The Role of Immune Cells in Oxi-Inflamm-Aging
Abstract
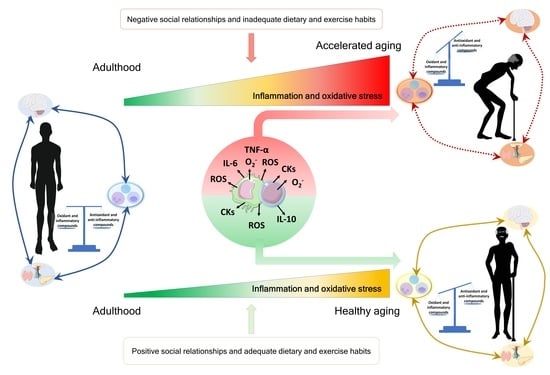
:1. Introduction
2. Following the Free Radical and Mitochondrial Theory of Aging
3. mitROS, the First in the Aging Process
4. Oxidation and Inflammation, Always Together. Oxi-Inflamm-Aging
5. Impact of Immunosenescence in Oxi-Inflamm-Aging
6. Can Immunosenescence Be a Marker of the Rate of Aging in Each Individual?
7. Lifestyle Situations Modulating the Rate of Aging: Friends or Foes?
7.1. Social Environment
7.2. Nutrition Conditions
7.3. Physical Exercise
7.4. Age-Related Diseases
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- López-Otín, C.; Kroemer, G. Hallmarks of Health. Cell 2021, 184, 33–63. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Miquel, J. An update of the oxidation-inflammation theory of aging: The involvement of the immune system in oxi-inflamm-aging. Curr. Pharm. Des. 2009, 15, 3003–3026. [Google Scholar] [CrossRef] [PubMed]
- Del Rey, A.; Besedovsky, H.O. Immune-Neuro-Endocrine Reflexes, Circuits, and Networks: Physiologic and Evolutionary Implications. Front. Horm. Res. 2017, 48, 1–18. [Google Scholar] [CrossRef]
- De la Fuente, M. Oxidation and Inflammation in the Immune and Nervous Systems, a Link Between Aging and Anxiety. In Handbook of Immunosenescence; Fulop, T., Franceschi, C., Hirokawa, K., Pawelec, G., Eds.; Springer: Dordrecht, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Rattan, S.I. Healthy ageing, but what is health? Biogerontology 2013, 14, 673–677. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Molecular gerontology: From homeodynamics to hormesis. Curr. Pharm. Des. 2014, 20, 3036–3039. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. Aging: A theory based on free radical and radiation chemistry. J. Gerontol. 1956, 11, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Harman, D. The biologic clock: The mitochondria? J. Am. Geriatr. Soc. 1972, 20, 145–147. [Google Scholar] [CrossRef] [PubMed]
- Miquel, J.; Economos, A.C.; Fleming, J.; Johnson, J.E., Jr. Mitochondrial role in cell aging. Exp. Gerontol. 1980, 15, 575–591. [Google Scholar] [CrossRef]
- Miquel, J. An update on the oxygen stress-mitochondrial mutation theory of aging: Genetic and evolutionary implications. Exp. Gerontol. 1998, 33, 113–126. [Google Scholar] [CrossRef]
- Barja, G. Updating the mitochondrial free radical theory of aging: An integrated view, key aspects, and confounding concepts. Antioxid. Redox Signal. 2013, 19, 1420–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Wang, X.; Vikash, V.; Ye, Q.; Wu, D.; Liu, Y.; Dong, W. ROS and ROS-Mediated Cellular Signaling. Oxid. Med. Cell. Longev. 2016, 2016, 4350965. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gladyshev, V.N. The free radical theory of aging is dead. Long live the damage theory! Antioxid. Redox Signal. 2014, 20, 727–731. [Google Scholar] [CrossRef]
- Sadowska-Bartosz, I.; Bartosz, G. Effect of antioxidants supplementation on aging and longevity. BioMed Res. Int. 2014, 2014, 404680. [Google Scholar] [CrossRef] [PubMed]
- Honda, Y.; Honda, S. Oxidative stress and life span determination in the nematode Caenorhabditis elegans. Ann. N. Y. Acad. Sci. 2002, 959, 466–474. [Google Scholar] [CrossRef] [PubMed]
- Ristow, M.; Schmeisser, S. Extending life span by increasing oxidative stress. Free Radic. Biol. Med. 2011, 51, 327–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mesquita, A.; Weinberger, M.; Silva, A.; Sampaio-Marques, B.; Almeida, B.; Leão, C.; Costa, V.; Rodrigues, F.; Burhans, W.C.; Ludovico, P. Caloric restriction or catalase inactivation extends yeast chronological lifespan by inducing H2O2 and superoxide dismutase activity. Proc. Natl. Acad. Sci. USA 2010, 107, 15123–15128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.; Hekimi, S. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 2010, 8, e1000556. [Google Scholar] [CrossRef] [Green Version]
- Schulz, T.J.; Zarse, K.; Voigt, A.; Urban, N.; Birringer, M.; Ristow, M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metab. 2007, 6, 280–293. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barja, G. The flux of free radical attack through mitochondrial DNA is related to aging rate. Aging 2000, 12, 342–355. [Google Scholar] [CrossRef] [PubMed]
- Jang, Y.C.; Pérez, V.I.; Song, W.; Lustgarten, M.S.; Salmon, A.B.; Mele, J.; Qi, W.; Liu, Y.; Liang, H.; Chaudhuri, A.; et al. Overexpression of Mn superoxide dismutase does not increase life span in mice. J. Gerontol. A Biol. Sci. Med. Sci. 2009, 64, 1114–1125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mockett, R.J.; Sohal, B.H.; Sohal, R.S. Expression of multiple copies of mitochondrially targeted catalase or genomic Mn superoxide dismutase transgenes does not extend the life span of Drosophila melanogaster. Free Radic. Biol. Med. 2010, 49, 2028–2031. [Google Scholar] [CrossRef] [Green Version]
- Schriner, S.E.; Linford, N.J.; Martin, G.M.; Treuting, P.; Ogburn, C.E.; Emond, M.; Coskun, P.E.; Ladiges, W.; Wolf, N.; Van Remmen, H.; et al. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science 2005, 308, 1909–1911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An Overview from Discovery, Molecular Mechanisms to Physiology and Pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Konno, T.; Melo, E.P.; Chambers, J.E.; Avezov, E. Intracellular Sources of ROS/H2O2 in Health and Neurodegeneration: Spotlight on Endoplasmic Reticulum. Cells 2021, 10, 233. [Google Scholar] [CrossRef] [PubMed]
- Knaus, U.G. Oxidants in Physiological Processes. Handb. Exp. Pharmacol. 2021, 264, 27–47. [Google Scholar] [CrossRef]
- Moghadam, Z.M.; Henneke, P.; Kolter, J. From Flies to Men: ROS and the NADPH Oxidase in Phagocytes. Front. Cell Dev. Biol. 2021, 9, 628991. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Pachouri, U.C.; Khaidem, D.C.; Kundu, A.; Chopra, C.; Singh, P. Mitochondrial DNA Damage and Diseases. F1000Research 2015, 4, 176. [Google Scholar] [CrossRef]
- Puertas, M.J.; González-Sánchez, M. Insertions of mitochondrial DNA into the nucleus-effects and role in cell evolution. Genome 2020, 63, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Barja, G. Towards a unified mechanistic theory of aging. Exp. Gerontol. 2019, 124, 110627. [Google Scholar] [CrossRef]
- Tuboly, E.; Mcllroy, D.; Briggs, G.; Lott, N.; Balogh, Z.J. Clinical implications and pathological associations of circulating mitochondrial DNA. Front. Biosci. 2017, 22, 1011–1022. [Google Scholar] [CrossRef] [Green Version]
- Boyapati, R.K.; Tamborska, A.; Dorward, D.A.; Ho, G.T. Advances in the understanding of mitochondrial DNA as a pathogenic factor in inflammatory diseases. F1000Research 2017, 6, 169. [Google Scholar] [CrossRef] [Green Version]
- López-Otín, C.; Blasco, M.A.; Partridge, L.; Serrano, M.; Kroemer, G. The hallmarks of aging. Cell 2013, 153, 1194–1217. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Bonafè, M.; Valensin, S.; Olivieri, F.; De Luca, M.; Ottaviani, E.; De Benedictis, G. Inflamm-aging. An evolutionary perspective on immunosenescence. Ann. N. Y. Acad. Sci. 2000, 908, 244–254. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; González, E.M.; De la Fuente, M. Increase of oxidation and inflammation in nervous and immune systems with aging and anxiety. Curr. Pharm. Des. 2014, 20, 4656–4678. [Google Scholar] [CrossRef] [PubMed]
- Fülöp, T.; Larbi, A.; Witkowski, J.M. Human Inflammaging. Gerontology 2019, 65, 495–504. [Google Scholar] [CrossRef] [PubMed]
- Khansari, N.; Shakiba, Y.; Mahmoudi, M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Pat. Inflamm. Allergy Drug Discov. 2009, 3, 73–80. [Google Scholar] [CrossRef]
- Federico, A.; Morgillo, F.; Tuccillo, C.; Ciardiello, F.; Loguercio, C. Chronic inflammation and oxidative stress in human carcinogenesis. Int. J. Cancer 2007, 121, 2381–2386. [Google Scholar] [CrossRef] [PubMed]
- Su, Y.J.; Wang, P.W.; Weng, S.W. The Role of Mitochondria in Immune-Cell-Mediated Tissue Regeneration and Ageing. Int. J. Mol. Sci. 2021, 22, 2668. [Google Scholar] [CrossRef] [PubMed]
- Picca, A.; Calvani, R.; Coelho-Junior, H.J.; Marzetti, E. Cell Death and Inflammation: The Role of Mitochondria in Health and Disease. Cells 2021, 10, 537. [Google Scholar] [CrossRef] [PubMed]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mitochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef]
- Cannizzo, E.S.; Clement, C.C.; Sahu, R.; Follo, C.; Santambrogio, L. Oxidative stress, inflamm-aging and immunosenescence. J. Proteom. 2011, 74, 2313–2323. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile inflammation: Sensing and reacting to damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, R.; Tardivel, A.; Thorens, B.; Choi, I.; Tschopp, J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 2010, 11, 136–140. [Google Scholar] [CrossRef] [PubMed]
- Vida, C.; de Toda, I.M.; Cruces, J.; Garrido, A.; Gonzalez-Sanchez, M.; De la Fuente, M. Role of macrophages in age-related oxidative stress and lipofuscin accumulation in mice. Redox Biol. 2017, 12, 423–437. [Google Scholar] [CrossRef] [Green Version]
- Pawelec, G. Age and immunity: What is “immunosenescence”? Exp. Gerontol. 2018, 105, 4–9. [Google Scholar] [CrossRef]
- Feehan, J.; Tripodi, N.; Apostolopoulos, V. The twilight of the immune system: The impact of immunosenescence in aging. Maturitas 2021, 147, 7–13. [Google Scholar] [CrossRef]
- Rodriguez, I.J.; Lalinde Ruiz, N.; Llano León, M.; Martínez Enríquez, L.; Montilla Velásquez, M.D.P.; Ortiz Aguirre, J.P.; Rodríguez Bohórquez, O.M.; Velandia Vargas, E.A.; Hernández, E.D.; Parra López, C.A. Immunosenescence Study of T Cells: A Systematic Review. Front. Immunol. 2021, 11, 604591. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, L.P.; Teixeira, V.R.; Alencar-Silva, T.; Simonassi-Paiva, B.; Pereira, R.W.; Pogue, R.; Carvalho, J.L. Hallmarks of aging and immunosenescence: Connecting the dots. Cytokine Growth Factor Rev. 2021, 59, 9–21. [Google Scholar] [CrossRef]
- Solana, R.; Mariani, E. NK and NK/T cells in human senescence. Vaccine 2000, 18, 1613–1620. [Google Scholar] [CrossRef]
- Hazeldine, J.; Lord, J.M. The impact of ageing on natural killer cell function and potential consequences for health in older adults. Ageing Res. Rev. 2013, 12, 1069–1078. [Google Scholar] [CrossRef]
- Hashimoto, K.; Kouno, T.; Ikawa, T.; Hayatsu, N.; Miyajima, Y.; Yabukami, H.; Terooatea, T.; Sasaki, T.; Suzuki, T.; Valentine, M.; et al. Single-cell transcriptomics reveals expansion of cytotoxic CD4 T cells in supercentenarians. Proc. Natl. Acad. Sci. USA 2019, 116, 24242–24251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso-Fernández, P.; Puerto, M.; Maté, I.; Ribera, J.M.; de la Fuente, M. Neutrophils of centenarians show function levels similar to those of young adults. J. Am. Geriatr. Soc. 2008, 56, 2244–2251. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Maté, I.; Vida, C.; Cruces, J.; De la Fuente, M. Immune function parameters as markers of biological age and predictors of longevity. Aging 2016, 8, 3110–3119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van der Loo, B.; Schildknecht, S.; Zee, R.; Bachschmid, M.M. Signalling processes in endothelial ageing in relation to chronic oxidative stress and their potential therapeutic implications in humans. Exp. Physiol. 2009, 94, 305–310. [Google Scholar] [CrossRef]
- Stout-Delgado, H.W.; Du, W.; Shirali, A.C.; Booth, C.J.; Goldstein, D.R. Aging promotes neutrophil-induced mortality by augmenting IL-17 production during viral infection. Cell Host Microbe 2009, 6, 446–456. [Google Scholar] [CrossRef] [Green Version]
- Sendama, W. The effect of ageing on the resolution of inflammation. Ageing Res. Rev. 2020, 57, 101000. [Google Scholar] [CrossRef] [PubMed]
- Van Beek, A.A.; Van den Bossche, J.; Mastroberardino, P.G.; de Winther, M.P.J.; Leenen, P.J.M. Metabolic Alterations in Aging Macrophages: Ingredients for Inflammaging? Trends Immunol. 2019, 40, 113–127. [Google Scholar] [CrossRef]
- Kale, A.; Sharma, A.; Stolzing, A.; Desprez, P.Y.; Campisi, J. Role of immune cells in the removal of deleterious senescent cells. Immun. Ageing 2020, 17, 16. [Google Scholar] [CrossRef] [PubMed]
- Cope, A.P. Studies of T-cell activation in chronic inflammation. Arthritis Res. 2002, 3, S197–S211. [Google Scholar] [CrossRef] [PubMed]
- Thorén, F.B.; Betten, A.; Romero, A.I.; Hellstrand, K. Cutting edge: Antioxidative properties of myeloid dendritic cells: Protection of T cells and NK cells from oxygen radical-induced inactivation and apoptosis. J. Immunol. 2007, 179, 21–25. [Google Scholar] [CrossRef] [Green Version]
- Ogata, K.; An, E.; Shioi, Y.; Nakamura, K.; Luo, S.; Yokose, N.; Minami, S.; Dan, K. Association between natural killer cell activity and infection in immunologically normal elderly people. Clin. Exp. Immunol. 2001, 124, 392–397. [Google Scholar] [CrossRef]
- Aw, D.; Silva, A.B.; Palmer, D.B. Immunosenescence: Emerging challenges for an ageing population. Immunology 2007, 120, 435–446. [Google Scholar] [CrossRef]
- Brighton, P.J.; Maruyama, Y.; Fishwick, K.; Vrljicak, P.; Tewary, S.; Fujihara, R.; Muter, J.; Lucas, E.S.; Yamada, T.; Woods, L.; et al. Clearance of senescent decidual cells by uterine natural killer cells in cycling human endometrium. eLife 2017, 6, e31274. [Google Scholar] [CrossRef] [PubMed]
- Sagiv, A.; Biran, A.; Yon, M.; Simon, J.; Lowe, S.W.; Krizhanovsky, V. Granule exocytosis mediates immune surveillance of senescent cells. Oncogene 2013, 32, 1971–1977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovadya, Y.; Landsberger, T.; Leins, H.; Vadai, E.; Gal, H.; Biran, A.; Yosef, R.; Sagiv, A.; Agrawal, A.; Shapira, A.; et al. Impaired immune surveillance accelerates accumulation of senescent cells and aging. Nat. Commun. 2018, 9, 5435. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ben-Shmuel, A.; Biber, G.; Barda-Saad, M. Unleashing Natural Killer Cells in the Tumor Microenvironment-The Next Generation of Immunotherapy? Front. Immunol. 2020, 11, 275. [Google Scholar] [CrossRef] [Green Version]
- Song, S.; Tchkonia, T.; Jiang, J.; Kirkland, J.L.; Sun, Y. Targeting Senescent Cells for a Healthier Aging: Challenges and Opportunities. Adv. Sci. 2020, 7, 2002611. [Google Scholar] [CrossRef]
- Borghesan, M.; Hoogaars, W.M.H.; Varela-Eirin, M.; Talma, N.; Demaria, M. A Senescence-Centric View of Aging: Implications for Longevity and Disease. Trends Cell Biol. 2020, 30, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Childs, B.G.; Baker, D.J.; Kirkland, J.L.; Campisi, J.; van Deursen, J.M. Senescence and apoptosis: Dueling or complementary cell fates? EMBO Rep. 2014, 15, 1139–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandrasekaran, A.; Idelchik, M.D.P.S.; Melendez, J.A. Redox control of senescence and age-related disease. Redox Biol. 2017, 11, 91–102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pawelec, G. Does immunosenescence drive organismal ageing via inflammageing? Immun. Ageing 2021, 18, 31. [Google Scholar] [CrossRef]
- Hall, B.M.; Balan, V.; Gleiberman, A.S.; Strom, E.; Krasnov, P.; Virtuoso, L.P.; Rydkina, E.; Vujcic, S.; Balan, K.; Gitlin, I.; et al. Aging of mice is associated with p16(Ink4a)- and β-galactosidase-positive macrophage accumulation that can be induced in young mice by senescent cells. Aging 2016, 8, 1294–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prattichizzo, F.; Bonafè, M.; Olivieri, F.; Franceschi, C. Senescence associated macrophages and “macroph-aging”: Are they pieces of the same puzzle? Aging 2016, 8, 3159–3160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arranz, L.; Caamaño, J.H.; Lord, J.M.; De la Fuente, M. Preserved immune functions and controlled leukocyte oxidative stress in naturally long-lived mice: Possible role of nuclear factor kappa B. J. Gerontol. A Biol. Sci. Med. Sci. 2010, 65, 941–950. [Google Scholar] [CrossRef] [Green Version]
- Desdín-Micó, G.; Soto-Heredero, G.; Aranda, J.F.; Oller, J.; Carrasco, E.; Gabandé-Rodríguez, E.; Blanco, E.M.; Alfranca, A.; Cussó, L.; Desco, M.; et al. T cells with dysfunctional mitochondria induce multimorbidity and premature senescence. Science 2020, 368, 1371–1376. [Google Scholar] [CrossRef] [PubMed]
- Blasco, M.A. Telomere length, stem cells and aging. Nat. Chem. Biol. 2007, 3, 640–649. [Google Scholar] [CrossRef]
- Horvath, S. DNA methylation age of human tissues and cell types. Genome Biol. 2013, 14, R115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Enroth, S.; Enroth, S.B.; Johansson, Å.; Gyllensten, U. Protein profiling reveals consequences of lifestyle choices on predicted biological aging. Sci. Rep. 2015, 5, 17282. [Google Scholar] [CrossRef]
- Martínez de Toda, I.; Vida, C.; Garrido, A.; De la Fuente, M. Redox Parameters as Markers of the Rate of Aging and Predictors of Life Span. J. Gerontol. A Biol. Sci. Med. Sci. 2020, 75, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Vida, C.; Sanz San Miguel, L.; De la Fuente, M. Function, Oxidative, and Inflammatory Stress Parameters in Immune Cells as Predictive Markers of Lifespan throughout Aging. Oxid. Med. Cell. Longev. 2019, 2019, 4574276. [Google Scholar] [CrossRef] [PubMed]
- Martínez de Toda, I.; Vida, C.; Díaz-Del Cerro, E.; De la Fuente, M. The Immunity Clock. J. Gerontol. A Biol. Sci. Med. Sci. 2021, glab136, Epub ahead of print. [Google Scholar] [CrossRef]
- Alpert, A.; Pickman, Y.; Leipold, M.; Rosenberg-Hasson, Y.; Ji, X.; Gaujoux, R.; Rabani, H.; Starosvetsky, E.; Kveler, K.; Schaffert, S.; et al. A clinically meaningful metric of immune age derived from high-dimensional longitudinal monitoring. Nat. Med. 2019, 25, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Sayed, N.; Huang, Y.; Nguyen, K.; Krejciova-Rajaniemi, Z.; Grawe, A.P.; Gao, T.; Tibshirani, R.; Hastie, T.; Alpert, A.; Cui, L.; et al. An inflammatory aging clock (iAge) based on deep learning tracks multimorbidity, immunosenescence, frailty and cardiovascular aging. Nat. Aging 2021, 1, 598–615. [Google Scholar] [CrossRef]
- Mariotti, A. The effects of chronic stress on health: New insights into the molecular mechanisms of brain-body communication. Future Sci. OA 2015, 1, FSO23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, S.; Wei, F.; Li, G. The evolution of the concept of stress and the framework of the stress system. Cell Stress 2021, 5, 76–85. [Google Scholar] [CrossRef] [PubMed]
- Arranz, L.; Guayerbas, N.; De la Fuente, M. Impairment of several immune functions in anxious women. J. Psychosom. Res. 2007, 62, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Rohleder, N. Acute and chronic stress induced changes in sensitivity of peripheral inflammatory pathways to the signals of multiple stress systems—2011 Curt Richter Award Winner. Psychoneuroendocrinology 2012, 37, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Cruces, J.; Venero, C.; Pereda-Pérez, I.; De la Fuente, M. The effect of psychological stress and social isolation on neuroimmunoendocrine communication. Curr. Pharm. Des. 2014, 20, 4608–4628. [Google Scholar] [CrossRef]
- de Toda, I.M.; Miguélez, L.; Siboni, L.; Vida, C.; De la Fuente, M. High perceived stress in women is linked to oxidation, inflammation and immunosenescence. Biogerontology 2019, 20, 823–835. [Google Scholar] [CrossRef]
- Garrido, A.; Cruces, J.; Ceprián, N.; Vara, E.; de la Fuente, M. Oxidative-Inflammatory Stress in Immune Cells from Adult Mice with Premature Aging. Int. J. Mol. Sci. 2019, 20, 769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Alvarez, L.; Baeza, I.; Arranz, L.; Marco, E.M.; Borcel, E.; Guaza, C.; Viveros, M.P.; De la Fuente, M. Behavioral, endocrine and immunological characteristics of a murine model of premature aging. Dev. Comp. Immunol. 2005, 29, 965–976. [Google Scholar] [CrossRef] [PubMed]
- Viveros, M.P.; Arranz, L.; Hernanz, A.; Miquel, J.; De la Fuente, M. A model of premature aging in mice based on altered stress-related behavioral response and immunosenescence. Neuroimmunomodulation 2007, 14, 157–162. [Google Scholar] [CrossRef] [PubMed]
- Martinez de Toda, I.; Garrido, A.; Vida, C.; Gomez-Cabrera, M.C.; Viña, J.; De la Fuente, M. Frailty Quantified by the “Valencia Score” as a Potential Predictor of Lifespan in Mice. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 1323–1329. [Google Scholar] [CrossRef] [PubMed]
- Rattan, S.I. Hormesis in aging. Ageing Res. Rev. 2008, 7, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Dhabhar, F.S. The short-term stress response−Mother nature’s mechanism for enhancing protection and performance under conditions of threat, challenge, and opportunity. Front. Neuroendocrinol. 2018, 49, 175–192. [Google Scholar] [CrossRef]
- Du Preez, A.; Onorato, D.; Eiben, I.; Musaelyan, K.; Egeland, M.; Zunszain, P.A.; Fernandes, C.; Thuret, S.; Pariante, C.M. Chronic stress followed by social isolation promotes depressive-like behaviour, alters microglial and astrocyte biology and reduces hippocampal neurogenesis in male mice. Brain Behav. Immun. 2021, 91, 24–47. [Google Scholar] [CrossRef] [PubMed]
- Farbstein, D.; Hollander, N.; Peled, O.; Apter, A.; Fennig, S.; Haberman, Y.; Gitman, H.; Yaniv, I.; Shkalim, V.; Pick, C.G.; et al. Social isolation in mice: Behavior, immunity, and tumor growth. Stress 2021, 24, 229–238. [Google Scholar] [CrossRef]
- Gorenko, J.A.; Moran, C.; Flynn, M.; Dobson, K.; Konnert, C. Social Isolation and Psychological Distress Among Older Adults Related to COVID-19: A Narrative Review of Remotely-Delivered Interventions and Recommendations. J. Appl. Gerontol. 2021, 40, 3–13. [Google Scholar] [CrossRef]
- Guner, T.A.; Erdogan, Z.; Demir, I. The Effect of Loneliness on Death Anxiety in the Elderly During the COVID-19 Pandemic. Omega 2021, 302228211010587, Epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Palermo-Neto, J.; Alves, G.J. Neuroimmune interactions and psychologycal stress induced by cohabitation with a sick partner: A review. Curr. Pharm. Des. 2014, 20, 4629–4641. [Google Scholar] [CrossRef]
- Machado, T.R.; Alves, G.J.; Quinteiro-Filho, W.M.; Palermo-Neto, J. Cohabitation with an Ehrlich tumor-bearing cagemate induces immune but not behavioral changes in male mice. Physiol. Behav. 2017, 169, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.R.; McMahon, E.K.; Boner, W.; Haussmann, M.F. Oxytocin administration prevents cellular aging caused by social isolation. Psychoneuroendocrinology 2019, 103, 52–60. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Xia, N. The role of oxidative stress in cardiovascular disease caused by social isolation and loneliness. Redox Biol. 2020, 37, 101585. [Google Scholar] [CrossRef] [PubMed]
- Hamasato, E.K.; de Lima, A.P.; de Oliveira, A.P.; dos Santos Franco, A.L.; de Lima, W.T.; Palermo-Neto, J. Cohabitation with a sick partner increases allergic lung inflammatory response in mice. Brain Behav. Immun. 2014, 42, 109–117. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.J.; Gavey, S.; Riddell, N.E.; Kontari, P.; Victor, C. The association between loneliness, social isolation and inflammation: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2020, 112, 519–541. [Google Scholar] [CrossRef]
- Holt-Lunstad, J.; Smith, T.B.; Baker, M.; Harris, T.; Stephenson, D. Loneliness and social isolation as risk factors for mortality: A meta-analytic review. Perspect. Psychol. Sci. 2015, 10, 227–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seeman, T.E.; Crimmins, E. Social environment effects on health and aging: Integrating epidemiologic and demographic approaches and perspectives. Ann. N. Y. Acad. Sci. 2001, 954, 88–117. [Google Scholar] [CrossRef] [PubMed]
- DeVries, A.C. Interaction among social environment, the hypothalamic-pituitary-adrenal axis, and behavior. Horm. Behav. 2002, 41, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Garrido, A.; Cruces, J.; Ceprián, N.; De la Fuente, M. Improvements in Behavior and Immune Function and Increased Life Span of Old Mice Cohabiting With Adult Animals. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 73, 873–881. [Google Scholar] [CrossRef]
- Garrido, A.; Cruces, J.; Ceprián, N.; Díaz-Del Cerro, E.; Félix, J.; De la Fuente, M. The ratio of prematurely aging to non-prematurely aging mice cohabiting, conditions their behavior, immunity and lifespan. J. Neuroimmunol. 2020, 343, 577240. [Google Scholar] [CrossRef] [PubMed]
- Holt-Lunstad, J. The Major Health Implications of Social Connection. Curr. Dir. Psychol. Sci. 2021, 30, 251–259. [Google Scholar] [CrossRef]
- Carruba, G.; Cocciadiferro, L.; Di Cristina, A.; Granata, O.M.; Dolcemascolo, C.; Campisi, L.; Zarcone, M.; Cinquegrani, C.; Traina, A. Nutrition, aging and cancer: Lessons from dietary intervention studies. Immun. Ageing 2016, 13, 13. [Google Scholar] [CrossRef]
- Kritchevsky, S.B. Nutrition and Healthy Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 1303–1305. [Google Scholar] [CrossRef] [PubMed]
- Van Gaal, L.F.; Mertens, I.L.; De Block, C.E. Mechanisms linking obesity with cardiovascular disease. Nature 2006, 444, 875–880. [Google Scholar] [CrossRef] [PubMed]
- Julien, C.; Tremblay, C.; Phivilay, A.; Berthiaume, L.; Emond, V.; Julien, P.; Calon, F. High-fat diet aggravates amyloid-beta and tau pathologies in the 3xTg-AD mouse model. Neurobiol. Aging 2010, 31, 1516–1531. [Google Scholar] [CrossRef] [PubMed]
- Cai, D. Neuroinflammation and neurodegeneration in overnutrition-induced diseases. Trends Endocrinol. Metab. 2013, 24, 40–47. [Google Scholar] [CrossRef] [Green Version]
- Bastien, M.; Poirier, P.; Lemieux, I.; Després, J.P. Overview of epidemiology and contribution of obesity to cardiovascular disease. Prog. Cardiovasc Dis. 2014, 56, 369–381. [Google Scholar] [CrossRef]
- Rath, E.; Haller, D. Inflammation and cellular stress: A mechanistic link between immune-mediated and metabolically driven pathologies. Eur. J. Nutr. 2011, 50, 219–233. [Google Scholar] [CrossRef]
- Sheridan, P.A.; Paich, H.A.; Handy, J.; Karlsson, E.A.; Hudgens, M.G.; Sammon, A.B.; Holland, L.A.; Weir, S.; Noah, T.L.; Beck, M.A. Obesity is associated with impaired immune response to influenza vaccination in humans. Int. J. Obes. 2012, 36, 1072–1077. [Google Scholar] [CrossRef] [Green Version]
- Hunsche, C.; Hernandez, O.; De la Fuente, M. Impaired Immune Response in Old Mice Suffering from Obesity and Premature Immunosenescence in Adulthood. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 983–991. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunsche, C.; de Toda, I.M.; De la Fuente, M. Impacts of the late adulthood diet-induced obesity onset on behavior, immune function, redox state and life span of male and female mice. Brain Behav. Immun. 2019, 78, 65–77. [Google Scholar] [CrossRef]
- Schipper, H.S.; Prakken, B.; Kalkhoven, E.; Boes, M. Adipose tissue-resident immune cells: Key players in immunometabolism. Trends Endocrinol. Metab. 2012, 23, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Klöting, N.; Blüher, M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev. Endocr. Metab. Disord. 2014, 15, 277–287. [Google Scholar] [CrossRef] [PubMed]
- Kalache, A.; de Hoogh, A.I.; Howlett, S.E.; Kennedy, B.; Eggersdorfer, M.; Marsman, D.S.; Shao, A.; Griffiths, J.C. Nutrition interventions for healthy ageing across the lifespan: A conference report. Eur. J. Nutr. 2019, 58, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mareschal, J.; Genton, L.; Collet, T.H.; Graf, C. Nutritional Intervention to Prevent the Functional Decline in Community-Dwelling Older Adults: A Systematic Review. Nutrients 2020, 12, 2820. [Google Scholar] [CrossRef]
- Heilbronn, L.K.; Ravussin, E. Calorie restriction and aging: Review of the literature and implications for studies in humans. Am. J. Clin. Nutr. 2003, 78, 361–369. [Google Scholar] [CrossRef]
- Masoro, E.J. Subfield history: Caloric restriction, slowing aging, and extending life. Sci. Aging Knowl. Environ. 2003, 2003, RE2. [Google Scholar] [CrossRef] [Green Version]
- Gensous, N.; Franceschi, C.; Santoro, A.; Milazzo, M.; Garagnani, P.; Bacalini, M.G. The Impact of Caloric Restriction on the Epigenetic Signatures of Aging. Int. J. Mol. Sci. 2019, 20, 2022. [Google Scholar] [CrossRef] [Green Version]
- Pifferi, F.; Aujard, F. Caloric restriction, longevity and aging: Recent contributions from human and non-human primate studies. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2019, 95, 109702. [Google Scholar] [CrossRef]
- Caristia, S.; Vito, M.; Sarro, A.; Leone, A.; Pecere, A.; Zibetti, A.; Filigheddu, N.; Zeppegno, P.; Prodam, F.; Faggiano, F.; et al. Is Caloric Restriction Associated with Better Healthy Aging Outcomes? A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Nutrients 2020, 12, 2290. [Google Scholar] [CrossRef] [PubMed]
- Solon-Biet, S.M.; McMahon, A.C.; Ballard, J.W.; Ruohonen, K.; Wu, L.E.; Cogger, V.C.; Warren, A.; Huang, X.; Pichaud, N.; Melvin, R.G.; et al. The ratio of macronutrients, not caloric intake, dictates cardiometabolic health, aging, and longevity in ad libitum-fed mice. Cell Metab. 2014, 19, 418–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senior, A.M.; Solon-Biet, S.M.; Cogger, V.C.; Le Couteur, D.G.; Nakagawa, S.; Raubenheimer, D.; Simpson, S.J. Dietary macronutrient content, age-specific mortality and lifespan. Proc. Biol. Sci. 2019, 286, 20190393. [Google Scholar] [CrossRef] [Green Version]
- Duncan, S.H.; Flint, H.J. Probiotics and prebiotics and health in ageing populations. Maturitas 2013, 75, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Vaiserman, A.M.; Koliada, A.K.; Marotta, F. Gut microbiota: A player in aging and a target for anti-aging intervention. Ageing Res. Rev. 2017, 35, 36–45. [Google Scholar] [CrossRef]
- Hunsche, C.; Cruces, J.; De la Fuente, M. Improvement of Redox State and Functions of Immune Cells as Well as of Behavioral Response in Aged Mice After Two-Week Supplementation of Fermented Milk with Probiotics. Curr. Microbiol. 2019, 76, 1278–1289. [Google Scholar] [CrossRef]
- De la Fuente, M.; Hernanz, A.; Guayerbas, N.; Victor, V.M.; Arnalich, F. Vitamin E ingestion improves several immune functions in elderly men and women. Free Radic. Res. 2008, 42, 272–280. [Google Scholar] [CrossRef]
- De la Fuente, M.; Sánchez, C.; Vallejo, C.; Díaz-Del Cerro, E.; Arnalich, F.; Hernanz, Á. Vitamin C and vitamin C plus E improve the immune function in the elderly. Exp. Gerontol. 2020, 142, 111118. [Google Scholar] [CrossRef]
- Alvarado, C.; Alvarez, P.; Puerto, M.; Gausserès, N.; Jiménez, L.; De la Fuente, M. Dietary supplementation with antioxidants improves functions and decreases oxidative stress of leukocytes from prematurely aging mice. Nutrition 2006, 22, 767–777. [Google Scholar] [CrossRef] [PubMed]
- Monti, D.A.; Zabrecky, G.; Kremens, D.; Liang, T.W.; Wintering, N.A.; Bazzan, A.J.; Zhong, L.; Bowens, B.K.; Chervoneva, I.; Intenzo, C.; et al. N-Acetyl Cysteine Is Associated With Dopaminergic Improvement in Parkinson’s Disease. Clin. Pharmacol. Ther. 2019, 106, 884–890. [Google Scholar] [CrossRef]
- Pahlavani, M.A. Influence of caloric restriction on aging immune system. J. Nutr. Health Aging 2004, 8, 38–47. [Google Scholar]
- Alvarez, P.; Alvarado, C.; Puerto, M.; Schlumberger, A.; Jiménez, L.; De la Fuente, M. Improvement of leukocyte functions in prematurely aging mice after five weeks of diet supplementation with polyphenol-rich cereals. Nutrition 2006, 22, 913–921. [Google Scholar] [CrossRef] [PubMed]
- Yaqoob, P. Ageing alters the impact of nutrition on immune function. Proc. Nutr. Soc. 2017, 76, 347–351. [Google Scholar] [CrossRef] [PubMed]
- Hunsche, C.; Hernandez, O.; Gheorghe, A.; Díaz, L.E.; Marcos, A.; De la Fuente, M. Immune dysfunction and increased oxidative stress state in diet-induced obese mice are reverted by nutritional supplementation with monounsaturated and n-3 polyunsaturated fatty acids. Eur. J. Nutr. 2018, 57, 1123–1135. [Google Scholar] [CrossRef] [PubMed]
- Weyh, C.; Krüger, K.; Strasser, B. Physical Activity and Diet Shape the Immune System during Aging. Nutrients 2020, 12, 622. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baeza, I.; De Castro, N.M.; Arranz, L.; De la Fuente, M. Soybean and green tea polyphenols improve immune function and redox status in very old ovariectomized mice. Rejuvenation Res. 2010, 13, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Roman, I.; Barja, G. Regulation of longevity and oxidative stress by nutritional interventions: Role of methionine restriction. Exp. Gerontol. 2013, 48, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Levine, M.E.; Suarez, J.A.; Brandhorst, S.; Balasubramanian, P.; Cheng, C.W.; Madia, F.; Fontana, L.; Mirisola, M.G.; Guevara-Aguirre, J.; Wan, J.; et al. Low protein intake is associated with a major reduction in IGF-1, cancer, and overall mortality in the 65 and younger but not older population. Cell Metab. 2014, 19, 407–417. [Google Scholar] [CrossRef] [Green Version]
- Wallace, M.A.; Aguirre, N.W.; Marcotte, G.R.; Marshall, A.G.; Baehr, L.M.; Hughes, D.C.; Hamilton, K.L.; Roberts, M.N.; Lopez-Dominguez, J.A.; Miller, B.F.; et al. The ketogenic diet preserves skeletal muscle with aging in mice. Aging Cell 2021, 20, e13322. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Victor, V.M. Anti-oxidants as modulators of immune function. Immunol. Cell Biol. 2000, 78, 49–54. [Google Scholar] [CrossRef]
- De la Fuente, M. Effects of antioxidants on immune system ageing. Eur. J. Clin. Nutr. 2002, 56, S5–S8. [Google Scholar] [CrossRef]
- Arranz, L.; Fernández, C.; Rodríguez, A.; Ribera, J.M.; De la Fuente, M. The glutathione precursor N-acetylcysteine improves immune function in postmenopausal women. Free Radic. Biol. Med. 2008, 45, 1252–1262. [Google Scholar] [CrossRef] [PubMed]
- Guayerbas, N.; Puerto, M.; Ferrández, M.D.; De La Fuente, M. A diet supplemented with thiolic anti-oxidants improves leucocyte function in two strains of prematurely ageing mice. Clin. Exp. Pharmacol. Physiol. 2002, 29, 1009–1014. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.; Kapila, R.; Kapasiya, M.; Saliganti, V.; Dass, G.; Kapila, S. Dietary supplementation of milk fermented with probiotic Lactobacillus fermentum enhances systemic immune response and antioxidant capacity in aging mice. Nutr. Res. 2014, 34, 968–981. [Google Scholar] [CrossRef]
- Sichetti, M.; De Marco, S.; Pagiotti, R.; Traina, G.; Pietrella, D. Anti-inflammatory effect of multistrain probiotic formulation (L. rhamnosus, B. lactis, and B. longum). Nutrition 2018, 53, 95–102. [Google Scholar] [CrossRef]
- Azad, M.A.K.; Sarker, M.; Wan, D. Immunomodulatory Effects of Probiotics on Cytokine Profiles. BioMed Res. Int. 2018, 2018, 8063647. [Google Scholar] [CrossRef] [Green Version]
- De Moreno de LeBlanc, A.; Chaves, S.; Carmuega, E.; Weill, R.; Antóine, J.; Perdigón, G. Effect of long-term continuous consumption of fermented milk containing probiotic bacteria on mucosal immunity and the activity of peritoneal macrophages. Immunobiology 2008, 213, 97–108. [Google Scholar] [CrossRef] [PubMed]
- Dong, H.; Rowland, I.; Thomas, L.V.; Yaqoob, P. Immunomodulatory effects of a probiotic drink containing Lactobacillus casei Shirota in healthy older volunteers. Eur. J. Nutr. 2013, 52, 1853–1863. [Google Scholar] [CrossRef]
- Maneerat, S.; Lehtinen, M.J.; Childs, C.E.; Forssten, S.D.; Alhoniemi, E.; Tiphaine, M.; Yaqoob, P.; Ouwehand, A.C.; Rastall, R.A. Consumption of Bifidobacterium lactis Bi-07 by healthy elderly adults enhances phagocytic activity of monocytes and granulocytes. J. Nutr. Sci. 2014, 2, e44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kimoto-Nira, H.; Suzuki, C.; Kobayashi, M.; Sasaki, K.; Kurisaki, J.; Mizumachi, K. Anti-ageing effect of a lactococcal strain: Analysis using senescence-accelerated mice. Br. J. Nutr. 2007, 98, 1178–1186. [Google Scholar] [CrossRef] [Green Version]
- Grompone, G.; Martorell, P.; Llopis, S.; González, N.; Genovés, S.; Mulet, A.P.; Fernández-Calero, T.; Tiscornia, I.; Bollati-Fogolín, M.; Chambaud, I.; et al. Anti-inflammatory Lactobacillus rhamnosus CNCM I-3690 strain protects against oxidative stress and increases lifespan in Caenorhabditis elegans. PLoS ONE 2012, 7, e52493. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhao, L.; Zheng, X.; Fu, T.; Guo, H.; Ren, F. Lactobacillus salivarius strain FDB89 induced longevity in Caenorhabditis elegans by dietary restriction. J. Microbiol. 2013, 51, 183–188. [Google Scholar] [CrossRef] [PubMed]
- Vargas-Mendoza, N.; Morales-González, Á.; Madrigal-Santillán, E.O.; Madrigal-Bujaidar, E.; Álvarez-González, I.; García-Melo, L.F.; Anguiano-Robledo, L.; Fregoso-Aguilar, T.; Morales-Gonzalez, J.A. Antioxidant and Adaptative Response Mediated by Nrf2 during Physical Exercise. Antioxidants 2019, 8, 196. [Google Scholar] [CrossRef] [Green Version]
- Pierre, N.; Appriou, Z.; Gratas-Delamarche, A.; Derbré, F. From physical inactivity to immobilization: Dissecting the role of oxidative stress in skeletal muscle insulin resistance and atrophy. Free Radic. Biol. Med. 2016, 98, 197–207. [Google Scholar] [CrossRef] [PubMed]
- Thirupathi, A.; Pinho, R.A.; Ugbolue, U.C.; He, Y.; Meng, Y.; Gu, Y. Effect of Running Exercise on Oxidative Stress Biomarkers: A Systematic Review. Front. Physiol. 2021, 11, 610112. [Google Scholar] [CrossRef] [PubMed]
- Cerqueira, É.; Marinho, D.A.; Neiva, H.P.; Lourenço, O. Inflammatory Effects of High and Moderate Intensity Exercise-A Systematic Review. Front. Physiol. 2020, 10, 1550. [Google Scholar] [CrossRef]
- Przewłócka, K.; Folwarski, M.; Kaźmierczak-Siedlecka, K.; Skonieczna-Żydecka, K.; Kaczor, J.J. Gut-Muscle Axis Exists and May Affect Skeletal Muscle Adaptation to Training. Nutrients 2020, 12, 1451. [Google Scholar] [CrossRef]
- Gleeson, M.; Nieman, D.C.; Pedersen, B.K. Exercise, nutrition and immune function. J. Sports Sci. 2004, 22, 115–125. [Google Scholar] [CrossRef]
- Radak, Z.; Chung, H.Y.; Koltai, E.; Taylor, A.W.; Goto, S. Exercise, oxidative stress and hormesis. Ageing Res. Rev. 2008, 7, 34–42. [Google Scholar] [CrossRef]
- Jin, C.H.; Paik, I.Y.; Kwak, Y.S.; Jee, Y.S.; Kim, J.Y. Exhaustive submaximal endurance and resistance exercises induce temporary immunosuppression via physical and oxidative stress. J. Exerc. Rehabil. 2015, 11, 198–203. [Google Scholar] [CrossRef]
- Yuan, X.; Xu, S.; Huang, H.; Liang, J.; Wu, Y.; Li, C.; Yuan, H.; Zhao, X.; Lai, X.; Hou, S. Influence of excessive exercise on immunity, metabolism, and gut microbial diversity in an overtraining mice model. Scand. J. Med. Sci. Sports. 2018, 28, 1541–1551. [Google Scholar] [CrossRef]
- Scartoni, F.R.; Sant’Ana, L.O.; Murillo-Rodriguez, E.; Yamamoto, T.; Imperatori, C.; Budde, H.; Vianna, J.M.; Machado, S. Physical Exercise and Immune System in the Elderly: Implications and Importance in COVID-19 Pandemic Period. Front. Psychol. 2020, 11, 593903. [Google Scholar] [CrossRef]
- Scheffer, D.D.L.; Latini, A. Exercise-induced immune system response: Anti-inflammatory status on peripheral and central organs. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165823. [Google Scholar] [CrossRef] [PubMed]
- Radak, Z.; Chung, H.Y.; Goto, S. Exercise and hormesis: Oxidative stress-related adaptation for successful aging. Biogerontology 2005, 6, 71–75. [Google Scholar] [CrossRef] [PubMed]
- De la Fuente, M.; Cruces, J.; Hernandez, O.; Ortega, E. Strategies to improve the functions and redox state of the immune system in aged subjects. Curr. Pharm. Des. 2011, 17, 3966–3993. [Google Scholar] [CrossRef]
- Rebelo-Marques, A.; De Sousa Lages, A.; Andrade, R.; Ribeiro, C.F.; Mota-Pinto, A.; Carrilho, F.; Espregueira-Mendes, J. Aging Hallmarks: The Benefits of Physical Exercise. Front. Endocrinol. 2018, 9, 258. [Google Scholar] [CrossRef]
- Carapeto, P.V.; Aguayo-Mazzucato, C. Effects of exercise on cellular and tissue aging. Aging 2021, 13, 14522–14543. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Hoffman-Goetz, L. Exercise and the immune system: Regulation, integration, and adaptation. Physiol. Rev. 2000, 80, 1055–1081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De la Fuente, M.; Hernanz, A.; Vallejo, M.C. The immune system in the oxidative stress conditions of aging and hypertension: Favorable effects of antioxidants and physical exercise. Antioxid. Redox Signal. 2005, 7, 1356–1366. [Google Scholar] [CrossRef] [PubMed]
- Giraldo, E.; Martin-Cordero, L.; Garcia, J.J. Gerhmann, M.; Multhoff, G.; Ortega, E. Exercise-induced extracellular 72 kDa heat shock protein (Hsp72) stimulates neutrophil phagocytic and fungicidal capacities via TLR-2. Eur. J. Appl. Physiol. 2010, 108, 217–225. [Google Scholar] [CrossRef]
- De Souza Teixeira, A.A.; Lira, F.S.; Rosa-Neto, J.C. Aging with rhythmicity. Is it possible? Physical exercise as a pacemaker. Life Sci. 2020, 261, 118453. [Google Scholar] [CrossRef] [PubMed]
- Ludlow, A.T.; Roth, S.M. Physical activity and telomere biology: Exploring the link with aging-related disease prevention. J. Aging Res. 2011, 2011, 790378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef]
- Sallam, N.; Laher, I. Exercise Modulates Oxidative Stress and Inflammation in Aging and Cardiovascular Diseases. Oxid. Med. Cell. Longev. 2016, 2016, 7239639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franceschi, C.; Campisi, J. Chronic inflammation (inflammaging) and its potential contribution to age-associated diseases. J. Gerontol. A Biol. Sci. Med. Sci. 2014, 69, S4–S9. [Google Scholar] [CrossRef] [PubMed]
- Bauer, M.E.; De La Fuente, M. The role of oxidative and inflammatory stress and persistent viral infections in immunosenescence. Mech. Ageing Dev. 2016, 158, 27–37. [Google Scholar] [CrossRef]
- Fulop, T.; Larbi, A.; Dupuis, G.; Le Page, A.; Frost, E.H.; Cohen, A.A.; Witkowski, J.M.; Franceschi, C. Immunosenescence and Inflamm-Aging As Two Sides of the Same Coin: Friends or Foes? Front. Immunol. 2018, 8, 1960. [Google Scholar] [CrossRef] [Green Version]
- Vida, C.; Martinez de Toda, I.; Garrido, A.; Carro, E.; Molina, J.A.; De la Fuente, M. Impairment of Several Immune Functions and Redox State in Blood Cells of Alzheimer’s Disease Patients. Relevant Role of Neutrophils in Oxidative Stress. Front. Immunol. 2018, 8, 1974. [Google Scholar] [CrossRef] [Green Version]
- Vida, C.; Kobayashi, H.; Garrido, A.; Martínez de Toda, I.; Carro, E.; Molina, J.A.; De la Fuente, M. Lymphoproliferation Impairment and Oxidative Stress in Blood Cells from Early Parkinson’s Disease Patients. Int. J. Mol. Sci. 2019, 20, 771. [Google Scholar] [CrossRef] [Green Version]
- Martínez de Toda, I.; Miguélez, L.; Vida, C.; Carro, E.; De la Fuente, M. Altered Redox State in Whole Blood Cells from Patients with Mild Cognitive Impairment and Alzheimer’s Disease. J. Alzheimers Dis. 2019, 71, 153–163. [Google Scholar] [CrossRef]
- Maté, I.; Martínez de Toda, I.; Arranz, L.; Álvarez-Sala, J.L.; De la Fuente, M. Accelerated immunosenescence, oxidation and inflammation lead to a higher biological age in COPD patients. Exp. Gerontol. 2021, 154, 111551. [Google Scholar] [CrossRef] [PubMed]
- Franceschi, C.; Garagnani, P.; Morsiani, C.; Conte, M.; Santoro, A.; Grignolio, A.; Monti, D.; Capri, M.; Salvioli, S. The Continuum of Aging and Age-Related Diseases: Common Mechanisms but Different Rates. Front. Med. 2018, 5, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martínez de Toda, I.; Ceprián, N.; Díaz-Del Cerro, E.; De la Fuente, M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells 2021, 10, 2974. https://doi.org/10.3390/cells10112974
Martínez de Toda I, Ceprián N, Díaz-Del Cerro E, De la Fuente M. The Role of Immune Cells in Oxi-Inflamm-Aging. Cells. 2021; 10(11):2974. https://doi.org/10.3390/cells10112974
Chicago/Turabian StyleMartínez de Toda, Irene, Noemi Ceprián, Estefanía Díaz-Del Cerro, and Mónica De la Fuente. 2021. "The Role of Immune Cells in Oxi-Inflamm-Aging" Cells 10, no. 11: 2974. https://doi.org/10.3390/cells10112974