Bioinspired Histidine–Zn2+ Coordination for Tuning the Mechanical Properties of Self-Healing Coiled Coil Cross-Linked Hydrogels
Abstract
:1. Introduction
2. Materials and Methods
2.1. Peptide Synthesis and Purification
2.2. Circular Dichroism Spectroscopy
2.3. Confocal Raman Spectroscopy
2.4. Hydrogel Preparation
2.5. Oscillatory Shear Rheology
3. Results
3.1. Secondary Structure and Metal Coordination Mode of the His-Modified Coiled Coil
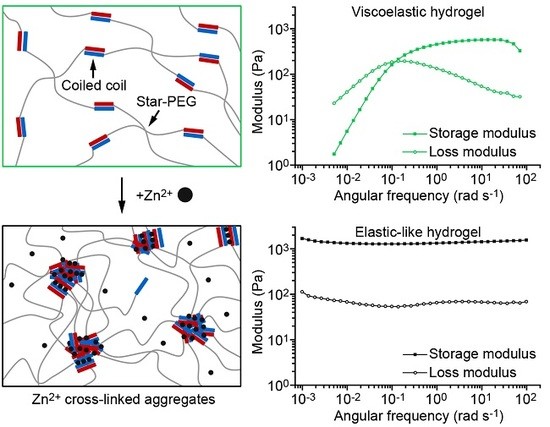
3.2. Switching the Coiled Coil Cross-Linked Hydrogel from Viscoelastic to Elastic Using His–Zn2+ Coordination
3.3. Tuning the Relaxation Time of the Coiled Coil Cross-Linked Hydrogel with Different Zn2+:His Ratios
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Degtyar, E.; Harrington, M.J.; Politi, Y.; Fratzl, P. The mechanical role of metal ions in biogenic protein-based materials. Angew. Chem. Int. Ed. 2014, 53, 12026–12044. [Google Scholar] [CrossRef] [PubMed]
- Broomell, C.C.; Mattoni, M.A.; Zok, F.W.; Waite, J.H. Critical role of zinc in hardening of Nereis jaws. J. Exp. Biol. 2006, 209, 3219–3225. [Google Scholar] [CrossRef] [PubMed]
- Priemel, T.; Degtyar, E.; Dean, M.N.; Harrington, M.J. Rapid self-assembly of complex biomolecular architectures during mussel byssus biofabrication. Nat. Commun. 2017, 8, 14539. [Google Scholar] [CrossRef] [PubMed]
- Waite, J.H.; Qin, X.X.; Coyne, K.J. The peculiar collagens of mussel byssus. Matrix Biol. 1998, 17, 93–106. [Google Scholar] [CrossRef]
- Reinecke, A.; Brezesinski, G.; Harrington, M.J. pH-responsive self-organization of metal-binding protein motifs from biomolecular junctions in mussel byssus. Adv. Mater. Interfaces 2017, 4, 1600416. [Google Scholar] [CrossRef]
- Reinecke, A.; Bertinetti, L.; Fratzl, P.; Harrington, M.J. Cooperative behavior of a sacrificial bond network and elastic framework in providing self-healing capacity in mussel byssal threads. J. Struct. Biol. 2016, 196, 329–339. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, C.N.Z.; Politi, Y.; Reinecke, A.; Harrington, M.J. Role of sacrificial protein–metal bond exchange in mussel byssal thread self-healing. Biomacromolecules 2015, 16, 2852–2861. [Google Scholar] [CrossRef]
- Schmidt, S.; Reinecke, A.; Wojcik, F.; Pussak, D.; Hartmann, L.; Harrington, M.J. Metal-mediated molecular self-healing in histidine-rich mussel peptides. Biomacromolecules 2014, 15, 1644–1652. [Google Scholar] [CrossRef]
- Fullenkamp, D.E.; He, L.; Barrett, D.G.; Burghardt, W.R.; Messersmith, P.B. Mussel-inspired histidine-based transient network metal coordination hydrogels. Macromolecules 2013, 46, 1167–1174. [Google Scholar] [CrossRef] [PubMed]
- Grindy, S.C.; Learsch, R.; Mozhdehi, D.; Cheng, J.; Barrett, D.G.; Guan, Z.; Messersmith, P.B.; Holten-Andersen, N. Control of hierarchical polymer mechanics with bioinspired metal-coordination dynamics. Nat. Mater. 2015, 14, 1210–1216. [Google Scholar] [CrossRef]
- Grindy, S.C.; Holten-Andersen, N. Bio-inspired metal-coordinate hydrogels with programmable viscoelastic material functions controlled by longwave UV light. Soft Matter 2017, 13, 4057–4065. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barrett, D.G.; Messersmith, P.B.; Holten-Andersen, N. Controlling hydrogel mechanics via bio-inspired polymer-nanoparticle bond dynamics. ACS Nano 2016, 10, 1317–1324. [Google Scholar] [CrossRef] [PubMed]
- Enke, M.; Bode, S.; Vitz, J.; Schacher, F.H.; Harrington, M.J.; Hager, M.D.; Schubert, U.S. Self-healing response in supramolecular polymers based on reversible zinc–histidine interactions. Polymer 2015, 69, 274–282. [Google Scholar] [CrossRef]
- Enke, M.; Jehle, F.; Bode, S.; Vitz, J.; Harrington, M.J.; Hager, M.D.; Schubert, U.S. Histidine–zinc interactions investigated by isothermal titration calorimetry (ITC) and their application in self-healing polymers. Macromol. Chem. Phys. 2017, 218, 1600458. [Google Scholar] [CrossRef]
- Wegner, S.V.; Schenk, F.C.; Witzel, S.; Bialas, F.; Spatz, J.P. Cobalt cross-linked redox-responsive PEG hydrogels: From viscoelastic liquids to elastic solids. Macromolecules 2016, 49, 4229–4235. [Google Scholar] [CrossRef]
- Wang, C.; Kopeček, J.; Stewart, R.J. Hybrid hydrogels cross-linked by genetically engineered coiled-coil block proteins. Biomacromolecules 2001, 2, 912–920. [Google Scholar] [CrossRef]
- Yang, J.; Xu, C.; Wang, C.; Kopeček, J. Refolding hydrogels self-assembled from N-(2-hydroxypropyl)methacrylamide graft copolymers by antiparallel coiled-coil formation. Biomacromolecules 2006, 7, 1187–1195. [Google Scholar] [CrossRef]
- Dušek, K.; Dušková-Smrčková, M.; Yang, J.; Kopeček, J. Coiled-coil hydrogels: Effect of grafted copolymer composition and cyclization on gelation. Macromolecules 2009, 42, 2265–2274. [Google Scholar] [CrossRef] [PubMed]
- Aronsson, C.; Dånmark, S.; Zhou, F.; Öberg, P.; Enander, K.; Su, H.; Aili, D. Self-sorting heterodimeric coiled coil peptides with defined and tuneable self-assembly properties. Sci. Rep. 2015, 5, 14063. [Google Scholar] [CrossRef]
- Burkoth, T.S.; Benzinger, T.L.S.; Urban, V.; Lynn, D.G.; Meredith, S.C.; Thiyagarajan, P. Self-assembly of Aβ(10-35)-PEG block copolymer fibrils. J. Am. Chem. Soc. 1999, 121, 7429–7430. [Google Scholar] [CrossRef]
- Radu, L.C.; Yang, J.; Kopeček, J. Self-assembling diblock copolymers of poly[N-(2-hydroxypropyl)methacrylamide] and a β-sheet peptide. Macromol. Biosci. 2009, 9, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Apostolovic, B.; Danial, M.; Klok, H.-A. Coiled coils: Attractive protein folding motifs for the fabrication of self-assembled, responsive and bioactive materials. Chem. Soc. Rev. 2010, 39, 3541–3575. [Google Scholar] [CrossRef] [PubMed]
- Mason, J.M.; Arndt, K.M. Coiled coil domains: Stability, specificity, and biological implications. Chembiochem 2004, 5, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Tunn, I.; de Léon, A.S.; Blank, K.G.; Harrington, M.J. Tuning coiled coil stability with histidine-metal coordination. Nanoscale 2018, 10, 22725–22729. [Google Scholar] [CrossRef] [PubMed]
- Thomas, F.; Boyle, A.L.; Burton, A.J.; Woolfson, D.N. A set of de novo designed parallel heterodimeric coiled coils with quantified dissociation constants in the micromolar to sub-nanomolar regime. J. Am. Chem. Soc. 2013, 135, 5161–5166. [Google Scholar] [CrossRef]
- Goktas, M.; Luo, C.; Sullan, R.M.A.; Bergues-Pupo, A.E.; Lipowsky, R.; Vila Verde, A.; Blank, K.G. Molecular mechanics of coiled coils loaded in the shear geometry. Chem. Sci. 2018, 9, 4610–4621. [Google Scholar] [CrossRef]
- Lupas, A.N.; Bassler, J.; Dunin-Horkawicz, S. The structure and topology of α-helical coiled coils. Subcell. Biochem. 2017, 82, 95–129. [Google Scholar]
- See, R.F.; Kruse, R.A.; Strub, W.M. Metal–ligand bond distances in first-row transition metal coordination compounds: Coordination number, oxidation state, and specific ligand effects. Inorg. Chem. 1998, 37, 5369–5375. [Google Scholar] [CrossRef]
- Wood, C.W.; Woolfson, D.N. CCBuilder 2.0: Powerful and accessible coiled-coil modeling. Protein Sci. 2018, 27, 103–111. [Google Scholar] [CrossRef]
- Zheng, H.; Chruszcz, M.; Lasota, P.; Lebioda, L.; Minor, W. Data mining of metal ion environments present in protein structures. J. Inorg. Biochem. 2008, 102, 1765–1776. [Google Scholar] [CrossRef]
- Kelly, S.M.; Jess, T.J.; Price, N.C. How to study proteins by circular dichroism. Biochim. Biophys. Acta 2005, 1751, 119–139. [Google Scholar] [CrossRef] [PubMed]
- Palmer, R.G.; Stein, D.L.; Abrahams, E.; Anderson, P.W. Models of hierarchically constrained dynamics for glassy relaxation. Phys. Rev. Lett. 1984, 53, 958–961. [Google Scholar] [CrossRef]
- Cathébras, N.; Collet, A.; Viguier, M.; Berret, J.-F. Synthesis and linear viscoelasticity of fluorinated hydrophobically modified ethoxylated urethanes (F-HEUR). Macromolecules 1998, 31, 1305–1311. [Google Scholar] [CrossRef]
- Lau, S.Y.; Taneja, A.K.; Hodges, R.S. Synthesis of a model protein of defined secondary and quaternary structure. Effect of chain length on the stabilization and formation of two-stranded α-helical coiled-coils. J. Biol. Chem. 1984, 259, 13253–13261. [Google Scholar] [PubMed]
- Zhou, N.E.; Kay, C.M.; Hodges, R.S. Synthetic model proteins: The relative contribution of leucine residues at the nonequivalent positions of the 3-4 hydrophobic repeat to the stability of the two-stranded α-helical coiled-coil. Biochemistry 1992, 31, 5739–5746. [Google Scholar] [CrossRef] [PubMed]
- Brandenburg, E.; Berlepsch, H.V.; Leiterer, J.; Emmerling, F.; Koksch, B. Formation of α-helical nanofibers by mixing β-structured and α-helical coiled coil peptides. Biomacromolecules 2012, 13, 3542–3551. [Google Scholar] [CrossRef] [PubMed]
- Bromley, E.H.C.; Channon, K.J.; King, P.J.S.; Mahmoud, Z.N.; Banwell, E.F.; Butler, M.F.; Crump, M.P.; Dafforn, T.R.; Hicks, M.R.; Hirst, J.D.; et al. Assembly pathway of a designed α-helical protein fiber. Biophys. J. 2010, 98, 1668–1676. [Google Scholar] [CrossRef]
- Pandya, M.J.; Spooner, G.M.; Sunde, M.; Thorpe, J.R.; Rodger, A.; Woolfson, D.N. Sticky-end assembly of a designed peptide fiber provides insight into protein fibrillogenesis. Biochemistry 2000, 39, 8728–8734. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Paramonov, S.E.; Hartgerink, J.D. Self-assembly of α-helical coiled coil nanofibers. J. Am. Chem. Soc. 2008, 130, 13691–13695. [Google Scholar] [CrossRef] [PubMed]
- Pagel, K.; Wagner, S.C.; Rezaei Araghi, R.; von Berlepsch, H.; Böttcher, C.; Koksch, B. Intramolecular charge interactions as a tool to control the coiled-coil-to-amyloid transformation. Chem. Eur. J. 2008, 14, 11442–11451. [Google Scholar] [CrossRef]
- Movasaghi, Z.; Rehman, S.; Rehman, D.I.U. Raman spectroscopy of biological tissues. Appl. Spectrosc. Rev. 2007, 42, 493–541. [Google Scholar] [CrossRef]
- Zhang, G.; Senak, L.; Moore, D.J. Measuring changes in chemistry, composition, and molecular structure within hair fibers by infrared and Raman spectroscopic imaging. J. Biomed. Opt. 2011, 16, 056009. [Google Scholar] [CrossRef]
- Paquin, R.; Colomban, P. Nanomechanics of single keratin fibres: A Raman study of the α-helix →β-sheet transition and the effect of water. J. Raman Spectrosc. 2007, 38, 504–514. [Google Scholar] [CrossRef]
- Takeuchi, H. Raman structural markers of tryptophan and histidine side chains in proteins. Biopolymers 2003, 72, 305–317. [Google Scholar] [CrossRef]
- Jehle, F.; Fratzl, P.; Harrington, M.J. Metal-tunable self-assembly of hierarchical structure in mussel-inspired peptide films. ACS Nano 2018, 12, 2160–2168. [Google Scholar] [CrossRef]
- Mezger, T.G. The Rheology Handbook, 4th ed.; Vincentz Network: Hannover, Germany, 2014; p. 150. [Google Scholar]
- Aili, D.; Enander, K.; Rydberg, J.; Nesterenko, I.; Björefors, F.; Baltzer, L.; Liedberg, B. Folding induced assembly of polypeptide decorated gold nanoparticles. J. Am. Chem. Soc. 2008, 130, 5780–5788. [Google Scholar] [CrossRef]
- Lindsey, C.P.; Patterson, G.D. Detailed comparison of the William–Watts and Cole–Davidson functions. J. Chem. Phys. 1980, 73, 3348–3357. [Google Scholar] [CrossRef]
- Lin, S.; Gu, L. Influence of crosslink density and stiffness on mechanical properties of type I collagen gel. Materials 2015, 8, 551–560. [Google Scholar] [CrossRef]
- Gupta, M.K.; Becknell, K.A.; Crosby, M.G.; Bedford, N.M.; Wright, J.; Dennis, P.B.; Naik, R.R. Programmable mechanical properties from a worm jaw-derived biopolymer through hierarchical ion exposure. ACS Appl. Mater. Interfaces 2018, 10, 31928–31937. [Google Scholar] [CrossRef] [PubMed]
- Harrington, M.J.; Gupta, H.S.; Fratzl, P.; Waite, J.H. Collagen insulated from tensile damage by domains that unfold reversibly: In situ X-ray investigation of mechanical yield and damage repair in the mussel byssus. J. Struct. Biol. 2009, 167, 47–54. [Google Scholar] [CrossRef] [PubMed]
- Carrington, E.; Gosline, J.M. Mechanical design of mussel byssus: Load cycle and strain rate dependence. Am. Malacol. Bull. 2004, 18, 135–142. [Google Scholar]
- Grindy, S.C.; Lenz, M.; Holten-Andersen, N. Engineering elasticity and relaxation time in metal-coordinate cross-linked hydrogels. Macromolecules 2016, 49, 8306–8312. [Google Scholar] [CrossRef]







© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tunn, I.; Harrington, M.J.; Blank, K.G. Bioinspired Histidine–Zn2+ Coordination for Tuning the Mechanical Properties of Self-Healing Coiled Coil Cross-Linked Hydrogels. Biomimetics 2019, 4, 25. https://doi.org/10.3390/biomimetics4010025
Tunn I, Harrington MJ, Blank KG. Bioinspired Histidine–Zn2+ Coordination for Tuning the Mechanical Properties of Self-Healing Coiled Coil Cross-Linked Hydrogels. Biomimetics. 2019; 4(1):25. https://doi.org/10.3390/biomimetics4010025
Chicago/Turabian StyleTunn, Isabell, Matthew J. Harrington, and Kerstin G. Blank. 2019. "Bioinspired Histidine–Zn2+ Coordination for Tuning the Mechanical Properties of Self-Healing Coiled Coil Cross-Linked Hydrogels" Biomimetics 4, no. 1: 25. https://doi.org/10.3390/biomimetics4010025