Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats
Abstract
:1. Introduction
2. Materials and Methods
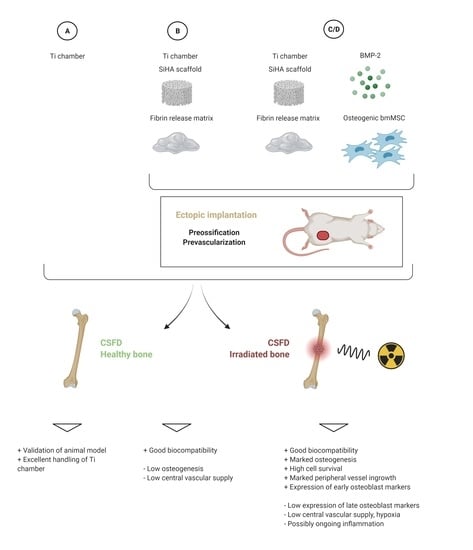
2.1. Experimental Design
2.2. Animals
2.3. Isolation and Culture of Mesenchymal Stem Cells
2.4. Preparation of Donors
2.5. Irradiation of Acceptor Animals
2.6. CSFD Surgery
Explantation of Chambers and Processing of Constructs
2.7. RNA Isolation and Gene Expression Analysis
2.8. Microcomputertomography
2.9. Histological Processing
2.10. Statistics
3. Results
3.1. Evaluation of the Animal Model
3.2. Bone Formation
3.3. Vascularization
3.4. Cell Survival
3.5. Gene Expression Analysis
4. Discussion
4.1. Animal Model
4.2. Gene Expression Analysis
4.3. Bone Formation
4.4. Vascularization and Cell Survival
4.5. Inflammation
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. Supplemental Information: Dosages of Medication for Acceptor and Donor Animals
Appendix A.1.1. Implantation of Titanium Chamber for Preossification/Prevascularization
Appendix A.1.2. Irradiation of Acceptor Animals
Appendix A.1.3. CSFD Surgery
References
- Toogood, P.; Miclau, T. Critical-Sized Bone Defects: Sequence and Planning. J. Orthop. Trauma 2017, 31, S23–S26. [Google Scholar] [CrossRef]
- Landau, M.J.; Badash, I.; Yin, C.; Alluri, R.K.; Patel, K.M. Free vascularized fibula grafting in the operative treatment of malignant bone tumors of the upper extremity: A systematic review of outcomes and complications. J. Surg. Oncol. 2018, 117, 1432–1439. [Google Scholar] [CrossRef] [PubMed]
- Niu, Y.; Bai, Y.; Xu, S.; Wu, D.; Liu, X.; Wang, P.; Zhang, C.; Li, M. Treatment of bone nonunion and bone defects associated with unsuccessful humeral condylar fracture repair with autogenous iliac bone reconstruction. J. Shoulder Elb. Surg. 2012, 21, 985–991. [Google Scholar] [CrossRef]
- Qiu, X.-S.; Chen, Y.-X.; Qi, X.-Y.; Shi, H.-F.; Wang, J.-F.; Xiong, J. Outcomes of cement beads and cement spacers in the treatment of bone defects associated with post-traumatic osteomyelitis. BMC Musculoskelet. Disord. 2017, 18, 256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Moucha, C.S.; Renard, R.L.; Gandhi, A.; Lin, S.S.; Tuan, R.S. Bone Allograft Safety and Performance. In Engineering of Functional Skeletal Tissue; Bronner, F., Farach-Carson, M.C., Mikos, A.G., Eds.; Springer: London, UK, 2007; pp. 46–54. [Google Scholar]
- Colnot, C. Cell Sources for Bone Tissue Engineering: Insights from Basic Science. Tissue Eng. Part B Rev. 2011, 17, 449–457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verboket, R.; Irrle, T.; Busche, Y.; Schaible, A.; Schröder, K.; Brune, J.; Marzi, I.; Nau, C.; Henrich, D. Fibrous Demineralized Bone Matrix (DBM) Improves Bone Marrow Mononuclear Cell (BMC)-Supported Bone Healing in Large Femoral Bone Defects in Rats. Cells 2021, 10, 1249. [Google Scholar] [CrossRef] [PubMed]
- McGovern, J.A.; Griffin, M.; Hutmacher, D.W. Animal models for bone tissue engineering and modelling disease. Dis. Model. Mech. 2018, 11, dmm033084. [Google Scholar] [CrossRef] [Green Version]
- Arkudas, A.; Lipp, A.; Buehrer, G.; Arnold, I.; Dafinova, D.; Brandl, A.; Beier, J.P.; Körner, C.; Lyer, S.; Alexiou, C.; et al. Pedicled Transplantation of Axially Vascularized Bone Constructs in a Critical Size Femoral Defect. Tissue Eng. Part A 2018, 24, 479–492. [Google Scholar] [CrossRef] [PubMed]
- Rottensteiner-Brandl, U.; Distel, L.; Stumpf, M.; Fey, T.; Köhn, K.; Bertram, U.; Lingens, L.F.; Greil, P.; Horch, R.E.; Arkudas, A. Influence of Different Irradiation Protocols on Vascularization and Bone Formation Parameters in Rat Femora. Tissue Eng. Part C Methods 2017, 23, 583–591. [Google Scholar] [CrossRef]
- Arkudas, A.; Tjiawi, J.; Saumweber, A.; Beier, J.P.; Polykandriotis, E.; Bleiziffer, O.; Horch, R.E.; Kneser, U. Evaluation of blood vessel ingrowth in fibrin gel subject to type and concentration of growth factors. J. Cell. Mol. Med. 2009, 13, 2864–2874. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, H.d.A.; Villar, R.C. Radiotherapy and immune response: The systemic effects of a local treatment. Clinics 2018, 73. [Google Scholar] [CrossRef] [PubMed]
- Ponnaiya, B.; Amundson, S.A.; Ghandhi, S.A.; Smilenov, L.B.; Geard, C.R.; Buonanno, M.; Brenner, D.J. Single-cell responses to ionizing radiation. Radiat. Environ. Biophys. 2013, 52, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Banda, M.; Bommineni, A.; Thomas, R.A.; Luckinbill, L.S.; Tucker, J.D. Evaluation and validation of housekeeping genes in response to ionizing radiation and chemical exposure for normalizing RNA expression in real-time PCR. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 649, 126–134. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xu, L.; Wang, J.; Bai, J.; Zhai, J.; Zhu, G. Radiation induces primary osteocyte senescence phenotype and affects osteoclastogenesis in vitro. Int. J. Mol. Med. 2021, 47, 76. [Google Scholar] [CrossRef]
- Su, X.; Yao, X.; Sun, Z.; Han, Q.; Zhao, R.C. Optimization of Reference Genes for Normalization of Reverse Transcription Quantitative Real-Time Polymerase Chain Reaction Results in Senescence Study of Mesenchymal Stem Cells. Stem Cells Dev. 2016, 25, 1355–1365. [Google Scholar] [CrossRef]
- González-Bermúdez, L.; Anglada, T.; Genescà, A.; Martín, M.; Terradas, M. Identification of reference genes for RT-qPCR data normalisation in aging studies. Sci. Rep. 2019, 9, 8570. [Google Scholar] [CrossRef] [Green Version]
- Buehrer, G.; Balzer, A.; Arnold, I.; Beier, J.P.; Körner, C.; Bleiziffer, O.; Brandl, A.; Weis, C.; Horch, R.E.; Kneser, U.; et al. Combination of BMP2 and MSCs Significantly Increases Bone Formation in the Rat Arterio-Venous Loop Model. Tissue Eng. Part A 2015, 21, 96–105. [Google Scholar] [CrossRef] [Green Version]
- Coughlan, M.; Davies, M.; Mostert, A.K.; Nanda, D.; Willems, P.C.; Rosenberg, G.; Ferch, R. A Prospective, Randomized, Multicenter Study Comparing Silicated Calcium Phosphate versus BMP-2 Synthetic Bone Graft in Posterolateral Instrumented Lumbar Fusion for Degenerative Spinal Disorders. Spine 2018, 43, E860–E868. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.S.; Yang, S.-S.; Yoon, J.-H.; Lee, J. Enhanced bone regeneration by silicon-substituted hydroxyapatite derived from cuttlefish bone. Clin. Oral Implants Res. 2017, 28, 49–56. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Calcium Phosphate-Based Osteoinductive Materials. Chem. Rev. 2008, 108, 4742–4753. [Google Scholar] [CrossRef]
- LeGeros, R.Z. Properties of Osteoconductive Biomaterials: Calcium Phosphates. Clin. Orthop. Relat. Res. 2002, 395, 81–98. [Google Scholar] [CrossRef] [PubMed]
- Coathup, M.J.; Samizadeh, S.; Fang, Y.S.; Buckland, T.; Hing, K.A.; Blunn, G.W. The Osteoinductivity of Silicate-Substituted Calcium Phosphate. J. Bone Jt. Surg. Am. Vol. 2011, 93, 2219–2226. [Google Scholar] [CrossRef] [PubMed]
- Costa, S.; Reagan, M.R. Therapeutic Irradiation: Consequences for Bone and Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drake, M.T.; Clarke, B.L.; Khosla, S. Bisphosphonates: Mechanism of Action and Role in Clinical Practice. Mayo Clin. Proc. 2008, 83, 1032–1045. [Google Scholar] [CrossRef] [Green Version]
- Krempien, R.; Huber, P.E.; Harms, W.; Treiber, M.; Wannenmacher, M.; Krempien, B. Combination of early bisphosphonate administration and irradiation leads to improved remineralization and restabilization of osteolytic bone metastases in an animal tumor model. Cancer 2003, 98, 1318–1324. [Google Scholar] [CrossRef]
- Costa, S.; Fairfield, H.; Reagan, M.R. Inverse correlation between trabecular bone volume and bone marrow adipose tissue in rats treated with osteoanabolic agents. Bone 2019, 123, 211–223. [Google Scholar] [CrossRef]
- Costa, S.; Fairfield, H.; Farrell, M.; Murphy, C.S.; Soucy, A.; Vary, C.; Holdsworth, G.; Reagan, M.R. Sclerostin antibody increases trabecular bone and bone mechanical properties by increasing osteoblast activity damaged by whole-body irradiation in mice. Bone 2021, 147, 115918. [Google Scholar] [CrossRef]
- Chandra, A.; Lin, T.; Zhu, J.; Tong, W.; Huo, Y.; Jia, H.; Zhang, Y.; Liu, X.S.; Cengel, K.; Xia, B.; et al. PTH1–34 Blocks Radiation-induced Osteoblast Apoptosis by Enhancing DNA Repair through Canonical Wnt Pathway. J. Biol. Chem. 2015, 290, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Botelho, C.; Brooks, R.; Best, S.; Lopes, M.A.; Santos, J.D.; Rushton, N.; Bonfield, W. Human osteoblast response to silicon-substituted hydroxyapatite. J. Biomed. Mater. Res. Part A 2006, 79, 723–730. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Stensløkken, K.-O.; Vaage, I.J. Osteoblast Differentiation at a Glance. Med. Sci. Monit. Basic Res. 2016, 22, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Rubin, D.B.; Griem, M.L. The Radiation Biology of the Vascular Endothelium; CRC Press: Boca Raton, FL, USA, 1998. [Google Scholar]
- Arkudas, A.; Pryymachuk, G.; Beier, J.P.; Weigel, L.; Körner, C.; Singer, R.F.; Bleiziffer, O.; Polykandriotis, E.; Horch, R.E.; Kneser, U. Combination of Extrinsic and Intrinsic Pathways Significantly Accelerates Axial Vascularization of Bioartificial Tissues. Plast. Reconstr. Surg. 2012, 129, 55e–65e. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Wan, Q.; Ye, X.; Cheng, Y.; Pathak, J.L.; Li, Z. Cellular hypoxia promotes osteogenic differentiation of mesenchymal stem cells and bone defect healing via STAT3 signaling. Cell. Mol. Biol. Lett. 2019, 24, 64. [Google Scholar] [CrossRef] [PubMed]
- Mayer, H.; Bertram, H.; Lindenmaier, W.; Korff, T.; Weber, H.; Weich, H. Vascular endothelial growth factor (VEGF-A) expression in human mesenchymal stem cells: Autocrine and paracrine role on osteoblastic and endothelial differentiation. J. Cell. Biochem. 2005, 95, 827–839. [Google Scholar] [CrossRef] [PubMed]
- Mutschall, H.; Winkler, S.; Weisbach, V.; Arkudas, A.; Horch, R.E.; Steiner, D. Bone tissue engineering using adipose-derived stem cells and endothelial cells: Effects of the cell ratio. J. Cell. Mol. Med. 2020, 24, 7034–7043. [Google Scholar] [CrossRef]
- Hong, H.S.; Kim, S.; Jin, Y.; Son, Y. Substance P enhances the therapeutic effect of MSCs by modulating their angiogenic potential. J. Cell. Mol. Med. 2020, 24, 12560–12571. [Google Scholar] [CrossRef]
- Sasaki, J.-I.; Hashimoto, M.; Yamaguchi, S.; Itoh, Y.; Yoshimoto, I.; Matsumoto, T.; Imazato, S. Fabrication of Biomimetic Bone Tissue Using Mesenchymal Stem Cell-Derived Three-Dimensional Constructs Incorporating Endothelial Cells. PLoS ONE 2015, 10, e0129266. [Google Scholar] [CrossRef] [Green Version]
- Winkler, S.; Mutschall, H.; Biggemann, J.; Fey, T.; Greil, P.; Körner, C.; Weisbach, V.; Meyer-Lindenberg, A.; Arkudas, A.; Horch, R.E.; et al. Human Umbilical Vein Endothelial Cell Support Bone Formation of Adipose-Derived Stem Cell-Loaded and 3D-Printed Osteogenic Matrices in the Arteriovenous Loop Model. Tissue Eng. Part A 2021, 27, 413–423. [Google Scholar] [CrossRef]
- Li, Q.; Yu, T.; Wang, F.; Liu, X.; Wang, Z. Endothelial progenitor cells with stem cells enhance osteogenic efficacy. Am. J. Transl. Res. 2020, 12, 2409–2424. [Google Scholar]
- Glass, G.E.; Chan, J.K.; Freidin, A.; Feldmann, M.; Horwood, N.; Nanchahal, J. TNF- promotes fracture repair by augmenting the recruitment and differentiation of muscle-derived stromal cells. Proc. Natl. Acad. Sci. USA 2011, 108, 1585–1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, J.K.; Glass, G.E.; Ersek, A.; Freidin, A.; Williams, G.A.; Gowers, K.; Santo, A.I.E.; Jeffery, R.; Otto, W.R.; Poulsom, R.; et al. Low-dose TNF augments fracture healing in normal and osteoporotic bone by up-regulating the innate immune response. EMBO Mol. Med. 2015, 7, 547–561. [Google Scholar] [CrossRef]
- Karnes, J.M.; Daffner, S.D.; Watkins, C.M. Multiple roles of tumor necrosis factor-alpha in fracture healing. Bone 2015, 78, 87–93. [Google Scholar] [CrossRef]
- Maruyama, M.; Rhee, C.; Utsunomiya, T.; Zhang, N.; Ueno, M.; Yao, Z.; Goodman, S.B. Modulation of the Inflammatory Response and Bone Healing. Front. Endocrinol. 2020, 11, 386. [Google Scholar] [CrossRef] [PubMed]
- Rottensteiner, U. Knochen-Tissue-Engineering im Critical Sized Femurdefektmodell der Ratte nach Bestrahlung; Universitätsbibliothek: Gießen, Germany, 2016. [Google Scholar]










| Group | Prevascularization/Preossification in Donors | Chamber Content | # of Donor Animals | Irradiation of Acceptors | Vasc/oss in CSFD | # of Acceptor Animals |
|---|---|---|---|---|---|---|
| A | No | empty | 0 | no | 12 weeks | 7 |
| A-irr | No | empty | 0 | yes (4 weeks before CSFD) | 12 weeks | 10 |
| B | yes (6 weeks) | Sc/Fib | 11 | no | 12 weeks | 11 |
| B-irr | yes (6 weeks) | Sc/Fib | 10 | yes (4 weeks before CSFD) | 12 weeks | 10 |
| C | yes (6 weeks) | Sc/Fib/BMP/MSC | 9 | no | 12 weeks | 10 |
| C-irr | yes (6 weeks) | Sc/Fib/BMP/MSC | 10 | yes (4 weeks before CSFD) | 12 weeks | 10 |
| D | yes (6 weeks) | Sc/Fib/BMP/MSC | 10 | no | 10 days | 9 |
| D-irr | yes (6 weeks) | Sc/Fib/BMP/MSC | 10 | yes (4 weeks before CSFD) | 10 days | 10 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rottensteiner-Brandl, U.; Bertram, U.; Lingens, L.F.; Köhn, K.; Distel, L.; Fey, T.; Körner, C.; Horch, R.E.; Arkudas, A. Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats. Cells 2021, 10, 2256. https://doi.org/10.3390/cells10092256
Rottensteiner-Brandl U, Bertram U, Lingens LF, Köhn K, Distel L, Fey T, Körner C, Horch RE, Arkudas A. Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats. Cells. 2021; 10(9):2256. https://doi.org/10.3390/cells10092256
Chicago/Turabian StyleRottensteiner-Brandl, Ulrike, Ulf Bertram, Lara F. Lingens, Katrin Köhn, Luitpold Distel, Tobias Fey, Carolin Körner, Raymund E. Horch, and Andreas Arkudas. 2021. "Free Transplantation of a Tissue Engineered Bone Graft into an Irradiated, Critical-Size Femoral Defect in Rats" Cells 10, no. 9: 2256. https://doi.org/10.3390/cells10092256