B Cells and Tertiary Lymphoid Structures Influence Survival in Lung Cancer Patients with Resectable Tumors
Abstract
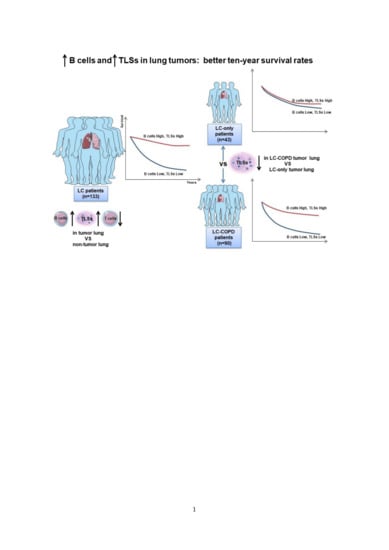
:Simple Summary
Abstract
1. Introduction
2. Results
2.1. Clinical Characteristics of the Study Patients
2.2. Number and Area of TLSs and Number of GCs in Lung Samples
2.2.1. Differences between LC-COPD and LC in Either Tumor or Non-Tumor Lung Samples
2.2.2. Differences between Tumor and Non-Tumor Lung Samples in LC-COPD and LC Patients
2.3. T and B Cell Levels in Lung Samples
2.3.1. Differences between LC-COPD and LC in either Tumor or Non-Tumor Lung Samples
2.3.2. Differences between Tumor and Non-Tumor Lung Samples in LC-COPD and LC Patients
2.4. Associations of TLSs with OS in LC and LC-COPD Patients
2.5. Associations of B and T Cells with Survival in LC and LC-COPD Patients
3. Discussion
4. Materials and Methods
4.1. Study Design and Ethics
4.2. Clinical Assessment
4.3. Sample Collection and Preservation
4.4. Identification of B Cells, T Cells, and TLSs in the Lung Specimens
4.5. Identification of GCs in TLSs of Lung Specimens
4.6. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ferlay, J.; Shin, H.R.; Bray, F.; Forman, D.; Mathers, C.; Parkin, D.M. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int. J. Cancer 2010, 127, 2893–2917. [Google Scholar] [CrossRef]
- Thomas, A.; Chen, Y.; Yu, T.; Jakopovic, M.; Giaccone, G. Trends and characteristics of young non-small cell lung cancer patients in the United States. Front. Oncol. 2015, 5, 113. [Google Scholar] [CrossRef] [Green Version]
- Soler-Cataluña, J.J.; Novella, L.; Soler, C.; Nieto, M.L.; Esteban, V.; Sánchez-Toril, F.; Miravitlles, M. Clinical Characteristics and Risk of Exacerbations Associated With Different Diagnostic Criteria of Asthma-COPD Overlap. Arch. Bronconeumol. 2019. [Google Scholar] [CrossRef]
- Pleguezuelos, E.; Gimeno-Santos, E.; Hernández, C.; Mata, M.d.C.; Palacios, L.; Piñera, P.; Molina, J.; Chiner, E.; Miravitlles, M. Recommendations on non-Pharmacological Treatment in Chronic Obstructive Pulmonary Disease From the Spanish COPD Guidelines (GesEPOC 2017). Arch. Bronconeumol. 2018, 54, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Miravitlles, M.; Soler-Cataluña, J.J.; Calle, M.; Molina, J.; Almagro, P.; Quintano, J.A.; Trigueros, J.A.; Cosío, B.G.; Casanova, C.; Antonio Riesco, J.; et al. Spanish Guidelines for Management of Chronic Obstructive Pulmonary Disease (GesEPOC) 2017. Pharmacological Treatment of Stable Phase. Arch. Bronconeumol. 2017, 53, 324–335. [Google Scholar] [CrossRef]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2019. CA. Cancer J. Clin. 2019, 69, 7–34. [Google Scholar] [CrossRef] [Green Version]
- Seijo, L.M.; Trujillo, J.C.; Zulueta, J.J. Screening in Lung Cancer: The Latest Evidence. Arch. Bronconeumol. 2020, 56, 7–8. [Google Scholar] [CrossRef]
- González-Marrón, A.; Martín-Sánchez, J.C.; Garcia-Alemany, F.; Martínez-Martín, E.; Matilla-Santander, N.; Cartanyà-Hueso, À.; Vidal, C.; García, M.; Martínez-Sánchez, J.M. Estimation of the Risk of Lung Cancer in Women Participating in a Population-Based Breast Cancer Screening Program. Arch. Bronconeumol. 2019. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Mao, K.; Guo, X. Clinical significance of serum T helper 1/T helper 2 cytokine shift in patients with non-small cell lung cancer. Oncol. Lett. 2014, 8, 1682–1686. [Google Scholar] [CrossRef] [Green Version]
- Mateu-Jimenez, M.; Curull, V.; Pijuan, L.; Sánchez-Font, A.; Rivera-Ramos, H.; Rodríguez-Fuster, A.; Aguiló, R.; Gea, J.; Barreiro, E. Systemic and Tumor Th1 and Th2 Inflammatory Profile and Macrophages in Lung Cancer: Influence of Underlying Chronic Respiratory Disease. J. Thorac. Oncol. 2017, 12. [Google Scholar] [CrossRef] [Green Version]
- Becker, Y. Respiratory syncytial virus(RSV)-induced allergy may be controlled by IL-4 and CX3C fractalkine antagonists and CpG ODN as adjuvant: Hypothesis and implications for treatment. Virus Genes 2006, 33, 253–264. [Google Scholar] [CrossRef]
- Conway, E.M.; Pikor, L.A.; Kung, S.H.Y.; Hamilton, M.J.; Lam, S.; Lam, W.L.; Bennewith, K.L. Macrophages, Inflammation, and Lung Cancer. Am. J. Respir. Crit. Care Med. 2016, 193, 116–130. [Google Scholar] [CrossRef] [PubMed]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salazar-Degracia, A.; Granado-Martínez, P.; Millán-Sánchez, A.; Tang, J.; Pons-Carreto, A.; Barreiro, E. Reduced lung cancer burden by selective immunomodulators elicits improvements in muscle proteolysis and strength in cachectic mice. J. Cell. Physiol. 2019, 234, 18041–18052. [Google Scholar] [CrossRef]
- van der Strate, B.W.A.; Postma, D.S.; Brandsma, C.-A.; Melgert, B.N.; Luinge, M.A.; Geerlings, M.; Hylkema, M.N.; van den Berg, A.; Timens, W.; Kerstjens, H.A.M. Cigarette smoke-induced emphysema: A role for the B cell? Am. J. Respir. Crit. Care Med. 2006, 173, 751–758. [Google Scholar] [CrossRef]
- Polverino, F.; Cosio, B.G.; Pons, J.; Laucho-Contreras, M.; Tejera, P.; Iglesias, A.; Rios, A.; Jahn, A.; Sauleda, J.; Divo, M.; et al. B cell-activating factor an orchestrator of lymphoid follicles in severe chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2015, 192, 695–705. [Google Scholar] [CrossRef] [Green Version]
- Dieu-Nosjean, M.C.; Goc, J.; Giraldo, N.A.; Sautès-Fridman, C.; Fridman, W.H. Tertiary lymphoid structures in cancer and beyond. Trends Immunol. 2014, 35, 571–580. [Google Scholar] [CrossRef]
- Fridman, W.H.; Remark, R.; Goc, J.; Giraldo, N.A.; Becht, E.; Hammond, S.A.; Damotte, D.; Dieu-Nosjean, M.C.; Sautès-Fridman, C. The immune microenvironment: A major player in human cancers. Int. Arch. Allergy Immunol. 2014, 164, 13–26. [Google Scholar] [CrossRef]
- Germain, C.; Gnjatic, S.; Tamzalit, F.; Knockaert, S.; Remark, R.; Goc, J.; Lepelley, A.; Becht, E.; Katsahian, S.; Bizouard, G.; et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am. J. Respir. Crit. Care Med. 2014, 189, 832–844. [Google Scholar] [CrossRef]
- Bracke, K.R.; Verhamme, F.M.; Seys, L.J.M.; Bantsimba-Malanda, C.; Cunoosamy, D.M.; Herbst, R.; Hammad, H.; Lambrecht, B.N.; Joos, G.F.; Brusselle, G.G. Role of CXCL13 in cigarette smoke-induced lymphoid follicle formation and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 2013, 188, 343–355. [Google Scholar] [CrossRef]
- Moyron-Quiroz, J.E.; Rangel-Moreno, J.; Kusser, K.; Hartson, L.; Sprague, F.; Goodrich, S.; Woodland, D.L.; Lund, F.E.; Randall, T.D. Role of inducible bronchus associated lymphoid tissue (iBALT) in respiratory immunity. Nat. Med. 2004, 10, 927–934. [Google Scholar] [CrossRef] [PubMed]
- Hogg, J.C. Pathophysiology of airflow limitation in chronic obstructive pulmonary disease. Lancet (London, England) 2004, 364, 709–721. [Google Scholar] [CrossRef]
- Hogg, J.C.; Chu, F.; Utokaparch, S.; Woods, R.; Elliott, W.M.; Buzatu, L.; Cherniack, R.M.; Rogers, R.M.; Sciurba, F.C.; Coxson, H.O.; et al. The nature of small-airway obstruction in chronic obstructive pulmonary disease. N. Engl. J. Med. 2004, 350, 2645–2653. [Google Scholar] [CrossRef] [PubMed]
- Bracke, K.R.; D’hulst, A.I.; Maes, T.; Moerloose, K.B.; Demedts, I.K.; Lebecque, S.; Joos, G.F.; Brusselle, G.G. Cigarette Smoke-Induced Pulmonary Inflammation and Emphysema Are Attenuated in CCR6-Deficient Mice. J. Immunol. 2006, 177, 4350–4359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dieu-Nosjean, M.C.; Antoine, M.; Danel, C.; Heudes, D.; Wislez, M.; Poulot, V.; Rabbe, N.; Laurans, L.; Tartour, E.; De Chaisemartin, L.; et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J. Clin. Oncol. 2008, 26, 4410–4417. [Google Scholar] [CrossRef] [Green Version]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020, 577, 556–560. [Google Scholar] [CrossRef] [PubMed]
- Biton, J.; Ouakrim, H.; Dechartres, A.; Alifano, M.; Mansuet-Lupo, A.; Si, H.; Halpin, R.; Creasy, T.; Bantsimba-Malanda, C.; Arrondeau, J.; et al. Impaired tumor-infiltrating t cells in patients with chronic obstructive pulmonary disease impact lung cancer response to PD-1 blockade. Am. J. Respir. Crit. Care Med. 2018, 198, 928–940. [Google Scholar] [CrossRef]
- Mark, N.M.; Kargl, J.; Busch, S.E.; Yang, G.H.Y.; Metz, H.E.; Zhang, H.; Hubbard, J.J.; Pipavath, S.N.J.; Madtes, D.K.; Houghton, A.M. Chronic Obstructive Pulmonary Disease Alters Immune Cell Composition and Immune Checkpoint Inhibitor Efficacy in Non-Small Cell Lung Cancer. Am. J. Respir. Crit. Care Med. 2018, 197, 325–336. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020, 577, 549–555. [Google Scholar] [CrossRef]
- Cabrita, R.; Lauss, M.; Sanna, A.; Donia, M.; Skaarup Larsen, M.; Mitra, S.; Johansson, I.; Phung, B.; Harbst, K.; Vallon-Christersson, J.; et al. Tertiary lymphoid structures improve immunotherapy and survival in melanoma. Nature 2020, 577, 561–565. [Google Scholar] [CrossRef]
- Dy, S.M.; Sharkey, P.; Herbert, R.; Haddad, K.; Wu, A.W. Comorbid illnesses and health care utilization among Medicare beneficiaries with lung cancer. Crit. Rev. Oncol. Hematol. 2006, 59, 218–225. [Google Scholar] [CrossRef] [PubMed]
- Zhai, R.; Yu, X.; Shafer, A.; Wain, J.C.; Christiani, D.C. The impact of coexisting COPD on survival of patients with early-stage non-small cell lung cancer undergoing surgical resection. Chest 2014, 145, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, Y.H.; Guan, W.J.; Liu, Q.; Wang, H.Q.; Zhu, Y.N.; Chen, R.C.; Zhang, G.J. Impact of COPD and emphysema on survival of patients with lung cancer: A meta-analysis of observational studies. Respirology 2016, 21, 269–279. [Google Scholar] [CrossRef] [PubMed]
- Iachina, M.; Jakobsen, E.; Møller, H.; Lüchtenborg, M.; Mellemgaard, A.; Krasnik, M.; Green, A. The Effect of Different Comorbidities on Survival of Non-small Cells Lung Cancer Patients. Lung 2015, 193, 291–297. [Google Scholar] [CrossRef] [PubMed]
- Uk Lim, J.; Yeo, C.D.; Rhee, C.K.; Kang, H.S.; Park, C.K.; Kim, J.S.; Kim, J.W.; Kim, S.J.; Yoon, H.K.; Lee, S.H. Comparison of clinical characteristics and overall survival between spirometrically diagnosed chronic obstructive pulmonary disease (COPD) and non-COPD never-smoking stage I-IV non-small cell lung cancer patients. Int. J. COPD 2019, 14, 929–938. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, B.; Dunn, L. The Declaration of Helsinki on Medical Research involving Human Subjects: A Review of Seventh Revision. J. Nepal Health Res. Counc. 2020, 17, 548–552. [Google Scholar] [CrossRef] [PubMed]
- Slatore, C.G.; Horeweg, N.; Jett, J.R.; Midthun, D.E.; Powell, C.A.; Wiener, R.S.; Wisnivesky, J.P.; Gould, M.K. An Official American Thoracic Society research statement: A research framework for pulmonary nodule evaluation and management. Am. J. Respir. Crit. Care Med. 2015, 192, 500–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozower, B.D.; Larner, J.M.; Detterbeck, F.C.; Jones, D.R. Special treatment issues in non-small cell lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest 2013, 143, e369S–e399S. [Google Scholar] [CrossRef] [Green Version]
- Detterbeck, F.C.; Boffa, D.J.; Kim, A.W.; Tanoue, L.T. The Eighth Edition Lung Cancer Stage Classification. Chest 2017, 151, 193–203. [Google Scholar] [CrossRef]
- Bousema, J.E.; Dijkgraaf, M.G.W.; Papen-Botterhuis, N.E.; Schreurs, H.W.; Maessen, J.G.; Van Der Heijden, E.H.; Steup, W.H.; Braun, J.; Noyez, V.J.J.M.; Hoeijmakers, F.; et al. MEDIASTinal staging of non-small cell lung cancer by endobronchial and endoscopic ultrasonography with or without additional surgical mediastinoscopy (MEDIASTrial): Study protocol of a multicenter randomised controlled trial. BMC Surg. 2018, 18. [Google Scholar] [CrossRef]
- De leyn, P.; Dooms, C.; Kuzdzal, J.; Lardinois, D.; Passlick, B.; Rami-Porta, R.; Turna, A.; Van Schil, P.; Venuta, F.; Waller, D.; et al. Revised ests guidelines for preoperative mediastinal lymph node staging for non-small-cell lung cancer. Eur. J. Cardio-Thoracic Surg. 2014, 45, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, P.; Congleton, J.; Fountain, S.W.; Jagoe, T.; McAuley, D.F.; Macmahon, J.; Muers, M.F.; Page, R.D.; Plant, P.K.; Roland, M.; et al. Guidelines on the selection of patients with lung cancer for surgery. Thorax 2001, 56, 89–108. [Google Scholar] [CrossRef] [Green Version]
- Vogelmeier, C.F.; Criner, G.J.; Martínez, F.J.; Anzueto, A.; Barnes, P.J.; Bourbeau, J.; Celli, B.R.; Chen, R.; Decramer, M.; Fabbri, L.M.; et al. Global Strategy for the Diagnosis, Management, and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Arch. Bronconeumol. 2017, 53, 128–149. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.; Ramis-Cabrer, D.; Curull, V.; Wang, X.; Qin, L.; Mateu-Jiménez, M.; Duran, X.; Pijuan, L.; Rodríguez-Fuster, A.; Espases, R.A.; et al. Immune cell subtypes and cytokines in lung tumor microenvironment: Influence of COPD. Cancers 2020, 12, 1217. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, E.; Tang, J.; Ramis-Cabrer, D.; Wang, X. Immunotherapy with monoclonal antibodies in lung cancer of mice: Oxidative stress and other biological events. Cancers 2019, 11, 1301. [Google Scholar] [CrossRef] [Green Version]
- Mateu-Jiménez, M.; Cucarull-Martínez, B.; Yelamos, J.; Barreiro, E. Reduced tumor burden through increased oxidative stress in lung adenocarcinoma cells of PARP-1 and PARP-2 knockout mice. Biochimie 2016, 121. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mateu-Jimenez, M.; Fermoselle, C.; Rojo, F.; Mateu, J.; Peña, R.; Urtreger, A.J.; Diament, M.J.; Joffé, E.D.B.K.; Pijuan, L.; De Herreros, A.G.; et al. Pharmacological approaches in an experimental model of non-small cell lung cancer: Effects on tumor biology. Curr. Pharm. Des. 2016, 22. [Google Scholar] [CrossRef] [Green Version]
- Budczies, J.; Klauschen, F.; Sinn, B.V.; Gyorffy, B.; Schmitt, W.D.; Darb-Esfahani, S.; Denkert, C. Cutoff Finder: A Comprehensive and Straightforward Web Application Enabling Rapid Biomarker Cutoff Optimization. PLoS ONE 2012, 7, e51862. [Google Scholar] [CrossRef] [Green Version]
- Lin, F.; Marcelo, K.L.; Rajapakshe, K.; Coarfa, C.; Dean, A.; Wilganowski, N.; Robinson, H.; Sevick, E.; Bissig, K.D.; Goldie, L.C.; et al. The CaMKK2/CaMKIV relay is an essential regulator of hepatic cancer. Hepatology 2015, 62, 505–520. [Google Scholar] [CrossRef]
- Depeille, P.; Henricks, L.M.; van de Ven, R.A.H.; Lemmens, E.; Wang, C.-Y.; Matli, M.; Werb, Z.; Haigis, K.M.; Donner, D.; Warren, R.; et al. RasGRP1 opposes proliferative EGFR-SOS1-Ras signals and restricts intestinal epithelial cell growth. Nat. Cell Biol. 2015, 17, 804–815. [Google Scholar] [CrossRef] [Green Version]





| Anthropometric Variables | Lung Cancer (n = 43) | Lung Cancer-COPD (n = 90) |
|---|---|---|
| Age, years | 65 (12) | 67 (8) |
| Male, N/Female, N | 17/26 | 78/12 *** |
| BMI, kg/m2 | 27 (4) | 26 (4) |
| Smoking history | ||
| Current: N, % | 13, 30 | 43, 48 |
| Ex-smoker: N, % | 8, 19 | 44, 49 ** |
| Never smoker: N, % | 22, 51 | 3, 3 *** |
| Pack-years | 17 (22) | 56 (25) *** |
| Lung function parameters | ||
| FEV1 | 90 (12) | 67 (15) *** |
| FEV1/FVC, % | 75 (6) | 61 (9) *** |
| DLco, % | 85 (14) | 67 (18) *** |
| Kco, % | 85 (12) | 69 (17) *** |
| GOLD Stage | ||
| GOLD Stage I: N, % | NA | 19, 21 |
| GOLD Stage II: N, % | NA | 63, 70 |
| GOLD Stage III: N, % | NA | 8, 9 |
| TNM staging | ||
| Stage 0–II: N, % | 37, 86 | 73, 81.1 |
| Stage III: N, % | 6, 14 | 13, 14.5 |
| Stage IV: N, % | 0, 0 | 4, 4.4 |
| Histological diagnosis | ||
| Squamous cell carcinoma: N, % | 5, 12 | 16, 17.8 |
| Adenocarcinoma: N, % | 32, 74 | 68, 75.6 |
| Others: N, % | 6, 14 | 6, 6.7 |
| Blood parameters | ||
| Total leucocytes/μL | 7.39 (2.42) × 103 | 9.17 (2.93) × 103 *** |
| Total neutrophils/μL | 4.82 (2.49) × 103 | 6.01 (2.61) × 103 ** |
| Total lymphocytes/μL | 1.76 (0.78) × 103 | 2.32 (1.61) × 103 * |
| Albumin (g/dL) | 4.3 (0.4) | 4.1 (0.6) |
| Total proteins (g/dL) | 7.0 (0.6) | 6.8 (0.8) |
| Fibrinogen (mg/dL) | 420 (130) | 454 (151) |
| CRP (mg/dL) | 6.5 (8.3) | 7.5 (13.1) |
| GSV (mm/h) | 27 (14) | 27 (16) |
| Body weight loss, kg | ||
| 0, N, % | 40, 93 | 82, 91 |
| 1–5, N, % | 1, 2 | 3, 3 |
| 6–10, N, % | 2, 5 | 5, 6 |
| Germinal Centers | Lung Cancer (n = 18) | Lung Cancer-COPD (n = 43) | ||
|---|---|---|---|---|
| NT Lung | T Lung | NT Lung | T Lung | |
| 0, n (%) | 17 (94) | 10 (56) * | 43 (100) | 36 (84) **,§ |
| >1, n (%) | 1 (6) | 8 (44) * | 0 (0) | 7 (16) **,§ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Ramis-Cabrer, D.; Curull, V.; Wang, X.; Mateu-Jiménez, M.; Pijuan, L.; Duran, X.; Qin, L.; Rodríguez-Fuster, A.; Aguiló, R.; et al. B Cells and Tertiary Lymphoid Structures Influence Survival in Lung Cancer Patients with Resectable Tumors. Cancers 2020, 12, 2644. https://doi.org/10.3390/cancers12092644
Tang J, Ramis-Cabrer D, Curull V, Wang X, Mateu-Jiménez M, Pijuan L, Duran X, Qin L, Rodríguez-Fuster A, Aguiló R, et al. B Cells and Tertiary Lymphoid Structures Influence Survival in Lung Cancer Patients with Resectable Tumors. Cancers. 2020; 12(9):2644. https://doi.org/10.3390/cancers12092644
Chicago/Turabian StyleTang, Jun, Daniel Ramis-Cabrer, Víctor Curull, Xuejie Wang, Mercé Mateu-Jiménez, Lara Pijuan, Xavier Duran, Liyun Qin, Alberto Rodríguez-Fuster, Rafael Aguiló, and et al. 2020. "B Cells and Tertiary Lymphoid Structures Influence Survival in Lung Cancer Patients with Resectable Tumors" Cancers 12, no. 9: 2644. https://doi.org/10.3390/cancers12092644