Glycerol Electro-Oxidation in Alkaline Media and Alkaline Direct Glycerol Fuel Cells
Abstract
:1. Introduction
2. Glycerol Electro-Oxidation in Alkaline Media
2.1. Precious Metal Catalysts
2.1.1. Pure Pt, Pd and Au Catalysts and Binary PtAu and PdAu Catalysts
2.1.2. Pt- and Pd- and Au- Based Binary/Ternary Catalysts
2.2. Non-Precious Metal Catalysts
3. Alkaline Direct Glycerol Fuel Cells (ADGFCs)
3.1. ADGFCs for Energy Production
3.2. ADGFC for Value-Added Chemical and Energy Production
4. Conclusions
Funding
Conflicts of Interest
Abbreviations
| AAEM | alkaline anion-exchange membrane |
| ADAFC | alkaline direct alcohol fuel cell |
| ADGFC | alkaline direct glycerol fuel cell |
| C3,C2,C1 | glycerol oxidation products with 3, 2 and 1 carbon atoms, respectively |
| DFT | density functional theory |
| DHA | dihydroxyacetone |
| FA | formic acid |
| GA | glycolic acid |
| GALD | glyceraldehyde |
| GLA | glyceric acid |
| GLY | glycerol |
| GOA | glyoxylic acid |
| GOR | glycerol oxidation reaction |
| HPA | hydroxypyruvic acid |
| LA | lactic acid |
| MOA | mesoxalic acid |
| MPD | maximum power density |
| OA | oxalic acid |
| TA | tartronic acid |
References
- Quispe, C.A.G.; Coronado, C.J.R.; Carvalho, J.A., Jr. Glycerol: Production, consumption, prices, characterization and new trends in combustion. Renew. Sustain. Energy Rev. 2013, 27, 475–493. [Google Scholar] [CrossRef]
- Coutanceau, C.; Baranton, S.; Bitty Kouamé, R.S. Selective electrooxidation of glycerol into value-added chemicals: A short overview. Front. Chem. 2019, 7, 100. [Google Scholar] [CrossRef] [PubMed]
- Behr, A.A.; Eilting, J.; Irawadi, K.; Leschinski, J.; Lindner, F. Improved utilisation of renewable resources: New important derivatives of glycerol. Green Chem. 2008, 10, 13–30. [Google Scholar] [CrossRef]
- Pagliaro, M.; Ciriminna, R.; Kimura, H.; Rossi, M.; Della Pina, C. From glycerol to value-added products. Angew. Chem. Int. Ed. 2007, 46, 4434–4440. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Saha, R.; Ghosh, A.; Mukherjee, K.; Saha, B. Micellar catalysis on 1,10-phenanthroline promoted chromic acid oxidation of glycerol in aqueous media. Tenside Surf. Deterg. 2012, 49, 370–375. [Google Scholar] [CrossRef]
- Gonzalez-Pajuelo, M.; Meynial-Salles, I.; Mendes, F.; Soucaille, P.; Vasconcelos, I. Microbial Conversion of Glycerol to 1,3-Propanediol: Physiological Comparison of a Natural Producer, Clostridium butyricum VPI 3266, and an Engineered Strain, Clostridium acetobutylicum DG1(pSPD5). Appl. Environ. Microbiol. 2006, 72, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Carrettin, S.; McMorn, P.; Johnston, P.; Griffin, K.; Kiely, C.J.; Attard, G.A.; Hutchings, G.J. Oxidation of glycerol using supported gold catalysts. Top. Catal. 2004, 27, 131–136. [Google Scholar] [CrossRef]
- Katryniok, B.; Kimura, H.; Skrzynska, E.; Girardon, J.S.; Fongarland, P.; Capron, M.; Ducoulombier, R.; Nimura, N.; Paul, S.; Dumeigni, F. Selective catalytic oxidation of glycerol: Perspectives for high value chemicals. Green Chem. 2011, 13, 1960–1979. [Google Scholar] [CrossRef]
- Villa, A.; Dimitratos, N.; Chan-Thaw, C.E.; Hammond, C.; Prati, L.; Hutchings, G.J. Glycerol oxidation using gold-containing catalysts. Acc. Chem. Res. 2015, 48, 1403–1412. [Google Scholar] [CrossRef]
- Kwon, Y.; Koper, M.T.M. Combining voltammetry with HPLC to electro-oxidation of glycerol. Anal. Chem. 2010, 82, 5420–5424. [Google Scholar] [CrossRef]
- Kwon, Y.Y.; Schouten, K.J.P.; Koper, M.T.M. Mechanism of the catalytic oxidation of glycerol on polycrystalline gold and platinum electrodes. ChemCatChem 2011, 3, 1176–1185. [Google Scholar] [CrossRef]
- Inoue, H.; Kimura, S.; Teraoka, Y.; Chiku, M.; Higuchi, E.; Lam, B.T.X. Mechanism of glycerol oxidation reaction on silver-modified palladium electrode in alkaline medium. Int. J. Hydrogen Energy 2018, 43, 18664–18671. [Google Scholar] [CrossRef]
- Benipal, N.; Qi, J.; Liu, Q.; Li, W. Carbon nanotube supported PdAg nanoparticles for electrocatalytic oxidation of glycerol in anion exchange membrane fuel cells. Appl. Catal. B Environ. 2017, 210, 121–130. [Google Scholar] [CrossRef]
- Wang, Y.; Li, L.; Hu, L.; Zhuang, L.; Lu, J.; Xu, B. A feasibility analysis for alkaline membrane direct methanol fuel cell: Thermodynamic disadvantages versus kinetic advantages. Electrochem. Commun. 2003, 5, 662–666. [Google Scholar] [CrossRef]
- Kwon, Y.; Lai, S.C.S.; Rodriguez, P.; Koper, M. Electrocatalytic oxidation of alcohols on gold in alkaline media: Base or gold catalysis? J. Am. Chem. Soc. 2011, 133, 6914–6917. [Google Scholar] [CrossRef]
- Garcia, R.; Besson, M.; Gallezot, P. Chemoselective catalytic oxidation of glycerol with air on platinum metals. Appl. Catal. A Gen. 1995, 127, 165–176. [Google Scholar] [CrossRef]
- Roquet, L.; Belgsir, E.M.; Leger, J.M.; Lamy, C. Kinetics and mechanism of the electrocatalytic oxidation of glycerol as investigated by chromatographic analysis of the reaction products: Potential and pH effects. Electrochim. Acta 1994, 39, 2387–2394. [Google Scholar] [CrossRef]
- Simoes, M.; Baranton, S.; Coutenceau, C. Electro-oxidation of glycerol at Pd based nano-catalysts for an application in alkaline fuel cells for chemicals and energy cogeneration. Appl. Catal. B Environ. 2010, 93, 354–362. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Qi, J.; Chadderdon, D.J.; Sun, K.; Warsko, K.M.; Li, W. Selective electro-oxidation of glycerol to tartronate or mesoxalate on Au nanoparticle catalyst via electrode potential tuning in anion-exchange membrane electro-catalytic flow reactor. Appl. Catal. B Environ. 2014, 147, 871–878. [Google Scholar] [CrossRef]
- Zhou, Y.; Shena, Y.; Xi, J. Seed-mediated synthesis of PtxAuy@Ag electrocatalysts for the selective oxidation of glycerol. Appl. Catal. B Environ. 2019, 245, 604–612. [Google Scholar] [CrossRef]
- Velazquez-Hernandez, I.; Oropeza-Guzman, M.T.; Guerra-Balcazar, M.; Alvarez-Contreras, L.; Arjona, N. Electrocatalytic promotion of Pt nanoparticles by incorporation of Ni(OH)2 for glycerol electro-oxidation: Analysis of activity and reaction pathway. ChemNanoMat 2019, 5, 68–78. [Google Scholar] [CrossRef]
- Chen, W.; Zhou, Y.; Shen, Y. Product distribution of glycerol electro-oxidation over platinum-ceria/graphene nanosheet. Electrochemistry 2019, 87, 30–34. [Google Scholar] [CrossRef]
- Marchionni, A.; Bevilacqua, M.; Bianchini, C.; Chen, Y.X.; Filippi, J.; Fornasiero, P.; Lavacchi, A.; Miller, H.; Wang, L.; Vizza, F. Electrooxidation of ethylene glycol and glycerol on Pd-(Ni-Zn)/C anodes in direct alcohol fuel cells. ChemSusChem 2013, 6, 518–528. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, R.G.; Aquino Neto, S.; Kokoh, K.B.; Rodrigues De Andrade, A. Electroconversion of glycerol in alkaline medium: From generation of energy to formation of value-added products. J. Power Sources 2017, 351, 174–182. [Google Scholar] [CrossRef]
- Sandrini, R.M.L.M.; Sempionatto, J.R.; Tremiliosi-Filho, G.; Herrero, E.; Feliu, J.M.; Souza-Garcia, J.; Angelucci, C. Electrocatalytic oxidation of glycerol on platinum single crystals in alkaline media. ChemElectroChem 2019, 6, 4238–4245. [Google Scholar] [CrossRef]
- Zalineeva, A.; Baranton, S.; Coutanceau, C. How do Bi-modified palladium nanoparticles work towards glycerol electrooxidation? An in situ FTIR study. Electrochim. Acta 2015, 176, 705–717. [Google Scholar] [CrossRef]
- Gomes, J.F.; Tremiliosi-Filho, G. Spectroscopic studies of the glycerol electrooxidation on polycrystalline Au and Pt surfaces in acidic and alkaline Media. Electrocatalysis 2011, 2, 96–105. [Google Scholar] [CrossRef]
- Gomes, J.F.; Garcia, A.C.; Gasparotto, L.H.S.; De Souza, N.E.; Ferreira, E.B.; Pires, C.C.; Tremiliosi-Filho, G. Influence of silver on the glycerol electro-oxidation over AuAg/C catalysts in alkaline medium: A cyclic voltammetry and in situ FTIR spectroscopy study. Electrochim. Acta 2014, 144, 361–368. [Google Scholar] [CrossRef]
- Dai, C.; Sun, L.; Liao, H.; Khezri, B.; Webster, R.D.; Fisher, A.C.; Xu, Z.J. Electrochemical production of lactic acid from glycerol oxidation catalyzed by AuPt nanoparticles. J. Catal. 2017, 56, 14–21. [Google Scholar] [CrossRef]
- Jeffery, D.Z.; Camara, G.A. The formation of carbon dioxide during glycerol electrooxidation in alkaline media: First spectroscopic evidences. Electrochem. Comm. 2010, 12, 1129–1132. [Google Scholar] [CrossRef]
- Zhang, J.; Liang, Y.; Li, N.; Li, Z.; Xu, C.; Jiang, S.P. A remarkable activity of glycerol electrooxidation on gold in alkaline medium. Electrochim. Acta 2012, 59, 156–159. [Google Scholar] [CrossRef]
- De Souza, N.E.; Gomes, J.F.; Tremiliosi-Filho, G. Reactivity of 3-carbon-atom chain alcohols on gold electrode: A comparison to understand the glycerol electro-oxidation. J. Electroanal. Chem. 2017, 800, 106–113. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Qi, J.; Chadderdon, D.J.; Li, W. Supported Pt, Pd and Au nanoparticle anode catalysts for anion-exchange membrane fuel cells with glycerol and crude glycerol fuels. Appl. Catal. B Environ. 2013, 136, 29–39. [Google Scholar] [CrossRef]
- Habibi, E.; Razmi, H. Glycerol electrooxidation on Pd, Pt and Au nanoparticles supported on carbon ceramic electrode in alkaline media. Int. J. Hydrogen Energy 2012, 37, 16800–16809. [Google Scholar] [CrossRef]
- Frota, E., Jr.; Purgatto, A.; Linares, J.J. Pt/C, Au/C and Pd/C catalysts for alkaline-based direct glycerol fuel cells. Chem. Eng. Trans. 2014, 41, 253–258. [Google Scholar]
- Burke, L.D.; Nugent, P.F. The electrochemistry of gold: I The redox behaviour of the metal in aqueous media. Gold Bull. 1997, 30, 43–53. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Tao, L.; Cheng, Y.; Yang, F.; Jin, Y.; Zhou, C.; Yu, H.; Yang, Y. Electrocatalytic oxidation of small molecule alcohols over Pt, Pd, and Au catalysts: The Effect of alcohol’s hydrogen bond donation ability and molecular structure properties. Catalysts 2019, 9, 387. [Google Scholar] [CrossRef] [Green Version]
- Li, D.; Zhu, Y.; Wang, H.; Ding, Y. Nanoporous gold as an active low temperature catalyst toward CO oxidation in hydrogen-rich stream. Sci. Rep. 2013, 3, 1–7. [Google Scholar] [CrossRef]
- Roy, C.B.; Nandy, D.K.; Rao, K.V. Electrocatalytic oxidation of methanol, ethanol, isopropanol, glycol & glycerol on Pt-Au electrodes. Ind. J. Chem. 1985, 24, 742–744. [Google Scholar]
- Li, N.; Xia, W.Y.; Xu, C.W.; Chen, S. Pt/C and Pd/C catalysts promoted by Au for glycerol and CO electrooxidation in alkaline medium. J. Energy Inst. 2017, 90, 725–733. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Da Silva, S.G.; De Souza, R.F.B.; Oliveira Neto, A. PtAu electrocatalyst for glycerol oxidation reaction using a ATR-FTIR/single direct alkaline glycerol/air Cell in situ study. Electrocatalysis 2016, 7, 22–32. [Google Scholar] [CrossRef]
- Jin, C.; Sun, C.; Dong, R.; Chen, Z. Electrocatalytic activity of PtAu/C catalysts for glycerol oxidation. J. Nanosci. Nanotech. 2012, 12, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Mougenot, M.; Caillard, A.; Simoes, M.; Baranton, S.; Coutanceau, C.; Brault, P. PdAu/C catalysts prepared by plasma sputtering for the electro-oxidation of Glycerol. Appl. Catal. B Environ. 2011, 107, 372–379. [Google Scholar] [CrossRef] [Green Version]
- Geraldes, A.N.; Da Silva, D.F.; De Andrade e Silva, L.G.; Spinace, E.V.; Oliveira Neto, A.; Dos Santos, M.C. Binary and ternary palladium based electrocatalysts for alkaline direct glycerol fuel cell. J. Power Sources 2015, 293, 823–830. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Da Silva, S.G.; De Souza, R.F.B.; Oliveira Neto, A. Glycerol oxidation reaction using PdAu/C electrocatalysts. Ionics 2016, 22, 1167–1175. [Google Scholar] [CrossRef]
- Yahya, N.; Kamarudin, S.K.; Karim, N.A.; Masdar, M.S.; Loh, K.S. Enhanced performance of a novel anodic PdAu/VGCNF catalyst for electro-oxidation in a glycerol fuel cell. Nanoscale Res. Lett. 2017, 12, 605–615. [Google Scholar] [CrossRef] [Green Version]
- Yahya, N.; Kamarudin, S.K.; Karim, N.A.; Masdar, M.S.; Loh, K.S.; Lim, K.L. Durability and performance of direct glycerol fuel cell with palladium aurum/vapor grown carbon nanofiber support. Energy Conv. Manag. 2019, 188, 120–130. [Google Scholar] [CrossRef]
- Rezaei, B.; Havakeshian, E.; Ensafi, A.A. Electrocatalytic activity of bimetallic Pd\\Au nanostructure supported on nanoporous stainless steel surface using galvanic replacement reaction toward the glycerol oxidation in alkaline media. J. Electroanal. Chem. 2016, 782, 108–116. [Google Scholar] [CrossRef]
- Dong, Y.; Wang, W.; Wang, Y.; Wang, P.; Zhang, L.; Lei, Z. PdAu nanoparticles anchored on P and Se codoped carbon support as an efficacious electrocatalyst towards glycerol electrooxidation. J. Taiwan Inst. Chem. Eng. 2018, 93, 500–508. [Google Scholar] [CrossRef]
- Lam, B.T.X.; Chiku, M.; Higuchi, E.; Inoue, H. Preparation of PdAg and PdAu nanoparticle-loaded carbon black catalysts and their electrocatalytic activity for the glycerol oxidation reaction in alkaline medium. J. Power Sources 2015, 297, 149–157. [Google Scholar] [CrossRef]
- Yang, G.Y.; Shao, S.; Ke, Y.H.; Liu, C.L.; Ren, H.F.; Dong, W.S. PtAu alloy nanoparticles supported on thermally expanded graphene oxide as a catalyst for the selective oxidation of glycerol. RSC Adv. 2015, 5, 37112–37118. [Google Scholar] [CrossRef]
- Zhou, Y.; Shen, Y.; Piao, J. Sustainable conversion of glycerol into value-added chemicals by selective electro-oxidation on Pt-based catalysts. ChemElectroChem 2018, 5, 1636–1643. [Google Scholar] [CrossRef]
- Moraes, M.C.; Junco, G.G.; Messa Moreira, T.F.; Gomes Pinheiro, C.J.; Olivi, P.; Profeti, D.; Profeti, L.P.R. NiO-promoted Pt electrocatalysts prepared by thermal decomposition of polymeric precursors for oxidation of glycerol in alkaline medium. J. Environ. Chem. Eng. 2019, 7, 102922. [Google Scholar] [CrossRef]
- Qi, J.; Xin, L.; Zhang, Z.; Sun, K.; He, H.; Wang, F.; Chadderdon, D.; Qiu, Y.; Liang, C.; Li, W. Surface dealloyed PtCo nanoparticles supported on carbon nanotube: Facile synthesis and promising application for anion exchange membrane direct crude glycerol fuel cell. Green Chem. 2013, 15, 1133–1137. [Google Scholar] [CrossRef]
- Ottoni, C.A.; Ramos, C.E.D.; De Souza, R.F.B.; Da Silva, S.G.; Spinace, E.V.; Neto, A.O. Glycerol and ethanol oxidation in alkaline medium using PtCu/C electrocatalysts. Int. J. Electrochem. Sci. 2018, 13, 1893–1904. [Google Scholar] [CrossRef]
- Garcia, A.C.; Ferreira, E.B.; Silva de Barros, V.V.; Linares, J.J.; Tremiliosi-Filho, G. PtAg/MnOx/C as a promising electrocatalyst for glycerol electro-oxidation in alkaline medium. J. Electroanal. Chem. 2017, 793, 188–196. [Google Scholar] [CrossRef]
- Simoes, M.; Baranton, S.; Coutanceau, C. Enhancement of catalytic properties for glycerol electrooxidation on Pt and Pd nanoparticles induced by Bi surface modification. Appl. Catal. B Environ. 2011, 110, 40–49. [Google Scholar] [CrossRef]
- Coutanceau, C.; Zalineeva, A.; Baranton, S.; Simoes, M. Modification of palladium surfaces by bismuth adatoms or clusters: Effect on electrochemical activity and selectivity towards polyol electrooxidation. Int. J. Hydrogen Energy 2014, 39, 15877–15886. [Google Scholar] [CrossRef]
- Gonzalez-Cobos, J.; Baranton, S.; Coutanceau, C. Development of Bismuth-Modified PtPd Nanocatalysts for the Electrochemical Reforming of Polyols into Hydrogen and Value-Added Chemicals. ChemElectroChem 2016, 3, 1694–1704. [Google Scholar] [CrossRef]
- De Souza, M.B.C.; Vicente, R.A.; Yukuhiro, V.Y.; Pires, C.T.G.; Cheuquepán, W.; Bott-Neto, J.L.; Solla-Gullón, J.; Fernández, P.S. Bi-modified Pt electrodes toward glycerol electrooxidation in alkaline solution: Effects on activity and selectivity. ACS Catal. 2019, 9, 5104–5110. [Google Scholar] [CrossRef]
- Xu, C.; Zeng, R.; Shen, P.K.; Wei, Z.D. Synergistic effect of CeO2 modified Pt/C catalysts on the alcohols oxidation. Electrochim. Acta 2005, 51, 1031–1035. [Google Scholar] [CrossRef]
- Zhai, C.; Hu, J.; Gao, H.; Zeng, L.; Xue, M.; Liu, Z.Q.; Zhu, M. Nano-engineered hexagonal PtCuCo nanocrystals with enhanced catalytic activity for ethylene glycol and glycerol electrooxidation. J. Taiwan Inst. Chem. Eng. 2018, 93, 477–484. [Google Scholar] [CrossRef]
- De Araujo, V.M.F.; Antolini, E.; Pocrifka, L.; Passos, R.R. Electro-oxidation of glycerol on carbon supported Pt75CoxNi25-x (x = 0, 0.9, 12.5, 24.1 and 25) catalysts in an alkaline medium. Electrocatalysis 2018, 9, 673–681. [Google Scholar] [CrossRef]
- Ferreira, R.S., Jr.; Giz, M.J.; Camara, G.A. Influence of the local pH on the electrooxidation of glycerol on Palladium–Rhodium electrodeposits. J. Electroanal. Chem. 2013, 697, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Ran, B.; Liu, S.; Zhao, J.; Tan, T.; Liang, B.; Wang, W. An adenine-originated N-doped carbon supporting Pd3Ru nanoparticle with high performance for glycerol electrooxidation. J. Mater. Sci. 2019, 54, 4579–4588. [Google Scholar] [CrossRef]
- Munoz, F.; Hua, C.; Kwong, T.; Tran, L.; Nguyen, T.Q.; Haan, J.L. Palladium–copper electrocatalyst for the promotion of the electrochemical oxidation of polyalcohol fuels in the alkaline direct alcohol fuel cell. Appl. Catal. B Environ. 2015, 174, 323–328. [Google Scholar] [CrossRef]
- Wang, W.; Jing, W.; Sheng, L.; Chai, D.; Kang, Y.; Lei, Z. Pd3Cu coupling with nitrogen-doped mesoporous carbon to boost performance in glycerol oxidation. Appl. Catal. A Gen. 2017, 538, 123–130. [Google Scholar] [CrossRef]
- Muneeb, O.; Estrada, J.; Tran, L.; Nguyen, K.; Flores, J.; Hu, S.; Fry-Petit, A.M.; Scudiero, L.; Ha, S.; Haan, J.L. Electrochemical oxidation of polyalcohols in alkaline media on palladium catalysts promoted by the addition of copper. Electrochim. Acta 2016, 218, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Rostami, H.; Omrani, A.; Rostami, A.A. On the role of electrodeposited nanostructured Pd-Co alloy on Au for the electrocatalytic oxidation of glycerol in alkaline media. Int. J. Hydrogen Energy 2015, 40, 9444–9451. [Google Scholar] [CrossRef]
- Fathirad, F.; Mostafavi, A.; Afzali, D. Bimetallic Pd–Mo nanoalloys supported on Vulcan XC-72R carbon as anode catalysts for direct alcohol fuel cell. Int. J. Hydrogen Energy 2017, 42, 3215–3221. [Google Scholar] [CrossRef]
- Carvalho, L.L.; Colmati, F.; Tanaka, A.A. Nickel–palladium electrocatalysts for methanol, ethanol, and glycerol oxidation reactions. Int. J. Hydrogen Energy 2017, 42, 16118–16126. [Google Scholar] [CrossRef]
- Zalineeva, A.; Serov, A.; Padilla, M.; Martinez, U.; Artyushkova, K.; Baranton, S.; Coutanceau, C.; Atanassov, P.B. Glycerol electrooxidation on self-supported Pd1Snx nanoparticules. Appl. Catal. B Environ. 2015, 176, 429–435. [Google Scholar] [CrossRef]
- Xu, C.; Tian, Z.; Shen, P.K.; Jiang, S.P. Oxide (CeO2, NiO, Co3O4 and Mn3O4)-promoted Pd/C electrocatalysts for alcohol electrooxidation in alkaline media. Electrochim. Acta 2008, 53, 2610–2618. [Google Scholar] [CrossRef]
- Hong, W.; Wang, J.; Wang, E. Synthesis of hollow PdRuCo nanoparticles with enhanced electrocatalytic activity. RSC Adv. 2015, 5, 46935–46940. [Google Scholar] [CrossRef]
- Kang, Y.; Wang, W.; Pu, Y.; Li, J.; Chai, D.; Lei, Z. An effective Pd-NiOx-P composite catalyst for glycerol electrooxidation: Co-existed phosphorus and nickel oxide to enhance performance of Pd. Chem. Eng. J. 2017, 308, 419–427. [Google Scholar] [CrossRef]
- Fashedemi, O.O.; Ozoemena, K.I. Comparative electrocatalytic oxidation of ethanol, ethylene glycol and glycerol in alkaline medium at Pd-decorated FeCo@Fe/C core-shell nanocatalysts. Electrochim. Acta 2014, 128, 279–286. [Google Scholar] [CrossRef]
- Sadiki, A.; Vo, P.; Hu, S.; Copenhaver, T.S.; Scudiero, L.; Ha, S.; Haan, J.L. Increased electrochemical oxidation rate of alcohols in alkaline media on palladium surfaces electrochemically modified by antimony, lead, and tin. Electrochim. Acta 2014, 139, 302–307. [Google Scholar] [CrossRef]
- Zanata, C.R.; Martins, C.A.; Teixeira-Neto, E.; Giz, M.J.; Camara, G.A. Two-step synthesis of Ir-decorated Pd nanocubes and their impact on the glycerol electrooxidation. J. Catal. 2019, 377, 358–366. [Google Scholar] [CrossRef]
- Garcia, A.; Caliman, J.; Ferreira, E.B.; Tremilios-Filho, G.; Linares, J.J. Promotional effect of Ag on the catalytic activity of Au for glycerol electrooxidation in alkaline medium. ChemElectroChem 2015, 2, 1036–1041. [Google Scholar] [CrossRef]
- Thia, L.; Xie, M.; Kim, D.; Wang, X. Ag containing porous Au structures as highly selective catalysts for glycolate and formate. Catal. Sci. Technol. 2017, 7, 874–881. [Google Scholar] [CrossRef]
- Yongprapat, S.; Therthianwong, A.; Therdthianwong, S. Improvement of catalytic performance of AuAg/C catalysts prepared by galvanic displacement technique for glycerol electrooxidation in alkaline medium. J. Appl. Electrochem. 2018, 48, 317–328. [Google Scholar] [CrossRef]
- Song, J.H.; Yu, J.Y.; Zhang, M.Z.; Liang, Y.J.; Xu, C.W. Glycerol electrooxidation on Au/Ni core/shell three-dimensional structure catalyst. Int. J. Electrochem. Sci. 2012, 7, 4362–4368. [Google Scholar]
- Pittayaporn, N.; Therdthianwong, A.; Therdthianwong, S. Au/C catalysts promoted with Ni for glycerol electrooxidation in alkaline media. J. Appl. Electrochem. 2018, 48, 251–262. [Google Scholar] [CrossRef]
- Thia, L.; Xie, M.; Liu, Z.; Ge, X.; Lu, Y.; Fong, W.E.; Wang, X. Cu modified Au nanoparticles as highly selective catalysts for glycerol electro-oxidation in alkaline solution. ChemCatChem 2016, 8, 3272–3278. [Google Scholar] [CrossRef]
- Xu, H.; Yan, B.; Wang, J.; Zhang, K.; Li, S.; Xiong, Z.; Wang, C.; Shiraishi, Y.; Du, Y.; Yang, P. Self-supported porous 2D AuCu triangular nanoprisms as model electrocatalysts for ethylene glycol and glycerol oxidation. J. Mater. Chem. A 2017, 5, 15932–15939. [Google Scholar] [CrossRef]
- Padayachee, D.; Golovko, V.; Marshall, A.T. The effect of MnO2 loading on the glycerol electrooxidation activity of Au/MnO2/C catalysts. Electrochim. Acta 2013, 98, 208–217. [Google Scholar] [CrossRef]
- Zhang, J.; Zhu, T.; Liang, Y.; Zhang, C.; Shi, S.; Xu, C. CeO2 promoted Au/C catalyst for glycerol electro-oxidation in alkaline medium. J. Energy Inst. 2016, 89, 325–329. [Google Scholar] [CrossRef]
- Su, Y.; Xu, Q.; Zhong, Q.; Zhang, C.; Shi, S.; Xu, C. Oxide (Co3O4, NiO, Mn3O4, MgO) promoted Au/C catalyst for glycerol electrooxidation in alkaline medium. Mater. Res. Bull. 2015, 64, 301–305. [Google Scholar] [CrossRef]
- Pérez, A.E.E.; Ribadeneira, R. Modeling with DFT and Chemical Descriptors Approach for the Development of Catalytic Alloys for PEMFCs. In Density Functional Theory; Glossman-Mitnik, D., Ed.; Wiley: Hoboken, NJ, USA, 2019; Chapter 2. [Google Scholar]
- Kwon, Y.; BirDja, Y.; Spanos, I.; Rodriguez, P.; Koper, M.T.M. Highly selective electro-oxidation of glycerol to dihydroxyacetone on platinum in the presence of bismuth. ACS Catal. 2012, 2, 759–764. [Google Scholar] [CrossRef]
- Antolini, E.; Gonzalez, E.R. Alkaline direct alcohol fuel cells. J. Power Sources 2010, 195, 3431–3450. [Google Scholar] [CrossRef]
- Fleshmann, M.; Korinek, K.; Pletcher, D. The oxidation of organic compounds at a nickel anode in alkaline solution. J. Electroanal. Chem. 1971, 31, 39–49. [Google Scholar] [CrossRef]
- Yeo, B.S.; Bell, A.T. In situ Raman study of nickel oxide and gold-supported nickel oxide catalysts for the electrochemical evolution of oxygen. J. Phys. Chem. C 2012, 116, 8394–8400. [Google Scholar] [CrossRef] [Green Version]
- El-Shafei, A.A. Electrocatalytic oxidation of methanol at a nickel hydroxide/glassy carbon modified electrode in alkaline medium. J. Electroanal. Chem. 1999, 471, 89–95. [Google Scholar] [CrossRef]
- Casella, I.G.; Cataldi, T.R.I.; Salvi, A.M.; Desimoni, E. Electrocatalytic oxidation and liquid chromatographic detection of aliphatic alcohols at a nickel-based glassy carbon modified electrode. Anal. Chem. 1993, 85, 3143–3160. [Google Scholar] [CrossRef]
- Tehrani, R.M.A.; Ab Ghani, S. Electrocatalysis of free glycerol at a nanonickel modified graphite electrode and its determination in biodiesel. Electrochim. Acta 2012, 70, 153–157. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Cossar, E.; Ntais, S.; Baranova, E.A. Electrochemical modification of nickel surfaces for efficient glycerol electrooxidation. J. Power Sources 2018, 375, 310–319. [Google Scholar] [CrossRef]
- Stradiotto, N.R.; Toghill, K.E.; Xiao, L.; Moshar, A.; Compton, R.G. The fabrication and characterization of a nickel nanoparticle modified boron doped diamond electrode for electrocatalysis of primary alcohol oxidation. Electroanalysis 2009, 21, 2627–2633. [Google Scholar] [CrossRef]
- Lin, Q.; Wei, Y.; Liu, W.; Yu, Y.; Hu, J. Electrocatalytic oxidation of ethylene glycol and glycerol on nickel ion implanted-modified indium tin oxide electrode. Int. J. Hydrogen Energy 2017, 42, 1403–1411. [Google Scholar] [CrossRef] [Green Version]
- Sivasakthi, P.; Sangaranarayanan, M.V. Pulse electrodeposited nickel with structure directing agents as an electrocatalyst for oxidation of glycerol. New J. Chem. 2019, 43, 8352–8362. [Google Scholar] [CrossRef]
- Bott-Neto, J.L.; Martins, T.S.; Machado, S.A.S.; Ticianelli, E.A. Electrocatalytic oxidation of methanol, ethanol, and glycerol on Ni(OH)2 nanoparticles encapsulated with poly[Ni(salen)] film. ACS Appl. Mater. Int. 2019, 11, 30810–30818. [Google Scholar] [CrossRef]
- Oliveira, V.L.; Morais, C.; Servat, K.; Napporn, T.W.; Tremiliosi-Filho, G.; Kokoh, K.B. Glycerol oxidation on nickel based nanocatalysts in alkaline medium—Identification of the reaction products. J. Electroanal. Chem. 2013, 703, 56–62. [Google Scholar] [CrossRef] [Green Version]
- Oliveira, V.L.; Morais, C.; Servat, K.; Napporn, T.W.; Tremiliosi-Filho, G.; Kokoh, K.B. Studies of the reaction products resulted from glycerol electrooxidation on Ni-based materials in alkaline medium. Electrochim. Acta 2014, 117, 255–262. [Google Scholar] [CrossRef]
- Habibi, B.; Delnavz, N. Electrooxidation of glycerol on nickel and nickel alloy (Ni–Cu and Ni–Co) nanoparticles in alkaline media. RSC Adv. 2016, 6, 31797–31806. [Google Scholar] [CrossRef]
- Ashok, A.; Kumar, A.; Ponra, J.; Mansour, S.A.; Tarlochan, F. Single step synthesis of porous NiCoO2 for effective electrooxidation of glycerol in alkaline medium. J. Electrochem. Soc. 2018, 165, J3301–J3309. [Google Scholar] [CrossRef]
- Houache, M.S.E.; Hughes, K.; Ahmed, A.; Safari, R.; Liu, H.; Gianluigi, A.; Botton, G.A.; Baranova, E.A. Electrochemical valorization of glycerol on Ni-rich bimetallic NiPd nanoparticles: Insight into product selectivity using in situ polarization modulation infrared-reflection absorption spectroscopy. ACS Sustain. Chem. Eng. 2019, 7, 14425–14434. [Google Scholar] [CrossRef]
- Han, J.; Kim, Y.; Kim, H.W.; Jackson, D.H.K.; Lee, D.; Chang, H.; Chae, H.J.; Lee, K.Y.; Kim, H.J. Effect of atomic-layer-deposited TiO2 on carbon-supported Ni catalysts for electrocatalytic glycerol oxidation in alkaline media. Electrochem. Comm. 2017, 83, 46–50. [Google Scholar] [CrossRef]
- Pletcher, D.; Fleischmann, M.; Korinek, K. The oxidation of organic compounds at a cobalt electrode in alkaline media. J. Electroanal. Chem. 1971, 33, 478–479. [Google Scholar] [CrossRef]
- Oshitani, M.; Sasaki, Y.; Takashima, K. Development of a nickel electrode having stable performance at various charge and discharge rates over a wide temperature range. J. Power Sources 1984, 12, 219–231. [Google Scholar] [CrossRef]
- Asgari, M.; Ghannadi Maragheh, M.; Davarkhah, R.; Lohrasbi, E.; Nozad Golikand, A. Electrocatalytic oxidation of methanol on the nickel–cobalt modified glassy carbon electrode in alkaline medium. Electrochim. Acta 2012, 59, 284–289. [Google Scholar] [CrossRef]
- Cui, X.; Guo, W.; Zhou, M.; Yang, Y.; Li, Y.; Xiao, P.; Zhang, Y.; Zhang, X. Promoting effect of Co in Ni m Con (m + n = 4) bimetallic electrocatalysts for methanol oxidation reaction. ACS Appl. Mater. Int. 2015, 7, 493–503. [Google Scholar] [CrossRef]
- Poreddy, R.; Engelbrekt, C.; Riisager, A. Copper oxide as efficient catalyst for oxidative dehydrogenation of alcohols with air. Catal. Sci. Technol. 2015, 5, 2467–2477. [Google Scholar] [CrossRef] [Green Version]
- Dodekatos, G.; Tüysüz, H. Effect of post-treatment on structure and catalytic activity of CuCo-based materials for glycerol oxidation. ChemCatChem 2017, 9, 610–619. [Google Scholar] [CrossRef]
- Deng, X.; Dodekatos, G.; Pupovac, K.; Weidenthaler, C.; Schmidt, W.N.; Schuth, F.; Tüysüz, H. Pseudomorphic generation of supported catalysts for glycerol oxidation. ChemCatChem 2015, 7, 3832–3837. [Google Scholar] [CrossRef]
- Dodekatos, G.; Ternieden, J.; Schünemann, S.; Weidenthaler, C.; Tüysüz, H. Promoting effect of solvent on Cu/CoO catalyst for selective glycerol oxidation under alkaline conditions. Catal. Sci. Technol. 2018, 8, 4891–4899. [Google Scholar] [CrossRef] [Green Version]
- Schünemann, S.; Schüth, F.; Harun Tüysüz, H. Selective glycerol oxidation over ordered mesoporous copper aluminum oxide catalysts. Catal. Sci. Technol. 2017, 7, 5614–5624. [Google Scholar] [CrossRef] [Green Version]
- Xin, J.; Zhao, M.; Zeng, C.; Yan, W.; Song, Z.; Thapa, P.S.; Subramaniam, B.; Chaudari, R.V. Oxidation of glycerol to dicarboxylic acids using cobalt catalysts. ACS Catal. 2016, 6, 4576–4583. [Google Scholar]
- Xin, Y.; Shen, P.K. Tantalum carbide doped by fluorine as non-precious metal anodic electrocatalyst superior to Pt/C for glycerol-oxidation. Electrochim. Acta 2017, 227, 267–274. [Google Scholar] [CrossRef]
- Fujiwara, N.; Siroma, Z.; Yamazaki, S.; Ioroi, T.; Senoh, H.; Yasuda, K. Direct ethanol fuel cells using an anion exchange membrane. J. Power Sources 2008, 185, 621–626. [Google Scholar] [CrossRef]
- Kim, J.; Momma, T.; Osaka, T. Cell performance of Pd–Sn catalyst in passive direct methanol alkaline fuel cell using anion exchange membrane. J. Power Sources 2009, 189, 999–1002. [Google Scholar] [CrossRef]
- Cifrain, M.; Kordesch, K.V. Advances, aging mechanism and lifetime in AFCs with circulating electrolytes. J. Power Sources 2004, 127, 234–242. [Google Scholar] [CrossRef]
- Matsuoka, K.; Iriyama, Y.; Abe, T.; Matsuoka, M.; Ogumi, Z. Alkaline direct alcohol fuel cells using an anion exchange membrane. J. Power Sources 2005, 150, 27–31. [Google Scholar] [CrossRef]
- Bambagioni, V.; Bianchini, C.; Marchionni, A.; Filippi, J.; Vizza, F.; Teddy, J.; Serp, P.; Zhiani, M. Pd and Pt–Ru anode electrocatalysts supported on multi-walled carbon nanotubes and their use in passive and active direct alcohol fuel cells with an anion-exchange membrane (alcohol =methanol, ethanol, glycerol). J. Power Sources 2009, 190, 241–251. [Google Scholar] [CrossRef]
- Ilie, A.; Simoes, M.; Baranton, S.; Coutanceau, C.; Martemianov, S. Influence of operational parameters and of catalytic materials on electrical performance of Direct Glycerol Solid Alkaline Membrane Fuel Cells. J. Power Sources 2011, 196, 4965–4971. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Li, W. Electrocatalytic oxidation of glycerol on Pt/C in anion-exchange membrane fuel cell: Cogeneration of electricity and valuable chemicals. Appl. Catal. B Environ. 2012, 119, 40–48. [Google Scholar] [CrossRef]
- Zhang, Z.; Xin, L.; Li, W. Supported gold nanoparticles as anode catalyst for anion-exchange membrane-direct glycerol fuel cell (AEM-DGFC). Int. J. Hydrogen Energy 2012, 37, 9393–9401. [Google Scholar] [CrossRef]
- Zhiani, M.; Rostami, H.; Majidi, S.; Karami, K. Bis (dibenzylidene acetone) palladium (0) catalyst for glycerol oxidation in half cell and in alkaline direct glycerol fuel cell. Int. J. Hydrogen Energy 2013, 38, 5435–5441. [Google Scholar] [CrossRef]
- Han, X.; Chadderdon, D.J.; Qi, J.; Xin, L.; Li, W.; Zhou, W. Numerical analysis of anion-exchange membrane direct glycerol fuel cells under steady state and dynamic operations. Int. J. Hydrogen Energy 2014, 39, 19767–19779. [Google Scholar] [CrossRef]
- Wang, Z.; Xin, L.; Zhao, X.; Qiu, Y.; Zhang, Z.; Baturina, O.A.; Li, W. Carbon supported Ag nanoparticles with different particle size as cathode catalysts for anion exchange membrane direct glycerol fuel cells. Renew. Energy 2014, 62, 556–562. [Google Scholar] [CrossRef]
- Nunes Couto, R.; Linares, J.J. KOH-doped polybenzimidazole for alkaline direct glycerol fuel cells. J. Membrane Sci. 2015, 486, 239–247. [Google Scholar] [CrossRef]
- Nascimento, A.P.; Linares, J.J. Performance of a direct glycerol fuel cell using KOH doped polybenzimidazole as electrolyte. J. Braz. Chem. Soc. 2014, 25, 509–516. [Google Scholar] [CrossRef]
- Qi, J.; Benipal, N.; Liang, C.; Li, W. PdAg/CNT catalyzed alcohol oxidation reaction for high-performanceanion exchange membrane direct alcohol fuel cell (alcohol = methanol, ethanol, ethylene glycol and glycerol). Appl. Catal. B Environ. 2016, 199, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Benipal, N.; Qi, J.; Gentile, J.C.; Li, W. Direct glycerol fuel cell with polytetrafluoroethylene (PTFE) thin film separator. Renew. Energy 2017, 105, 647–655. [Google Scholar] [CrossRef] [Green Version]
- Frota, E.F., Jr.; Silva de Barros, V.V.; de Araujo, B.R.S.; Purgatto, A.G.; Linares, J.J. Pt/C containing different platinum loadings for use as electrocatalysts in alkaline PBI-based direct glycerol fuel cells. Int. J. Hydrogen Energy 2017, 42, 23095–23106. [Google Scholar] [CrossRef]
- Xin, L.; Zhang, Z.; Wang, Z.; Li, W. Simultaneous generation of mesoxalic acid and electricity from glycerol on a gold anode catalyst in anion-exchange membrane fuel cells. ChemCatChem 2012, 4, 1105–1114. [Google Scholar] [CrossRef]
- Olivares-Ramırez, J.M.; Dector, A.; Banuelos-Dıas, J.A.; Amaya-Cruz, D.M.; Ortiz-Verdın, A.; Jimenez-Sandoval, O.; Sabaté, N.; Esquivel, J.P. Evaluation of a passive anion-exchange membrane micro fuel cell using glycerol from several sources. Fuel Cells 2019, 19, 10–18. [Google Scholar] [CrossRef] [Green Version]
- Maya-Cornejo, J.; Ortiz-Ortega, E.; Alvarez-Contreras, L.; Arjona, N.; Guerra-Balcazar, M.; Ledesma-Garcia, J.; Arriaga, L.G. Copper–palladium core–shell as an anode in a multi-fuel membraneless nanofluidic fuel cell: Toward a new era of small energy conversion devices. Chem. Commun. 2015, 51, 2536–2539. [Google Scholar] [CrossRef] [PubMed]
- Maya-Cornejo, J.; Guerra-Balcazar, M.; Arjona, N.; Alvarez-Contreras, L.; Rodriguez Valadez, F.J.; Gurrola, M.P.; Ledesma-Garcia, J.; Arriaga, L.G. Elecrooxidation of crude glycerol as waste from biodiesel in a nanofluidic fuel cell using Cu@Pd/C and Cu@Pt/C. Fuel 2016, 183, 195–205. [Google Scholar] [CrossRef]
- Tseng, Y.J.; Scott, D. A simple, membrane-free, direct glycerol fuel cell utilizing a precious metal-free cathode and gold-plated anode surfaces. Energies 2018, 11, 2259. [Google Scholar] [CrossRef] [Green Version]
- Martins, C.A.; Ibrahim, O.A.; Pei, P.; Kjeang, E. In situ decoration of metallic catalysts in flow-through electrodes: Application of Fe/Pt/C for glycerol oxidation in a microfluidic fuel cell. Electrochim. Acta 2019, 305, 47–55. [Google Scholar] [CrossRef]
- Rasmussen, M.; Serov, A.; Artyushkova, K.; Chen, D.; Rose, T.C.; Atanassov, P.; Harris, J.M.; Minteer, S.D. Enhancement of electrocatalytic oxidation of glycerol by plasmonics. ChemElectroChem 2019, 6, 241–245. [Google Scholar] [CrossRef]
- Martins, C.A.; Ibrahim, O.A.; Pei, P.; Kjeang, E. Towards a fuel-flexible direct alcohol microfluidic fuel cell with flow-through porous electrodes: Assessment of methanol, ethylene glycol and glycerol fuels. Electrochim. Acta 2018, 271, 537–543. [Google Scholar] [CrossRef]
- Tran, K.; Nguyen, T.Q.; Bartron, A.M.; Sadiki, A.; Haan, J.L. A Fuel-Flexible Alkaline Direct Liquid Fuel Cell. Fuel Cells 2014, 14, 834–841. [Google Scholar] [CrossRef]
- Zakaria, K.; McKay, M.; Thimmappa, R.; Hasan, M.; Mamlouk, M.; Scott, K. Direct Glycerol Fuel Cells: Comparison with Direct Methanol and Ethanol Fuel Cells. ChemElectroChem 2019, 6, 2578–2585. [Google Scholar] [CrossRef]
- Caselli, G.; Mantovanini, M.; Gandolfi, C.A.; Allegretti, M.; Fiorentino, S.; Pellegrini, L.; Melillo, G.; Bertini, R.; Sabbatini, W.; Anacardio, R.; et al. Tartronates: A new generation of drugs affecting bone metabolism. J. Bone Miner. Res. 1997, 12, 972–981. [Google Scholar] [CrossRef] [PubMed]
- Kosaka, K.; Akanuma, Y. Historical changes in diabetes therapy in Japan. Diabetes Res. Clin. Pract. 1994, 24, S221–S227. [Google Scholar] [CrossRef]
- Davis, W.R.; Tomsho, J.W.; Nikam, S.; Peliska, J. Inhibition of HIV-1 reverse transcriptase-catalyzed DNA strand transfer reactions by 4-chlorophenylhydrazone of mesoxalic acid. Biochemistry 2000, 39, 14279–14291. [Google Scholar] [CrossRef]
- Qi, J.; Xin, L.; Chadderdon, D.J.; Qiu, Y.; Jiang, Y.; Benipal, N.; Liang, C.; Li, W. Electrocatalytic selective oxidation of glycerol to tartronate on Au/C anode catalysts in anion exchange membrane fuel cells with electricity cogeneration. Appl. Catal. B Environ. 2014, 154, 360–368. [Google Scholar] [CrossRef]
- Pan, Z.F.; Chen, R.; An, L.; Li, Y.S. Alkaline anion exchange membrane fuel cells for cogeneration of electricity and valuable chemicals. J. Power Sources 2017, 365, 430–445. [Google Scholar] [CrossRef]











| Catalyst | GOR Activity | Selectivity (E,V) | Reference |
|---|---|---|---|
| Pt1−xAux@Ag, x: 0.3–0.8 | Pt1−xAux > Pt/C, max: x = 0.60 | C3 (0.5, 0.7, 0.9 and 1.3 V vs. RHE) | [20] |
| Pt1−xAux, x: 0.10–0.75 | Pt1−xAux > Pt, max: x = 0.35 | - | [39] |
| Pt1−xAux, x: 0.10–0.75 | Pt1−xAux > Pt, max: x = 0.85 | LA (0.45–0.6 V vs. RHE) | [29] |
| Pt1−xAux/C, x: 0.15–0.33 | Pt1−xAux/C > Pt/C, max: x = 0.20 | - | [40] |
| Pt1−xAux /C, x: 0.10–0.50 | Pt1−xAux/C > Pt/C, max: x = 0.50 | - | [41] |
| PtAu/C | PtAu/C Pt/C | - | [42] |
| Pd1−xAux/C, x: 0.15–0.33 | Pd1−xAux/C > Pd/C, max: x = 0.20 | - | [40] |
| Pd1−xAux, x: 0.5, 0.7 | Pd1−xAux > Pd, max: x = 0.50 | - | [18] |
| Pd0.7Au0.3 | Pd0.7Au0.3 > Pd | - | [43] |
| Pd0.5Au0.5/C | Pd0.5Au0.5/C > Pd/C | - | [44] |
| Pd1−xAux/C, x: 0.25, 0.5, 0.75 | Pd1−xAux/C > Pd/C, max: x = 0.50 | C2,C1 (−0.4–0.05 V vs. Ag/AgCl) | [45] |
| Pd1−xAux/VGNCF, x: 0.25, 0.33, 0.50 | Pd1−xAux/VGNCF > Pd/VGCNF, max = 0.50 | - | [47] |
| PdAu/NPSS | PdAu/NPSS > Pd/NPSS | - | [48] |
| Pd1−xAux/P-Se-C, x: 0.25, 0.33, 0.50 | Pd1−xAux/P-Se-C > Pd/P-Se-C, max = 0.50 | - | [49] |
| Pd1−xAux/C, x: 0.25, 0.5 | Pd1−xAux/C > Pd/C, max: x = 0.50 | - | [50] |
| Catalyst | GOR Activity | Selectivity (E,V) | Reference |
|---|---|---|---|
| PtRh/GNS, PtRhNi/GNS | PtRh/GNS, PtRhNi/GNS > Pt/GNS | C2 (−0.4 V vs. SCE) | [52] |
| PtNi/GNS | PtNi/GNS > Pt/GNS | TA (−0.4 V vs. SCE) | [52] |
| PtNi(OH)2/C | PtNi(OH)2/C > Pt/C | MOA,GA,FA (−011 V to 0.43 V vs. NHE) | [21] |
| PtNiO/Ti | Pt0.8Ni0.2O/Ti > Pt/Ti, Pt0.5Ni0.5O/Ti, Pt0.2Ni0.8O/Ti < Pt/Ti, | - | [53] |
| PtCo/CNT | PtCo/CNT > Pt/CNT | - | [54] |
| PtRu/GNS | PtRu/GNS > Pt/GNS | C3 (−0.4 V vs. SCE) | [52] |
| PtCu | Pt1Cu1, Pt7Cu3 > Pt, Pt9Cu1 < Pt | - | [55] |
| PtAg/C, PtAg/MnOx/C | PtAg/MnOx/C > PtAg/C > Pt/C | C1 (0.5–0.6 V vs. RHE) | [56] |
| Pt9Bi1/C | Pt9Bi1/C > Pt/C | - | [57] |
| PtBi | PtBi > Pt | C3 (0.25–0.60 V vs. RHE) | [58] |
| Pt9Bi1/C | Pt9Bi1/C > Pt/C | C3 (0.3–0.7 V vs. RHE) | [59] |
| PtBi | PtBi > Pt | GLA (0.7–0.85 V vs. RHE) | [60] |
| PtCeO2/C | PtCeO2/C > Pt/C | - | [61] |
| PtCeO2/C | PtCeO2/C > Pt/C | C3 (−0.4 V vs. SCE) | [22] |
| PtCuCo | PtCuCo > PtCu > PtCo > Pt/C | - | [62] |
| PtCoNi/C | PtCoNi/C > PtCo/C, PtNi/C > Pt/C | - | [63] |
| PdRh | PdRh > Pd | CO32− (0.3–0.9 V vs. RHE) | [64] |
| Pd3Ru/NC | Pd3Ru/NC > Pd/NC | - | [65] |
| Pd3Ag1/C, Pd1Ag1/C | Pd3Ag1/C, Pd1Ag1/C >Pd/C | - | [50] |
| Pd9Cu1/C | Pd9Cu1/C >Pd/C | - | [66] |
| Pd3Cu/NMC | Pd3Cu/NMC/Pd/C | - | [67] |
| PdCu/C | PdCu/C > Pd/C | - | [68] |
| PdAg, PdAg3 | PdAg, PdAg3 > Pd/C | C2 (n.d.) | [13] |
| PdCo/Au | PdCo/Au > Pd/Au | - | [69] |
| PdMo/C | PdMo/C > Pd/C | - | [70] |
| PdNi/C | PdNi/C > Pd/C | - | [18] |
| PdNi/C | PdNi/C > Pd/C | - | [71] |
| Pd9Bi1/C | Pt/C = Pd9Bi1/C > Pd/C | - | [57] |
| PdBi | PdBi > Pd | - | [58] |
| PdBi | PdBi > Pd | DHA (0.35–0.7 V vs. RHE) CO32−, HPA (0.7–1.0 V vs. RHE) | [26] |
| Pd1Sn1, Pd1Sn2, Pd1Sn3 | Pd1Sn1, Pd1Sn2 > Pd, Pd1Sn3 < Pd | C3 (0.5–0.9 V vs. RHE) | [72] |
| PdMOx/C, (MOx = CeO2, NiO, Co3O4, Mn3O4) | PdMxOy > Pd/C | - | [73] |
| PdRuCo | PdRuCo > Pd/C | - | [74] |
| PdAu/C, PdSn/C, PdAuSn/C | PdAu/C = Pd5Au4Sn1/C > Pd5Au1Sn4/C > Pd PdSn/C < Pd/C | - | [44] |
| PdNiOP/C | PdNiOP/C > Pd/C | - | [75] |
| FeCo@Fe@Pd/C | FeCo@Fe@Pd/C > Pd/C | - | [76] |
| PdM (M = Sb, Sn, or Pb) | PdM > Pd | - | [77] |
| PdIr | PdIr lower onset potential and j than Pd | CO32− (0.5–1.0 V vs. RHE) | [78] |
| AuAg/C | AuAg/C > Au/C | - | [79] |
| AuAg/C | AuAg/C < Au/C | C1 (0.5–1.6 V vs. RHE) | [28] |
| AuAg | AuAg/C > Au/C | C1,C2 (n.d.) | [80] |
| AuAg/C (5–30 wt% Au) | 5, 10 wt% AuAg > Au, 30 wt% AuAg < Au | - | [81] |
| Au/Ni | Au/Ni > Au | - | [82] |
| Au2Ni/C | Au2Ni/C > Au/C | - | [83] |
| Au/Cu/C | n.d. | C3 (GLA,TA; 0.0–0.1 V vs. Ag/AgCl) | [84] |
| AuCu | AuCu > Au | - | [85] |
| AuMnO2/C | AuMnO2(5,9%)/C > Au, AuMnO2(16,23%)/C < Au | - | [86] |
| AuCeO2/C | AuCeO2C > Au/C | - | [87] |
| Au-Co3O4, -NiO, -Mn3O4 and -MgO/C | Au-Co3O4, -NiO, -Mn3O4 and -MgO/C >Au/C | - | [88] |
| Catalyst | GOR Activity | Selectivity (E,V) | Reference |
|---|---|---|---|
| electrochemically treated Ni | Treated Ni > untreated Ni | GALD (0.34–0.54 V vs. Hg/HgO) | [97] |
| Ni-boron doped diamond | Ni-BDD > bulk Ni macro electrode | - | [98] |
| pulse electrodeposited Ni-Ca, Ni-TBr | pulse electrodeposited Ni-Ca, Ni-TBr > bare Ni | - | [100] |
| poly[Ni(salen)] encapsulated Ni(OH)2 nanoparticles | - | FA (n.d.) | [101] |
| NiCo/C | NiCo/C > Ni/C | C2,C1 (1.3–1.9 V vs. RHE) | [102] |
| NiCo/C, NiFe/C | NiCo > Ni/C, NiFe/C < Ni/C | C2,C1(1.2–1.9 V vs. RHE) | [103] |
| NiCo/CCE, NiCu/CCE | NiCo/CCE, NiFe/CCE > Ni/CCE | - | [104] |
| NiCoO2 | NiCoO2 > NiO, Co3O4 | - | [105] |
| NiPd | NiPd > Ni | GALD (0.34 V vs. Hg/HgO) | [106] |
| Ni-TiO2/C | Ni-TiO2/C > Ni/C | - | [107] |
| CuCo | - | C2,C1 (n.d.) | [113] |
| Cu/CoO | - | C2,C1 (n.d.) | [114,115] |
| Ordered mesoporous Cu-Al2O3 | Cu-Al2O3 > Al2O3, Ordered Cu-Al2O3 > non-ordered Cu-Al2O3 | C2,C1 (n.d.) | [116] |
| Co/Mg3Al(OH)y(CO3)z | - | TA (64%), OXA (24%) (n.d.) | [117] |
| TaCxFyOz/C | TaCxFyOz/C > Pt | - | [118] |
| Fuel | Anode | Cathode | Electrolyte Membrane | Temp. °C | MPD mW cm−2 | Refs |
|---|---|---|---|---|---|---|
| 1 M GLY/1 M KOH | PtRu/C | Pt/C | Koei Chemical Co., M.W. ca. 102,000 4-VP | 50 | 6.7 | [122] |
| 5 wt% Gly/2 M KOH | Pd/CNT | Fe-Co HypermecTM K14 | Tokuyama A-006 | 20–22, 25, 40, 60, 80 | 6 (passive ADGFC), 16, 35, 55,78 | [123] |
| 1 M GLY/2–6 M NaOH, 2 M Gly/2–6 M NaOH, 3 M Gly/2–6 M NaOH, 1 M Gly/4 M NaOH, 1 M Gly/4 M NaOH | Pt/C, Pt9Bi1/C, Pd/C, Pd9Bi1/C, Pt5Pd5/C | Pt/C | ADP® Solvay, Fumapem® FAA Fumatech | 25 | 8–11, 9–7, 4–0, 8, 10, 5.5, 6, 8.5 | [124] |
| 1 M Gly/2 M KOH | Pt/C | Fe-Cu-N4/C HypermecTM | Tokuyama A201 | 50, 80 | 59, 125 | [125] |
| 1 M Gly/2 M KOH | Au/C | Fe-Cu-N4/C HypermecTM | Tokuyama A201 | 50, 60, 70, 80 | 18, 26, 37, 58 | [126] |
| 1 M Gly/2 M KOH | Pt/C, Pd/C, Au/C | HypermecTM (Fe-Cu-N4/C, Acta) | Tokuyama A201 | 50, 80, 80, 50, 80, 80, 50, 80, 80 | 59, 125, 121 (crude Gly), 38, 72, 61 (crude Gly), 18, 58, 31 (crude Gly) | [33] |
| 5 wt% Gly/2 M KOH | Pd(NiZn)/C | Fe-Co/C | Tokuyama A-201 | 25, 80 | 17 (passive ADGFC), 119 | [23] |
| 5 wt% Gly/10 wt% KOH | Pd(DBA)2 | Fe-Co HypermecTM K14 | Tokuyama A-006 | 25 | 24 (passive ADGFC) | [127] |
| 3 M Gly/6 M KOH | PtCo/CNT, Pt/CNT | Fe-Cu-N4/C HypermecTM | Tokuyama A901 | 80 | 285, 269 (crude Gly), 229 (crude Gly) | [54] |
| 1 M Gly/8 M KOH | Au/C | Fe-Cu-N4/C HypermecTM | Tokuyama A901 | 60 | 45 | [128] |
| 1 M Gly/2 M KOH | Pt/C | Ag/C, nanocapsule, Ag/C | Tokuyama A-201 | 80 | 86, 66 (crude Gly), 45, 38 (crude Gly) | [129] |
| 2 M Gly/2 M KOH | Pd/C, Pd1Au1C, Pd1Sn1/C, Pd5Au4Sn1/C | Pd/C | Fumasep-FAA3-PEEK | 85 (max. values) | 34, 28, 17, 51 | [44] |
| 1 M Gly/4 M KOH | Pt3Sn/C | Pt/C | PBI/KOH | 30, 45, 60, 75 | 8, 17, 22, 34 | [130] |
| 1 M Gly/4 M KOH | Pt/C | Pt/C | PBI/KOH | 30, 45, 60, 75, 90 | 5, 8, 12, 18, 16 | [131] |
| 1 M Gly/2 M KOH | Pd87Cu13/C, Pd/C | Pt black | Tokuyama A-201 | 60 | 70, 40 | [66] |
| 3 M Gly/6 M KOH | Pd/CNT, PdAg/CNT | Fe-Cu-N4/C HypermecTM | Tokuyama A901 | 80 | 180, 276 | [132] |
| 1 M Gly/6 M KOH, 3 M Gly/6 M KOH | PdAg/CNT | Fe-Cu-N4/C HypermecTM | PTFE (225 μm), PTFE (0.45 μm) | 80 | 130, 227 | [133] |
| 1 M Gly/2 M KOH | Pd/CNT, PdAg/CNT, PdAg3/CNT | Fe-Cu-N4/C HypermecTM | Tokuyama A-201 | 60 | 54, 77, 70 | [13] |
| 1 M Gly/4 M KOH | Pt/C 20%, Pt/C 30%, Pt/C 40%, Pt/C 60% | Pt/C | PBI/KOH | 60 | 20, 23, 27, 36 | [134] |
| 1 M Gly/1 M KOH | Pt/C, Pt9Cu1/C, Pt7Cu3/C, Pt1Cu1/C | Pt/C | KOH treated Nafion | 90 | 8, 18, 16, 11 | [55] |
| 2 M Gly/5 M NaOH | PdAu/CNF, Pd black | Pt/C | Fumasep FAA-3-PK-130 | 25 | 7 (passive DGFC), 5.8 | [47] |
| 1 M Gly/4 M KOH | PtAgMnO/C, PtAg/C, Pt/C | Pt/C | PBI | 60, 90, 60, 90, 60, 90 | 46, 103, 34, 77, 23, 59 | [56] |
| 1 M Gly/21 M KOH | Au/C, Pt/C | Fe-Cu-N4/C | Tokuyama A-201 | 50, 80, 50 | 18, 58, 60 | [135] |
| 0.1 M Gly/0.3 M KOH (micro fuel cell) | PtRu/C | Pt/C | Tokuyama A-201 | 25 | 1.01 (passiveADGFC), 0.94 (saponif. GLy), 0.86 (crude Gly) | [136] |
| 0.1 M Gly/0.3 M KOH | Cu@Pd/C | Pt/C | None | 25 | 20.4 | [137] * |
| 5 vol%Gly/0.3 M | Cu@Pd/C, Cu@Pt/C | Pt/C | None | 25 | 17.4, 23.2 | [138] * |
| 1.4 M Gly/8 M KOH | Au-plated Pt | Ag-plated Ni | None | 25 | 1.3 | [139] * |
| 0.05 M Gly/1 M KOH | Pt/C | Pt/C, 0.15% Fe Pt/C | None | 25 | 39, 54 | [140] * |
| 0.1 M Gly/0.1 M NaOH | AgAu and, TBAB-modified Nafion | Pt on CC | Nafion 212 | 25 | 7 × 10−3 (dark), 15 × 10−3 (light) | [141] ** |
| Anode Catalyst | Fuel | Temp. °C | Cell Potential V | Selectivity % | Power Density mW cm−2 | Refs |
|---|---|---|---|---|---|---|
| Au/C-NC (1 mgAu cm−2) | 1 M Gly/2 M KOH | 50 | 0.5 | 75 C3, 49 TA | 2 (t = 0 h) | [126] |
| 0.3 | 75 C3, 39 TA | 10 | ||||
| 0.1 | 78 C3, 37 TA | 15 | ||||
| Au/C-NC (1 mgAu cm−2) | 1 M Gly/8 M KOH | 60 | 0.1 | 89 C3, 69 TA | 15 (t = 0 h) | [148] |
| Au/C-AQ (1 mgAu cm−2) | 85 C3, 64 TA | 20 | ||||
| Au/C-NC (5 mgAu cm−2) | 1 M Gly/2 M KOH | 50 | 0.5 | 73 C3, 32 TA | 6 (t = 0 h) | [135] |
| 0.3 | 78 C3, 46 MOA | 23 | ||||
| 0.1 | 70 C3, 34 MOA | 14 | ||||
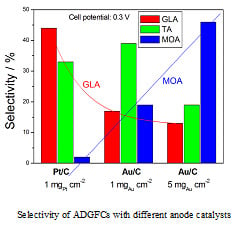
| Pt/C (1 mgPt cm−2) | 1 M Gly/2 M KOH 1 M Gly/4 M KOH 1 M Gly/0.5 MKOH 0.1 M Gly/2 M KOH | 50 | 0.7 0.5 0.3 0.1 0.7 0.5 0.3 0.7 0.5 0.3 0.7 0.5 0.3 | 84 C3, 47 GLA 81 C3, 41 GLA 79 C3, 44 GLA 70 C3, 34 GLA 83 C3, 46 GLA 87 C3, 45 TA 85 C3, 42 TA 78 C3, 44 GLA 71 C3, 38 GLA 70 C3, 49 GLA 91 C3, 50 TA 76 C3, 40 GLA 62 C3, 34 TA | 5 (averaged 2 h) 25 48 33 5 27 58 1 9 21 1 8 17 | [125] |
| Pt/C (20 wt%; 2 mgPt cm−2) | 1 M Gly/4 M KOH | 60 | 0.4 | 80 C3, 75 TA | 20 | [134] |
| 90 | 0.5 | 72 C3, 67 TA | 24 | |||
| 90 | 0.3 | 63 C3, 60 TA | 30 | |||
| Pd/CNT (3 mgPt cm−2) | 1 M Gly/4 M KOH | 60 | 0.1 | 66 C3, 40 TA | 12 (t = 0) | [13] |
| PdAg/CNT (3 mgPt cm−2) | 0.1 | 57 C2, 36 OA | 12 | |||
| PdAg3/CNT(3 mgPt cm−2) | 0.1 | 77 C2, 39 OA | 12 |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Antolini, E. Glycerol Electro-Oxidation in Alkaline Media and Alkaline Direct Glycerol Fuel Cells. Catalysts 2019, 9, 980. https://doi.org/10.3390/catal9120980
Antolini E. Glycerol Electro-Oxidation in Alkaline Media and Alkaline Direct Glycerol Fuel Cells. Catalysts. 2019; 9(12):980. https://doi.org/10.3390/catal9120980
Chicago/Turabian StyleAntolini, Ermete. 2019. "Glycerol Electro-Oxidation in Alkaline Media and Alkaline Direct Glycerol Fuel Cells" Catalysts 9, no. 12: 980. https://doi.org/10.3390/catal9120980