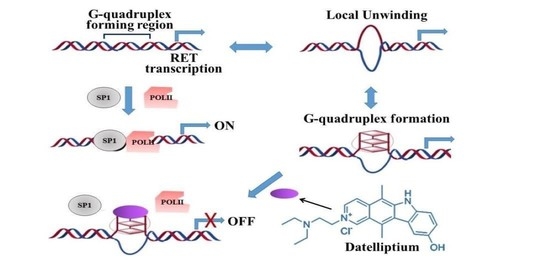
Selective Antitumor Activity of Datelliptium toward Medullary Thyroid Carcinoma by Downregulating RET Transcriptional Activity
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Cell Culture and Media
2.3. Western Blotting
2.4. Immunofluorescence Analysis
2.5. Wound Healing Scratch Assay
2.6. Spheroid Formation
2.7. Tumor Xenograft Study
2.8. Statistical Analysis
3. Results
3.1. Effect of Datelliptium on RET Protein Expression in MTC Cell Lines
3.2. Effects of Datelliptium on EMT in TT Cells
3.3. Effects of Datelliptium on Migration in MTC
3.4. Effects of Datelliptium on Preformed CSCs from TT Cells
3.5. Effects of Datelliptium on Cyclin D1 Expression in TT and MZ-CRC-1 Cells
3.6. In Vivo Antitumor Activity of Datelliptium in a MTC Xenograft Model
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabanillas, E.M.; McFadden, D.G.; Durante, C. Thyroid cancer. Lancet 2016, 388, 2783–2795. [Google Scholar] [CrossRef]
- Elisei, R. Thyroid Carcinoma. In Encyclopedia of Endocrine Diseases; Elsevier BV: Amsterdam, The Netherlands, 2018; pp. 573–585. [Google Scholar]
- Nikiforov, Y.E. Thyroid carcinoma: Molecular pathways and therapeutic targets. Mod. Pathol. 2008, 21, S37–S43. [Google Scholar] [CrossRef] [Green Version]
- Priya, S.R.; Dravid, C.S.; Digumarti, R.; Dandekar, M. Targeted Therapy for Medullary Thyroid Cancer: A Review. Front. Oncol. 2017, 7, 238. [Google Scholar] [CrossRef] [Green Version]
- Wells, S.A.; Pacini, F.; Robinson, B.G.; Santoro, M. Multiple Endocrine Neoplasia Type 2 and Familial Medullary Thyroid Carcinoma: An Update. J. Clin. Endocrinol. Metab. 2013, 98, 3149–3164. [Google Scholar] [CrossRef]
- Roskoski, R.; Sadeghi-Nejad, A. Role of RET protein-tyrosine kinase inhibitors in the treatment RET-driven thyroid and lung cancers. Pharmacol. Res. 2018, 128, 1–17. [Google Scholar] [CrossRef]
- Drilon, A.; Hu, Z.I.; Lai, G.G.Y.; Tan, D.S.W. Targeting RET-driven cancers: Lessons from evolving preclinical and clinical landscapes. Nat. Rev. Clin. Oncol. 2018, 15, 151–167. [Google Scholar] [CrossRef]
- Drosten, M.; Hilken, G.; Rödicker, F.; Mise, N.; Cranston, A.N.; Dahmen, U.; Ponder, B.A.J.; Bockmann, M.; Pützer, B.M. Role of MEN2A-Derived RET in Maintenance and Proliferation of Medullary Thyroid Carcinoma. J. Natl. Cancer Inst. 2004, 96, 1231–1239. [Google Scholar] [CrossRef]
- De Groot, J.W.B.; Links, T.P.; Plukker, J.T.M.; Lips, C.J.M.; Hofstra, R.M.W. RET as a Diagnostic and Therapeutic Target in Sporadic and Hereditary Endocrine Tumors. Endocr. Rev. 2006, 27, 535–560. [Google Scholar] [CrossRef] [Green Version]
- Grabowski, P.; Briest, F.; Baum, R.; Zaknun, J.; Kulkarni, H.; Zeitz, M.; Hörsch, D. Vandetanib therapy in medullary thyroid cancer. Drugs Today 2012, 48, 723. [Google Scholar] [CrossRef]
- Markham, A. Selpercatinib: First Approval. Drugs 2020, 80, 1119–1124. [Google Scholar] [CrossRef]
- Markham, A. Pralsetinib: First Approval. Drugs 2020, 80, 1865–1870. [Google Scholar] [CrossRef] [PubMed]
- Fagin, J.A.; Wells, S.A. Biologic and Clinical Perspectives on Thyroid Cancer. N. Engl. J. Med. 2016, 375, 1054–1067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, Y.-J.; Kumarasamy, V.; Camacho, D.; Sun, D. Involvement of G-quadruplex structures in regulation of human RET gene expression by small molecules in human medullary thyroid carcinoma TT cells. Oncogene 2014, 34, 1292–1299. [Google Scholar] [CrossRef]
- Sun, D.; Guo, K.; Rusche, J.J.; Hurley, L.H. Facilitation of a structural transition in the polypurine/polypyrimidine tract within the proximal promoter region of the human VEGF gene by the presence of potassium and G-quadruplex-interactive agents. Nucleic Acids Res. 2005, 33, 6070–6080. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, S.; Hurley, L.H.; Neidle, S. Targeting G-quadruplexes in gene promoters: A novel anticancer strategy? Nat. Rev. Drug Discov. 2011, 10, 261–275. [Google Scholar] [CrossRef] [Green Version]
- Kumarasamy, V.M.; Shin, Y.-J.; White, J.; Sun, D. Selective repression of RET proto-oncogene in medullary thyroid carcinoma by a natural alkaloid berberine. BMC Cancer 2015, 15, 599. [Google Scholar] [CrossRef] [Green Version]
- Kumarasamy, V.M.; Sun, D. Demonstration of a potent RET transcriptional inhibitor for the treatment of medullary thyroid carcinoma based on an ellipticine derivative. Int. J. Oncol. 2017, 51, 145–157. [Google Scholar] [CrossRef] [Green Version]
- Khayat, D.; Borel, C.; Azab, M.; Paraisot, D.; Malaurie, E.; Bouloux, C.; Weil, M. Phase I study of Datelliptium chloride, hydrochloride given by 24-h continuous intravenous infusion. Cancer Chemother. Pharmacol. 1992, 30, 226–228. [Google Scholar] [CrossRef]
- Shakib, H.; Rajabi, S.; Dehghan, M.H.; Mashayekhi, F.J.; Safari-Alighiarloo, N.; Hedayati, M. Epithelial-to-mesenchymal transition in thyroid cancer: A comprehensive review. Endocrine 2019, 66, 435–455. [Google Scholar] [CrossRef]
- Cockburn, J.G.; Richardson, D.; Gujral, T.; Mulligan, L.M. RET-Mediated Cell Adhesion and Migration Require Multiple Integrin Subunits. J. Clin. Endocrinol. Metab. 2010, 95, E342–E346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Castellone, M.D.; Melillo, R.M. RET-mediated modulation of tumor microenvironment and immune response in multiple endocrine neoplasia type 2 (MEN2). Endocr. Relat. Cancer 2018, 25, T105–T119. [Google Scholar] [CrossRef] [Green Version]
- Alqahtani, T.; Kumarasamy, V.M.; Huczyński, A.; Sun, D. Salinomycin and its derivatives as potent RET transcriptional inhibitors for the treatment of medullary thyroid carcinoma. Int. J. Oncol. 2019, 56, 348–358. [Google Scholar] [CrossRef]
- Tsai, J.H.; Yang, J. Epithelial-mesenchymal plasticity in carcinoma metastasis. Genes Dev. 2013, 27, 2192–2206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garg, M. Epithelial-mesenchymal transition-activating transcription factors-multifunctional regulators in cancer. World J. Stem Cells 2013, 5, 188–195. [Google Scholar] [CrossRef]
- Battaglia, R.A.; Delic, S.; Herrmann, H.; Snider, N.T. Vimentin on the move: New developments in cell migration. F1000Research 2018, 7, 1796. [Google Scholar] [CrossRef] [Green Version]
- Suh, Y.H.; Yoon, C.-H.; Kim, R.-K.; Lim, E.-J.; Oh, Y.S.; Hwang, S.-G.; An, S.; Yoon, G.; Gye, M.C.; Yi, J.-M.; et al. Claudin-1 induces epithelial–mesenchymal transition through activation of the c-Abl-ERK signaling pathway in human liver cells. Oncogene 2012, 32, 4873–4882. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.K.; Kwon, Y.; Jang, M.; Park, M.; Kim, J.; Cho, S.; Jang, D.G.; Lee, W.-B.; Jung, S.H.; Choi, H.J.; et al. β-catenin activation down-regulates cell-cell junction-related genes and induces epithelial-to-mesenchymal transition in colorectal cancers. Sci. Rep. 2019, 9, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Da, C.; Wu, K.; Yue, C.; Bai, P.; Wang, R.; Wang, G.; Zhao, M.; Lv, Y.; Hou, P. N-cadherin promotes thyroid tumorigenesis through modulating major signaling pathways. Oncotarget 2016, 8, 8131–8142. [Google Scholar] [CrossRef] [Green Version]
- Mulligan, L.M. GDNF and the RET Receptor in Cancer: New Insights and Therapeutic Potential. Front. Physiol. 2019, 9, 1873. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.-L.; Yuan, B.-Q.; Shen, G.-S. Mechanism of RET gene mediated EGFR signaling pathway onepithelial-mesenchymal transition, proliferation and apoptosis of papillary thyroid carcinoma cells. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 8036–8047. [Google Scholar] [CrossRef]
- Veschi, V.; Verona, F.; Iacono, M.L.; D’Accardo, C.; Porcelli, G.; Turdo, A.; Gaggianesi, M.; Forte, S.; Giuffrida, D.; Memeo, L.; et al. Cancer Stem Cells in Thyroid Tumors: From the Origin to Metastasis. Front. Endocrinol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Mulligan, L.M. RET revisited: Expanding the oncogenic portfolio. Nat. Rev. Cancer 2014, 14, 173–186. [Google Scholar] [CrossRef]
- Jijiwa, M.; Kawai, K.; Fukihara, J.; Nakamura, A.; Hasegawa, M.; Suzuki, C.; Sato, T.; Enomoto, A.; Asai, N.; Murakumo, Y.; et al. GDNF-mediated signaling via RET tyrosine 1062 is essential for maintenance of spermatogonial stem cells. Genes Cells 2008, 13, 365–374. [Google Scholar] [CrossRef]
- Wang, S.; Lloyd, R.V.; Hutzler, M.J.; Safran, M.S.; Patwardhan, N.A.; Khan, A. The Role of Cell Cycle Regulatory Protein, Cyclin D1, in the Progression of Thyroid Cancer. Mod. Pathol. 2000, 13, 882–887. [Google Scholar] [CrossRef] [Green Version]
- Sporny, S.; Slowinska-Klencka, D.; Ratynska, M. Cyclin D1 expression in primary thyroid carcinomas. Neuro Endocrinol. Lett. 2005, 26, 815–818. [Google Scholar]
- Averous, J.; Fonseca, B.D.; Proud, C. Regulation of cyclin D1 expression by mTORC1 signaling requires eukaryotic initiation factor 4E-binding protein 1. Oncogene 2007, 27, 1106–1113. [Google Scholar] [CrossRef] [PubMed]
- Takuwa, N.; Fukui, Y.; Takuwa, Y. Cyclin D1 Expression Mediated by Phosphatidylinositol 3-Kinase through mTOR-p70 S6K -Independent Signaling in Growth Factor-Stimulated NIH 3T3 Fibroblasts. Mol. Cell. Biol. 1999, 19, 1346–1358. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zhou, L.; Xie, N.; Nice, E.C.; Zhang, T.; Cui, Y.; Huang, C. Overcoming cancer therapeutic bottleneck by drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 1–25. [Google Scholar] [CrossRef]
- Glassberg, B.; Khan, S.; Pemov, A.; Hawley, R.; Widemann, B.C.; Khan, J.; Glod, J. Molecular mechanism(s) of resistance to vandetanib in medullary thyroid carcinoma. J. Clin. Oncol. 2020, 38, e15628. [Google Scholar] [CrossRef]
- Maciel, L.M.Z.; Magalhães, P.K.R. Medullary thyroid carcinoma-Adverse events during systemic treatment: Risk-benefit ratio. Arch. Endocrinol. Metab. 2017, 61, 398–402. [Google Scholar] [CrossRef] [Green Version]
- Skoura, E. Depicting Medullary Thyroid Cancer Recurrence: The Past and the Future of Nuclear Medicine Imaging. Int. J. Endocrinol. Metab. 2013, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maxwell, J.E.; Sherman, S.; O’Dorisio, T.; Howe, J.R. Medical management of metastatic medullary thyroid cancer. Cancer 2014, 120, 3287–3301. [Google Scholar] [CrossRef] [Green Version]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, B.; Xie, C.; Ye, D. RET Proto-oncogene Gene Mutation Is Related to Cervical Lymph Node Metastasis in Medullary Thyroid Carcinoma. Endocr. Pathol. 2019, 30, 297–304. [Google Scholar] [CrossRef]
- Pozo, K.; Hillmann, A.; Augustyn, A.; Plattner, F.; Hai, T.; Singh, T.; Ramezani, S.; Sun, X.; Pfragner, R.; Minna, J.D.; et al. Differential expression of cell cycle regulators in CDK5-dependent medullary thyroid carcinoma tumorigenesis. Oncotarget 2015, 6, 12080–12093. [Google Scholar] [CrossRef]
- Tagliati, F.; Zatelli, M.C.; Bottoni, A.; Piccin, D.; Luchin, A.; Culler, M.D.; degli Uberti, E. Role of Complex Cyclin D1/Cdk4 in Somatostatin Subtype 2 Receptor-Mediated Inhibition of Cell Proliferation of a Medullary Thyroid Carcinoma Cell Line in Vitro. Endocrinology 2006, 147, 3530–3538. [Google Scholar] [CrossRef]
- Yang, L.; Shi, P.; Zhao, G.; Xu, J.; Peng, W.; Zhang, J.; Zhang, G.; Wang, X.; Dong, Z.; Chen, F.; et al. Targeting cancer stem cell pathways for cancer therapy. Signal Transduct. Target. Ther. 2020, 5, 1–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, W.; Jiao, J.; Huang, L.; Ye, F.; Shi, M.; Xiaodong, C.; Wang, X.; Hu, D.; Xie, X. Cyclin D1 affects epithelial–mesenchymal transition in epithelial ovarian cancer stem cell-like cells. Onco Targets Ther. 2013, 6, 667–677. [Google Scholar] [CrossRef] [Green Version]
- Mucci-LoRusso, P.; Polin, L.; Biernat, L.A.; Valeriote, F.A.; Corbett, T.H. Activity of datelliptium acetate (NSC 311152; SR 95156A) against solid tumors of mice. Investig. New Drugs 1990, 8, 253–261. [Google Scholar] [CrossRef]
- Boven, E.; Winograd, B.; Berger, D.P.; Dumont, M.P.; Braakhuis, B.J.; Fodstad, O.; Langdon, S.; Fiebig, H.H. Phase II preclinical drug screening in human tumor xenografts: A first European multicenter collaborative study. Cancer Res. 1992, 52, 5940–5947. [Google Scholar]
- Ducrocq, C.; Wendling, F.; Chermann, J.C.; Tourbez-Perrin, M.; Rivalle, C.; Tambourin, P.; Pochon, F.; Bisagni, E. Structure-activity relationship in a series of newly synthesized 1-amino-substituted ellipticine derivatives. J. Med. Chem. 1980, 23, 1212–1216. [Google Scholar] [CrossRef]
- Subbiah, V.; Shen, T.; Terzyan, S.; Liu, X.; Hu, X.; Patel, K.; Hu, M.; Cabanillas, M.; Behrang, A.; Meric-Bernstam, F.; et al. Structural basis of acquired resistance to selpercatinib and pralsetinib mediated by non-gatekeeper RET mutations. Ann. Oncol. 2021, 32, 261–268. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, T.; Alswied, A.; Sun, D. Selective Antitumor Activity of Datelliptium toward Medullary Thyroid Carcinoma by Downregulating RET Transcriptional Activity. Cancers 2021, 13, 3288. https://doi.org/10.3390/cancers13133288
Alqahtani T, Alswied A, Sun D. Selective Antitumor Activity of Datelliptium toward Medullary Thyroid Carcinoma by Downregulating RET Transcriptional Activity. Cancers. 2021; 13(13):3288. https://doi.org/10.3390/cancers13133288
Chicago/Turabian StyleAlqahtani, Tariq, Abdullah Alswied, and Daekyu Sun. 2021. "Selective Antitumor Activity of Datelliptium toward Medullary Thyroid Carcinoma by Downregulating RET Transcriptional Activity" Cancers 13, no. 13: 3288. https://doi.org/10.3390/cancers13133288