The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants
Abstract
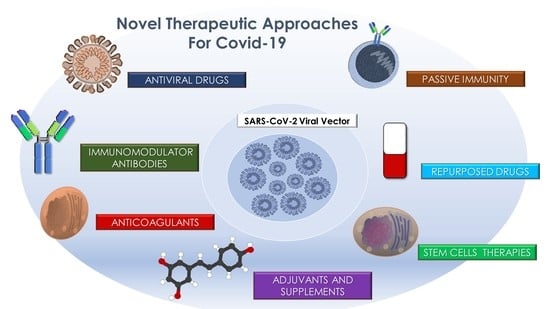
:1. Introduction
2. Materials and Methods
3. Results
3.1. Antiviral Drugs
3.2. Repurposed Drugs
3.2.1. Antibiotics
3.2.2. Interferon
3.3. Passive Immunity
Convalescent Plasma
3.4. Immunomodulators and Antibodies
3.4.1. Tocilizumab
3.4.2. Baricitinib
3.4.3. Regen-Cov
3.4.4. Bamlanivimab
3.4.5. Bamlanivimab/Etesevimab
3.4.6. AZD7442
3.4.7. MabCo19
3.4.8. VIR-7831
3.4.9. ANAKINRA
3.4.10. Corticosteroids
3.5. Anticoagulants
3.6. Stem Cells Autologous and Allograft
3.7. Adjuvants and Antioxidants
3.7.1. Lattoferrin
3.7.2. Vitamin D
3.7.3. Melatonin
3.7.4. Lianhuaqingwen (Lhqw)
3.7.5. Gamma Oryzanol
3.7.6. Resveratrol
4. Discussion
Future Orientation for Primary Prevention
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int (accessed on 16 April 2021).
- Dong, E.; Du, H.; Gardner, L. An Interactive Web-Based Dashboard to Track COVID-19 in Real Time. Lancet Infect. Dis. 2020, 20, 533–534. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; De Michele, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; et al. An Alternative “Trojan Horse” Hypothesis for COVID-19: Immune Deficiency of IL-10 and SARS-CoV-2 Biology. Endocr. Metab. Immune Disord. Drug Targets 2021. [Google Scholar] [CrossRef] [PubMed]
- Han, Q.; Lin, Q.; Ni, Z.; You, L. Uncertainties about the Transmission Routes of 2019 Novel Coronavirus. Influenza Other Respir. Viruses 2020, 14, 470–471. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Facial Skin Temperature and Discomfort When Wearing Protective Face Masks: Thermal Infrared Imaging Evaluation and Hands Moving the Mask. Int. J. Environ. Res. Public Health 2020, 17, 4624. [Google Scholar] [CrossRef] [PubMed]
- Scarano, A.; Inchingolo, F.; Rapone, B.; Festa, F.; Tari, S.R.; Lorusso, F. Protective Face Masks: Effect on the Oxygenation and Heart Rate Status of Oral Surgeons during Surgery. Int. J. Environ. Res. Public Health 2021, 18, 2363. [Google Scholar] [CrossRef]
- Hindson, J. COVID-19: Faecal-Oral Transmission? Nat. Rev. Gastroenterol. Hepatol. 2020, 17, 259. [Google Scholar] [CrossRef]
- Bai, Y.; Yao, L.; Wei, T.; Tian, F.; Jin, D.-Y.; Chen, L.; Wang, M. Presumed Asymptomatic Carrier Transmission of COVID-19. JAMA 2020, 323, 1406–1407. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balzanelli, G.M.; Distratis, P.; Aityan, S.K.; Amatulli, F.; Catucci, O.; Cefalo, A.; Dipalma, G.; Inchingolo, F.; Lazzaro, R.; Nguyen, K.C. COVID-19 and COVID-like Patients: A Brief Analysis and Findings of Two Deceased Cases. Open Access Maced. J. Med. Sci. 2020, 8, 490–495. [Google Scholar] [CrossRef]
- Bellocchio, L.; Bordea, I.R.; Ballini, A.; Lorusso, F.; Hazballa, D.; Isacco, C.G.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Inchingolo, F.; et al. Environmental Issues and Neurological Manifestations Associated with COVID-19 Pandemic: New Aspects of the Disease? Int. J. Environ. Res. Public Health 2020, 17, 8049. [Google Scholar] [CrossRef]
- Lorusso, F.; Inchingolo, F.; Scarano, A. The Impact of The Novel Covid-19 On The Scientific Production Spread: A Five-Month Bibliometric Report Of The Worldwide Research Community. Acta Med. Mediter. 2020, 36, 1–4. [Google Scholar]
- Scarano, A.; Inchingolo, F.; Lorusso, F. Environmental Disinfection of a Dental Clinic during the Covid-19 Pandemic: A Narrative Insight. Biomed. Res. Int. 2020, 2020, 8896812. [Google Scholar] [CrossRef]
- Balzanelli, G.M.; Distratis, P.; Amatulli, F.; Catucci, O.; Cefalo, A.; Lazzaro, R.; Palazzo, D.; Aityan, K.S.; Dipalma, G.; Inchingolo, F. Clinical Features in Predicting COVID-19. Biomed. J. Sci. Tech. Res. 2020, 29, 22921–22926. [Google Scholar]
- Balzanelli, M.; Distratis, P.; Catucci, O.; Amatulli, F.; Cefalo, A.; Lazzaro, R.; Aityan, K.S.; Dalagni, G.; Nico, A.; De Michele, A.; et al. Clinical and Diagnostic Findings in COVID-19 Patients: An Original Research from SG Moscati Hospital in Taranto Italy. J. Biol. Regul. Homeost. Agents 2021, 35. [Google Scholar] [CrossRef]
- Santacroce, L.; Inchingolo, F.; Topi, S.; Del Prete, R.; Di Cosola, M.; Charitos, I.A.; Montagnani, M. Potential Beneficial Role of Probiotics on the Outcome of COVID-19 Patients: An Evolving Perspective. Diabetes Metab. Synd. Clin. Res. Rev. 2021, 15, 295–301. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Hu, B.; Hu, C.; Zhu, F.; Liu, X.; Zhang, J.; Wang, B.; Xiang, H.; Cheng, Z.; Xiong, Y.; et al. Clinical Characteristics of 138 Hospitalized Patients With 2019 Novel Coronavirus-Infected Pneumonia in Wuhan, China. JAMA 2020, 323, 1061–1069. [Google Scholar] [CrossRef]
- Lorusso, F.; Postiglione, F.; Delvecchio, M.; Rapone, B.; Scarano, A. The impact of diabetes in implant oral rehabilitations: A bibliometric study and literature review. Acta Med. 2020, 36, 3333. [Google Scholar]
- Dalamaga, M.; Christodoulatos, G.S.; Karampela, I.; Vallianou, N.; Apovian, C.M. Understanding the Co-Epidemic of Obesity and COVID-19: Current Evidence, Comparison with Previous Epidemics, Mechanisms, and Preventive and Therapeutic Perspectives. Curr. Obes. Rep. 2021. [Google Scholar] [CrossRef] [PubMed]
- Ballini, A.; Gnoni, A.; De Vito, D.; Dipalma, G.; Cantore, S.; Gargiulo Isacco, C.; Saini, R.; Santacroce, L.; Topi, S.; Scarano, A.; et al. Effect of Probiotics on the Occurrence of Nutrition Absorption Capacities in Healthy Children: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 8645–8657. [Google Scholar] [CrossRef] [PubMed]
- Bottalico, L.; Castellaneta, F.; Charitos, I.A. From hydrotherapy to the discovery of the gut microbiota: The historical gastrointestinal health Concept. Gut 2020, 11, 82–90. [Google Scholar]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X.; Liu, L.; Shan, H.; Lei, C.L.; Hui, D.S.C.; et al. Clinical characteristics of coronavirus disease. N. Engl. J. Med. 2019, 1708–1720. [Google Scholar] [CrossRef]
- Chen, L.; Liu, H.G.; Liu, W.; Liu, J.; Liu, K.; Shang, J.; Deng, Y.; Wei, S. Analysis of clinical features of 29 patients with 2019 novel coronavirus pneumonia. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, E005. [Google Scholar] [CrossRef]
- Solimando, A.G.; Susca, N.; Borrelli, P.; Prete, M.; Lauletta, G.; Pappagallo, F.; Buono, R.; Inglese, G.; Forina, B.M.; Bochicchio, D.; et al. Short-Term Variations in Neutrophil-to-Lymphocyte and Urea-to-Creatinine Ratios Anticipate Intensive Care Unit Admission of COVID-19 Patients in the Emergency Department. Front. Med. 2020, 7, 625176. [Google Scholar] [CrossRef]
- Gasparro, R.; Scandurra, C.; Maldonato, N.M.; Dolce, P.; Bochicchio, V.; Valletta, A.; Sammartino, G.; Sammartino, P.; Mariniello, M.; di Lauro, A.E.; et al. Perceived Job Insecurity and Depressive Symptoms among Italian Dentists: The Moderating Role of Fear of COVID-19. Int. J. Environ. Res. Public Health 2020, 17, 5338. [Google Scholar] [CrossRef] [PubMed]
- Prompetchara, E.; Ketloy, C.; Palaga, T. Immune Responses in COVID-19 and Potential Vaccines: Lessons Learned from SARS and MERS Epidemic. Asian Pac. J. Allergy Immunol. 2020, 38, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Pang, J.; Wang, M.X.; Ang, I.Y.H.; Tan, S.H.X.; Lewis, R.F.; Chen, J.I.-P.; Gutierrez, R.A.; Gwee, S.X.W.; Chua, P.E.Y.; Yang, Q.; et al. Potential Rapid Diagnostics, Vaccine and Therapeutics for 2019 Novel Coronavirus (2019-NCoV): A Systematic Review. J. Clin. Med. 2020, 9, 623. [Google Scholar] [CrossRef] [Green Version]
- Shanmugaraj, B.; Malla, A.; Phoolcharoen, W. Emergence of Novel Coronavirus 2019-NCoV: Need for Rapid Vaccine and Biologics Development. Pathogens 2020, 9, 148. [Google Scholar] [CrossRef] [Green Version]
- Santacroce, L.; Charitos, I.A.; Carretta, D.M.; De Nitto, E.; Lovero, R. The Human Coronaviruses (HCoVs) and the Molecular Mechanisms of SARS-CoV-2 Infection. J. Mol. Med. 2020, 1–14. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Topi, S.; Saini, R.; De Vito, D.; Inchingolo, F. Probiotics Efficacy on Oxidative Stress Values in Inflammatory Bowel Disease: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Endocr. Metab. Immune Disord. Drug Targets 2019, 19, 373–381. [Google Scholar] [CrossRef]
- Ballini, A.; Santacroce, L.; Cantore, S.; Bottalico, L.; Dipalma, G.; Vito, D.D.; Saini, R.; Inchingolo, F. Probiotics Improve Urogenital Health in Women. Open Access Maced. J. Med. Sci. 2018, 6, 1845–1850. [Google Scholar] [CrossRef] [Green Version]
- Brooks, S.K.; Webster, R.K.; Smith, L.E.; Woodland, L.; Wessely, S.; Greenberg, N.; Rubin, G.J. The Psychological Impact of Quarantine and How to Reduce It: Rapid Review of the Evidence. Lancet 2020, 395, 912–920. [Google Scholar] [CrossRef] [Green Version]
- Parmet, W.E.; Sinha, M.S. Covid-19—The Law and Limits of Quarantine. N. Engl. J. Med. 2020, 382, e28. [Google Scholar] [CrossRef]
- Singhal, T. A Review of Coronavirus Disease-2019 (COVID-19). Indian J. Pediatr. 2020, 87, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Ashour, H.M.; Elkhatib, W.F.; Rahman, M.M.; Elshabrawy, H.A. Insights into the Recent 2019 Novel Coronavirus (SARS-CoV-2) in Light of Past Human Coronavirus Outbreaks. Pathogens 2020, 9, 186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Peng, F.; Wang, R.; Yange, M.; Guan, K.; Jiang, T.; Xu, G.; Sun, J.; Chang, C. The Deadly Coronaviruses: The 2003 SARS Pandemic and the 2020 Novel Coronavirus Epidemic in China. J. Autoimmun. 2020, 109, 102434. [Google Scholar] [CrossRef]
- Zhang, T.; He, Y.; Xu, W.; Ma, A.; Yang, Y.; Xu, K.-F. Clinical Trials for the Treatment of Coronavirus Disease 2019 (COVID-19): A Rapid Response to Urgent Need. Sci. China Life Sci. 2020, 63, 774–776. [Google Scholar] [CrossRef] [Green Version]
- Ruan, Q.; Yang, K.; Wang, W.; Jiang, L.; Song, J. Clinical Predictors of Mortality Due to COVID-19 Based on an Analysis of Data of 150 Patients from Wuhan, China. Intens. Care Med. 2020, 46, 846–848. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Coronavirus Disease (COVID-19) Technical Guidance: Patient Management; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Russell, C.D.; Millar, J.E.; Baillie, J.K. Clinical Evidence Does Not Support Corticosteroid Treatment for 2019-NCoV Lung Injury. Lancet 2020, 395, 473–475. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Liu, Y.; Tian, D.; Wang, C.; Wang, S.; Cheng, J.; Hu, M.; Fang, M.; Gao, Y. Potential Benefits of Precise Corticosteroids Therapy for Severe 2019-NCoV Pneumonia. Signal Transduct. Target Ther. 2020, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- O’Dowd, K.; Nair, K.M.; Forouzandeh, P.; Mathew, S.; Grant, J.; Moran, R.; Bartlett, J.; Bird, J.; Pillai, S.C. Face Masks and Respirators in the Fight against the COVID-19 Pandemic: A Review of Current Materials, Advances and Future Perspectives. Materials 2020, 13, 3363. [Google Scholar] [CrossRef]
- Charitos, I.A.; Ballini, A.; Bottalico, L.; Cantore, S.; Passarelli, P.C.; Inchingolo, F.; D’Addona, A.; Santacroce, L. Special Features of SARS-CoV-2 in Daily Practice. WJCC 2020, 8, 3920–3933. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.C.D.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L. The Pivotal Role of Oral Microbiota in Health and Disease. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Lin, L.; Lu, L.; Cao, W.; Li, T. Hypothesis for Potential Pathogenesis of SARS-CoV-2 Infection-a Review of Immune Changes in Patients with Viral Pneumonia. Emerg. Microb. Infect. 2020, 9, 727–732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thanh Le, T.; Andreadakis, Z.; Kumar, A.; Gómez Román, R.; Tollefsen, S.; Saville, M.; Mayhew, S. The COVID-19 Vaccine Development Landscape. Nat. Rev. Drug Discov. 2020, 19, 305–306. [Google Scholar] [CrossRef] [PubMed]
- Triggle, C.R.; Bansal, D.; Farag, E.A.B.A.; Ding, H.; Sultan, A.A. COVID-19: Learning from Lessons to Guide Treatment and Prevention Interventions. mSphere 2020, 5. [Google Scholar] [CrossRef]
- Dong, L.; Hu, S.; Gao, J. Discovering Drugs to Treat Coronavirus Disease 2019 (COVID-19). Drug Discov. Ther. 2020, 14, 58–60. [Google Scholar] [CrossRef] [Green Version]
- Arabi, Y.; Asseri, A.; Webb, S.; Marshall, J.; Al Moamary, M. Clinical Trials for Coronavirus Disease 2019: What Is Being Evaluated and What Is Not. Ann. Thorac. Med. 2020, 15, 49. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.A.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA Extension Statement for Reporting of Systematic Reviews Incorporating Network Meta-Analyses of Health Care Interventions: Checklist and Explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Chan, K.S.; Lai, S.T.; Chu, C.M.; Tsui, E.; Tam, C.Y.; Wong, M.M.L.; Tse, M.W.; Que, T.L.; Peiris, J.S.M.; Sung, J.; et al. Treatment of Severe Acute Respiratory Syndrome with Lopinavir/Ritonavir: A Multicentre Retrospective Matched Cohort Study. Hong Kong Med. J. 2003, 9, 399–406. [Google Scholar]
- Chu, C.M. Role of Lopinavir/Ritonavir in the Treatment of SARS: Initial Virological and Clinical Findings. Thorax 2004, 59, 252–256. [Google Scholar] [CrossRef] [Green Version]
- Lim, J.; Jeon, S.; Shin, H.Y.; Kim, M.J.; Seong, Y.M.; Lee, W.J.; Choe, K.W.; Kang, Y.M.; Lee, B.; Park, S.J. Case of the Index Patient Who Caused Tertiary Transmission of COVID-19 Infection in Korea: The Application of Lopinavir/Ritonavir for the Treatment of COVID-19 Infected Pneumonia Monitored by Quantitative RT-PCR. J. Kor. Med. Sci. 2020, 35, e79. [Google Scholar] [CrossRef]
- Young, B.E.; Ong, S.W.X.; Kalimuddin, S.; Low, J.G.; Tan, S.Y.; Loh, J.; Ng, O.-T.; Marimuthu, K.; Ang, L.W.; Mak, T.M.; et al. Epidemiologic Features and Clinical Course of Patients Infected With SARS-CoV-2 in Singapore. JAMA 2020, 323, 1488–1494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, B.; Wang, Y.; Wen, D.; Liu, W.; Wang, J.; Fan, G.; Ruan, L.; Song, B.; Cai, Y.; Wei, M.; et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe Covid-19. N. Engl. J. Med. 2020, 382, 1787–1799. [Google Scholar] [CrossRef] [PubMed]
- Furuta, Y.; Takahashi, K.; Fukuda, Y.; Kuno, M.; Kamiyama, T.; Kozaki, K.; Nomura, N.; Egawa, H.; Minami, S.; Watanabe, Y.; et al. In Vitro and in Vivo Activities of Anti-Influenza Virus Compound T-705. Antimicrob. Agents Chemother. 2002, 46, 977–981. [Google Scholar] [CrossRef] [Green Version]
- Furuta, Y.; Komeno, T.; Nakamura, T. Favipiravir (T-705), a Broad Spectrum Inhibitor of Viral RNA Polymerase. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2017, 93, 449–463. [Google Scholar] [CrossRef] [Green Version]
- Sissoko, D.; Laouenan, C.; Folkesson, E.; M’Lebing, A.-B.; Beavogui, A.-H.; Baize, S.; Camara, A.-M.; Maes, P.; Shepherd, S.; Danel, C.; et al. Experimental Treatment with Favipiravir for Ebola Virus Disease (the JIKI Trial): A Historically Controlled, Single-Arm Proof-of-Concept Trial in Guinea. PLoS Med. 2016, 13, e1001967. [Google Scholar] [CrossRef] [Green Version]
- Chinello, P.; Petrosillo, N.; Pittalis, S.; Biava, G.; Ippolito, G.; Nicastri, E. INMI Ebola Team QTc Interval Prolongation during Favipiravir Therapy in an Ebolavirus-Infected Patient. PLoS Negl. Trop. Dis. 2017, 11, e0006034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumagai, Y.; Murakawa, Y.; Hasunuma, T.; Aso, M.; Yuji, W.; Sakurai, T.; Noto, M.; Oe, T.; Kaneko, A. Lack of Effect of Favipiravir, a Novel Antiviral Agent, on QT Interval in Healthy Japanese Adults. Int. J. Clin. Pharmacol. Ther. 2015, 53, 866–874. [Google Scholar] [CrossRef]
- Agostini, M.L.; Andres, E.L.; Sims, A.C.; Graham, R.L.; Sheahan, T.P.; Lu, X.; Smith, E.C.; Case, J.B.; Feng, J.Y.; Jordan, R.; et al. Coronavirus Susceptibility to the Antiviral Remdesivir (GS-5734) Is Mediated by the Viral Polymerase and the Proofreading Exoribonuclease. mBio 2018, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheahan, T.P.; Sims, A.C.; Leist, S.R.; Schäfer, A.; Won, J.; Brown, A.J.; Montgomery, S.A.; Hogg, A.; Babusis, D.; Clarke, M.O.; et al. Comparative Therapeutic Efficacy of Remdesivir and Combination Lopinavir, Ritonavir, and Interferon Beta against MERS-CoV. Nat. Commun. 2020, 11, 222. [Google Scholar] [CrossRef] [Green Version]
- Yao, T.-T.; Qian, J.-D.; Zhu, W.-Y.; Wang, Y.; Wang, G.-Q. A Systematic Review of Lopinavir Therapy for SARS Coronavirus and MERS Coronavirus-A Possible Reference for Coronavirus Disease-19 Treatment Option. J. Med. Virol. 2020, 92, 556–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lombardia, S.R. Vademecum per La Cura Delle Persone Con Malattia Da COVI-19; Società Italiana di Malattie Infettive e Tropicali: Prato, Italy, 2020. [Google Scholar]
- Al-Tawfiq, J.A.; Al-Homoud, A.H.; Memish, Z.A. Remdesivir as a Possible Therapeutic Option for the COVID-19. Travel Med. Infect. Dis. 2020, 34, 101615. [Google Scholar] [CrossRef] [PubMed]
- Siegel, D.; Hui, H.C.; Doerffler, E.; Clarke, M.O.; Chun, K.; Zhang, L.; Neville, S.; Carra, E.; Lew, W.; Ross, B.; et al. Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f][Triazin-4-Amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses. J. Med. Chem. 2017, 60, 1648–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mulangu, S.; Dodd, L.E.; Davey, R.T.; Tshiani Mbaya, O.; Proschan, M.; Mukadi, D.; Lusakibanza Manzo, M.; Nzolo, D.; Tshomba Oloma, A.; Ibanda, A.; et al. A Randomized, Controlled Trial of Ebola Virus Disease Therapeutics. N. Engl. J. Med. 2019, 381, 2293–2303. [Google Scholar] [CrossRef]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and Chloroquine Effectively Inhibit the Recently Emerged Novel Coronavirus (2019-NCoV) in Vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef]
- Sheahan, T.P.; Sims, A.C.; Graham, R.L.; Menachery, V.D.; Gralinski, L.E.; Case, J.B.; Leist, S.R.; Pyrc, K.; Feng, J.Y.; Trantcheva, I.; et al. Broad-Spectrum Antiviral GS-5734 Inhibits Both Epidemic and Zoonotic Coronaviruses. Sci. Transl. Med. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Nicastri, E.; Petrosillo, N.; Ascoli Bartoli, T.; Lepore, L.; Mondi, A.; Palmieri, F.; D’Offizi, G.; Marchioni, L.; Murachelli, S.; Ippolito, G.; et al. National Institute for the Infectious Diseases “L. Spallanzani”, IRCCS. Recommendations for COVID-19 Clinical Management. Infect. Dis. Rep. 2020, 12, 8543. [Google Scholar] [CrossRef]
- Commissioner of the Coronavirus (COVID-19) Update: FDA Issues Emergency Use Authorization for Potential COVID-19 Treatment. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-issues-emergency-use-authorization-potential-covid-19-treatment (accessed on 4 January 2021).
- Hu, Y.; Meng, X.; Zhang, F.; Xiang, Y.; Wang, J. The in Vitro Antiviral Activity of Lactoferrin against Common Human Coronaviruses and SARS-CoV-2 Is Mediated by Targeting the Heparan Sulfate Co-Receptor. Emerg. Microb. Infect. 2021, 10, 317–330. [Google Scholar] [CrossRef]
- Berlutti, F.; Pantanella, F.; Natalizi, T.; Frioni, A.; Paesano, R.; Polimeni, A.; Valenti, P. Antiviral Properties of Lactoferrin—A Natural Immunity Molecule. Molecules 2011, 16, 6992–7018. [Google Scholar] [CrossRef] [Green Version]
- (Released by National Health Commission & National Administration of Traditional Chinese Medicine on March 3, 2020) Diagnosis and Treatment Protocol for Novel Coronavirus Pneumonia (Trial Version 7). Chin. Med. J. 2020, 133, 1087–1095. [CrossRef]
- Delang, L.; Abdelnabi, R.; Neyts, J. Favipiravir as a Potential Countermeasure against Neglected and Emerging RNA Viruses. Antivir. Res. 2018, 153, 85–94. [Google Scholar] [CrossRef] [PubMed]
- Bryner, J. Flu Drug Used in Japan Shows Promise in Treating COVID-19. Live Science, New York, NY. 2020. Available online: https://www.livescience.com/flu-drug-could-treat-coronavirus.html (accessed on 23 May 2021).
- Crump, A. Ivermectin: Enigmatic Multifaceted “wonder” Drug Continues to Surprise and Exceed Expectations. J. Antibiot. 2017, 70, 495–505. [Google Scholar] [CrossRef] [Green Version]
- Wagstaff, K.M.; Sivakumaran, H.; Heaton, S.M.; Harrich, D.; Jans, D.A. Ivermectin Is a Specific Inhibitor of Importin α/β-Mediated Nuclear Import Able to Inhibit Replication of HIV-1 and Dengue Virus. Biochem. J. 2012, 443, 851–856. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caly, L.; Druce, J.D.; Catton, M.G.; Jans, D.A.; Wagstaff, K.M. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antivir. Res. 2020, 178, 104787. [Google Scholar] [CrossRef] [PubMed]
- EMA Raccomanda Di Non Utilizzare Ivermectina per La Prevenzione o Il Trattamento Di COVID-19 al Di Fuori Degli Studi Clinici. Available online: https://www.aifa.gov.it/-/ema-raccomanda-di-non-utilizzare-ivermectina-per-la-prevenzione-o-il-trattamento-di-covid-19-al-di-fuori-degli-studi-clinici (accessed on 20 May 2021).
- Momekov, G.; Momekova, D. Ivermectin as a Potential COVID-19 Treatment from the Pharmacokinetic Point of View: Antiviral Levels Are Not Likely Attainable with Known Dosing Regimens. Biotechnol. Biotechnol. Equipm. 2020, 34, 469–474. [Google Scholar] [CrossRef]
- Costanzo, M.; De Giglio, M.A.R.; Roviello, G.N. SARS-CoV-2: Recent Reports on Antiviral Therapies Based on Lopinavir/Ritonavir, Darunavir/Umifenovir, Hydroxychloroquine, Remdesivir, Favipiravir and Other Drugs for the Treatment of the New Coronavirus. Curr. Med. Chem. 2020, 27, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-C.; Shih, T.-P.; Ko, W.-C.; Tang, H.-J.; Hsueh, P.-R. Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) and Coronavirus Disease-2019 (COVID-19): The Epidemic and the Challenges. Int. J. Antimicrob. Agents 2020, 55, 105924. [Google Scholar] [CrossRef] [PubMed]
- multicenter collaboration group of Department of Science and Technology of Guangdong Province and Health Commission of Guangdong Province for chloroquine in the treatment of novel coronavirus pneumonia [Expert consensus on chloroquine phosphate for the treatment of novel coronavirus pneumonia]. Zhonghua Jie He He Hu Xi Za Zhi 2020, 43, 185–188. [CrossRef]
- Yao, X.; Ye, F.; Zhang, M.; Cui, C.; Huang, B.; Niu, P.; Liu, X.; Zhao, L.; Dong, E.; Song, C.; et al. In Vitro Antiviral Activity and Projection of Optimized Dosing Design of Hydroxychloroquine for the Treatment of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). Clin. Infect. Dis. 2020, 71, 732–739. [Google Scholar] [CrossRef] [Green Version]
- Vincent, M.J.; Bergeron, E.; Benjannet, S.; Erickson, B.R.; Rollin, P.E.; Ksiazek, T.G.; Seidah, N.G.; Nichol, S.T. Chloroquine Is a Potent Inhibitor of SARS Coronavirus Infection and Spread. Virol. J. 2005, 2, 69. [Google Scholar] [CrossRef] [Green Version]
- Golden, E.B.; Cho, H.-Y.; Hofman, F.M.; Louie, S.G.; Schönthal, A.H.; Chen, T.C. Quinoline-Based Antimalarial Drugs: A Novel Class of Autophagy Inhibitors. Neurosurg. Focus 2015, 38, E12. [Google Scholar] [CrossRef]
- Gao, J.; Tian, Z.; Yang, X. Breakthrough: Chloroquine Phosphate Has Shown Apparent Efficacy in Treatment of COVID-19 Associated Pneumonia in Clinical Studies. Biosci. Trends 2020, 14, 72–73. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Hu, J.; Zhang, Z.; Jiang, S.; Han, S.; Yan, D.; Zhuang, R.; Hu, B.; Zhang, Z. Efficacy of Hydroxychloroquine in Patients with COVID-19: Results of a Randomized Clinical Trial. MedRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Gautret, P.; Lagier, J.-C.; Parola, P.; Hoang, V.T.; Meddeb, L.; Mailhe, M.; Doudier, B.; Courjon, J.; Giordanengo, V.; Vieira, V.E.; et al. Hydroxychloroquine and Azithromycin as a Treatment of COVID-19: Results of an Open-Label Non-Randomized Clinical Trial. Int. J. Antimicrob. Agents 2020, 56, 105949. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Cao, R.; Xu, M.; Wang, X.; Zhang, H.; Hu, H.; Li, Y.; Hu, Z.; Zhong, W.; Wang, M. Hydroxychloroquine, a Less Toxic Derivative of Chloroquine, Is Effective in Inhibiting SARS-CoV-2 Infection in Vitro. Cell Discov. 2020, 6, 16. [Google Scholar] [CrossRef] [Green Version]
- Farmaci Utilizzabili per il Trattamento della Malattia COVID19|Agenzia Italiana del Farmaco. Available online: https://aifa.gov.it/aggiornamento-sui-farmaci-utilizzabili-per-il-trattamento-della-malattia-covid19 (accessed on 14 February 2021).
- Abella, B.S.; Jolkovsky, E.L.; Biney, B.T.; Uspal, J.E.; Hyman, M.C.; Frank, I.; Hensley, S.E.; Gill, S.; Vogl, D.T.; Maillard, I.; et al. Efficacy and Safety of Hydroxychloroquine vs Placebo for Pre-Exposure SARS-CoV-2 Prophylaxis Among Health Care Workers. JAMA Intern. Med. 2020. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti, A.B.; Zampieri, F.G.; Rosa, R.G.; Azevedo, L.C.P.; Veiga, V.C.; Avezum, A.; Damiani, L.P.; Marcadenti, A.; Kawano-Dourado, L.; Lisboa, T.; et al. Hydroxychloroquine with or without Azithromycin in Mild-to-Moderate Covid-19. N. Engl. J. Med. 2020. [Google Scholar] [CrossRef]
- Brown, S.M.; Peltan, I.; Kumar, N.; Leither, L.; Webb, B.J.; Starr, N.; Grissom, C.K.; Buckel, W.R.; Srivastava, R.; Butler, A.M.; et al. Hydroxychloroquine vs. Azithromycin for Hospitalized Patients with COVID-19 (HAHPS): Results of a Randomized, Active Comparator Trial. Ann. ATS 2020. [Google Scholar] [CrossRef] [PubMed]
- Parsons, L. March 2020. UK Biotech Synairgen to Begin COVID-19 Trial of Lead Candidate; Synairgen plc: Southampton, UK, 2020. [Google Scholar]
- Chan, K.W.; Wong, V.T.; Tang, S.C.W. COVID-19: An Update on the Epidemiological, Clinical, Preventive and Therapeutic Evidence and Guidelines of Integrative Chinese-Western Medicine for the Management of 2019 Novel Coronavirus Disease. Am. J. Chin. Med. 2020, 48, 737–762. [Google Scholar] [CrossRef]
- Peiffer-Smadja, N.; Yazdanpanah, Y. Nebulised Interferon Beta-1a for Patients with COVID-19. Lancet Respir. Med. 2021, 9, 122–123. [Google Scholar] [CrossRef]
- Macdonald, L.E.; Karow, M.; Stevens, S.; Auerbach, W.; Poueymirou, W.T.; Yasenchak, J.; Frendewey, D.; Valenzuela, D.M.; Giallourakis, C.C.; Alt, F.W.; et al. Precise and in Situ Genetic Humanization of 6 Mb of Mouse Immunoglobulin Genes. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef] [Green Version]
- Murphy, A.J.; Macdonald, L.E.; Stevens, S.; Karow, M.; Dore, A.T.; Pobursky, K.; Huang, T.T.; Poueymirou, W.T.; Esau, L.; Meola, M.; et al. Mice with Megabase Humanization of Their Immunoglobulin Genes Generate Antibodies as Efficiently as Normal Mice. Proc. Natl. Acad. Sci. USA 2014, 111, 5153–5158. [Google Scholar] [CrossRef] [Green Version]
- Baum, A.; Fulton, B.O.; Wloga, E.; Copin, R.; Pascal, K.E.; Russo, V.; Giordano, S.; Lanza, K.; Negron, N.; Ni, M.; et al. Antibody Cocktail to SARS-CoV-2 Spike Protein Prevents Rapid Mutational Escape Seen with Individual Antibodies. Science 2020, 369. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Su, B.; Guo, X.; Sun, W.; Deng, Y.; Bao, L.; Zhu, Q.; Zhang, X.; Zheng, Y.; Geng, C.; et al. Potent Neutralizing Antibodies against SARS-CoV-2 Identified by High-Throughput Single-Cell Sequencing of Convalescent Patients’ B Cells. Cell 2020, 182, 73–84.e16. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Wang, F.; Shen, C.; Peng, W.; Li, D.; Zhao, C.; Li, Z.; Li, S.; Bi, Y.; Yang, Y.; et al. A Noncompeting Pair of Human Neutralizing Antibodies Block COVID-19 Virus Binding to Its Receptor ACE2. Science 2020, 368, 1274–1278. [Google Scholar] [CrossRef] [PubMed]
- Hansen, J.; Baum, A.; Pascal, K.E.; Russo, V.; Giordano, S.; Wloga, E.; Fulton, B.O.; Yan, Y.; Koon, K.; Patel, K.; et al. Studies in Humanized Mice and Convalescent Humans Yield a SARS-CoV-2 Antibody Cocktail. Science 2020, 369, 1010–1014. [Google Scholar] [CrossRef]
- Immunoglobulin Genes in Transgenic Mice—ScienceDirect. Available online: https://www.sciencedirect.com/science/article/abs/pii/0168952585900897 (accessed on 21 March 2021).
- AIFA Pubblica Parere CTS Su Anticorpi Monoclonali. Available online: https://www.aifa.gov.it/-/aifa-pubblica-parere-cts-su-anticorpi-monoclonali (accessed on 10 April 2021).
- Gazzetta Ufficiale. Available online: https://www.gazzettaufficiale.it/eli/id/2021/02/08/21A00788/sg (accessed on 10 April 2021).
- Pinho, A.C. EMA Reviewing Data on Monoclonal Antibody Use for COVID-19. Available online: https://www.ema.europa.eu/en/news/ema-reviewing-data-monoclonal-antibody-use-covid-19 (accessed on 10 April 2021).
- Commissioner of the Coronavirus (COVID-19) Update: FDA Authorizes Monoclonal Antibody for Treatment of COVID-19. Available online: https://www.fda.gov/news-events/press-announcements/coronavirus-covid-19-update-fda-authorizes-monoclonal-antibody-treatment-covid-19 (accessed on 21 March 2021).
- Canada, H. Bamlanivimab (Bamlanivimab). Available online: https://covid-vaccine.canada.ca/bamlanivimab/product-details (accessed on 21 March 2021).
- Chen, P.; Nirula, A.; Heller, B.; Gottlieb, R.L.; Boscia, J.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. SARS-CoV-2 Neutralizing Antibody LY-CoV555 in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 229–237. [Google Scholar] [CrossRef]
- Dodd, R.Y.; Foster, G.A.; Stramer, S.L. Keeping Blood Transfusion Safe From West Nile Virus: American Red Cross Experience, 2003 to 2012. Transfus. Med. Rev. 2015, 29, 153–161. [Google Scholar] [CrossRef]
- Hung, I.F.; To, K.K.; Lee, C.-K.; Lee, K.-L.; Chan, K.; Yan, W.-W.; Liu, R.; Watt, C.-L.; Chan, W.-M.; Lai, K.-Y.; et al. Convalescent Plasma Treatment Reduced Mortality in Patients with Severe Pandemic Influenza A (H1N1) 2009 Virus Infection. Clin. Infect. Dis. 2011, 52, 447–456. [Google Scholar] [CrossRef]
- Mair-Jenkins, J.; Saavedra-Campos, M.; Baillie, J.K.; Cleary, P.; Khaw, F.-M.; Lim, W.S.; Makki, S.; Rooney, K.D.; Nguyen-Van-Tam, J.S.; Beck, C.R.; et al. The Effectiveness of Convalescent Plasma and Hyperimmune Immunoglobulin for the Treatment of Severe Acute Respiratory Infections of Viral Etiology: A Systematic Review and Exploratory Meta-Analysis. J. Infect. Dis. 2015, 211, 80–90. [Google Scholar] [CrossRef] [Green Version]
- Kraft, C.S.; Hewlett, A.L.; Koepsell, S.; Winkler, A.M.; Kratochvil, C.J.; Larson, L.; Varkey, J.B.; Mehta, A.K.; Lyon, G.M.; Friedman-Moraco, R.J.; et al. The Use of TKM-100802 and Convalescent Plasma in 2 Patients with Ebola Virus Disease in the United States. Clin. Infect. Dis. 2015, 61, 496–502. [Google Scholar] [CrossRef]
- Van Griensven, J.; Edwards, T.; de Lamballerie, X.; Semple, M.G.; Gallian, P.; Baize, S.; Horby, P.W.; Raoul, H.; Magassouba, N.; Antierens, A.; et al. Evaluation of Convalescent Plasma for Ebola Virus Disease in Guinea. N. Engl. J. Med. 2016, 374, 33–42. [Google Scholar] [CrossRef]
- Duan, K.; Liu, B.; Li, C.; Zhang, H.; Yu, T.; Qu, J.; Zhou, M.; Chen, L.; Meng, S.; Hu, Y.; et al. Effectiveness of Convalescent Plasma Therapy in Severe COVID-19 Patients. Proc. Natl. Acad. Sci. USA 2020, 117, 9490–9496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Zhong, N.; Guan, Y. Treatment with Convalescent Plasma for Influenza A (H5N1) Infection. N. Engl. J. Med. 2007, 357, 1450–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burnouf, T.; Radosevich, M. Treatment of Severe Acute Respiratory Syndrome with Convalescent Plasma. Hong Kong Med. J. 2003, 9, 309. [Google Scholar] [PubMed]
- Sorrell, F.J.; Szklarz, M.; Abdul Azeez, K.R.; Elkins, J.M.; Knapp, S. Family-Wide Structural Analysis of Human Numb-Associated Protein Kinases. Structure 2016, 24, 401–411. [Google Scholar] [CrossRef] [Green Version]
- Roback, J.D.; Guarner, J. Convalescent Plasma to Treat COVID-19: Possibilities and Challenges. JAMA 2020, 323, 1561–1562. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, A.; Mukherjee, A.; Kumar, G.; Chatterjee, P.; Bhatnagar, T.; Malhotra, P. Convalescent Plasma in the Management of Moderate Covid-19 in Adults in India: Open Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial). BMJ 2020, 371, m3939. [Google Scholar] [CrossRef]
- Wu, F.; Wang, A.; Liu, M.; Wang, Q.; Chen, J.; Xia, S.; Ling, Y.; Zhang, Y.; Xun, J.; Lu, L.; et al. Neutralizing Antibody Responses to SARS-CoV-2 in a COVID-19 Recovered Patient Cohort and Their Implications. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Xia, X.; Li, K.; Wu, L.; Wang, Z.; Zhu, M.; Huang, B.; Li, J.; Wang, Z.; Wu, W.; Wu, M.; et al. Improved Clinical Symptoms and Mortality among Patients with Severe or Critical COVID-19 after Convalescent Plasma Transfusion. Blood 2020, 136, 755–759. [Google Scholar] [CrossRef]
- ISBT: COVID-19 Convalescent Plasma Document Library. Available online: https://www.isbtweb.org/covid-19resources/covid-19-convalescent-plasma-document-library/ (accessed on 18 March 2021).
- Sparrow, E.; Friede, M.; Sheikh, M.; Torvaldsen, S. Therapeutic Antibodies for Infectious Diseases. Bull. World Health Organ. 2017, 95, 235–237. [Google Scholar] [CrossRef]
- Graham, B.S.; Ambrosino, D.M. History of Passive Antibody Administration for Prevention and Treatment of Infectious Diseases. Curr. Opin. HIV AIDS 2015, 10, 129–134. [Google Scholar] [CrossRef]
- Lee, J.; Park, S.-S.; Kim, T.Y.; Lee, D.-G.; Kim, D.-W. Lymphopenia as a Biological Predictor of Outcomes in COVID-19 Patients: A Nationwide Cohort Study. Cancers 2021, 13, 471. [Google Scholar] [CrossRef] [PubMed]
- Jahanshahlu, L.; Rezaei, N. Monoclonal Antibody as a Potential Anti-COVID-19. Biomed. Pharmacother. 2020, 129, 110337. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.-M.; Hwang, Y.-C.; Liu, I.-J.; Lee, C.-C.; Tsai, H.-Z.; Li, H.-J.; Wu, H.-C. Development of Therapeutic Antibodies for the Treatment of Diseases. J. Biomed. Sci. 2020, 27. [Google Scholar] [CrossRef] [PubMed]
- Walker, L.M.; Burton, D.R. Passive Immunotherapy of Viral Infections: “super-Antibodies” Enter the Fray. Nat. Rev. Immunol. 2018, 18, 297–308. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.K.; Li, W.; Moore, M.J.; Choe, H.; Farzan, M. A 193-Amino Acid Fragment of the SARS Coronavirus S Protein Efficiently Binds Angiotensin-Converting Enzyme 2. J. Biol. Chem. 2004, 279, 3197–3201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molecular and Biological Characterization of Human Monoclonal Antibodies Binding to the Spike and Nucleocapsid Proteins of Severe Acute Respiratory Syndrome Coronavirus—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/15650189/ (accessed on 20 March 2021).
- Duan, J.; Yan, X.; Guo, X.; Cao, W.; Han, W.; Qi, C.; Feng, J.; Yang, D.; Gao, G.; Jin, G. A Human SARS-CoV Neutralizing Antibody against Epitope on S2 Protein. Biochem. Biophys. Res. Commun. 2005, 333, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Meulen, J.T.; van den Brink, E.N.; Poon, L.L.M.; Marissen, W.E.; Leung, C.S.W.; Cox, F.; Cheung, C.Y.; Bakker, A.Q.; Bogaards, J.A.; van Deventer, E.; et al. Human Monoclonal Antibody Combination against SARS Coronavirus: Synergy and Coverage of Escape Mutants. PLOS Med. 2006, 3, e237. [Google Scholar] [CrossRef] [Green Version]
- Shen, C.; Wang, Z.; Zhao, F.; Yang, Y.; Li, J.; Yuan, J.; Wang, F.; Li, D.; Yang, M.; Xing, L.; et al. Treatment of 5 Critically Ill Patients With COVID-19 With Convalescent Plasma. JAMA 2020, 323, 1582–1589. [Google Scholar] [CrossRef]
- Berry, J.D.; Gaudet, R.G. Antibodies in Infectious Diseases: Polyclonals, Monoclonals and Niche Biotechnology. N. Biotechnol. 2011, 28, 489–501. [Google Scholar] [CrossRef]
- Lillicrap, D. Disseminated Intravascular Coagulation in Patients with 2019-NCoV Pneumonia. J. Thromb. Haemost. 2020, 18, 786–787. [Google Scholar] [CrossRef] [Green Version]
- Cantore, S.; Mirgaldi, R.; Ballini, A.; Coscia, M.F.; Scacco, S.; Papa, F.; Inchingolo, F.; Dipalma, G.; De Vito, D. Cytokine Gene Polymorphisms Associate with Microbiogical Agents in Periodontal Disease: Our Experience. Int. J. Med. Sci. 2014, 11, 674–679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Tang, F.; Fontanet, A.; Zhan, L.; Zhao, Q.-M.; Zhang, P.-H.; Wu, X.-M.; Zuo, S.-Q.; Baril, L.; Vabret, A.; et al. Long-Term SARS Coronavirus Excretion from Patient Cohort, China. Emerg. Infect. Dis. 2004, 10, 1841–1843. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Han, M.; Li, T.; Sun, W.; Wang, D.; Fu, B.; Zhou, Y.; Zheng, X.; Yang, Y.; Li, X.; et al. Effective Treatment of Severe COVID-19 Patients with Tocilizumab. Proc. Natl. Acad. Sci. USA 2020, 117, 10970–10975. [Google Scholar] [CrossRef]
- COVID-19: Studio Randomizzato Italiano, Nessun Beneficio dal Tocilizumab. Available online: https://aifa.gov.it/-/covid-19-studio-randomizzato-italiano-nessun-beneficio-dal-tocilizumab (accessed on 10 April 2021).
- Weinreich, D.M.; Sivapalasingam, S.; Norton, T.; Ali, S.; Gao, H.; Bhore, R.; Musser, B.J.; Soo, Y.; Rofail, D.; Im, J.; et al. REGN-COV2, a Neutralizing Antibody Cocktail, in Outpatients with Covid-19. N. Engl. J. Med. 2021, 384, 238–251. [Google Scholar] [CrossRef] [PubMed]
- Richardson, P.; Griffin, I.; Tucker, C.; Smith, D.; Oechsle, O.; Phelan, A.; Stebbing, J. Baricitinib as Potential Treatment for 2019-NCoV Acute Respiratory Disease. Lancet 2020, 395, e30. [Google Scholar] [CrossRef] [Green Version]
- Coronavirus Covid-19: Il Baricitinib Costituisce uno Strumento in Più Nella Terapia delle Forme Serie e Critiche, Trial Americani Confermano Evidenze di uno Studio Italiano|AgenSIR; SIR—Servizio Informazione Religiosa: Rome, Italy, 2021.
- Casirivimab and Imdevimab. Available online: https://www.regeneron.com/casirivimab-imdevimab (accessed on 10 April 2021).
- Tuccori, M.; Ferraro, S.; Convertino, I.; Cappello, E.; Valdiserra, G.; Blandizzi, C.; Maggi, F.; Focosi, D. Anti-SARS-CoV-2 Neutralizing Monoclonal Antibodies: Clinical Pipeline. mAbs 2020, 12, 1854149. [Google Scholar] [CrossRef] [PubMed]
- Bethesda, M. Drugs and Lactation Database (LactMed); National Library of Medicine (US): Bethesda, MD, USA, 2006. [Google Scholar]
- Hussain, A.; Hasan, A.; Nejadi Babadaei, M.M.; Bloukh, S.H.; Chowdhury, M.E.H.; Sharifi, M.; Haghighat, S.; Falahati, M. Targeting SARS-CoV2 Spike Protein Receptor Binding Domain by Therapeutic Antibodies. Biomed Pharm. 2020, 130, 110559. [Google Scholar] [CrossRef] [PubMed]
- Gottlieb, R.L.; Nirula, A.; Chen, P.; Boscia, J.; Heller, B.; Morris, J.; Huhn, G.; Cardona, J.; Mocherla, B.; Stosor, V.; et al. Effect of Bamlanivimab as Monotherapy or in Combination With Etesevimab on Viral Load in Patients With Mild to Moderate COVID-19: A Randomized Clinical Trial. JAMA 2021, 325, 632. [Google Scholar] [CrossRef]
- An EUA for Bamlanivimab—A Monoclonal Antibody for COVID-19|The Medical Letter, Inc. Available online: https://secure.medicalletter.org/w1612a (accessed on 21 March 2021).
- Dong, J.; Zost, S.J.; Greaney, A.J.; Starr, T.N.; Dingens, A.S.; Chen, E.C.; Chen, R.E.; Case, J.B.; Sutton, R.E.; Gilchuk, P.; et al. Genetic and Structural Basis for Recognition of SARS-CoV-2 Spike Protein by a Two-Antibody Cocktail. bioRxiv 2021. [Google Scholar] [CrossRef]
- Toscana Life Sciences: Selezionati Tre Anticorpi Monoclonali per Test Clinici Su Covid-19. Available online: https://www.aboutpharma.com/blog/2020/07/17/toscana-life-sciences-selezionati-tre-anticorpi-monoclonali-per-test-clinici-su-covid-19/ (accessed on 10 April 2021).
- Monoclonal Antibody Discovery (MAD) LAB; Toscana Life Sciences: Siena, Italy, 2021.
- Covid. Anticorpo Monoclonale VIR-7831 Riduce Ospedalizzazione e Rischio Di Morte Nel Trattamento Precoce Degli Adulti—Quotidiano Sanità. Available online: http://www.quotidianosanita.it/scienza-e-farmaci/articolo.php?articolo_id=93402 (accessed on 10 April 2021).
- Cavalli, G.; Larcher, A.; Tomelleri, A.; Campochiaro, C.; Della-Torre, E.; Luca, G.D.; Farina, N.; Boffini, N.; Ruggeri, A.; Poli, A.; et al. Interleukin-1 and Interleukin-6 Inhibition Compared with Standard Management in Patients with COVID-19 and Hyperinflammation: A Cohort Study. Lancet Rheumatol. 2021. [Google Scholar] [CrossRef]
- Al San Raffaele Uno Studio per Le Cure Sperimentali Contro COVID-19. Available online: https://www.hsr.it/news/2020/marzo/cure-sperimentali-coronavirus (accessed on 21 March 2021).
- Campochiaro, C.; Della-Torre, E.; Cavalli, G.; De Luca, G.; Ripa, M.; Boffini, N.; Tomelleri, A.; Baldissera, E.; Rovere-Querini, P.; Ruggeri, A.; et al. Efficacy and Safety of Tocilizumab in Severe COVID-19 Patients: A Single-Centre Retrospective Cohort Study. Eur. J. Intern. Med. 2020, 76, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Stockman, L.J.; Bellamy, R.; Garner, P. SARS: Systematic Review of Treatment Effects. PLoS Med. 2006, 3, e343. [Google Scholar] [CrossRef] [Green Version]
- Arabi, Y.M.; Mandourah, Y.; Al-Hameed, F.; Sindi, A.A.; Almekhlafi, G.A.; Hussein, M.A.; Jose, J.; Pinto, R.; Al-Omari, A.; Kharaba, A.; et al. Corticosteroid Therapy for Critically Ill Patients with Middle East Respiratory Syndrome. Am. J. Respir. Crit. Care Med. 2018, 197, 757–767. [Google Scholar] [CrossRef]
- RECOVERY Collaborative Group; Horby, P.; Lim, W.S.; Emberson, J.R.; Mafham, M.; Bell, J.L.; Linsell, L.; Staplin, N.; Brightling, C.; Ustianowski, A.; et al. Dexamethasone in Hospitalized Patients with Covid-19. N. Engl. J. Med. 2021, 384, 693–704. [Google Scholar] [CrossRef]
- Shang, L.; Zhao, J.; Hu, Y.; Du, R.; Cao, B. On the Use of Corticosteroids for 2019-NCoV Pneumonia. Lancet 2020, 395, 683–684. [Google Scholar] [CrossRef] [Green Version]
- Inciardi, R.M.; Lupi, L.; Zaccone, G.; Italia, L.; Raffo, M.; Tomasoni, D.; Cani, D.S.; Cerini, M.; Farina, D.; Gavazzi, E.; et al. Cardiac Involvement in a Patient With Coronavirus Disease 2019 (COVID-19). JAMA Cardiol. 2020, 5, 819–824. [Google Scholar] [CrossRef] [Green Version]
- CZARSKA-THORLEY, D. EMA Endorses Use of Dexamethasone in COVID-19 Patients on Oxygen or Mechanical Ventilation. Available online: https://www.ema.europa.eu/en/news/ema-endorses-use-dexamethasone-covid-19-patients-oxygen-mechanical-ventilation (accessed on 10 April 2021).
- Salute, M. Della Covid-19, Indicazioni per L’assistenza a Domicilio e per L’accesso dei Visitatori Nelle Strutture Residenziali Sociosanitarie. Available online: http://www.salute.gov.it/portale/news/p3_2_1_1_1.jsp?lingua=italiano&menu=notizie&p=dalministero&id=5201 (accessed on 10 April 2021).
- Arachchillage, D.R.J.; Laffan, M. Abnormal Coagulation Parameters Are Associated with Poor Prognosis in Patients with Novel Coronavirus Pneumonia. J. Thromb. Haemost. 2020, 18, 1233–1234. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant Treatment Is Associated with Decreased Mortality in Severe Coronavirus Disease 2019 Patients with Coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Balzanelli, M.G.; Distratis, P.; Catucci, O.; Cefalo, A.; Lazzaro, R.; Inchingolo, F.; Tomassone, D.; Aityan, S.K.; Ballini, A.; Nguyen, K.C.D.; et al. Mesenchymal Stem Cells: The Secret Children’s Weapons against the SARS-CoV-2 Lethal Infection. Appl. Sci. 2021, 6, 1696. [Google Scholar] [CrossRef]
- Galluccio, F.; Ergonenc, T.; Garcia Martos, A.; Allam, A.E.-S.; Pérez-Herrero, M.; Aguilar, R.; Emmi, G.; Spinicci, M.; Terrancle Juan, I.; Fajardo-Pérez, M. Treatment Algorithm for COVID-19: A Multidisciplinary Point of View. Clin. Rheumatol. 2020, 39, 2077–2084. [Google Scholar] [CrossRef]
- Lang, J.; Yang, N.; Deng, J.; Liu, K.; Yang, P.; Zhang, G.; Jiang, C. Inhibition of SARS Pseudovirus Cell Entry by Lactoferrin Binding to Heparan Sulfate Proteoglycans. PLoS ONE 2011, 6, e23710. [Google Scholar] [CrossRef] [PubMed]
- Clausen, T.M.; Sandoval, D.R.; Spliid, C.B.; Pihl, J.; Painter, C.D.; Thacker, B.E.; Glass, C.A.; Narayanan, A.; Majowicz, S.A.; Zhang, Y.; et al. SARS-CoV-2 Infection Depends on Cellular Heparan Sulfate and ACE2. bioRxiv 2020. [Google Scholar] [CrossRef]
- Tandon, R.; Sharp, J.S.; Zhang, F.; Pomin, V.H.; Ashpole, N.M.; Mitra, D.; McCandless, M.G.; Jin, W.; Liu, H.; Sharma, P.; et al. Effective Inhibition of SARS-CoV-2 Entry by Heparin and Enoxaparin Derivatives. J. Virol. 2021, 95. [Google Scholar] [CrossRef]
- Mycroft-West, C.J.; Su, D.; Pagani, I.; Rudd, T.R.; Elli, S.; Gandhi, N.S.; Guimond, S.E.; Miller, G.J.; Meneghetti, M.C.Z.; Nader, H.B.; et al. Heparin Inhibits Cellular Invasion by SARS-CoV-2: Structural Dependence of the Interaction of the Spike S1 Receptor-Binding Domain with Heparin. Thromb. Haemost. 2020, 120, 1700–1715. [Google Scholar] [CrossRef] [PubMed]
- Tree, J.A.; Turnbull, J.E.; Buttigieg, K.R.; Elmore, M.J.; Coombes, N.; Hogwood, J.; Mycroft-West, C.J.; Lima, M.A.; Skidmore, M.A.; Karlsson, R.; et al. Unfractionated Heparin Inhibits Live Wild Type SARS-CoV-2 Cell Infectivity at Therapeutically Relevant Concentrations. Br. J. Pharmacol. 2021, 178, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, C.; Pham, V.H. Autologous Peripheral Blood Stem Cells Increase the Telomere Length in Patient: A Case Report of 13 Patients. J. Stem Cell Res. Ther. 2016, 6. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Jiang, W.; Chen, L.; Xu, Z.; Zhang, Q.; Zhu, M.; Ye, P.; Li, H.; Yu, L.; Zhou, X.; et al. Evaluation of the Safety and Efficacy of Using Human Menstrual Blood-Derived Mesenchymal Stromal Cells in Treating Severe and Critically Ill COVID-19 Patients: An Exploratory Clinical Trial. Clin. Transl. Med. 2021, 11, e297. [Google Scholar] [CrossRef]
- Shi, L.; Huang, H.; Lu, X.; Yan, X.; Jiang, X.; Xu, R.; Wang, S.; Zhang, C.; Yuan, X.; Xu, Z.; et al. Effect of Human Umbilical Cord-Derived Mesenchymal Stem Cells on Lung Damage in Severe COVID-19 Patients: A Randomized, Double-Blind, Placebo-Controlled Phase 2 Trial. Signal Transduct. Target Ther. 2021, 6, 58. [Google Scholar] [CrossRef]
- Lanzoni, G.; Linetsky, E.; Correa, D.; Messinger Cayetano, S.; Alvarez, R.A.; Kouroupis, D.; Alvarez Gil, A.; Poggioli, R.; Ruiz, P.; Marttos, A.C.; et al. Umbilical Cord Mesenchymal Stem Cells for COVID-19 Acute Respiratory Distress Syndrome: A Double-Blind, Phase 1/2a, Randomized Controlled Trial. Stem Cells Transl. Med. 2021, 10, 660–673. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.; Xu, R.; Wang, S.; Xu, Z.; Zhang, C.; Li, Y.; Yang, T.; Shi, L.; Fu, J.; Jiang, T.; et al. Human Umbilical Cord-Derived Mesenchymal Stem Cell Therapy in Patients with COVID-19: A Phase 1 Clinical Trial. Signal Transduct. Target Ther. 2020, 5, 172. [Google Scholar] [CrossRef]
- Parhizkar Roudsari, P.; Alavi-Moghadam, S.; Payab, M.; Sayahpour, F.A.; Aghayan, H.R.; Goodarzi, P.; Mohamadi-Jahani, F.; Larijani, B.; Arjmand, B. Auxiliary Role of Mesenchymal Stem Cells as Regenerative Medicine Soldiers to Attenuate Inflammatory Processes of Severe Acute Respiratory Infections Caused by COVID-19. Cell Tissue Bank 2020, 21, 405–425. [Google Scholar] [CrossRef] [PubMed]
- Gargiulo, C.; Hai, N.T.; Nguyen, K.C.; Van Phuc, P.; Abe, K.; Flores, V.; Shiffman, M. Isolation and Characterization of Multipotent and Pluripotent Stem Cells from Human Peripheral Blood. Stem Cell Discov. 2015, 5, 19. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; Inchingolo, F.; Murmura, G.; Traini, T.; Piattelli, A.; Lorusso, F. Three-Dimensional Architecture and Mechanical Properties of Bovine Bone Mixed with Autologous Platelet Liquid, Blood, or Physiological Water: An In Vitro Study. Int. J. Mol. Sci. 2018, 19, 1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, S.; Pittenger, M.F. Human Mesenchymal Stem Cells Modulate Allogeneic Immune Cell Responses. Blood 2005, 105, 1815–1822. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Corcione, A.; Benvenuto, F.; Ferretti, E.; Giunti, D.; Cappiello, V.; Cazzanti, F.; Risso, M.; Gualandi, F.; Mancardi, G.L.; Pistoia, V. Human Mesenchymal Stem Cells Modulate B-Cell Functions. Blood 2006, 107, 367–372. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.-X.; Zhang, Y.; Liu, B.; Zhang, S.-X.; Wu, Y.; Yu, X.-D.; Mao, N. Human Mesenchymal Stem Cells Inhibit Differentiation and Function of Monocyte-Derived Dendritic Cells. Blood 2005, 105, 4120–4126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasmusson, I.; Uhlin, M.; Le Blanc, K.; Levitsky, V. Mesenchymal Stem Cells Fail to Trigger Effector Functions of Cytotoxic T Lymphocytes. J. Leukoc. Biol. 2007, 82, 887–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spaggiari, G.M.; Capobianco, A.; Becchetti, S.; Mingari, M.C.; Moretta, L. Mesenchymal Stem Cell-Natural Killer Cell Interactions: Evidence That Activated NK Cells Are Capable of Killing MSCs, Whereas MSCs Can Inhibit IL-2-Induced NK-Cell Proliferation. Blood 2006, 107, 1484–1490. [Google Scholar] [CrossRef]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Charrier, J.-B. Selecting a Relevant in Vitro Cell Model for Testing and Comparing the Effects of a Choukroun’s Platelet-Rich Fibrin (PRF) Membrane and a Platelet-Rich Plasma (PRP) Gel: Tricks and Traps. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodont. 2010, 110, 409–411, author reply 411–413. [Google Scholar] [CrossRef] [PubMed]
- Németh, K.; Leelahavanichkul, A.; Yuen, P.S.T.; Mayer, B.; Parmelee, A.; Doi, K.; Robey, P.G.; Leelahavanichkul, K.; Koller, B.H.; Brown, J.M.; et al. Bone Marrow Stromal Cells Attenuate Sepsis via Prostaglandin E(2)-Dependent Reprogramming of Host Macrophages to Increase Their Interleukin-10 Production. Nat. Med. 2009, 15, 42–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franquesa, M.; Mensah, F.K.; Huizinga, R.; Strini, T.; Boon, L.; Lombardo, E.; DelaRosa, O.; Laman, J.D.; Grinyó, J.M.; Weimar, W.; et al. Human Adipose Tissue-Derived Mesenchymal Stem Cells Abrogate Plasmablast Formation and Induce Regulatory B Cells Independently of T Helper Cells. Stem Cells 2015, 33, 880–891. [Google Scholar] [CrossRef] [Green Version]
- Tabera, S.; Pérez-Simón, J.A.; Díez-Campelo, M.; Sánchez-Abarca, L.I.; Blanco, B.; López, A.; Benito, A.; Ocio, E.; Sánchez-Guijo, F.M.; Cañizo, C.; et al. The Effect of Mesenchymal Stem Cells on the Viability, Proliferation and Differentiation of B-Lymphocytes. Haematologica 2008, 93, 1301–1309. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; De Vito, D.; Martelli, F.S.; Georgakopoulos, I.; Almasri, M.; Dibello, V.; Altini, V.; Farronato, G.; Dipalma, G.; et al. Characterization of Human Apical Papilla-Derived Stem Cells. J. Biol. Regul. Homeost. Agents 2017, 31, 901–910. [Google Scholar]
- Ballini, A.; Cantore, S.; Scacco, S.; Perillo, L.; Scarano, A.; Aityan, S.K.; Contaldo, M.; Cd Nguyen, K.; Santacroce, L.; Syed, J.; et al. A Comparative Study on Different Stemness Gene Expression between Dental Pulp Stem Cells vs. Dental Bud Stem Cells. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 1626–1633. [Google Scholar] [CrossRef]
- Covid, Chi Sono i Long-Haulers: Infettati Non Ospedalizzati Che Non Riescono a Guarire. Le Ipotesi per Migliorare Le Loro Condizioni. Available online: Https://Www.Ilfattoquotidiano.It/2020/12/02/Covid-Chi-Sono-i-Long-Haulers-Infettati-Non-Ospedalizzati-Che-Non-Riescono-a-Guarire-Le-Ipotesi-per-Migliorare-Le-Loro-Condizioni/6021171/ (accessed on 23 May 2021).
- Montoya, J.G.; Anderson, J.N.; Adolphs, D.L.; Bateman, L.; Klimas, N.; Levine, S.M.; Garvert, D.; Kaiser, J.D. KPAX002 as a Treatment for Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS): A Prospective, Randomized Trial. Int. J. Clin. Exp. Med. 2018, 11, 2890–2900. [Google Scholar]
- Comhaire, F. Treating Patients Suffering from Myalgic Encephalopathy/Chronic Fatigue Syndrome (ME/CFS) with Sodium Dichloroacetate: An Open-Label, Proof-of-Principle Pilot Trial. Med. Hypotheses 2018, 114, 45–48. [Google Scholar] [CrossRef] [PubMed]
- Carr, A.C. A New Clinical Trial to Test High-Dose Vitamin C in Patients with COVID-19. Crit. Care 2020, 24, 133. [Google Scholar] [CrossRef] [Green Version]
- Rubin, R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA 2020, 324, 1381–1383. [Google Scholar] [CrossRef]
- Charitos, I.A.; Del Prete, R.; Inchingolo, F.; Mosca, A.; Carretta, D.; Ballini, A.; Santacroce, L. What We Have Learned for the Future about COVID-19 and Healthcare Management of It? Acta Biomed. 2020. [Google Scholar] [CrossRef]
- Baig, A.M. Chronic COVID Syndrome: Need for an Appropriate Medical Terminology for Long-COVID and COVID Long-Haulers. J. Med. Virol. 2020. [Google Scholar] [CrossRef]
- Gavriatopoulou, M.; Korompoki, E.; Fotiou, D.; Ntanasis-Stathopoulos, I.; Psaltopoulou, T.; Kastritis, E.; Terpos, E.; Dimopoulos, M.A. Organ-Specific Manifestations of COVID-19 Infection. Clin. Exp. Med. 2020, 20, 493–506. [Google Scholar] [CrossRef]
- Soto, M.E.; Guarner-Lans, V.; Soria-Castro, E.; Manzano Pech, L.; Pérez-Torres, I. Is Antioxidant Therapy a Useful Complementary Measure for Covid-19 Treatment? An Algorithm for Its Application. Medicina 2020, 56, 386. [Google Scholar] [CrossRef] [PubMed]
- Signorini, L. Probiotics May Modulate the Impact of Aging on Adults. J. Biol. Regul. Homeost. Agents 2020, 34. [Google Scholar] [CrossRef]
- Maestri, E.; Formoso, G.; Da Cas, R.; Mammarella, F.; Guerrizio, M.A.; Trotta, F. Vitamina D e Coronavirus: Un Nuovo Campo Di Impiego? Rec. Progr. Med. 2020, 111, 253–256. [Google Scholar]
- Alshahrani, F.; Aljohani, N. Vitamin D: Deficiency, Sufficiency and Toxicity. Nutrients 2013, 5, 3605–3616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aygun, H. Vitamin D Can Prevent COVID-19 Infection-Induced Multiple Organ Damage. Naunyn Schmiedebergs Arch. Pharmacol. 2020, 393, 1157–1160. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F.; Martelli, F.S.; Gargiulo Isacco, C.; Borsani, E.; Cantore, S.; Corcioli, F.; Boddi, A.; Nguyễn, K.C.D.; De Vito, D.; Aityan, S.K.; et al. Chronic Periodontitis and Immunity, Towards the Implementation of a Personalized Medicine: A Translational Research on Gene Single Nucleotide Polymorphisms (SNPs) Linked to Chronic Oral Dysbiosis in 96 Caucasian Patients. Biomedicines 2020, 8, 115. [Google Scholar] [CrossRef]
- Alesci, A.; Aragona, M.; Cicero, N.; Lauriano, E.R. Can Nutraceuticals Assist Treatment and Improve Covid-19 Symptoms? Nat. Prod. Res. 2021, 1–20. [Google Scholar] [CrossRef]
- Kritis, P.; Karampela, I.; Kokoris, S.; Dalamaga, M. The Combination of Bromelain and Curcumin as an Immune-Boosting Nutraceutical in the Prevention of Severe COVID-19. Metabol. Open 2020, 8, 100066. [Google Scholar] [CrossRef]
- Poe, F.L.; Corn, J. N-Acetylcysteine: A Potential Therapeutic Agent for SARS-CoV-2. Med. Hypoth. 2020, 109862. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, A.; Muecksch, F.; Lorenzi, J.C.C.; Leist, S.R.; Cipolla, M.; Bournazos, S.; Schmidt, F.; Gazumyan, A.; Baric, R.S.; Robbiani, D.F.; et al. Antibody Potency, Effector Function and Combinations in Protection from SARS-CoV-2 Infection in Vivo. bioRxiv 2020. [Google Scholar] [CrossRef]
- Afrin, L.B.; Weinstock, L.B.; Molderings, G.J. Covid-19 Hyperinflammation and Post-Covid-19 Illness May Be Rooted in Mast Cell Activation Syndrome. Int J. Infect. Dis. 2020, 100, 327–332. [Google Scholar] [CrossRef] [PubMed]
- Mazurier, J.; Spik, G. Comparative Study of the Iron-Binding Properties of Human Transferrins. I. Complete and Sequential Iron Saturation and Desaturation of the Lactotransferrin. Biochim. Biophys. Acta 1980, 629, 399–408. [Google Scholar] [CrossRef]
- Baker, E.N. Structure and Reactivity of Transferrins. In Advances in Inorganic Chemistry; Sykes, A.G., Ed.; Academic Press: Cambridge, MA, USA, 1994; Volume 41, pp. 389–463. [Google Scholar]
- Ganz, T. Iron and Infection. Int. J. Hematol. 2018, 107, 7–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peroni, D.G.; Fanos, V. Lactoferrin Is an Important Factor When Breastfeeding and COVID-19 Are Considered. Acta Paediatr. 2020. [Google Scholar] [CrossRef] [PubMed]
- Frontiers|The Biology of Lactoferrin, an Iron-Binding Protein That Can Help Defend Against Viruses and Bacteria|Immunology. Available online: https://www.frontiersin.org/articles/10.3389/fimmu.2020.01221/full (accessed on 28 March 2021).
- Rosa, L.; Cutone, A.; Lepanto, M.S.; Paesano, R.; Valenti, P. Lactoferrin: A Natural Glycoprotein Involved in Iron and Inflammatory Homeostasis. Int. J. Mol. Sci. 2017, 18, 1985. [Google Scholar] [CrossRef] [PubMed]
- Lepanto, M.S.; Rosa, L.; Paesano, R.; Valenti, P.; Cutone, A. Lactoferrin in Aseptic and Septic Inflammation. Molecules 2019, 24, 1323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manzoni, P.; Stolfi, I.; Messner, H.; Cattani, S.; Laforgia, N.; Romeo, M.G.; Bollani, L.; Rinaldi, M.; Gallo, E.; Quercia, M.; et al. Bovine Lactoferrin Prevents Invasive Fungal Infections in Very Low Birth Weight Infants: A Randomized Controlled Trial. Pediatrics 2012, 129, 116–123. [Google Scholar] [CrossRef]
- Enteral Lactoferrin Supplementation for Very Preterm Infants: A Randomised Placebo-Controlled Trial. Lancet 2019, 393, 423–433. [CrossRef] [Green Version]
- Li, F. Evidence for a Common Evolutionary Origin of Coronavirus Spike Protein Receptor-Binding Subunits. J. Virol. 2012, 86, 2856–2858. [Google Scholar] [CrossRef] [Green Version]
- Hasan, A.; Paray, B.A.; Hussain, A.; Qadir, F.A.; Attar, F.; Aziz, F.M.; Sharifi, M.; Derakhshankhah, H.; Rasti, B.; Mehrabi, M.; et al. A Review on the Cleavage Priming of the Spike Protein on Coronavirus by Angiotensin-Converting Enzyme-2 and Furin. J. Biomol. Struct. Dynam. 2020, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.-Y.; Xie, X.-L.; Peng, Y.-G.; Wu, M.-J.; Deng, X.-Z.; Wu, Y.; Xiong, L.-J.; Shang, L.-H. From SARS to COVID-19: What We Have Learned about Children Infected with COVID-19. Int J. Infect. Dis. 2020, 96, 710–714. [Google Scholar] [CrossRef]
- Bordea, I.R.; Xhajanka, E.; Candrea, S.; Bran, S.; Onișor, F.; Inchingolo, A.D.; Malcangi, G.; Pham, V.H.; Inchingolo, A.M.; Scarano, A.; et al. Coronavirus (SARS-CoV-2) Pandemic: Future Challenges for Dental Practitioners. Microorganisms 2020, 8, 1704. [Google Scholar] [CrossRef] [PubMed]
- Oliva, S.; Cucchiara, S.; Locatelli, F. Children and Fecal SARS-CoV-2 Shedding: Just the Tip of the Iceberg of Italian COVID-19 Outbreak? Dig. Liver Dis. 2020, 52, 1219–1221. [Google Scholar] [CrossRef] [PubMed]
- Inchingolo, F. Probiotics and EpiCor® in Human Health. J. Biol. Regul. Homeost. Agents 2019, 33. [Google Scholar] [CrossRef]
- Cantore, S.; Ballini, A.; De Vito, D.; Abbinante, A.; Altini, V.; Dipalma, G.; Inchingolo, F.; Saini, R. Clinical Results of Improvement in Periodontal Condition by Administration of Oral Probiotics. J. Biol. Regul. Homeost. Agents 2018, 32, 1329–1334. [Google Scholar] [PubMed]
- Dental Supplement; Gargiulo Isacco, C.; Ballini, A.; Paduanelli, G.; Inchingolo, A.D.; Nguyen, K.C.D.; Inchingolo, A.M.; Pham, V.H.; Aityan, S.K.; Schiffman, M.; et al. Bone Decay and beyond: How Can We Approach It Better. J. Biol. Regul. Homeost. Agents 2019, 33, 143–154. [Google Scholar]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Ballini, A.; Scacco, S.; Boccellino, M.; Santacroce, L.; Arrigoni, R. Microbiota and Obesity: Where Are We Now? Biology 2020, 9, 415. [Google Scholar] [CrossRef]
- Polimeno, L.; Barone, M.; Mosca, A.; Viggiani, M.T.; Joukar, F.; Mansour-Ghanaei, F.; Mavaddati, S.; Daniele, A.; Debellis, L.; Bilancia, M.; et al. Soy Metabolism by Gut Microbiota from Patients with Precancerous Intestinal Lesions. Microorganisms 2020, 8, 469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Santacroce, L.; Mavaddati, S.; Hamedi, J.; Zeinali, B.; Ballini, A.; Bilancia, M. Expressive Analysis of Gut Microbiota in Pre- and Post- Solid Organ Transplantation Using Bayesian Topic Models. In Computational Science and Its Applications—ICCSA 2020; Gervasi, O., Murgante, B., Misra, S., Garau, C., Blečić, I., Taniar, D., Apduhan, B.O., Rocha, A.M.A.C., Tarantino, E., Torre, C.M., et al., Eds.; Lecture Notes in Computer Science; Springer International Publishing: Cham, Switzerland, 2020; Volume 12252, pp. 150–165. ISBN 978-3-030-58810-6. [Google Scholar]
- Polimeno, L.; Barone, M.; Mosca, A.; Viggiani, M.T.; Di Leo, A.; Debellis, L.; Troisi, M.; Daniele, A.; Santacroce, L. Gut Microbiota Imbalance Is Related to Sporadic Colorectal Neoplasms. A Pilot Study. Appl. Sci. 2019, 9, 5491. [Google Scholar] [CrossRef] [Green Version]
- D’Elia, R.V.; Harrison, K.; Oyston, P.C.; Lukaszewski, R.A.; Clark, G.C. Targeting the “Cytokine Storm” for Therapeutic Benefit. Clin Vaccine Immunol 2013, 20, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Dohan Ehrenfest, D.M.; Del Corso, M.; Inchingolo, F.; Sammartino, G.; Charrier, J.-B. Platelet-Rich Plasma (PRP) and Platelet-Rich Fibrin (PRF) in Human Cell Cultures: Growth Factor Release and Contradictory Results. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodont. 2010, 110, 418–421, author reply 421–422. [Google Scholar] [CrossRef]
- D’Esposito, V.; Passaretti, F.; Perruolo, G.; Ambrosio, M.R.; Valentino, R.; Oriente, F.; Raciti, G.A.; Nigro, C.; Miele, C.; Sammartino, G.; et al. Platelet-Rich Plasma Increases Growth and Motility of Adipose Tissue-Derived Mesenchymal Stem Cells and Controls Adipocyte Secretory Function. J. Cell Biochem. 2015, 116, 2408–2418. [Google Scholar] [CrossRef] [Green Version]
- Kawakami, H.; Park, H.; Park, S.; Kuwata, H.; Shephard, R.J.; Aoyagi, Y. Effects of Enteric-Coated Lactoferrin Supplementation on the Immune Function of Elderly Individuals: A Randomised, Double-Blind, Placebo-Controlled Trial. Int. Dairy J. 2015, 47, 79–85. [Google Scholar] [CrossRef]
- Borodina, I.; Kenny, L.C.; McCarthy, C.M.; Paramasivan, K.; Pretorius, E.; Roberts, T.J.; van der Hoek, S.A.; Kell, D.B. The Biology of Ergothioneine, an Antioxidant Nutraceutical. Nutr. Res. Rev. 2020, 33, 190–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takeuchi, T.; Jyonotsuka, T.; Kamemori, N.; Kawano, G.; Shimizu, H.; Ando, K.; Harada, E. Enteric-Formulated Lactoferrin Was More Effectively Transported into Blood Circulation from Gastrointestinal Tract in Adult Rats. Exp. Physiol. 2006, 91, 1033–1040. [Google Scholar] [CrossRef] [PubMed]
- Lungs as Target of COVID-19 Infection: Protective Common Molecular Mechanisms of Vitamin D and Melatonin as a New Potential Synergistic Treatment. Available online: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7227533/ (accessed on 14 February 2021).
- Martineau, A.R.; Jolliffe, D.A.; Hooper, R.L.; Greenberg, L.; Aloia, J.F.; Bergman, P.; Dubnov-Raz, G.; Esposito, S.; Ganmaa, D.; Ginde, A.A.; et al. Vitamin D Supplementation to Prevent Acute Respiratory Tract Infections: Systematic Review and Meta-Analysis of Individual Participant Data. BMJ 2017, 356. [Google Scholar] [CrossRef] [Green Version]
- Ilie, P.C.; Stefanescu, S.; Smith, L. The Role of Vitamin D in the Prevention of Coronavirus Disease 2019 Infection and Mortality. Aging Clin. Exp. Res. 2020, 1–4. [Google Scholar] [CrossRef]
- Biesalski, H. Vitamin D Deficiency and Co-Morbidities in COVID-19 Patients—A Fatal Relationship? NFS J. 2020, 20. [Google Scholar] [CrossRef]
- Ali, N. Role of Vitamin D in Preventing of COVID-19 Infection, Progression and Severity. J. Infect. Public Health 2020, 13, 1373–1380. [Google Scholar] [CrossRef]
- Griffin, G.; Hewison, M.; Hopkin, J.; Kenny, R.A.; Quinton, R.; Rhodes, J.; Subramanian, S.; Thickett, D. Preventing Vitamin D Deficiency during the COVID-19 Pandemic: UK Definitions of Vitamin D Sufficiency and Recommended Supplement Dose Are Set Too Low. Clin. Med. 2021, 21, e48–e51. [Google Scholar] [CrossRef]
- Arboleda, J.F.; Urcuqui-Inchima, S. Vitamin D Supplementation: A Potential Approach for Coronavirus/COVID-19 Therapeutics? Front. Immunol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.A.; Bearden, A.; Striker, R. Vitamin D and the Anti-Viral State. J Clin Virol 2011, 50, 194–200. [Google Scholar] [CrossRef]
- Greiller, C.L.; Martineau, A.R. Modulation of the Immune Response to Respiratory Viruses by Vitamin D. Nutrients 2015, 7, 4240–4270. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inchingolo, A.D.; Inchingolo, A.M.; Bordea, I.R.; Malcangi, G.; Xhajanka, E.; Scarano, A.; Lorusso, F.; Farronato, M.; Tartaglia, G.M.; Isacco, C.G.; et al. SARS-CoV-2 Disease Adjuvant Therapies and Supplements Breakthrough for the Infection Prevention. Microorganisms 2021, 28, 525. [Google Scholar] [CrossRef]
- Zemb, P.; Bergman, P.; Camargo, C.A.; Cavalier, E.; Cormier, C.; Courbebaisse, M.; Hollis, B.; Joulia, F.; Minisola, S.; Pilz, S.; et al. Vitamin D Deficiency and the COVID-19 Pandemic. J. Glob. Antimicrob. Resist. 2020, 22, 133–134. [Google Scholar] [CrossRef] [PubMed]
- Manson, J.E.; Cook, N.R.; Lee, I.-M.; Christen, W.; Bassuk, S.S.; Mora, S.; Gibson, H.; Gordon, D.; Copeland, T.; D’Agostino, D.; et al. Vitamin D Supplements and Prevention of Cancer and Cardiovascular Disease. N. Engl. J. Med. 2019, 380, 33–44. [Google Scholar] [CrossRef]
- Pittas, A.G.; Dawson-Hughes, B.; Sheehan, P.; Ware, J.H.; Knowler, W.C.; Aroda, V.R.; Brodsky, I.; Ceglia, L.; Chadha, C.; Chatterjee, R.; et al. Vitamin D Supplementation and Prevention of Type 2 Diabetes. N. Engl. J. Med. 2019, 381, 520–530. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Signorini, L.; Ballini, A.; Arrigoni, R.; Leonardis, F.D.; Saini, R.; Cantore, S.; Vito, D.D.; Coscia, M.F.; Dipalma, G.; Inchingolo, L.S.F. Evaluation of a Nutraceutical Product with Probiotics, Vitamin d, Plus Banaba Leaf Extracts (Lagerstroemia Speciosa) in Glycemic Control. Available online: https://www.eurekaselect.com/187708/article (accessed on 28 November 2020).
- Melatonina. Available online: https://www.my-personaltrainer.it/fisiologia/ormoni/melatonina.html (accessed on 15 February 2021).
- Hardeland, R. Aging, Melatonin, and the Pro- and Anti-Inflammatory Networks. Int. J. Mol. Sci. 2019, 20, 1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melatonin Alleviates Radiation-Induced Lung Injury via Regulation of MiR-30e/NLRP3 Axis. Available online: https://www.hindawi.com/journals/omcl/2019/4087298/ (accessed on 15 February 2021).
- Miller, S.C.; Pandi, P.S.R.; Esquifino, A.I.; Cardinali, D.P.; Maestroni, G.J.M. The Role of Melatonin in Immuno-Enhancement: Potential Application in Cancer. Int. J. Exp. Pathol. 2006, 87, 81–87. [Google Scholar] [CrossRef]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A Narrative Review of the Histological and Histomorphometrical Evaluation of the Peri-Implant Bone in Loaded and Unloaded Dental Implants. A 30-Year Experience (1988–2018). Int. J. Environ. Res. Public Health 2020, 17, 2088. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tumedei, M.; Piattelli, A.; Degidi, M.; Mangano, C.; Iezzi, G. A 30-Year (1988–2018) Retrospective Microscopical Evaluation of Dental Implants Retrieved for Different Causes: A Narrative Review. Int. J. Periodont. Restor. Dent. 2020, 40, e211–e227. [Google Scholar] [CrossRef] [PubMed]
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant Primary Stability with an Osteocondensation Drilling Protocol in Different Density Polyurethane Blocks. Comp. Methods Biomech. Biomed. Eng. 2020, 1–7. [Google Scholar] [CrossRef]
- Fujiwara, S.; Kato, S.; Bengazi, F.; Urbizo Velez, J.; Tumedei, M.; Kotsu, M.; Botticelli, D. Healing at Implants Installed in Osteotomies Prepared Either with a Piezoelectric Device or Drills: An Experimental Study in Dogs. Oral Maxillofac. Surg. 2020. [Google Scholar] [CrossRef]
- Scarano, A.; Crincoli, V.; Di Benedetto, A.; Cozzolino, V.; Lorusso, F.; Podaliri Vulpiani, M.; Grano, M.; Kalemaj, Z.; Mori, G.; Grassi, F.R. Bone Regeneration Induced by Bone Porcine Block with Bone Marrow Stromal Stem Cells in a Minipig Model of Mandibular “Critical Size” Defect. Stem Cells Int. 2017, 2017, 9082869. [Google Scholar] [CrossRef]
- Scarano, A.; Lorusso, F.; Ravera, L.; Mortellaro, C.; Piattelli, A. Bone Regeneration in Iliac Crestal Defects: An Experimental Study on Sheep. BioMed Res. Int. 2016, 2016, 4086870. [Google Scholar] [CrossRef] [Green Version]
- Scarano, A.; de Oliveira, P.S.; Traini, T.; Lorusso, F. Sinus Membrane Elevation with Heterologous Cortical Lamina: A Randomized Study of a New Surgical Technique for Maxillary Sinus Floor Augmentation without Bone Graft. Materials 2018, 11, 1457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarano, A.; Murmura, G.; Vantaggiato, G.; Lauritano, D.; Silvestre-Rangil, J.; DI Cerbo, A.; Lorusso, F. Delayed Expansion of Atrophic Mandible (Deam): A Case Report. Oral Implantol. 2017, 10, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Kaur, C.; Ling, E.A. Effects of Melatonin on Macrophages/Microglia in Postnatal Rat Brain. J. Pineal Res. 1999, 26, 158–168. [Google Scholar] [CrossRef] [PubMed]
- Reiter, R.J.; Ma, Q.; Sharma, R. Treatment of Ebola and Other Infectious Diseases: Melatonin “Goes Viral”. Melatonin Res. 2020, 3, 43–57. [Google Scholar] [CrossRef]
- Sun, C.-K.; Lee, F.-Y.; Kao, Y.-H.; Chiang, H.-J.; Sung, P.-H.; Tsai, T.-H.; Lin, Y.-C.; Leu, S.; Wu, Y.-C.; Lu, H.-I.; et al. Systemic Combined Melatonin-Mitochondria Treatment Improves Acute Respiratory Distress Syndrome in the Rat. J. Pineal Res. 2015, 58, 137–150. [Google Scholar] [CrossRef] [PubMed]
- Lambert, D.W.; Clarke, N.E.; Hooper, N.M.; Turner, A.J. Calmodulin Interacts with Angiotensin-Converting Enzyme-2 (ACE2) and Inhibits Shedding of Its Ectodomain. FEBS Lett. 2008, 582, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Sehirli, A.O.; Sayiner, S.; Serakinci, N. Role of Melatonin in the Treatment of COVID-19; as an Adjuvant through Cluster Differentiation 147 (CD147). Mol. Biol. Rep. 2020, 47, 8229–8233. [Google Scholar] [CrossRef] [PubMed]
- Kuba, K.; Imai, Y.; Ohto-Nakanishi, T.; Penninger, J.M. Trilogy of ACE2: A Peptidase in the Renin–Angiotensin System, a SARS Receptor, and a Partner for Amino Acid Transporters. Pharmacol. Ther. 2010, 128, 119–128. [Google Scholar] [CrossRef]
- Feitosa, E.L.; Júnior, F.T.D.S.S.; Nery Neto, J.A.D.O.; Matos, L.F.L.; Moura, M.H.D.S.; Rosales, T.O.; De Freitas, G.B.L. COVID-19: Rational Discovery of the Therapeutic Potential of Melatonin as a SARS-CoV-2 Main Protease Inhibitor. Int. J. Med. Sci. 2020, 17, 2133–2146. [Google Scholar] [CrossRef]
- Haldar, C.; Yadav, R. Alipreeta, null Annual Reproductive Synchronization in Ovary and Pineal Gland Function of Female Short-Nosed Fruit Bat, Cynopterus Sphinx. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2006, 144, 395–400. [Google Scholar] [CrossRef]
- Rijal, S.; Cho, D.H.; Park, S.-A.; Jang, S.H.; Ábrahám, I.M.; Han, S.K. Melatonin Suppresses the Kainate Receptor-Mediated Excitation on Gonadotropin-Releasing Hormone Neurons in Female and Male Prepubertal Mice. Int. J. Mol. Sci. 2020, 21, 5991. [Google Scholar] [CrossRef]
- Ramírez-Rodríguez, G.B.; Olvera-Hernández, S.; Vega-Rivera, N.M.; Ortiz-López, L. Melatonin Influences Structural Plasticity in the Axons of Granule Cells in the Dentate Gyrus of Balb/C Mice. Int. J. Mol. Sci. 2019, 20, 73. [Google Scholar] [CrossRef] [Green Version]
- Perfilyeva, Y.V.; Ostapchuk, Y.O.; Abdolla, N.; Tleulieva, R.; Krasnoshtanov, V.C.; Belyaev, N.N. Exogenous Melatonin Up-Regulates Expression of CD62L by Lymphocytes in Aged Mice under Inflammatory and Non-Inflammatory Conditions. Immunol. Investig. 2019, 48, 632–643. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Melatonin and Chromatin. Melatonin Res. 2019, 2, 67–93. [Google Scholar] [CrossRef]
- Emet, M.; Ozcan, H.; Ozel, L.; Yayla, M.; Halici, Z.; Hacimuftuoglu, A. A Review of Melatonin, Its Receptors and Drugs. Euras. J. Med. 2016, 48, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Ren, J.; Zhang, A.-H.; Wang, X.-J. Traditional Chinese Medicine for COVID-19 Treatment. Pharmacol. Res. 2020, 155, 104743. [Google Scholar] [CrossRef] [PubMed]
- Bonucci, M. Prodotti Naturali per L’epidemia di Coronavirus: Il Punto di ARTOI sul Lianhua Qingwen|Oncologia e Covid19; ARTOI: Rome, Italy, 2020. [Google Scholar]
- Hu, K.; Guan, W.-J.; Bi, Y.; Zhang, W.; Li, L.; Zhang, B.; Liu, Q.; Song, Y.; Li, X.; Duan, Z.; et al. Efficacy and Safety of Lianhuaqingwen Capsules, a Repurposed Chinese Herb, in Patients with Coronavirus Disease 2019: A Multicenter, Prospective, Randomized Controlled Trial. Phytomedicine 2020, 153242. [Google Scholar] [CrossRef] [PubMed]
- Ding, Y.; Zeng, L.; Li, R.; Chen, Q.; Zhou, B.; Chen, Q.; Cheng, P.L.; Yutao, W.; Zheng, J.; Yang, Z.; et al. The Chinese Prescription Lianhuaqingwen Capsule Exerts Anti-Influenza Activity through the Inhibition of Viral Propagation and Impacts Immune Function. BMC Complement. Altern. Med. 2017, 17, 130. [Google Scholar] [CrossRef] [Green Version]
- Liu, M.; Gao, Y.; Yuan, Y.; Yang, K.; Shi, S.; Tian, J.; Zhang, J. Efficacy and Safety of Herbal Medicine (Lianhuaqingwen) for Treating COVID-19: A Systematic Review and Meta-Analysis. Integrat. Med. Res. 2021, 10, 100644. [Google Scholar] [CrossRef]
- Gamma Orizanolo. Available online: https://www.humanitas.it/enciclopedia/integratori-alimentari/gamma-orizanolo/ (accessed on 28 March 2021).
- Francisqueti-Ferron, F.V.; Garcia, J.L.; Ferron, A.J.T.; Nakandakare-Maia, E.T.; Gregolin, C.S.; das Silva, J.P.C.; Dos Santos, K.C.; Lo, Â.T.C.; Siqueira, J.S.; Corrêa, C.R.; et al. Gamma-Oryzanol as a Potential Modulator of Oxidative Stress and Inflammation via PPAR-y in Adipose Tissue: A Hypothetical Therapeutic for Cytokine Storm in COVID-19? Mol. Cell Endocrinol. 2021, 520, 111095. [Google Scholar] [CrossRef]
- Gamma Oryzanol Can Prevent Cytokine Storm in Covid Patients: Experts—Daijiworld.Com. Available online: https://www.daijiworld.com/news/newsDisplay?newsID=812071 (accessed on 28 March 2021).
- Rungratanawanich, W.; Cenini, G.; Mastinu, A.; Sylvester, M.; Wilkening, A.; Abate, G.; Bonini, S.A.; Aria, F.; Marziano, M.; Maccarinelli, G.; et al. γ-Oryzanol Improves Cognitive Function and Modulates Hippocampal Proteome in Mice. Nutrients 2019, 11, 753. [Google Scholar] [CrossRef] [Green Version]
- Borsani, E.; Bonazza, V.; Buffoli, B.; Nocini, P.F.; Albanese, M.; Zotti, F.; Inchingolo, F.; Rezzani, R.; Rodella, L.F. Beneficial Effects of Concentrated Growth Factors and Resveratrol on Human Osteoblasts In Vitro Treated with Bisphosphonates. Biomed. Res. Int. 2018, 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neves, A.R.; Lúcio, M.; Martins, S.; Lima, J.L.C.; Reis, S. Novel Resveratrol Nanodelivery Systems Based on Lipid Nanoparticles to Enhance Its Oral Bioavailability. Int. J. Nanomed. 2013, 8, 177–187. [Google Scholar] [CrossRef] [Green Version]
- Campanella, V.; Syed, J.; Santacroce, L.; Saini, R.; Ballini, A.; Inchingolo, F. Oral Probiotics Influence Oral and Respiratory Tract Infections in Pediatric Population: A Randomized Double-Blinded Placebo-Controlled Pilot Study. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 8034–8041. [Google Scholar] [CrossRef] [PubMed]
- Santacroce, L.; Charitos, I.A.; Ballini, A.; Inchingolo, F.; Luperto, P.; De Nitto, E.; Topi, S. The Human Respiratory System and Its Microbiome at a Glimpse. Biology 2020, 9, 318. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.-T.; Kwon, D.Y.; Park, O.J.; Kim, M.S. Resveratrol Protects ROS-Induced Cell Death by Activating AMPK in H9c2 Cardiac Muscle Cells. Genes Nutr. 2008, 2, 323–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baldassarre, M.E.; Di Mauro, A.; Labellarte, G.; Pignatelli, M.; Fanelli, M.; Schiavi, E.; Mastromarino, P.; Capozza, M.; Panza, R.; Laforgia, N. Resveratrol plus Carboxymethyl-β-Glucan in Infants with Common Cold: A Randomized Double-Blind Trial. Heliyon 2020, 6, e03814. [Google Scholar] [CrossRef]
- Ballini, A.; Signorini, L.; Inchingolo, A.D.; Saini, R.; Gnoni, A.; Scacco, S.; Cantore, S.; Dipalma, G.; Inchingolo, F.; Santacroce, L. Probiotics May Improve Serum Folate Availability in Pregnant Women: A Pilot Study. Open Access Maced. J. Med. Sci. 2020, 8, 1124–1130. [Google Scholar] [CrossRef]
- Lin, S.-C.; Ho, C.-T.; Chuo, W.-H.; Li, S.; Wang, T.T.; Lin, C.-C. Effective Inhibition of MERS-CoV Infection by Resveratrol. BMC Infect. Dis. 2017, 17, 144. [Google Scholar] [CrossRef] [Green Version]
- Chachay, V.S.; Macdonald, G.A.; Martin, J.H.; Whitehead, J.P.; O’Moore–Sullivan, T.M.; Lee, P.; Franklin, M.; Klein, K.; Taylor, P.J.; Ferguson, M.; et al. Resveratrol Does Not Benefit Patients With Nonalcoholic Fatty Liver Disease. Clin. Gastroenterol. Hepatol. 2014, 12, 2092–2103.e6. [Google Scholar] [CrossRef] [Green Version]
- Abba, Y.; Abu Hassim, H.; Hamzah, H.; Noordin, M.M. Antiviral Activity of Resveratrol against Human and Animal Viruses. Adv. Virol. 2015, 184241. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, M.G.; Pires, P.R.; Ribeiro, F.V.; Pimentel, S.P.; Cirano, F.R.; Napimoga, M.H.; Casati, M.Z.; Casarin, R.C.V. Systemic Treatment with Resveratrol Reduces the Progression of Experimental Periodontitis and Arthritis in Rats. PLoS ONE 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Tiao, M.-M.; Lin, Y.-J.; Yu, H.-R.; Sheen, J.-M.; Lin, I.-C.; Lai, Y.-J.; Tain, Y.-L.; Huang, L.-T.; Tsai, C.-C. Resveratrol Ameliorates Maternal and Post-Weaning High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease via Renin-Angiotensin System. Lipids Health Dis. 2018, 17. [Google Scholar] [CrossRef] [Green Version]
- Petti, S.; Scully, C. Polyphenols, Oral Health and Disease: A Review. J. Dent. 2009, 37, 413–423. [Google Scholar] [CrossRef]
- Daglia, M.; Papetti, A.; Grisoli, P.; Aceti, C.; Dacarro, C.; Gazzani, G. Antibacterial Activity of Red and White Wine against Oral Streptococci. J. Agric. Food Chem. 2007, 55, 5038–5042. [Google Scholar] [CrossRef]
- Miglino, N. Resveratrolo via Aerosol, dalla Campania una Speranza Contro Covid-19. Available online: http://www.nutrientiesupplementi.it/attualita/item/898-resveratrolo-via-aereosol-dalla-campania-una-speranza-contro-covid-19 (accessed on 30 March 2021).
- Kim, Y.C.; Dema, B.; Reyes-Sandoval, A. COVID-19 Vaccines: Breaking Record Times to First-in-Human Trials. NPJ Vacc. 2020, 5, 34. [Google Scholar] [CrossRef] [PubMed]
- Sheridan, C. COVID-19 Spurs Wave of Innovative Diagnostics. Nat. Biotechnol. 2020, 38, 769–772. [Google Scholar] [CrossRef]
- Puhan, M.A.; Schünemann, H.J.; Murad, M.H.; Li, T.; Brignardello-Petersen, R.; Singh, J.A.; Kessels, A.G.; Guyatt, G.H. GRADE Working Group A GRADE Working Group Approach for Rating the Quality of Treatment Effect Estimates from Network Meta-Analysis. BMJ 2014, 349, g5630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pham, V.H.; Gargiulo Isacco, C.; Nguyen, K.C.D.; Le, S.H.; Tran, D.K.; Nguyen, Q.V.; Pham, H.T.; Aityan, S.; Pham, S.T.; Cantore, S.; et al. Rapid and Sensitive Diagnostic Procedure for Multiple Detection of Pandemic Coronaviridae Family Members SARS-CoV-2, SARS-CoV, MERS-CoV and HCoV: A Translational Research and Cooperation between the Phan Chau Trinh University in Vietnam and University of Bari “Aldo Moro” in Italy. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 7173–7191. [Google Scholar] [CrossRef]
- Hoffmann, M.; Kleine-Weber, H.; Schroeder, S.; Krüger, N.; Herrler, T.; Erichsen, S.; Schiergens, T.S.; Herrler, G.; Wu, N.-H.; Nitsche, A.; et al. SARS-CoV-2 Cell Entry Depends on ACE2 and TMPRSS2 and Is Blocked by a Clinically Proven Protease Inhibitor. Cell 2020, 181, 271–280.e8. [Google Scholar] [CrossRef] [PubMed]
- Tay, M.Z.; Poh, C.M.; Rénia, L.; MacAry, P.A.; Ng, L.F.P. The Trinity of COVID-19: Immunity, Inflammation and Intervention. Nat. Rev. Immunol. 2020, 20, 363–374. [Google Scholar] [CrossRef]
- Baronio, M.; Freni-Sterrantino, A.; Pinelli, M.; Natalini, G.; Tonini, G.; Marri, M.; Baglivo, M.; Sabatini, T.; Maltese, P.E.; Chiurazzi, P.; et al. Italian SARS-CoV-2 Patients in Intensive Care: Towards an Identikit for Subjects at Risk? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9698–9704. [Google Scholar] [CrossRef]
- Li, G.; Kim, J.; Huang, Z.; St Clair, J.R.; Brown, D.A.; London, E. Efficient Replacement of Plasma Membrane Outer Leaflet Phospholipids and Sphingolipids in Cells with Exogenous Lipids. Proc. Natl. Acad. Sci. USA 2016, 113, 14025–14030. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baglivo, M.; Baronio, M.; Natalini, G.; Beccari, T.; Chiurazzi, P.; Fulcheri, E.; Petralia, P.P.; Michelini, S.; Fiorentini, G.; Miggiano, G.A.; et al. Natural Small Molecules as Inhibitors of Coronavirus Lipid-Dependent Attachment to Host Cells: A Possible Strategy for Reducing SARS-COV-2 Infectivity? SARS-COV-2 Lipid-Dependent Attachment to Host Cells. Acta Bio Med. Atenei Parmens. 2020, 91, 161–164. [Google Scholar] [CrossRef]
- Yamada, K.; Ogawa, H.; Hara, A.; Yoshida, Y.; Yonezawa, Y.; Karibe, K.; Nghia, V.B.; Yoshimura, H.; Yamamoto, Y.; Yamada, M.; et al. Mechanism of the Antiviral Effect of Hydroxytyrosol on Influenza Virus Appears to Involve Morphological Change of the Virus. Antivir. Res. 2009, 83, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Bertelli, M.; Kiani, A.K.; Paolacci, S.; Manara, E.; Kurti, D.; Dhuli, K.; Bushati, V.; Miertus, J.; Pangallo, D.; Baglivo, M.; et al. Hydroxytyrosol: A Natural Compound with Promising Pharmacological Activities. J. Biotechnol. 2020, 309, 29–33. [Google Scholar] [CrossRef] [PubMed]
- Ergoren, M.C.; Paolacci, S.; Manara, E.; Dautaj, A.; Dhuli, K.; Anpilogov, K.; Camilleri, G.; Suer, H.K.; Sayan, M.; Tuncel, G.; et al. A Pilot Study on the Preventative Potential of Alpha-Cyclodextrin and Hydroxytyrosol against SARS-CoV-2 Transmission. Acta Bio Med Atenei Parmens. 2020, 91, e2020022. [Google Scholar] [CrossRef]
- Dallavilla, T.; Bertelli, M.; Morresi, A.; Bushati, V.; Stuppia, L.; Beccari, T.; Chiurazzi, P.; Marceddu, G. Bioinformatic Analysis Indicates That SARS-CoV-2 Is Unrelated to Known Artificial Coronaviruses. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 4558–4564. [Google Scholar] [CrossRef] [PubMed]
- Welcome—European Biotechnology Thematic Network Association. Available online: https://www.ebtna.eu/haber/the-best-anti-covid-product-award-to-drmatte-8773 (accessed on 11 April 2021).







| Electronic Search Keywords and Indicators | |
|---|---|
| Keywords: | Advanced keyword search: ((“COVID-19” OR “2019-nCoV” OR “coronavirus” OR “SARS-CoV-2”) AND drugs) AND (Therapy/Narrow[filter]) |
| Databases | PubMed (Medline), EMBASE, Google Scholar, UpToDate, and Web of Science |
| Antiviral Drugs | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Chan et al. [51] | Lopinavir, Ritonavir | Cohort study | Lopinavir 400 mg/Ritonavir 100 mg oral administration twice a day. | Decrease in the death rate 2.3% and 0% intubation rate. |
| Chu et al. [52] | Lopinavir, Ritonavir | Cohort study | Lopinavir 400 mg/Ritonavir 100 mg oral administration twice a day for 2 weeks. | Mild adverse reactions, favorable clinical response of Lopinavir/Ritonavir therapy. |
| Lim et al. [53] | Lopinavir, Ritonavir | Clinical report | Lopinavir 200 mg/Ritonavir 50 mg oral administration twice a day for 2 weeks. | Lopinavir/Ritonavir is recommended for high-risk population groups for COVID-19. |
| Young et al. [54] | Lopinavir, Ritonavir | Clinical report | Lopinavir 400 mg/Ritonavir 100 mg oral administration twice a day for 2 weeks. | No subjects with a severe respiratory pathology/1 subject supplemental oxygen administration. |
| Cao et al. [55] | Lopinavir, Ritonavir | Randomized clinical trial | Lopinavir 400 mg/Ritonavir 100 mg oral administration. | Mortality rate similar between the Lopinavir–Ritonavir protocol and the control group, but shorter time of clinical improvement. |
| Furuta et al. [56] | Favipiravir | In vivo animal study | Oral administration 100 mg/kg four times a day. | Favipiravir useful and selective against influenza virus infections. |
| Furuta et al. [57] | Favipiravir | In vivo animal study | Oral administration 30 mg/kg/twice a day and four times a day. | Anti-viral activities against influenza H3N2, H3N2 or H5N1. |
| Sissoko et al. [58] | Favipiravir | non-randomized clinical trial | Oral administration loading dose: 6000 mg; dose: 2400 mg/d for 9 days. | Mean decrease in Ebola viral load of 0.33 log10 copies/mL/day. |
| Chinello et al. [59] | Favipiravir | Case report | Oral administration loading dose: 6000 mg; dose: 1.200 mg/d for 9 days. | Mean decrease in Ebola viral load of 0.33 log10 copies/mL/day. |
| Kumagai et al. [60] | Favipiravir | QT study | Single oral doses 1200 and 2400 mg. | No detectable effects on the QT/QTc interval. |
| Repurposed Drugs | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Chen et al. [89] | Hydroxychloroquine | Randomized clinical trial | Dose: Hydroxychloroquine 400 mg/d for 5 days oral administration | Shorter time to clinical recovery and promotion the absorption of pneumonia. |
| Gautret et al. [90] | Hydroxychloroquine, Azithromycin | Non-randomized clinical trial | Dose: Hydroxychloroquine 600mg/d azithromycin 250 mg/d oral administration | Azithromycin/Hydroxychloroquine protocol was more efficient for virus elimination. |
| Momekov et al. [81] | Ivermectin | Pharmacokinetic study | Dose: 150–800 µg/kg up to 2000 µg/kg oral administration | Concentrations not attainable against SARS-CoV-2 |
| Convalescent Plasma | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Hung et al. [113] | Convalescent Plasma | Prospective cohort study | Convalescent plasma-neutralizing antibody titer ≥ 1:160 | Reduced respiratory tract viral load, cytokine response; additionally, mortality against H1N1 |
| Kraft et al. [115] | Convalescent Plasma, TKM-100802 | Clinical reports | TKM-100802 infusion 0.3 mg/kg/convalescent plasma | No serious long-term sequelae against Ebola virus disease |
| van Griensven et al. [116] | Convalescent Plasma | Non-randomized clinical trial | Convalescent plasma 500 mL, unknown neutralizing antibodies titer | Higher cycle-threshold values, a shorter duration of symptoms of Ebola virus disease |
| Duan et al. [117] | Convalescent Plasma | Clinical reports | Convalescent plasma 200 mL-neutralizing antibody titers 1:640 | Decreased symptoms and increase in oxyhemoglobin saturation within 3 days in severe COVID-19 subjects |
| Immunomodulators | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Xu et al. [128] | Tocilizumab | Clinical trial | Lopinavir/Ritonavir 200/50 mg twice a day, oral administration; IFN-α (5 million unit/2 mL aerosol twice a day; tocilizumab dose load: 4–8 mg/kg to 400 mg intravenous administration | Improved the clinical outcome in severe cases of COVID-19, reduced mortality rate |
| Corticosteroids | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Arabi et al. [160] | Corticosteroids; Hydrocortisone-equivalent doses (methylprednisolone, 1:5; dexamethasone, 1:25; prednisolone, 1:4) | Multicenter clinical trial | Corticosteroid therapy initiation | No difference of 90-day mortality rate if associated with delayed MERS-CoV RNA clearance |
| RECOVERY Collaborative Group [161] | Dexamethasone Methylprednisolone Prednisone | Randomized clinical trial | Dose of 6 mg (eq. 160 mg hydrocortisone, 32 mg Methylprednisolone, 40 mg Prednisone) EV daily up for 10 days | The drug protocol produced a decrease in 28-day mortality in severe intensive therapy patients |
| Anticoagulants | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Tang et al. [167] | Low molecular weight heparin | Clinical trial | Low molecular weight heparin for at least 7 days | Better prognosis in severe COVID-19 patients |
| Stem Cells Treatment | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Gargiulo et al. [175] | Autologous peripheral blood stem cells | Case report | Anti-retroviral therapy plus stem cell therapy with 0.5 mL of human placenta for 4 and 1/2 months of treatment | Decreased HIV viral activity with a total recovery of pneumonia and skin infection. |
| Xu et al. [176] | Menstrual blood-derived MSCs | Clinical trial | Allogeneic, menstrual 9 × 107 blood-derived MSC therapy, and concomitant therapies at 0, 3, and 5 days | A significant decrease in dyspnea as early response. No difference in adverse events (AEs) between test and control group. |
| Shi et al. [177] | Umbilical cord-mesenchymal stem cells (UC-MSCs) | Randomized clinical trial | 4 × 107 UC-MSCs per infusion and concomitant therapies on days 0, 3, and 6 | Decrease in lung lesion and solid component lesion volume after the treatment. Similar adverse events ratio compared to the placebo. |
| Lanzoni et al. [178] | Umbilical cord-mesenchymal stem cells (UC-MSCs) | Randomized clinical trial | 100 ± 20 × 106 UC-MSCs per infusion at day 0 and day 3 and concomitant therapies | No difference in adverse events. A significant decrease in inflammatory cytokines in the test groups. |
| Meng et al. [179] | Umbilical cord-mesenchymal stem cells (UC-MSCs) | Randomized clinical trial | 3 × 107 UC-MSCs per infusion | UC-MSCs infusion in moderate and severe COVID-19 subjects is safe and well tolerated. |
| Adjuvants and Antioxidants | ||||
|---|---|---|---|---|
| Authors | Drug | Study Design | Administration Protocol | Results |
| Montoya et al. [195] | Low-dose methylphenidate hydrochloride with a mitochondrial modulator | Randomized clinical trial | Methylphenidate hydrochloride oral 5 mg twice a day/1 week-10 mg twice a day/2 weeks; Mitochondrial modulator, oral 4 tablets twice a day | Decrease in fatigue and concentration disturbance related to myalgic encephalomyelitis and chronic fatigue syndrome |
| Comhaire et al. [196] | Sodium dichloroacetate | Pilot study | Dosage oral one tablet/day for 1 month | Benefit of nutriceutical treatment by sodium dichloroacetate against myalgic encephalopathy/chronic fatigue syndrome symptoms |
| Carr et al. [197] | High-dose vitamin C | Clinical trial | Oral dosage 24 g/day of IV vitamin C/7 days | A reduction in symptoms in the high-dose treatment. No difference among the prognosis and other clinical outcomes of COVID-19 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inchingolo, A.D.; Dipalma, G.; Inchingolo, A.M.; Malcangi, G.; Santacroce, L.; D’Oria, M.T.; Isacco, C.G.; Bordea, I.R.; Candrea, S.; Scarano, A.; et al. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants 2021, 10, 881. https://doi.org/10.3390/antiox10060881
Inchingolo AD, Dipalma G, Inchingolo AM, Malcangi G, Santacroce L, D’Oria MT, Isacco CG, Bordea IR, Candrea S, Scarano A, et al. The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants. Antioxidants. 2021; 10(6):881. https://doi.org/10.3390/antiox10060881
Chicago/Turabian StyleInchingolo, Alessio Danilo, Gianna Dipalma, Angelo Michele Inchingolo, Giuseppina Malcangi, Luigi Santacroce, Maria Teresa D’Oria, Ciro Gargiulo Isacco, Ioana Roxana Bordea, Sebastian Candrea, Antonio Scarano, and et al. 2021. "The 15-Months Clinical Experience of SARS-CoV-2: A Literature Review of Therapies and Adjuvants" Antioxidants 10, no. 6: 881. https://doi.org/10.3390/antiox10060881