, R.; Zhou, H.; Zhu, Y.; Zheng, X.; Tang, M.; Wu, X.; Yu, C.; Wang, G. Improving the utilization rate of foliar nitrogen fertilizers by surface roughness engineering of silica spheres. Environ. Sci. Nano 2020, 7, 3526. [Google Scholar] [CrossRef]
- Suriyaprabha, R.; Karunakaran, G.; Yuvakkumar, R.; Rajendran, V.; Kannan, N. Foliar application of silica nanoparticles on the phytochemical responses of maize (Zea mays L.) and its toxicological behavior. Synth. React. Inorg. Metal-Organ. Nano-Metal Chem. 2014, 44, 1128–1131. [Google Scholar] [CrossRef]
- Félix Alvarez, R.; de Mello Prado, R.; Felisberto, G.; Fernandes Deus, A.C.; Lima de Oliveira, R.L. Effects of soluble silicate and nanosilica application on rice nutrition in an oxisol. Pedosphere 2018, 28, 597–606. [Google Scholar] [CrossRef] [Green Version]
- Lima de Oliveira, R.L.; de Mello Prado, R.; Felisberto, G.; Rodrigues Cruz, F.J. Different sources of silicon by foliar spraying on the growth and gas exchange in Sorghum. J. Soil Sci. Plant Nutr. 2019, 19, 948–953. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Manivasakan, P.; Yuvakkumar, R.; Rajendran, V.; Prabu, P.; Kannan, N. Effect of nanosilica and silicon sources on plant growth promoting rhizobacteria, soil nutrients and maize seed germination. IET Nanobiotechnol. 2013, 7, 70–77. [Google Scholar] [CrossRef]
- Karunakaran, G.; Suriyaprabha, R.; Rajendran, V.; Kannan, N. Influence of ZrO2, SiO2, Al2O3 and TiO2 nanoparticles on maize seed germination under different growth conditions. IET Nanobiotechnol. 2016, 10, 171–177. [Google Scholar] [CrossRef]
- Siddiqui, M.H.; Al-Whaibi, M.H. Role of nano-SiO2 in germination of tomato (Lycopersicum esculentum seeds Mill.). Saudi J. Biol. Sci. 2014, 21, 13–17. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Hussain, H.I.; Yi, Z.; Rookes, J.E.; Kong, L.; Cahill, D.M. Mesoporous silica nanoparticles enhance seedling growth and photosynthesis in wheat and lupin. Chemosphere 2016, 152, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Khan, Z.; Ansari, M.Y.K. Impact of engineered Si nanoparticles on seed germination, vigour index and genotoxicity assessment via DNA damage of root tip cells in Lens culinaris. Plant Biochem. Physiol. 2018, 6, 1–5. [Google Scholar] [CrossRef]
- Dos Santos, L.C.N.; Marques Teixeira, G.C.; de Mello Prado, R.; Rocha, A.M.S.; dos Santos Pinto, R.C. Response of pre-sprouted sugarcane seedlings to foliar spraying of potassium silicate, sodium and potassium silicate, nanosilica and monosilicic acid. Sugar Tech. 2020, 22, 773–781. [Google Scholar] [CrossRef]
- Piras, A.M.; Maisetta, G.; Sandreschi, S.; Esin, S.; Gazzarri, M.; Batoni, G.; Chiellini, F. Preparation, physical–chemical and biological characterization of chitosan nanoparticles loaded with lysozyme. Int. J. Biol. Macromol. 2014, 67, 124–131. [Google Scholar] [CrossRef]
- Kumaraswamy, R.V.; Kumari, S.; Choudhary, R.C.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Engineered chitosan based nanomaterials: Bioactivities, mechanisms and perspectives in plant protection and growth. Int. J. Biol. Macromol. 2018, 113, 494–506. [Google Scholar] [CrossRef]
- Chandra, S.; Chakraborty, N.; Dasgupta, A.; Sarkar, J.; Panda, K.; Acharya, K. Chitosan Nanoparticles: A Positive Modulator of Innate Immune Responses in Plants. Sci. Rep. 2015, 5, 15195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chouhan, D.; Mandal, P. Applications of chitosan and chitosan based metallic nanoparticles in agrosciences—A review. Int. J. Biol. Macromol. 2021, 166, 1554–1569. [Google Scholar] [CrossRef] [PubMed]
- Katiyar, D.; Hemantaranjan, A.; Singh, B. Chitosan as a promising natural compound to enhance potential physiological responses in plant: A review. Indian J. Plant Physiol. 2015, 20, 1–9. [Google Scholar] [CrossRef]
- Guan, Y.; Hu, J.; Wang, X.; Shao, C. Seed priming with chitosan improves maize germination and seedling growth in relation to physiological changes under low temperature stress*. J. Zhejiang Univ. Sci. B 2009, 10, 427–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Synthesis, characterization, and application of chitosan nanomaterials loaded with zinc and copper for plant growth and protection. In Nanotechnology; Prasad, R., Kumar, M., Kumar, V., Eds.; Springer: Singapore, 2017; pp. 227–247. [Google Scholar]
- Saharan, V.; Kumaraswamy, R.V.; Choudhary, R.C.; Kumari, S.; Pal, A.; Raliya, R.; Biswas, P. Cu-chitosan nanoparticle mediated sustainable approach to enhance seedling growth in maize by mobilizing reserved food. J. Agric. Food Chem. 2016, 64, 6148–6155. [Google Scholar] [CrossRef]
- Sathiyabama, M.; Parthasarathy, R. Biological preparation of chitosan nanoparticles and its in vitro antifungal efficacy against some phytopathogenic fungi. Carbohydr. Polym. 2016, 151, 321–325. [Google Scholar] [CrossRef]
- Saharan, V.; Sharma, G.; Yadav, M.; Choudhary, M.K.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P. Synthesis and in vitro antifungal efficacy of Cu–chitosan nanoparticles against pathogenic fungi of tomato. Int. J. Biol. Macromol. 2015, 75, 346–353. [Google Scholar] [CrossRef]
- Nguyen Van, S.; Dinh Minh, H.; Nguyen Anh, D. Study on chitosan nanoparticles on biophysical characteristics and growth of robusta coffee in green house. Biocatal. Agric. Biotechnol. 2013, 2, 289–294. [Google Scholar] [CrossRef]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Cu-chitosan nanoparticle boost defense responses and plant growth in maize (Zea mays L.). Sci. Rep. 2017, 7, 9754. [Google Scholar] [CrossRef] [PubMed]
- Sathiyabama, M.; Charles, R.E. Fungal cell wall polymer based nanoparticles in protection of tomato plants from wilt disease caused by Fusarium Oxysporum f.sp. lycopersici. Carbohydr. Polym. 2015, 133, 400–407. [Google Scholar] [CrossRef]
- Ditta, A.; Arshad, M. Applications and perspectives of using nanomaterials for sustainable plant nutrition. Nanotechnol. Rev. 2016, 5, 209–229. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, P.; Dapkekar, A.; Oak, M.; Paknikar, K.; Rajwade, J. Nanocarrier-mediated foliar zinc fertilization influences expression of metal homeostasis related genes in flag leaves and enhances gluten content in durum wheat. PLoS ONE 2018, 13, e0191035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deshpande, P.; Dapkekar, A.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc complexed chitosan/TPP nanoparticles: A promising micronutrient nanocarrier suited for foliar application. Carbohydr. Polym. 2017, 165, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Dapkekar, A.; Deshpande, P.; Oak, M.D.; Paknikar, K.M.; Rajwade, J.M. Zinc use efficiency is enhanced in wheat through nanofertilization. Sci. Rep. 2018, 8, 6832. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, R.C.; Kumaraswamy, R.V.; Kumari, S.; Sharma, S.S.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Zinc encapsulated chitosan nanoparticle to promote maize crop yield. Int. J. Biol. Macromol. 2019, 127, 126–135. [Google Scholar] [CrossRef]
- Kanjana, D. Preparation and characterization of nanoformulated zinc fertilizer by using biopolymer and their effects on cotton. Int. J. Chem. Stud. 2019, 7, 1530–1537. [Google Scholar]
- Corradini, E.; de Moura, M.R.; Mattoso, L.H.C. A preliminary study of the incorporation of NPK fertilizer into chitosan nanoparticles. Express Polym. Lett. 2010, 4, 509–515. [Google Scholar] [CrossRef]
- Abdel-Aziz, H.M.M.; Hasaneen, M.N.A.; Omer, A.M. Nano chitosan-NPK fertilizer enhances the growth and productivity of wheat plants grown in sandy soil. Span. J. Agric. Res. 2016, 14, e0902. [Google Scholar] [CrossRef]
- Yen, M.-T.; Yang, J.-H.; Mau, J.-L. Physicochemical characterization of chitin and chitosan from crab chells. Carbohydr. Polym. 2009, 75, 15–21. [Google Scholar] [CrossRef]
- Sharma, G.; Kumar, A.; Devi, K.A.; Prajapati, D.; Bhagat, D.; Pal, A.; Raliya, R.; Biswas, P.; Saharan, V. Chitosan nanofertilizer to foster source activity in maize. Int. J. Biol. Macromol. 2020, 145, 226–234. [Google Scholar] [CrossRef]
- Dhlamini, B.; Paumo, H.K.; Katata-Seru, L.; Kutu, F.R. Sulphate-supplemented NPK nanofertilizer and its effect on maize growth. Mater. Res. Express 2020, 7, 095011. [Google Scholar] [CrossRef]
- Leonardi, M.; Caruso, G.M.; Carroccio, S.C.; Boninelli, S.; Curcuruto, G.; Zimbone, M.; Allegra, M.; Torrisi, B.; Ferlito, F.; Miritello, M. Smart nanocomposites of chitosan/alginate nanoparticles loaded with copper oxides as alternative nanofertilizers. Environ. Sci. Nano 2021, 8, 174–187. [Google Scholar] [CrossRef]
- Grillo, R.; Mattos, B.D.; Antunes, D.R.; Forini, M.M.L.; Monikh, F.A.; Rojas, O.J. Foliage adhesion and interactions with particulate delivery systems for plant nanobionics and intelligent agriculture. Nano Today 2021, 37, 101078. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fellet, G.; Pilotto, L.; Marchiol, L.; Braidot, E. Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures. Agronomy 2021, 11, 1239. https://doi.org/10.3390/agronomy11061239
Fellet G, Pilotto L, Marchiol L, Braidot E. Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures. Agronomy. 2021; 11(6):1239. https://doi.org/10.3390/agronomy11061239
Chicago/Turabian StyleFellet, Guido, Laura Pilotto, Luca Marchiol, and Enrico Braidot. 2021. "Tools for Nano-Enabled Agriculture: Fertilizers Based on Calcium Phosphate, Silicon, and Chitosan Nanostructures" Agronomy 11, no. 6: 1239. https://doi.org/10.3390/agronomy11061239