A Novel Four-Gene Prognostic Signature for Prediction of Survival in Patients with Soft Tissue Sarcoma
Abstract
:Simple Summary
Abstract
1. Introduction
2. Materials and Methods
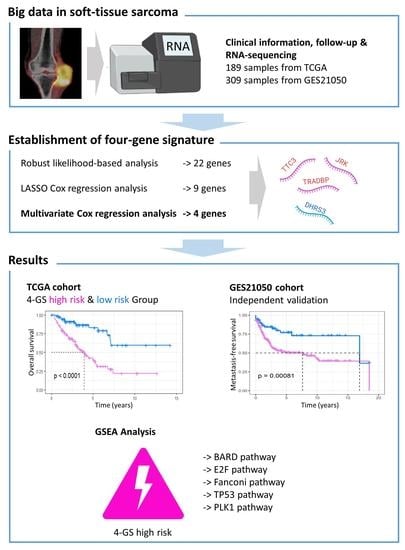
2.1. Patient Data
2.2. Establishment of Prognostic Gene Signature
2.3. Establishment of Predictive Nomograms
2.4. Gene Set Enrichment Analysis
2.5. Statistical Analysis
3. Results
3.1. Establishment of Four-Gene Prognostic Signature
3.2. The Predictive Value of Four-Gene Signature on the OS of STS Patients
3.3. The Predictive Value of Four-Gene Signature on the Relapse Free Survival (RFS) and Metastasis Free Survival (MFS) of STS Patients
3.4. Univariate and Multivariate Analyses of the Four-Gene Signature Prognostic Role
3.5. Establishment of Nomograms for Predicting OS and RFS in STS Patients
3.6. Identification of Four-Gene Signature Related Biological Pathways and Processes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Massarweh, N.N.; Dickson, P.V.; Anaya, D.A. Soft tissue sarcomas: Staging principles and prognostic nomograms. J. Surg. Oncol. 2015, 111, 532–539. [Google Scholar] [CrossRef]
- Ferrari, A.; Sultan, I.; Huang, T.T.; Rodriguez-Galindo, C.; Shehadeh, A.; Meazza, C.; Ness, K.K.; Casanova, M.; Spunt, S.L. Soft tissue sarcoma across the age spectrum: A population-based study from the surveillance epidemiology and end results database. Pediatr. Blood Cancer 2011, 57, 943–949. [Google Scholar] [CrossRef] [Green Version]
- Blay, J.-Y. Treatment of advanced soft tissue sarcoma by histological subtype: Wish, prediction or reality? Future Oncol. 2019, 15, 5–10. [Google Scholar] [CrossRef]
- Kim, J.; Kim, J.H.; Kang, H.G.; Park, S.Y.; Yu, J.Y.; Lee, E.Y.; Oh, S.E.; Kim, Y.H.; Yun, T.; Park, C.; et al. Integrated molecular characterization of adult soft tissue sarcoma for therapeutic targets. BMC Med. Genet. 2018, 19, 216. [Google Scholar] [CrossRef]
- Savina, M.; Le Cesne, A.; Blay, J.-Y.; Ray-Coquard, I.; Mir, O.; Toulmonde, M.; Cousin, S.; Terrier, P.; Ranchere-Vince, D.; Meeus, P.; et al. Patterns of care and outcomes of patients with METAstatic soft tissue SARComa in a real-life setting: The METASARC observational study. BMC Med. 2017, 15, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widemann, B.C.; Italiano, A. Biology and Management of Undifferentiated Pleomorphic Sarcoma, Myxofibrosarcoma, and Malignant Peripheral Nerve Sheath Tumors: State of the Art and Perspectives. J. Clin. Oncol. 2018, 36, 160–167. [Google Scholar] [CrossRef]
- Spunt, S.L.; Million, L.; Chi, Y.-Y.; Anderson, J.; Tian, J.; Hibbitts, E.; Coffin, C.; McCarville, M.B.; Randall, R.L.; Parham, D.M.; et al. A risk-based treatment strategy for non-rhabdomyosarcoma soft-tissue sarcomas in patients younger than 30 years (ARST0332): A Children’s Oncology Group prospective study. Lancet Oncol. 2020, 21, 145–161. [Google Scholar] [CrossRef]
- Bourcier, K.; Le Cesne, A.; Tselikas, L.; Adam, J.; Mir, O.; Honore, C.; De Baere, T. Basic Knowledge in Soft Tissue Sarcoma. Cardiovasc. Interv. Radiol. 2019, 42, 1255–1261. [Google Scholar] [CrossRef] [PubMed]
- De Mello, R.A.; Tavares, Á.; Mountzios, G. International Manual of Oncology Practice; Springer International Publishing: Basel, Switzerland, 2015. [Google Scholar]
- Casanova, M.; Ferrari, A. Pharmacotherapy for pediatric soft-tissue sarcomas. Expert Opin. Pharmacother. 2011, 12, 517–531. [Google Scholar] [CrossRef]
- Guillou, L.; Coindre, J.M.; Bonichon, F.; Nguyen, B.B.; Terrier, P.; Collin, F.; O Vilain, M.; Mandard, A.M.; Le Doussal, V.; Leroux, A.; et al. Comparative study of the National Cancer Institute and French Federation of Cancer Centers Sarcoma Group grading systems in a population of 410 adult patients with soft tissue sarcoma. J. Clin. Oncol. 1997, 15, 350–362. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Lei, C.; Song, S.; Jing, W.; Jin, C.; Gong, S.; Tian, H.; Guo, T. Immune checkpoint blockade in the treatment of malignant tumor: Current statue and future strategies. Cancer Cell Int. 2021, 21, 1–14. [Google Scholar] [CrossRef]
- Aggerholm-Pedersen, N.; Sørensen, B.S.; Overgaard, J.; Toustrup, K.; Baerentzen, S.; Nielsen, O.S.; Maretty-Kongstad, K.; Nordsmark, M.; Alsner, J.; Safwat, A. A prognostic profile of hypoxia-induced genes for localised high-grade soft tissue sarcoma. Br. J. Cancer 2016, 115, 1096–1104. [Google Scholar] [CrossRef] [Green Version]
- Merry, E.; Thway, K.; Jones, R.L.; Huang, P.H. Predictive and prognostic transcriptomic biomarkers in soft tissue sarcomas. NPJ Precis. Oncol. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Zuo, S.; Wei, M.; Zhang, H.; Chen, A.; Wu, J.; Wei, J.; Dong, J. A robust six-gene prognostic signature for prediction of both disease-free and overall survival in non-small cell lung cancer. J. Transl. Med. 2019, 17, 152. [Google Scholar] [CrossRef]
- Xiao, K.; Liu, Q.; Peng, G.; Su, J.; Qin, C.-Y.; Wang, X.-Y. Identification and validation of a three-gene signature as a candidate prognostic biomarker for lower grade glioma. PeerJ 2020, 8, e8312. [Google Scholar] [CrossRef] [PubMed]
- Abeshouse, A.; Adebamowo, C.; Adebamowo, S.N.; Akbani, R.; Akeredolu, T.; Ally, A.; Anderson, M.L.; Anur, P.; Appelbaum, E.L.; Armenia, J.; et al. Comprehensive and Integrated Genomic Characterization of Adult Soft Tissue Sarcomas. Cell 2017, 171, 950–965.e28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chibon, F.; Lagarde, P.; Salas, S.; Pérot, G.; Brouste, V.; Tirode, F.; Lucchesi, C.; De Reynies, A.; Kauffmann, A.; Bui, B.; et al. Validated prediction of clinical outcome in sarcomas and multiple types of cancer on the basis of a gene expression signature related to genome complexity. Nat. Med. 2010, 16, 781–787. [Google Scholar] [CrossRef] [PubMed]
- Cho, H.; Yu, A.; Kim, S.; Kang, J.; Hong, S.-M. Robust Likelihood-Based Survival Modeling with Microarray Data. J. Stat. Softw. 2009, 29, 1–16. [Google Scholar] [CrossRef]
- Subramanian, A.; Tamayo, P.; Mootha, V.K.; Mukherjee, S.; Ebert, B.L.; Gillette, M.A.; Paulovich, A.; Pomeroy, S.L.; Golub, T.R.; Lander, E.S.; et al. Gene set enrichment analysis: A knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA 2005, 102, 15545–15550. [Google Scholar] [CrossRef] [Green Version]
- Story, M.D.; Durante, M. Radiogenomics. Med. Phys. 2018, 45, e1111–e1122. [Google Scholar] [CrossRef]
- Kandimalla, R.; Tomihara, H.; Banwait, J.K.; Yamamura, K.; Singh, G.; Baba, H.; Goel, A. A 15-Gene Immune, Stromal, and Proliferation Gene Signature that Significantly Associates with Poor Survival in Patients with Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2020, 26, 3641–3648. [Google Scholar] [CrossRef] [Green Version]
- Liu, G.-M.; Zeng, H.-D.; Zhang, C.-Y.; Xu, J.-W. Identification of a six-gene signature predicting overall survival for hepatocellular carcinoma. Cancer Cell Int. 2019, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Shen, R.; Liu, B.; Li, X.; Yu, T.; Xu, K.; Ma, J. Development and validation of an immune gene-set based prognostic signature for soft tissue sarcoma. BMC Cancer 2021, 21, 1–14. [Google Scholar] [CrossRef]
- de Boer, E.M.J.; Orie, V.K.; Williams, T.; Baker, M.R.; De Oliveira, H.M.; Polvikoski, T.; Silsby, M.; Menon, P.; Bos, M.V.D.; Halliday, G.M.; et al. TDP-43 proteinopathies: A new wave of neurodegenerative diseases. J. Neurol. Neurosurg. Psychiatry 2021, 92, 86–95. [Google Scholar] [CrossRef] [PubMed]
- Neumann, M.; Sampathu, D.M.; Kwong, L.K.; Truax, A.C.; Micsenyi, M.C.; Chou, T.T.; Bruce, J.; Schuck, T.; Grossman, M.; Clark, C.M.; et al. Ubiquitinated TDP-43 in Frontotemporal Lobar Degeneration and Amyotrophic Lateral Sclerosis. Science 2006, 314, 130–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, E.B.; Lee, V.M.Y.; Trojanowski, J.Q. Gains or losses: Molecular mechanisms of TDP43-mediated neurodegeneration. Nat. Rev. Neurosci. 2012, 13, 38–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tauffenberger, A.; Vaccaro, A.; Aulas, A.; Velde, C.V.; Parker, J.A. Glucose delays age-dependent proteotoxicity. Aging Cell 2012, 11, 856–866. [Google Scholar] [CrossRef] [Green Version]
- Chiang, P.-M.; Ling, J.; Jeong, Y.H.; Price, D.L.; Aja, S.M.; Wong, P.C. Deletion of TDP-43 down-regulates Tbc1d1, a gene linked to obesity, and alters body fat metabolism. Proc. Natl. Acad. Sci. USA 2010, 107, 16320–16324. [Google Scholar] [CrossRef] [Green Version]
- Ayala, Y.M.; Misteli, T.; Baralle, F.E. TDP-43 regulates retinoblastoma protein phosphorylation through the repression of cyclin-dependent kinase 6 expression. Proc. Natl. Acad. Sci. USA 2008, 105, 3785–3789. [Google Scholar] [CrossRef] [Green Version]
- Teittinen, K.J.; Kärkkäinen, P.; Salonen, J.; Rönnholm, G.; Korkeamäki, H.; Vihinen, M.; Kalkkinen, N.; Lohi, O. Nucleolar proteins with altered expression in leukemic cell lines. Leuk. Res. 2012, 36, 232–236. [Google Scholar] [CrossRef] [PubMed]
- Postel-Vinay, S.; Véron, A.S.; Tirode, F.; Pierron, G.; Reynaud, S.; Kovar, H.; Oberlin, O.; Lapouble, E.; Ballet, S.; Lucchesi, C.; et al. Common variants near TARDBP and EGR2 are associated with susceptibility to Ewing sarcoma. Nat. Genet. 2012, 44, 323–327. [Google Scholar] [CrossRef] [PubMed]
- Park, Y.-Y.; Kim, S.-B.; Han, H.D.; Sohn, B.H.; Kim, J.H.; Liang, J.; Lu, Y.; Rodriguez-Aguayo, C.; Lopez-Berestein, G.; Mills, G.B.; et al. Tat-activating regulatory DNA-binding protein regulates glycolysis in hepatocellular carcinoma by regulating the platelet isoform of phosphofructokinase through microRNA 520. Hepatology 2013, 58, 182–191. [Google Scholar] [CrossRef] [PubMed]
- Benchabane, H.; Xin, N.; Tian, A.; Hafler, B.P.; Nguyen, K.; Ahmed, A.; Ahmed, Y. Jerky/Earthbound facilitates cell-specific Wnt/Wingless signalling by modulating β-catenin-TCF activity. EMBO J. 2011, 30, 1444–1458. [Google Scholar] [CrossRef] [Green Version]
- Pangon, L.; Ng, I.; Giry-Laterriere, M.; Currey, N.; Morgan, A.D.; Benthani, F.; Tran, P.N.; Alsohaily, S.; Segelov, E.; Parker, B.L.; et al. JRK is a positive regulator of β-catenin transcriptional activity commonly overexpressed in colon, breast and ovarian cancer. Oncogene 2016, 35, 2834–2841. [Google Scholar] [CrossRef] [PubMed]
- Suizu, F.; Hiramuki, Y.; Okumura, F.; Matsuda, M.; Okumura, A.J.; Hirata, N.; Narita, M.; Kohno, T.; Yokota, J.; Bohgaki, M.; et al. The E3 Ligase TTC3 Facilitates Ubiquitination and Degradation of Phosphorylated Akt. Dev. Cell 2009, 17, 800–810. [Google Scholar] [CrossRef]
- Kim, J.-H.; Ham, S.; Lee, Y.; Suh, G.Y.; Lee, Y.-S. TTC3 contributes to TGF-β1-induced epithelial−mesenchymal transition and myofibroblast differentiation, potentially through SMURF2 ubiquitylation and degradation. Cell Death Dis. 2019, 10, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Dey-Guha, I.; Alves, C.; Yeh, A.C.; Salony; Sole, X.; Darp, R.; Ramaswamy, S. A Mechanism for Asymmetric Cell Division Resulting in Proliferative Asynchronicity. Mol. Cancer Res. 2015, 13, 223–230. [Google Scholar] [CrossRef] [Green Version]
- Tang, X.-H.; Gudas, L.J. Retinoids, Retinoic Acid Receptors, and Cancer. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 345–364. [Google Scholar] [CrossRef]
- Oler, G.; Camacho, C.P.; Hojaij, F.C.; Michaluart, P.; Riggins, G.J.; Cerutti, J.M. Gene expression profiling of papillary thyroid carcinoma identifies transcripts correlated with BRAF mutational status and lymph node metastasis. Clin. Cancer Res. 2008, 14, 4735–4742. [Google Scholar] [CrossRef] [Green Version]
- Kamei, N.; Hiyama, K.; Yamaoka, H.; Kamimatsuse, A.; Onitake, Y.; Sueda, T.; Hiyama, E. Evaluation of genes identified by microarray analysis in favorable neuroblastoma. Pediatr. Surg. Int. 2009, 25, 931–937. [Google Scholar] [CrossRef]
- D’Alterio, C.; Scala, S.; Sozzi, G.; Roz, L.; Bertolini, G. Paradoxical effects of chemotherapy on tumor relapse and metastasis promotion. Semin. Cancer Biol. 2020, 60, 351–361. [Google Scholar] [CrossRef]
- Istl, A.C.; Ruck, J.M.; Morris, C.D.; Levin, A.S.; Meyer, C.F.; Johnston, F.M. Call for improved design and reporting in soft tissue sarcoma studies: A systematic review and meta-analysis of chemotherapy and survival outcomes in resectable STS. J. Surg. Oncol. 2019, 119, 824–835. [Google Scholar] [CrossRef]
- Tanaka, K.; Kawano, M.; Iwasaki, T.; Itonaga, I.; Tsumura, H. A meta-analysis of randomized controlled trials that compare standard doxorubicin with other first-line chemotherapies for advanced/metastatic soft tissue sarcomas. PLoS ONE 2019, 14, e0210671. [Google Scholar] [CrossRef]
- Plaks, V.; Kong, N.; Werb, Z. The Cancer Stem Cell Niche: How Essential Is the Niche in Regulating Stemness of Tumor Cells? Cell Stem Cell 2015, 16, 225–238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakahashi, N.; Emori, M.; Tsuchie, H.; Sonoda, T.; Takada, K.; Miyajima, M.; Watanabe, A.; Shimada, Y.; Yamashita, T. Treatment outcome of chest wall soft tissue sarcomas: Analysis of prognostic factors. J. Surg. Oncol. 2019, 120, 1235–1240. [Google Scholar] [CrossRef]
- Zer, A.; Prince, R.M.; Amir, E.; Razak, A.R.A. Multi-agent chemotherapy in advanced soft tissue sarcoma (STS)—A systematic review and meta-analysis. Cancer Treat. Rev. 2018, 63, 71–78. [Google Scholar] [CrossRef]
- Iasonos, A.; Schrag, D.; Raj, G.V.; Panageas, K.S. How To Build and Interpret a Nomogram for Cancer Prognosis. J. Clin. Oncol. 2008, 26, 1364–1370. [Google Scholar] [CrossRef] [PubMed]
- Callegaro, D.; Miceli, R.; Mariani, L.; Raut, C.P.; Gronchi, A. Soft tissue sarcoma nomograms and their incorporation into practice. Cancer 2017, 123, 2802–2820. [Google Scholar] [CrossRef] [Green Version]
- de Cárcer, G.; Venkateswaran, S.V.; Salgueiro, L.; El Bakkali, A.; Somogyi, K.; Rowald, K.; Montañés, P.; Sanclemente, M.; Escobar, B.; de Martino, A.; et al. Plk1 overexpression induces chromosomal instability and suppresses tumor development. Nat. Commun. 2018, 9, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeggo, P.A.; Pearl, L.; Carr, A.M. DNA repair, genome stability and cancer: A historical perspective. Nat. Rev. Cancer 2016, 16, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed]
- Bracken, A.; Ciro, M.; Cocito, A.; Helin, K. E2F target genes: Unraveling the biology. Trends Biochem. Sci. 2004, 29, 409–417. [Google Scholar] [CrossRef] [PubMed]








| Characteristics | All (n = 189) | Training Set (n = 95) | Testing Set (n = 94) |
|---|---|---|---|
| Age group (Median) | |||
| ≤60 years | 96 (50.79%) | 48 (50.53%) | 48 (51.06%) |
| >60 years | 93 (49.21%) | 47 (49.47%) | 46 (48.94%) |
| Gender | |||
| Male | 87 (46.03%) | 45 (47.37%) | 42 (44.68%) |
| Female | 102 (53.97%) | 50 (52.63%) | 52 (55.32%) |
| Pathologic tumour size | |||
| ≤10.5 cm | 95 (50.26%) | 52 (54.74%) | 43 (45.74%) |
| >10.5 cm | 94 (49.74%) | 43 (45.26%) | 51 (54.26%) |
| Radiotherapy | |||
| Yes | 54 (28.57%) | 34 (35.79%) | 20 (21.28%) |
| No | 135 (71.43%) | 61 (64.21%) | 74 (78.72%) |
| Pharmaceutical therapy | |||
| Yes | 45 (23.81%) | 26 (27.37%) | 19 (20.21%) |
| No | 144 (76.19%) | 69 (72.63%) | 75 (79.79%) |
| FNCLCC grade | |||
| 1 | 10 (5.29%) | 5 (5.26%) | 5 (5.32%) |
| 2 | 105 (55.56%) | 49 (51.58%) | 56 (59.57%) |
| 3 | 74 (39.15%) | 41 (43.16%) | 33 (35.11%) |
| Vital status | |||
| Alive | 117 (61.90%) | 62 (65.26%) | 55 (58.51%) |
| Dead | 72 (38.10%) | 33 (34.74%) | 39 (41.49%) |
| Relapse status | |||
| Relapse | 114 (60.32%) | 61 (64.21%) | 53 (56.38%) |
| Non-Relapse | 75 (39.68%) | 34 (35.79%) | 41 (43.62%) |
| Histological type | |||
| DDLPS | 49 (25.93%) | 23 (24.21%) | 26 (27.66%) |
| LMS | 68 (35.98%) | 34 (35.79%) | 34 (36.17%) |
| UPS | 41 (21.69%) | 24 (25.26%) | 17 (18.09%) |
| MFS | 17 (8.99%) | 6 (6.32%) | 11 (11.70%) |
| SS | 10 (5.29%) | 5 (5.26%) | 5 (5.32%) |
| MPNST | 4 (2.12%) | 3 (3.16%) | 1 (1.06%) |
| Tumour site | |||
| Retroperitoneum | 81 (42.8%) | 39 (41.1%) | 42 (44.7%) |
| Upper/Lower Extremity | 57 (30.2%) | 30 (31.6%) | 27 (28.7%) |
| Superficial Trunk | 9 (4.8%) | 4 (4.2%) | 5 (5.3%) |
| Chest | 10 (5.3%) | 4 (4.2%) | 6 (6.4%) |
| Uterus | 19 (10.1%) | 11 (11.6%) | 8 (8.5%) |
| Other | 13 (6.8%) | 7 (7.3%) | 6 (6.4%) |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Age (Continuous) | 1.020 | 1.003~1.039 | 0.025 | 1.025 | 1.007~1.043 | 0.005 |
| Gender (Male vs. Female) | 1.015 | 0.637~1.619 | 0.949 | |||
| Pathological tumour size (cm) | 1.043 | 1.016~1.070 | 0.002 | 1.065 | 1.037~1.094 | <0.001 |
| Radiotherapy (Yes vs. No) | 0.758 | 0.444~1.293 | 0.309 | |||
| Pharmaceutical therapy (Yes vs. No) | 1.291 | 0.769~2.167 | 0.335 | |||
| FNCLCC grade (3 vs. 1/2) | 1.583 | 0.995~2.519 | 0.053 | |||
| Tumour site | ||||||
| Upper/Lower extremity | (Reference) | |||||
| Retroperitoneum | 1.100 | 0.630~1.917 | 0.738 | |||
| Superficial trunk | 1.023 | 0.302~3.462 | 0.971 | |||
| Chest | 1.418 | 0.532~3.782 | 0.485 | |||
| Uterus | 1.436 | 0.652~3.161 | 0.369 | |||
| Other | 0.507 | 0.118~2.171 | 0.360 | |||
| Histological subtype | ||||||
| DDLPS | (Reference) | |||||
| LMS | 0.669 | 0.379~1.183 | 0.167 | |||
| UPS | 0.768 | 0.387~1.527 | 0.452 | |||
| MFS | 0.829 | 0.354~1.945 | 0.668 | |||
| Other | 0.649 | 0.224~1.886 | 0.428 | |||
| 4GS-Risk score (High vs. Low) | 3.813 | 2.084~6.975 | <0.001 | 5.141 | 2.756~9.589 | <0.001 |
| Characteristics | Univariate Analysis | Multivariate Analysis | ||||
|---|---|---|---|---|---|---|
| HR | 95%CI | p-Value | HR | 95%CI | p-Value | |
| Age (Continuous) | 1.002 | 0.989~1.015 | 0.754 | |||
| Gender (Male vs. Female) | 1.176 | 0.813~1.699 | 0.390 | |||
| Pathological tumour size (cm) | 1.032 | 1.010~1.054 | 0.004 | 1.052 | 1.028~1.076 | <0.001 |
| Radiotherapy (Yes vs. No) | 1.033 | 0.685~1.558 | 0.875 | |||
| Pharmaceutical therapy (Yes vs. No) | 1.723 | 1.146~2.591 | 0.009 | 1.528 | 1.001~2.332 | 0.049 |
| FNCLCC grade (3 vs. 1/2) | 1.465 | 1.009~2.126 | 0.045 | 1.592 | 1.089~2.328 | 0.016 |
| Tumour site | ||||||
| Upper/Lower extremity | (Reference) | |||||
| Retroperitoneum | 0.896 | 0.585~1.375 | 0.618 | |||
| Superficial trunk | 0.473 | 0.145~1.545 | 0.215 | |||
| Chest | 0.761 | 0.321~1.809 | 0.537 | |||
| Uterus | 1.260 | 0.667~2.380 | 0.369 | |||
| Other | 0.653 | 0.256~1.666 | 0.476 | |||
| Histological subtype | ||||||
| DDLPS | (Reference) | |||||
| LMS | 0.869 | 0.553~1.367 | 0.545 | |||
| UPS | 0.829 | 0.479~1.435 | 0.504 | |||
| MFS | 0.847 | 0.417~1.723 | 0.648 | |||
| Other | 0.595 | 0.249~1.421 | 0.243 | |||
| 4GS-Risk score (High vs. Low) | 1.978 | 1.322~2.959 | <0.001 | 2.173 | 1.405~3.362 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, C.; Gong, S.; Osterhoff, G.; Schopow, N. A Novel Four-Gene Prognostic Signature for Prediction of Survival in Patients with Soft Tissue Sarcoma. Cancers 2021, 13, 5837. https://doi.org/10.3390/cancers13225837
Wu C, Gong S, Osterhoff G, Schopow N. A Novel Four-Gene Prognostic Signature for Prediction of Survival in Patients with Soft Tissue Sarcoma. Cancers. 2021; 13(22):5837. https://doi.org/10.3390/cancers13225837
Chicago/Turabian StyleWu, Changwu, Siming Gong, Georg Osterhoff, and Nikolas Schopow. 2021. "A Novel Four-Gene Prognostic Signature for Prediction of Survival in Patients with Soft Tissue Sarcoma" Cancers 13, no. 22: 5837. https://doi.org/10.3390/cancers13225837