Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks
Abstract
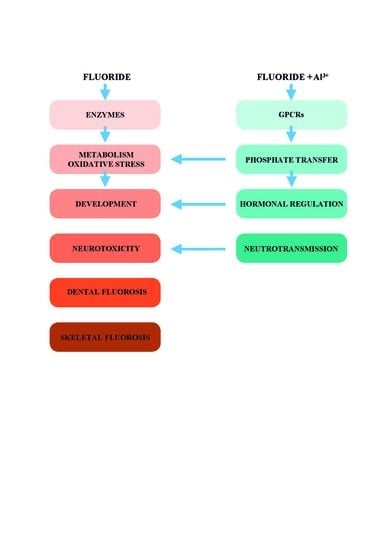
:1. Introduction
2. Methods
3. Mechanisms of Fluoride Toxicity on Cellular Level
3.1. Fluoride Inhibits Enzymes through Competition with Magnesium
3.2. Fluoride Inhibits Phosphoryl Transfer Reactions
3.3. Fluoride Inhibits Adenosine Triphosphatases by Multifactorial Mechanisms
3.4. What Do the Studies of Fluoride Effects on Enzymes In Vitro Tell Us?
3.5. Fluoride Effects on Transmembrane Signaling: A Link to Understanding Integrative Networks in Fluoride Toxicity
3.5.1. G Protein Signaling Cascade
3.5.2. Aluminofluoride Complexes
4. The Implication of Fluoride Toxicity on Human Health
4.1. Oxidative Stress
4.2. The Implication of Fluoride Interactions with G Protein-Coupled Receptors for Human Health
4.3. Disturbances of the Thyroid Gland
4.4. Melatonin
4.5. Fluoride-Induced Neurotoxicity
4.5.1. Mechanisms of Fluoride Neurotoxicity on the Cellular Level
4.5.2. Developmental Neurotoxicity of Fluoride in Children
4.5.3. The Role of Fluoride in the Etiopathogenesis of Autism Spectrum Disorder (ASD)
4.5.4. Fluoride in Fetal and Infant Development
4.6. The Potential Risk of Novel Fluorinated Drugs
5. The Economic Consequences of Fluoride Toxicity
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ghosh, A.; Mukherjee, K.; Ghosh, S.K.; Saha, B. Sources and toxicity of fluoride in the environment. Res. Chem. Intermed. 2013, 39, 2881–2915. [Google Scholar] [CrossRef]
- Vithanage, M.; Bhattacharya, P. Fluoride in the environment: Sources, distribution and defluoridation. Env. Chem. Lett. 2015, 13, 131–147. [Google Scholar] [CrossRef]
- Strunecká, A.; Patocka, J.; Blaylock, R.; Chinoy, N. Fluoride interactions: From molecules to disease. Curr. Signal. Transd. Ther. 2007, 2, 190–213. [Google Scholar] [CrossRef]
- Hirzy, J.; Connett, P.; Xiang, Q.; Spittle, B.; Kennedy, D. Developmental neurotoxicity of fluoride: A quantitative risk analysis toward establishing a safe dose for children. Fluoride 2016, 49, 379–400. [Google Scholar]
- Strunecká, A.; Strunecký, O.; Guan, Z. The resemblance of fluorosis pathology to that of autism spectrum disorder: A mini-review. Fluoride 2019, 52, 105–115. [Google Scholar]
- Saeed, M.; Malik, R.N.; Kamal, A. Fluorosis and cognitive development among children (6–14 years of age) in the endemic areas of the world: A review and critical analysis. Environ. Sci. Pollut. Res. Int. 2020, 27, 2566–2579. [Google Scholar] [CrossRef]
- Dean, H.T. Post-war implications of fluorine and dental health: Epidemiological aspects. J. Public Health 1944, 34, 133–143. [Google Scholar] [CrossRef]
- Arnold, F.A., Jr.; Dean, H.T.; Jay, P.; Knutson, J.W. Effect of fluoridated public water supplies on dental caries prevalence. Public Health Rep. 1956, 71, 652–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whelton, H.P.; Spencer, A.J.; Do, L.G.; Rugg-Gunn, A.J. Fluoride revolution and dental caries: Evolution of policies for global use. J. Dent. Res. 2019, 98, 837–846. [Google Scholar] [CrossRef] [PubMed]
- Fawell, J.; Bailey, K.; Chilton, J.; Dahi, E.; Fewtrell, L.; Magara, Y. Fluoride in Drinking-Water; World Health Organization: Geneva, Switzerland, 2006. [Google Scholar]
- EFSA Panel on Dietetic Products, Nutrition, and Allergies. Scientific opinion on dietary reference values for fluoride, 2013. EFSA J. 2013, 11, 3332. [Google Scholar]
- CDC. Basics of Oral Health. Available online: https://www.cdc.gov/oralhealth/basics/index.html (accessed on 1 September 2020).
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef]
- Dickens, F.; Simer, F. Observations on tissue glycolysis: The effect of fluoride and some other substances. Biochem. J. 1929, 23, 936–958. [Google Scholar] [CrossRef]
- Warburg, O.; Christian, W. Isolierung und Kristallisation des Gärungsferments Enolase. Naturwissenschaften 2005, 29, 589–590. [Google Scholar] [CrossRef]
- Brewer, J.M. Yeast enolase: Mechanism of activation by metal ions. CRC. Crit. Rev. Biochem. 1981, 11, 209–254. [Google Scholar] [CrossRef] [PubMed]
- Feig, S.A.; Segel, G.B.; Shohet, S.B.; Nathan, D.G. Energy metabolism in human erythrocytes. II. Effects of glucose depletion. J. Clin. Investig. 1972, 51, 1547–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weston, J. Biochemistry of Magnesium. In The Chemistry of Organomagnesium Compounds; Rappoport, Z., Ilan, M., Eds.; John Wiley & Sons Ltd.: West Sussex, UK, 2008; pp. 315–367. [Google Scholar] [CrossRef]
- Baxter, N.J.; Olguin, L.F.; Golicnik, M.; Feng, G.; Hounslow, A.M.; Bermel, W.; Blackburn, G.M.; Hollfelder, F.; Waltho, J.P.; Williams, N.H. A Trojan horse transition state analogue generated by MgF3− formation in an enzyme active site. Proc. Natl. Acad. Sci. USA 2006, 103, 14732–14737. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jin, Y.; Richards, N.G.; Waltho, J.P.; Blackburn, G.M. Metal fluorides as analogues for studies on phosphoryl transfer enzymes. Angew. Chem. Int. Ed. Engl. 2017, 56, 4110–4128. [Google Scholar] [CrossRef]
- Antonny, B.; Sukumar, M.; Bigay, J.; Chabre, M.; Higashijima, T. The mechanism of aluminum-independent G-protein activation by fluoride and magnesium. 31P NMR spectroscopy and fluorescence kinetic studies. J. Biol. Chem. 1993, 268, 2393–2402. [Google Scholar]
- Swanson, M.A. Phosphatases of the liver. III. “Neutral” pyrophosphatase. J. Biol. Chem. 1952, 194, 685–693. [Google Scholar]
- Baykov, A.A.; Cooperman, B.S.; Goldman, A.; Lahti, R. Cytoplasmic inorganic pyrophosphatase. In Inorganic Polyphosphates: Biochemistry, Biology, Biotechnology; Schröder, H.C., Müller, W.E.G., Eds.; Springer: Berlin/Heidelberg, Germany, 1999; pp. 127–150. [Google Scholar] [CrossRef]
- Kajander, T.; Kellosalo, J.; Goldman, A. Inorganic pyrophosphatases: One substrate, three mechanisms. FEBS Lett. 2013, 587, 1863–1869. [Google Scholar] [CrossRef] [Green Version]
- Waugh, D.T. Fluoride exposure induces inhibition of sodium-and potassium-activated adenosine triphosphatase (Na+, K+-ATPase) enzyme activity: Molecular mechanisms and implications for public health. Int. J. Environ. Res. Public Health 2019, 16, 1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, A.J.; Coll, R.J. Fluoride is a slow, tight-binding inhibitor of the calcium ATPase of sarcoplasmic reticulum. J. Biol. Chem. 1992, 267, 5229–5235. [Google Scholar] [PubMed]
- National Research Council. Fluoride in Drinking Water: A Scientific Review of EPA; Standards; The National Academies Press: Washington, DC, USA, 2006. [Google Scholar] [CrossRef]
- American Dental Association. ADA Fluoridation Policy. Available online: https://www.ada.org/en/public-programs/advocating-for-the-public/fluoride-and-fluoridation/ada-fluoridation-policy (accessed on 1 September 2020).
- Guth, S.; Hüser, S.; Roth, A.; Degen, G.; Diel, P.; Edlund, K.; Eisenbrand, G.; Engel, K.H.; Epe, B.; Grune, T.; et al. Toxicity of fluoride: Critical evaluation of evidence for human developmental neurotoxicity in epidemiological studies, animal experiments and in vitro analyses. Arch. Toxicol. 2020, 94, 1375–1415. [Google Scholar] [CrossRef] [PubMed]
- Rafique, T.; Ahmed, I.; Soomro, F.Y.; Khan, M.; Shirin, K. Fluoride levels in urine, blood plasma and serum of people living in an endemic fluorosis area in the Thar Desert, Pakistan. J. Chem. Soc. Pak. 2015, 37, 1223–1230. [Google Scholar]
- Burgstahler, A.W. Paradoxical dose-response effects of fluoride. Fluoride 2002, 35, 143–147. [Google Scholar]
- Zakrzewska, H.; Udała, J.; Błaszczyk, B. In vitro influence of sodium fluoride on ram semen quality and enzyme activities. Fluoride 2002, 35, 153–160. [Google Scholar]
- McQueen, C. Comprehensive Toxicology, 3rd ed.; McQueen, C., Ed.; Elsevier Science: Amsterdam, The Netherlands, 2017; pp. 77–82. [Google Scholar]
- Lau, K.H.; Farley, J.R.; Freeman, T.K.; Baylink, D.J. A proposed mechanism of the mitogenic action of fluoride on bone cells. Inhibition of the activity of an osteoblastic acid phosphatase. Metabolism 1989, 38, 858–868. [Google Scholar]
- Moonga, B.S.; Pazianas, M.; Alam, A.S.; Shankar, V.S.; Huang, C.L.; Zaidi, M. Stimulation of a Gs-like protein in the osteoclast inhibits bone resorption but enhances tartrate-resistant acid phosphatase secretion. Biochem. Biophys. Res. Commun. 1993, 190, 496–501. [Google Scholar] [CrossRef]
- Oguro, A.; Kawase, T.; Orikasa, M. NaF induces early differentiation of murine bone marrow cells along the granulocytic pathway but not the monocytic or preosteoclastic pathway in vitro. Cell. Dev. Biol. Anim. 2003, 39, 243–248. [Google Scholar] [CrossRef]
- Partanen, S. Inhibition of human renal acid phosphatases by nephrotoxic micromolar concentrations of fluoride. Exp. Toxicol. Pathol. 2002, 54, 231–237. [Google Scholar] [CrossRef]
- Arellano, M.; Malet-Martino, M.; Martino, R.; Spector, T. 5-Ethynyluracil (GW776): Effects on the formation of the toxic catabolites of 5-fluorouracil, fluoroacetate and fluorohydroxypropionic acid in the isolated perfused rat liver model. Br. J. Cancer 1997, 76, 1170–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackmore, P.F.; Bocckino, S.B.; Waynick, L.E.; Exton, J.H. Role of a guanine nucleotide-binding regulatory protein in the hydrolysis of hepatocyte phosphatidylinositol 4,5-bisphosphate by calcium-mobilizing hormones and the control of cell calcium. Studies utilizing aluminum fluoride. J. Biol. Chem. 1985, 260, 14477–14483. [Google Scholar] [PubMed]
- Magnaldo, I.; Pouyssegur, J.; Paris, S. Thrombin exerts a dual effect on stimulated adenylate cyclase in hamster fibroblasts, an inhibition via a GTP-binding protein and a potentiation via activation of protein kinase C. Biochem. J. 1988, 253, 711–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strunecká, A.; Patočka, J. Pharmacological and toxicological effects of aluminofluoride complexes. Fluoride 1999, 32, 230–242. [Google Scholar]
- Zhao, X.L.; Wu, J.H. Actions of sodium fluoride on acetylcholinesterase activities in rats. Biomed. Environ. Sci. 1998, 11, 1–6. [Google Scholar]
- Hara, K.; Yu, M. Effect of fluoride on human salivary amylase activity. Fluoride 1995, 28, 71–74. [Google Scholar]
- Tormanen, C.D. Substrate inhibition of rat liver and kidney arginase with fluoride. J. Inorg. Biochem. 2003, 93, 243–246. [Google Scholar] [CrossRef]
- Gutiérrez-Salinas, J.; García-Ortíz, L.; Morales González, J.A.; Hernández-Rodríguez, S.; Ramírez-García, S.; Núñez-Ramos, N.R.; Madrigal-Santillán, E. In vitro effect of sodium fluoride on malondialdehyde concentration and on superoxide dismutase, catalase, and glutathione peroxidase in human erythrocytes. Sci. World J. 2013, 2013, 864718. [Google Scholar] [CrossRef] [Green Version]
- Goschorska, M.; Gutowska, I.; Olszewska, M.; Baranowska-Bosiacka, I.; Rać, M.; Olszowski, T.; Chlubek, D. Effect of sodium fluoride on the catalase activity in THP-1 macrophages. Fluoride 2015, 48, 274–282. [Google Scholar]
- Stohr, J.; Novotny, J.; Bourova, L.; Svoboda, P. Modulation of adenylyl cyclase activity in young and adult rat brain cortex. Identification of suramin as a direct inhibitor of adenylyl cyclase. J. Cell. Mol. Med. 2005, 9, 940–952. [Google Scholar] [CrossRef]
- Lebioda, L.; Zhang, E.; Lewinski, K.; Brewer, J.M. Fluoride inhibition of yeast enolase: Crystal structure of the enolase-Mg2+-F−-Pi complex at 2.6 A resolution. Proteins 1993, 16, 219–225. [Google Scholar] [CrossRef] [PubMed]
- Feig, S.A.; Shohet, S.B.; Nathan, D.G. Energy metabolism in human erythrocytes. I. Effects of sodium fluoride. J. Clin. Investig. 1971, 50, 1731–1737. [Google Scholar] [CrossRef] [PubMed]
- Shahed, A.R.; Miller, A.; Allmann, D.W. Effect of fluorine containing compounds on the activity of glycolytic enzymes in rat hepatocytes. Biochem. Biophys. Res. Commun. 1980, 94, 901–908. [Google Scholar] [CrossRef]
- Nishikimi, A.; Uekawa, N.; Yamada, M. Involvement of glycolytic metabolism in developmental inhibition of rat two-cell embryos by phosphate. J. Exp. Zool. 2000, 287, 503–509. [Google Scholar] [CrossRef]
- Van Loveren, C. Antimicrobial activity of fluoride and its in vivo importance: Identification of research questions. Caries. Res. 2001, 35 (Suppl. 1), 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lunardi, J.; Dupuis, A.; Garin, J.; Issartel, J.P.; Michel, L.; Chabre, M.; Vignais, P.V. Inhibition of H+-transporting ATPase by formation of a tight nucleoside diphosphate-fluoroaluminate complex at the catalytic site. Proc. Natl. Acad. Sci. USA 1988, 85, 8958–8962. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lange, A.J.; Arion, W.J.; Burchell, A.; Burchell, B. Aluminum ions are required for stabilization and inhibition of hepatic microsomal glucose-6-phosphatase by sodium fluoride. J. Biol. Chem. 1986, 261, 101–107. [Google Scholar]
- Blackmore, P.; Exton, J. Studies on the hepatic calcium-mobilizing activity of aluminum fluoride and glucagon. Modulation by cAMP and phorbol myristate acetate. J. Biol. Chem. 1986, 261, 11056–11063. [Google Scholar]
- Zhang, B.; Wu, D. Effect of fluoride on proliferation and differentiation in rat and mouse embryo bud cell in vitro. Hua Xi Yi Ke Da Xue Xue Bao 1998, 29, 256–268. [Google Scholar]
- Loevenhart, A.S.; Peirce, G. The inhibiting effect of sodium fluoride on the action of lipase. J. Biol. Chem. 1907, 2, 397–413. [Google Scholar]
- McClure, F.J. A review of fluorine and its physiological effects. Physiol. Rev. 1933, 13, 277–300. [Google Scholar] [CrossRef]
- Ratz, P.H.; Lattanzio, F.A. L-type Ca2+ channel desensitization by F- reduces PhE-induced increase in [Ca2+]i but not stress. Am. J. Physiol. 1992, 262, C621–C627. [Google Scholar] [CrossRef] [PubMed]
- Ratz, P.H.; Blackmore, P.F. Differential activation of rabbit femoral arteries by aluminium fluoride and sodium fluoride. J. Pharmacol. Exp. Ther. 1990, 254, 514–520. [Google Scholar] [PubMed]
- Heikinheimo, P.; Tuominen, V.; Ahonen, A.K.; Teplyakov, A.; Cooperman, B.S.; Baykov, A.A.; Lahti, R.; Goldman, A. Toward a quantum-mechanical description of metal-assisted phosphoryl transfer in pyrophosphatase. Proc. Natl. Acad. Sci. USA 2001, 98, 3121–3126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saralakumari, D.; Rao, P.R. Red blood cell glucose metabolism in human chronic fluoride toxicity. Bull. Environ. Contam. Toxicol. 1991, 47, 834–839. [Google Scholar] [CrossRef]
- Slater, E.C.; Felberg, N.T.; Holler, T. The effect of fluoride on the succinic oxidase system. Biochem. J. 1952, 52, 185–196. [Google Scholar] [CrossRef]
- Sullivan, W. The in vitro and in vivo efects of fluoride on succinic dehydrogenase activity. Fluoride 1969, 2, 168–174. [Google Scholar]
- Sampson, E.J.; Baird, M.A. Chemical inhibition used in a kinetic urease/glutamate dehydrogenase method for urea in serum. Clin. Chem. 1979, 25, 1721–1729. [Google Scholar] [CrossRef]
- Sutherland, E.W.; Rall, T.W.; Menon, T. Adenyl cylase. I. Distribution, preparation, and properties. J. Biol. Chem. 1962, 237, 1220–1227. [Google Scholar]
- Hrbasova, M.; Novotny, J.; Hejnova, L.; Kolar, F.; Neckar, J.; Svoboda, P. Altered myocardial Gs protein and adenylyl cyclase signaling in rats exposed to chronic hypoxia and normoxic recovery. J. Appl. Physiol. 2003, 94, 2423–2432. [Google Scholar] [CrossRef]
- Nair, B.G.; Parikh, B.; Milligan, G.; Patel, T.B. Cardiac adenylate cyclase. J. Biol. Chem. 1990, 263, 21317–21322. [Google Scholar]
- Sternweis, P.C.; Gilman, A.G. Aluminum: A requirement for activation of the regulatory component of adenylate cyclase by fluoride. Proc. Natl. Acad. Sci. USA 1982, 79, 4888–4891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, J.; Sims, C.; Chang, C.H.; Berti Mattera, L.; Hopfer, U.; Douglas, J. Proximal tubular epithelial cells posses a novel 42-kDa guanine nucleotide - binding regulatory protein. Proc. Natl. Acad. Sci. USA 1990, 87, 7532–7535. [Google Scholar] [CrossRef] [Green Version]
- Chae, H.J.; Chae, S.W.; Kang, J.S.; Kim, D.E.; Kim, H.R. Mechanism of mitogenic effect of fluoride on fetal rat osteoblastic cells: Evidence for Shc, Grb2 and P-CREB-dependent pathways. Res. Commun. Mol. Pathol. Pharmacol. 1999, 105, 185–199. [Google Scholar] [PubMed]
- Hawkins, C.; Xu, A.; Narayanan, N. Comparison of the effects of fluoride on the calcium pumps of cardiac and fast skeletal muscle sarcoplasmic reticulum: Evidence for tissue-specific qualitative difference in calcium-induced pump conformation. J. Biol. Chem. 1994, 269, 31198–31206. [Google Scholar] [CrossRef]
- Yatani, A.; Brown, A.M. Mechanism of fluoride activation of G protein-gated muscarinic atrial K+ channels. J. Biol. Chem. 1991, 366, 22872–22877. [Google Scholar]
- Terzic, A.; Tung, R.T.; Inanobe, A.; Katada, T.; Kurachi, Y. G proteins activate ATP-sensitive K+ channels by antagonizing ATP-dependent gating. Neuron 1994, 12, 885–893. [Google Scholar] [CrossRef]
- Hughes, B.P.; Barritt, G.J. The stimulation by sodium fluoride of plasma-membrane Ca2+ inflow in isolated hepatocytes. Evidence that a GTP-binding regulatory protein is involved in the hormonal stimulation of Ca2+ inflow. Biochem. J. 1987, 245, 41–47. [Google Scholar] [CrossRef] [Green Version]
- Caverzasio, J.; Montessuit, C.H.; Bonjour, J.P. Functional role of Pi transport in osteogenic cells. News. Physiol. Sci. 1996, 11, 119–125. [Google Scholar] [CrossRef]
- Thomas, A.B.; Hashimoto, H.; Baylink, D.J.; Lau, K.H. Fluoride at mitogenic concentrations increases the steady state phosphotyrosyl phosphorylation level of cellular proteins in human bone cells. J. Clin. Endocrinol. Metab. 1996, 81, 2570–2578. [Google Scholar]
- Rall, T.W.; Sutherland, E.W. Formation of a cyclic adenine ribonucleotide by tissue particles. J. Biol. Chem. 1958, 232, 1065–1076. [Google Scholar] [PubMed]
- Rodbell, M. Signal transduction: Evolution of an idea. Environ. Health Perspect. 1995, 103, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Gilman, A.G. G proteins: Transducers of receptor-generated signals. Annu. Rev. Biochem. 1987, 56, 615–649. [Google Scholar] [CrossRef] [PubMed]
- Kleuss, C.; Raw, A.S.; Lee, E.; Sprang, S.R.; Gilman, A.G. Mechanism of GTP hydrolysis by G-protein alpha subunits. Proc. Natl. Acad. Sci. USA 1994, 91, 9828–9831. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprang, S.R. Invited review: Activation of G proteins by GTP and the mechanism of Galpha-catalyzed GTP hydrolysis. Biopolymers 2016, 105, 449–462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oldham, W.M.; Hamm, H.E. Heterotrimeric G protein activation by G-protein-coupled receptors. Nat. Rev. Mol. Cell. Biol. 2008, 9, 60–71. [Google Scholar] [CrossRef]
- Syrovatkina, V.; Alegre, K.O.; Dey, R.; Huang, X.Y. Regulation, signaling, and physiological functions of G-proteins. J. Mol. Biol. 2016, 428, 3850–3868. [Google Scholar] [CrossRef] [Green Version]
- Bigay, J.; Deterre, P.; Pfister, C.; Chabre, M. Fluoroaluminates activate transducin-GDP by mimicking the γ-phosphate of GTP in its binding site. FEBS Lett. 1985, 191, 181–185. [Google Scholar] [CrossRef] [Green Version]
- Sondek, J.; Lambright, D.G.; Noel, J.P.; Hamm, H.E.; Sigler, P.B. GTPase mechanism of Gproteins from the 1.7-A crystal structure of transducin alpha-GDP-AIF-4. Nature 1994, 372, 276–279. [Google Scholar] [CrossRef]
- Martin, R.B. Ternary hydroxide complexes in neutral solutions of Al3+ and F−. Biochem. Biophys. Res. Commun. 1988, 155, 1194–1200. [Google Scholar] [CrossRef]
- Xu, Y.W.; Moréra, S.; Janin, J.; Cherfils, J. AlF3 mimics the transition state of protein phosphorylation in the crystal structure of nucleoside diphosphate kinase and MgADP. Proc. Natl. Acad. Sci. USA 1997, 94, 3579–3583. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabre, M. Aluminofluoride and beryllofluoride complexes: New phosphate analogues in enzymology. Trends Biochem. Sci. 1990, 15, 6–10. [Google Scholar] [CrossRef]
- Waugh, D.T.; Godfrey, M.; Limeback, H.; Potter, W. Black tea source, production, and consumption: Assessment of health risks of fluoride intake in New Zealand. J. Environ. Public Health 2017, 2017, 5120504. [Google Scholar] [CrossRef] [PubMed]
- Hattab, F. An update on fluorides and fluorosis with reference to oral health status in the Gulf Region: Review. Asian. J. Dent. Sc. 2020, 3, 27–48. [Google Scholar]
- WHO. Guidelines for Drinking-Water Quality, 2nd Edition: Volume 1—Recommendations; World Health Organisation: Geneva, Switzerland, 1993. [Google Scholar]
- Neurath, C.; Limeback, H.; Osmunson, B.; Connett, M.; Kanter, V.; Wells, C.R. Dental fluorosis trends in US oral health surveys: 1986 to 2012. JDR Clin. Trans. Res. 2019, 4, 298–308. [Google Scholar] [CrossRef]
- Grandjean, P. Developmental fluoride neurotoxicity: An updated review. Environ. Health 2019, 18, 110. [Google Scholar] [CrossRef] [Green Version]
- Srivastava, S.; Flora, S.J.S. Fluoride in drinking water and skeletal fluorosis: A review of the global impact. Curr. Environ. Health Rep. 2020, 7, 140–146. [Google Scholar] [CrossRef]
- Waldbott, G.L.; Burgstahler, A.W.; McKinney, H.L. Fluoridation: The great dilemma. In Fluoridation: The Great Dilemma; Coronado Press: Lawrence, KS, USA, 1978; pp. 1–152. [Google Scholar]
- Varol, E.; Icli, A.; Aksoy, F.; Bas, H.A.; Sutcu, R.; Ersoy, I.H.; Varol, S.; Ozaydin, M. Evaluation of total oxidative status and total antioxidant capacity in patients with endemic fluorosis. Toxicol. Ind. Health 2013, 29, 175–180. [Google Scholar] [CrossRef]
- Barbier, O.; Arreola-Mendoza, L.; Del Razo, L.M. Molecular mechanisms of fluoride toxicity. Chem. Biol. Interact. 2010, 188, 319–333. [Google Scholar] [CrossRef]
- Ailani, V.; Gupta, R.C.; Gupta, S.K.; Gupta, K. Oxidative stress in cases of chronic fluoride intoxication. Indian J. Clin. Biochem. 2009, 24, 426–429. [Google Scholar] [CrossRef] [Green Version]
- Shivarajashankara, Y.M.; Shivarajashankara, A.R.; Bhat, P.G.; Rao, S.H. Effect of fluoridation intoxication on lipid peroxidation and antioxidant systems. Fluoride 2001, 34, 108–113. [Google Scholar]
- James, S.J.; Cutler, P.; Melnyk, S.; Jernigan, S.; Janak, L.; Gaylor, D.W.; Neubrander, J.A. Metabolic biomarkers of increased oxidative stress and impaired methylation capacity in children with autism. Am. J. Clin. Nutr. 2004, 80, 1611–1617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Strunecka, A.; Strunecky, O. Chronic fluoride exposure and the risk of autism spectrum disorder. Int. J. Environ. Res. Public Health 2019, 16, 3431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonola-Gallardo, I.; Irigoyen-Camacho, M.E.; Vera-Robles, L.; Campero, A.; Gómez-Quiroz, L. Enzymatic activity of glutathione s-transferase and dental fluorosis among children receiving two different levels of naturally fluoridated water. Biol. Trace. Elem. Res. 2017, 176, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Kostenis, E.; Pfeil, E.M.; Annala, S. Heterotrimeric Gq proteins as therapeutic targets? J. Biol. Chem. 2020, 295, 5206–5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, T. G-Protein coupled receptors: Answers from simulations. Beilstein J. Org. Chem. 2017, 13, 1071–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sriram, K.; Insel, P.A. G protein-coupled receptors as targets for approved drugs: How many targets and how many drugs? Mol. Pharmacol. 2018, 93, 251–258. [Google Scholar] [CrossRef] [Green Version]
- Flock, T.; Hauser, A.S.; Lund, N.; Gloriam, D.E.; Balaji, S.; Babu, M.M. Selectivity determinants of GPCR-G-protein binding. Nature 2017, 545, 317–322. [Google Scholar] [CrossRef]
- Kheradpisheh, Z.; Mahvi, A.H.; Mirzaei, M.; Mokhtari, M.; Azizi, R.; Fallahzadeh, H.; Ehrampoush, M.H. Correlation between drinking water fluoride and TSH hormone by ANNs and ANFIS. J. Environ. Health Sci. Eng. 2018, 16, 11–18. [Google Scholar] [CrossRef]
- Sadia, Z.; Humayun, A.; Shafiq ur, R.; Shan, E.; Amer, S.; Farhat, Y.; Shehnila, A. Effect of excess fluoride consumption on urine-serum fluorides, dental state and thyroid hormones among children in “Talab Sarai” Punjab Pakistan. Open Chem. 2020, 18, 119–128. [Google Scholar] [CrossRef]
- Susheela, A.K.; Bhatnagar, M.; Vig, K.; Mondal, N.K. Excess fluoride ingestion and thyroid hormone derangements in children living in Delhi, India. Fluoride 2005, 38, 98–108. [Google Scholar]
- Singh, N.; Verma, K.G.; Verma, P.; Sidhu, G.K.; Sachdeva, S. A comparative study of fluoride ingestion levels, serum thyroid hormone & TSH level derangements, dental fluorosis status among school children from endemic and non-endemic fluorosis areas. SpringerPlus 2014, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yasmin, S.; Ranjan, S.; D’Souza, D. Effect of excess fluoride ingestion on human thyroid function in Gaya region, Bihar, India. Toxicol. Environ. Chem. 2013, 95, 1235–1243. [Google Scholar] [CrossRef]
- Shashi, A.; Singla, S. Syndrome of low triiodothyronine in chronic fluorosis. Int. J. Appl. Basic Med. Res. 2013, 3, 152–160. [Google Scholar]
- Wang, M.; Liu, L.; Li, H.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Li, P.; Zhao, Q.; Dong, L.; et al. Thyroid function, intelligence, and low-moderate fluoride exposure among Chinese school-age children. Environ. Int. 2020, 134, 105229. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Payan, A.; Duarte-Gardea, M.; Ortiz, M.; Gardea-Torresdey, J.L. Chronic effects of fluoride on growth, blood chemistry, and thyroid hormones in adolescents residing in three communities in Northern Mexico. Fluoride 2005, 38, 246–249. [Google Scholar]
- Peckham, S.; Lowery, D.; Spencer, S. Are fluoride levels in drinking water associated with hypothyroidism prevalence in England? A large observational study of GP practice data and fluoride levels in drinking water. J. Epidemiol Community Health 2015, 69, 619–624. [Google Scholar] [CrossRef] [Green Version]
- Malin, A.J.; Riddell, J.; McCague, H.; Till, C. Fluoride exposure and thyroid function among adults living in Canada: Effect modification by iodine status. Environ. Int. 2018, 121, 667–674. [Google Scholar] [CrossRef]
- Rovet, J.F. The role of thyroid hormones for brain development and cognitive function. Endocr. Dev. 2014, 26, 26–43. [Google Scholar] [CrossRef]
- Reiter, R.J.; Rosales-Corral, S.A.; Tan, D.X.; Acuna-Castroviejo, D.; Qin, L.; Yang, S.F.; Xu, K. Melatonin, a full service anti-cancer agent: Inhibition of initiation, progression and metastasis. Int. J. Mol. Sci. 2017, 18, 843. [Google Scholar] [CrossRef]
- Luke, J. The Effect of Fluoride on the Physiology of the Pineal Gland. Ph.D. Thesis, University of Surrey, Guildford, UK, 1997. [Google Scholar]
- Luke, J. Fluoride deposition in the aged human pineal gland. Caries Res. 2001, 35, 125–128. [Google Scholar] [CrossRef] [PubMed]
- Kunz, D.; Schmitz, S.; Mahlberg, R.; Mohr, A.; Stoter, C.; Wolf, K.J.; Herrmann, W.M. A new concept for melatonin deficit: On pineal calcification and melatonin excretion. Neuropsychopharmacology 1999, 21, 765–772. [Google Scholar] [CrossRef]
- Schlesinger, E.R.; Overton, D.E.; Chase, H.C.; Cantwell, K.T. Newburgh-Kingston caries-fluorine study. XIII. Pediatric findings after ten years. J. Am. Dent. Assoc. 1956, 52, 296–306. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Jiang, S.; Han, M.; Yang, Z.; Lv, J.; Deng, C.; Reiter, R.J.; Yang, Y. Exogenous melatonin as a treatment for secondary sleep disorders: A systematic review and meta-analysis. Front. Neuroendocrinol. 2019, 52, 22–28. [Google Scholar] [CrossRef]
- Veatch, O.J.; Goldman, S.E.; Adkins, K.W.; Malow, B.A. Melatonin in children with autism spectrum disorders: How does the evidence fit together? J. Nat. Sci. 2015, 1, e125. [Google Scholar]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev. Med. Child. Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Pagan, C.; Goubran-Botros, H.; Delorme, R.; Benabou, M.; Lemière, N.; Murray, K.; Amsellem, F.; Callebert, J.; Chaste, P.; Jamain, S.; et al. Disruption of melatonin synthesis is associated with impaired 14-3-3 and miR-451 levels in patients with autism spectrum disorders. Sci. Rep. 2017, 7, 2096. [Google Scholar] [CrossRef]
- Pagan, C.; Delorme, R.; Callebert, J.; Goubran-Botros, H.; Amsellem, F.; Drouot, X.; Boudebesse, C.; Le Dudal, K.; Ngo-Nguyen, N.; Laouamri, H.; et al. The serotonin-N-acetylserotonin-melatonin pathway as a biomarker for autism spectrum disorders. Transl. Psychiatry 2014, 4, e479. [Google Scholar] [CrossRef]
- Lu, F.; Zhang, Y.; Trivedi, A.; Jiang, X.; Chandra, D.; Zheng, J.; Nakano, Y.; Abduweli Uyghurturk, D.; Jalai, R.; Onur, S.G.; et al. Fluoride related changes in behavioral outcomes may relate to increased serotonin. Physiol. Behav. 2019, 206, 76–83. [Google Scholar] [CrossRef]
- Malin, A.J.; Bose, S.; Busgang, S.A.; Gennings, C.; Thorpy, M.; Wright, R.O.; Wright, R.J.; Arora, M. Fluoride exposure and sleep patterns among older adolescents in the United States: A cross-sectional study of NHANES 2015-2016. Environ. Health 2019, 18, 106. [Google Scholar] [CrossRef] [Green Version]
- Johns, J. Association between high pineal fluoride content and pineal calcification in a low fluoride area. Fluoride 2016, 49, 472–484. [Google Scholar]
- Chlubek, D.; Sikora, M. Fluoride and pineal gland. Appl. Sci. 2020, 10, 2885. [Google Scholar] [CrossRef]
- Farland, W.H.; Savitz, D.A.; Crofton, K.M.; Ghassabian, A.; Klotz, J.B.; Lam, J.; Lein, P.J.; Louis, G.M.B.; Pennell, M.L.; Steinmaus, C.; et al. NTP Monograph on the Systematic Review of Fluoride. Review of Fluoride Exposure and Neurodevelopmental and Cognitive Health Effects; National Academies Press: Washington, DC, USA, 2020. [Google Scholar]
- Spittle, B. Fluoride Fatigue. Fluoride Poisoning: Is Fluoride in Your Drinking Water, and from Other Sources, Making You Sick? Paua Press Limited: Dunedin, New Zealand, 2008; Volume 1, p. 78. [Google Scholar]
- Spittle, B. Psychopharmacology of fluoride: A review. Clin. Psychopharmacol. 1994, 9, 79–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duan, Q.; Jiao, J.; Chen, X.; Wang, X. Association between water fluoride and the level of children’s intelligence: A dose-response meta-analysis. Public Health 2018, 154, 87–97. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zhang, Q.; Liu, Y.; Han, L.; Wang, Q.; Chen, P.; Zhang, S.; Wang, A.; Zhou, X. Fluoride induces apoptosis via inhibiting SIRT1 activity to activate mitochondrial p53 pathway in human neuroblastoma SH-SY5Y cells. Toxicol. Appl. Pharmacol. 2018, 347, 60–69. [Google Scholar] [CrossRef]
- Strunecka, A.; Blaylock, R.L.; Strunecky, O. Fluoride, aluminum, and aluminofluoride complexes in pathogenesis of the autism spectrum disorders: A possible role of immunoexcitotoxicity. J. Appl. Biomed. 2016, 14, 171–176. [Google Scholar] [CrossRef]
- Strunecka, A.; Blaylock, R.L.; Patocka, J.; Strunecky, O. Immunoexcitotoxicity as the central mechanism of etiopathology and treatment of autism spectrum disorders: A possible role of fluoride and aluminum. Surg. Neurol. Int. 2018, 9, 74. [Google Scholar] [CrossRef]
- Goschorska, M.; Baranowska-Bosiacka, I.; Gutowska, I.; Metryka, E.; Skórka-Majewicz, M.; Chlubek, D. Potential role of fluoride in the etiopathogenesis of Alzheimer’s disease. Int. J. Mol. Sci. 2018, 19, 3965. [Google Scholar] [CrossRef] [Green Version]
- Strunecká, A.; Patočka, J. Aluminofluoride complexes in the etiology of Alzheimer´s disease. In Structure and Bonding. New Developments in Biological Aluminum Chemistry; Atwood, D., Roesky, C., Eds.; Springer: Berlin/Heidelberg, Germany, 2003; pp. 139–181. [Google Scholar]
- Hu, Y.H.; Wu, S.S. Fluoride in cerebrospinal fluid of patients with fluorosis. J. Neurol. Neurosurg. Psychiatry 1988, 51, 1591–1593. [Google Scholar] [CrossRef] [Green Version]
- Du, L. The effect of fluorine on the developing human brain. Fluoride 2008, 41, 327–330, Translated from Zhonghua Bing Li Xue Za Zhi 1992, 21, 218–220. [Google Scholar]
- Grandjean, P.; Landrigan, P.J. Neurobehavioural effects of developmental toxicity. Lancet Neurol. 2014, 13, 330–338. [Google Scholar] [CrossRef] [Green Version]
- Rocha-Amador, D.; Navarro, M.E.; Carrizales, L.; Morales, R.; Calderón, J. Decreased intelligence in children and exposure to fluoride and arsenic in drinking water. Cad. Saude. Publica 2007, 23 (Suppl. 4), S579–S587. [Google Scholar] [CrossRef] [PubMed]
- Seraj, B.; Shahrabi, M.; Shadfar, M.; Ahmadi, R.; Fallahzadeh, M.; Eslamlu, H.F.; Kharazifard, M.J. Effect of high water fluoride concentration on the intellectual development of children in Makoo, Iran. J. Dent. 2012, 9, 221–229. [Google Scholar]
- Aravind, A.; Dhanya, R.S.; Narayan, A.; Sam, G.; Adarsh, V.J.; Kiran, M. Effect of fluoridated water on intelligence in 10-12-year-old school children. J. Int. Soc. Prev. Community Dent. 2016, 6, S237–S242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, X.; Chen, J.; Li, Y.; Liu, H.; Hou, C.; Zeng, Q.; Cui, Y.; Zhao, L.; Li, P.; Zhou, Z.; et al. Threshold effects of moderately excessive fluoride exposure on children’s health: A potential association between dental fluorosis and loss of excellent intelligence. Environ. Int. 2018, 118, 116–124. [Google Scholar] [CrossRef] [PubMed]
- Razdan, P.; Patthi, B.; Kumar, J.K.; Agnihotri, N.; Chaudhari, P.; Prasad, M. Effect of fluoride concentration in drinking water on intelligence quotient of 12–14-year-old children in mathura district: A cross-sectional study. J. Int. Soc. Prev. Commun. Dent. 2017, 7, 252–258. [Google Scholar] [CrossRef]
- Bashash, M.; Thomas, D.; Hu, H.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Ettinger, A.S.; Wright, R.; Zhang, Z.; et al. Prenatal fluoride exposure and cognitive outcomes in children at 4 and 6–12 years of age in Mexico. Environ. Health Perspect. 2017, 125, 097017. [Google Scholar] [CrossRef]
- Jin, T.X.; Hua, Z.; Guan, Z.Z. The historical review and development strategies on prevention and control of coal-burning type of endemic fluorosis in Liupanshui, Guizhou of China. Fluoride 2019, 52, 94–95. [Google Scholar]
- Tang, Q.Q.; Du, J.; Ma, H.H.; Jiang, S.J.; Zhou, X.J. Fluoride and children’s intelligence: A meta-analysis. Biol. Trace Elem. Res. 2008, 126, 115–120. [Google Scholar] [CrossRef]
- Choi, A.L.; Sun, G.; Zhang, Y.; Grandjean, P. Developmental fluoride neurotoxicity: A systematic review and meta-analysis. Environ. Health Perspect. 2012, 120, 1362–1368. [Google Scholar] [CrossRef] [Green Version]
- Bashash, M.; Marchand, M.; Hu, H.; Till, C.; Martinez-Mier, E.A.; Sanchez, B.N.; Basu, N.; Peterson, K.E.; Green, R.; Schnaas, L.; et al. Prenatal fluoride exposure and attention deficit hyperactivity disorder (ADHD) symptoms in children at 6–12 years of age in Mexico City. Environ. Int. 2018, 121, 658–666. [Google Scholar] [CrossRef]
- Green, R.; Lanphear, B.; Hornung, R.; Flora, D.; Martinez-Mier, E.A.; Neufeld, R.; Ayotte, P.; Muckle, G.; Till, C. Association between maternal fluoride exposure during pregnancy and IQ scores in offspring in Canada. JAMA Pediatr. 2019, 173, 940–948. [Google Scholar] [CrossRef] [PubMed]
- Till, C.; Green, R.; Flora, D.; Hornung, R.; Martinez-Mier, E.A.; Blazer, M.; Farmus, L.; Ayotte, P.; Muckle, G.; Lanphear, B. Fluoride exposure from infant formula and child IQ in a Canadian birth cohort. Environ. Int. 2020, 134, 105315. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.Y.; Liang, Y.X.; Chen, L.S.; Wang, C.S.; Chen, B.H.; Chen, X.D.; Zhou, M. Effect of fluoride in drinking water on intelligence in children. Fluoride 2003, 36, 84–94. [Google Scholar]
- Spittle, B. Reviews of developmental fluoride neurotoxicity by Grandjean and Guth et al. Fluoride 2020, 53, 204–219. [Google Scholar]
- Introduction to the 65 IQ Studies. Available online: http://fluoridealert.org/studies/brain01a/ (accessed on 1 September 2020).
- Nakamoto, T.; Rawls, H.R. Fluoride exposure in early life as the possible root cause of disease in later life. J. Clin. Pediatr. Dent. 2018, 42, 325–330. [Google Scholar] [CrossRef]
- Horowitz, H.S. The 2001 CDC recommendations for using fluoride to prevent and control dental caries in the United States. J. Public Health Dent. 2003, 63, 3–8; discussion 9–10. [Google Scholar] [CrossRef]
- Canadian Paediatric Society. The use of fluoride in infants and children. Paediatr. Child. Health 2002, 7, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Yerien, D.E.; Bonesi, S.; Postigo, A. Fluorination methods in drug discovery. Org. Biomol. Chem. 2016, 14, 8398–8427. [Google Scholar] [CrossRef] [Green Version]
- Kharasch, E.D. Metabolism and toxicity of the new anesthetic agents. Acta Anaesthesiol. Belg. 1996, 47, 7–14. [Google Scholar]
- Halothane Hepatotoxicity. Available online: https://emedicine.medscape.com/article/166232-overview (accessed on 1 September 2020).
- Furberg, C.D.; Pitt, B. Withdrawal of cerivastatin from the world market. Curr. Control. Trials Cardiovasc. Med. 2001, 2, 205–207. [Google Scholar] [CrossRef] [PubMed]
- Kuhlmann, C.R.; Gast, C.; Li, F.; Schäfer, M.; Tillmanns, H.; Waldecker, B.; Wiecha, J. Cerivastatin activates endothelial calcium-activated potassium channels and thereby modulates endothelial nitric oxide production and cell proliferation. J. Am. Soc. Nephrol. 2004, 15, 868–875. [Google Scholar] [CrossRef] [Green Version]
- Goncharov, N.V.; Jenkins, R.O.; Radilov, A.S. Toxicology of fluoroacetate: A review, with possible directions for therapy research. J. Appl. Toxicol. 2006, 26, 148–161. [Google Scholar] [CrossRef] [PubMed]
- de Forni, M.; Malet-Martino, M.C.; Jaillais, P.; Shubinski, R.E.; Bachaud, J.M.; Lemaire, L.; Canal, P.; Chevreau, C.; Carrié, D.; Soulié, P.; et al. Cardiotoxicity of high-dose continuous infusion fluorouracil: A prospective clinical study. J. Clin. Oncol. 1992, 10, 1795–1801. [Google Scholar] [CrossRef] [PubMed]
- Reis-Mendes, A.F.; Sousa, E.; de Lourdes Bastos, M.; Costa, V.M. The role of the metabolism of anticancer drugs in their induced-cardiotoxicity. Curr. Drug. Metab. 2015, 17, 75–90. [Google Scholar] [CrossRef] [PubMed]
- Polk, A.; Vistisen, K.; Vaage-Nilsen, M.; Nielsen, D.L. A systematic review of the pathophysiology of 5-fluorouracil-induced cardiotoxicity. BMC Pharmacol. Toxicol. 2014, 15, 47. [Google Scholar] [CrossRef] [Green Version]
- CDC. Cost Savings of Community Water Fluoridation. Available online: https://www.cdc.gov/fluoridation/basics/cost.htm (accessed on 1 September 2020).
- Leigh, J.P.; Du, J. Brief report: Forecasting the economic burden of autism in 2015 and 2025 in the United States. J. Autism. Dev. Disord. 2015, 45, 4135–4139. [Google Scholar] [CrossRef]
- Cakir, J.; Frye, R.E.; Walker, S.J. The lifetime social cost of autism: 1990–2029. RASD 2020, 72, 101502. [Google Scholar] [CrossRef]






| ENZYME | SOURCE | FLUORIDE Concentration | REFERENCES |
|---|---|---|---|
| acid phosphatase | ram semen | 20–200 μM | [32] |
| osteoblasts | mM | [34] | |
| osteoclasts | mM | [35,36] | |
| kidney | 10 μM | [37] | |
| aconitase | liver | mM | [38] |
| adenylyl cyclase | liver | 5–10 mM | [39] |
| fibroblasts | 5 mM | [40] | |
| acetylcholinesterase | red blood cells | 0.01–10 mM | [41] |
| brain | 5–50 mM | [42] | |
| amylase | human saliva | 50–500 mM | [43] |
| arginase | liver, kidney | >4 mM | [44] |
| Ca-ATPase | sarcoplasmic reticulum | 5–10 mM | [26] |
| catalase | red blood cells | μM | [45] |
| macrophages | 0.5–05 mM | [46] | |
| cytochrome-c-oxidase | liver, heart | 0.01–10 mM | [47] |
| enolase | red blood cells | 1–50 mM | [48,49] |
| hepatocytes | 3 mM | [50] | |
| embryonic cells | 50 μM | [51] | |
| oral bacteria | 16–54 μM | [52] | |
| F(1)F(o)-ATP synthase | mitochondria | mM | [53] |
| glucose-6-phosphatase | liver | μM | [19,54] |
| glutathione peroxidase | red blood cells | μM | [45] |
| glycogen synthase | hepatocytes | 2–15 mM | [55] |
| lactate dehydrogenase | ram semen | 20–200 μM | [32] |
| fetal osteoblast | 6–60 μM | [56] | |
| bone marrow | <0.5 mM | [36] | |
| lipase | pancreas, liver | 5 μM–5 mM | [57,58] |
| L-Ca2+channels | heart | 10 mM | [59] |
| Na+/K+ ATPase | plasma membrane | 1–10 mM | [25] |
| protein phosphatase | liver | 10–50 mM | [60] |
| bone | μM | [24] | |
| pyrophosphatase | yeast | 5 mM | [24,61] |
| pyruvate kinase | red blood cells | 10–50 mM | [62] |
| succinate dehydrogenase | heart, liver, kidney | 1–15 mM | [63,64] |
| superoxide dismutase | red blood cells | μM | [45] |
| urease | human | 250 mM | [58,65] |
| ENZYME | SOURCE | FLUORIDE Concentration | REFERENCES |
|---|---|---|---|
| acid phosphatase | ram semen | 100 mM | [32] |
| adenylyl cyclase | heart, liver, brain | 1–10 mM | [47,66,67,68] |
| smooth muscle | 10 mM | [60] | |
| lymphoma cell | 10 mM | [69] | |
| kidney | 5 mM | [70] | |
| alkaline phosphatase | bone cells | 10–100 µM | [71] |
| aspartate transaminase | ram semen | 20–200 µM | [32] |
| Ca2+-ATPase | sarcoplasmic reticulum | 1–10 mM | [72] |
| glutamyl S-transferase | ram semen | 20–200 μM | [32] |
| K+[ACh]M channel | heart | >1 mM | [73] |
| K+ATP channel | heart | mM | [74] |
| lactate dehydrogenase | hepatocytes | 1–30 mM | [50] |
| ram semen | 100 mM | [32] | |
| L-type Ca2+ channel | rabbit femoral artery | 10 mM | [60] |
| glycogen phosphorylase | hepatocytes | 1–50 mM | [39,75] |
| tyrosine kinase | osteoblasts | 1–10 mM | [76] |
| 10–200 µM | [71,77] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Strunecka, A.; Strunecky, O. Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Appl. Sci. 2020, 10, 7100. https://doi.org/10.3390/app10207100
Strunecka A, Strunecky O. Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks. Applied Sciences. 2020; 10(20):7100. https://doi.org/10.3390/app10207100
Chicago/Turabian StyleStrunecka, Anna, and Otakar Strunecky. 2020. "Mechanisms of Fluoride Toxicity: From Enzymes to Underlying Integrative Networks" Applied Sciences 10, no. 20: 7100. https://doi.org/10.3390/app10207100