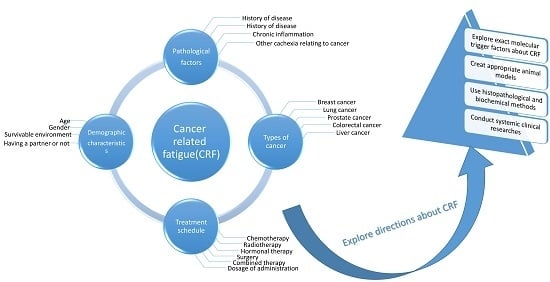
A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis
Abstract
:1. Background
2. Prevalence of CRF and Its Association with Cancer and Cancer Therapy
3. Skeletal Muscular and Mitochondrial Dysfunction
4. Peripheral Immune Activation and Inflammation Dysfunction
5. Neuron and Central Nervous System (CNS) Disorder
6. Conclusion and Discussion
Author Contributions
Funding
Conflicts of Interest
References
- Yancey, J.R.; Thomas, S.M. Chronic fatigue syndrome: Diagnosis and treatment. Am. Fam. Physician 2012, 86, 741–746. [Google Scholar] [PubMed]
- Hawley, J.A.; Reilly, T. Fatigue revisited. J. Sports Sci. 1997, 15, 245–246. [Google Scholar] [PubMed]
- Urrila, A.S.; Paunio, T.; Palomaki, E.; Marttunen, M. Sleep in adolescent depression: Physiological perspectives. Acta Physiol. 2015, 213, 758–777. [Google Scholar] [CrossRef] [PubMed]
- Ce, E.; Rampichini, S.; Limonta, E.; Esposito, F. Fatigue effects on the electromechanical delay components during the relaxation phase after isometric contraction. Acta Physiol. 2014, 211, 82–96. [Google Scholar] [CrossRef] [PubMed]
- Cancer-related fatigue. Clinical practice guidelines in oncology. J. Natl. Compr. Canc. Netw. 2003, 1, 308–331.
- Goldstein, D.; Bennett, B.K.; Webber, K.; Boyle, F.; de Souza, P.L.; Wilcken, N.R.; Scott, E.M.; Toppler, R.; Murie, P.; O’Malley, L.; et al. Cancer-related fatigue in women with breast cancer: Outcomes of a 5-year prospective cohort study. J. Clin. Oncol. 2012, 30, 1805–1812. [Google Scholar] [CrossRef] [PubMed]
- Servaes, P.; Gielissen, M.F.; Verhagen, S.; Bleijenberg, G. The course of severe fatigue in disease-free breast cancer patients: A longitudinal study. Psychooncology 2007, 16, 787–795. [Google Scholar] [CrossRef] [PubMed]
- Andrykowski, M.A.; Schmidt, J.E.; Salsman, J.M.; Beacham, A.O.; Jacobsen, P.B. Use of a case definition approach to identify cancer-related fatigue in women undergoing adjuvant therapy for breast cancer. J. Clin. Oncol. 2005, 23, 6613–6622. [Google Scholar] [CrossRef]
- Thong, M.S.; Mols, F.; Wang, X.S.; Lemmens, V.E.; Smilde, T.J.; van de Poll-Franse, L.V. Quantifying fatigue in (long-term) colorectal cancer survivors: A study from the population-based patient reported outcomes following initial treatment and long term evaluation of survivorship registry. Eur. J. Cancer 2013, 49, 1957–1966. [Google Scholar] [CrossRef]
- Bower, J.E.; Lamkin, D.M. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behav. Immun. 2013, 30, S48–S57. [Google Scholar] [CrossRef]
- Prue, G.; Rankin, J.; Allen, J.; Gracey, J.; Cramp, F. Cancer-related fatigue: A critical appraisal. Eur. J. Cancer 2006, 42, 846–863. [Google Scholar] [CrossRef] [PubMed]
- Evans, W.J.; Morley, J.E.; Argiles, J.; Bales, C.; Baracos, V.; Guttridge, D.; Jatoi, A.; Kalantar-Zadeh, K.; Lochs, H.; Mantovani, G.; et al. Cachexia: A new definition. Clin. Nutr. 2008, 27, 793–799. [Google Scholar] [CrossRef] [PubMed]
- Fearon, K.; Strasser, F.; Anker, S.D.; Bosaeus, I.; Bruera, E.; Fainsinger, R.L.; Jatoi, A.; Loprinzi, C.; MacDonald, N.; Mantovani, G.; et al. Definition and classification of cancer cachexia: An international consensus. Lancet Oncol. 2011, 12, 489–495. [Google Scholar] [CrossRef]
- Vanhoutte, G.; van de Wiel, M.; Wouters, K.; Sels, M.; Bartolomeeussen, L.; de Keersmaecker, S.; Verschueren, E.; de Vroey, V.; de Wilde, A.; Smits, E.; et al. Cachexia in cancer: What is in the definition? BMJ. Open Gastroenterol. 2016, 3, e000097. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Busquets, S.; Stemmler, B.; Lopez-Soriano, F.J. Cancer cachexia: Understanding the molecular basis. Nat. Rev. Cancer 2014, 14, 754–762. [Google Scholar] [CrossRef]
- Grimble, R.F. Nutritional therapy for cancer cachexia. Gut 2003, 52, 1391–1392. [Google Scholar] [CrossRef] [PubMed]
- Gullett, N.P.; Mazurak, V.C.; Hebbar, G.; Ziegler, T.R. Nutritional interventions for cancer-induced cachexia. Curr. Probl. Cancer 2011, 35, 58–90. [Google Scholar] [CrossRef]
- Miller, M.; Maguire, R.; Kearney, N. Patterns of fatigue during a course of chemotherapy: Results from a multi-centre study. Eur. J. Oncol. Nurs. 2007, 11, 126–132. [Google Scholar] [CrossRef]
- Cleeland, C.S.; Bennett, G.J.; Dantzer, R.; Dougherty, P.M.; Dunn, A.J.; Meyers, C.A.; Miller, A.H.; Payne, R.; Reuben, J.M.; Wang, X.S. Are the symptoms of cancer and cancer treatment due to a shared biologic mechanism? Cancer 2003, 97, 2919–2925. [Google Scholar] [CrossRef]
- Abrahams, H.J.; Gielissen, M.F.; Schmits, I.C.; Verhagen, C.A.; Rovers, M.M.; Knoop, H. Risk factors, prevalence, and course of severe fatigue after breast cancer treatment: A meta-analysis involving 12,327 breast cancer survivors. Ann. Oncol. 2016, 27, 965–974. [Google Scholar] [CrossRef]
- Prigozin, A.; Uziely, B.; Musgrave, C.F. The relationship between symptom severity and symptom interference, education, age, marital status, and type of chemotherapy treatment in Israeli women with early-stage breast cancer. Oncol. Nurs. Forum 2010, 37, 411–418. [Google Scholar] [CrossRef] [PubMed]
- Berger, A.M.; Lockhart, K.; Agrawal, S. Variability of patterns of fatigue and quality of life over time based on different breast cancer adjuvant chemotherapy regimens. Oncol. Nurs. Forum 2009, 36, 563–570. [Google Scholar] [CrossRef] [PubMed]
- Gilliam, L.A.; St Clair, D.K. Chemotherapy-induced weakness and fatigue in skeletal muscle: The role of oxidative stress. Antioxid. Redox Signal. 2011, 15, 2543–2563. [Google Scholar] [CrossRef] [PubMed]
- Larsen, S.; Nielsen, J.; Hansen, C.N.; Nielsen, L.B.; Wibrand, F.; Stride, N.; Schroder, H.D.; Boushel, R.; Helge, J.W.; Dela, F.; et al. Biomarkers of mitochondrial content in skeletal muscle of healthy young human subjects. J. Physiol. 2012, 590, 3349–3360. [Google Scholar] [CrossRef] [PubMed]
- Argiles, J.M.; Lopez-Soriano, F.J.; Busquets, S. Muscle wasting in cancer: The role of mitochondria. Curr. Opin. Clin. Nutr. Metab. Care 2015, 18, 221–225. [Google Scholar] [CrossRef] [PubMed]
- Agudelo, D.; Bourassa, P.; Berube, G.; Tajmir-Riahi, H.A. Intercalation of antitumor drug doxorubicin and its analogue by DNA duplex: Structural features and biological implications. Int. J. Biol. Macromol. 2014, 66, 144–150. [Google Scholar] [CrossRef] [PubMed]
- Quach, B.; Birk, A.; Szeto, H. Mechanism of preventing doxorubicin-induced mitochondrial toxicity with cardiolipin-targeted peptide, SS-31. FASEB J. 2014, 28, 966. [Google Scholar]
- Cheregi, B.; Timpani, C.; Nurgali, K.; Hayes, A.; Rybalka, E. Chemotherapy-induced mitochondrial respiratory dysfunction, oxidant production and death in healthy skeletal muscle C2C12 myoblast and myotube models. Neuromuscul. Disord. 2015, 25, S202. [Google Scholar] [CrossRef]
- Deavall, D.G.; Martin, E.A.; Horner, J.M.; Roberts, R. Drug-induced oxidative stress and toxicity. J. Toxicol. 2012, 2012, 645460. [Google Scholar] [CrossRef]
- Sawyer, D.B.; Peng, X.; Chen, B.; Pentassuglia, L.; Lim, C.C. Mechanisms of anthracycline cardiac injury: Can we identify strategies for cardioprotection? Prog. Cardiovasc. Dis. 2010, 53, 105–113. [Google Scholar] [CrossRef]
- Sarosiek, K.A.; Ni Chonghaile, T.; Letai, A. Mitochondria: Gatekeepers of response to chemotherapy. Trends Cell Biol. 2013, 23, 612–619. [Google Scholar] [CrossRef] [PubMed]
- Wallace, D.C. Mitochondria and cancer. Nat. Rev. Cancer 2012, 12, 685–698. [Google Scholar] [CrossRef] [Green Version]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial reactive oxygen species (ROS) and ROS-induced ROS release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [PubMed]
- Dirks-Naylor, A.J.; Tran, N.T.; Yang, S.; Mabolo, R.; Kouzi, S.A. The effects of acute doxorubicin treatment on proteome lysine acetylation status and apical caspases in skeletal muscle of fasted animals. J. Cachexia Sarcopenia Muscle 2013, 4, 239–243. [Google Scholar] [CrossRef]
- Gilliam, L.A.; Moylan, J.S.; Callahan, L.A.; Sumandea, M.P.; Reid, M.B. Doxorubicin causes diaphragm weakness in murine models of cancer chemotherapy. Muscle Nerve 2011, 43, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Ismail, H.M.; Dorchies, O.M.; Perozzo, R.; Strosova, M.K.; Scapozza, L.; Ruegg, U.T. Inhibition of iPLA2 beta and of stretch-activated channels by doxorubicin alters dystrophic muscle function. Br. J. Pharmacol. 2013, 169, 1537–1550. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Walder, K.; Maes, M. Central pathways causing fatigue in neuro-inflammatory and autoimmune illnesses. BMC Med. 2015, 13, 28. [Google Scholar] [CrossRef]
- Bai, P.; Canto, C.; Oudart, H.; Brunyanszki, A.; Cen, Y.; Thomas, C.; Yamamoto, H.; Huber, A.; Kiss, B.; Houtkooper, R.H.; et al. PARP-1 inhibition increases mitochondrial metabolism through SIRT1 activation. Cell Metab. 2011, 13, 461–468. [Google Scholar] [CrossRef]
- Zong, W.X.; Ditsworth, D.; Bauer, D.E.; Wang, Z.Q.; Thompson, C.B. Alkylating DNA damage stimulates a regulated form of necrotic cell death. Genes Dev. 2004, 18, 1272–1282. [Google Scholar] [CrossRef] [Green Version]
- Niere, M.; Kernstock, S.; Koch-Nolte, F.; Ziegler, M. Functional localization of two poly(ADP-ribose)-degrading enzymes to the mitochondrial matrix. Mol. Cell. Biol. 2008, 28, 814–824. [Google Scholar] [CrossRef]
- Gourdier, I.; Crabbe, L.; Andreau, K.; Pau, B.; Kroemer, G. Oxaliplatin-induced mitochondrial apoptotic response of colon carcinoma cells does not require nuclear DNA. Oncogene 2004, 23, 7449–7457. [Google Scholar] [CrossRef] [Green Version]
- Lutsenko, S.; Barnes, N.L.; Bartee, M.Y.; Dmitriev, O.Y. Function and regulation of human copper-transporting ATPases. Physiol. Rev. 2007, 87, 1011–1046. [Google Scholar] [CrossRef]
- Neel, B.A.; Lin, Y.; Pessin, J.E. Skeletal muscle autophagy: A new metabolic regulator. Trends Endocrinol. Metab. 2013, 24, 635–643. [Google Scholar] [CrossRef]
- Lind, M.J. Principles of cytotoxic chemotherapy. Medicine 2004, 32, 20–25. [Google Scholar] [CrossRef]
- Singh, R.; Teel, C.; Sabus, C.; McGinnis, P.; Kluding, P. Fatigue in Type 2 Diabetes: Impact on Quality of Life and Predictors. PLoS ONE 2016, 11, e0165652. [Google Scholar] [CrossRef]
- Lazzarino, G.; Amorini, A.M.; Eikelenboom, M.J.; Killestein, J.; Belli, A.; di Pietro, V.; Tavazzi, B.; Barkhof, F.; Polman, C.H.; Uitdehaag, B.M.; et al. Cerebrospinal fluid ATP metabolites in multiple sclerosis. Mult. Scler. J. 2010, 16, 549–554. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. Mitochondrial dysfunctions in myalgic encephalomyelitis/chronic fatigue syndrome explained by activated immuno-inflammatory, oxidative and nitrosative stress pathways. Metab. Brain Dis. 2014, 29, 19–36. [Google Scholar] [CrossRef]
- Booth, N.E.; Myhill, S.; McLaren-Howard, J. Mitochondrial dysfunction and the pathophysiology of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS). Int. J. Clin. Exp. Med. 2012, 5, 208–220. [Google Scholar]
- Myhill, S.; Booth, N.E.; McLaren-Howard, J. Targeting mitochondrial dysfunction in the treatment of Myalgic Encephalomyelitis/Chronic Fatigue Syndrome (ME/CFS)—A clinical audit. Int. J. Clin. Exp. Med. 2013, 6, 1. [Google Scholar]
- Behan, W.M.; Mcdonald, M.; Darlington, L.G.; Stone, T.W. Oxidative stress as a mechanism for quinolinic acid-induced hippocampal damage: Protection by melatonin and deprenyl. Br. J. Pharmacol. 1999, 128, 1754–1760. [Google Scholar] [CrossRef]
- Hollingsworth, K.G.; Jones, D.E.; Taylor, R.; Blamire, A.M.; Newton, J.L. Impaired cardiovascular response to standing in chronic fatigue syndrome. Eur. J. Clin. Investig. 2010, 40, 608–615. [Google Scholar] [CrossRef]
- Jones, D.E.; Hollingsworth, K.G.; Taylor, R.; Blamire, A.M.; Newton, J.L. Abnormalities in pH handling by peripheral muscle and potential regulation by the autonomic nervous system in chronic fatigue syndrome. J. Intern. Med. 2010, 267, 394–401. [Google Scholar] [CrossRef]
- Perl, A.; Hanczko, R.; Doherty, E. Assessment of mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. Methods Mol. Biol. 2012, 900, 61–89. [Google Scholar]
- Perl, A.; Nagy, G.; Gergely, P.; Puskas, F.; Qian, Y.; Banki, K. Apoptosis and mitochondrial dysfunction in lymphocytes of patients with systemic lupus erythematosus. Methods Mol. Med. 2004, 102, 87–114. [Google Scholar]
- Nagy, G.; Koncz, A.; Fernandez, D.; Perl, A. Nitric oxide, mitochondrial hyperpolarization, and T cell activation. Free Radic. Biol. Med. 2007, 42, 1625–1631. [Google Scholar] [CrossRef] [Green Version]
- Morris, G.; Maes, M. Oxidative and Nitrosative Stress and Immune-Inflammatory Pathways in Patients with Myalgic Encephalomyelitis (ME)/Chronic Fatigue Syndrome (CFS). Curr. Neuropharmacol. 2014, 12, 168–185. [Google Scholar] [CrossRef]
- Lutz, N.W.; Cozzone, P.J. Metabolic profiling in multiple sclerosis and other disorders by quantitative analysis of cerebrospinal fluid using nuclear magnetic resonance spectroscopy. Curr. Pharm. Biotechnol. 2011, 12, 1016–1025. [Google Scholar] [CrossRef]
- Lutz, N.W.; Viola, A.; Malikova, I.; Confort-Gouny, S.; Audoin, B.; Ranjeva, J.P.; Pelletier, J.; Cozzone, P.J. Inflammatory multiple-sclerosis plaques generate characteristic metabolic profiles in cerebrospinal fluid. PLoS ONE 2007, 2, e595. [Google Scholar] [CrossRef]
- Reinke, S.N.; Broadhurst, D.L.; Sykes, B.D.; Baker, G.B.; Catz, I.; Warren, K.G.; Power, C. Metabolomic profiling in multiple sclerosis: Insights into biomarkers and pathogenesis. Mult. Scler. J. 2014, 20, 1396–1400. [Google Scholar] [CrossRef]
- Miaskowski, C.; Aouizerat, B.E.; Dodd, M.; Cooper, B. Conceptual issues in symptom clusters research and their implications for quality-of-life assessment in patients with cancer. J. Natl. Cancer Inst. Monogr. 2007, 2007, 39–46. [Google Scholar] [CrossRef]
- Teunissen, S.C.; Wesker, W.; Kruitwagen, C.; de Haes, H.C.; Voest, E.E.; de Graeff, A. Symptom prevalence in patients with incurable cancer: A systematic review. J. Pain Symptom Manag. 2007, 34, 94–104. [Google Scholar] [CrossRef]
- Molfino, A.; Formiconi, A.; Rossi Fanelli, F.; Muscaritoli, M. Ghrelin: From discovery to cancer cachexia therapy. Curr. Opin. Clin. Nutr. Metab. Care 2014, 17, 471–476. [Google Scholar] [CrossRef]
- Kim, H.J.; Kim, H.J.; Yun, J.; Kim, K.H.; Kim, S.H.; Lee, S.C.; Bae, S.B.; Kim, C.K.; Lee, N.S.; Lee, K.T.; et al. Pathophysiological role of hormones and cytokines in cancer cachexia. J. Korean Med. Sci. 2012, 27, 128–134. [Google Scholar] [CrossRef]
- Mak, R.H.; Cheung, W.W.; Gertler, A. Exploiting the therapeutic potential of leptin signaling in cachexia. Curr. Opin. Support. Palliat. Care 2014, 8, 352–357. [Google Scholar] [CrossRef]
- Wolf, I.; Sadetzki, S.; Kanety, H.; Kundel, Y.; Pariente, C.; Epstein, N.; Oberman, B.; Catane, R.; Kaufman, B.; Shimon, I. Adiponectin, ghrelin, and leptin in cancer cachexia in breast and colon cancer patients. Cancer 2006, 106, 966–973. [Google Scholar] [CrossRef]
- Morris, G.; Anderson, G.; Galecki, P.; Berk, M.; Maes, M. A narrative review on the similarities and dissimilarities between myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS) and sickness behavior. BMC Med. 2013, 11, 64. [Google Scholar] [CrossRef]
- Norheim, K.B.; Jonsson, G.; Omdal, R. Biological mechanisms of chronic fatigue. Rheumatology 2011, 50, 1009–1018. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.N.; Dantzer, R.; Langley, K.E.; Bennett, G.J.; Dougherty, P.M.; Dunn, A.J.; Meyers, C.A.; Miller, A.H.; Payne, R.; Reuben, J.M.; et al. A cytokine-based neuroimmunologic mechanism of cancer-related symptoms. Neuroimmunomodulation 2004, 11, 279–292. [Google Scholar] [CrossRef]
- Miaskowski, C.; Aouizerat, B.E. Is there a biological basis for the clustering of symptoms? Semin. Oncol. Nurs. 2007, 23, 99–105. [Google Scholar] [CrossRef]
- Dantzer, R. Cytokine, sickness behavior, and depression. Neurol. Clin. 2006, 24, 441–460. [Google Scholar] [CrossRef]
- Fernandez-Gonzalo, R.; de Paz, J.A.; Rodriguez-Miguelez, P.; Cuevas, M.J.; Gonzalez-Gallego, J. Effects of eccentric exercise on toll-like receptor 4 signaling pathway in peripheral blood mononuclear cells. J. Appl. Physiol. 2012, 112, 2011–2018. [Google Scholar] [CrossRef] [Green Version]
- Jialal, I.; Kaur, H.; Devaraj, S. Toll-like receptor status in obesity and metabolic syndrome: A translational perspective. J. Clin. Endocrinol. Metab. 2014, 99, 39–48. [Google Scholar] [CrossRef]
- Lucas, K.; Maes, M. Role of the Toll Like receptor (TLR) radical cycle in chronic inflammation: Possible treatments targeting the TLR4 pathway. Mol. Neurobiol. 2013, 48, 190–204. [Google Scholar] [CrossRef]
- Ader, R. Psychoneuroimmunology. ILAR J. 1998, 39, 27–29. [Google Scholar] [CrossRef] [Green Version]
- Li, M.C.; He, S.H. IL-10 and its related cytokines for treatment of inflammatory bowel disease. World J. Gastroenterol. 2004, 10, 620–625. [Google Scholar] [CrossRef]
- Gilbertson-White, S.; Aouizerat, B.E.; Miaskowski, C. Methodologic issues in the measurement of cytokines to elucidate the biological basis for cancer symptoms. Biol. Res. Nurs. 2011, 13, 15–24. [Google Scholar] [CrossRef]
- Goedendorp, M.M.; Tack, C.J.; Steggink, E.; Bloot, L.; Bazelmans, E.; Knoop, H. Chronic fatigue in type 1 diabetes: Highly prevalent but not explained by hyperglycemia or glucose variability. Diabetes Care 2014, 37, 73–80. [Google Scholar] [CrossRef]
- Willems, L.M.; Kwakkenbos, L.; Leite, C.C.; Thombs, B.D.; van den Hoogen, F.H.; Maia, A.C.; Vliet Vlieland, T.P.; van den Ende, C.H. Frequency and impact of disease symptoms experienced by patients with systemic sclerosis from five European countries. Clin. Exp. Rheumatol. 2014, 32, 88–93. [Google Scholar]
- Hewlett, S.; Chalder, T.; Choy, E.; Cramp, F.; Davis, B.; Dures, E.; Nicholls, C. Kirwan, J. Fatigue in rheumatoid arthritis: Time for a conceptual model. Rheumatology 2011, 50, 1004–1006. [Google Scholar] [CrossRef]
- Meyers, C.A.; Albitar, M.; Estey, E. Cognitive impairment, fatigue, and cytokine levels in patients with acute myelogenous leukemia or myelodysplastic syndrome. Cancer 2005, 104, 788–793. [Google Scholar] [CrossRef]
- Reyes-Gibby, C.C.; Wang, J.; Spitz, M.; Wu, X.; Yennurajalingam, S.; Shete, S. Genetic variations in interleukin-8 and interleukin-10 are associated with pain, depressed mood, and fatigue in lung cancer patients. J. Pain Symptom Manag. 2013, 46, 161–172. [Google Scholar] [CrossRef]
- Miaskowski, C.; Cooper, B.A.; Dhruva, A.; Dunn, L.B.; Langford, D.J.; Cataldo, J.K.; Baggott, C.R.; Merriman, J.D.; Dodd, M.; Lee, K.; et al. Evidence of Associations between Cytokine Genes and Subjective Reports of Sleep Disturbance in Oncology Patients and Their Family Caregivers. PLoS ONE 2012, 7, e40560. [Google Scholar] [CrossRef]
- Irwin, M.R. Inflammation at the intersection of behavior and somatic symptoms. Psychiatr. Clin. N. Am. 2011, 34, 605–620. [Google Scholar] [CrossRef]
- Flachenecker, P.; Bihler, I.; Weber, F.; Gottschalk, M.; Toyka, K.V.; Rieckmann, P. Cytokine mRNA expression in patients with multiple sclerosis and fatigue. Mult. Scler. J. 2004, 10, 165–169. [Google Scholar] [CrossRef]
- Heesen, C.; Nawrath, L.; Reich, C.; Bauer, N.; Schulz, K.H.; Gold, S.M. Fatigue in multiple sclerosis: An example of cytokine mediated sickness behaviour? J. Neurol. Neurosurg. Psychiatry 2006, 77, 34–39. [Google Scholar] [CrossRef]
- Bower, J.E. Cancer-related fatigue: Links with inflammation in cancer patients and survivors. Brain Behav. Immun. 2007, 21, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Bower, J.E. Cancer-related fatigue—Mechanisms, risk factors, and treatments. Nat. Rev. Clin. Oncol. 2014, 11, 597–609. [Google Scholar] [CrossRef]
- Lee, Y.C.; Frits, M.L.; Iannaccone, C.K.; Weinblatt, M.E.; Shadick, N.A.; Williams, D.A.; Cui, J. Subgrouping of patients with rheumatoid arthritis based on pain, fatigue, inflammation, and psychosocial factors. Arthritis Rheumatol. 2014, 66, 2006–2014. [Google Scholar] [CrossRef]
- Norden, D.M.; Bicer, S.; Clark, Y.; Jing, R.F.; Henry, C.J.; Wold, L.E.; Reiser, P.J.; Godbout, J.P.; McCarthy, D.O. Tumor growth increases neuroinflammation, fatigue and depressive-like behavior prior to alterations in muscle function. Brain Behav. Immun. 2015, 43, 76–85. [Google Scholar] [CrossRef]
- Arnett, S.V.; Clark, I.A. Inflammatory fatigue and sickness behavior—Lessons for the diagnosis and management of chronic fatigue syndrome. J. Affect Disord. 2012, 141, 130–142. [Google Scholar] [CrossRef]
- Bluthe, R.M.; Beaudu, C.; Kelley, K.W.; Dantzer, R. Differential effects of IL-1ra on sickness behavior and weight loss induced by IL-1 in rats. Brain Res. 1995, 677, 171–176. [Google Scholar] [CrossRef]
- Heinzelmann, M.; Lee, H.; Rak, H.; Livingston, W.; Barr, T.; Baxter, T.; Scattergood-Keepper, L.; Mysliwiec, V.; Gill, J. Sleep restoration is associated with reduced plasma C-reactive protein and depression symptoms in military personnel with sleep disturbance after deployment. Sleep Med. 2014, 15, 1565–1570. [Google Scholar] [CrossRef]
- Chauffier, K.; Salliot, C.; Berenbaum, F.; Sellam, J. Effect of biotherapies on fatigue in rheumatoid arthritis: A systematic review of the literature and meta-analysis. Rheumatology 2012, 51, 60–68. [Google Scholar] [CrossRef]
- Chrousos, G.P. The hypothalamic-pituitary-adrenal axis and immune-mediated inflammation. N. Engl. J. Med. 1995, 332, 1351–1362. [Google Scholar] [CrossRef]
- Masson, C. Rheumatoid anemia. Jt. Bone Spine 2011, 78, 131–137. [Google Scholar] [CrossRef] [Green Version]
- Schmidt, C.; Peng, B.; Li, Z.; Sclabas, G.M.; Fujioka, S.; Niu, J.; Schmidt-Supprian, M.; Evans, D.B.; Abbruzzese, J.L.; Chiao, P.J. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol. Cell 2003, 12, 1287–1300. [Google Scholar] [CrossRef]
- Tabruyn, S.P.; Memet, S.; Ave, P.; Verhaeghe, C.; Mayo, K.H.; Struman, I.; Martial, J.A.; Griffioen, A.W. NF-kappaB activation in endothelial cells is critical for the activity of angiostatic agents. Mol. Cancer Ther. 2009, 8, 2645–2654. [Google Scholar] [CrossRef]
- Anrather, J.; Racchumi, G.; Iadecola, C. NF-kappaB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J. Biol. Chem. 2006, 281, 5657–5667. [Google Scholar] [CrossRef]
- Nakata, S.; Tsutsui, M.; Shimokawa, H.; Yamashita, T.; Tanimoto, A.; Tasaki, H.; Ozumi, K.; Sabanai, K.; Morishita, T.; Suda, O.; et al. Statin treatment upregulates vascular neuronal nitric oxide synthase through Akt/NF-kappaB pathway. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 92–98. [Google Scholar] [CrossRef]
- Sultani, M.; Stringer, A.M.; Bowen, J.M.; Gibson, R.J. Anti-inflammatory cytokines: Important immunoregulatory factors contributing to chemotherapy-induced gastrointestinal mucositis. Chemother. Res. Pract. 2012, 2012, 490804. [Google Scholar] [CrossRef]
- Sonis, S.T. A biological approach to mucositis. J. Support. Oncol. 2004, 2, 21–32. [Google Scholar]
- Sonis, S.T. Pathobiology of oral mucositis: Novel insights and opportunities. J. Support. Oncol. 2007, 5, 3–11. [Google Scholar]
- Maes, M.; Kubera, M.; Obuchowiczwa, E.; Goehler, L.; Brzeszcz, J. Depression’s multiple comorbidities explained by (neuro)inflammatory and oxidative & nitrosative stress pathways. Neuroendocr. Endocrinol. Lett. 2011, 32, 7–24. [Google Scholar]
- Maes, M.; Mihaylova, I.; Leunis, J.C. Chronic fatigue syndrome is accompanied by an IgM-related immune response directed against neopitopes formed by oxidative or nitrosative damage to lipids and proteins. Neuroendocr. Endocrinol. Lett. 2006, 27, 615–621. [Google Scholar]
- Kuper, H.; Adami, H.O.; Trichopoulos, D. Infections as a major preventable cause of human cancer. J. Intern. Med. 2001, 249, 61–74. [Google Scholar] [CrossRef]
- Morris, G.; Maes, M. A neuro-immune model of Myalgic Encephalomyelitis/Chronic fatigue syndrome. Metab. Brain Dis. 2013, 28, 523–540. [Google Scholar] [CrossRef]
- Burfeind, K.G.; Michaelis, K.A.; Marks, D.L. The central role of hypothalamic inflammation in the acute illness response and cachexia. Semin. Cell Dev. Biol. 2016, 54, 42–52. [Google Scholar] [CrossRef]
- Berk, M.; Williams, L.J.; Jacka, F.N.; O’Neil, A.; Pasco, J.A.; Moylan, S.; Allen, N.B.; Stuart, A.L.; Hayley, A.C.; Byrne, M.L.; et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013, 11, 200. [Google Scholar] [CrossRef]
- Kreisel, T.; Frank, M.G.; Licht, T.; Reshef, R.; Ben-Menachem-Zidon, O.; Baratta, M.V.; Maier, S.F.; Yirmiya, R. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol. Psychiatry 2014, 19, 699–709. [Google Scholar] [CrossRef]
- Maes, M.; Berk, M.; Goehler, L.; Song, C.; Anderson, G.; Galecki, P.; Leonard, B. Depression and sickness behavior are Janus-faced responses to shared inflammatory pathways. BMC Med. 2012, 10, 66. [Google Scholar] [CrossRef]
- Steiner, J.; Walter, M.; Gos, T.; Guillemin, G.J.; Bernstein, H.G.; Sarnyai, Z.; Mawrin, C.; Brisch, R.; Bielau, H.; Schwabedissen, L.M.Z.; et al. Severe depression is associated with increased microglial quinolinic acid in subregions of the anterior cingulate gyrus: Evidence for an immune-modulated glutamatergic neurotransmission? J. Neuroinflamm. 2013, 10, 34. [Google Scholar] [CrossRef]
- Ratel, S.; Kluka, V.; Vicencio, S.G.; Jegu, A.G.; Cardenoux, C.; Morio, C.; Coudeyre, E.; Martin, V. Insights into the Mechanisms of Neuromuscular Fatigue in Boys and Men. Med. Sci. Sport Exerc. 2015, 47, 2319–2328. [Google Scholar] [CrossRef]
- Amann, M.; Blain, G.M.; Proctor, L.T.; Sebranek, J.J.; Pegelow, D.F.; Dempsey, J.A. Implications of group III and IV muscle afferents for high-intensity endurance exercise performance in humans. J. Physiol. 2011, 589, 5299–5309. [Google Scholar] [CrossRef]
- Perry, V.H.; Cunningham, C.; Boche, D. Atypical inflammation in the central nervous system in prion disease. Curr. Opin. Neurol. 2002, 15, 349–354. [Google Scholar] [CrossRef]
- Bodnar, R.J.; Pasternak, G.W.; Mann, P.E.; Paul, D.; Warren, R.; Donner, D.B. Mediation of anorexia by human recombinant tumor necrosis factor through a peripheral action in the rat. Cancer Res. 1989, 49, 6280–6284. [Google Scholar]
- Cone, R.D.; Cowley, M.A.; Butler, A.A.; Fan, W.; Marks, D.L.; Low, M.J. The arcuate nucleus as a conduit for diverse signals relevant to energy homeostasis. Int. J. Obes. 2002, 25, S63. [Google Scholar] [CrossRef]
- Grossberg, A.J.; Scarlett, J.M.; Zhu, X.; Bowe, D.D.; Batra, A.K.; Braun, T.P.; Marks, D.L. Arcuate nucleus proopiomelanocortin neurons mediate the acute anorectic actions of leukemia inhibitory factor via gp130. Endocrinology 2010, 151, 606–616. [Google Scholar] [CrossRef]
- Lawrence, C.B.; Rothwell, N.J. Anorexic but Not Pyrogenic Actions of Interleukin-1 are Modulated by Central Melanocortin-3/4 Receptors in the Rat. J. Neuroendocrinol. 2001, 13, 490–495. [Google Scholar] [CrossRef]
- Sonti, G.; Ilyin, S.E.; Plata-Salamán, C.R. Anorexia induced by cytokine interactions at pathophysiological concentrations. Am. J. Physiol. 1996, 270, 1394–1402. [Google Scholar] [CrossRef]
- Morton, G.J.; Cummings, D.E.; Baskin, D.G.; Barsh, G.S.; Schwartz, M.W. Central nervous system control of food intake and body weight. Nature 2006, 443, 289–295. [Google Scholar] [CrossRef]
- Millington, G.W. The role of proopiomelanocortin (POMC) neurones in feeding behaviour. Nutr. Metab. 2007, 4, 18. [Google Scholar] [CrossRef]
- Murphy, K.G. Dissecting the role of cocaine- and amphetamine-regulated transcript (CART) in the control of appetite. Brief. Funct. Genom. Proteom. 2005, 4, 95–111. [Google Scholar] [CrossRef]
- Cowley, M.A.; Dinulescu, D.M.; Pronchuk, N.; Fan, W.; Colmers, W.F.; Cone, R.D. Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: Evidence of a cellular basis for the adipostat. Neuron 1999, 24, 155–163. [Google Scholar] [CrossRef]
- Pritchard, L.E.; Armstrong, D.; Davies, N.; Oliver, R.L.; Schmitz, C.A.; Brennand, J.C.; Wikinson, G.F.; White, A. Agouti-related protein (83-132) is a competitive antagonist at the human melanocortin-4 receptor: No evidence for differential interactions with pro-opiomelanocortin-derived ligands. J. Endocrinol. 2004, 180, 183–191. [Google Scholar] [CrossRef]
- Kuo, L.E.; Kitlinska, J.B.; Tilan, J.U.; Li, L.; Baker, S.B.; Johnson, M.D.; Lee, E.W.; Burnett, M.S.; Fricke, S.T.; Kvetnansky, R.; et al. Neuropeptide Y acts directly in the periphery on fat tissue and mediates stress-induced obesity and metabolic syndrome. Nat. Med. 2007, 13, 803–811. [Google Scholar] [CrossRef]
- Tatemoto, K.; Carlquist, M.; Mutt, V. Neuropeptide Y—A novel brain peptide with structural similarities to peptide YY and pancreatic polypeptide. Nature 1982, 296, 659–660. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Jobst, E.E.; Enriori, P.J.; Bowe, D.D.; Batra, A.K.; Grant, W.F.; Cowley, M.A.; Marks, D.L. Regulation of central melanocortin signaling by interleukin-1 beta. Endocrinology 2007, 148, 4217–4225. [Google Scholar] [CrossRef]
- Scarlett, J.M.; Zhu, X.; Enriori, P.J.; Bowe, D.D.; Batra, A.K.; Levasseur, P.R.; Grant, W.F.; Meguid, M.M.; Cowley, M.A.; Marks, D.L. Regulation of agouti-related protein messenger ribonucleic acid transcription and peptide secretion by acute and chronic inflammation. Endocrinology 2008, 149, 4837–4845. [Google Scholar] [CrossRef]
- Wisse, B.E.; Ogimoto, K.; Tang, J.; Harris, M.K., Jr.; Raines, E.W.; Schwartz, M.W. Evidence that lipopolysaccharide-induced anorexia depends upon central, rather than peripheral, inflammatory signals. Endocrinology 2007, 148, 5230–5237. [Google Scholar] [CrossRef]
- Braun, T.P.; Marks, D.L. The regulation of muscle mass by endogenous glucocorticoids. Front. Physiol. 2015, 6, 12. [Google Scholar] [CrossRef]
- Braun, T.P.; Szumowski, M.; Levasseur, P.R.; Grossberg, A.J.; Zhu, X.; Agarwal, A.; Marks, D.L. Muscle atrophy in response to cytotoxic chemotherapy is dependent on intact glucocorticoid signaling in skeletal muscle. PLoS ONE 2014, 9, e106489. [Google Scholar] [CrossRef]
- Johns, N.; Stephens, N.A.; Fearon, K.C. Muscle wasting in cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2215–2229. [Google Scholar] [CrossRef]
- Finsterer, J. Biomarkers of peripheral muscle fatigue during exercise. BMC Musculoskelet. Disord. 2011, 13, 218. [Google Scholar] [CrossRef]
- Keyser, R.E. Peripheral fatigue: High-energy phosphates and hydrogen ions. PM & R 2010, 2, 347–358. [Google Scholar]
- Vanhatalo, A.; Fulford, J.; DiMenna, F.J.; Jones, A.M. Influence of hyperoxia on muscle metabolic responses and the power-duration relationship during severe-intensity exercise in humans: A 31P magnetic resonance spectroscopy study. Exp. Physiol. 2010, 95, 528–540. [Google Scholar] [CrossRef]
- Guertin, P.A. Central pattern generator for locomotion: Anatomical, physiological, and pathophysiological considerations. Front. Neurol. 2013, 3, 183. [Google Scholar] [CrossRef]
- Green, H.J. Mechanisms of muscle fatigue in intense exercise. J. Sports Sci. 1997, 15, 247–256. [Google Scholar] [CrossRef]
- Zajac, A.; Chalimoniuk, M.; Maszczyk, A.; Golas, A.; Lngfort, J. Central and Peripheral Fatigue During Resistance Exercise—A Critical Review. J. Hum. Kinet. 2015, 49, 159–169. [Google Scholar] [CrossRef]
- Chalimoniuk, M.; Chrapusta, S.J.; Lukacova, N.; Langfort, J. Endurance training upregulates the nitric oxide/soluble guanylyl cyclase/cyclic guanosine 3’,5’-monophosphate pathway in the striatum, midbrain and cerebellum of male rats. Brain Res. 2015, 1618, 29–40. [Google Scholar] [CrossRef]
- Galdino, G.S.; Xavier, C.H.; Almeida, R.; Silva, G.; Fontes, M.A.; Menezes, G.; Duarte, I.D.; Perez, A.C. The Nitric oxide/CGMP/KATP pathway mediates systemic and central antinociception induced by resistance exercise in rats. Int. J. Neurosci. 2015, 125, 765–773. [Google Scholar] [CrossRef]
- Meeusen, R.; Watson, P. Amino acids and the brain: Do they play a role in “central fatigue”? Int. J. Sport Nutr. Exerc. Metab. 2007, 17, 37–46. [Google Scholar] [CrossRef]
- Meeusen, R.; Watson, P.; Hasegawa, H.; Roelands, B.; Piacentini, M.F. Central fatigue: The serotonin hypothesis and beyond. Sports Med. 2006, 36, 881–909. [Google Scholar] [CrossRef]
- Sutoo, D.; Akiyama, K. Regulation of brain function by exercise. Neurobiol. Dis. 2003, 13, 1–14. [Google Scholar] [CrossRef]
- Bouret, S.; Sara, S.J. Network reset: A simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005, 28, 574–582. [Google Scholar] [CrossRef]
- Romero-Gomez, M.; Jover, M.; Galan, J.J.; Ruiz, A. Gut ammonia production and its modulation. Metab. Brain Dis. 2009, 24, 147–157. [Google Scholar] [CrossRef]
- DeLuca, J.; Genova, H.M.; Capili, E.J.; Wylie, G.R. Functional neuroimaging of fatigue. Phys. Med. Rehabil. Clin. N. Am. 2009, 20, 325–337. [Google Scholar] [CrossRef]
- Genova, H.M.; Rajagopalan, V.; Deluca, J.; Das, A.; Binder, A.; Arjunan, A.; Chiaravalloti, N.; Wylie, G. Examination of cognitive fatigue in multiple sclerosis using functional magnetic resonance imaging and diffusion tensor imaging. PLoS ONE 2013, 8, e78811. [Google Scholar] [CrossRef]
- Kohl, A.D.; Wylie, G.R.; Genova, H.M.; Hillary, F.G.; Deluca, J. The neural correlates of cognitive fatigue in traumatic brain injury using functional MRI. Brain Inj. 2009, 23, 420–432. [Google Scholar] [CrossRef]
- Chaudhuri, A.; Behan, P.O. Fatigue in neurological disorders. Lancet 2004, 363, 978–988. [Google Scholar] [CrossRef]
- Filippi, M.; Rocca, M.A. MR imaging of gray matter involvement in multiple sclerosis: Implications for understanding disease pathophysiology and monitoring treatment efficacy. Am. J. Neuroradiol. 2010, 31, 1171–1177. [Google Scholar] [CrossRef]
- Messina, S.; Patti, F. Gray matters in multiple sclerosis: Cognitive impairment and structural MRI. Mult. Scler. Int. 2014, 2014, 609694. [Google Scholar] [CrossRef]
- Ceccarelli, A.; Rocca, M.A.; Pagani, E.; Colombo, B.; Martinelli, V.; Comi, G.; Filippi, M. A voxel-based morphometry study of grey matter loss in MS patients with different clinical phenotypes. Neuroimage 2008, 42, 315–322. [Google Scholar] [CrossRef]
- Henry, R.G.; Shieh, M.; Okuda, D.T.; Evangelista, A.; Gorno-Tempini, M.L.; Pelletier, D. Regional grey matter atrophy in clinically isolated syndromes at presentation. J. Neurol. Neurosurg. Psychiatry 2008, 79, 1236–1244. [Google Scholar] [CrossRef] [Green Version]
- Inglese, M.; Oesingmann, N.; Casaccia, P.; Fleysher, L. Progressive multiple sclerosis and gray matter pathology: An MRI perspective. Mt. Sinai J. Med. 2011, 78, 258–267. [Google Scholar] [CrossRef]
- Inglese, M.; Park, S.J.; Johnson, G.; Babb, J.S.; Miles, L.; Jaggi, H.; Herbert, J.; Grossman, R.I. Deep gray matter perfusion in multiple sclerosis: Dynamic susceptibility contrast perfusion magnetic resonance imaging at 3 T. Arch. Neurol. 2007, 64, 196–202. [Google Scholar] [CrossRef]
- Pellicano, C.; Gallo, A.; Li, X.; Ikonomidou, V.N.; Evangelou, I.E.; Ohayon, J.M.; Stern, S.K.; Ehrmantraut, M.; Cantor, F.; McFarland, H.F.; et al. Relationship of cortical atrophy to fatigue in patients with multiple sclerosis. Arch. Neurol. 2010, 67, 447–453. [Google Scholar] [CrossRef]
- Téllez, N.; Alonso, J.; Río, J.; Tintoré, M.; Nos, C.; Montalban, X.; Rovira, A. The basal ganglia: A substrate for fatigue in multiple sclerosis. Neuroradiology 2008, 50, 17–23. [Google Scholar] [CrossRef]
- Oberheim, N.A.; Goldman, S.A.; Nedergaard, M. Heterogeneity of astrocytic form and function. Methods Mol. Biol. 2012, 814, 23–45. [Google Scholar]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef]
- Stobart, J.L.; Anderson, C.M. Multifunctional role of astrocytes as gatekeepers of neuronal energy supply. Front. Cell. Neurosci. 2013, 7, 38. [Google Scholar] [CrossRef]
- Haider, L.; Simeonidou, C.; Steinberger, G.; Hametner, S.; Grigoriadis, N.; Deretzi, G.; Kovacs, G.G.; Kutzelnigg, A.; Lassmann, H.; Frischer, J.M. Multiple sclerosis deep grey matter: The relation between demyelination, neurodegeneration, inflammation and iron. J. Neurol. Neurosurg. Psychiatry 2014, 85, 1386–1395. [Google Scholar] [CrossRef]
- Cella, D.; Peterman, A.; Passik, S.; Jacobsen, P.; Breitbart, W. Progress toward guidelines for the management of fatigue. Oncology 1998, 12, 369–377. [Google Scholar]
- Sugihara, A.Q.; Rolle, C.E.; Lesniak, M.S. Regulatory T cells actively infiltrate metastatic brain tumors. Int. J. Oncol. 2009, 34, 1533–1540. [Google Scholar] [Green Version]
- Lu, F.; Selak, M.; O’Connor, J.; Croul, S.; Lorenzana, C.; Butunoi, C.; Kalman, B. Oxidative damage to mitochondrial DNA and activity of mitochondrial enzymes in chronic active lesions of multiple sclerosis. J. Neurol. Sci. 2000, 177, 95–103. [Google Scholar] [CrossRef]
- Raudonis, B.M.; Kelley, I.H.; Rowe, N.; Ellis, J. A Pilot Study of Proinflammatory Cytokines and Fatigue in Women with Breast Cancer During Chemotherapy. Cancer Nurs. 2017, 40, 323–331. [Google Scholar] [CrossRef]
- Bower, J.E.; Ganz, P.A.; Irwin, M.R.; Castellon, S.; Arevalo, J.; Cole, S.W. Cytokine Genetic Variations and Fatigue Among Patients with Breast Cancer. J. Clin. Oncol. 2013, 31, 1656–1661. [Google Scholar] [CrossRef]
- Cai, B.; Allexandre, D.; Rajagopalan, V.; Jiang, Z.; Siemionow, V.; Ranganathan, V.K.; Davis, M.P.; Walsh, D.; Dai, K.; Yue, G.H. Evidence of Significant Central Fatigue in Patients with Cancer-Related Fatigue during Repetitive Elbow Flexions till Perceived Exhaustion. PLoS ONE 2014, 9, e115370. [Google Scholar] [CrossRef]
- Janda, M.; Gerstner, N.; Obermair, A.; Fuerst, A.; Wachter, S.; Dieckmann, K.; Pötter, R. Quality of life changes during conformal radiation therapy for prostate carcinoma. J. Cancer 2000, 89, 1322–1328. [Google Scholar] [CrossRef] [Green Version]
- Ng, A.V. The underrecognized role of impaired muscle function in cancer-related fatigue. J. Support. Oncol. 2010, 8, 177. [Google Scholar]
- Mortimer, J.E.; Waliany, S.; Dieli-Conwright, C.M.; Patel, S.K.; Hurria, A.; Chao, J.; Tiep, B.; Behrendt, C.E. Objective physical and mental markers of self-reported fatigue in women undergoing (neo)adjuvant chemotherapy for early-stage breast cancer. Cancer 2017, 123, 1810–1816. [Google Scholar] [CrossRef]
- Kanzaki, A.; Okauchi, T.; Hu, D.; Shingaki, T.; Katayama, Y.; Koyama, H.; Watanabe, Y.; Cui, Y. Extension of recovery time from fatigue by repeated rest with short-term sleep during continuous fatigue load: Development of chronic fatigue model. J. Neurosci. Res. 2016, 94, 424–429. [Google Scholar] [CrossRef]




© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, S.; Chu, S.; Gao, Y.; Ai, Q.; Liu, Y.; Li, X.; Chen, N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells 2019, 8, 738. https://doi.org/10.3390/cells8070738
Yang S, Chu S, Gao Y, Ai Q, Liu Y, Li X, Chen N. A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis. Cells. 2019; 8(7):738. https://doi.org/10.3390/cells8070738
Chicago/Turabian StyleYang, Songwei, Shifeng Chu, Yan Gao, Qidi Ai, Yingjiao Liu, Xun Li, and Naihong Chen. 2019. "A Narrative Review of Cancer-Related Fatigue (CRF) and Its Possible Pathogenesis" Cells 8, no. 7: 738. https://doi.org/10.3390/cells8070738