Recent Developments in Dielectric Barrier Discharge Plasma-Assisted Catalytic Dry Reforming of Methane over Ni-Based Catalysts
Abstract
:1. Introduction
2. Overview of Non-Thermal Plasma
2.1. Characteristics of Non-Thermal Plasma (NTP)
2.2. Non-Thermal Plasma Generation
2.3. Mechanism of DRM Catalyzed by DBD Plasma
2.4. Synergy of Non-Thermal Plasma and Catalyst
3. Ni-Based Catalyst Assisted with DBD Plasma for DRM
3.1. Pure Ni Catalysts with Different Supports
3.1.1. Ni/SiO2
3.1.2. Ni/Al2O3
3.1.3. Ni/ZrO2
3.1.4. Ni/AC
3.2. Ni-Based Catalysts with Doping
3.2.1. Transition Metals
3.2.2. Alkali and Alkaline Earth Metals
3.2.3. Rare Earth Metals
| Catalyst | Parameters | Conversion(%) | Selectivity(%) | T (°C) | SIE * (J/mL) | Energy Efficiency (mmol/kJ) | Remarks | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| CH4/ CO2 Ratio | Catalyst Loading (g) | Flow Rate (mL/min) | Power (W) | CH4 | CO2 | H2 | CO | ||||||
| LaNiO3@SiO2 | 1:1 | 0.2 | 40 | 150 | 88.31 | 77.76 | 83.65 | 92.43 | 200 | 225 | 0.17 | Integrated with DBD, LaNiO3@SiO2 shows improved conversions and selectivity. | [82] |
| Ni/SiO2 | 1:1 | 0.2 | 50 | 86 | 26 | 16 | 47.4 | 52.9 | 110 | 103.2 | N.A. | The combination of DBD and Ni/SiO2 catalyst enhance the activity of DRM due to the reaction between carbon-containing intermediates and oxygen radicals. | [59] |
| Ni/Al2O3 | 1:1 | 0.3 | 56 | 70 | 60 | 77 | 70–75 | 80 | 550 | 75 | 39% | The charge recombination on the Ni/Al2O3 catalyst surface will enhance the diffusion of carbon through the Ni catalyst and promote its oxidation by CO2. | [49] |
| Ni/Al2O3 | 4:1 | 6.4 | 50 | 1600 | 8.3 | 7.6 | 69 | 20 | Ambient temperature | 4.6 eV/molecule | 4.5% | Ni addition enhanced the H2/CO ratio and reduced coke formation with the help of DBD. | [63] |
| Ni/γ-Al2O3 | 1:1 | 1.0 | 50 | 50 | 56.4 | 30.2 | 31 | 52.4 | <150 | 60 | 0.32 | The combination of plasma and Ni/γ-Al2O3 can increase the conversion rate of CH4. | [27] |
| Ni/ La2O3-MgAl2O4 | 1:1 | 0.5 | 20 | 100 | 86 | 84.5 | 50 | 49.5 | 350 | 300 | 0.13 | La2O3 inhibits the RWGS * reaction, improves H2 selectivity and yield, and the formed intermediate carbonate (La2O2CO3) inhibits carbon deposition. | [54] |
| Ni/La2O3 | 1:1 | 0.2 | 50 | 160 | 63 | 54 | 71 | 85 | 150 | 240 | 0.14 | Ni/LaO3 nanoparticles show excellent thermal stability in the DBD plasma reactor. La contributes to the formation of intermediates, which are responsible for activating CO2 and inhibiting carbon deposition. | [83] |
| Ni/ZrO2 | 1:1 | 0.6 | 50 | 200 | 53.57 | 60.81 | 82 | 95 | 650 | 240 | N.A. | The Ni/ZrO2 catalyst prepared by the DBD plasma decomposition method greatly improves its activity due to its high dispersion and increased oxygen vacancies. | [55] |
| Ni-Co/ Al2O3-ZrO2 | 1:1 | 0.3 | 40 | N.A. | 58 | 62 | 95 | 100 | 850 | N.A. | N.A. | The Ni-Co/Al2O3-ZrO2 catalyst after plasma treatment shows high catalytic activity due to its narrow particle size distribution, large surface area and strong metal-support interaction. | [68] |
| Ni-Mn/γ-Al2O3 | 1:1 | 0.5 | 30 | 2.2 | 28.4 | 13.2 | 23.2 | 40.5 | N.A. | 4.2 | 2.76 | A higher activity and energy efficiency is achieved by the integrated plasma and Ni-Mn bimetallic catalyst system. | [20] |
| Ni-Mg/Al2O3 | 1.6:1 | 0.4 | 50 | 16 | 32 | 16 | 41.7 | 29.5 | 160 | 19.2 | 0.58 | K promoted catalyst shows the best performance and enhances the energy efficiency of plasma process because it contains more active sites. | [61] |
| Ni-K/Al2O3 | 1.6:1 | 0.4 | 50 | 16 | 34 | 23 | 43.3 | 31.3 | 160 | 19.2 | 0.67 | ||
| Ni-Ce/Al2O3 | 1.6:1 | 0.4 | 50 | 16 | 32 | 22 | 41.8 | 31.1 | 160 | 19.2 | 0.63 | ||
| NiMgAlCe | 4:6 | 0.45 | 90 | 48 | 36.1 | 22.5 | N.A. | N.A. | N.A. | 32 | N.A. | Mg and Ce promoted the CO2 adsorption and increased the discharge area by partially tuning the filament discharge into surface discharge. | [75] |
| Mg,Ce-Ni/γ- Al2O3 | 1:1 | N.A. | 30 | 2.7 | 34.7 | 13 | 35 | 53.7 | N.A. | 5.4 | 1.97 | Mobile oxygen and surface basicity effectively removed coke during methane activation by Ni and DBD. | [80] |
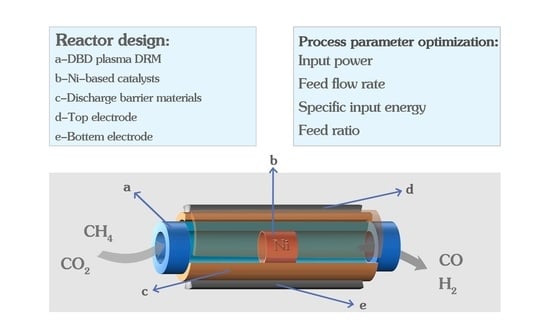
4. DBD Plasma Reactor Design
4.1. Configuration of DBD Plasma Reactor
4.2. Medium Material
4.3. Discharge Volume
5. Effects of Process Parameter
5.1. Input Power
5.2. The Feed Flow Rate
5.3. Specific Input Energy
5.4. The Feed Ratio
6. Conclusions and Outlook
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Snoeckx, R.; Zeng, Y.X.; Tu, X.; Bogaerts, A. Plasma-based dry reforming: Improving the conversion and energy efficiency in a dielectric barrier discharge. RSC Adv. 2015, 5, 29799–29808. [Google Scholar] [CrossRef]
- Harinarayanan, P.; Damjan, L.J.; Dasireddy, V.D.B.C.; Likozar, B. A review of plasma-assisted catalytic conversion of gaseous carbon dioxide and methane into value-added platform chemicals and fuels. RSC Adv. 2018, 8, 27481–27508. [Google Scholar]
- Gao, X.; Liu, H.; Hidajat, K.; Kawi, S. Anti-Coking Ni/SiO2Catalyst for Dry Reforming of Methane: Role of Oleylamine/Oleic Acid Organic Pair. ChemCatChem 2015, 7, 4188–4196. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Thitsartarn, W.; Sutthiumporn, K.; Kawi, S. Inverse NiAl2O4 on LaAlO3–Al2O3: Unique Catalytic Structure for Stable CO2 Reforming of Methane. J. Phys. Chem. C 2013, 117, 8120–8130. [Google Scholar] [CrossRef]
- Gao, X.; Hidajat, K.; Kawi, S. Facile synthesis of Ni/SiO 2 catalyst by sequential hydrogen/air treatment: A superior anti-coking catalyst for dry reforming of methane. J. CO2 Util. 2016, 15, 146–153. [Google Scholar] [CrossRef]
- Kawi, S.; Kathiraser, Y.; Ni, J.; Oemar, U.; Li, Z.; Saw, E.T. Progress in Synthesis of Highly Active and Stable Nickel-Based Catalysts for Carbon Dioxide Reforming of Methane. ChemSusChem 2015, 8, 3556–3575. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Ashok, J.; Widjaja, S.; Hidajat, K.; Kawi, S. Ni/SiO2 catalyst prepared via Ni-aliphatic amine complexation for dry reforming of methane: Effect of carbon chain number and amine concentration. Appl. Catal. A Gen. 2015, 503, 34–42. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Recent developments in non-thermal catalytic DBD plasma reactor for dry reforming of methane. Energy Convers. Manag. 2019, 183, 529–560. [Google Scholar] [CrossRef]
- Chung, W.-C.; Chang, M.-B. Review of catalysis and plasma performance on dry reforming of CH4 and possible synergistic effects. Renew. Sustain. Energy Rev. 2016, 62, 13–31. [Google Scholar] [CrossRef]
- Uytdenhouwen, Y.; Hereijgers, J.; Breugelmans, T.; Cool, P.; Bogaerts, A. How gas flow design can influence the performance of a DBD plasma reactor for dry reforming of methane. Chem. Eng. J. 2021, 405, 6618. [Google Scholar] [CrossRef]
- Bian, Z.; Das, S.; Wai, M.H.; Hongmanorom, P.; Kawi, S. A Review on Bimetallic Nickel-Based Catalysts for CO2 Reforming of Methane. ChemPhysChem 2017, 18, 3117–3134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Li, M.; Bian, Z.; Kathiraser, Y.; Kawi, S. Design of highly stable and selective core/yolk–shell nanocatalysts—A review. Appl. Catal. B: Environ. 2016, 188, 324–341. [Google Scholar] [CrossRef]
- Kathiraser, Y.; Oemar, U.; Saw, E.T.; Li, Z.; Kawi, S. Kinetic and mechanistic aspects for CO2 reforming of methane over Ni based catalysts. Chem. Eng. J. 2015, 278, 62–78. [Google Scholar] [CrossRef]
- Li, Z.; Das, S.; Hongmanorom, P.; Dewangan, N.; Wai, M.H.; Kawi, S. Silica-based micro- and mesoporous catalysts for dry reforming of methane. Catal. Sci. Technol. 2018, 8, 2763–2778. [Google Scholar] [CrossRef]
- Bian, Z.; Kawi, S. Preparation, characterization and catalytic application of phyllosilicate: A review. Catal. Today 2020, 339, 3–23. [Google Scholar] [CrossRef]
- Gao, X.; Ashok, J.; Kawi, S. Smart Designs of Anti-Coking and Anti-Sintering Ni-Based Catalysts for Dry Reforming of Methane: A Recent Review. Reactions 2020, 1, 162–194. [Google Scholar] [CrossRef]
- Jang, W.-J.; Shim, J.-O.; Kim, H.-M.; Yoo, S.-Y.; Roh, H.-S. A review on dry reforming of methane in aspect of catalytic properties. Catal. Today 2019, 324, 15–26. [Google Scholar] [CrossRef]
- Li, K.; Liu, J.-L.; Li, X.-S.; Lian, H.-Y.; Zhu, X.; Bogaerts, A.; Zhu, A.-M. Novel power-to-syngas concept for plasma catalytic reforming coupled with water electrolysis. Chem. Eng. J. 2018, 353, 297–304. [Google Scholar] [CrossRef]
- Uytdenhouwen, Y.; Bal, K.; Neyts, E.; Meynen, V.; Cool, P.; Bogaerts, A. On the kinetics and equilibria of plasma-based dry reforming of methane. Chem. Eng. J. 2021, 405, 6630. [Google Scholar] [CrossRef]
- Ray, D.; Reddy, P.K.R.; Subrahmanyam, C. Ni-Mn/γ-Al2O3 assisted plasma dry reforming of methane. Catal. Today 2018, 309, 212–218. [Google Scholar] [CrossRef]
- Brune, L.; Ozkan, A.; Genty, E.; De Bocarmé, T.V.; Reniers, F. Dry reforming of methane via plasma-catalysis: Influence of the catalyst nature supported on alumina in a packed-bed DBD configuration. J. Phys. D Appl. Phys. 2018, 51, 4002. [Google Scholar] [CrossRef]
- Rico, V.J.; Hueso, J.L.; Cotrino, J.; Gonzalez-Elipe, A.R. Evaluation of different dielectric barrier discharge plasma configura-tions as an alternative technology for green C1 chemistry in the carbon dioxide reforming of methane and the direct decom-position of methanol. J. Phys. Chem. A. 2010, 114, 4009–4016. [Google Scholar] [CrossRef]
- Vakili, R.; Gholami, R.; Stere, C.E.; Chansai, S.; Chen, H.; Holmes, S.M.; Jiao, Y.; Hardacre, C.; Fan, X. Plasma-assisted catalytic dry reforming of methane (DRM) over metal-organic frameworks (MOFs)-based catalysts. Appl. Catal. B Environ. 2020, 260, 8195. [Google Scholar] [CrossRef]
- Abiev, R.S.; Sladkovskiy, D.A.; Semikin, K.V.; Murzin, D.Y.; Rebrov, E.V. Non-Thermal Plasma for Process and Energy In-tensification in Dry Reforming of Methane. Catalysts 2020, 10, 1358. [Google Scholar] [CrossRef]
- Li, Z.; Lin, Q.; Li, M.; Cao, J.; Liu, F.; Pan, H.; Wang, Z.; Kawi, S. Recent advances in process and catalyst for CO2 reforming of methane. Renew. Sustain. Energy Rev. 2020, 134, 312. [Google Scholar] [CrossRef]
- George, A.; Shen, B.; Craven, M.; Wang, Y.; Kang, D.; Wu, C.; Tu, X. A Review of Non-Thermal Plasma Technology: A novel solution for CO2 conversion and utilization. Renew. Sustain. Energy Rev. 2021, 135, 9702. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhu, X.; Mei, D.; Ashford, B.; Tu, X. Plasma-catalytic dry reforming of methane over γ-Al2O3 supported metal catalysts. Catal. Today 2015, 256, 80–87. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, H.; Dalai, A.K. Effects of metal content on activity and stability of Ni-Co bimetallic catalysts for CO2 reforming of CH. Appl. Catal. A Gen. 2008, 339, 121–129. [Google Scholar] [CrossRef]
- Theofanidis, S.A.; Galvita, V.V.; Poelman, H.; Marin, G.B. Enhanced Carbon-Resistant Dry Reforming Fe-Ni Catalyst: Role of Fe. ACS Catal. 2015, 5, 3028–3039. [Google Scholar] [CrossRef]
- Fan, M.S.; Abdullah, A.Z.; Bhatia, S. Kinetic Evaluation of Methane−Carbon Dioxide Reforming Process Based on the Reaction Steps. Chem. Sus. Chem. 2011, 4, 1643–1653. [Google Scholar] [CrossRef]
- Li, Z.; Kathiraser, Y.; Ashok, J.; Oemar, U.; Kawi, S. Simultaneous Tuning Porosity and Basicity of Nickel@Nickel–Magnesium Phyllosilicate Core–Shell Catalysts for CO2 Reforming of CH. Langmuir 2014, 30, 14694–14705. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Tan, Z.; Hidajat, K.; Kawi, S. Highly reactive Ni-Co/SiO2 bimetallic catalyst via complexation with oleylamine/oleic acid organic pair for dry reforming of methane. Catal. Today 2017, 281, 250–258. [Google Scholar] [CrossRef]
- Oemar, U.; Kathiraser, Y.; Mo, L.; Ho, X.K.; Kawi, S. CO2reforming of methane over highly active La-promoted Ni supported on SBA-15 catalysts: Mechanism and kinetic modelling. Catal. Sci. Technol. 2016, 6, 1173–1186. [Google Scholar] [CrossRef]
- Ni, J.; Zhao, J.; Chen, L.; Lin, J.; Kawi, S. Lewis Acid Sites Stabilized Nickel Catalysts for Dry (CO2) Reforming of Methane. ChemCatChem 2016, 8, 3732–3739. [Google Scholar] [CrossRef]
- Mo, L.; Leong, K.K.M.; Kawi, S. A highly dispersed and anti-coking Ni–La2O3/SiO2 catalyst for syngas production from dry carbon dioxide reforming of methane. Catal. Sci. Technol. 2014, 4, 2107–2114. [Google Scholar] [CrossRef]
- Ni, J.; Chen, L.; Lin, J.; Schreyer, M.K.; Wang, Z.; Kawi, S. High performance of Mg–La mixed oxides supported Ni catalysts for dry reforming of methane: The effect of crystal structure. Int. J. Hydrog. Energy 2013, 38, 13631–13642. [Google Scholar] [CrossRef]
- Tu, X.; Whitehead, J.C. Plasma-catalytic dry reforming of methane in an atmospheric dielectric barrier discharge: Under-standing the synergistic effect at low temperature. Appl. Catal. B Environ. 2012, 125, 439–448. [Google Scholar] [CrossRef]
- Mei, D.; Ashford, B.; He, Y.-L.; Tu, X. Plasma-catalytic reforming of biogas oversupported Ni catalysts in a dielectric barrier discharge reactor: Effect of catalyst supports. Plasma Process. Polym. 2017, 14, 1600076. [Google Scholar] [CrossRef] [Green Version]
- Bogaerts, A.; Centi, G. Plasma Technology for CO2 Conversion: A Personal Perspective on Prospects and Gaps. Front. Energy Res. 2020, 8, 111. [Google Scholar] [CrossRef]
- Jennifer, M.-C.; Coulombe, S.; Kopyscinski, J. Influence of Operating Parameters on Plasma-Assisted Dry Reforming of Methane in a Rotating Gliding Arc Reactor. Plasma Chem. Plasma Process. 2020, 40, 857–881. [Google Scholar]
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2003, 23, 1–46. [Google Scholar] [CrossRef]
- Zhang, X.; Wenren, Y.; Zhou, W.; Han, J.; Lu, H.; Zhu, Z.; Wu, Z.; Cha, M.S. Dry reforming of methane in a temperature-controlled dielectric barrier discharge reactor: Disclosure of reactant effect. J. Phys. D Appl. Phys. 2020, 53, 4002. [Google Scholar] [CrossRef] [Green Version]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Cold plasma dielectric barrier discharge reactor for dry reforming of methane over Ni/γ-Al2O3-MgO nanocomposite. Fuel Process. Technol. 2018, 178, 166–179. [Google Scholar] [CrossRef]
- Andersen, J.; Christensen, J.; Østberg, M.; Bogaerts, A.; Jensen, A. Plasma-catalytic dry reforming of methane: Screening of catalytic materials in a coaxial packed-bed DBD reactor. Chem. Eng. J. 2020, 397, 5519. [Google Scholar] [CrossRef]
- Michielsen, I.; Uytdenhouwen, Y.; Bogaerts, A.; Meynen, V. Altering Conversion and Product Selectivity of Dry Reforming of Methane in a Dielectric Barrier Discharge by Changing the Dielectric Packing Material. Catalysts 2019, 9, 51. [Google Scholar] [CrossRef] [Green Version]
- Chun, S.M.; Shin, D.H.; Ma, S.H.; Yang, G.W.; Hong, Y.C. CO2 Microwave Plasma—Catalytic Reactor for Efficient Reforming of Methane to Syngas. Catalysts 2019, 9, 292. [Google Scholar] [CrossRef] [Green Version]
- Holzer, F.; Kopinke, F.D.; Roland, U. Influence of Ferroelectric Materials and Catalysts on the Performance of Non-Thermal Plasma (NTP) for the Removal of Air Pollutants. Plasma Chem. Plasma Process. 2005, 25, 595–611. [Google Scholar] [CrossRef]
- Li, H.; Yuan, G.; Shan, B.; Zhang, X.; Ma, H.; Tian, Y.; Lu, H.; Liu, J. Chemical Vapor Deposition of Vertically Aligned Carbon Nanotube Arrays: Critical Effects of Oxide Buffer Layers. Nanoscale Res. Lett. 2019, 14, 106. [Google Scholar] [CrossRef] [PubMed]
- Kameshima, S.; Tamura, K.; Ishibashi, Y.; Nozaki, T. Pulsed dry methane reforming in plasma-enhanced catalytic reaction. Catal. Today 2015, 256, 67–75. [Google Scholar] [CrossRef]
- Slaets, J.; Aghaei, M.; Ceulemans, S.; Van Alphen, S.; Bogaerts, A. CO2 and CH4 conversion in “real” gas mixtures in a gliding arc plasmatron: How do N2 and O2 affect the performance? Green Chem. 2020, 22, 1366–1377. [Google Scholar] [CrossRef]
- Damjan, L.J.; Lin, J.-L.; Pohar, A.; Likozar, B. Methane Dry Reforming over Ni/Al2O3 Catalyst in Spark Plasma Reactor: Linking Computational Fluid Dynamics (CFD) with Reaction Kinetic Modelling. Catal. Today 2021, 362, 11–12. [Google Scholar]
- Yap, D.; Tatibouët, J.-C.; Catherine, B.-D. Catalyst assisted by non-thermal plasma in dry reforming of methane at low tem-perature. Catal. Today 2018, 299, 263–271. [Google Scholar] [CrossRef]
- Ray, D.; Nepak, D.; Vinodkumar, T.; Subrahmanyam, C. g-C3N4 promoted DBD plasma assisted dry reforming of methane. Energy 2019, 183, 630–638. [Google Scholar] [CrossRef]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Evaluating the Performance of a Ni Catalyst Supported on La2O3-MgAl2O4 for Dry Reforming of Methane in a Packed Bed Dielectric Barrier Discharge Plasma Reactor. Energy Fuels 2019, 33, 11630–11647. [Google Scholar] [CrossRef]
- Hu, X.; Jia, X.; Zhang, X.; Liu, Y.; Liu, C.-J. Improvement in the activity of Ni/ZrO2 by cold plasma decomposition for dry reforming of methane. Catal. Commun. 2019, 128, 5720. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, G.; Liu, J.; Zhao, P.; Hou, P.; Xu, Y.; Zhang, R. Effect of different activated carbon support on CH4-CO2 re-forming over Co-based catalysts. Int. J. Hydr. Energy 2018, 43, 1497–1507. [Google Scholar] [CrossRef]
- Effendi, A.; Hellgardt, K.; Zhang, Z.-G.; Yoshida, T. Characterisation of carbon deposits on Ni/SiO2 in the reforming of CH4–CO2 using fixed- and fluidised-bed reactors. Catal. Commun. 2003, 4, 203–207. [Google Scholar] [CrossRef]
- Luo, Y.-R. Comprehensive Handbook of Chemical Bond Energies; Chapman and Hall/CRC: London, UK; Boca Raton, FL, USA, 2007. [Google Scholar]
- Zhang, K.; Mukhriza, T.; Liu, X.; Greco, P.P.; Chiremba, E. A study on CO2 and CH4 conversion to synthesis gas and higher hydrocarbons by the combination of catalysts and dielectric-barrier discharges. Appl. Catal. A Gen. 2015, 502, 138–149. [Google Scholar] [CrossRef]
- Wang, H.; Han, J.; Bo, Z.; Qin, L.; Wang, Y.; Yu, F. Non-thermal plasma enhanced dry reforming of CH4 with CO2 over activated carbon supported Ni catalysts. Mol. Catal. 2019, 475, 486. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, L.; Wu, C.; Wang, J.; Shen, B.; Tu, X. Low temperature reforming of biogas over K-, Mg- and Ce-promoted Ni/Al2O3 catalysts for the production of hydrogen rich syngas: Understanding the plasma-catalytic synergy. Appl. Catal. B Environ. 2018, 224, 469–478. [Google Scholar] [CrossRef] [Green Version]
- Ray, D.; Nepak, D.; Janampelli, S.; Goshal, P.; Subrahmanyam, C.; Ghosal, P.; Subrahmanyam, C. Dry Reforming of Methane in DBD Plasma over Ni-Based Catalysts: Influence of Process Conditions and Support on Performance and Durability. Energy Technol. 2019, 7, 1008. [Google Scholar] [CrossRef]
- Suttikul, T.; Nuchdang, S.; Rattanaphra, D.; Phalakornkule, C. Influence of Operating Parameters, Al2O3 and Ni/Al2O3 Cata-lysts on Plasma Assisted CO2 Reforming of CH4 in a Parallel Plate Dielectric Barrier Discharge for High H2/CO Ratio Syngas Production. Plasma Chem. Plasma Process. 2020, 40, 1445–1463. [Google Scholar] [CrossRef]
- Kameshima, S.; Mizukami, R.; Yamazaki, T.; Prananto, L.A.; Nozaki, T. Interfacial reactions between DBD and porous catalyst in dry methane reforming. J. Phys. D: Appl. Phys. 2018, 51, 4006. [Google Scholar] [CrossRef]
- Nagaraja, B.M.; Bulushev, D.A.; Beloshapkin, S.; Ross, J.R.H. The effect of potassium on the activity and stability of Ni-MgO-ZrO2 catalysts for the dry reforming of methane to give synthesis gas. Catal. Today 2011, 178, 132–136. [Google Scholar] [CrossRef]
- Vasiliades, M.A.; Djinović, P.; Pintar, A.; Kovač, J.; Efstathiou, A.M. The effect of CeO2–ZrO2 structural differences on the origin and reactivity of carbon formed during methane dry reforming over NiCo/CeO2–ZrO2 catalysts studied by transient techniques. Catal. Sci. Technol. 2017, 7, 5422–5434. [Google Scholar] [CrossRef]
- Hou, Z.; Yashima, T. Small Amounts of Rh-Promoted Ni Catalysts for Methane Reforming with CO. Catal. Lett. 2003, 89, 193–197. [Google Scholar] [CrossRef]
- Rahemi, N.; Haghighi, M.; Babaluo, A.A.; Allahyari, S.; Estifaee, P.; Jafari, M.F. Plasma-Assisted Dispersion of Bimetallic Ni–Co over Al2O3–ZrO2 for CO2 Reforming of Methane: Influence of Voltage on Catalytic Properties. Top. Catal. 2017, 60, 145–854. [Google Scholar] [CrossRef]
- Mei, D.; Liu, S.; Tu, X. CO2 reforming with methane for syngas production using a dielectric barrier discharge plasma coupled with Ni/γ-Al2O3 catalysts: Process optimization through response surface methodology. J. CO2 Util. 2017, 21, 314–326. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Galvez, M.E.; Da Costa, P.; Grzybek, T. Catalytic activity of hydrotalcite-derived catalysts in the dry reforming of methane: On the effect of Ce promotion and feed gas composition. React. Kinet. Mech. Catal. 2017, 121, 185–208. [Google Scholar] [CrossRef] [Green Version]
- Yan, X.; Hu, T.; Liu, P.; Li, S.; Zhao, B.; Zhang, Q.; Jiao, W.; Chen, S.; Wang, P.; Lu, J.; et al. Highly efficient and stable Ni/CeO2-SiO2 catalyst for dry reforming of methane: Effect of interfacial structure of Ni/CeO2 on SiO. Appl. Catal. B: Environ. 2019, 246, 221–231. [Google Scholar] [CrossRef]
- Jin, L.; Li, Y.; Lin, P.; Hu, H. CO2 reforming of methane on Ni/γ-Al2O3 catalyst prepared by dielectric barrier discharge hydrogen plasm. Int. J. Hydrog. Energy 2014, 39, 5756–5763. [Google Scholar] [CrossRef]
- Chen, H.-W.; Wang, C.-Y.; Yu, C.-H.; Tseng, L.-T.; Liao, P.-H. Carbon dioxide reforming of methane reaction catalyzed by stable nickel copper catalysts. Catal. Today 2004, 97, 173–180. [Google Scholar] [CrossRef]
- Dębek, R.; Motak, M.; Grzybek, T.; Galvez, M.E.; Da Costa, P. A Short Review on the Catalytic Activity of Hydrotalcite-Derived Materials for Dry Reforming of Methane. Catalysts 2017, 7, 32. [Google Scholar] [CrossRef] [Green Version]
- Tao, X.; Yang, C.; Huang, L.; Xu, D. DBD plasma combined with catalysts derived from NiMgAlCe hydrotalcite for CO2 reforming of CH. Mater. Chem. Phys. 2020, 250, 3118. [Google Scholar] [CrossRef]
- Sengupta, S.; Deo, G. Modifying alumina with CaO or MgO in supported Ni and Ni–Co catalysts and its effect on dry reforming of CH. J. CO2 Util. 2015, 10, 67–77. [Google Scholar] [CrossRef]
- Özkara-Aydınoğlu, Ş.; Aksoylu, A.E. Carbon dioxide reforming of methane over Co-X/ZrO2 catalysts (X = La, Ce, Mn, Mg, K). Catal. Commun. 2010, 11, 1165–1170. [Google Scholar] [CrossRef]
- Gurav, H.R.; Dama, S.; Samuel, V.; Chilukuri, S. Influence of preparation method on activity and stability of Ni catalysts supported on Gd doped ceria in dry reforming of methane. J. CO2 Util. 2017, 20, 357–367. [Google Scholar] [CrossRef]
- Bacariza, M.; Biset-Peiró, M.; Graça, I.; Guilera, J.; Morante, J.; Lopes, J.; Andreu, T.; Henriques, C. DBD plasma-assisted CO2 methanation using zeolite-based catalysts: Structure composition-reactivity approach and effect of Ce as promoter. J. CO2 Util. 2018, 26, 202–211. [Google Scholar] [CrossRef]
- Ray, D.; Chawdhury, P.; Subrahmanyam, C. Promising Utilization of CO2 for Syngas Production over Mg2+- and Ce2+- Pro-moted Ni/γ-Al2O3 Assisted by Nonthermal Plasma. ACS Omega 2020, 5, 14040–14050. [Google Scholar] [CrossRef]
- Sheng, Z.; Kim, H.-H.; Yao, S.; Nozaki, T. Plasma-chemical promotion of catalysis for CH4 dry reforming: Unveiling plas-ma-enabled reaction mechanisms. Phys. Chem. Chem. Phys. 2020, 22, 19349–19358. [Google Scholar] [CrossRef]
- Zheng, X.; Tan, S.; Dong, L.; Li, S.; Chen, H. LaNiO3@SiO2 core–shell nano-particles for the dry reforming of CH4 in the dielectric barrier discharge plasma. Int. J. Hydrog. Energy 2014, 39, 11360–11367. [Google Scholar] [CrossRef]
- Zheng, X.-G.; Tan, S.-Y.; Dong, L.-C.; Li, S.-B.; Chen, H.-M.; Wei, S.-A. Experimental and kinetic investigation of the plasma catalytic dry reforming of methane over perovskite LaNiO3 nanoparticles. Fuel Process. Technol. 2015, 137, 250–258. [Google Scholar] [CrossRef]
- Choi, J.H.; Il, L.T.; Han, I.; Oh, B.Y.; Jeong, M.C.; Myoung, J.M. Improvement of plasma uniformity using ZnO-coated dielectric barrier discharge in open air. Appl. Phys. Lett. 2006, 89, 8150. [Google Scholar] [CrossRef]
- Holzer, F.; Roland, U.; Kopinke, F.D. Combination of non-thermal plasma and heterogeneous catalysis for oxidation of volatile organic compounds: Part 1. accessibility of the intra-particle volume. Appl. Catal. B. 2002, 38, 163–181. [Google Scholar] [CrossRef]
- Ozkan, A.; Dufour, T.; Bogaerts, A.; Reniers, F. How do the barrier thickness and dielectric material influence the filamentary mode and CO2 conversion in a flowing DBD? Plasma Sources Sci. Technol. 2016, 25, 5016. [Google Scholar] [CrossRef] [Green Version]
- Khoja, A.H.; Tahir, M.; Amin, N.A.S. Dry reforming of methane using different dielectric materials and DBD plasma reactor configurations. Energy Convers. Manag. 2017, 144, 262–274. [Google Scholar] [CrossRef]
- Kundu, S.K.; Kennedy, E.M.; Gaikwad, V.V.; Molloy, T.S.; Dlugogorski, B.Z. Experimental investigation of alumina and quartz as dielectrics for a cylindrical double dielectric barrier discharge reactor in argon diluted methane plasma. Chem. Eng. J. 2012, 180, 178–189. [Google Scholar] [CrossRef]
- Yan, X.; Zhao, B.; Liu, Y.; Li, Y. Dielectric barrier discharge plasma for preparation of Ni-based catalysts with enhanced coke resistance: Current status and perspective. Catal. Today 2015, 256, 29–40. [Google Scholar] [CrossRef]
- Duan, X.; Li, Y.; Ge, W.; Wang, B. Degradation of CO2through dielectric barrier discharge microplasma. Greenh. Gases: Sci. Technol. 2015, 5, 131–140. [Google Scholar] [CrossRef]
- Pinhão, N.; Moura, A.; Branco, J.; Neves, J. Influence of gas expansion on process parameters in non-thermal plasma plug-flow reactors: A study applied to dry reforming of methane. Int. J. Hydrog. Energy 2016, 41, 9245–9255. [Google Scholar] [CrossRef]
- Chawdhury, P.; Ray, D.; Subrahmanyam, C. Single step conversion of methane to methanol assisted by nonthermal plasma. Fuel Process. Technol. 2018, 179, 32–41. [Google Scholar] [CrossRef]
- Mao, S.; Tan, Z.; Zhang, L.; Huang, Q. Plasma-assisted biogas reforming to syngas at room temperature condition. J. Energy Inst. 2018, 91, 172–183. [Google Scholar] [CrossRef]
- Liu, C.; Li, M.; Wang, J.; Zhou, X.; Guo, Q.; Yan, J.; Li, Y. Plasma methods for preparing green catalysts: Current status and perspective. Chin. J. Catal. 2016, 37, 340–348. [Google Scholar] [CrossRef]
- Michielsen, I.; Uytdenhouwen, Y.; Pype, J.; Mertens, J.; Reniers, F.; Meynen, V.; Bogaerts, A. CO2 dissociation in a packed bed DBD reactor: First steps towards a better understanding of plasma catalysis. Chem. Eng. J. 2017, 326, 477–488. [Google Scholar] [CrossRef]
- Zhang, X.; Cha, M.S. Electron-induced dry reforming of methane in a tempera-ture-controlled dielectric barrier discharge reactor. J. Phys. D Appl. Phys. 2013, 46, 5205. [Google Scholar] [CrossRef]
- Snoeckx, R.; Bogaerts, A. Plasma technology–A novel solution for CO2 conversion? Chem. Soc. Rev. 2017, 46, 5805–5863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, Z.; Kameshima, S.; Sakata, K.; Nozaki, T. Plasma-Enabled Dry Methane Reforming. Plasma Chem. Gas Convers. 2018. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, X.; Lin, Z.; Li, T.; Huang, L.; Zhang, J.; Askari, S.; Dewangan, N.; Jangam, A.; Kawi, S. Recent Developments in Dielectric Barrier Discharge Plasma-Assisted Catalytic Dry Reforming of Methane over Ni-Based Catalysts. Catalysts 2021, 11, 455. https://doi.org/10.3390/catal11040455
Gao X, Lin Z, Li T, Huang L, Zhang J, Askari S, Dewangan N, Jangam A, Kawi S. Recent Developments in Dielectric Barrier Discharge Plasma-Assisted Catalytic Dry Reforming of Methane over Ni-Based Catalysts. Catalysts. 2021; 11(4):455. https://doi.org/10.3390/catal11040455
Chicago/Turabian StyleGao, Xingyuan, Ziting Lin, Tingting Li, Liuting Huang, Jinmiao Zhang, Saeed Askari, Nikita Dewangan, Ashok Jangam, and Sibudjing Kawi. 2021. "Recent Developments in Dielectric Barrier Discharge Plasma-Assisted Catalytic Dry Reforming of Methane over Ni-Based Catalysts" Catalysts 11, no. 4: 455. https://doi.org/10.3390/catal11040455