Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders
Abstract
:1. Introduction
2. Existential Phenomenological Psychotherapy
2.1. Existential Psychotherapy’s Approach for the Treatment of Mood and Anxiety Disorders
2.2. Clinical Evidence of Meaning-Centered Psychotherapy
3. Neurological Sciences’ Approach to Mood and Anxiety Disorders
3.1. Neuroimaging
3.1.1. Functional Magnetic Resonance Imaging
The Default Mode Network
The Executive Control Network
The Salience Network
3.1.2. Task-Related Functional Magnetic Resonance Imaging
3.2. Other Relevant Biomarkers and Therapeutic Targets
4. Future Perspective: Bridging the Expertise
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AAI | animal-assisted intervention |
| ALFF | amplitude of low-frequency fluctuation |
| dlPFC | dorsolateral prefrontal cortex |
| DMN | default mode network |
| ECN | executive control network |
| EPP | existential phenomenological psychotherapy |
| FC | functional connectivity |
| fMRI | functional magnetic resonance imaging |
| ICA | independent component analysis |
| IMCP | individual meaning-centered psychotherapy |
| KYN | kynurenines |
| KYNA | kynurenic acid |
| LLD | late-life depression |
| MAOI | monoamine oxidase inhibitors |
| MCGP | meaning-centered group psychotherapy |
| MCP | meaning-centered psychotherapy |
| NMDA | N-methyl-D-aspartate |
| mPFC | medial prefrontal cortices |
| PCC | posterior cingulate cortex |
| PRI | pet-robot intervention |
| PTSD | posttraumatic stress disorder |
| ReHo | regional homogeneity |
| SNRI | selective norepinephrine reuptake inhibitor |
| SSRI | selective serotonin reuptake inhibitor |
| SN | salience network |
| TRD | treatment-resistant depression |
| TRP | tryptophan |
References
- Mental Health—Our World in Data. Available online: https://ourworldindata.org/mental-health#:~:text=Globally%20an%20estimated%20284%20million,experience%20anxiety%20disorders%20than%20men (accessed on 14 December 2020).
- Zhou, X.; Teng, T.; Zhang, Y.; Del Giovane, C.; Furukawa, T.A.; Weisz, J.R.; Li, X.; Cuijpers, P.; Coghill, D.; Xiang, Y.; et al. Comparative efficacy and acceptability of antidepressants, psychotherapies, and their combination for acute treatment of children and adolescents with depressive disorder: A systematic review and network meta-analysis. Lancet Psychiatry 2020, 7, 581–601. [Google Scholar] [CrossRef]
- InformedHealth.org. Cologne, Germany: Institute for Quality and Efficiency in Health Care (IQWiG). Depression: How Effective Are Antidepressants? 2020. Available online: https://www.ncbi.nlm.nih.gov/books/NBK361016/ (accessed on 14 December 2020).
- Depression and Other Common Mental Disorders Global Health Estimates. Available online: https://apps.who.int/iris/bitstream/handle/10665/254610/WHO-MSD-MER-2017.2-eng.pdf (accessed on 14 December 2020).
- Smolderen, K.G.; Buchanan, D.M.; Gosch, K.; Whooley, M.; Chan, P.S.; Vaccarino, V.; Parashar, S.; Shah, A.J.; Ho, P.M.; Spertus, J.A. Depression Treatment and 1-Year Mortality After Acute Myocardial Infarction: Insights from the TRIUMPH Registry (Translational Research Investigating Underlying Disparities in Acute Myocardial Infarction Patients’ Health Status). Circulation 2017, 135, 1681–1689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, E. Antidepressants in Patients with Advanced Cancer: When They’re Warranted and How to Choose Therapy. Oncol. Williston Park 2019, 33, 62–68. [Google Scholar]
- Cuijpers, P.; van Straten, A.; van Oppen, P.; Andersson, G. Are psychological and pharmacologic interventions equally effective in the treatment of adult depressive disorders? A meta-analysis of comparative studies. J. Clin. Psychiatry 2008, 69, 1675–1685. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cuijpers, P.; Noma, H.; Karyotaki, E.; Vinkers, C.H.; Cipriani, A.; Furukawa, T.A. A network meta-analysis of the effects of psychotherapies, pharmacotherapies and their combination in the treatment of adult depression. World Psychiatry 2020, 19, 92–107. [Google Scholar] [CrossRef] [PubMed]
- Castanheira, L.; Silva, C.; Cheniaux, E.; Telles-Correia, D. Neuroimaging Correlates of Depression-Implications to Clinical Practice. Front. Psychiatry 2019, 10, 703. [Google Scholar] [CrossRef] [PubMed]
- Pound, P. Are Animal Models Needed to Discover, Develop and Test Pharmaceutical Drugs for Humans in the 21st Century? Animals 2020, 10, 2455. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, A.; Alberini, C.M. Editorial: Neurobiological Models of Psychotherapy. Front. Behav. Neurosci. 2019, 13, 144. [Google Scholar] [CrossRef] [PubMed]
- Wrathall, M.A.; Dreyfus, H.L.; Dreyfus, H.L. A Brief Introduction to Phenomenology and Existentialism. In A Companion to Phenomenology and Existentialism; Dreyfus, H.L., Wrathall, M.A., Eds.; Malden Blackwell Publishing: Oxford, UK, 2006; pp. 1–6. [Google Scholar]
- Spiegelberg, H. Phenomenology in Psychology and Psychiatry: A Historical Introduction; Northwestern University Press: Evanston, IL, USA, 1972. [Google Scholar]
- Moran, D. Introduction to Phenomenology; Routledge: London, UK; New York, NY, USA, 2000. [Google Scholar]
- Wiggins, O.P.; Schwartz, M.A. Psychiatry. In Encyclopedia of Phenomenology; Embree, L., Ed.; Springer Science + Business Media: Dordrecht, The Netherlands, 1997; pp. 562–568. [Google Scholar]
- Wertz, F.J. Phenomenological Currents in Twentieth-Century Psychology. In A Companion to Phenomenology and Existentialism; Dreyfus, H.L., Wrathall, M.A., Eds.; Malden Blackwell Publishing: Oxford, UK, 2006; pp. 394–411. [Google Scholar]
- Spinelli, E. Practising Existential Psychotherapy: The Relational World; SAGE Publications: Los Angeles, CA, USA; London, UK; New Delhi, India; Singapore, 2007. [Google Scholar]
- Längle, A. The Viennese School of Existential Analysis: The Search for Meaning and Affirmation of Life. In Existential Therapy: Legacy, Vibrancy and Dialogue; Barnett, L., Madison, G., Eds.; Routledge: London, UK; New York, NY, USA, 2012; pp. 159–170. [Google Scholar]
- Frankl, E.V. On the Theory and Therapy of Mental Disorders: An Introduction to Logotherapy and Existential Analysis; Dubois, J.M., Ed.; Brunner Routledge: New York, NY, USA, 2004. [Google Scholar]
- Ameli, M. Reason, Meaning, and Resilience in the Treatment of Depression: Logotherapy as a Bridge Between Cognitive-Behavior Therapy and Positive Psychology. In Clinical Perspectives on Meaning: Positive and Existential Psychotherapy; Russo-Netzer, P., Schulenberg, S.E., Batthyany, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 223–244. [Google Scholar]
- Costanza, A.; Baertschi, M.; Weber, K.; Canuto, A. Maladies neurologiques et suicide: De la neurobiologie au manque d’espoir [Neurological diseases and suicide: From neurobiology to hopelessness]. Rev. Med. Suisse 2015, 11, 402–405. [Google Scholar]
- Costanza, A.; Amerio, A.; Aguglia, A.; Escelsior, A.; Serafini, G.; Berardelli, I.; Pompili, M.; Amore, M. When Sick Brain and Hopelessness Meet: Some Aspects of Suicidality in the Neurological Patient. CNS Neurol Disord Drug Targets 2020, 19, 257–263. [Google Scholar] [CrossRef]
- Vos, J. Working with Meaning in Life in Mental Health Care: A Systematic Literature Review of the Practices and Effectiveness of Meaning-Centred Therapies. In Clinical Perspectives on Meaning: Positive and Existential Psychotherapy; Russo-Netzer, P., Schulenberg, S.E., Batthyany, A., Eds.; Springer: Cham, Switzerland, 2016; pp. 59–87. [Google Scholar]
- Fraguell-Hernando, C.; Limonero, J.T.; Gil, F. Psychological intervention in patients with advanced cancer at home through Individual Meaning-Centered Psychotherapy-Palliative Care: A pilot study. Support Care Cancer 2020, 28, 4803–4811. [Google Scholar] [CrossRef]
- Breitbart, W.; Poppito, S.; Rosenfeld, B.; Vickers, A.J.; Li, Y.; Abbey, J.; Olden, M.; Pessin, H.; Lichtenthal, W.; Sjoberg, D.; et al. Pilot randomized controlled trial of individual meaning-centered psychotherapy for patients with advanced cancer. J. Clin. Oncol. 2012, 30, 1304–1309. [Google Scholar] [CrossRef] [Green Version]
- Breitbart, W.; Rosenfeld, B.; Gibson, C.; Pessin, H.; Poppito, S.; Nelson, C.; Tomarken, A.; Timm, A.K.; Berg, A.; Jacobson, C.; et al. Meaning-centered group psychotherapy for patients with advanced cancer: A pilot randomized controlled trial. Psychooncology 2010, 19, 21–28. [Google Scholar] [CrossRef] [Green Version]
- Breitbart, W.; Pessin, H.; Rosenfeld, B.; Applebaum, A.J.; Lichtenthal, W.G.; Li, Y.; Saracino, R.M.; Marziliano, A.M.; Masterson, M.; Tobias, K.; et al. Individual meaning-centered psychotherapy for the treatment of psychological and existential distress: A randomized controlled trial in patients with advanced cancer. Cancer 2018, 124, 3231–3239. [Google Scholar] [CrossRef] [Green Version]
- Breitbart, W.; Rosenfeld, B.; Pessin, H.; Applebaum, A.; Kulikowski, J.; Lichtenthal, W.G. Meaning-centered group psychotherapy: An effective intervention for improving psychological well-being in patients with advanced cancer. J. Clin. Oncol. 2015, 33, 749–754. [Google Scholar] [CrossRef] [Green Version]
- Rosenfeld, B.; Cham, H.; Pessin, H.; Breitbart, W. Why is Meaning-Centered Group Psychotherapy (MCGP) effective? Enhanced sense of meaning as the mechanism of change for advanced cancer patients. Psychooncology 2018, 27, 654–660. [Google Scholar] [CrossRef]
- Applebaum, A.J.; Buda, K.L.; Schofield, E.; Farberov, M.; Teitelbaum, N.D.; Evans, K.; Cowens-Alvarado, R.; Cannady, R.S. Exploring the cancer caregiver’s journey through web-based Meaning-Centered Psychotherapy. Psychooncology 2018, 27, 847–856. [Google Scholar] [CrossRef] [PubMed]
- Holtmaat, K.; van der Spek, N.; Lissenberg-Witte, B.; Breitbart, W.; Cuijpers, P.; Verdonck-de Leeuw, I. Long-term efficacy of meaning-centered group psychotherapy for cancer survivors: 2-Year follow-up results of a randomized controlled trial. Psychooncology 2020, 29, 711–718. [Google Scholar] [CrossRef] [PubMed]
- Van der Spek, N.; Vos, J.; van Uden-Kraan, C.F.; Breitbart, W.; Cuijpers, P.; Knipscheer-Kuipers, K.; Willemsen, V.; Tollenaar, R.A.; van Asperen, C.J.; Verdonck-de Leeuw, I.M. Effectiveness and cost-effectiveness of meaning-centered group psychotherapy in cancer survivors: Protocol of a randomized controlled trial. BMC Psychiatry 2014, 14, 22. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthal, W.G.; Catarozoli, C.; Masterson, M.; Slivjak, E.; Schofield, E.; Roberts, K.E.; Neimeyer, R.A.; Wiener, L.; Prigerson, H.G.; Kissane, D.W.; et al. An open trial of meaning-centered grief therapy: Rationale and preliminary evaluation. Palliat. Support Care 2019, 17, 2–12. [Google Scholar] [CrossRef] [PubMed]
- Fillion, L.; Duval, S.; Dumont, S.; Gagnon, P.; Tremblay, I.; Bairati, I.; Breitbart, W.S. Impact of a meaning-centered intervention on job satisfaction and on quality of life among palliative care nurses. Psychooncology 2009, 18, 1300–13310. [Google Scholar] [CrossRef]
- Vos, J.; Vitali, D. The effects of psychological meaning-centered therapies on quality of life and psychological stress: A metaanalysis. Palliat Support Care 2018, 16, 608–632. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg, M.D.; Finn, E.S.; Scheinost, D.; Papademetris, X.; Shen, X.; Constable, R.T.; Chun, M.M. A neuromarker of sustained attention from whole-brain functional connectivity. Nat. Neurosci. 2016, 19, 165–171. [Google Scholar] [CrossRef] [Green Version]
- Hsu, W.-T.; Rosenberg, M.D.; Scheinost, D.; Constable, R.T.; Chun, M.M. Resting-state functional connectivity predicts neuroticism and extraversion in novel individuals. Soc. Cogn. Affect. Neurosci. 2018, 13, 224–232. [Google Scholar] [CrossRef] [Green Version]
- Jiang, R.; Calhoun, V.D.; Zuo, N.; Lin, D.; Li, J.; Fan, L.; Qi, S.; Sun, H.; Fu, Z.; Song, M.; et al. Connectome-based individualized prediction of temperament trait scores. Neuroimage 2018, 183, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Beaty, R.E.; Kenett, Y.N.; Christensen, A.P.; Rosenberg, M.D.; Benedek, M.; Chen, Q.; Fink, A.; Qiu, J.; Kwapil, T.R.; Kane, M.J.; et al. Robust prediction of individual creative ability from brain functional connectivity. Proc. Natl. Acad. Sci. USA 2018, 115, 1087–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kühn, S.; Vanderhasselt, M.-A.; De Raedt, R.; Gallinat, J. Why ruminators won’t stop: The structural and resting state correlates of rumination and its relation to depression. J. Affect. Disord. 2012, 141, 352–360. [Google Scholar] [CrossRef] [Green Version]
- Brakowski, J.; Spinelli, S.; Dörig, N.; Bosch, O.G.; Manoliu, A.; Holtforth, M.G.; Seifritz, E. Resting state brain network function in major depression—Depression symptomatology, antidepressant treatment effects, future research. J. Psychiatr. Res. 2017, 92, 147–159. [Google Scholar] [CrossRef]
- Raichle, M.E.; MacLeod, A.M.; Snyder, A.Z.; Powers, W.J.; Gusnard, D.A.; Shulman, G.L. A default mode of brain function. Proc. Natl. Acad. Sci. USA 2001, 98, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Buckner, R.L.; Andrews-Hanna, J.R.; Schacter, D.L. The Brain’s Default Network. Ann. N. Y. Acad. Sci. 2008, 1124, 1–38. [Google Scholar] [CrossRef] [Green Version]
- Mohan, A.; Roberto, A.J.; Mohan, A.; Lorenzo, A.; Jones, K.; Carney, M.J.; Liogier-Weyback, L.; Hwang, S.; Lapidus, K.A.B. The Significance of the Default Mode Network (DMN) in Neurological and Neuropsychiatric Disorders: A Review. Yale J. Biol. Med. 2016, 89, 49–57. [Google Scholar]
- Kyeong, S.; Kim, J.; Kim, J.; Kim, E.J.; Kim, H.E.; Kim, J.-J. Differences in the modulation of functional connectivity by self-talk tasks between people with low and high life satisfaction. Neuroimage 2020, 217, 116929. [Google Scholar] [CrossRef]
- Spreng, R.N.; Mar, R.A.; Kim, A.S.N. The common neural basis of autobiographical memory, prospection, navigation, theory of mind, and the default mode: A quantitative meta-analysis. J. Cogn. Neurosci. 2009, 21, 489–510. [Google Scholar] [CrossRef] [PubMed]
- Raichle, M.E. The Brain’s Default Mode Network. Annu. Rev. Neurosci. 2015, 38, 433–447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrews-Hanna, J.R.; Smallwood, J.; Spreng, R.N. The default network and self-generated thought: Component processes, dynamic control, and clinical relevance. Ann. N. Y. Acad. Sci. 2014, 1316, 29–52. [Google Scholar] [CrossRef] [PubMed]
- Leech, R.; Sharp, D.J. The role of the posterior cingulate cortex in cognition and disease. Brain 2014, 137, 12–32. [Google Scholar] [CrossRef] [Green Version]
- Posner, J.; Hellerstein, D.J.; Gat, I.; Mechling, A.; Klahr, K.; Wang, Z.; McGrath, P.J.; Stewart, J.W.; Peterson, B.S. Antidepressants Normalize the Default Mode Network in Patients with Dysthymia. JAMA Psychiatry 2013, 70, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Greicius, M.D.; Gennatas, E.D.; Growdon, M.E.; Jang, J.Y.; Rabinovici, G.D.; Kramer, J.H.; Weiner, M.; Miller, B.L.; Seeley, W.W. Divergent network connectivity changes in behavioural variant frontotemporal dementia and Alzheimer’s disease. Brain 2010, 133, 1352–1367. [Google Scholar] [CrossRef] [Green Version]
- Yuan, Y.; Zhang, Z.; Bai, F.; Yu, H.; Shi, Y.; Qian, Y.; Liu, W.; You, J.; Zhang, X.; Liu, Z. Abnormal neural activity in the patients with remitted geriatric depression: A resting-state functional magnetic resonance imaging study. J. Affect. Disord. 2008, 111, 145–152. [Google Scholar] [CrossRef]
- Chen, J.; Liu, F.; Xun, G.; Chen, H.; Hu, M.; Guo, X.; Xiao, C.; Wooderson, S.C.; Guo, W.; Zhao, J. Early and late onset, first-episode, treatment-naive depression: Same clinical symptoms, different regional neural activities. J. Affect. Disord. 2012, 143, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Mulders, P.C.; van Eijndhoven, P.F.; Schene, A.H.; Beckmann, C.F.; Tendolkar, I. Resting-state functional connectivity in major depressive disorder: A review. Neurosci. Biobehav. Rev. 2015, 56, 330–344. [Google Scholar] [CrossRef]
- Li, B.; Liu, L.; Friston, K.J.; Shen, H.; Wang, L.; Zeng, L.-L.; Hu, D. A Treatment-Resistant Default Mode Subnetwork in Major Depression. Biol. Psychiatry 2013, 74, 48–54. [Google Scholar] [CrossRef]
- Guo, W.; Liu, F.; Zhang, J.; Zhang, Z.; Yu, L.; Liu, J.; Chen, H.; Xiao, C. Abnormal default-mode network homogeneity in first-episode, drug-naive major depressive disorder. PLoS ONE 2014, 9, e91102. [Google Scholar] [CrossRef] [Green Version]
- Sheline, Y.I.; Price, J.L.; Yan, Z.; Mintun, M.A. Resting-state functional MRI in depression unmasks increased connectivity between networks via the dorsal nexus. Proc. Natl. Acad. Sci. USA 2010, 107, 11020–11025. [Google Scholar] [CrossRef] [Green Version]
- Van Tol, M.J.; Li, M.; Metzger, C.D.; Hailla, N.; Horn, D.I.; Li, W.; Heinze, H.J.; Bogerts, B.; Steiner, J.; He, H.; et al. Local cortical thinning links to resting-statedisconnectivity in major depressive disorder. Psychol. Med. 2014, 44, 2053–2065. [Google Scholar] [CrossRef]
- Wu, M.; Andreescu, C.; Butters, M.A.; Tamburo, R.; Reynolds, C.F.; Aizenstein, H. Default-mode network connectivity and white matter burden in late-life depression. Psychiatry Res. Neuroimaging 2011, 194, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Andreescu, C.; Tudorascu, D.L.; Butters, M.A.; Tamburo, E.; Patel, M.; Price, J.; Karp, J.F.; Reynolds, C.F.; Aizenstein, H. Resting state functional connectivity and treatment response in late-life depression. Psychiatry Res. Neuroimaging 2013, 214, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.K.; Barrett, F.S.; May, D.G.; Cosimano, M.P.; Sepeda, N.D.; Johnson, M.W.; Finan, P.H.; Griffiths, R.G. Effects of Psilocybin-Assisted Therapy on Major Depressive Disorder: A Randomized Clinical Trial. JAMA Psychiatry 2020. [Google Scholar] [CrossRef]
- Duffau, H. Functional Mapping before and after Low-Grade Glioma Surgery: A New Way to Decipher Various Spatiotemporal Patterns of Individual Neuroplastic Potential in Brain Tumor Patients. Cancers 2020, 12, 2611. [Google Scholar] [CrossRef]
- Shen, K.K.; Welton, T.; Lyon, M.; McCorkindale, A.N.; Sutherland, G.T.; Burnham, S.; Fripp, J.; Martins, R.; Grieve, S.M. Structural core of the executive control network: A high angular resolution diffusion MRI study. Hum. Brain Mapp. 2020, 41, 1226–1236. [Google Scholar] [CrossRef]
- Zhu, Z.; Johnson, N.F.; Kim, C.; Gold, B.T. Reduced frontal cortex efficiency is associated with lower white matter integrity in aging. Cereb. Cortex 2015, 25, 138–146. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg-Katz, K.; Herman, T.; Jacob, Y.; Mirelman, A.; Giladi, N.; Hendler, T.; Hausdorff, J.M. Fall risk is associated with amplified functional connectivity of the central executive network in patients with Parkinson’s disease. J. Neurol. 2015, 262, 2448–2456. [Google Scholar] [CrossRef]
- Cai, S.; Peng, Y.; Chong, T.; Zhang, Y.; von Deneen, K.M.; Huang, L. Differentiated Effective Connectivity Patterns of the Executive Control Network in Progressive MCI: A Potential Biomarker for Predicting AD. Curr. Alzheimer Res. 2017, 14, 937–950. [Google Scholar] [CrossRef]
- Zhao, Q.; Lu, H.; Metmer, H.; Li, W.X.Y.; Lu, J. Evaluating functional connectivity of executive control network and frontoparietal network in Alzheimer’s disease. Brain Res. 2018, 1678, 262–272. [Google Scholar] [CrossRef]
- Cieri, F.; Esposito, R.; Cera, N.; Pieramico, V.; Tartaro, A.; Di Giannantonio, M. Late-life depression: Modifications of brain resting state activity. J. Geriatr. Psychiatry Neurol. 2017, 30, 140–150. [Google Scholar] [CrossRef]
- Respino, M.; Hoptman, M.J.; Victoria, L.W.; Alexopoulos, G.S.; Solomonov, N.; Stein, A.T.; Coluccio, M.; Morimoto, S.S.; Blau, C.J.; Abreu, L.; et al. Cognitive Control Network Homogeneity and Executive Functions in Late-Life Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2020, 5, 213–221. [Google Scholar] [CrossRef]
- Manning, K.; Wang, L.; Steffens, D. Recent advances in the use of imaging in psychiatry: Functional magnetic resonance imaging of large-scale brain networks in late-life depression. F1000Research 2019, 8, 1–9. [Google Scholar] [CrossRef]
- Lockwood, K.A.; Alexopoulos, G.S.; van Gorp, W.G. Executive dysfunction in geriatric depression. Am. J. Psychiatry 2002, 159, 1119–1126. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Hoptman, M.J.; Kanellopoulos, D.; Murphy, C.F.; Lim, K.O.; Gunning, F.M. Functional connectivity in the cognitive control network and the default mode network in late-life depression. J. Affect. Disord. 2012, 139, 56–65. [Google Scholar] [CrossRef] [Green Version]
- Alalade, E.; Denny, K.; Potter, G.; Steffens, D.; Wang, L. Altered Cerebellar-Cerebral Functional Connectivity in Geriatric Depression. PLoS ONE 2011, 6, e20035. [Google Scholar] [CrossRef]
- Yin, Y.; Hou, Z.; Wang, X.; Sui, Y.; Yuan, Y. Association between altered resting-state cortico-cerebellar functional connectivity networks and mood/cognition dysfunction in late-onset depression. J. Neural Transm. 2015, 122, 887–889. [Google Scholar] [CrossRef]
- Li, W.; Wang, Y.; Ward, B.D.; Antuono, P.G.; Li, S.-J.; Goveas, J.S. Intrinsic inter-network brain dysfunction correlates with symptom dimensions in late-life depression. J. Psychiatr. Res. 2017, 87, 71–80. [Google Scholar] [CrossRef] [Green Version]
- Yue, Y.; Jia, X.; Hou, Z.; Zang, Y.; Yuan, Y. Frequency-dependent amplitude alterations of resting-state spontaneous fluctuations in late-onset depression. Biomed. Res. Int. 2015, 2015, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Yue, Y.; Yuan, Y.; Hou, Z.; Jiang, W.; Bai, F.; Zhang, Z. Abnormal Functional Connectivity of Amygdala in Late- Onset Depression Was Associated with Cognitive Deficits. PLoS ONE 2013, 8, e75058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Yuan, Y.; Bai, F.; Shu, H.; You, J.; Li, L.; Zhang, Z. Altered functional connectivity networks of hippocampal subregions in remitted late-onset depression: A longitudinal resting-state study. Neurosci. Bull. 2015, 31, 13–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, K.J.; Alexopoulos, G.S.; Mcgovern, A.R.; Morimoto, S.S.; Yuen, G.; Kanellopoulos, T.; Gunning, F.M. Executive functioning in late-life depression. Psychiatr. Ann. 2014, 44, 143–146. [Google Scholar] [CrossRef]
- Gandelman, J.A.; Albert, K.; Boyd, B.D.; Park, J.W.; Riddle, M.; Woodward, N.D.; Kang, H.; Landman, B.A.; Taylor, W.D. Intrinsic Functional Network Connectivity Is Associated With Clinical Symptoms and Cognition in Late-Life Depression. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2019, 4, 160–170. [Google Scholar] [CrossRef]
- Alexopoulos, G.S.; Kiosses, D.N.; Klimstra, S.; Kalayam, B.; Bruce, M.L. Clinical Presentation of the “Depression–Executive Dysfunction Syndrome” of Late Life. Am. J. Geriatr. Psychiatry 2002, 10, 98–106. [Google Scholar] [PubMed]
- Alexopoulos, G.S.; Kiosses, D.N.; Heo, M.; Murphy, C.F.; Shanmugham, B.; Gunning-Dixon, F. Executive Dysfunction and the Course of Geriatric Depression. Biol. Psychiatry 2005, 58, 204–210. [Google Scholar] [CrossRef]
- Manning, K.J.; Alexopoulos, G.S.; Banerjee, S.; Morimoto, S.S.; Seirup, J.K.; Klimstra, S.A.; Yuen, G.; Kanellopoulos, T.; Gunning-Dixon, F. Executive functioning complaints and escitalopram treatment response in late-life depression. Am. J. Geriatr. Psychiatry 2015, 23, 440–445. [Google Scholar] [CrossRef] [Green Version]
- Morimoto, S.S.; Kanellopoulos, D.; Manning, K.J.; Alexopoulos, G.S. Diagnosis and treatment of depression and cognitive impairment in late life. Ann. N. Y. Acad. Sci. 2015, 1345, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Yin, Y.; He, X.; Xu, M.; Hou, Z.; Song, X.; Sui, Y.; Liu, Z.; Jiang, W.; Yue, Y.; Zhang, Y.; et al. Structural and functional connectivity of default mode network underlying the cognitive impairment in late-onset depression. Sci. Rep. 2016, 6, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.; Kim, Y.-K. Crosstalk between Depression and Dementia with Resting-State fMRI Studies and Its Relationship with Cognitive Functioning. Biomedicines 2021, 9, 82. [Google Scholar] [CrossRef] [PubMed]
- Menon, V.; Uddin, L.Q. Saliency, switching, attention and control: A network model of insula function. Brain Struct. Funct. 2010, 214, 655–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeley, X.W.W. The Salience Network: A Neural System for Perceiving and Responding to Homeostatic Demands. J. Neurosci. 2019, 39, 9878–9882. [Google Scholar] [CrossRef] [PubMed]
- Touroutoglou, A.; Hollenbeck, M.; Dickerson, B.C.; Feldman Barrett, L. Dissociable large-scale networks anchored in the right anterior insula subserve affective experience and attention. Neuroimage 2012, 60, 1947–1958. [Google Scholar] [CrossRef] [Green Version]
- Seeley, W.W.; Menon, V.; Schatzberg, A.F.; Keller, J.; Glover, G.H.; Kenna, H.; Reiss, A.L.; Greicius, M.D. 973 Dissociable Intrinsic Connectivity Networks for Salience Processing and Executive Control. J. Neurosci. 2007, 27, 2349–2356. [Google Scholar] [CrossRef]
- Chand, G.B.; Wu, J.; Hajjar, I.; Qiu, D. Interactions of the Salience Network and Its Subsystems with the Default-Mode and the Central-Executive. Brain Connect. 2017, 7, 401–412. [Google Scholar] [CrossRef]
- Elton, A.; Gao, W. Divergent task-dependent functional connectivity of executive control and salience networks. Cortex 2014, 51, 56–66. [Google Scholar] [CrossRef]
- Cullen, K.R.; Westlund, M.K.; Klimes-Dougan, B.; Mueller, B.A.; Houri, A.; Eberly, L.E.; Lim, K.O. Abnormal Amygdala Resting-State Functional Connectivity in Adolescent Depression. JAMA Psychiatry 2014, 71, 1138–1147. [Google Scholar] [CrossRef]
- Luking, K.R.; Repovs, G.; Belden, A.C.; Gaffrey, M.S.; Botteron, K.N.; Luby, J.L.; Barch, D.M. Functional Connectivity of the Amygdala in Early-Childhood-Onset Depression. J. Am. Acad. Child Adolesc. Psychiatry 2011, 50, 1027–1041. [Google Scholar] [CrossRef] [Green Version]
- Dai, L.; Zhou, H.; Xu, X.; Zuo, Z. Brain structural and functional changes in patients with major depressive disorder: A literature review. PeerJ 2019, 7, e8170. [Google Scholar] [CrossRef]
- Connolly, C.G.; Wu, J.; Ho, T.C.; Hoeft, F.; Wolkowitz, O.; Eisendrath, S.; Frank, G.; Hendren, R.; Max, J.E.; Paulus, M.P.; et al. Resting-state functional connectivity of subgenual anterior cingulate cortex in depressed adolescents. Biol. Psychiatry 2013, 74, 898–907. [Google Scholar] [CrossRef] [Green Version]
- Davey, C.G.; Whittle, S.; Harrison, B.J.; Simmons, J.G.; Byrne, M.L.; Schwartz, O.S.; Allen, N.B. Functional brain-imaging correlates of negative affectivity and the onset of first-episode depression. Psychol. Med. 2015, 45, 1001–1009. [Google Scholar] [CrossRef]
- Yuen, G.S.; Gunning-Dixon, F.M.; Hoptman, M.J.; AbdelMalak, B.; McGovern, A.R.; Seirup, J.K.; Alexopoulos, G.S. The salience network in the apathy of late-life depression. Int. J. Geriatr. Psychiatry 2014, 29, 1116–1124. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Li, L.; Wu, M.; Chen, Z.; Hu, X.; Chen, Y.; Zhu, H.; Jia, Z.; Gong, Q. Brain gray matter alterations in first episodes of depression: A meta-analysis of whole-brain studies. Neurosci. Biobehav. Rev. 2016, 60, 43–50. [Google Scholar] [CrossRef]
- Steffens, D.C.; Wang, L.; Pearlson, G.D. Functional connectivity predictors of acute depression treatment outcome. Int. Psychogeriatr. 2019, 31, 1831–1835. [Google Scholar] [CrossRef]
- Janiri, D.; Moser, D.A.; Doucet, G.E.; Luber, M.J.; Rasgon, A.; Lee, W.H.; Murrough, J.W.; Sani, G.; Eickhoff, S.B.; Frangou, S. Shared Neural Phenotypes for Mood and Anxiety Disorders: A Meta-analysis of 226 Task-Related Functional Imaging Studies. JAMA Psychiatry 2020, 77, 172–179. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Telegdy, G. Neurotransmissions of antidepressant-like effects of neuromedin U-23 in mice. Behav. Brain Res. 2014, 259, 196–199. [Google Scholar] [CrossRef]
- Tanaka, M.; Kádár, K.; Tóth, G.; Telegdy, G. Antidepressant-like effects of urocortin 3 fragments. Brain Res. Bull. 2011, 84, 414–418. [Google Scholar] [CrossRef]
- Telegdy, G.; Tanaka, M.; Schally, A.V. Effects of the LHRH antagonist Cetrorelix on the brain function in mice. Neuropeptides 2009, 43, 229–234. [Google Scholar] [CrossRef]
- Tanaka, M.; Schally, A.V.; Telegdy, G. Neurotransmission of the antidepressant-like effects of the growth hormone-releasing hormone antagonist MZ-4-71. Behav. Brain Res. 2012, 228, 388–391. [Google Scholar] [CrossRef] [PubMed]
- Vargas, A.S.; Luís, Â.; Barroso, M.; Gallardo, E.; Pereira, L. Psilocybin as a New Approach to Treat Depression and Anxiety in the Context of Life-Threatening Diseases—A Systematic Review and Meta-Analysis of Clinical Trials. Biomedicines 2020, 8, 331. [Google Scholar] [CrossRef] [PubMed]
- Ibos, K.E.; Bodnár, É.; Bagosi, Z.; Bozsó, Z.; Tóth, G.; Szabó, G.; Csabafi, K. Kisspeptin-8 Induces Anxiety-Like Behavior and Hypolocomotion by Activating the HPA Axis and Increasing GABA Release in the Nucleus Accumbens in Rats. Biomedicines 2021, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Caruso, G.; Godos, J.; Castellano, S.; Micek, A.; Murabito, P.; Galvano, F.; Ferri, R.; Grosso, G.; Caraci, F. The Therapeutic Potential of Carnosine/Anserine Supplementation against Cognitive Decline: A Systematic Review with Meta-Analysis. Biomedicines 2021, 9, 253. [Google Scholar] [CrossRef]
- Kim, I.B.; Park, S.-C. Neural Circuitry–Neurogenesis Coupling Model of Depression. Int. J. Mol. Sci. 2021, 22, 2468. [Google Scholar] [CrossRef]
- Małgorzata, P.; Paweł, K.; Iwona, M.L.; Brzostek, T.; Andrzej, P. Glutamatergic dysregulation in mood disorders: Opportunities for the discovery of novel drug targets. Expert Opin. Ther. Targets 2020, 3, 1–23. [Google Scholar]
- Tanaka, M.; Bohár, Z.; Vécsei, L. Are Kynurenines Accomplices or Principal Villains in Dementia? Maintenance of Kynurenine Metabolism. Molecules 2020, 25, 564. [Google Scholar] [CrossRef] [Green Version]
- Pochwat, B.; Nowak, G.; Szewczyk, B. An update on NMDA antagonists in depression. Expert Rev. Neurother. 2019, 19, 1055–1067. [Google Scholar] [CrossRef]
- Shin, C.; Kim, Y.K. Ketamine in Major Depressive Disorder: Mechanisms and Future Perspectives. Psychiatry Investig. 2020, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Zanos, P.; Gould, T.D. Mechanisms of ketamine action as an antidepressant. Mol. Psychiatry 2018, 23, 801–811. [Google Scholar] [CrossRef]
- Encyclopedia. The Tryptophan-Kynurenine Metabolic Pathway. Available online: https://encyclopedia.pub/8633 (accessed on 24 March 2021).
- Tanaka, M.; Toldi, J.; Vécsei, L. Exploring the Etiological Links behind Neurodegenerative Diseases: Inflammatory Cytokines and Bioactive Kynurenines. Int. J. Mol. Sci. 2020, 21, 2431. [Google Scholar] [CrossRef] [Green Version]
- Török, N.; Tanaka, M.; Vécsei, L. Searching for Peripheral Biomarkers in Neurodegenerative Diseases: The Tryptophan-Kynurenine Metabolic Pathway. Int. J. Mol. Sci. 2020, 21, 9338. [Google Scholar] [CrossRef]
- Erabi, H.; Okada, G.; Shibasaki, C.; Setoyama, D.; Kang, D.; Takamura, M.; Yoshino, A.; Fuchikami, M.; Kurata, A.; Kato, T.A.; et al. Kynurenic acid is a potential overlapped biomarker between diagnosis and treatment response for depression from metabolome analysis. Sci. Rep. 2020, 10, 16822. [Google Scholar] [CrossRef]
- Hunt, C.; Macedo e Cordeiro, T.; Suchting, R.; de Dios, C.; Cuellar Leal, V.A.; Soares, J.C.; Dantzer, R.; Teixeira, A.L.; Selvaraj, S. Effect of immune activation on the kynurenine pathway and depression symptoms—A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 118, 514. [Google Scholar] [CrossRef] [PubMed]
- Serafini, G.; Adavastro, G.; Canepa, G.; Capobianco, L.; Conigliaro, C.; Pittaluga, F.; Murri, M.B.; Valchera, A.; De Berardis, D.; Pompili, M.; et al. Abnormalities in Kynurenine Pathway Metabolism in Treatment-Resistant Depression and Suicidality: A Systematic Review. CNS Neurol. Disord. Drug Targets 2017, 16, 440–453. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.J.; Cryan, J.F.; Dinan, T.G.; Clarke, G. Kynurenine pathway metabolism and the microbiota-gut-brain axis. Neuropharmacology 2017, 112, 399–412. [Google Scholar] [CrossRef]
- Kim, E.Y.; Ahn, H.-S.; Lee, M.Y.; Yu, J.; Yeom, J.; Jeong, H.; Min, H.; Lee, H.J.; Kim, K.; Ahn, Y.M. An Exploratory Pilot Study with Plasma Protein Signatures Associated with Response of Patients with Depression to Antidepressant Treatment for 10 Weeks. Biomedicines 2020, 8, 455. [Google Scholar] [CrossRef] [PubMed]
- López-Gambero, A.J.; Sanjuan, C.; Serrano-Castro, P.J.; Suárez, J.; Rodríguez de Fonseca, F. The Biomedical Uses of Inositols: A Nutraceutical Approach to Metabolic Dysfunction in Aging and Neurodegenerative Diseases. Biomedicines 2020, 8, 295. [Google Scholar] [CrossRef]
- Cantón-Habas, V.; Rich-Ruiz, M.; Romero-Saldaña, M.; Carrera-González, M.P. Depression as a Risk Factor for Dementia and Alzheimer’s Disease. Biomedicines 2020, 8, 45. [Google Scholar] [CrossRef]
- Kowalska, K.; Krzywoszański, Ł.; Droś, J.; Pasińska, P.; Wilk, A.; Klimkowicz-Mrowiec, A. Early Depression Independently of Other Neuropsychiatric Conditions, Influences Disability and Mortality after Stroke (Research Study—Part of PROPOLIS Study). Biomedicines 2020, 8, 509. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-Mora, P.; Pérez-De la Cruz, V.; Estrada-Cortés, B.; Toussaint-González, P.; Martínez-Cortéz, J.A.; Rodríguez-Barragán, M.; Quinzaños-Fresnedo, J.; Rangel-Caballero, F.; Gamboa-Coria, G.; Sánchez-Vázquez, I.; et al. Serum Kynurenines Correlate with Depressive Symptoms and Disability in Poststroke Patients: A Cross-sectional Study. Neurorehabilit. Neural Repair 2020, 154596832095367. [Google Scholar]
- Park, S.; Bak, A.; Kim, S.; Nam, Y.; Kim, H.; Yoo, D.-H.; Moon, M. Animal-Assisted and Pet-Robot Interventions for Ameliorating Behavioral and Psychological Symptoms of Dementia: A Systematic Review and Meta-Analysis. Biomedicines 2020, 8, 150. [Google Scholar] [CrossRef] [PubMed]
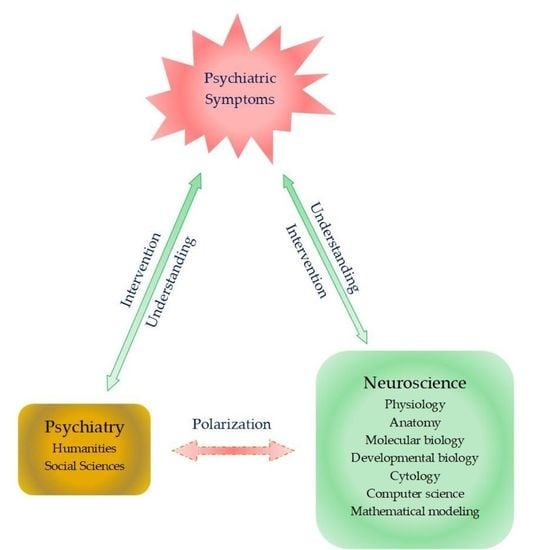
- Di Nicola, V.; Stoyanov, D.S. Psychiatry in Crisis At the Crossroads of Social Sciences, the Humanities, and Neuroscience; Springer Nature Switzerland AG: Cham, Switzerland, 2021. [Google Scholar] [CrossRef]





| Name of the Technique | How Does It Work? | Application |
|---|---|---|
| Paradoxical intention | Resolves to opt for an attitude that is diametrically opposed to that which they would originally want to adopt as a “natural” reaction to perceived psychological difficulty | Anxiety disorders Depression [18]. |
| Dereflection | Redirect the attention from the self, towards other people or other phenomena in the world | Anxiety disorders Depression [18]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balogh, L.; Tanaka, M.; Török, N.; Vécsei, L.; Taguchi, S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines 2021, 9, 340. https://doi.org/10.3390/biomedicines9040340
Balogh L, Tanaka M, Török N, Vécsei L, Taguchi S. Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders. Biomedicines. 2021; 9(4):340. https://doi.org/10.3390/biomedicines9040340
Chicago/Turabian StyleBalogh, Lehel, Masaru Tanaka, Nóra Török, László Vécsei, and Shigeru Taguchi. 2021. "Crosstalk between Existential Phenomenological Psychotherapy and Neurological Sciences in Mood and Anxiety Disorders" Biomedicines 9, no. 4: 340. https://doi.org/10.3390/biomedicines9040340