Alzheimer’s Disease and Diabetes: Role of Diet, Microbiota and Inflammation in Preclinical Models
Abstract
:1. Alzheimer’s Disease
2. Prediabetes and Type 2 Diabetes Mellitus
3. AD-T2DM Relationship
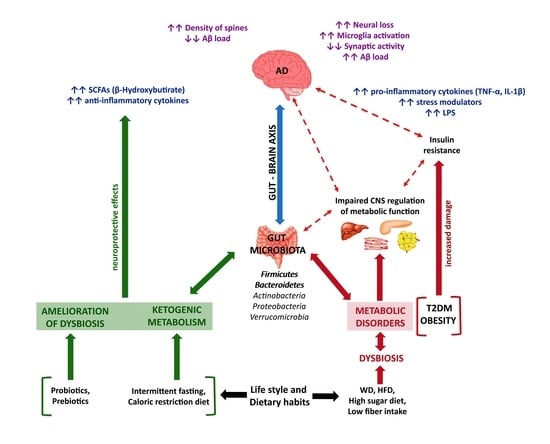
3.1. Inflammation
3.2. Diet
3.3. Gut Microbiota
4. Diet, Inflammation and Gut Microbiota in AD Models
5. Diet, Inflammation and Gut Microbiota in T2DM Models
6. Microbiota, Inflammation and Diet in Preclinical Mixed Models in AD-T2DM
7. Common Microbiota Alterations in AD, T2DM and Mixed Preclinical Models
8. Conclusions
Author Contributions
Funding

Conflicts of Interest
References
- Arvanitakis, Z.; Shah, R.C.; Bennett, D.A. Diagnosis and Management of Dementia: Review. JAMA 2019, 322, 1589–1599. [Google Scholar] [CrossRef] [PubMed]
- Alzheimer’s_Association. 2017 Alzheimer’s disease facts and figures. Alzheimer’s Dement. 2017, 13, 325–373. [Google Scholar] [CrossRef]
- Arnoriaga-Rodríguez, M.; Mayneris-Perxachs, J.; Burokas, A.; Contreras-Rodríguez, O.; Blasco, G.; Coll, C.; Biarnés, C.; Miranda-Olivos, R.; Latorre, J.; Moreno-Navarrete, J.M.; et al. Obesity Impairs Short-Term and Working Memory through Gut Microbial Metabolism of Aromatic Amino Acids. Cell Metab. 2020, 32, 548–560.e547. [Google Scholar] [CrossRef]
- Bloom, G.S. Amyloid-beta and tau: The trigger and bullet in Alzheimer disease pathogenesis. JAMA Neurol. 2014, 71, 505–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spires-Jones, T.L.; Hyman, B.T. The intersection of amyloid beta and tau at synapses in Alzheimer’s disease. Neuron 2014, 82, 756–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calvo-Rodriguez, M.; de la Fuente, C.; Garcia-Durillo, M.; Garcia-Rodriguez, C.; Villalobos, C.; Nunez, L. Aging and amyloid beta oligomers enhance TLR4 expression, LPS-induced Ca(2+) responses, and neuron cell death in cultured rat hippocampal neurons. J. Neuroinflamm. 2017, 14, 24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological alterations in Alzheimer disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef] [PubMed]
- Esquerda-Canals, G.; Montoliu-Gaya, L.; Guell-Bosch, J.; Villegas, S. Mouse Models of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 57, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Heneka, M.T.; Carson, M.J.; El Khoury, J.; Landreth, G.E.; Brosseron, F.; Feinstein, D.L.; Jacobs, A.H.; Wyss-Coray, T.; Vitorica, J.; Ransohoff, R.M.; et al. Neuroinflammation in Alzheimer’s disease. Lancet Neurol. 2015, 14, 388–405. [Google Scholar] [CrossRef] [Green Version]
- Calsolaro, V.; Edison, P. Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimer’s Dement. 2016, 12, 719–732. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; McCabe, C.; Medina, S.; Varshavsky, M.; Kitsberg, D.; Dvir-Szternfeld, R.; Green, G.; Dionne, D.; Nguyen, L.; Marshall, J.L.; et al. Disease-associated astrocytes in Alzheimer’s disease and aging. Nat. Neurosci. 2020, 23, 701–706. [Google Scholar] [CrossRef]
- Sankar, S.B.; Infante-Garcia, C.; Weinstock, L.D.; Ramos-Rodriguez, J.J.; Hierro-Bujalance, C.; Fernandez-Ponce, C.; Wood, L.B.; Garcia-Alloza, M. Amyloid beta and diabetic pathology cooperatively stimulate cytokine expression in an Alzheimer’s mouse model. J. Neuroinflamm. 2020, 17, 38. [Google Scholar] [CrossRef] [PubMed]
- Wes, P.D.; Sayed, F.A.; Bard, F.; Gan, L. Targeting microglia for the treatment of Alzheimer’s Disease. Glia 2016, 64, 1710–1732. [Google Scholar] [CrossRef] [PubMed]
- Zhao, R.; Hu, W.; Tsai, J.; Li, W.; Gan, W.B. Microglia limit the expansion of beta-amyloid plaques in a mouse model of Alzheimer’s disease. Mol. Neurodegener. 2017, 12, 47. [Google Scholar] [CrossRef]
- Hickman, S.E.; Allison, E.K.; El Khoury, J. Microglial dysfunction and defective beta-amyloid clearance pathways in aging Alzheimer’s disease mice. J. Neurosci. 2008, 28, 8354–8360. [Google Scholar] [CrossRef]
- Lucin, K.M.; Wyss-Coray, T. Immune activation in brain aging and neurodegeneration: Too much or too little? Neuron 2009, 64, 110–122. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, Z.; Yang, R.; Wang, W.; Qi, L.; Huang, T. Associations between gut microbiota and Alzheimer’s disease, major depressive disorder, and schizophrenia. J. Neuroinflamm. 2020, 17, 288. [Google Scholar] [CrossRef] [PubMed]
- Goyal, D.; Ali, S.A.; Singh, R.K. Emerging role of gut microbiota in modulation of neuroinflammation and neurodegeneration with emphasis on Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry 2020, 110112. [Google Scholar] [CrossRef]
- Zhuang, Z.Q.; Shen, L.L.; Li, W.W.; Fu, X.; Zeng, F.; Gui, L.; Lu, Y.; Cai, M.; Zhu, C.; Tan, Y.L.; et al. Gut Microbiota is Altered in Patients with Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 63, 1337–1346. [Google Scholar] [CrossRef] [Green Version]
- Laiteerapong, N.; Ham, S.A.; Gao, Y.; Moffet, H.H.; Liu, J.Y.; Huang, E.S.; Karter, A.J. The Legacy Effect in Type 2 Diabetes: Impact of Early Glycemic Control on Future Complications (The Diabetes & Aging Study). Diabetes Care 2019, 42, 416–426. [Google Scholar] [CrossRef] [Green Version]
- Pratley, R.E. Type 2 diabetes is one of the leading causes of premature morbidity and mortality. Introduction. Am. J. Med. 2013, 126, S1. [Google Scholar] [CrossRef] [PubMed]
- Saeedi, P.; Petersohn, I.; Salpea, P.; Malanda, B.; Karuranga, S.; Unwin, N.; Colagiuri, S.; Guariguata, L.; Motala, A.A.; Ogurtsova, K.; et al. Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: Results from the International Diabetes Federation Diabetes Atlas, 9(th) edition. Diabetes Res. Clin. Pract. 2019, 157, 107843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabak, A.G.; Herder, C.; Rathmann, W.; Brunner, E.J.; Kivimaki, M. Prediabetes: A high-risk state for diabetes development. Lancet 2012, 379, 2279–2290. [Google Scholar] [CrossRef] [Green Version]
- Esser, N.; Utzschneider, K.M.; Kahn, S.E. Early beta cell dysfunction vs insulin hypersecretion as the primary event in the pathogenesis of dysglycaemia. Diabetologia 2020, 63, 2007–2021. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Y.; Ley, S.H.; Hu, F.B. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat. Rev. Endocrinol. 2018, 14, 88–98. [Google Scholar] [CrossRef]
- Fazeli Farsani, S.; van der Aa, M.P.; van der Vorst, M.M.; Knibbe, C.A.; de Boer, A. Global trends in the incidence and prevalence of type 2 diabetes in children and adolescents: A systematic review and evaluation of methodological approaches. Diabetologia 2013, 56, 1471–1488. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Ren, Z.H.; Qiang, H.; Wu, J.; Shen, M.; Zhang, L.; Lyu, J. Trends in the incidence of diabetes mellitus: Results from the Global Burden of Disease Study 2017 and implications for diabetes mellitus prevention. BMC Public Health 2020, 20, 1415. [Google Scholar] [CrossRef]
- Liu, C.; Feng, X.; Li, Q.; Wang, Y.; Li, Q.; Hua, M. Adiponectin, TNF-alpha and inflammatory cytokines and risk of type 2 diabetes: A systematic review and meta-analysis. Cytokine 2016, 86, 100–109. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.C.; Chien, Y.W.; Yang, S.C. The alteration of gut microbiota in newly diagnosed type 2 diabetic patients. Nutrition 2019, 63–64, 51–56. [Google Scholar] [CrossRef]
- Hur, K.Y.; Lee, M.S. Gut Microbiota and Metabolic Disorders. Diabetes Metab. J. 2015, 39, 198–203. [Google Scholar] [CrossRef] [Green Version]
- Palacios, T.; Vitetta, L.; Coulson, S.; Madigan, C.D.; Denyer, G.S.; Caterson, I.D. The effect of a novel probiotic on metabolic biomarkers in adults with prediabetes and recently diagnosed type 2 diabetes mellitus: Study protocol for a randomized controlled trial. Trials 2017, 18, 7. [Google Scholar] [CrossRef] [Green Version]
- Vallianou, N.G.; Stratigou, T.; Tsagarakis, S. Microbiome and diabetes: Where are we now? Diabetes Res. Clin. Pract. 2018, 146, 111–118. [Google Scholar] [CrossRef]
- Grasset, E.; Burcelin, R. The gut microbiota to the brain axis in the metabolic control. Rev. Endocr. Metab. Disord. 2019, 20, 427–438. [Google Scholar] [CrossRef] [Green Version]
- Farre, R.; Fiorani, M.; Abdu Rahiman, S.; Matteoli, G. Intestinal Permeability, Inflammation and the Role of Nutrients. Nutrients 2020, 12, 1185. [Google Scholar] [CrossRef]
- Sharma, S.; Tripathi, P. Gut microbiome and type 2 diabetes: Where we are and where to go? J. Nutr. Biochem. 2019, 63, 101–108. [Google Scholar] [CrossRef] [PubMed]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef]
- Sircana, A.; Framarin, L.; Leone, N.; Berrutti, M.; Castellino, F.; Parente, R.; De Michieli, F.; Paschetta, E.; Musso, G. Altered Gut Microbiota in Type 2 Diabetes: Just a Coincidence? Curr. Diabetes Rep. 2018, 18, 98. [Google Scholar] [CrossRef]
- Lin, H.V.; Frassetto, A.; Kowalik, E.J., Jr.; Nawrocki, A.R.; Lu, M.M.; Kosinski, J.R.; Hubert, J.A.; Szeto, D.; Yao, X.; Forrest, G.; et al. Butyrate and propionate protect against diet-induced obesity and regulate gut hormones via free fatty acid receptor 3-independent mechanisms. PLoS ONE 2012, 7, e35240. [Google Scholar] [CrossRef] [PubMed]
- Kellow, N.J.; Coughlan, M.T.; Savige, G.S.; Reid, C.M. Effect of dietary prebiotic supplementation on advanced glycation, insulin resistance and inflammatory biomarkers in adults with pre-diabetes: A study protocol for a double-blind placebo-controlled randomised crossover clinical trial. BMC Endocr. Disord. 2014, 14, 55. [Google Scholar] [CrossRef] [Green Version]
- Kandimalla, R.; Thirumala, V.; Reddy, P.H. Is Alzheimer’s disease a Type 3 Diabetes? A critical appraisal. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1078–1089. [Google Scholar] [CrossRef]
- Andreu-Reinon, M.E.; Huerta, J.M.; Gavrila, D.; Amiano, P.; Mar, J.; Tainta, M.; Ardanaz, E.; Larumbe, R.; Navarro, C.; Colorado-Yohar, S.M.; et al. Incidence of Dementia and Associated Factors in the EPIC-Spain Dementia Cohort. J. Alzheimer’s Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- de Bruijn, R.F.; Bos, M.J.; Portegies, M.L.; Hofman, A.; Franco, O.H.; Koudstaal, P.J.; Ikram, M.A. The potential for prevention of dementia across two decades: The prospective, population-based Rotterdam Study. BMC Med. 2015, 13, 132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frison, E.; Dufouil, C.; Helmer, C.; Berr, C.; Auriacombe, S.; Chene, G. Diabetes-Associated Dementia Risk and Competing Risk of Death in the Three-City Study. J. Alzheimer’s Dis. 2019, 71, 1339–1350. [Google Scholar] [CrossRef]
- Luchsinger, J.A.; Palta, P.; Rippon, B.; Sherwood, G.; Soto, L.; Ceballos, F.; Laing, K.; Igwe, K.; He, H.; Razlighi, Q.; et al. Pre-Diabetes, but not Type 2 Diabetes, Is Related to Brain Amyloid in Late Middle-Age. J. Alzheimer’s Dis. 2020, 75, 1241–1252. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Mudher, A. Alzheimer’s Disease and Type 2 Diabetes: A Critical Assessment of the Shared Pathological Traits. Front. Neurosci. 2018, 12, 383. [Google Scholar] [CrossRef] [Green Version]
- Mittal, K.; Katare, D.P. Shared links between type 2 diabetes mellitus and Alzheimer’s disease: A review. Diabetes Metab. Syndr. 2016, 10, S144–S149. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.E.; Park, Y.G.; Han, K.; Kim, M.K.; Koh, E.S.; Kim, E.S.; Lee, M.K.; Kim, B.; Hong, O.K.; Kwon, H.S. BMI, Weight Change, and Dementia Risk in Patients with New-Onset Type 2 Diabetes: A Nationwide Cohort Study. Diabetes Care 2019, 42, 1217–1224. [Google Scholar] [CrossRef] [PubMed]
- Wium-Andersen, I.K.; Osler, M.; Jorgensen, M.B.; Rungby, J.; Wium-Andersen, M.K. Antidiabetic medication and risk of dementia in patients with type 2 diabetes: A nested case-control study. Eur. J. Endocrinol. 2019, 181, 499–507. [Google Scholar] [CrossRef]
- Guilliams, M.; Mildner, A.; Yona, S. Developmental and Functional Heterogeneity of Monocytes. Immunity 2018, 49, 595–613. [Google Scholar] [CrossRef] [Green Version]
- Chaney, A.; Williams, S.R.; Boutin, H. In vivo molecular imaging of neuroinflammation in Alzheimer’s disease. J. Neurochem. 2019, 149, 438–451. [Google Scholar] [CrossRef] [Green Version]
- Ozben, T.; Ozben, S. Neuro-inflammation and anti-inflammatory treatment options for Alzheimer’s disease. Clin. Biochem. 2019, 72, 87–89. [Google Scholar] [CrossRef]
- Di Filippo, M.; Chiasserini, D.; Gardoni, F.; Viviani, B.; Tozzi, A.; Giampa, C.; Costa, C.; Tantucci, M.; Zianni, E.; Boraso, M.; et al. Effects of central and peripheral inflammation on hippocampal synaptic plasticity. Neurobiol. Dis. 2013, 52, 229–236. [Google Scholar] [CrossRef]
- Condello, C.; Yuan, P.; Grutzendler, J. Microglia-Mediated Neuroprotection, TREM2, and Alzheimer’s Disease: Evidence from Optical Imaging. Biol. Psychiatry 2018, 83, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Tejera, D.; Heneka, M.T. Microglia in Alzheimer’s disease: The good, the bad and the ugly. Curr. Alzheimer Res. 2016, 13, 370–380. [Google Scholar] [CrossRef]
- Barroeta-Espar, I.; Weinstock, L.D.; Perez-Nievas, B.G.; Meltzer, A.C.; Siao Tick Chong, M.; Amaral, A.C.; Murray, M.E.; Moulder, K.L.; Morris, J.C.; Cairns, N.J.; et al. Distinct cytokine profiles in human brains resilient to Alzheimer’s pathology. Neurobiol. Dis. 2019, 121, 327–337. [Google Scholar] [CrossRef]
- Park, K.W.; Baik, H.H.; Jin, B.K. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J. Immunol 2009, 183, 4666–4674. [Google Scholar] [CrossRef]
- Zhou, J.; Yu, W.; Zhang, M.; Tian, X.; Li, Y.; Lu, Y. Imbalance of Microglial TLR4/TREM2 in LPS-Treated APP/PS1 Transgenic Mice: A Potential Link Between Alzheimer’s Disease and Systemic Inflammation. Neurochem. Res. 2019, 44, 1138–1151. [Google Scholar] [CrossRef]
- Swardfager, W.; Lanctot, K.; Rothenburg, L.; Wong, A.; Cappell, J.; Herrmann, N. A meta-analysis of cytokines in Alzheimer’s disease. Biol. Psychiatry 2010, 68, 930–941. [Google Scholar] [CrossRef] [PubMed]
- Newcombe, E.A.; Camats-Perna, J.; Silva, M.L.; Valmas, N.; Huat, T.J.; Medeiros, R. Inflammation: The link between comorbidities, genetics, and Alzheimer’s disease. J. Neuroinflamm. 2018, 15, 276. [Google Scholar] [CrossRef] [PubMed]
- Haghani, K.; Pashaei, S.; Vakili, S.; Taheripak, G.; Bakhtiyari, S. TNF-alpha knockdown alleviates palmitate-induced insulin resistance in C2C12 skeletal muscle cells. Biochem. Biophys. Res. Commun. 2015, 460, 977–982. [Google Scholar] [CrossRef] [PubMed]
- Copps, K.D.; White, M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia 2012, 55, 2565–2582. [Google Scholar] [CrossRef] [Green Version]
- Infante-Garcia, C.; Ramos-Rodriguez, J.J.; Galindo-Gonzalez, L.; Garcia-Alloza, M. Long-term central pathology and cognitive impairment are exacerbated in a mixed model of Alzheimer’s disease and type 2 diabetes. Psychoneuroendocrinology 2016, 65, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Jimenez-Palomares, M.; Murillo-Carretero, M.I.; Infante-Garcia, C.; Berrocoso, E.; Hernandez-Pacho, F.; Lechuga-Sancho, A.M.; Cozar-Castellano, I.; Garcia-Alloza, M. Central vascular disease and exacerbated pathology in a mixed model of type 2 diabetes and Alzheimer’s disease. Psychoneuroendocrinology 2015, 62, 69–79. [Google Scholar] [CrossRef] [PubMed]
- Ramos-Rodriguez, J.J.; Ortiz-Barajas, O.; Gamero-Carrasco, C.; de la Rosa, P.R.; Infante-Garcia, C.; Zopeque-Garcia, N.; Lechuga-Sancho, A.M.; Garcia-Alloza, M. Prediabetes-induced vascular alterations exacerbate central pathology in APPswe/PS1dE9 mice. Psychoneuroendocrinology 2014, 48, 123–135. [Google Scholar] [CrossRef]
- Scarmeas, N.; Anastasiou, C.A.; Yannakoulia, M. Nutrition and prevention of cognitive impairment. Lancet Neurol. 2018, 17, 1006–1015. [Google Scholar] [CrossRef]
- Spector, R. Nutrient transport systems in brain: 40 years of progress. J. Neurochem. 2009, 111, 315–320. [Google Scholar] [CrossRef]
- Hooshmand, B.; Mangialasche, F.; Kalpouzos, G.; Solomon, A.; Kareholt, I.; Smith, A.D.; Refsum, H.; Wang, R.; Muhlmann, M.; Ertl-Wagner, B.; et al. Association of Vitamin B12, Folate, and Sulfur Amino Acids with Brain Magnetic Resonance Imaging Measures in Older Adults: A Longitudinal Population-Based Study. JAMA Psychiatry 2016, 73, 606–613. [Google Scholar] [CrossRef] [Green Version]
- Van der Zwaluw, N.L.; Brouwer-Brolsma, E.M.; van de Rest, O.; van Wijngaarden, J.P.; In’t Veld, P.H.; Kourie, D.I.; Swart, K.M.; Enneman, A.W.; van Dijk, S.C.; van der Velde, N.; et al. Folate and Vitamin B12-Related Biomarkers in Relation to Brain Volumes. Nutrients 2016, 9, 8. [Google Scholar] [CrossRef] [Green Version]
- Szczechowiak, K.; Diniz, B.S.; Leszek, J. Diet and Alzheimer’s dementia—Nutritional approach to modulate inflammation. Pharm. Biochem. Behav. 2019, 184, 172743. [Google Scholar] [CrossRef]
- Valls-Pedret, C.; Sala-Vila, A.; Serra-Mir, M.; Corella, D.; de la Torre, R.; Martinez-Gonzalez, M.A.; Martinez-Lapiscina, E.H.; Fito, M.; Perez-Heras, A.; Salas-Salvado, J.; et al. Mediterranean Diet and Age-Related Cognitive Decline: A Randomized Clinical Trial. JAMA Intern Med. 2015, 175, 1094–1103. [Google Scholar] [CrossRef] [Green Version]
- Rusek, M.; Pluta, R.; Ulamek-Koziol, M.; Czuczwar, S.J. Ketogenic Diet in Alzheimer’s Disease. Int. J. Mol. Sci. 2019, 20, 3892. [Google Scholar] [CrossRef] [Green Version]
- Morrill, S.J.; Gibas, K.J. Ketogenic diet rescues cognition in ApoE4+ patient with mild Alzheimer’s disease: A case study. Diabetes Metab. Syndr. 2019, 13, 1187–1191. [Google Scholar] [CrossRef]
- Ota, M.; Matsuo, J.; Ishida, I.; Takano, H.; Yokoi, Y.; Hori, H.; Yoshida, S.; Ashida, K.; Nakamura, K.; Takahashi, T.; et al. Effects of a medium-chain triglyceride-based ketogenic formula on cognitive function in patients with mild-to-moderate Alzheimer’s disease. Neurosci. Lett. 2019, 690, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Witte, A.V.; Fobker, M.; Gellner, R.; Knecht, S.; Floel, A. Caloric restriction improves memory in elderly humans. Proc. Natl. Acad. Sci. USA 2009, 106, 1255–1260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Molina, L.; Lewis-Mikhael, A.M.; Riquelme-Gallego, B.; Cano-Ibanez, N.; Oliveras-Lopez, M.J.; Bueno-Cavanillas, A. Improving type 2 diabetes mellitus glycaemic control through lifestyle modification implementing diet intervention: A systematic review and meta-analysis. Eur. J. Nutr. 2020, 59, 1313–1328. [Google Scholar] [CrossRef]
- Al-Rashed, F.; Alghaith, A.; Azim, R.; AlMekhled, D.; Thomas, R.; Sindhu, S.; Ahmad, R. Increasing the Duration of Light Physical Activity Ameliorates Insulin Resistance Syndrome in Metabolically Healthy Obese Adults. Cells 2020, 9, 1189. [Google Scholar] [CrossRef]
- Tucker, L.A. Fiber Intake and Insulin Resistance in 6374 Adults: The Role of Abdominal Obesity. Nutrients 2018, 10, 237. [Google Scholar] [CrossRef] [Green Version]
- Greco, M.; Chiefari, E.; Montalcini, T.; Accattato, F.; Costanzo, F.S.; Pujia, A.; Foti, D.; Brunetti, A.; Gulletta, E. Early effects of a hypocaloric, Mediterranean diet on laboratory parameters in obese individuals. Mediat. Inflamm. 2014, 2014, 750860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barnard, N.D.; Cohen, J.; Jenkins, D.J.; Turner-McGrievy, G.; Gloede, L.; Jaster, B.; Seidl, K.; Green, A.A.; Talpers, S. A low-fat vegan diet improves glycemic control and cardiovascular risk factors in a randomized clinical trial in individuals with type 2 diabetes. Diabetes Care 2006, 29, 1777–1783. [Google Scholar] [CrossRef] [Green Version]
- Lee, Y.; Park, K. Adherence to a Vegetarian Diet and Diabetes Risk: A Systematic Review and Meta-Analysis of Observational Studies. Nutrients 2017, 9, 603. [Google Scholar] [CrossRef] [Green Version]
- Valdes, A.M.; Walter, J.; Segal, E.; Spector, T.D. Role of the gut microbiota in nutrition and health. BMJ 2018, 361, k2179. [Google Scholar] [CrossRef] [Green Version]
- Khosravi, A.; Mazmanian, S.K. Disruption of the gut microbiome as a risk factor for microbial infections. Curr. Opin. Microbiol. 2013, 16, 221–227. [Google Scholar] [CrossRef] [Green Version]
- Voth, E.; Khanna, S. Fecal microbiota transplantation for treatment of patients with recurrent Clostridioides difficile infection. Expert Rev. Anti Infect. Ther. 2020, 1–8. [Google Scholar] [CrossRef]
- Rinninella, E.; Cintoni, M.; Raoul, P.; Lopetuso, L.R.; Scaldaferri, F.; Pulcini, G.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. Food Components and Dietary Habits: Keys for a Healthy Gut Microbiota Composition. Nutrients 2019, 11, 2393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; Batto, J.M.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.A.D.; Gasbarrini, A.; Mele, M.C. What is the Healthy Gut Microbiota Composition? A Changing Ecosystem across Age, Environment, Diet, and Diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, F.; Li, N.; Shi, J.; Li, H.; Yue, Y.; Jiao, W.; Wang, N.; Song, Y.; Huo, G.; Li, B. Lactobacillus acidophilus alleviates type 2 diabetes by regulating hepatic glucose, lipid metabolism and gut microbiota in mice. Food Funct. 2019, 10, 5804–5815. [Google Scholar] [CrossRef] [PubMed]
- Ejtahed, H.S.; Hoseini-Tavassol, Z.; Khatami, S.; Zangeneh, M.; Behrouzi, A.; Ahmadi Badi, S.; Moshiri, A.; Hasani-Ranjbar, S.; Soroush, A.R.; Vaziri, F.; et al. Main gut bacterial composition differs between patients with type 1 and type 2 diabetes and non-diabetic adults. J. Diabetes Metab. Disord. 2020, 19, 265–271. [Google Scholar] [CrossRef]
- Zinöcker, M.K.; Lindseth, I.A. The Western Diet-Microbiome-Host Interaction and Its Role in Metabolic Disease. Nutrients 2018, 10, 365. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Fang, L.; Chen, S.; Zhou, H.; Fan, Y.; Lin, L.; Li, J.; Xu, J.; Chen, Y.; Ma, Y.; et al. Gut Microbiome Alterations Precede Cerebral Amyloidosis and Microglial Pathology in a Mouse Model of Alzheimer’s Disease. Biomed. Res. Int. 2020, 2020, 8456596. [Google Scholar] [CrossRef]
- Minato, T.; Maeda, T.; Fujisawa, Y.; Tsuji, H.; Nomoto, K.; Ohno, K.; Hirayama, M. Progression of Parkinson’s disease is associated with gut dysbiosis: Two-year follow-up study. PLoS ONE 2017, 12, e0187307. [Google Scholar] [CrossRef] [Green Version]
- Vogt, N.M.; Kerby, R.L.; Dill-McFarland, K.A.; Harding, S.J.; Merluzzi, A.P.; Johnson, S.C.; Carlsson, C.M.; Asthana, S.; Zetterberg, H.; Blennow, K.; et al. Gut microbiome alterations in Alzheimer’s disease. Sci. Rep. 2017, 7, 13537. [Google Scholar] [CrossRef]
- Cattaneo, A.; Cattane, N.; Galluzzi, S.; Provasi, S.; Lopizzo, N.; Festari, C.; Ferrari, C.; Guerra, U.P.; Paghera, B.; Muscio, C.; et al. Association of brain amyloidosis with pro-inflammatory gut bacterial taxa and peripheral inflammation markers in cognitively impaired elderly. Neurobiol. Aging 2017, 49, 60–68. [Google Scholar] [CrossRef] [Green Version]
- Strandwitz, P.; Kim, K.H.; Terekhova, D.; Liu, J.K.; Sharma, A.; Levering, J.; McDonald, D.; Dietrich, D.; Ramadhar, T.R.; Lekbua, A.; et al. GABA-modulating bacteria of the human gut microbiota. Nat. Microbiol. 2019, 4, 396–403. [Google Scholar] [CrossRef]
- Huang, D.; Liu, D.; Yin, J.; Qian, T.; Shrestha, S.; Ni, H. Glutamate-glutamine and GABA in brain of normal aged and patients with cognitive impairment. Eur. Radiol. 2017, 27, 2698–2705. [Google Scholar] [CrossRef] [PubMed]
- Riccio, P.; Rossano, R. Undigested Food and Gut Microbiota May Cooperate in the Pathogenesis of Neuroinflammatory Diseases: A Matter of Barriers and a Proposal on the Origin of Organ Specificity. Nutrients 2019, 11, 2714. [Google Scholar] [CrossRef] [Green Version]
- Hugenholtz, F.; de Vos, W.M. Mouse models for human intestinal microbiota research: A critical evaluation. Cell Mol. Life Sci. 2018, 75, 149–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, T.L.; Vieira-Silva, S.; Liston, A.; Raes, J. How informative is the mouse for human gut microbiota research? Dis. Model Mech. 2015, 8, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Feng, Q.; Liang, S.; Sonne, S.B.; Xia, Z.; Qiu, X.; Li, X.; Long, H.; Zhang, J.; Zhang, D.; et al. A catalog of the mouse gut metagenome. Nat. Biotechnol. 2015, 33, 1103–1108. [Google Scholar] [CrossRef] [PubMed]
- Ogata, H.; Goto, S.; Sato, K.; Fujibuchi, W.; Bono, H.; Kanehisa, M. KEGG: Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 1999, 27, 29–34. [Google Scholar] [CrossRef] [Green Version]
- Chung, Y.W.; Gwak, H.J.; Moon, S.; Rho, M.; Ryu, J.H. Functional dynamics of bacterial species in the mouse gut microbiome revealed by metagenomic and metatranscriptomic analyses. PLoS ONE 2020, 15, e0227886. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senghor, B.; Sokhna, C.; Ruimy, R.; Lagier, J.-C. Gut microbiota diversity according to dietary habits and geographical provenance. Hum. Microbiome J. 2018, 7–8, 1–9. [Google Scholar] [CrossRef]
- Fransen, F.; van Beek, A.A.; Borghuis, T.; Aidy, S.E.; Hugenholtz, F.; van der Gaast-de Jongh, C.; Savelkoul, H.F.J.; De Jonge, M.I.; Boekschoten, M.V.; Smidt, H.; et al. Aged Gut Microbiota Contributes to Systemical Inflammaging after Transfer to Germ-Free Mice. Front. Immunol. 2017, 8, 1385. [Google Scholar] [CrossRef] [Green Version]
- Elderman, M.; Hugenholtz, F.; Belzer, C.; Boekschoten, M.; van Beek, A.; de Haan, B.; Savelkoul, H.; de Vos, P.; Faas, M. Sex and strain dependent differences in mucosal immunology and microbiota composition in mice. Biol. Sex Differ. 2018, 9, 26. [Google Scholar] [CrossRef] [PubMed]
- Maldonado Weng, J.; Parikh, I.; Naqib, A.; York, J.; Green, S.J.; Estus, S.; LaDu, M.J. Synergistic effects of APOE and sex on the gut microbiome of young EFAD transgenic mice. Mol. Neurodegener. 2019, 14, 47. [Google Scholar] [CrossRef]
- Sanguinetti, E.; Collado, M.C.; Marrachelli, V.G.; Monleon, D.; Selma-Royo, M.; Pardo-Tendero, M.M.; Burchielli, S.; Iozzo, P. Microbiome-metabolome signatures in mice genetically prone to develop dementia, fed a normal or fatty diet. Sci. Rep. 2018, 8, 4907. [Google Scholar] [CrossRef] [Green Version]
- Bauerl, C.; Collado, M.C.; Diaz Cuevas, A.; Vina, J.; Perez Martinez, G. Shifts in gut microbiota composition in an APP/PSS1 transgenic mouse model of Alzheimer’s disease during lifespan. Lett. Appl. Microbiol. 2018, 66, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Wu, L.; Peng, G.; Han, Y.; Tang, R.; Ge, J.; Zhang, L.; Jia, L.; Yue, S.; Zhou, K.; et al. Altered microbiomes distinguish Alzheimer’s disease from amnestic mild cognitive impairment and health in a Chinese cohort. Brain Behav. Immun. 2019, 80, 633–643. [Google Scholar] [CrossRef]
- Stadlbauer, V.; Engertsberger, L.; Komarova, I.; Feldbacher, N.; Leber, B.; Pichler, G.; Fink, N.; Scarpatetti, M.; Schippinger, W.; Schmidt, R.; et al. Dysbiosis, gut barrier dysfunction and inflammation in dementia: A pilot study. BMC Geriatr. 2020, 20, 248. [Google Scholar] [CrossRef]
- Peng, W.; Yi, P.; Yang, J.; Xu, P.; Wang, Y.; Zhang, Z.; Huang, S.; Wang, Z.; Zhang, C. Association of gut microbiota composition and function with a senescence-accelerated mouse model of Alzheimer’s Disease using 16S rRNA gene and metagenomic sequencing analysis. Aging 2018, 10, 4054–4065. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Y.; Xiayu, X.; Shi, C.; Chen, W.; Song, N.; Fu, X.; Zhou, R.; Xu, Y.F.; Huang, L.; et al. Altered Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2017, 60, 1241–1257. [Google Scholar] [CrossRef]
- Lukiw, W.J. Gastrointestinal (GI) Tract Microbiome-Derived Neurotoxins-Potent Neuro-Inflammatory Signals From the GI Tract via the Systemic Circulation into the Brain. Front. Cell. Infect. Microbiol. 2020, 10, 22. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Jaber, V.; Lukiw, W.J. Secretory Products of the Human GI Tract Microbiome and Their Potential Impact on Alzheimer’s Disease (AD): Detection of Lipopolysaccharide (LPS) in AD Hippocampus. Front. Cell. Infect. Microbiol. 2017, 7, 318. [Google Scholar] [CrossRef] [PubMed]
- Hoogland, I.C.M.; Westhoff, D.; Engelen-Lee, J.Y.; Melief, J.; Valls Seron, M.; Houben-Weerts, J.; Huitinga, I.; van Westerloo, D.J.; van der Poll, T.; van Gool, W.A.; et al. Microglial Activation after Systemic Stimulation with Lipopolysaccharide and Escherichia coli. Front. Cell. Neurosci. 2018, 12, 110. [Google Scholar] [CrossRef] [Green Version]
- Luu, M.; Weigand, K.; Wedi, F.; Breidenbend, C.; Leister, H.; Pautz, S.; Adhikary, T.; Visekruna, A. Regulation of the effector function of CD8(+) T cells by gut microbiota-derived metabolite butyrate. Sci. Rep. 2018, 8, 14430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silva, Y.P.; Bernardi, A.; Frozza, R.L. The Role of Short-Chain Fatty Acids from Gut Microbiota in Gut-Brain Communication. Front. Endocrinol. 2020, 11, 25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, M.; van Esch, B.; Wagenaar, G.T.M.; Garssen, J.; Folkerts, G.; Henricks, P.A.J. Pro- and anti-inflammatory effects of short chain fatty acids on immune and endothelial cells. Eur. J. Pharm. 2018, 831, 52–59. [Google Scholar] [CrossRef]
- Ho, L.; Ono, K.; Tsuji, M.; Mazzola, P.; Singh, R.; Pasinetti, G.M. Protective roles of intestinal microbiota derived short chain fatty acids in Alzheimer’s disease-type beta-amyloid neuropathological mechanisms. Expert Rev. Neurother. 2018, 18, 83–90. [Google Scholar] [CrossRef] [Green Version]
- Syeda, T.; Sanchez-Tapia, M.; Pinedo-Vargas, L.; Granados, O.; Cuervo-Zanatta, D.; Rojas-Santiago, E.; Diaz-Cintra, S.A.; Torres, N.; Perez-Cruz, C. Bioactive Food Abates Metabolic and Synaptic Alterations by Modulation of Gut Microbiota in a Mouse Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2018, 66, 1657–1682. [Google Scholar] [CrossRef]
- Burokas, A.; Arboleya, S.; Moloney, R.D.; Peterson, V.L.; Murphy, K.; Clarke, G.; Stanton, C.; Dinan, T.G.; Cryan, J.F. Targeting the Microbiota-Gut-Brain Axis: Prebiotics Have Anxiolytic and Antidepressant-like Effects and Reverse the Impact of Chronic Stress in Mice. Biol. Psychiatry 2017, 82, 472–487. [Google Scholar] [CrossRef] [PubMed]
- Lundberg, R.; Toft, M.F.; Metzdorff, S.B.; Hansen, C.H.F.; Licht, T.R.; Bahl, M.I.; Hansen, A.K. Human microbiota-transplanted C57BL/6 mice and offspring display reduced establishment of key bacteria and reduced immune stimulation compared to mouse microbiota-transplantation. Sci. Rep. 2020, 10, 7805. [Google Scholar] [CrossRef]
- Erny, D.; Hrabe de Angelis, A.L.; Jaitin, D.; Wieghofer, P.; Staszewski, O.; David, E.; Keren-Shaul, H.; Mahlakoiv, T.; Jakobshagen, K.; Buch, T.; et al. Host microbiota constantly control maturation and function of microglia in the CNS. Nat. Neurosci. 2015, 18, 965–977. [Google Scholar] [CrossRef]
- Matcovitch-Natan, O.; Winter, D.R.; Giladi, A.; Vargas Aguilar, S.; Spinrad, A.; Sarrazin, S.; Ben-Yehuda, H.; David, E.; Zelada Gonzalez, F.; Perrin, P.; et al. Microglia development follows a stepwise program to regulate brain homeostasis. Science 2016, 353, aad8670. [Google Scholar] [CrossRef] [PubMed]
- Harach, T.; Marungruang, N.; Duthilleul, N.; Cheatham, V.; Mc Coy, K.D.; Frisoni, G.; Neher, J.J.; Fak, F.; Jucker, M.; Lasser, T.; et al. Reduction of Abeta amyloid pathology in APPPS1 transgenic mice in the absence of gut microbiota. Sci. Rep. 2017, 7, 41802. [Google Scholar] [CrossRef] [PubMed]
- Manocha, G.D.; Floden, A.M.; Miller, N.M.; Smith, A.J.; Nagamoto-Combs, K.; Saito, T.; Saido, T.C.; Combs, C.K. Temporal progression of Alzheimer’s disease in brains and intestines of transgenic mice. Neurobiol. Aging 2019, 81, 166–176. [Google Scholar] [CrossRef]
- Pellegrini, C.; Daniele, S.; Antonioli, L.; Benvenuti, L.; D’Antongiovanni, V.; Piccarducci, R.; Pietrobono, D.; Citi, V.; Piragine, E.; Flori, L.; et al. Prodromal Intestinal Events in Alzheimer’s Disease (AD): Colonic Dysmotility and Inflammation Are Associated with Enteric AD-Related Protein Deposition. Int. J. Mol. Sci. 2020, 21, 3523. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.H.; Kim, J.; Lee, M.J.; Kim, Y. Abnormalities of plasma cytokines and spleen in senile APP/PS1/Tau transgenic mouse model. Sci. Rep. 2015, 5, 15703. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.S.; Kim, Y.; Choi, H.; Kim, W.; Park, S.; Lee, D.; Kim, D.K.; Kim, H.J.; Choi, H.; Hyun, D.W.; et al. Transfer of a healthy microbiota reduces amyloid and tau pathology in an Alzheimer’s disease animal model. Gut 2020, 69, 283–294. [Google Scholar] [CrossRef]
- Minter, M.R.; Zhang, C.; Leone, V.; Ringus, D.L.; Zhang, X.; Oyler-Castrillo, P.; Musch, M.W.; Liao, F.; Ward, J.F.; Holtzman, D.M.; et al. Antibiotic-induced perturbations in gut microbial diversity influences neuro-inflammation and amyloidosis in a murine model of Alzheimer’s disease. Sci. Rep. 2016, 6, 30028. [Google Scholar] [CrossRef]
- Sun, J.; Xu, J.; Ling, Y.; Wang, F.; Gong, T.; Yang, C.; Ye, S.; Ye, K.; Wei, D.; Song, Z.; et al. Fecal microbiota transplantation alleviated Alzheimer’s disease-like pathogenesis in APP/PS1 transgenic mice. Transl. Psychiatry 2019, 9, 189. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Ahn, E.H.; Kang, S.S.; Liu, X.; Alam, A.; Ye, K. Gut dysbiosis contributes to amyloid pathology, associated with C/EBPbeta/AEP signaling activation in Alzheimer’s disease mouse model. Sci. Adv. 2020, 6, eaba0466. [Google Scholar] [CrossRef]
- Shen, L.; Liu, L.; Ji, H.F. Alzheimer’s Disease Histological and Behavioral Manifestations in Transgenic Mice Correlate with Specific Gut Microbiome State. J. Alzheimer’s Dis. 2017, 56, 385–390. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Guilherme, M.; Todorov, H.; Osterhof, C.; Mollerke, A.; Cub, K.; Hankeln, T.; Gerber, S.; Endres, K. Impact of Acute and Chronic Amyloid-beta Peptide Exposure on Gut Microbial Commensals in the Mouse. Front. Microbiol. 2020, 11, 1008. [Google Scholar] [CrossRef]
- Lee, H.J.; Lee, K.E.; Kim, J.K.; Kim, D.H. Suppression of gut dysbiosis by Bifidobacterium longum alleviates cognitive decline in 5XFAD transgenic and aged mice. Sci. Rep. 2019, 9, 11814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.; Jiang, W.; Ouyang, T.; Shen, X.Y.; Wang, F.; Qu, Y.H.; Zhang, M.; Luo, T.; Wang, H.Q. Jatrorrhizine Balances the Gut Microbiota and Reverses Learning and Memory Deficits in APP/PS1 transgenic mice. Sci. Rep. 2019, 9, 19575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, J.Y.; Choi, J.; Lee, Y.; Lee, J.E.; Lee, E.H.; Kwon, H.J.; Yang, J.; Jeong, B.R.; Kim, Y.K.; Han, P.L. Metagenome Analysis of Bodily Microbiota in a Mouse Model of Alzheimer Disease Using Bacteria-derived Membrane Vesicles in Blood. Exp. Neurobiol. 2017, 26, 369–379. [Google Scholar] [CrossRef]
- Kaur, H.; Nagamoto-Combs, K.; Golovko, S.; Golovko, M.Y.; Klug, M.G.; Combs, C.K. Probiotics ameliorate intestinal pathophysiology in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2020, 92, 114–134. [Google Scholar] [CrossRef]
- Honarpisheh, P.; Reynolds, C.R.; Blasco Conesa, M.P.; Moruno Manchon, J.F.; Putluri, N.; Bhattacharjee, M.B.; Urayama, A.; McCullough, L.D.; Ganesh, B.P. Dysregulated Gut Homeostasis Observed Prior to the Accumulation of the Brain Amyloid-beta in Tg2576 Mice. Int. J. Mol. Sci. 2020, 21, 1711. [Google Scholar] [CrossRef] [Green Version]
- Brandscheid, C.; Schuck, F.; Reinhardt, S.; Schafer, K.H.; Pietrzik, C.U.; Grimm, M.; Hartmann, T.; Schwiertz, A.; Endres, K. Altered Gut Microbiome Composition and Tryptic Activity of the 5xFAD Alzheimer’s Mouse Model. J. Alzheimer’s Dis. 2017, 56, 775–788. [Google Scholar] [CrossRef]
- Sun, J.; Liu, S.; Ling, Z.; Wang, F.; Ling, Y.; Gong, T.; Fang, N.; Ye, S.; Si, J.; Liu, J. Fructooligosaccharides Ameliorating Cognitive Deficits and Neurodegeneration in APP/PS1 Transgenic Mice through Modulating Gut Microbiota. J. Agric. Food Chem. 2019, 67, 3006–3017. [Google Scholar] [CrossRef]
- Parada Venegas, D.; De la Fuente, M.K.; Landskron, G.; Gonzalez, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short Chain Fatty Acids (SCFAs)-Mediated Gut Epithelial and Immune Regulation and Its Relevance for Inflammatory Bowel Diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef] [Green Version]
- Palm, N.W.; de Zoete, M.R.; Cullen, T.W.; Barry, N.A.; Stefanowski, J.; Hao, L.; Degnan, P.H.; Hu, J.; Peter, I.; Zhang, W.; et al. Immunoglobulin A coating identifies colitogenic bacteria in inflammatory bowel disease. Cell 2014, 158, 1000–1010. [Google Scholar] [CrossRef] [Green Version]
- Dinh, D.M.; Volpe, G.E.; Duffalo, C.; Bhalchandra, S.; Tai, A.K.; Kane, A.V.; Wanke, C.A.; Ward, H.D. Intestinal microbiota, microbial translocation, and systemic inflammation in chronic HIV infection. J. Infect. Dis. 2015, 211, 19–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wakita, Y.; Shimomura, Y.; Kitada, Y.; Yamamoto, H.; Ohashi, Y.; Matsumoto, M. Taxonomic classification for microbiome analysis, which correlates well with the metabolite milieu of the gut. BMC Microbiol. 2018, 18, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.S.; Wang, R.X.; Alexeev, E.E.; Lanis, J.M.; Battista, K.D.; Glover, L.E.; Colgan, S.P. Hypoxanthine is a checkpoint stress metabolite in colonic epithelial energy modulation and barrier function. J. Biol. Chem. 2018, 293, 6039–6051. [Google Scholar] [CrossRef] [Green Version]
- Kaakoush, N.O. Sutterella Species, IgA-degrading Bacteria in Ulcerative Colitis. Trends Microbiol. 2020, 28, 519–522. [Google Scholar] [CrossRef]
- Wang, L.; Christophersen, C.T.; Sorich, M.J.; Gerber, J.P.; Angley, M.T.; Conlon, M.A. Increased abundance of Sutterella spp. and Ruminococcus torques in feces of children with autism spectrum disorder. Mol. Autism 2013, 4, 42. [Google Scholar] [CrossRef] [Green Version]
- Hiippala, K.; Kainulainen, V.; Kalliomaki, M.; Arkkila, P.; Satokari, R. Mucosal Prevalence and Interactions with the Epithelium Indicate Commensalism of Sutterella spp. Front. Microbiol. 2016, 7, 1706. [Google Scholar] [CrossRef] [Green Version]
- Zhai, R.; Xue, X.; Zhang, L.; Yang, X.; Zhao, L.; Zhang, C. Strain-Specific Anti-inflammatory Properties of Two Akkermansia muciniphila Strains on Chronic Colitis in Mice. Front. Cell. Infect. Microbiol. 2019, 9, 239. [Google Scholar] [CrossRef]
- Haran, J.P.; Bhattarai, S.K.; Foley, S.E.; Dutta, P.; Ward, D.V.; Bucci, V.; McCormick, B.A. Alzheimer’s Disease Microbiome Is Associated with Dysregulation of the Anti-Inflammatory P-Glycoprotein Pathway. mBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sanchez, B.; Delgado, S.; Blanco-Miguez, A.; Lourenco, A.; Gueimonde, M.; Margolles, A. Probiotics, gut microbiota, and their influence on host health and disease. Mol. Nutr. Food Res. 2017, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonfili, L.; Cecarini, V.; Berardi, S.; Scarpona, S.; Suchodolski, J.S.; Nasuti, C.; Fiorini, D.; Boarelli, M.C.; Rossi, G.; Eleuteri, A.M. Microbiota modulation counteracts Alzheimer’s disease progression influencing neuronal proteolysis and gut hormones plasma levels. Sci. Rep. 2017, 7, 2426. [Google Scholar] [CrossRef]
- Abraham, D.; Feher, J.; Scuderi, G.L.; Szabo, D.; Dobolyi, A.; Cservenak, M.; Juhasz, J.; Ligeti, B.; Pongor, S.; Gomez-Cabrera, M.C.; et al. Exercise and probiotics attenuate the development of Alzheimer’s disease in transgenic mice: Role of microbiome. Exp. Gerontol. 2019, 115, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Amakye, W.K.; Guo, L.; Gong, C.; Zhao, Y.; Yao, M.; Ren, J. Walnut-Derived Peptide PW5 Ameliorates Cognitive Impairments and Alters Gut Microbiota in APP/PS1 Transgenic Mice. Mol. Nutr. Food Res. 2019, 63, e1900326. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, N.B.; Bryrup, T.; Allin, K.H.; Nielsen, T.; Hansen, T.H.; Pedersen, O. Alterations in fecal microbiota composition by probiotic supplementation in healthy adults: A systematic review of randomized controlled trials. Genome Med. 2016, 8, 52. [Google Scholar] [CrossRef] [Green Version]
- Shokryazdan, P.; Faseleh Jahromi, M.; Navidshad, B.; Liang, J.B. Effects of prebiotics on immune system and cytokine expression. Med. Microbiol. Immunol. 2017, 206, 1–9. [Google Scholar] [CrossRef]
- Wahl, D.; Solon-Biet, S.M.; Wang, Q.P.; Wali, J.A.; Pulpitel, T.; Clark, X.; Raubenheimer, D.; Senior, A.M.; Sinclair, D.A.; Cooney, G.J.; et al. Comparing the Effects of Low-Protein and High-Carbohydrate Diets and Caloric Restriction on Brain Aging in Mice. Cell Rep. 2018, 25, 2234–2243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattson, M.P.; Moehl, K.; Ghena, N.; Schmaedick, M.; Cheng, A. Intermittent metabolic switching, neuroplasticity and brain health. Nat. Rev. Neurosci. 2018, 19, 63–80. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Arumugam, T.V. Hallmarks of Brain Aging: Adaptive and Pathological Modification by Metabolic States. Cell Metab. 2018, 27, 1176–1199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Cabo, R.; Mattson, M.P. Effects of Intermittent Fasting on Health, Aging, and Disease. N. Engl. J. Med. 2019, 381, 2541–2551. [Google Scholar] [CrossRef]
- Nasaruddin, M.L.; Syed Abd Halim, S.A.; Kamaruzzaman, M.A. Studying the Relationship of Intermittent Fasting and beta-Amyloid in Animal Model of Alzheimer’s Disease: A Scoping Review. Nutrients 2020, 12, 3215. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Q.; Zhou, J.; Wu, X.; Zhu, Q. beta hydroxybutyrate levels in serum and cerebrospinal fluid under ketone body metabolism in rats. Exp. Anim. 2017, 66, 177–182. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Zhan, Z.; Zhang, J.; Zhou, F.; An, L. beta-Hydroxybutyrate Ameliorates Abeta-Induced Downregulation of TrkA Expression by Inhibiting HDAC1/3 in SH-SY5Y Cells. Am. J. Alzheimer’s Dis. Other Demen. 2020, 35, 1533317519883496. [Google Scholar] [CrossRef] [Green Version]
- Xie, G.; Tian, W.; Wei, T.; Liu, F. The neuroprotective effects of beta-hydroxybutyrate on Abeta-injected rat hippocampus in vivo and in Abeta-treated PC-12 cells in vitro. Free Radic. Res. 2015, 49, 139–150. [Google Scholar] [CrossRef] [PubMed]
- Hu, E.; Du, H.; Shang, S.; Zhang, Y.; Lu, X. Beta-Hydroxybutyrate Enhances BDNF Expression by Increasing H3K4me3 and Decreasing H2AK119ub in Hippocampal Neurons. Front. Neurosci. 2020, 14, 591177. [Google Scholar] [CrossRef] [PubMed]
- Dabke, P.; Das, A.M. Mechanism of Action of Ketogenic Diet Treatment: Impact of Decanoic Acid and Beta-Hydroxybutyrate on Sirtuins and Energy Metabolism in Hippocampal Murine Neurons. Nutrients 2020, 12, 2379. [Google Scholar] [CrossRef]
- Lazic, D.; Tesic, V.; Jovanovic, M.; Brkic, M.; Milanovic, D.; Zlokovic, B.V.; Kanazir, S.; Perovic, M. Every-other-day feeding exacerbates inflammation and neuronal deficits in 5XFAD mouse model of Alzheimer’s disease. Neurobiol. Dis. 2020, 136, 104745. [Google Scholar] [CrossRef]
- Zhang, J.; Zhan, Z.; Li, X.; Xing, A.; Jiang, C.; Chen, Y.; Shi, W.; An, L. Intermittent Fasting Protects against Alzheimer’s Disease Possible through Restoring Aquaporin-4 Polarity. Front. Mol. Neurosci. 2017, 10, 395. [Google Scholar] [CrossRef]
- Liu, Y.; Cheng, A.; Li, Y.J.; Yang, Y.; Kishimoto, Y.; Zhang, S.; Wang, Y.; Wan, R.; Raefsky, S.M.; Lu, D.; et al. SIRT3 mediates hippocampal synaptic adaptations to intermittent fasting and ameliorates deficits in APP mutant mice. Nat. Commun. 2019, 10, 1886. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Zhang, Y.; Wei, X.; Zang, J.; Liu, Y.; Wang, Y.; Gong, C.X.; Wei, W. Intermittent fasting promotes adult hippocampal neuronal differentiation by activating GSK-3beta in 3xTg-AD mice. J. Neurochem. 2020. [Google Scholar] [CrossRef]
- Kashiwaya, Y.; Bergman, C.; Lee, J.H.; Wan, R.; King, M.T.; Mughal, M.R.; Okun, E.; Clarke, K.; Mattson, M.P.; Veech, R.L. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol. Aging 2013, 34, 1530–1539. [Google Scholar] [CrossRef] [Green Version]
- Schnurr, T.M.; Jakupovic, H.; Carrasquilla, G.D.; Angquist, L.; Grarup, N.; Sorensen, T.I.A.; Tjonneland, A.; Overvad, K.; Pedersen, O.; Hansen, T.; et al. Obesity, unfavourable lifestyle and genetic risk of type 2 diabetes: A case-cohort study. Diabetologia 2020, 63, 1324–1332. [Google Scholar] [CrossRef]
- Tremblay, J.; Hamet, P. Environmental and genetic contributions to diabetes. Metabolism 2019, 100S, 153952. [Google Scholar] [CrossRef]
- Lindenberg, F.C.B.; Ellekilde, M.; Thorn, A.C.; Kihl, P.; Larsen, C.S.; Hansen, C.H.F.; Metzdorff, S.B.; Aalbaek, B.; Hansen, A.K. Dietary LPS traces influences disease expression of the diet-induced obese mouse. Res. Vet Sci. 2019, 123, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Bingxuan Wang, P.; Chandrasekera, C.; Pippin, J.J. Leptin- and Leptin Receptor-Deficient Rodent Models: Relevance for Human Type 2 Diabetes. Curr. Diabetes Rev. 2014, 10, 131–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avtanski, D.; Pavlov, V.A.; Tracey, K.J.; Poretsky, L. Characterization of inflammation and insulin resistance in high-fat diet-induced male C57BL/6J mouse model of obesity. Anim. Model. Exp. Med. 2019, 2, 252–258. [Google Scholar] [CrossRef]
- Cao, Y.; Sun, Y.; Zou, S.; Duan, B.; Sun, M.; Xu, X. Yeast beta-Glucan Suppresses the Chronic Inflammation and Improves the Microenvironment in Adipose Tissues of ob/ob Mice. J Agric Food Chem 2018, 66, 621–629. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.L.; Bridson, T.L.; Alim, M.A.; Rush, C.M.; Rudd, D.M.; Govan, B.L.; Ketheesan, N. Development of a diet-induced murine model of diabetes featuring cardinal metabolic and pathophysiological abnormalities of type 2 diabetes. Biol. Open 2016, 5, 1149–1162. [Google Scholar] [CrossRef] [Green Version]
- Preguica, I.; Alves, A.; Nunes, S.; Gomes, P.; Fernandes, R.; Viana, S.D.; Reis, F. Diet-Induced Rodent Models of Diabetic Peripheral Neuropathy, Retinopathy and Nephropathy. Nutrients 2020, 12, 250. [Google Scholar] [CrossRef] [Green Version]
- Showalter, M.R.; Nonnecke, E.B.; Linderholm, A.L.; Cajka, T.; Sa, M.R.; Lonnerdal, B.; Kenyon, N.J.; Fiehn, O. Obesogenic diets alter metabolism in mice. PLoS ONE 2018, 13, e0190632. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez-Correa, E.; Gonzalez-Perez, I.; Clavel-Perez, P.I.; Contreras-Vargas, Y.; Carvajal, K. Biochemical and nutritional overview of diet-induced metabolic syndrome models in rats: What is the best choice? Nutr. Diabetes 2020, 10, 24. [Google Scholar] [CrossRef]
- Gilani, A.; Pandey, V.; Garcia, V.; Agostinucci, K.; Singh, S.P.; Schragenheim, J.; Bellner, L.; Falck, J.R.; Paudyal, M.P.; Capdevila, J.H.; et al. High-fat diet-induced obesity and insulin resistance in CYP4a14(-/-) mice is mediated by 20-HETE. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2018, 315, R934–R944. [Google Scholar] [CrossRef] [Green Version]
- Rocha, V.D.S.; Claudio, E.R.G.; da Silva, V.L.; Cordeiro, J.P.; Domingos, L.F.; da Cunha, M.R.H.; Mauad, H.; do Nascimento, T.B.; Lima-Leopoldo, A.P.; Leopoldo, A.S. High-Fat Diet-Induced Obesity Model Does Not Promote Endothelial Dysfunction via Increasing Leptin/Akt/eNOS Signaling. Front. Physiol. 2019, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Ussar, S.; Griffin, N.W.; Bezy, O.; Fujisaka, S.; Vienberg, S.; Softic, S.; Deng, L.; Bry, L.; Gordon, J.I.; Kahn, C.R. Interactions between Gut Microbiota, Host Genetics and Diet Modulate the Predisposition to Obesity and Metabolic Syndrome. Cell Metab. 2015, 22, 516–530. [Google Scholar] [CrossRef] [Green Version]
- You-Hua, X.; Chen-Lin, G.; Heng-Li, G.; Wen-Qian, Z.; Wei Huang, S.-S.T.; Wen-Jun, G.; Yong, X.; Hua, Z.; Quan, Z. Sodium Butyrate Supplementation Ameliorates Diabetic Inflammation in db/db Mice. J. Endocrinol. 2018, 238, 231–244. [Google Scholar]
- Burke, S.J.; Batdorf, H.M.; Burk, D.H.; Noland, R.C.; Eder, A.E.; Boulos, M.S.; Karlstad, M.D.; Collier, J.J. db/db Mice Exhibit Features of Human Type 2 Diabetes That Are Not Present in Weight-Matched C57BL/6J Mice Fed a Western Diet. J. Diabetes Res. 2017, 2017, 8503754. [Google Scholar] [CrossRef] [Green Version]
- Dey, A.; Hao, S.; Erion, J.R.; Wosiski-Kuhn, M.; Stranahan, A.M. Glucocorticoid sensitization of microglia in a genetic mouse model of obesity and diabetes. J. Neuroimmunol. 2014, 269, 20–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, W.; Xu, J.H.; Yu, T.; Chen, Q.K. Effects of berberine and metformin on intestinal inflammation and gut microbiome composition in db/db mice. Biomed. Pharm. 2019, 118, 109131. [Google Scholar] [CrossRef] [PubMed]
- Cui, S.; Qiao, L.; Yu, S.; Men, L.; Li, Y.; Li, F.; Du, J. The Antagonist of CXCR1 and CXCR2Protects db/db mice from Metabolic Diseasesthrough Modulating Inflammation. Am. J. Physiol. Endocrinol. Metab. 2019, 317, E1205–E1217. [Google Scholar] [CrossRef] [PubMed]
- Petersen, C.; Bharat, D.; Cutler, B.R.; Gholami, S.; Denetso, C.; Mueller, J.E.; Cho, J.M.; Kim, J.S.; Symons, J.D.; Anandh Babu, P.V. Circulating metabolites of strawberry mediate reductions in vascular inflammation and endothelial dysfunction in db/db mice. Int. J. Cardiol. 2018, 263, 111–117. [Google Scholar] [CrossRef]
- Steven, S.; Dib, M.; Hausding, M.; Kashani, F.; Oelze, M.; Kröller-Schön, S.; Hanf, A.; Daub, S.; Roohani, S.; Gramlich, Y.; et al. CD40L controls obesity-associated vascular inflammation, oxidative stress, and endothelial dysfunction in high fat diet-treated and db/db mice. Cardiovasc. Res. 2018, 114, 312–323. [Google Scholar] [CrossRef] [Green Version]
- Mracek, T.; Gao, D.; Tzanavari, T.; Bao, Y.; Xiao, X.; Stocker, C.; Trayhurn, P.; Bing, C. Downregulation of zinc-{alpha}2-glycoprotein in adipose tissue and liver of obese ob/ob mice and by tumour necrosis factor-alpha in adipocytes. J. Endocrinol. 2010, 204, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Sutter, A.G.; Palanisamy, A.P.; Lench, J.H.; Jessmore, A.P.; Chavin, K.D. Development of steatohepatitis in Ob/Ob mice is dependent on Toll-like receptor 4. Ann. Hepatol. 2015, 14, 735–743. [Google Scholar] [CrossRef]
- Ye, C.; Wang, R.; Wang, M.; Huang, Z.; Tang, C. Leptin alleviates intestinal mucosal barrier injury and inflammation in obese mice with acute pancreatitis. Int. J. Obes. 2018, 42, 1471–1479. [Google Scholar] [CrossRef] [PubMed]
- Sartori, M.; Conti, F.F.; Dias, D.D.S.; Dos Santos, F.; Machi, J.F.; Palomino, Z.; Casarini, D.E.; Rodrigues, B.; De Angelis, K.; Irigoyen, M.C. Association between Diastolic Dysfunction with Inflammation and Oxidative Stress in Females ob/ob Mice. Front. Physiol. 2017, 8, 572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Son, M.; Oh, S.; Choi, J.; Jang, J.T.; Choi, C.H.; Park, K.Y.; Son, K.H.; Byun, K. Attenuation of Inflammation and Leptin Resistance by Pyrogallol-Phloroglucinol-6,6-Bieckol on in the Brain of Obese Animal Models. Nutrients 2019, 11, 2773. [Google Scholar] [CrossRef] [Green Version]
- Zheng, W.; Zhou, J.; Song, S.; Kong, W.; Xia, W.; Chen, L.; Zeng, T. Dipeptidyl-Peptidase 4 Inhibitor Sitagliptin Ameliorates Hepatic Insulin Resistance by Modulating Inflammation and Autophagy in ob/ob Mice. Int. J. Endocrinol. 2018, 2018, 8309723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cani, P.D.; Possemiers, S.; Van de Wiele, T.; Guiot, Y.; Everard, A.; Rottier, O.; Geurts, L.; Naslain, D.; Neyrinck, A.; Lambert, D.M.; et al. Changes in gut microbiota control inflammation in obese mice through a mechanism involving GLP-2-driven improvement of gut permeability. Gut 2009, 58, 1091–1103. [Google Scholar] [CrossRef] [Green Version]
- Jena, P.K.; Sheng, L.; Di Lucente, J.; Jin, L.W.; Maezawa, I.; Wan, Y.Y. Dysregulated bile acid synthesis and dysbiosis are implicated in Western diet-induced systemic inflammation, microglial activation, and reduced neuroplasticity. FASEB J. 2018, 32, 2866–2877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heijden, R.A.v.d.; Sheedfar, F.; Morrison, M.C.; Hommelberg, P.P.; Kor, D.; Kloosterhuis, N.J.; Gruben, N.; Youssef, S.A.; Bruin, A.D.; Hofker, M.H.; et al. High-fat diet induced obesity primes inflammation in adipose tissue prior to liver in C57BL/6j mice. Aging 2015, 7, 256–267. [Google Scholar] [CrossRef] [Green Version]
- He, M.Q.; Wang, J.Y.; Wang, Y.; Sui, J.; Zhang, M.; Ding, X.; Zhao, Y.; Chen, Z.Y.; Ren, X.X.; Shi, B.Y. High-fat diet-induced adipose tissue expansion occurs prior to insulin resistance in C57BL/6J mice. Chronic Dis. Transl. Med. 2020, 6, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Tsuru, H.; Osaka, M.; Hiraoka, Y.; Yoshida, M. HFD-induced hepatic lipid accumulation and inflammation are decreased in Factor D deficient mouse. Sci. Rep. 2020, 10, 17593. [Google Scholar] [CrossRef]
- Schroeder, B.O.; Birchenough, G.M.H.; Pradhan, M.; Nystrom, E.E.L.; Henricsson, M.; Hansson, G.C.; Backhed, F. Obesity-associated microbiota contributes to mucus layer defects in genetically obese mice. J. Biol. Chem. 2020. [Google Scholar] [CrossRef]
- Singh, H.; Miyamoto, S.; Darshi, M.; Torralba, M.G.; Kwon, K.; Sharma, K.; Pieper, R. Gut Microbial Changes in Diabetic db/db Mice and Recovery of Microbial Diversity upon Pirfenidone Treatment. Microorganisms 2020, 8, 1347. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yao, G.; Sheng, Y.; Yang, L.; Wang, Z.; Yang, Z.; Zhuang, P.; Zhang, Y. JinQi Jiangtang Tablet Regulates Gut Microbiota and Improve Insulin Sensitivity in Type 2 Diabetes Mice. J. Diabetes Res. 2019, 2019, 1872134. [Google Scholar] [CrossRef] [Green Version]
- Nagpal, R.; Mishra, S.P.; Yadav, H. Unique Gut Microbiome Signatures Depict Diet-Versus Genetically Induced Obesity in Mice. Int. J. Mol. Sci. 2020, 21, 3434. [Google Scholar] [CrossRef]
- Liu, Z.; Dai, X.; Zhang, H.; Shi, R.; Hui, Y.; Jin, X.; Zhang, W.; Wang, L.; Wang, Q.; Wang, D.; et al. Gut microbiota mediates intermittent-fasting alleviation of diabetes-induced cognitive impairment. Nat. Commun. 2020, 11, 855. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, Z.; Shen, P.; Cheng, P.; Lu, Y.; Wang, A.; Sun, Z. Gut Bacteria Selectively Altered by Sennoside a Alleviate Type 2 Diabetes and Obesity Traits. Oxid. Med. Cell. Longev. 2020, 2020, 2375676. [Google Scholar] [CrossRef]
- Deng, N.; He, Z.; Guo, R.; Zheng, B.; Li, T.; Liu, R.H. Highland Barley Whole Grain (Hordeum vulgare L.) Ameliorates Hyperlipidemia by Modulating Cecal Microbiota, miRNAs, and AMPK Pathways in Leptin Receptor-Deficient db/db Mice. J. Agric. Food Chem. 2020, 68, 11735–11746. [Google Scholar] [CrossRef] [PubMed]
- Cai, T.T.; Ye, X.L.; Li, R.R.; Chen, H.; Wang, Y.Y.; Yong, H.J.; Pan, M.L.; Lu, W.; Tang, Y.; Miao, H.; et al. Resveratrol Modulates the Gut Microbiota and Inflammation to Protect against Diabetic Nephropathy in Mice. Front. Pharm. 2020, 11, 1249. [Google Scholar] [CrossRef] [PubMed]
- Chen, Q.; Ren, D.; Wu, J.; Yu, H.; Chen, X.; Wang, J.; Zhang, Y.; Liu, M.; Wang, T. Shenyan Kangfu tablet alleviates diabetic kidney disease through attenuating inflammation and modulating the gut microbiota. J. Nat. Med. 2020. [Google Scholar] [CrossRef]
- Yu, F.; Han, W.; Zhan, G.; Li, S.; Xiang, S.; Zhu, B.; Jiang, X.; Yang, L.; Luo, A.; Hua, F.; et al. Abnormal gut microbiota composition contributes to cognitive dysfunction in streptozotocin-induced diabetic mice. Aging (Albany N.Y.) 2019, 11, 3262–3279. [Google Scholar] [CrossRef]
- Bagarolli, R.A.; Tobar, N.; Oliveira, A.G.; Araujo, T.G.; Carvalho, B.M.; Rocha, G.Z.; Vecina, J.F.; Calisto, K.; Guadagnini, D.; Prada, P.O.; et al. Probiotics modulate gut microbiota and improve insulin sensitivity in DIO mice. J. Nutr. Biochem. 2017, 50, 16–25. [Google Scholar] [CrossRef] [Green Version]
- Kocsis, T.; Molnar, B.; Nemeth, D.; Hegyi, P.; Szakacs, Z.; Balint, A.; Garami, A.; Soos, A.; Marta, K.; Solymar, M. Probiotics have beneficial metabolic effects in patients with type 2 diabetes mellitus: A meta-analysis of randomized clinical trials. Sci. Rep. 2020, 10, 11787. [Google Scholar] [CrossRef]
- Di Renzo, L.; Gualtieri, P.; Romano, L.; Marrone, G.; Noce, A.; Pujia, A.; Perrone, M.A.; Aiello, V.; Colica, C.; De Lorenzo, A. Role of Personalized Nutrition in Chronic-Degenerative Diseases. Nutrients 2019, 11, 1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Yuan, T.; Dai, X.; Shi, L.; Liu, X. Intermittent Fasting Alleviates Diabetes-induced Cognitive Decline via Gut Microbiota-metabolites-brain Axis (OR32-04-19). Curr. Dev. Nutr. 2019, 3. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; You, L.J.; Huang, Q.; Fu, X.; Zhang, B.; Liu, R.H.; Li, C. Modulation of gut microbiota by mulberry fruit polysaccharide treatment of obese diabetic db/db mice. Food Funct. 2018, 9, 3732–3742. [Google Scholar] [CrossRef] [PubMed]
- Xie, Y.; Xiao, M.; Ni, Y.; Jiang, S.; Feng, G.; Sang, S.; Du, G. Alpinia oxyphylla Miq. Extract Prevents Diabetes in Mice by Modulating Gut Microbiota. J. Diabetes Res. 2018, 2018, 4230590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, M.; Liu, Y.; Li, M.; Cheng, M.; Zhang, Y.; Deng, G.; Kang, X.; Liu, H. Baicalin improves intestinal microecology and abnormal metabolism induced by high-fat diet. Eur. J. Pharm. 2019, 857, 172457. [Google Scholar] [CrossRef] [PubMed]
- Beli, E.; Yan, Y.; Moldovan, L.; Vieira, C.P.; Gao, R.; Duan, Y.; Prasad, R.; Bhatwadekar, A.; White, F.A.; Townsend, S.D.; et al. Restructuring of the Gut Microbiome by Intermittent Fasting Prevents Retinopathy and Prolongs Survival in db/db Mice. Diabetes 2018, 67, 1867–1879. [Google Scholar] [CrossRef] [Green Version]
- Biessels, G.J.; Despa, F. Cognitive decline and dementia in diabetes mellitus: Mechanisms and clinical implications. Nat. Rev. Endocrinol. 2018, 14, 591–604. [Google Scholar] [CrossRef]
- Craft, S. The role of metabolic disorders in Alzheimer disease and vascular dementia: Two roads converged. Arch. Neurol. 2009, 66, 300–305. [Google Scholar] [CrossRef] [Green Version]
- Moyse, E.; Haddad, M.; Benlabiod, C.; Ramassamy, C.; Krantic, S. Common Pathological Mechanisms and Risk Factors for Alzheimer’s Disease and Type-2 Diabetes: Focus on Inflammation. Curr. Alzheimer Res. 2019, 16, 986–1006. [Google Scholar] [CrossRef]
- Salas, I.H.; De Strooper, B. Diabetes and Alzheimer’s Disease: A Link not as Simple as it Seems. Neurochem. Res. 2019, 44, 1271–1278. [Google Scholar] [CrossRef] [Green Version]
- Davari, S.; Talaei, S.A.; Alaei, H.; Salami, M. Probiotics treatment improves diabetes-induced impairment of synaptic activity and cognitive function: Behavioral and electrophysiological proofs for microbiome-gut-brain axis. Neuroscience 2013, 240, 287–296. [Google Scholar] [CrossRef] [PubMed]
- Gao, K.; Yang, R.; Zhang, J.; Wang, Z.; Jia, C.; Zhang, F.; Li, S.; Wang, J.; Murtaza, G.; Xie, H.; et al. Effects of Qijian mixture on type 2 diabetes assessed by metabonomics, gut microbiota and network pharmacology. Pharm. Res. 2018, 130, 93–109. [Google Scholar] [CrossRef] [PubMed]
- Miklossy, J.; McGeer, P.L. Common mechanisms involved in Alzheimer’s disease and type 2 diabetes: A key role of chronic bacterial infection and inflammation. Aging 2016, 8, 575–588. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agusti, A.; Garcia-Pardo, M.P.; Lopez-Almela, I.; Campillo, I.; Maes, M.; Romani-Perez, M.; Sanz, Y. Interplay Between the Gut-Brain Axis, Obesity and Cognitive Function. Front. Neurosci. 2018, 12, 155. [Google Scholar] [CrossRef] [PubMed]
- Naseer, M.I.; Bibi, F.; Alqahtani, M.H.; Chaudhary, A.G.; Azhar, E.I.; Kamal, M.A.; Yasir, M. Role of gut microbiota in obesity, type 2 diabetes and Alzheimer’s disease. CNS Neurol. Disord. Drug Targets 2014, 13, 305–311. [Google Scholar] [CrossRef] [PubMed]
- Chan, E.S.; Chen, C.; Soong, T.W.; Wong, B.S. Differential Binding of Human ApoE Isoforms to Insulin Receptor is Associated with Aberrant Insulin Signaling in AD Brain Samples. Neuromol. Med. 2018, 20, 124–132. [Google Scholar] [CrossRef]
- Levy, O.; Lavalette, S.; Hu, S.J.; Housset, M.; Raoul, W.; Eandi, C.; Sahel, J.A.; Sullivan, P.M.; Guillonneau, X.; Sennlaub, F. APOE Isoforms Control Pathogenic Subretinal Inflammation in Age-Related Macular Degeneration. J. Neurosci. 2015, 35, 13568–13576. [Google Scholar] [CrossRef]
- Soto, I.; Graham, L.C.; Richter, H.J.; Simeone, S.N.; Radell, J.E.; Grabowska, W.; Funkhouser, W.K.; Howell, M.C.; Howell, G.R. APOE Stabilization by Exercise Prevents Aging Neurovascular Dysfunction and Complement Induction. PLoS Biol. 2015, 13, e1002279. [Google Scholar] [CrossRef] [Green Version]
- Zhao, N.; Liu, C.C.; Van Ingelgom, A.J.; Martens, Y.A.; Linares, C.; Knight, J.A.; Painter, M.M.; Sullivan, P.M.; Bu, G. Apolipoprotein E4 Impairs Neuronal Insulin Signaling by Trapping Insulin Receptor in the Endosomes. Neuron 2017, 96, 115–129.e115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youmans, K.L.; Tai, L.M.; Nwabuisi-Heath, E.; Jungbauer, L.; Kanekiyo, T.; Gan, M.; Kim, J.; Eimer, W.A.; Estus, S.; Rebeck, G.W.; et al. APOE4-specific changes in Abeta accumulation in a new transgenic mouse model of Alzheimer disease. J. Biol. Chem. 2012, 287, 41774–41786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, T.; Chu, C.; Shi, R.; Cui, T.; Zhang, X.; Zhao, Y.; Shi, X.; Hui, Y.; Pan, J.; Qian, R.; et al. ApoE-Dependent Protective Effects of Sesamol on High-Fat Diet-Induced Behavioral Disorders: Regulation of the Microbiome-Gut-Brain Axis. J. Agric. Food Chem. 2019, 67, 6190–6201. [Google Scholar] [CrossRef]
- Kothari, V.; Luo, Y.; Tornabene, T.; O’Neill, A.M.; Greene, M.W.; Geetha, T.; Babu, J.R. High fat diet induces brain insulin resistance and cognitive impairment in mice. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 499–508. [Google Scholar] [CrossRef] [PubMed]
- Janssen, C.I.; Jansen, D.; Mutsaers, M.P.; Dederen, P.J.; Geenen, B.; Mulder, M.T.; Kiliaan, A.J. The Effect of a High-Fat Diet on Brain Plasticity, Inflammation and Cognition in Female ApoE4-Knockin and ApoE-Knockout Mice. PLoS ONE 2016, 11, e0155307. [Google Scholar] [CrossRef] [Green Version]
- Theriault, P.; ElAli, A.; Rivest, S. High fat diet exacerbates Alzheimer’s disease-related pathology in APPswe/PS1 mice. Oncotarget 2016, 7, 67808–67827. [Google Scholar] [CrossRef] [Green Version]
- Medrano-Jimenez, E.; Jimenez-Ferrer Carrillo, I.; Pedraza-Escalona, M.; Ramirez-Serrano, C.E.; Alvarez-Arellano, L.; Cortes-Mendoza, J.; Herrera-Ruiz, M.; Jimenez-Ferrer, E.; Zamilpa, A.; Tortoriello, J.; et al. Malva parviflora extract ameliorates the deleterious effects of a high fat diet on the cognitive deficit in a mouse model of Alzheimer’s disease by restoring microglial function via a PPAR-gamma-dependent mechanism. J. Neuroinflamm. 2019, 16, 143. [Google Scholar] [CrossRef]
- Matsuda, T.; Hisatsune, T. Cholinergic Modification of Neurogenesis and Gliosis Improves the Memory of AbetaPPswe/PSEN1dE9 Alzheimer’s Disease Model Mice Fed a High-Fat Diet. J. Alzheimer’s Dis. 2017, 56, 1–23. [Google Scholar] [CrossRef]
- Ramos-Rodriguez, J.J.; Spires-Jones, T.; Pooler, A.M.; Lechuga-Sancho, A.M.; Bacskai, B.J.; Garcia-Alloza, M. Progressive Neuronal Pathology and Synaptic Loss Induced by Prediabetes and Type 2 Diabetes in a Mouse Model of Alzheimer’s Disease. Mol. Neurobiol. 2017, 54, 3428–3438. [Google Scholar] [CrossRef] [PubMed]
- Nakandakari, S.; Munoz, V.R.; Kuga, G.K.; Gaspar, R.C.; Sant’Ana, M.R.; Pavan, I.C.B.; da Silva, L.G.S.; Morelli, A.P.; Simabuco, F.M.; da Silva, A.S.R.; et al. Short-term high-fat diet modulates several inflammatory, ER stress, and apoptosis markers in the hippocampus of young mice. Brain Behav. Immun. 2019, 79, 284–293. [Google Scholar] [CrossRef] [PubMed]
- Sah, S.K.; Lee, C.; Jang, J.H.; Park, G.H. Effect of high-fat diet on cognitive impairment in triple-transgenic mice model of Alzheimer’s disease. Biochem. Biophys. Res. Commun. 2017, 493, 731–736. [Google Scholar] [CrossRef]
- Fang, F.; Lue, L.F.; Yan, S.; Xu, H.; Luddy, J.S.; Chen, D.; Walker, D.G.; Stern, D.M.; Yan, S.; Schmidt, A.M.; et al. RAGE-dependent signaling in microglia contributes to neuroinflammation, Abeta accumulation, and impaired learning/memory in a mouse model of Alzheimer’s disease. FASEB J. 2010, 24, 1043–1055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Valente, T.; Gella, A.; Fernandez-Busquets, X.; Unzeta, M.; Durany, N. Immunohistochemical analysis of human brain suggests pathological synergism of Alzheimer’s disease and diabetes mellitus. Neurobiol. Dis. 2010, 37, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Knight, E.M.; Martins, I.V.; Gumusgoz, S.; Allan, S.M.; Lawrence, C.B. High-fat diet-induced memory impairment in triple-transgenic Alzheimer’s disease (3xTgAD) mice is independent of changes in amyloid and tau pathology. Neurobiol. Aging 2014, 35, 1821–1832. [Google Scholar] [CrossRef]
- Olgiati, P.; Politis, A.; Malitas, P.; Albani, D.; Dusi, S.; Polito, L.; De Mauro, S.; Zisaki, A.; Piperi, C.; Stamouli, E.; et al. APOE epsilon-4 allele and cytokine production in Alzheimer’s disease. Int. J. Geriatr. Psychiatry 2010, 25, 338–344. [Google Scholar] [CrossRef]
- Hoffman, J.D.; Yanckello, L.M.; Chlipala, G.; Hammond, T.C.; McCulloch, S.D.; Parikh, I.; Sun, S.; Morganti, J.M.; Green, S.J.; Lin, A.L. Dietary inulin alters the gut microbiome, enhances systemic metabolism and reduces neuroinflammation in an APOE4 mouse model. PLoS ONE 2019, 14, e0221828. [Google Scholar] [CrossRef]
- Gale, S.C.; Gao, L.; Mikacenic, C.; Coyle, S.M.; Rafaels, N.; Murray Dudenkov, T.; Madenspacher, J.H.; Draper, D.W.; Ge, W.; Aloor, J.J.; et al. APOepsilon4 is associated with enhanced in vivo innate immune responses in human subjects. J. Allergy Clin. Immunol. 2014, 134, 127–134. [Google Scholar] [CrossRef] [Green Version]
- Parikh, I.J.; Estus, J.L.; Zajac, D.J.; Malik, M.; Maldonado Weng, J.; Tai, L.M.; Chlipala, G.E.; LaDu, M.J.; Green, S.J.; Estus, S. Murine Gut Microbiome Association with APOE Alleles. Front. Immunol. 2020, 11, 200. [Google Scholar] [CrossRef]
- Vitek, M.P.; Brown, C.M.; Colton, C.A. APOE genotype-specific differences in the innate immune response. Neurobiol. Aging 2009, 30, 1350–1360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruce-Keller, A.J.; Fernandez-Kim, S.O.; Townsend, R.L.; Kruger, C.; Carmouche, R.; Newman, S.; Salbaum, J.M.; Berthoud, H.R. Maternal obese-type gut microbiota differentially impact cognition, anxiety and compulsive behavior in male and female offspring in mice. PLoS ONE 2017, 12, e0175577. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, K.; Mulak, A. Brain-Gut-Microbiota Axis in Alzheimer’s Disease. J. Neurogastroenterol. Motil. 2019, 25, 48–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwangbo, D.S.; Lee, H.Y.; Abozaid, L.S.; Min, K.J. Mechanisms of Lifespan Regulation by Calorie Restriction and Intermittent Fasting in Model Organisms. Nutrients 2020, 12, 1194. [Google Scholar] [CrossRef]
- Sohal, R.S.; Forster, M.J. Caloric restriction and the aging process: A critique. Free Radic. Biol. Med. 2014, 73, 366–382. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Li, S.; Yang, L.; Huang, P.; Li, W.; Wang, S.; Zhao, G.; Zhang, M.; Pang, X.; Yan, Z.; et al. Structural modulation of gut microbiota in life-long calorie-restricted mice. Nat. Commun. 2013, 4, 2163. [Google Scholar] [CrossRef] [Green Version]
- Liu, C.; Zhou, N.; Du, M.X.; Sun, Y.T.; Wang, K.; Wang, Y.J.; Li, D.H.; Yu, H.Y.; Song, Y.; Bai, B.B.; et al. The Mouse Gut Microbial Biobank expands the coverage of cultured bacteria. Nat. Commun. 2020, 11, 79. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Zhang, L.; Xue, J.; Yang, X.; Dong, X.; Sha, L.; Lei, H.; Zhang, X.; Zhu, L.; Wang, Z.; et al. Dietary inulin alleviates diverse stages of type 2 diabetes mellitus via anti-inflammation and modulating gut microbiota in db/db mice. Food Funct. 2019, 10, 1915–1927. [Google Scholar] [CrossRef]
| Phylum | Class | Family | AD Mice | Ref. |
|---|---|---|---|---|
| Actinobacteria | Actinobacteria | Turicibacteriaceae | = in APP/PS1 mice at 6–24 months of age | [107] |
| Coriobacteriaceae | ↑ in APP/PS1 mice at 6–8 months of age | [132] | ||
| Bacteroidetes | Bacteroidia | Rikenellaceae | ↓ in APP/PS1 mice at 6–24 months of age | [90,107] |
| Bacteroidaceae (Genus Prevotella) | ↓ in APP/PS1 mice at 3–6–8 months of age | [90,132] | ||
| ↓ in 5xFAD mice at 6–12 months of age | [134] | |||
| ↑ in 3xTg-AD at 11 months of age | [119] | |||
| Firmicutes | Erysipelotrichia | Erysipelotrichaceae | ↑ in APP/PS1 mice at 24 months of age | [107,111] |
| Clostridia | Ruminococcaceae | ↓ Genus Ruminiclostridium in tg2576 mice at 6–15 months of age | [138] | |
| ↓ Genus Ruminococcus in APP/PS1 mice at 6–8 months of age | [111,132] | |||
| ↑ Genus Ruminococcus in 5xFAD mice at 6–12 months of age | [134] | |||
| Clostridiaceae | ↓ Butyricicoccus pullicaecorum in APP/PS1 mice at 8–12 months of age | [111] | ||
| ↑ transiently Clostridium leptum in 5xFAD mice at 9 weeks of age | [139] | |||
| Lachnospiraceae | ↑ in APP/PS1 mice at 3 months of age | [90] | ||
| ↑ in 5xFAD mice at 6 months of age | [134] | |||
| Bacilli | Lactobacillaceae (Genus Lactobacillus) | ↑ in Tg2576 at 6 months of age/= at 15 months of age | [138] | |
| ↓ in APP/PS1 mice at 8 months of age | [140] | |||
| ↑ in 3xTg-AD mice at 11 months of age | [119] | |||
| ↓ in 5xFAD mice at 6 months of age | [134] | |||
| Proteobacteria | β-proteobacteria | Sutterellaceae (Genus Sutterella) | ↑ in APP/PS1 mice at 6 months of age | [107] |
| ↑ in 5xFAD mice at 6–12 months of age | [134] | |||
| δ-proteobacteria | Desulfovibrionaceae (Genus Desulfovibrio) | ↑ in APP/PS1 mice at 5–9 months of age | [111,130,132] | |
| ↑ in 5xFAD mice at 6–12 months of age | [134] | |||
| ε-proteobacteria | Helicobacteraceae | ↑ in APP/PS1 mice at 3–6–8 months of age | [132] | |
| ↑ in 5xFAD mice at 6 months of age/↓ at 12 months of age | [134] | |||
| γ-proteobacteria | Enterobacteriaceae | ↑ in APP/PS1 mice at 6 months of age | [90] | |
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiaceae (Akkermansia spp.) | ↑↑ in APP/PS1 at 2–12 months of age | [90,111,130] |
| N.D. in 5xFAD mice at 9 weeks of age/↑ at 12 months of age | [134,139] |
| Phylum | Class | Family | Diabetes mice (db) | Reference |
|---|---|---|---|---|
| Actinobacteria | Actinobacteria | Coriobacteriaceae | ↑ in db/db mice at 8–12–18 weeks of age | [205] |
| Dermabacteraceae (Genus Brachybacterium) | ↑ in db/db mice at 8–12–18 weeks of age | [205] | ||
| Pseudonocardiaceae (Genus Pseudonocardia) | ↑ in db/db mice at 8–12–18 weeks of age | [205] | ||
| Bacteroidetes | Bacteroidia | Muribaculaceae | ↓ in db/db mice by 8 months of age | [209] |
| Rickenellaceae | ↑ Genus Rikenella in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] | ||
| ↑ Genus Rikenella in db/db mice by 6 months of age ↓Genus Alistipes in db/db mice by 20 weeks -8 months of age | [209,210,211] | |||
| Bacteroidaceae (Genus Bacteroides) | ↓ in db/db mice by 20 weeks of age/ ↑ by 6-8 months of age | [204,209,210,211] | ||
| ↓ in ob/ob mice by 8 weeks of age | [211] | |||
| Bacteroidetes | Prevotellaceae | ↑ Genus Paraprevotella in db/db mice by 6 months of age ↑ Genus Alloprevotella in db/db mice by 6 months of age /↓ by 8 months of age | [209,211] | |
| Odoribacteraceae | ↑ in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] | ||
| Firmicutes | Bacilli | Streptococcaceae (Genus: Lactococcus) | ↑ in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] |
| Enterococcaceae (Genus Enterococcus) | ↑ in db/db mice by 6 months of age | [211] | ||
| Planococcaceae (Genus Kurthia) | ↑ in db/db mice by 6 months of age | [211] | ||
| Lactobacillaceae (Genus Lactobacillus) | ↑ in db/db mice by 14 weeks of age | [206] | ||
| Clostridia | Ruminococcaceae | ↑ in db/db by 8 weeks | [205] | |
| ↑ ob/ob mice by 8 weeks of age ↑ Genera Ruminococcus_1 and Oscillibacter in ob/ob mice by 15–17 weeks of age | [203,206,211] | |||
| Lachnospiraceae | ↑ Genus Coprococcus in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] | ||
| ↑ Genera Lachnospiraceae_NK4A136 and Agathobacter in ob/ob mice by 15–17 weeks of age | [203,206] | |||
| ↓ in db/db mice by 20 weeks of age | [204] | |||
| Clostridiaceae (Genus Bacteroides) | ↑ in ob/ob mice by 8 weeks of age | [211] | ||
| Proteobacteria | α-Proteobacteria | Bradyrhizobiaceae | ↑ in db/db mice at 8-12-18 weeks of age | [205] |
| γ-Proteobacteria | Enterobacteriaceae (Genus Klebsiella) | ↑in db/db mice by 6 months of age | [211] | |
| Moraxellaceae | ↑ in ob/ob mice at 8-12-18 weeks of age | [205] | ||
| δ-Proteobacteria | Desulfovibrionaceae (Genus Desulfovibrio) | ↑ in db/db mice by 12 weeks | [205] | |
| ↑ in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] | |||
| ε-Proteobacteria | Helicobacteraceae | ↓ Genus Helicobacter in STZ-induced diabetic mice with cognitive disorder | [212] | |
| ↑ in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] | |||
| Deferribacteres | Deferribacteres | Deferribacteraceae | ↑ Genera Deferribacter and Mucispirillum in HFD induced diabetic mice by 14 weeks of age, after 8 weeks of feeding | [206] |
| Phylum | Class | Family | Mixed-Model (AD-Metabolic Disease) | Ref. |
|---|---|---|---|---|
| Bacteroidetes | Bacteroidia | Muribaculaceae | ↓ in 3xTg-AD-HFD mice | [106] |
| Bacteroidetes | Prevotellaceae | ↓Genus Prevotella in E4FAD mice | [248] | |
| Firmicutes | Erysipelotrichia | Erysipelotrichaceae | ↑ Genus Turicibacter in E4FAD mice | [248] |
| Clostridia | Clostridiaceae | ↑↑ Genus Clostridium in 3xTg-AD-HFD mice | [106] | |
| Dehalobacteriaceae | ↓ in 3xTg-AD-HFD mice | [106] | ||
| Peptococcaceae | ↓ in 3xTg-AD-HFD mice | [106] | ||
| Bacilli | Lactobacillaceae | ↓ Genus Lactobacillus in E4FAD mice | [248] | |
| Streptococcaceae | Genus Lactococcus not present in APOE-/- mice | [235] | ||
| Proteobacteria | γ-proteobacteria | Enterobacteriaceae | ↑ Genus Escherichia in E4FAD mice | [248] |
| Verrucomicrobia | Verrucomicrobiae | Verrucomicrobiaceae (Akkermansia spp.) | ↑ in E4FAD mice | [248] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Carranza-Naval, M.J.; Vargas-Soria, M.; Hierro-Bujalance, C.; Baena-Nieto, G.; Garcia-Alloza, M.; Infante-Garcia, C.; del Marco, A. Alzheimer’s Disease and Diabetes: Role of Diet, Microbiota and Inflammation in Preclinical Models. Biomolecules 2021, 11, 262. https://doi.org/10.3390/biom11020262
Carranza-Naval MJ, Vargas-Soria M, Hierro-Bujalance C, Baena-Nieto G, Garcia-Alloza M, Infante-Garcia C, del Marco A. Alzheimer’s Disease and Diabetes: Role of Diet, Microbiota and Inflammation in Preclinical Models. Biomolecules. 2021; 11(2):262. https://doi.org/10.3390/biom11020262
Chicago/Turabian StyleCarranza-Naval, Maria Jose, Maria Vargas-Soria, Carmen Hierro-Bujalance, Gloria Baena-Nieto, Monica Garcia-Alloza, Carmen Infante-Garcia, and Angel del Marco. 2021. "Alzheimer’s Disease and Diabetes: Role of Diet, Microbiota and Inflammation in Preclinical Models" Biomolecules 11, no. 2: 262. https://doi.org/10.3390/biom11020262