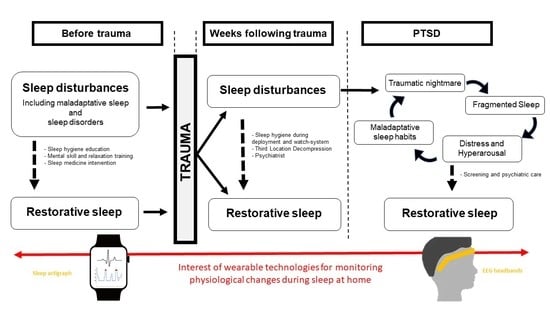
Sleep and PTSD in the Military Forces: A Reciprocal Relationship and a Psychiatric Approach
Abstract
:1. Introduction
2. Sleep in the Military Forces: Epidemiology and Recommendations
2.1. Sleep Schedules, Quantity and Quality
2.2. Sleep Disorders
3. PTSD in the Military Forces
3.1. Clinical Specific Aspects and Prevalence
3.2. Sleep and PTSD in Military Forces
3.2.1. Sleep Characteristics in Military PTSD
3.2.2. Dynamic Link between Sleep and the Emergence of Military PTSD
4. A Reciprocal Relationship
4.1. The Reciprocal Relationship Model: A Reality
4.2. Physiological Hypotheses
5. Clinical and Research Perspectives
6. Conclusions and Implications for Non-Military PTSD Disorders
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- American Psychiatric Association. DSM-5, Diagnostic and Statistical Manual of Mental Disorders; American Psychiatric Publishing: Arlington, VA, USA, 2013; pp. 271–280. [Google Scholar]
- Breslau, N. The Epidemiology of Trauma, PTSD, and Other Posttrauma Disorders. Trauma Violence Abus. 2009, 10, 198–210. [Google Scholar] [CrossRef]
- Germain, A. Sleep disturbances as the hallmark of PTSD: Where are we now? Am. J. Psychiatry 2013, 170, 372–382. [Google Scholar] [CrossRef]
- Pillar, G.; Malhotra, A.; Lavie, P. Post-traumatic stress disorder and sleep-what a nightmare! Sleep Med. Rev. 2000, 4, 183–200. [Google Scholar] [CrossRef] [PubMed]
- Rosen, R.C.; Cikesh, B.; Fang, S.; Trachtenberg, F.L.; Seal, K.H.; Magnavita, A.M.; Bovin, M.J.; Green, J.D.; Bliwise, D.L.; Marx, B.P.; et al. Posttraumatic Stress Disorder Severity and Insomnia-Related Sleep Disturbances: Longitudinal Associations in a Large, Gender-Balanced Cohort of Combat-Exposed Veterans. J. Trauma Stress 2019, 32, 936–945. [Google Scholar] [CrossRef] [PubMed]
- Eugene, A.R.; Masiak, J. The Neuroprotective Aspects of Sleep. MEDtube Sci. 2015, 3, 35–40. [Google Scholar] [PubMed]
- Chennaoui, M.; Léger, D.; Gomez-Merino, D. Sleep and the GH/IGF-1 axis: Consequences and countermeasures of sleep loss/disorders. Sleep Med. Rev. 2020, 49, 101223. [Google Scholar] [CrossRef] [PubMed]
- Troxel, W.M.; Shih, R.A.; Pedersen, E.R.; Geyer, L.; Fisher, M.P.; Griffin, B.A.; Haas, A.C.; Kurz, J.; Steinberg, P.S. Sleep in the Military: Promoting Healthy Sleep Among U.S. Servicemembers. Rand Health Q. 2015, 5, 19. [Google Scholar]
- Mantua, J.; Bessey, A.; Sowden, W.J.; Chabuz, R.; Brager, A.J.; Capaldi, V.F.; Simonelli, G. A Review of Environmental Barriers to Obtaining Adequate Sleep in the Military Operational Context. Mil. Med. 2019, 184, e259–e266. [Google Scholar] [CrossRef] [Green Version]
- Watson, N.F.; Badr, M.S.; Belenky, G.; Bliwise, D.L.; Buxton, O.M.; Buysse, D.; Dinges, D.F.; Gangwisch, J.; Grandner, M.A.; Kushida, C.; et al. Joint Consensus Statement of the American Academy of Sleep Medicine and Sleep Research Society on the Recommended Amount of Sleep for a Healthy Adult: Methodology and Discussion. Sleep 2015, 38, 1161–1183. [Google Scholar] [CrossRef]
- Good, C.H.; Brager, A.J.; Capaldi, V.F.; Mysliwiec, V. Sleep in the United States Military. Neuropsychopharmacology 2020, 45, 176–191. [Google Scholar] [CrossRef] [Green Version]
- Luxton, D.D.; Greenburg, D.; Ryan, J.; Niven, A.; Wheeler, G.; Mysliwiec, V. Prevalence and impact of short sleep duration in redeployed OIF soldiers. Sleep 2011, 34, 1189–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seelig, A.D.; Jacobson, I.G.; Smith, B.; Hooper, T.I.; Boyko, E.J.; Gackstetter, G.D.; Gehrman, P.; Macera, C.A.; Smith, T.C.; Millennium Cohort Study Team. Sleep patterns before, during, and after deployment to Iraq and Afghanistan. Sleep 2010, 33, 1615–1622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harrison, E.; Glickman, G.L.; Beckerley, S.; Taylor, M.K. Self-Reported Sleep During U.S. Navy Operations and the Impact of Deployment-Related Factors. Mil. Med. 2017, 182, 189–194. [Google Scholar] [CrossRef] [Green Version]
- Miller, N.L.; Shattuck, L.G.; Matsangas, P. Longitudinal study of sleep patterns of United States Military Academy cadets. Sleep 2010, 33, 1623–1631. [Google Scholar] [CrossRef] [Green Version]
- Danker-Hopfe, H.; Sauter, C.; Kowalski, J.T.; Kropp, S.; Ströhle, A.; Wesemann, U.; Zimmermann, P.L. Sleep quality of German soldiers before, during and after deployment in Afghanistan-a prospective study. J. Sleep Res. 2017, 26, 353–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunt, E.J.F.; Greenberg, N.; Jones, N. Poor sleep after military deployment: Associations with mental health difficulties. Occup. Med. 2016, 66, 669–675. [Google Scholar] [CrossRef] [Green Version]
- Beaumont, M.; Batéjat, D.; Piérard, C.; Van Beers, P.; Denis, J.B.; Coste, O.; Doireau, P.; Chauffard, F.; French, J.; Lagarde, D. Caffeine or melatonin effects on sleep and sleepiness after rapid eastward transmeridian travel. J. Appl. Physiol. 2004, 96, 50–58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trousselard, M.; Leger, D.; van Beers, P.; Coste, O.; Vicard, A.; Pontis, J.; Crosnier, S.-N.; Chennaoui, M. Sleeping under the Ocean: Despite Total Isolation, Nuclear Submariners Maintain Their Sleep and Wake Patterns throughout Their Under Sea Mission. PLoS ONE 2015, 10, e0126721. [Google Scholar] [CrossRef] [Green Version]
- Maiguy, A.; Verret, C.; Sauvet, F.; Léger, D.; Baert, P.; Minaberry, S.; Champier, G.; Guidicci, J.-B.; Perret, M.-R.; Chennaoui, M. Étude de la prévalence des troubles du sommeil chez les militaires de la place de Paris. Médecine du Sommeil 2014, 11, 24–25. [Google Scholar] [CrossRef]
- Soyere, G.; Sauvet, F.; Traccard, C.; Van Beers, P.; Fusaï, T.; Bonin, O.; Boissy, J.M.; Lamotte, F.; Chennaoui, M. Prévalence des troubles du sommeil et évaluation d’une formation d’éducation thérapeutique aux règles d’hygiène du sommeil chez des militaires français. Médecine du Sommeil 2018, 15, 45–46. [Google Scholar] [CrossRef]
- Pierres, V.; Lefloch, H.; Sauvet, F.; Chennaoui, M. Évaluation de l’impact des troubles du sommeil au sein de la Brigade des sapeurs-pompiers de Paris (BSPP). Médecine du Sommeil 2016, 13, 19–20. [Google Scholar] [CrossRef]
- LoPresti, M.L.; Anderson, J.A.; Saboe, K.N.; McGurk, D.L.; Balkin, T.J.; Sipos, M.L. The Impact of Insufficient Sleep on Combat Mission Performance. Mil. Behav. Health 2016, 4, 356–363. [Google Scholar] [CrossRef]
- Haran, F.J.; Schumacher, P.; Markwald, R.; Handy, J.D.; Tsao, J.W. Relationships between sleepiness, mood, and neurocognitive performance in military personnel. Front. Neurol. 2019, 10, 64. [Google Scholar] [CrossRef]
- Mantua, J.; Bessey, A.F.; Mickelson, C.; Choynowski, J.J.; Noble, J.; Burke, T.M.; McKeon, A.B.; Sowden, W.J. Sleep and high-risk behavior in military Service Members: A mega-analysis of four diverse U.S. Army units. Sleep 2021, 44, zsaa221. [Google Scholar] [CrossRef]
- Grier, T.; Dinkeloo, E.; Reynolds, M.; Jones, B.H. Sleep duration and musculoskeletal injury incidence in physically active men and women: A study of U.S. Army Special Operation Forces soldiers. Sleep Health 2020, 6, 344–349. [Google Scholar] [CrossRef]
- Peterson, A.L.; Goodie, J.L.; Satterfield, W.A.; Brim, W.L. Sleep disturbance during military deployment. Mil. Med. 2008, 173, 230–235. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mysliwiec, V.; Matsangas, P.; Baxter, T.; McGraw, L.; Bothwell, N.E.; Roth, B.J. Comorbid insomnia and obstructive sleep apnea in military personnel: Correlation with polysomnographic variables. Mil. Med. 2014, 179, 294–300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogers, A.E.; Stahlman, S.; Hunt, D.J.; Oh, G.-T.; Clark, L.L. Obstructive sleep apnea and associated attrition, active component, U.S. Armed Forces, January 2004–May 2016. MSMR 2016, 23, 2–11. [Google Scholar]
- Bramoweth, A.D.; Germain, A. Deployment-related insomnia in military personnel and veterans. Curr. Psychiatry Rep. 2013, 15, 401. [Google Scholar] [CrossRef]
- Erickson, E.A.; Stahlman, S.; McNellis, M.G. Insomnia and motor vehicle accident-related injuries, active component, U.S. Armed Forces, 2007–2016. MSMR 2017, 24, 2–11. [Google Scholar]
- Capener, D.C.; Brock, M.S.; Hansen, S.L.; Matsangas, P.; Mysliwiec, V. An Initial Report of Sleep Disorders in Women in the U.S. Military. Mil. Med. 2018, 183, e266–e271. [Google Scholar] [CrossRef] [Green Version]
- Vanderperre, G.; Nguyen-Huy-Thui, G.; Marsan, P.; Desjeux, G.; Marcaillou, M. Impact of the obstructive sleep hypopnea-apnea syndrom treated on employment for military employment. Rev. Epidemiol. Sante Publique 2018, 66, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Gehrman, P.; Seelig, A.D.; Jacobson, I.G.; Boyko, E.J.; Hooper, T.I.; Gackstetter, G.D.; Ulmer, C.S.; Smith, T.C. Predeployment Sleep Duration and Insomnia Symptoms as Risk Factors for New-Onset Mental Health Disorders Following Military Deployment. Sleep 2013, 36, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Seelig, A.D.; Jacobson, I.G.; Donoho, C.J.; Trone, D.W.; Crum-Cianflone, N.F.; Balkin, T.J. Sleep and Health Resilience Metrics in a Large Military Cohort. Sleep 2016, 39, 1111–1120. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.E.; Campbell-Sills, L.; Kessler, R.C.; Sun, X.; Heeringa, S.G.; Nock, M.K.; Ursano, R.J.; Jain, S.; Stein, M.B. Pre-deployment insomnia is associated with post-deployment post-traumatic stress disorder and suicidal ideation in US Army soldiers. Sleep 2019, 42, zsy229. [Google Scholar] [CrossRef]
- MacGregor, A.J.; Markwald, R.R.; Dougherty, A.L.; Seda, G. The relationship between military occupation and diagnosed insomnia following combat deployment. J. Clin. Sleep Med. 2020, 16, 1125–1132. [Google Scholar] [CrossRef] [PubMed]
- Wardat, I. Troubles psychiques en relation avec un événement traumatisant dans les armées: Facteurs associés aux conduites addictives et à la prise en charge psychiatrique avant la déclaration. Bull. Épidémiol. Armées 2014, 4, 2–7. [Google Scholar]
- Davis, J.L.; Rhudy, J.L.; Pruiksma, K.E.; Byrd, P.; Williams, A.E.; McCabe, K.M.; Bartley, E.J. Physiological predictors of response to exposure, relaxation, and rescripting therapy for chronic nightmares in a randomized clinical trial. J. Clin. Sleep Med. 2011, 7, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schreuder, B.J.; Kleijn, W.C.; Rooijmans, H.G. Nocturnal re-experiencing more than forty years after war trauma. J. Trauma Stress 2000, 13, 453–463. [Google Scholar] [CrossRef]
- Alonso, J.; Angermeyer, M.C.; Bernert, S.; Bruffaerts, R.; Brugha, T.S.; Bryson, H.; de Girolamo, G.; Graaf, R.; Demyttenaere, K.; Gasquet, I.; et al. Prevalence of mental disorders in Europe: Results from the European Study of the Epidemiology of Mental Disorders ( ESEMeD ) project. Acta Psychiatr. Scand. Suppl. 2004, 420, 21–27. [Google Scholar] [CrossRef]
- Gradus, J.L. Epidemiology of PTSD from the National Center for Post-Traumatic Stress Disorder, Department of Veterans Affairs. From the National Center for Post-Traumatic Stress Disorder, Department of Veterans Affairs. Available online: www.ptsd.va.gov (accessed on 10 June 2021).
- Spitzer, R.L.; First, M.B.; Wakefield, J.C. Saving PTSD from itself in DSM-V. J. Anxiety Disord. 2007, 21, 233–241. [Google Scholar] [CrossRef]
- Vallet, D.; Arvers, P. Etude Exploratoire sur l’Etat de Stress Post-Traumatique dans Deux Unites Operationnelles de l’Armee de Terre (Exploratory Study of the Condition of Post-Traumatic Stress Disorder from Two Operational Units of Ground Forces). Méd. Armées 2006, 33, 441–445. [Google Scholar]
- Berry, X.; Marimoutou, C.; Pommier de Santi, V.; Rabatel, E.; Deparis, X.; Paul, F. Etats de stress post-traumatique au retour d’Afghanistan. Stress Trauma 2011, 11, 75–84. [Google Scholar]
- Varker, T.; Kartal, D.; Watson, L.; Freijah, I.; Donnell, M.O.; Forbes, D.; Phelps, A.; Hopwood, M.; Mcfarlane, A.; Cooper, J.; et al. Defining response and nonresponse to posttraumatic stress disorder treatments: A systematic review. Clin. Psychol. Sci. Pract. 2020, 27. [Google Scholar] [CrossRef]
- Friedman, M.J.; Marmar, C.R.; Baker, D.G.; Sikes, C.R.; Farfel, G.M. Randomized, double-blind comparison of sertraline and placebo for posttraumatic stress disorder in a department of veterans affairs setting. J. Clin. Psychiatry 2007, 68, 711–720. [Google Scholar] [CrossRef] [PubMed]
- Tucker, P.; Zaninelli, R.; Yehuda, R.; Ruggiero, L.; Dillingham, K.; Pitts, C.D. Paroxetine in the treatment of chronic posttraumatic stress disorder: Results of a placebo-controlled, flexible-dosage trial. J. Clin. Psychiatry 2001, 62, 860–868. [Google Scholar] [CrossRef]
- Huang, Z.D.; Zhao, Y.F.; Li, S.; Gu, H.Y.; Lin, L.L.; Yang, Z.Y.; Niu, Y.-M.; Zhang, C.; Luo, J. Comparative efficacy and acceptability of pharmaceutical management for adults with post-traumatic stress disorder: A systematic review and meta-analysis. Front. Pharmacol. 2020, 8, 559. [Google Scholar] [CrossRef]
- Stein, M.B.; Kline, N.A.; Matloff, J.L. Adjunctive olanzapine for SSRI-resistant combat-related PTSD: A double-blind, placebo-controlled study. Am. J. Psychiatry 2002, 159, 1777–1779. [Google Scholar] [CrossRef] [Green Version]
- Colas, M.-D.; Lahutte, B. Le stress au sein de la population militaire: Du stress opérationnel à l’état de stress post-traumatique. Méd. Armées 2009, 37, 399–410. [Google Scholar]
- Alexander, W. Pharmacotherapy for Post-traumatic Stress Disorder In Combat Veterans: Focus on Antidepressants and Atypical Antipsychotic Agents. Pharm. Ther. 2012, 37, 32–38. [Google Scholar]
- Nappi, C.M.; Drummond, S.P.A.; Hall, J.M.H. Treating nightmares and insomnia in posttraumatic stress disorder: A review of current evidence. Neuropharmacology 2012, 62, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Creamer, J.L.; Brock, M.S.; Matsangas, P.; Motamedi, V.; Mysliwiec, V. Nightmares in United States Military Personnel with Sleep Disturbances. J. Clin. Sleep Med. 2018, 14, 419–426. [Google Scholar] [CrossRef] [Green Version]
- Detweiler, M.B.; Pagadala, B.; Candelario, J.; Boyle, J.S.; Detweiler, J.G.; Lutgens, B.W. Treatment of Post-Traumatic Stress Disorder Nightmares at a Veterans Affairs Medical Center. J. Clin. Med. 2016, 5, 117. [Google Scholar] [CrossRef] [Green Version]
- Livezey, J.; Oliver, T.; Cantilena, L. Prolonged Neuropsychiatric Symptoms in a Military Service Member Exposed to Mefloquine. Drug Saf.-Case Rep. 2016, 3, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nevin, R.L. Screening for Symptomatic Mefloquine Exposure Among Veterans with Chronic Psychiatric Symptoms. Fed. Pract. 2017, 34, 12–14. [Google Scholar] [PubMed]
- Eick-Cost, A.A.; Hu, Z.; Rohrbeck, P.; Clark, L.L. Neuropsychiatric Outcomes after Mefloquine Exposure among U.S. Military Service Members. Am. J. Trop. Med. Hyg. 2017, 96, 159–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woodward, S.H.; Arsenault, N.J.; Murray, C.; Bliwise, D.L. Laboratory sleep correlates of nightmare complaint in PTSD inpatients. Biol. Psychiatry 2000, 48, 1081–1087. [Google Scholar] [CrossRef]
- Brock, M.S.; Powell, T.A.; Creamer, J.L.; Moore, B.A.; Mysliwiec, V. Trauma Associated Sleep Disorder: Clinical Developments 5 Years After Discovery. Curr. Psychiatry Rep. 2019, 21, 80. [Google Scholar] [CrossRef]
- Phelps, A.J.; Forbes, D.; Hopwood, M.; Creamer, M. Trauma-related dreams of Australian veterans with PTSD: Content, affect and phenomenology. Aust. N. Z. J. Psychiatry 2011, 45, 853–860. [Google Scholar] [CrossRef] [PubMed]
- Mysliwiec, V.; Brock, M.S.; Creamer, J.L.; O’Reilly, B.M.; Germain, A.; Roth, B.J. Trauma associated sleep disorder: A parasomnia induced by trauma. Sleep Med. Rev. 2018, 37, 94–104. [Google Scholar] [CrossRef]
- Phelps, A.J.; Forbes, D.; Creamer, M. Understanding posttraumatic nightmares: An empirical and conceptual review. Clin. Psychol. Rev. 2008, 28, 338–355. [Google Scholar] [CrossRef]
- Onton, J.A.; Kang, D.Y.; Coleman, T.P. Visualization of Whole-Night Sleep EEG From 2-Channel Mobile Recording Device Reveals Distinct Deep Sleep Stages with Differential Electrodermal Activity. Front. Hum. Neurosci. 2016, 10, 605. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Ren, R.; Sanford, L.D.; Yang, L.; Zhou, J.; Zhang, J.; Wing, Y.-K.; Shi, J.; Lu, L.; Tang, X. Sleep in posttraumatic stress disorder: A systematic review and meta-analysis of polysomnographic findings. Sleep Med. Rev. 2019, 48, 101210. [Google Scholar] [CrossRef] [PubMed]
- Mellman, T.A.; Bustamante, V.; Fins, A.I.; Pigeon, W.R.; Nolan, B. REM sleep and the early development of posttraumatic stress disorder. Am. J. Psychiatry 2002, 159, 1696–1701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Laxminarayan, S.; Ramakrishnan, S.; Dovzhenok, A.; Cashmere, J.D.; Germain, A.; Reifman, J. Increased oscillatory frequency of sleep spindles in combat-exposed veteran men with post-traumatic stress disorder. Sleep 2020, 43, zsaa064. [Google Scholar] [CrossRef] [PubMed]
- Rousseau, P.F.; Vallat, R.; Coste, O.; Cadis, H.; Nicolas, F.; Trousselard, M.; Ruby, P.; Khalfa, S. Sleep parameters improvement in PTSD soldiers after symptoms remission. Sci. Rep. 2021, 11, 8873. [Google Scholar] [CrossRef]
- De Boer, M.; Nijdam, M.J.; Jongedijk, R.A.; Bangel, K.A.; Olff, M.; Hofman, W.F.; Talamini, L.M. The spectral fingerprint of sleep problems in post-traumatic stress disorder. Sleep 2020, 43, zsz269. [Google Scholar] [CrossRef] [Green Version]
- Wang, C.; Ramakrishnan, S.; Laxminarayan, S.; Dovzhenok, A.; Cashmere, J.D.; Germain, A.; REifman, J. An attempt to identify reproducible high-density EEG markers of PTSD during sleep. Sleep 2020, 43, zsz207. [Google Scholar] [CrossRef]
- Kobayashi, I.; Lavela, J.; Bell, K.; Mellman, T.A. The Impact of Posttraumatic Stress Disorder Versus Resilience on Nocturnal Autonomic Nervous System Activity as Functions of Sleep Stage and Time of Sleep. Physiol. Behav. 2016, 164 Pt A, 11–18. [Google Scholar] [CrossRef] [Green Version]
- Germain, A.; McKeon, A.B.; Campbell, R.L. Sleep in PTSD: Conceptual model and novel directions in brain-based research and interventions. Curr. Opin. Psychol. 2017, 14, 84–89. [Google Scholar] [CrossRef]
- Coventry, P.A.; Meader, N.; Melton, H.; Temple, M.; Dale, H.; Wright, K.; Cloitre, M.; Karatzias, T.; Bisson, J.; Roberts, N.P.; et al. Psychological and pharmacological interventions for posttraumatic stress disorder and comorbid mental health problems following complex traumatic events: Systematic review and component network meta-analysis. PLoS Med. 2020, 17, e1003262S. [Google Scholar] [CrossRef]
- Colvonen, P.J.; Straus, L.D.; Stepnowsky, C.; McCarthy, M.J.; Goldstein, L.A.; Norman, S.B. Recent Advancements in Treating Sleep Disorders in Co-Occurring PTSD. Curr. Psychiatry Rep. 2018, 20, 48. [Google Scholar] [CrossRef]
- Pruiksma, K.E.; Taylor, D.J.; Wachen, J.S.; Mintz, J.; Young-McCaughan, S.; Peterson, A.L.; Yarvis, J.S.; Borah, E.V.; Dondanville, K.A.; Litz, B.T.; et al. Residual sleep disturbances following PTSD treatment in active duty military personnel. Psychol. Trauma 2016, 8, 697–701. [Google Scholar] [CrossRef] [PubMed]
- Levin, R.; Nielsen, T.A. Disturbed Dreaming, Posttraumatic Stress Disorder, and Affect Distress: A Review and Neurocognitive Model. Psychol. Bull. 2007, 133, 482–528. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McLay, R.N.; Klam, W.P.; Volkert, S.L. Insomnia is the most commonly reported symptom and predicts other symptoms of post-traumatic stress disorder in U.S. service members returning from military deployments. Mil. Med. 2010, 175, 759–762. [Google Scholar] [CrossRef] [Green Version]
- Pruiksma, K.E.; Wachen, J.S.; Wardle, S.; Resick, P.A. Psychotherapy Interventions for Comorbid Sleep Disorders and Posttraumatic Stress Disorder BT. In Sleep and Combat-Related Post Traumatic Stress Disorder; Vermetten, E., Germain, A., Neylan, T.C., Eds.; Springer: New York, NY, USA, 2018; pp. 277–292. [Google Scholar] [CrossRef]
- Harb, G.C.; Cook, J.M.; Phelps, A.J.; Gehrman, P.R.; Forbes, D.; Localio, R.; Harpaz-Rotem, I.; Gur, R.C.; Ross, R.J. Randomized Controlled Trial of Imagery Rehearsal for Posttraumatic Nightmares in Combat Veterans. J. Clin. Sleep Med. 2019, 15, 757–767. [Google Scholar] [CrossRef]
- Van Liempt, S.; van Zuiden, M.; Westenberg, H.; Super, A.; Vermetten, E. Impact of impaired sleep on the development of PTSD symptoms in combat veterans: A pospective longitudinal cohort study. Depress. Anxiety 2013, 30, 469–474. [Google Scholar] [CrossRef]
- Bryant, R.A.; Creamer, M.; O’Donnell, M.; Silove, D.; McFarlane, A.C. Sleep disturbance immediately prior to trauma predicts subsequent psychiatric disorder. Sleep 2010, 33, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Pace-Schott, E.F.; Germain, A.; Milad, M.R. Sleep and REM sleep disturbance in the pathophysiology of PTSD: The role of extinction memory. Biol. Mood Anxiety Disord. 2015, 5, 165–167. [Google Scholar] [CrossRef] [Green Version]
- Porcheret, K.; Iyadurai, L.; Bonsall, M.B.; Goodwin, G.M.; Beer, S.A.; Darwent, M.; Holmes, E.A. Sleep and intrusive memories immediately after a traumatic event in emergency department patients. Sleep 2020, 43, zsaa033. [Google Scholar] [CrossRef] [PubMed]
- Mellman, T.A.; Pigeon, W.R.; Nowell, P.D.; Nolan, B. Relationships between REM sleep findings and PTSD symptoms during the early aftermath of trauma. J. Trauma Stress 2007, 20, 893–901. [Google Scholar] [CrossRef] [PubMed]
- Khazaie, H.; Masoudi, M. Sleep disturbances in veterans with chronic war-induced PTSD. J. Inj. Violence Res. 2016, 8, 99–107. [Google Scholar] [CrossRef]
- Krakow, B.J.; Ulibarri, V.A.; Moore, B.A.; Mciver, N.D. Posttraumatic stress disorder and sleep-disordered breathing: A review of comorbidity research. Sleep Med. Rev. 2015, 24, 37–45. [Google Scholar] [CrossRef]
- Nevin, R.L. Induced Hypoglossal Dysfunction as a Cause of Obstructive Sleep Apnea in Mefloquine-Exposed Veterans. Laryngoscope 2020, 130, E949. [Google Scholar] [CrossRef] [PubMed]
- Wright, K.M.; Britt, T.W.; Bliese, P.D.; Adler, A.B.; Picchioni, D.; Moore, D. Insomnia as predictor versus outcome of PTSD and depression among Iraq combat veterans. J. Clin. Psychol. 2011, 67, 1240–1258. [Google Scholar] [CrossRef]
- Sauvet, F.; Gomez, D.; Rabat, A.; Chennaoui, M. Guide Pratique. Gestion du Cycle Veille—Sommeil en Milieu Militaire; IRBA: Brétigny-sur-Orge, France, EAN: 9782914558938; 2020; p. 83. [Google Scholar]
- Perreaut-Pierre, E. Comprendre et Pratiquer les Techniques d’Optimisation; InterEditions: Paris, France, 2012. [Google Scholar]
- Debellemaniere, E.; Gomez-Merino, D.; Erblang, M.; Dorey, R.; Genot, M.; Perreaut-Pierre, E.; Pisani, A.; Rocco, L.; Sauvet, F.; Léger, D.; et al. Using relaxation techniques to improve sleep during naps. Ind. Health 2018, 56, 220–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arrêté du 20 Décembre 2012 Relatif à la Détermination et au Contrôle de l’Aptitude Médicale à Servir du Personnel Militaire. NOR DEFK1243552A JORF no0015 du 18 Janvier 2013. Available online: https://www.legifrance.gouv.fr/loda/id/JORFTEXT000026952134/ (accessed on 10 June 2021).
- Hughes, J.; Earnshaw, N.; Greenberg, N.; Eldridge, R.; Fear, N.; French, C.; Deahl, M.P.; Wessely, S. Use of psychological decompression in military operational environments. Mil. Med. 2008, 173, 534–538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garber, B.G.; Zamorski, M.A. Evaluation of a third-location decompression program for Canadian forces members returning from Afghanistan. Mil. Med. 2012, 177, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Vautier, V. Le SAS de décompression à Chypre. Description, critiques et perspectives. Méd. Armées 2012, 40, 315–319. [Google Scholar]
- Dworkin, E.R.; Bergman, H.E.; Walton, T.O.; Walker, D.D.; Kaysen, D.L. Co-Occurring Post-Traumatic Stress Disorder and Alcohol Use Disorder in U.S. Military and Veteran Populations. Alcohol Res. 2018, 39, 161–169. [Google Scholar]
- Hoge, C.W.; Castro, C.A.; Messer, S.C.; McGurk, D.; Cotting, D.I.; Koffman, R.L. Combat duty in Iraq and Afghanistan, mental health problems, and barriers to care. US Army Med. Dep. J. Jul-Sep. 2008, 7–17. [Google Scholar] [CrossRef] [Green Version]
- Miller, K.E.; Brownlow, J.A.; Gehrman, P.R. Sleep in PTSD: Treatment approaches and outcomes. Curr. Opin. Psychol. 2020, 34, 12–17. [Google Scholar] [CrossRef]
- Roehrs, T.; Merlotti, L.; Petrucelli, N.; Stepanski, E.; Roth, T. Experimental sleep fragmentation. Sleep 1994, 17, 438–443. [Google Scholar] [CrossRef] [Green Version]
- Lipinska, G.; Thomas, K.G.F. Rapid eye movement fragmentation, not slow-wave sleep, predicts neutral declarative memory consolidation in posttraumatic stress disorder. J. Sleep Res. 2019, 28, e12846. [Google Scholar] [CrossRef]
- Stepanski, E.J. The effect of sleep fragmentation on daytime function. Sleep 2002, 25, 268–276. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, M.H.; Arand, D.L. Clinical effects of sleep fragmentation versus sleep deprivation. Sleep Med. Rev. 2003, 7, 297–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosseland, R.; Pallesen, S.; Nordhus, I.H.; Matre, D.; Blågestad, T. Effects of Sleep Fragmentation and Induced Mood on Pain Tolerance and Pain Sensitivity in Young Healthy Adults. Front. Psychol. 2018, 9, 2089. [Google Scholar] [CrossRef]
- Roehrs, T.A.; Roth, T. Sleep Disturbance in Substance Use Disorders. Psychiatr. Clin. N. Am. 2015, 38, 793–803. [Google Scholar] [CrossRef] [Green Version]
- Jaoude, P.; Vermont, L.N.; Porhomayon, J.; El-Solh, A.A. Sleep-disordered breathing in patients with post-traumatic stress disorder. Ann. Am. Thorac. Soc. 2015, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Medic, G.; Wille, M.; Hemels, M.E. Short- and long-term health consequences of sleep disruption. Nat. Sci. Sleep 2017, 9, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Wilhelm, I.; Azza, Y.; Brennwald, K.; Schäfer, Y.E.; Seifritz, E.; Kleim, B. Investigating the effect of a nap following experimental trauma on analogue PTSD symptoms. Sci. Rep. 2021, 11, 4710. [Google Scholar] [CrossRef]
- Kobayashi, I.; Boarts, J.M.; Delahanty, D.L. Polysomnographically measured sleep abnormalities in PTSD: A meta-analytic review. Psychophysiology 2007, 44, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Van der Kolk, B.; Blitz, R.; Burr, W.; Sherry, S.; Hartmann, E. Nightmares and trauma: A comparison of nightmares after combat with lifelong nightmares in veterans. Am. J. Psychiatry 1984, 141, 187–190. [Google Scholar] [CrossRef]
- Dagan, Y.; Lavie, P.; Bleich, A. Elevated awakening thresholds in sleep stage 3–4 in war-related post-traumatic stress disorder. Biol. Psychiatry 1991, 30, 618–622. [Google Scholar] [CrossRef]
- Breslau, N.; Roth, T.; Burduvali, E.; Kapke, A.; Schultz, L.; Roehrs, T. Sleep in lifetime posttraumatic stress disorder: A community-based polysomnographic study. Arch. Gen. Psychiatry 2004, 61, 508–516. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.J.; Ball, W.A.; Dinges, D.F.; Kribbs, N.B.; Morrison, A.R.; Silver, S.M.; Mulvaney, F.D. Rapid eye movement sleep disturbance in posttraumatic stress disorder. Biol. Psychiatry 1994, 35, 195–202. [Google Scholar] [CrossRef]
- Habukawa, M.; Uchimura, N.; Maeda, M.; Ogi, K.; Hiejima, H.; Kakuma, T. Differences in rapid eye movement (REM) sleep abnormalities between posttraumatic stress disorder (PTSD) and major depressive disorder patients: REM interruption correlated with nightmare complaints in PTSD. Sleep Med. 2018, 43, 34–39. [Google Scholar] [CrossRef]
- Mellman, T.A.; Kobayashi, I.; Lavela, J.; Wilson, B.; Hall Brown, T.S. A relationship between REM sleep measures and the duration of posttraumatic stress disorder in a young adult urban minority population. Sleep 2014, 37, 1321–1326. [Google Scholar] [CrossRef] [Green Version]
- Raskind, M.A.; Peskind, E.R.; Chow, B.; Harris, C.; Davis-Karim, A.; Holmes, H.A.; Hart, K.L.; McFall, M.; Mellman, T.A.; Reist, C.; et al. Trial of Prazosin for Post-Traumatic Stress Disorder in Military Veterans. N. Engl. J. Med. 2018, 378, 507–517. [Google Scholar] [CrossRef] [PubMed]
- Rusch, H.L.; Guardado, P.; Baxter, T.; Mysliwiec, V.; Army, M.; Stress, L.B. Improved Sleep Quality is Associated with Reductions in Depression and PTSD Arousal Symptoms and Increases in IGF-1 Concentrations. J. Clin. Sleep Med. 2015, 11, 615–623. [Google Scholar] [CrossRef] [Green Version]
- Van Liempt, S.; Vermetten, E.; Lentjes, E.; Arends, J.; Westenberg, H. Decreased nocturnal growth hormone secretion and sleep fragmentation in combat-related posttraumatic stress disorder; potential predictors of impaired memory consolidation. Psychoneuroendocrinology 2011, 36, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Colvonen, P.J.; Ellison, J.; Haller, M.; Norman, S.B. Examining Insomnia and PTSD Over Time in Veterans in Residential Treatment for Substance Use Disorders and PTSD. Behav. Sleep Med. 2019, 17, 524–535. [Google Scholar] [CrossRef] [PubMed]
- Kanady, J.C.; Talbot, L.S.; Maguen, S.; Straus, L.D.; Richards, A.; Ruoff, L.; Metzler, T.J.; Neylane, T.C. Cognitive Behavioral Therapy for Insomnia Reduces Fear of Sleep in Individuals with Posttraumatic Stress Disorder. J. Clin. Sleep Med. 2018, 14, 1193–1203. [Google Scholar] [CrossRef] [PubMed]
- Ross, R.J. The Changing REM Sleep Signature of Posttraumatic Stress Disorder. Sleep 2014, 37, 1281–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onton, J.A.; Matthews, S.C.; Kang, D.Y.; Coleman, T.P. In-Home Sleep Recordings in Military Veterans with Posttraumatic Stress Disorder Reveal Less REM and Deep Sleep <1 Hz. Front. Hum. Neurosci. 2018, 12, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnal, P.J.; Thorey, V.; Debellemaniere, E.; Ballard, M.E.; Hernandez, A.B.; Guillot, A.; Jourde, H.; Harris, M.; Guillard, M.; Van Beers, P.; et al. The dreem headband compared to polysomnography for electroencephalographic signal acquisition and sleep staging. Sleep 2020, 43, zsaa097. [Google Scholar] [CrossRef] [PubMed]
- Cohen Maril, M. How a Vibrating Smartwatch Could be Used to Stop Nightmares. 1 December 2020. Available online: https://www.ncbi.nlm.nih.gov/search/research-news/12106/ (accessed on 10 June 2021).
- Andrillon, T.; Solelhac, G.; Bouchequet, P.; Romano, F.; Le Brun, M.; Brigham, M.; Chennaoui, M.; Léger, D. Revisiting the value of polysomnographic data in insomnia: More than meets the eye. Sleep Med. 2020, 66, 184–200. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saguin, E.; Gomez-Merino, D.; Sauvet, F.; Leger, D.; Chennaoui, M. Sleep and PTSD in the Military Forces: A Reciprocal Relationship and a Psychiatric Approach. Brain Sci. 2021, 11, 1310. https://doi.org/10.3390/brainsci11101310
Saguin E, Gomez-Merino D, Sauvet F, Leger D, Chennaoui M. Sleep and PTSD in the Military Forces: A Reciprocal Relationship and a Psychiatric Approach. Brain Sciences. 2021; 11(10):1310. https://doi.org/10.3390/brainsci11101310
Chicago/Turabian StyleSaguin, Emeric, Danielle Gomez-Merino, Fabien Sauvet, Damien Leger, and Mounir Chennaoui. 2021. "Sleep and PTSD in the Military Forces: A Reciprocal Relationship and a Psychiatric Approach" Brain Sciences 11, no. 10: 1310. https://doi.org/10.3390/brainsci11101310