Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries
Abstract
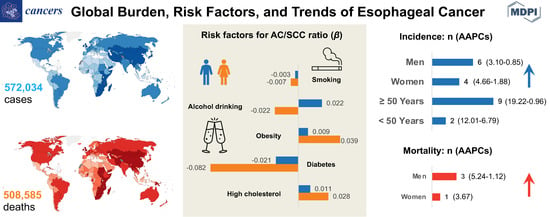
:Simple Summary
Abstract
1. Introduction
2. Methods
2.1. Data Source
2.2. Statistical Analysis
3. Results
3.1. Global Burden
3.1.1. Incidence
3.1.2. Mortality
3.1.3. Incidence by Histologic Subtypes
3.2. Associations between Risk Factors and AC/SCC Incidence Ratio
3.3. Temporal Trends
3.3.1. Incidence Trend
3.3.2. Mortality Trend
3.3.3. Incidence Trend by Age Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AAPC | average annual percent change |
| AC | adenocarcinoma |
| ASR | age-standardized rates |
| CI | confidence interval |
| CI5 | Cancer Incidence in Five Continents |
| CROSS | ChemoRadiotherapy for Esophageal cancer followed by Surgery Study |
| FLOT | fluorouracil plus leucovorin, oxaliplatin, and docetaxel |
| GERD | gastro-esophageal reflux disease |
| GHO | Global Health Observatory |
| NORDCAN | Nordic Cancer Registries |
| SCC | squamous cell carcinoma |
| SEER | surveillance, epidemiology, and end results |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA A Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [Green Version]
- American Cancer Society. Survival Rates for Esophageal Cancer. 2020. Available online: https://www.cancer.org/cancer/esophagus-cancer/detection-diagnosis-staging/survival-rates.html#references (accessed on 10 June 2020).
- Shapiro, J.; van Lanschot, J.J.B.; Hulshof, M.; van Hagen, P.; van Berge Henegouwen, M.I.; Wijnhoven, B.P.L.; van Laarhoven, H.W.M.; Nieuwenhuijzen, G.A.P.; Hospers, G.A.P.; Bonenkamp, J.J.; et al. Neoadjuvant chemoradiotherapy plus surgery versus surgery alone for oesophageal or junctional cancer (CROSS): Long-term results of a randomised controlled trial. Lancet Oncol. 2015, 16, 1090–1098. [Google Scholar] [CrossRef]
- Al-Batran, S.E.; Homann, N.; Pauligk, C.; Goetze, T.O.; Meiler, J.; Kasper, S.; Kopp, H.G.; Mayer, F.; Haag, G.M.; Luley, K.; et al. Perioperative chemotherapy with fluorouracil plus leucovorin, oxaliplatin, and docetaxel versus fluorouracil or capecitabine plus cisplatin and epirubicin for locally advanced, resectable gastric or gastro-oesophageal junction adenocarcinoma (FLOT4): A randomised, phase 2/3 trial. Lancet 2019, 393, 1948–1957. [Google Scholar] [CrossRef] [PubMed]
- Wong, M.C.S.; Hamilton, W.; Whiteman, D.C.; Jiang, J.Y.; Qiao, Y.; Fung, F.D.H.; Wang, H.H.X.; Chiu, P.W.Y.; Ng, E.K.W.; Wu, J.C.Y.; et al. Global Incidence and mortality of oesophageal cancer and their correlation with socioeconomic indicators temporal patterns and trends in 41 countries. Sci. Rep. 2018, 8, 4522. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, C.; Dicker, D.; Pain, A.; Hamavid, H.; Moradi-Lakeh, M.; MacIntyre, M.F.; Allen, C.; Hansen, G.; Woodbrook, R.; Wolfe, C.; et al. The Global Burden of Cancer 2013. JAMA Oncol. 2015, 1, 505–527. [Google Scholar] [CrossRef] [PubMed]
- Pennathur, A.; Gibson, M.K.; Jobe, B.A.; Luketich, J.D. Oesophageal carcinoma. Lancet 2013, 381, 400–412. [Google Scholar] [CrossRef] [Green Version]
- Lepage, C.; Rachet, B.; Jooste, V.; Faivre, J.; Coleman, M.P. Continuing rapid increase in esophageal adenocarcinoma in England and Wales. Am. J. Gastroenterol. 2008, 103, 2694–2699. [Google Scholar] [CrossRef]
- Eslick, G.D. Epidemiology of esophageal cancer. Gastroenterol. Clin. N. Am. 2009, 38, 17–25. [Google Scholar] [CrossRef]
- Abnet, C.C.; Arnold, M.; Wei, W.Q. Epidemiology of Esophageal Squamous Cell Carcinoma. Gastroenterology 2018, 154, 360–373. [Google Scholar] [CrossRef]
- Coleman, H.G.; Xie, S.H.; Lagergren, J. The Epidemiology of Esophageal Adenocarcinoma. Gastroenterology 2018, 154, 390–405. [Google Scholar] [CrossRef]
- Xie, S.H.; Lagergren, J. Risk factors for oesophageal cancer. Best Pr. Res. Clin. Gastroenterol. 2018, 36, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.L.; Xie, S.H.; Wahlin, K.; Lagergren, J. Global time trends in the incidence of esophageal squamous cell carcinoma. Clin. Epidemiol. 2018, 10, 717–728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Soerjomataram, I.; Ferlay, J.; Forman, D. Global incidence of oesophageal cancer by histological subtype in 2012. Gut 2015, 64, 381–387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Ferlay, J.; van Berge Henegouwen, M.I.; Soerjomataram, I. Global burden of oesophageal and gastric cancer by histology and subsite in 2018. Gut 2020, 69, 1564–1571. [Google Scholar] [CrossRef] [PubMed]
- Malhotra, G.K.; Yanala, U.; Ravipati, A.; Follet, M.; Vijayakumar, M.; Are, C. Global trends in esophageal cancer. J. Surg. Oncol. 2017, 115, 564–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arnold, M.; Laversanne, M.; Brown, L.M.; Devesa, S.S.; Bray, F. Predicting the Future Burden of Esophageal Cancer by Histological Subtype: International Trends in Incidence up to 2030. Am. J. Gastroenterol. 2017, 112, 1247–1255. [Google Scholar] [CrossRef]
- GBD 2017 Oesophageal Cancer Collaborators. The global, regional, and national burden of oesophageal cancer and its attributable risk factors in 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Gastroenterol. Hepatol. 2020, 5, 582–597. [Google Scholar] [CrossRef]
- Wong, M.C.; Goggins, W.B.; Wang, H.H.; Fung, F.D.; Leung, C.; Wong, S.Y.; Ng, C.F.; Sung, J.J. Global Incidence and Mortality for Prostate Cancer: Analysis of Temporal Patterns and Trends in 36 Countries. Eur. Urol. 2016, 70, 862–874. [Google Scholar] [CrossRef] [Green Version]
- Wong, M.C.; Huang, J.; Lok, V.; Wang, J.; Fung, F.; Ding, H.; Zheng, Z.J. Differences in Incidence and Mortality Trends of Colorectal Cancer, Worldwide, Based on Sex, Age, and Anatomic Location. Clin. Gastroenterol. Hepatol. 2020. [Google Scholar] [CrossRef]
- Cancer Incidence in Five Continents. Volume XI. Available online: https://ci5.iarc.fr (accessed on 10 May 2020).
- Surveillance, Epidemiology, and End Results (SEER) Program. Available online: https://seer.cancer.gov/about/ (accessed on 10 May 2020).
- The NORDCAN Project. Available online: https://www-dep.iarc.fr/NORDCAN/english/frame.asp (accessed on 10 May 2020).
- Engholm, G.; Ferlay, J.; Christensen, N.; Bray, F.; Gjerstorff, M.L.; Klint, A.; Kotlum, J.E.; Olafsdottir, E.; Pukkala, E.; Storm, H.H. NORDCAN—a Nordic tool for cancer information, planning, quality control and research. Acta Oncol. 2010, 49, 725–736. [Google Scholar] [CrossRef]
- World Health Organization. WHO Mortality Database. Available online: https://www.who.int/healthinfo/mortality_data/en/ (accessed on 10 May 2020).
- Mathers, C.D.; Fat, D.M.; Inoue, M.; Rao, C.; Lopez, A.D. Counting the dead and what they died from: An assessment of the global status of cause of death data. Bull World Health Organ 2005, 83, 171–177. [Google Scholar]
- International Agency for Research on Cancer, W.H.O. Data Visualization Tools for Exploring the Global Cancer Burden in 2018. Available online: https://gco.iarc.fr/today/home (accessed on 10 May 2020).
- World Health Organization. International Statistical Classification of Diseases and Related Health Problems 10th Revision. Available online: https://icd.who.int/browse10/2016/en (accessed on 10 May 2020).
- Segi, M.; Fujisaku, S.; Kurihara, M. Geographical observation on cancer mortality by selected sites on the basis of standardised death rate. Gan 1957, 48, 219–225. [Google Scholar] [PubMed]
- Kim, H.J.; Fay, M.P.; Feuer, E.J.; Midthune, D.N. Permutation tests for joinpoint regression with applications to cancer rates. Stat. Med. 2000, 19, 335–351. [Google Scholar] [CrossRef]
- Division of Health Informatics, C.o.P. Cancer Trend Analysis Using Joinpoint Regression. Available online: https://www.health.pa.gov/topics/HealthStatistics/Statistical-Resources/UnderstandingHealthStats/Documents/Cancer_Trend_Analysis_Using_Joinpoint_Regression_Part_1_The_Basics.pdf (accessed on 15 December 2020).
- Clegg, L.X.; Hankey, B.F.; Tiwari, R.; Feuer, E.J.; Edwards, B.K. Estimating average annual per cent change in trend analysis. Stat. Med. 2009, 28, 3670–3682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kmet, J.; Mahboubi, E. Esophageal cancer in the Caspian littoral of Iran: Initial studies. Science 1972, 175, 846–853. [Google Scholar] [CrossRef]
- Zhang, H.-Z.; Jin, G.-F.; Shen, H.-B. Epidemiologic differences in esophageal cancer between Asian and Western populations. Chin. J. Cancer 2012, 31, 281–286. [Google Scholar] [CrossRef] [Green Version]
- Middleton, D.R.S.; Bouaoun, L.; Hanisch, R.; Bray, F.; Dzamalala, C.; Chasimpha, S.; Menya, D.; Mbalawa, C.G.; N’Da, G.; Woldegeorgis, M.A.; et al. Esophageal cancer male to female incidence ratios in Africa: A systematic review and meta-analysis of geographic, time and age trends. Cancer Epidemiol. 2018, 53, 119–128. [Google Scholar] [CrossRef]
- Lagergren, J.; Lagergren, P. Recent developments in esophageal adenocarcinoma. CA A Cancer J. Clin. 2013, 63, 232–248. [Google Scholar] [CrossRef]
- Xie, S.H.; Lagergren, J. The Male Predominance in Esophageal Adenocarcinoma. Clin. Gastroenterol. Hepatol. 2016, 14, 338–347. [Google Scholar] [CrossRef] [Green Version]
- Xie, S.H.; Ness-Jensen, E.; Rabbani, S.; Langseth, H.; Gislefoss, R.E.; Mattsson, F.; Lagergren, J. Circulating Sex Hormone Levels and Risk of Esophageal Adenocarcinoma in a Prospective Study in Men. Am. J. Gastroenterol. 2020, 115, 216–223. [Google Scholar] [CrossRef]
- Cronin-Fenton, D.P.; Murray, L.J.; Whiteman, D.C.; Cardwell, C.; Webb, P.M.; Jordan, S.J.; Corley, D.A.; Sharp, L.; Lagergren, J. Reproductive and sex hormonal factors and oesophageal and gastric junction adenocarcinoma: A pooled analysis. Eur. J. Cancer 2010, 46, 2067–2076. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindkvist, B.; Johansen, D.; Stocks, T.; Concin, H.; Bjørge, T.; Almquist, M.; Häggström, C.; Engeland, A.; Hallmans, G.; Nagel, G.; et al. Metabolic risk factors for esophageal squamous cell carcinoma and adenocarcinoma: A prospective study of 580,000 subjects within the Me-Can project. BMC Cancer 2014, 14, 103. [Google Scholar] [CrossRef] [Green Version]
- Agrawal, K.; Markert, R.J.; Agrawal, S. Risk factors for adenocarcinoma and squamous cell carcinoma of the esophagus and lung. Hypertension 2018, 61. [Google Scholar] [CrossRef]
- Pandeya, N.; Olsen, C.M.; Whiteman, D.C. Sex differences in the proportion of esophageal squamous cell carcinoma cases attributable to tobacco smoking and alcohol consumption. Cancer Epidemiol. 2013, 37, 579–584. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renehan, A.G.; Tyson, M.; Egger, M.; Heller, R.F.; Zwahlen, M. Body-mass index and incidence of cancer: A systematic review and meta-analysis of prospective observational studies. Lancet 2008, 371, 569–578. [Google Scholar] [CrossRef]
- Steffen, A.; Schulze, M.B.; Pischon, T.; Dietrich, T.; Molina, E.; Chirlaque, M.D.; Barricarte, A.; Amiano, P.; Quirós, J.R.; Tumino, R.; et al. Anthropometry and esophageal cancer risk in the European prospective investigation into cancer and nutrition. Cancer Epidemiol. Biomark. Prev. 2009, 18, 2079–2089. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lahmann, P.H.; Pandeya, N.; Webb, P.M.; Green, A.C.; Whiteman, D.C. Body mass index, long-term weight change, and esophageal squamous cell carcinoma: Is the inverse association modified by smoking status? Cancer 2012, 118, 1901–1909. [Google Scholar] [CrossRef]
- World Health Organization. Obesity and Overweight. 2019. Available online: https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight (accessed on 10 May 2020).
- Wong, M.C.; Huang, J.; Wang, J.; Chan, P.S.; Lok, V.; Chen, X.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Global, regional and time-trend prevalence of central obesity: A systematic review and meta-analysis of 13.2 million subjects. Eur. J. Epi. 2020. [Google Scholar] [CrossRef]
- Wong, M.C.; Huang, J.; Pang, T.W.; Lok, V.; Chen, X.; Choi, P.; Leung, C.; Wang, H.H.X.; Lao, X.Q.; Zheng, Z.J. Worldwide incidence and prevalence of metabolic syndrome: A systematic review and meta-analysis of 14.6 million individuals. Gastroenterology 2020, 158, S-1003. [Google Scholar]
- Lagergren, J.; Bergström, R.; Lindgren, A.; Nyrén, O. Symptomatic gastroesophageal reflux as a risk factor for esophageal adenocarcinoma. N. Engl. J. Med. 1999, 340, 825–831. [Google Scholar] [CrossRef]
- Lagergren, J. Influence of obesity on the risk of esophageal disorders. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 340–347. [Google Scholar] [CrossRef] [PubMed]
- McColl, K.E.; Going, J.J. Aetiology and classification of adenocarcinoma of the gastro-oesophageal junction/cardia. Gut 2010, 59, 282–284. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, M.M.; Sharaf, R.R.; Aziz, R.K. Helicobacter pylori: A poor man’s gut pathogen? Gut Pathog. 2010, 2, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thrumurthy, S.G.; Chaudry, M.A.; Thrumurthy, S.S.D.; Mughal, M. Oesophageal cancer: Risks, prevention, and diagnosis. BMJ 2019, 366, l4373. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.; Yoshida, K.; Suetsugu, T.; Imai, T.; Matsuhashi, N.; Yamaguchi, K. Recent advancements in esophageal cancer treatment in Japan. Ann. Gastroenterol. Surg. 2018, 2, 253–265. [Google Scholar] [CrossRef]
- Wang, Q.-L.; Xie, S.-H.; Li, W.-T.; Lagergren, J. Smoking Cessation and Risk of Esophageal Cancer by Histological Type: Systematic Review and Meta-analysis. Jnci J. Natl. Cancer Inst. 2017, 109. [Google Scholar] [CrossRef] [Green Version]
- di Pietro, M.; Canto, M.I.; Fitzgerald, R.C. Endoscopic Management of Early Adenocarcinoma and Squamous Cell Carcinoma of the Esophagus: Screening, Diagnosis, and Therapy. Gastroenterology 2018, 154, 421–436. [Google Scholar] [CrossRef]
- Fitzgerald, R.C.; di Pietro, M.; O’Donovan, M.; Maroni, R.; Muldrew, B.; Debiram-Beecham, I.; Gehrung, M.; Offman, J.; Tripathi, M.; Smith, S.G.; et al. Cytosponge-trefoil factor 3 versus usual care to identify Barrett’s oesophagus in a primary care setting: A multicentre, pragmatic, randomised controlled trial. Lancet 2020, 396, 333–344. [Google Scholar] [CrossRef]
- Roshandel, G.; Merat, S.; Sotoudeh, M.; Khoshnia, M.; Poustchi, H.; Lao-Sirieix, P.; Malhotra, S.; O’Donovan, M.; Etemadi, A.; Nickmanesh, A.; et al. Pilot study of cytological testing for oesophageal squamous cell dysplasia in a high-risk area in Northern Iran. Br. J. Cancer 2014, 111, 2235–2241. [Google Scholar] [CrossRef] [Green Version]
- Wei, W.Q.; Chen, Z.F.; He, Y.T.; Feng, H.; Hou, J.; Lin, D.M.; Li, X.Q.; Guo, C.L.; Li, S.S.; Wang, G.Q.; et al. Long-Term Follow-Up of a Community Assignment, One-Time Endoscopic Screening Study of Esophageal Cancer in China. J. Clin. Oncol. 2015, 33, 1951–1957. [Google Scholar] [CrossRef]
- Yang, J.; Wei, W.Q.; Niu, J.; Liu, Z.C.; Yang, C.X.; Qiao, Y.L. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J. Gastroenterol. 2012, 18, 2493–2501. [Google Scholar] [CrossRef] [PubMed]
- Xie, S.H.; Ness-Jensen, E.; Medefelt, N.; Lagergren, J. Assessing the feasibility of targeted screening for esophageal adenocarcinoma based on individual risk assessment in a population-based cohort study in Norway (The HUNT Study). Am. J. Gastroenterol. 2018, 113, 829–835. [Google Scholar] [CrossRef] [PubMed]
- Maringe, C.; Spicer, J.; Morris, M.; Purushotham, A.; Nolte, E.; Sullivan, R.; Rachet, B.; Aggarwal, A. The impact of the COVID-19 pandemic on cancer deaths due to delays in diagnosis in England, UK: A national, population-based, modelling study. Lancet Oncol. 2020, 21, 1023–1034. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Koulaouzidis, A.; Marlicz, W.; Lok, V.; Chu, C.; Ngai, C.H.; Zhang, L.; Chen, P.; Wang, S.; Yuan, J.; et al. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers 2021, 13, 141. https://doi.org/10.3390/cancers13010141
Huang J, Koulaouzidis A, Marlicz W, Lok V, Chu C, Ngai CH, Zhang L, Chen P, Wang S, Yuan J, et al. Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries. Cancers. 2021; 13(1):141. https://doi.org/10.3390/cancers13010141
Chicago/Turabian StyleHuang, Junjie, Anastasios Koulaouzidis, Wojciech Marlicz, Veeleah Lok, Cedric Chu, Chun Ho Ngai, Lin Zhang, Ping Chen, Shanjuan Wang, Jinqiu Yuan, and et al. 2021. "Global Burden, Risk Factors, and Trends of Esophageal Cancer: An Analysis of Cancer Registries from 48 Countries" Cancers 13, no. 1: 141. https://doi.org/10.3390/cancers13010141