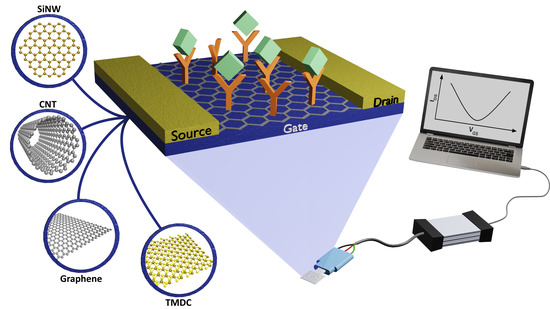
Addressing the Theoretical and Experimental Aspects of Low-Dimensional-Materials-Based FET Immunosensors: A Review
Abstract
:1. Introduction
2. Fundamentals
2.1. From Biosensors to Immunosensors: Definitions and Type of Transduction
2.2. Field-Effect Transistors as Biosensors—Principles of Operation
2.3. LDM Semiconductors in FET-Based Immunosensors
2.4. FET Based on 1D and 2D Materials: Detection Mechanisms
3. Recent Applications towards Biosensing
3.1. 1D Materials
3.1.1. Carbon Nanotubes
3.1.2. Nanowires—Silicon and Beyond
3.2. 2D Materials
3.2.1. Graphene
3.2.2. Transition Metal Dichalcogenides
3.2.3. Black Phosphorus and MXenes
3.3. Theory and Simulation of Biosensing
4. Outlook and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| Ab | Antibody |
| Ag | Antigen |
| BioFET | Field-effect-transistor-based biosensor |
| BP | Black phosphorus |
| CNT | Carbon nanotube |
| DEP | Dielectrophoresis |
| DFT | Density functional theory |
| DI | Deionized water |
| EDL | Electrical double layer |
| EI | Electrochemical immunosensor |
| FET | Field-effect transistor |
| GFET | Graphene field-effect transistor |
| IDS | Drain-source current |
| ION/IOFF | Ratio of drain-source current between on and off operation modes |
| ISFET | Ion sensitive field-effect transistor |
| LDM | Low-dimensional material |
| LOD | Limit of detection |
| MD | Molecular dynamics |
| N/A | Not available |
| NEGF | Non-equilibrium green’s functions |
| NR | Nanorod |
| NW | Nanowire |
| ox-SWCNT | Oxidized single-walled carbon nanotube |
| rGO | Reduced graphene oxide |
| sc-SWCNT | Semiconducting single-walled carbon nanotube |
| SGFET | Solution-gated field-effect transistor |
| SWCNT | Single-walled carbon nanotube |
| TMD | Transition metal dichalcogenides |
| VGS | Gate-source voltage |
| VTH | Threshold voltage |
| λD | Debye length |
References
- Scheller, F.W.; Hintsche, R.; Pfeiffer, D.; Schubert, F.; Riedel, K.; Kindervater, R. Biosensors: Fundamentals, applications and trends. Sens. Actuators B Chem. 1991, 4, 197–206. [Google Scholar] [CrossRef]
- Clark, L.C.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 2006, 102, 29–45. [Google Scholar] [CrossRef]
- Amorim, R.G.; Rocha, A.R.; Scheicher, R.H. Boosting DNA recognition sensitivity of graphene nanogaps through nitrogen edge functionalization. J. Phys. Chem. C 2016, 120, 19384–19388. [Google Scholar] [CrossRef]
- Prasongkit, J.; Feliciano, G.T.; Rocha, A.R.; He, Y.; Osotchan, T.; Ahuja, R.; Scheicher, R.H. Theoretical assessment of feasibility to sequence DNA through interlayer electronic tunneling transport at aligned nanopores in bilayer graphene. Sci. Rep. 2015, 5. [Google Scholar] [CrossRef]
- Cui, Y.; Wei, Q.; Park, H.; Lieber, C.M. Nanowire nanosensors for highly sensitive and selective detection of biological and chemical species. Science 2001, 293, 1289–1292. [Google Scholar] [CrossRef] [PubMed]
- Tran, T.T.; Clark, K.; Ma, W.; Mulchandani, A. Detection of a secreted protein biomarker for citrus Huanglongbing using a single-walled carbon nanotubes-based chemiresistive biosensor. Biosens. Bioelectron. 2020, 147, 111766. [Google Scholar] [CrossRef]
- Chakraborty, B.; Mondal, D.; Roychaudhuri, C. ZnO nanorod FET biosensors with enhanced sensing performance: Design issues for rational geometry selection. IEEE Sens. J. 2020, 20, 13451–13460. [Google Scholar] [CrossRef]
- Syu, Y.-C.; Hsu, W.-E.; Lin, C.-T. Review—field-effect transistor biosensing: Devices and clinical applications. ECS J. Solid State Sci. Technol. 2018, 7, Q3196–Q3207. [Google Scholar] [CrossRef]
- Mattioli, I.A.; Hassan, A.; Sanches, N.M.; Vieira, N.C.S.; Crespilho, F.N. Highly sensitive interfaces of graphene electrical-electrochemical vertical devices for on drop atto-molar DNA detection. Biosens. Bioelectron. 2021, 175, 112851. [Google Scholar] [CrossRef] [PubMed]
- Macedo, L.J.A.; Iost, R.M.; Hassan, A.; Balasubramanian, K.; Crespilho, F.N. Bioelectronics and interfaces using monolayer graphene. ChemElectroChem 2019, 6, 31–59. [Google Scholar] [CrossRef]
- Alves, A.P.P.; Meireles, L.M.; Ferrari, G.A.; Cunha, T.H.R.; Paraense, M.O.; Campos, L.C.; Lacerda, R.G. Highly sensitive and reusable ion-sensor based on functionalized graphene. Appl. Phys. Lett. 2020, 117, 33105. [Google Scholar] [CrossRef]
- Cipriano, T.; Knotts, G.; Laudari, A.; Bianchi, R.C.; Alves, W.A.; Guha, S. Bioinspired peptide nanostructures for organic field-effect transistors. ACS Appl. Mater. Interfaces 2014, 6, 21408–21415. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Guo, X. Carbon nanomaterials field-effect-transistor-based biosensors. NPG Asia Mater. 2012, 4, e23. [Google Scholar] [CrossRef] [Green Version]
- de Moraes, A.C.M.; Kubota, L.T. Recent trends in field-effect transistors-based immunosensors. Chemosensors 2016, 4, 20. [Google Scholar] [CrossRef] [Green Version]
- Vu, C.A.; Chen, W.Y. Field-effect transistor biosensors for biomedical applications: Recent advances and future prospects. Sensors 2019, 19, 4214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hwang, M.T.; Heiranian, M.; Kim, Y.; You, S.; Leem, J.; Taqieddin, A.; Faramarzi, V.; Jing, Y.; Park, I.; van der Zande, A.M.; et al. Ultrasensitive detection of nucleic acids using deformed graphene channel field-effect biosensors. Nat. Commun. 2020, 11, 1543. [Google Scholar] [CrossRef] [PubMed]
- Miernicki, M.; Hofmann, T.; Eisenberger, I.; von der Kammer, F.; Praetorius, A. Legal and practical challenges in classifying nanomaterials according to regulatory definitions. Nat. Nanotechnol. 2019, 14, 208–216. [Google Scholar] [CrossRef]
- ISO/TS 80004-2:2015 Nanotechnologies—Vocabulary—Part 2: Nano-objects. 2015, p. 10. Available online: https://www.iso.org/standard/54440.html (accessed on 17 February 2021).
- Holzinger, M.; Le Goff, A.; Cosnier, S.; Le Goff, A.; Cosnier, S. Nanomaterials for biosensing applications: A review. Front. Chem. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Tran, T.T.; Mulchandani, A. Carbon nanotubes and graphene nano field-effect transistor-based biosensors. TrAC Trends Anal. Chem. 2016, 79, 222–232. [Google Scholar] [CrossRef] [Green Version]
- Mao, S.; Chang, J.; Pu, H.; Lu, G.; He, Q.; Zhang, H.; Chen, J. Two-dimensional nanomaterial-based field-effect transistors for chemical and biological sensing. Chem. Soc. Rev. 2017, 46, 6872–6904. [Google Scholar] [CrossRef]
- Zheng, G.; Patolsky, F.; Cui, Y.; Wang, W.U.; Lieber, C.M. Multiplexed electrical detection of cancer markers with nanowire sensor arrays. Nat. Biotechnol. 2005, 23, 1294–1301. [Google Scholar] [CrossRef]
- Lee, M.; Lucero, A.; Kim, J. One-dimensional nanomaterials for field-effect transistor (FET) type biosensor applications. Trans. Electr. Electron. Mater. 2012, 13, 165–170. [Google Scholar] [CrossRef] [Green Version]
- Patolsky, F.; Lieber, C.M. Nanowire nanosensors. Mater. Today 2005, 8, 20–28. [Google Scholar] [CrossRef]
- Nguyen, E.P.; de Carvalho Castro Silva, C.; Merkoçi, A. Recent advancement in biomedical applications on the surface of two-dimensional materials: From biosensing to tissue engineering. Nanoscale 2020, 12, 19043–19067. [Google Scholar] [CrossRef] [PubMed]
- Shen, M.Y.; Li, B.R.; Li, Y.K. Silicon nanowire field-effect-transistor based biosensors: From sensitive to ultra-sensitive. Biosens. Bioelectron. 2014, 60, 101–111. [Google Scholar] [CrossRef]
- Wang, F.F.; Hu, X.Y.; Niu, X.X.; Xie, J.Y.; Chu, S.S.; Gong, Q.H. Low-dimensional materials-based field-effect transistors. J. Mater. Chem. C 2018, 6, 924–941. [Google Scholar] [CrossRef]
- da Silva, E.T.S.G.; Souto, D.E.P.; Barragan, J.T.C.; Giarola, J.d.F.; de Moraes, A.C.M.; Kubota, L.T. Electrochemical biosensors in point-of-care devices: Recent advances and future trends. ChemElectroChem 2017, 4, 778–794. [Google Scholar] [CrossRef]
- Mohanty, S.P.; Kougianos, E. Biosensors: A tutorial review. IEEE Potentials 2006, 25, 35–40. [Google Scholar] [CrossRef]
- Mehrotra, P. Biosensors and their applications—A review. J. Oral Biol. Craniofac. Res. 2016, 6, 153–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campàs, M.; Prieto-Simón, B.; Marty, J.-L. A review of the use of genetically engineered enzymes in electrochemical biosensors. Semin. Cell Dev. Biol. 2009, 20, 3–9. [Google Scholar] [CrossRef]
- Thévenot, D.R.; Toth, K.; Durst, R.A.; Wilson, G.S. Electrochemical biosensors: Recommended definitions and classification. International Union of Pure and Applied Chemistry: Physical Chemistry Division, Commission I.7 (Biophysical Chemistry); Analytical Chemistry Division, Commission V.5 (Electroanalytica). Biosens. Bioelectron. 2001, 16, 121–131. [Google Scholar] [CrossRef]
- Fatibello-Filho, O.; Lupetti, K.O.; Leite, O.D.; Vieira, I.C. Chapter 17 Electrochemical biosensors based on vegetable tissues and crude extracts for environmental, food and pharmaceutical analysis. In Electrochemical Sensor Analysis; Alegret, S., Merkoçi, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2007; Volume 49, pp. 357–377. ISBN 0166-526X. [Google Scholar]
- Li, Y.-C.E.; Lee, I.-C. The Current Trends of Biosensors in Tissue Engineering. Biosensors 2020, 10, 88. [Google Scholar] [CrossRef]
- Felix, F.S.; Angnes, L. Electrochemical immunosensors—A powerful tool for analytical applications. Biosens. Bioelectron. 2018, 102, 470–478. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Turner, A.P.F. Biosensors: Sense and sensibility. Chem. Soc. Rev. 2013, 42, 3184–3196. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, G.P.; Corrêa, C.C.; Kubota, L.T. A simple, sensitive and reduced cost paper-based device with low quantity of chemicals for the early diagnosis of Plasmodium falciparum malaria using an enzyme-based colorimetric assay. Sens. Actuators B Chem. 2018, 255, 2113–2120. [Google Scholar] [CrossRef]
- Roda, A.; Cavalera, S.; Di Nardo, F.; Calabria, D.; Rosati, S.; Simoni, P.; Colitti, B.; Baggiani, C.; Roda, M.; Anfossi, L. Dual lateral flow optical/chemiluminescence immunosensors for the rapid detection of salivary and serum IgA in patients with COVID-19 disease. Biosens. Bioelectron. 2021, 172, 112765. [Google Scholar] [CrossRef]
- Parolo, C.; Sena-Torralba, A.; Bergua, J.F.; Calucho, E.; Fuentes-Chust, C.; Hu, L.; Rivas, L.; Álvarez-Diduk, R.; Nguyen, E.P.; Cinti, S.; et al. Tutorial: Design and fabrication of nanoparticle-based lateral-flow immunoassays. Nat. Protoc. 2020, 15, 3788–3816. [Google Scholar] [CrossRef] [PubMed]
- Piguillem, S.V.; Regiart, M.; Bertotti, M.; Raba, J.; Messina, G.A.; Fernández-Baldo, M.A. Microfluidic fluorescence immunosensor using ZnONFs for invasive aspergillosis determination. Microchem. J. 2020, 159, 105371. [Google Scholar] [CrossRef]
- Guo, W.; Vilaplana, L.; Hansson, J.; Marco, M.P.; van der Wijngaart, W. Immunoassays on thiol-ene synthetic paper generate a superior fluorescence signal. Biosens. Bioelectron. 2020, 163, 112279. [Google Scholar] [CrossRef] [PubMed]
- Amin, N.; Torralba, A.S.; Álvarez-Diduk, R.; Afkhami, A.; Merkoçi, A. Lab in a tube: Point-of-care detection of Escherichia coli. Anal. Chem. 2020, 92, 4209–4216. [Google Scholar] [CrossRef]
- Giarola, J.d.F.; Souto, D.E.P.; Kubota, L.T. Evaluation of PAMAM dendrimers (G3, G4, and G5) in the construction of a SPR-based immunosensor for cardiac troponin T. Anal. Sci. 2021, 1–28. [Google Scholar] [CrossRef]
- Sharma, P.K.; Kumar, J.S.; Singh, V.V.; Biswas, U.; Sarkar, S.S.; Alam, S.I.; Dash, P.K.; Boopathi, M.; Ganesan, K.; Jain, R. Surface plasmon resonance sensing of Ebola virus: A biological threat. Anal. Bioanal. Chem. 2020, 412, 4101–4112. [Google Scholar] [CrossRef] [PubMed]
- Hampitak, P.; Jowitt, T.A.; Melendrez, D.; Fresquet, M.; Hamilton, P.; Iliut, M.; Nie, K.; Spencer, B.; Lennon, R.; Vijayaraghavan, A. A point-of-care immunosensor based on a quartz crystal microbalance with graphene biointerface for antibody assay. ACS Sens. 2020, 5, 3520–3532. [Google Scholar] [CrossRef]
- Pohanka, M. Immunoassay of interferon gamma by quartz crystal microbalance biosensor. Talanta 2020, 218, 121167. [Google Scholar] [CrossRef]
- Holford, T.R.J.; Davis, F.; Higson, S.P.J. Recent trends in antibody based sensors. Biosens. Bioelectron. 2012, 34, 12–24. [Google Scholar] [CrossRef]
- Bari, S.M.I.; Reis, L.G.; Nestorova, G.G. Calorimetric sandwich-type immunosensor for quantification of TNF-α. Biosens. Bioelectron. 2019, 126, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, L.M.; Towe, B.; Wolf, A.; Mertens, F.; Lerchner, J. Binding event measurement using a chip calorimeter coupled to magnetic beads. Sens. Actuators B Chem. 2010, 145, 239–245. [Google Scholar] [CrossRef]
- Bergveld, P. Development of an ion-sensitive solid-state device for neurophysiological measurements. IEEE Trans. Biomed. Eng. 1970, 17, 70–71. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Castro Silva, C.; Pinotti, L.F. Sensing materials: Electrolyte-gated organic field-effect transistor (EGOFETs). In Reference Module in Biomedical Sciences; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Kaisti, M. Detection principles of biological and chemical FET sensors. Biosens. Bioelectron. 2017, 98, 437–448. [Google Scholar] [CrossRef]
- Bergveld, P. Thirty years of ISFETOLOGY. Sens. Actuators B Chem. 2003, 88, 1–20. [Google Scholar] [CrossRef] [Green Version]
- Janissen, R.; Sahoo, P.K.; Santos, C.A.; da Silva, A.M.; von Zuben, A.A.G.; Souto, D.E.P.; Costa, A.D.T.; Celedon, P.; Zanchin, N.I.T.; Almeida, D.B.; et al. InP nanowire biosensor with tailored biofunctionalization: Ultrasensitive and highly selective disease biomarker detection. Nano Lett. 2017, 17, 5938–5949. [Google Scholar] [CrossRef] [Green Version]
- Allen, B.L.; Kichambare, P.D.; Star, A. Carbon nanotube field-effect-transistor-based biosensors. Adv. Mater. 2007, 19, 1439–1451. [Google Scholar] [CrossRef]
- Seo, G.; Lee, G.; Kim, M.J.; Baek, S.-H.H.; Choi, M.; Ku, K.B.; Lee, C.-S.S.; Jun, S.; Park, D.; Kim, H.G.; et al. Rapid detection of COVID-19 causative virus (SARS-CoV-2) in human nasopharyngeal swab specimens using field-effect transistor-based biosensor. ACS Nano 2020, 14, 5135–5142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sarkar, D.; Liu, W.; Xie, X.; Anselmo, A.C.; Mitragotri, S.; Banerjee, K. MoS2 field-effect transistor for next-generation label-free biosensors. ACS Nano 2014, 8, 3992–4003. [Google Scholar] [CrossRef]
- Picca, R.A.; Manoli, K.; Macchia, E.; Sarcina, L.; Di Franco, C.; Cioffi, N.; Blasi, D.; Österbacka, R.; Torricelli, F.; Scamarcio, G.; et al. Ultimately sensitive organic bioelectronic transistor sensors by materials and device structure design. Adv. Funct. Mater. 2020, 30, 1–23. [Google Scholar] [CrossRef]
- Kim, D.-S.; Park, J.-E.; Shin, J.-K.; Kim, P.K.; Lim, G.; Shoji, S. An extended gate FET-based biosensor integrated with a Si microfluidic channel for detection of protein complexes. Sens. Actuators B Chem. 2006, 117, 488–494. [Google Scholar] [CrossRef]
- Kim, J.; Rim, Y.S.; Chen, H.; Cao, H.H.; Nakatsuka, N.; Hinton, H.L.; Zhao, C.; Andrews, A.M.; Yang, Y.; Weiss, P.S. Fabrication of high-performance ultrathin In2O3 film field-effect transistors and biosensors using chemical lift-off lithography. ACS Nano 2015, 9, 4572–4582. [Google Scholar] [CrossRef] [Green Version]
- Parkula, V.; Berto, M.; Diacci, C.; Patrahau, B.; Di Lauro, M.; Kovtun, A.; Liscio, A.; Sensi, M.; Samorì, P.; Greco, P.; et al. Harnessing selectivity and sensitivity in electronic biosensing: A novel lab-on-chip multigate organic transistor. Anal. Chem. 2020, 92, 9330–9337. [Google Scholar] [CrossRef]
- Buth, F.; Donner, A.; Sachsenhauser, M.; Stutzmann, M.; Garrido, J.A. Biofunctional electrolyte-gated organic field-effect transistors. Adv. Mater. 2012, 24, 4511–4517. [Google Scholar] [CrossRef]
- Macchia, E.; Sarcina, L.; Picca, R.A.; Manoli, K.; Di Franco, C.; Scamarcio, G.; Torsi, L. Ultra-low HIV-1 p24 detection limits with a bioelectronic sensor. Anal. Bioanal. Chem. 2020, 412, 811–818. [Google Scholar] [CrossRef] [Green Version]
- Yin, P.T.; Shah, S.; Chhowalla, M.; Lee, K.B. Design, synthesis, and characterization of graphene-nanoparticle hybrid materials for bioapplications. Chem. Rev. 2015, 115, 2483–2531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically-transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Asiri, A.M.; Tang, Z.; Du, D.; Lin, Y. Graphene based materials for biomedical applications. Mater. Today 2013, 16, 365–373. [Google Scholar] [CrossRef]
- Chen, Y.; Ren, R.; Pu, H.; Chang, J.; Mao, S.; Chen, J. Field-effect transistor biosensors with two-dimensional black phosphorus nanosheets. Biosens. Bioelectron. 2017, 89, 505–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alabsi, S.S.; Ahmed, A.Y.; Dennis, J.O.; Khir, M.H.M.; Algamili, A.S. A Review of carbon nanotubes field effect-based biosensors. IEEE Access 2020, 8, 69509–69521. [Google Scholar] [CrossRef]
- Béraud, A.; Sauvage, M.; Bazán, C.M.; Tie, M.; Bencherif, A.; Bouilly, D. Graphene field-effect transistors as bioanalytical sensors: Design, operation and performance. Analyst 2021, 146, 403–428. [Google Scholar] [CrossRef]
- Fakih, I.; Mahvash, F.; Siaj, M.; Szkopek, T. Sensitive precise pH measurement with large-area graphene field-effect transistors at the quantum-capacitance limit. Phys. Rev. Appl. 2017, 8, 044022. [Google Scholar] [CrossRef]
- Ahmad, W.; Gong, Y.; Abbas, G.; Khan, K.; Khan, M.; Ali, G.; Shuja, A.; Tareen, A.K.; Khan, Q.; Li, D. Evolution of low-dimensional material-based field-effect transistors. Nanoscale 2021, 13, 5162–5186. [Google Scholar] [CrossRef]
- Bolotsky, A.; Butler, D.; Dong, C.; Gerace, K.; Glavin, N.R.; Muratore, C.; Robinson, J.A.; Ebrahimi, A. Two-dimensional materials in biosensing and healthcare: From in vitro diagnostics to optogenetics and beyond. ACS Nano 2019, 13, 9781–9810. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Xu, Y.; Hu, M.; Ling, H.; Zhu, X. MXenes: Focus on optical and electronic properties and corresponding applications. Nanophotonics 2020, 9, 1601–1620. [Google Scholar] [CrossRef]
- Jiang, X.; Kuklin, A.V.; Baev, A.; Ge, Y.; Ågren, H.; Zhang, H.; Prasad, P.N. Two-dimensional MXenes: From morphological to optical, electric, and magnetic properties and applications. Phys. Rep. 2020, 848, 1–58. [Google Scholar] [CrossRef]
- Zhang, X.; Zhao, X.; Wu, D.; Jing, Y.; Zhou, Z. High and anisotropic carrier mobility in experimentally possible Ti2CO2 (MXene) monolayers and nanoribbons. Nanoscale 2015, 7, 16020–16025. [Google Scholar] [CrossRef] [PubMed]
- Khan, R.; Andreescu, S. Mxenes-based bioanalytical sensors: Design, characterization, and applications. Sensors 2020, 20, 5434. [Google Scholar] [CrossRef] [PubMed]
- Hutagalung, S.D.; Fadhali, M.M.; Areshi, R.A.; Tan, F.D. Optical and electrical characteristics of silicon nanowires prepared by electroless etching. Nanoscale Res. Lett. 2017, 12, 425. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avouris, P.; Chen, Z.; Perebeinos, V. Carbon-based electronics. Nat. Nanotechnol. 2007, 2, 605–615. [Google Scholar] [CrossRef]
- Lee, M.; Jeon, Y.; Sik Son, K.; Hyung Shim, J.; Kim, S. Comparative performance analysis of silicon nanowire tunnel FETs and MOSFETs on plastic substrates in flexible logic circuit applications. Phys. Status Solid 2012, 209, 1350–1358. [Google Scholar] [CrossRef]
- Li, X.; Wang, X.; Zhang, L.; Lee, S.; Dai, H. Chemically derived, ultrasmooth graphene nanoribbon semiconductors. Science 2008, 319, 1229–1232. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H. Two-dimensional MoS2: Properties, preparation, and applications. J. Mater. 2015, 1, 33–44. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Yu, Y.; Ye, G.J.; Ge, Q.; Ou, X.; Wu, H.; Feng, D.; Chen, X.H.; Zhang, Y. Black phosphorus field-effect transistors. Nat. Nanotechnol. 2014, 9, 372–377. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Bi, Q.; Sajjad, M.; Wang, X.; Ren, Y.; Zhou, X.; Xu, W.; Liu, Z. One-dimensional porous silicon nanowires with large surface area for fast charge–discharge lithium-ion batteries. Nanomaterials 2018, 8, 285. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Wang, H.; Liu, J.; Bao, C. Measuring the specific surface area of monolayer graphene oxide in water. Mater. Lett. 2020, 261, 127098. [Google Scholar] [CrossRef]
- Cheng, J.; Gao, L.; Li, T.; Mei, S.; Wang, C.; Wen, B.; Huang, W.; Li, C.; Zheng, G.; Wang, H.; et al. Two-dimensional black phosphorus nanomaterials: Emerging advances in electrochemical energy storage science. Nano Micro Lett. 2020, 12, 179. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Fang, Y.; Ramasamy, R. Non-covalent functionalization of carbon nanotubes for electrochemical biosensor development. Sensors 2019, 19, 392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doucey, M.-A.; Carrara, S. Nanowire sensors in cancer. Trends Biotechnol. 2019, 37, 86–99. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.; Dak, P.; Lee, Y.; Park, H.; Choi, W.; Alam, M.A.; Kim, S. Two-dimensional layered MoS2 biosensors enable highly sensitive detection of biomolecules. Sci. Rep. 2015, 4, 7352. [Google Scholar] [CrossRef] [Green Version]
- Kukkar, M.; Tuteja, S.K.; Sharma, A.L.; Kumar, V.; Paul, A.K.; Kim, K.-H.; Sabherwal, P.; Deep, A. A new electrolytic synthesis method for few-layered MoS2 nanosheets and their robust biointerfacing with reduced antibodies. ACS Appl. Mater. Interfaces 2016, 8, 16555–16563. [Google Scholar] [CrossRef]
- Xu, B.; Zhi, C.; Shi, P. Latest advances in MXene biosensors. J. Phys. Mater. 2020, 3, 031001. [Google Scholar] [CrossRef]
- Patolsky, F.; Zheng, G.; Lieber, C.M. Fabrication of silicon nanowire devices for ultrasensitive, label-free, real-time detection of biological and chemical species. Nat. Protoc. 2006, 1, 1711–1724. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Chatterjee, K. Recent advances in the field of transition metal dichalcogenides for biomedical applications. Nanoscale 2018, 10, 16365–16397. [Google Scholar] [CrossRef]
- Naguib, M.; Mochalin, V.N.; Barsoum, M.W.; Gogotsi, Y. 25th Anniversary article: MXenes: A new family of two-dimensional materials. Adv. Mater. 2014, 26, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- de Freitas Martins, E.; Troiano Feliciano, G.; Hendrik Scheicher, R.; Reily Rocha, A. Simulating DNA Chip Design Using All-Electronic Graphene-Based Substrates. Molecules 2019, 24, 951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsang, D.K.H.; Lieberthal, T.J.; Watts, C.; Dunlop, I.E.; Ramadan, S.; del Rio Hernandez, A.E.; Klein, N. Chemically functionalised graphene FET biosensor for the label-free sensing of exosomes. Sci. Rep. 2019, 9, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Huang, W.; Diallo, A.K.; Dailey, J.L.; Besar, K.; Katz, H.E. Electrochemical processes and mechanistic aspects of field-effect sensors for biomolecules. J. Mater. Chem. C 2015, 3, 6445–6470. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Sedki, M.; Shen, Y.; Mulchandani, A. Nano-FET-enabled biosensors: Materials perspective and recent advances in North America. Biosens. Bioelectron. 2021, 176, 112941. [Google Scholar] [CrossRef] [PubMed]
- Martel, R.; Schmidt, T.; Shea, H.R.; Hertel, T.; Avouris, P. Single- and multi-wall carbon nanotube field-effect transistors. Appl. Phys. Lett. 1998, 73, 2447–2449. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.W.; Oh, J.S.; Yoo, S.H.; Choi, H.H.; Yoo, K.-H. Electrically refreshable carbon-nanotube-based gas sensors. Nanotechnology 2007, 18, 435504. [Google Scholar] [CrossRef]
- Melzer, K.; Bhatt, V.D.; Jaworska, E.; Mittermeier, R.; Maksymiuk, K.; Michalska, A.; Lugli, P. Enzyme assays using sensor arrays based on ion-selective carbon nanotube field-effect transistors. Biosens. Bioelectron. 2016, 84, 7–14. [Google Scholar] [CrossRef]
- Kuang, Z.; Kim, S.N.; Crookes-Goodson, W.J.; Farmer, B.L.; Naik, R.R. Biomimetic chemosensor: Designing peptide recognition elements for surface functionalization of carbon nanotube field-effect transistors. ACS Nano 2010, 4, 452–458. [Google Scholar] [CrossRef]
- Xuan, C.T.; Thuy, N.T.; Luyen, T.T.; Huyen, T.T.T.; Tuan, M.A. Carbon Nanotube Field-Effect Transistor for DNA Sensing. J. Electron. Mater. 2017, 46, 3507–3511. [Google Scholar] [CrossRef]
- Ko, J.W.; Woo, J.-M.; Jinhong, A.; Cheon, J.H.; Lim, J.H.; Kim, S.H.; Chun, H.; Kim, E.; Park, Y.J. Multi-Order dynamic range dna sensor using a gold decorated swcnt random network. ACS Nano 2011, 5, 4365–4372. [Google Scholar] [CrossRef]
- Shao, W.; Shurin, M.R.; Wheeler, S.E.; He, X.; Star, A. rapid detection of SARS-CoV-2 antigens using high-purity semiconducting single-walled carbon nanotube-based field-effect transistors. ACS Appl. Mater. Interfaces 2021. [Google Scholar] [CrossRef]
- Münzer, A.M.; Seo, W.; Morgan, G.J.; Michael, Z.P.; Zhao, Y.; Melzer, K.; Scarpa, G.; Star, A. Sensing reversible protein-ligand interactions with single-walled carbon nanotube field-effect transistors. J. Phys. Chem. C 2014, 118, 17193–17199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lapedus, M. Chasing after Carbon Nanotube FETs. Available online: https://semiengineering.com/chasing-after-carbon-nanotube-fets/ (accessed on 25 March 2021).
- Fischer, M.J.E. Amine coupling through EDC/NHS: A practical approach. In Surface Plasmon Resonance: Methods and Protocols; Mol, N.J., Fischer, M.J.E., Eds.; Humana Press: Totowa, NJ, USA, 2010; pp. 55–73. ISBN 978-1-60761-670-2. [Google Scholar]
- Amoretti, M.; Amsler, C.; Bonomi, G.; Bouchta, A.; Bowe, P.; Carraro, C.; Cesar, C.L.; Charlton, M.; Collier, M.J.T.; Doser, M.; et al. Production and detection of cold antihydrogen atoms. Nature 2002, 419, 456–459. [Google Scholar] [CrossRef]
- Alim, S.; Vejayan, J.; Yusoff, M.M.; Kafi, A.K.M. Recent uses of carbon nanotubes & gold nanoparticles in electrochemistry with application in biosensing: A review. Biosens. Bioelectron. 2018, 121, 125–136. [Google Scholar] [CrossRef] [PubMed]
- Sadighbayan, D.; Hasanzadeh, M.; Ghafar-Zadeh, E. Biosensing based on field-effect transistors (FET): Recent progress and challenges. TrAC Trends Anal. Chem. 2020, 133, 116067. [Google Scholar] [CrossRef] [PubMed]
- Mao, S.; Lu, G.; Yu, K.; Chen, J. Specific biosensing using carbon nanotubes functionalized with gold nanoparticle–antibody conjugates. Carbon N. Y. 2010, 48, 479–486. [Google Scholar] [CrossRef]
- Kim, J.P.; Lee, B.Y.; Lee, J.; Hong, S.; Sim, S.J. Enhancement of sensitivity and specificity by surface modification of carbon nanotubes in diagnosis of prostate cancer based on carbon nanotube field effect transistors. Biosens. Bioelectron. 2009, 24, 3372–3378. [Google Scholar] [CrossRef]
- Michael, Z.P.; Shao, W.; Sorescu, D.C.; Euler, R.W.; Burkert, S.C.; Star, A. Probing biomolecular interactions with gold nanoparticle-decorated single-walled carbon nanotubes. J. Phys. Chem. C 2017, 121, 20813–20820. [Google Scholar] [CrossRef]
- Ding, M.; Tang, Y.; Star, A. Understanding interfaces in metal–graphitic hybrid nanostructures. J. Phys. Chem. Lett. 2013, 4, 147–160. [Google Scholar] [CrossRef]
- Schmidt, V.; Wittemann, J.V.; Senz, S.; Gösele, U. Silicon nanowires: A review on aspects of their growth and their electrical properties. Adv. Mater. 2009, 21, 2681–2702. [Google Scholar] [CrossRef]
- Tran, D.P.; Wolfrum, B.; Stockmann, R.; Pai, J.-H.; Pourhassan-Moghaddam, M.; Offenhäusser, A.; Thierry, B. Complementary metal oxide semiconductor compatible silicon nanowires-on-a-chip: Fabrication and preclinical validation for the detection of a cancer prognostic protein marker in serum. Anal. Chem. 2015, 87, 1662–1668. [Google Scholar] [CrossRef]
- Gonchar, K.A.; Agafilushkina, S.N.; Moiseev, D.V.; Bozhev, I.V.; Manykin, A.A.; Kropotkina, E.A.; Gambaryan, A.S.; Osminkina, L.A. H1N1 influenza virus interaction with a porous layer of silicon nanowires. Mater. Res. Express 2020, 7, 035002. [Google Scholar] [CrossRef]
- Gao, A.; Yang, X.; Tong, J.; Zhou, L.; Wang, Y.; Zhao, J.; Mao, H.; Li, T. Multiplexed detection of lung cancer biomarkers in patients serum with CMOS-compatible silicon nanowire arrays. Biosens. Bioelectron. 2017, 91, 482–488. [Google Scholar] [CrossRef] [PubMed]
- Chartuprayoon, N.; Zhang, M.; Bosze, W.; Choa, Y.H.; Myung, N.V. One-dimensional nanostructures based bio-detection. Biosens. Bioelectron. 2015, 63, 432–443. [Google Scholar] [CrossRef] [PubMed]
- Mu, L.; Chang, Y.; Sawtelle, S.D.; Wipf, M.; Duan, X.; Reed, M.A. Silicon nanowire field-effect transistors—A versatile class of potentiometric nanobiosensors. IEEE Access 2015, 3, 287–302. [Google Scholar] [CrossRef]
- Chen, K.I.; Li, B.R.; Chen, Y.T. Silicon nanowire field-effect transistor-based biosensors for biomedical diagnosis and cellular recording investigation. Nano Today 2011, 6, 131–154. [Google Scholar] [CrossRef]
- Chang, S.-M.; Palanisamy, S.; Wu, T.-H.; Chen, C.-Y.; Cheng, K.-H.; Lee, C.-Y.; Yuan, S.-S.F.; Wang, Y.-M. Utilization of silicon nanowire field-effect transistors for the detection of a cardiac biomarker, cardiac troponin I and their applications involving animal models. Sci. Rep. 2020, 10, 22027. [Google Scholar] [CrossRef]
- Smith, R.; Geary, S.M.; Salem, A.K. Silicon nanowires and their impact on cancer detection and monitoring. ACS Appl. Nano Mater. 2020. [Google Scholar] [CrossRef]
- Tran, D.P.; Winter, M.A.; Wolfrum, B.; Stockmann, R.; Yang, C.-T.; Pourhassan-Moghaddam, M.; Offenhäusser, A.; Thierry, B. Toward intraoperative detection of disseminated tumor cells in lymph nodes with silicon nanowire field-effect transistors. ACS Nano 2016, 10, 2357–2364. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Zhang, Y.; To, S.; You, L.; Sun, Y. Effect of nanowire number, diameter, and doping density on nano-fet biosensor sensitivity. ACS Nano 2011, 5, 6661–6668. [Google Scholar] [CrossRef] [PubMed]
- Nair, P.R.; Alam, M.A. Design considerations of silicon nanowire biosensors. IEEE Trans. Electron Devices 2007, 54, 3400–3408. [Google Scholar] [CrossRef]
- Fathil, M.F.M.; Arshad, M.K.M.; Nuzaihan, M.N.M.; Gopinath, S.C.B.; Ruslinda, A.R.; Hashim, U. The ZnO-FET biosensor for cardiac troponin I. IOP Conf. Ser. Mater. Sci. Eng. 2018, 318. [Google Scholar] [CrossRef]
- Chakraborty, B.; Ghosh, S.; Das, N.; RoyChaudhuri, C. Liquid gated ZnO nanorod FET sensor for ultrasensitive detection of Hepatitis B surface antigen with vertical electrode configuration. Biosens. Bioelectron. 2018, 122, 58–67. [Google Scholar] [CrossRef] [PubMed]
- Ditshego, N.M.J. Zno nanowire field effect transistor for biosensing: A review. J. Nano Res. 2019, 60, 94–112. [Google Scholar] [CrossRef] [Green Version]
- Joyce, H.J.; Docherty, C.J.; Gao, Q.; Tan, H.H.; Jagadish, C.; Lloyd-Hughes, J.; Herz, L.M.; Johnston, M.B. Electronic properties of GaAs, InAs and InP nanowires studied by terahertz spectroscopy. Nanotechnology 2013, 24, 214006. [Google Scholar] [CrossRef]
- Ahn, M.S.; Ahmad, R.; Bhat, K.S.; Yoo, J.Y.; Mahmoudi, T.; Hahn, Y.B. Fabrication of a solution-gated transistor based on valinomycin modified iron oxide nanoparticles decorated zinc oxide nanorods for potassium detection. J. Colloid Interface Sci. 2018, 518, 277–283. [Google Scholar] [CrossRef]
- Novoselov, K.S. Electric Field Effect in Atomically Thin Carbon Films. Science 2004, 306, 666–669. [Google Scholar] [CrossRef] [Green Version]
- Schedin, F.; Geim, A.K.; Morozov, S.V.; Hill, E.W.; Blake, P.; Katsnelson, M.I.; Novoselov, K.S. Detection of individual gas molecules adsorbed on graphene. Nat. Mater. 2007, 6, 652–655. [Google Scholar] [CrossRef] [PubMed]
- Ang, P.K.; Chen, W.; Wee, A.T.S.; Loh, K.P. Solution-Gated Epitaxial Graphene as pH Sensor. J. Am. Chem. Soc. 2008, 130, 14392–14393. [Google Scholar] [CrossRef]
- Mohanty, N.; Berry, V. Graphene-based single-bacterium resolution biodevice and DNA transistor: Interfacing graphene derivatives with nanoscale and microscale biocomponents. Nano Lett. 2008, 8, 4469–4476. [Google Scholar] [CrossRef]
- Mao, S.; Lu, G.; Yu, K.; Bo, Z.; Chen, J. Specific Protein Detection Using Thermally Reduced Graphene Oxide Sheet Decorated with Gold Nanoparticle-Antibody Conjugates. Adv. Mater. 2010, 22, 3521–3526. [Google Scholar] [CrossRef]
- Ohno, Y.; Maehashi, K.; Matsumoto, K. Label-free biosensors based on aptamer-modified graphene field-effect transistors. J. Am. Chem. Soc. 2010, 132, 18012–18013. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Niu, F.; Zhu, C.; Yang, J.; Xi, N. Graphene-based FET detector for E. coli K12 real-time monitoring and its theoretical analysis. J. Sens. 2016, 2016. [Google Scholar] [CrossRef]
- Zhou, L.; Mao, H.; Wu, C.; Tang, L.; Wu, Z.; Sun, H.; Zhang, H.; Zhou, H.; Jia, C.; Jin, Q.; et al. Label-free graphene biosensor targeting cancer molecules based on non-covalent modification. Biosens. Bioelectron. 2017, 87, 701–707. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.; Shukla, S.; Bajpai, V.K.; Han, Y.K.; Huh, Y.S.; Kumar, A.; Ghosh, A.; Gandhi, S. A smart nanosensor for the detection of human immunodeficiency virus and associated cardiovascular and arthritis diseases using functionalized graphene-based transistors. Biosens. Bioelectron. 2019, 126, 792–799. [Google Scholar] [CrossRef]
- Kanai, Y.; Ohmuro-Matsuyama, Y.; Tanioku, M.; Ushiba, S.; Ono, T.; Inoue, K.; Kitaguchi, T.; Kimura, M.; Ueda, H.; Matsumoto, K. Graphene field effect transistor-based immunosensor for ultrasensitive noncompetitive detection of small antigens. ACS Sens. 2020, 5, 24–28. [Google Scholar] [CrossRef]
- Perez, A.; Amorim, R.G.; Villegas, C.E.P.; Rocha, A.R. Nanogap-based all-electronic DNA sequencing devices using MoS2 monolayers. Phys. Chem. Chem. Phys. 2020, 22, 27053–27059. [Google Scholar] [CrossRef] [PubMed]
- Mak, K.F.; Lee, C.; Hone, J.; Shan, J.; Heinz, T.F. Atomically thin MoS2: A new direct-gap semiconductor. Phys. Rev. Lett. 2010, 105, 136805. [Google Scholar] [CrossRef] [Green Version]
- Wang, L.; Wang, Y.; Wong, J.I.; Palacios, T.; Kong, J.; Yang, H.Y. Functionalized MoS2 nanosheet-based field-effect biosensor for label-free sensitive detection of cancer marker proteins in solution. Small 2014, 10, 1101–1105. [Google Scholar] [CrossRef]
- Nam, H.; Oh, B.-R.; Chen, P.; Chen, M.; Wi, S.; Wan, W.; Kurabayashi, K.; Liang, X. Multiple MoS2 Transistors for Sensing Molecule Interaction Kinetics. Sci. Rep. 2015, 5, 10546. [Google Scholar] [CrossRef] [Green Version]
- Nam, H.; Oh, B.-R.; Chen, P.; Yoon, J.S.; Wi, S.; Chen, M.; Kurabayashi, K.; Liang, X. Two different device physics principles for operating MoS2 transistor biosensors with femtomolar-level detection limits. Appl. Phys. Lett. 2015, 107, 012105. [Google Scholar] [CrossRef]
- Ryu, B.; Nam, H.; Oh, B.-R.; Song, Y.; Chen, P.; Park, Y.; Wan, W.; Kurabayashi, K.; Liang, X. Cyclewise operation of printed MoS2 transistor biosensors for rapid biomolecule quantification at femtomolar levels. ACS Sens. 2017, 2, 274–281. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Yang, S.; Pineda-Gómez, R.; Ibarlucea, B.; Ma, J.; Lohe, M.R.; Akbar, T.F.; Baraban, L.; Cuniberti, G.; Feng, X. Electrochemically exfoliated high-quality 2H-MoS2 for multiflake thin film flexible biosensors. Small 2019, 1901265. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Sando, S.; Cui, T. Biosensor Based on Layer by Layer Deposited Phosphorene Nanoparticles for Liver Cancer Detection. In Proceedings of the Volume 2: Advanced Manufacturing; American Society of Mechanical Engineers, Tampa, FL, USA, 3–9 November 2017. [Google Scholar]
- Naguib, M.; Kurtoglu, M.; Presser, V.; Lu, J.; Niu, J.; Heon, M.; Hultman, L.; Gogotsi, Y.; Barsoum, M.W. Two-dimensional nanocrystals produced by exfoliation of Ti3AlC2. Adv. Mater. 2011, 23, 4248–4253. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Peng, Z.; Holl, N.J.; Hassan, M.R.; Pappas, J.M.; Wei, C.; Izadi, O.H.; Wang, Y.; Dong, X.; Wang, C.; et al. MXene–graphene field-effect transistor sensing of influenza virus and SARS-CoV-2. ACS Omega 2021, 6, 6643–6653. [Google Scholar] [CrossRef]
- Rafat, N.; Satoh, P.; Calabrese Barton, S.; Worden, R.M. Integrated experimental and theoretical studies on an electrochemical immunosensor. Biosensors 2020, 10, 144. [Google Scholar] [CrossRef]
- De Freitas Martins, E.; Amorim, R.G.; Feliciano, G.T.; Scheicher, R.H.; Rocha, A.R. The role of water on the electronic transport in graphene nanogap devices designed for DNA sequencing. Carbon N. Y. 2020, 158, 314–319. [Google Scholar] [CrossRef]
- Kogikoski, S.; Sousa, C.P.; Liberato, M.S.; Andrade-Filho, T.; Prieto, T.; Ferreira, F.F.; Rocha, A.R.; Guha, S.; Alves, W.A. Multifunctional biosensors based on peptide–polyelectrolyte conjugates. Phys. Chem. Chem. Phys. 2016, 18, 3223–3233. [Google Scholar] [CrossRef] [PubMed]
- Lowe, B.M.; Skylaris, C.K.; Green, N.G.; Shibuta, Y.; Sakata, T. Molecular dynamics simulation of potentiometric sensor response: The effect of biomolecules, surface morphology and surface charge. Nanoscale 2018, 10, 8650–8666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mirzaii Babolghani, F.; Mohammadi-Manesh, E. Simulation and experimental study of FET biosensor to detect polycyclic aromatic hydrocarbons. Appl. Surf. Sci. 2019, 488, 662–670. [Google Scholar] [CrossRef]
- Stroud, A.; Leuty, G.; Muratore, C.; Derosa, P.A.; Berry, R. Molecular dynamics simulation of the interaction of HLL peptide and 2D materials with individual residue resolution. Comput. Mater. Sci. 2019, 169, 109112. [Google Scholar] [CrossRef]
- Badhe, Y.; Gupta, R.; Rai, B. In silico design of peptides with binding to the receptor binding domain (RBD) of the SARS-CoV-2 and their utility in bio-sensor development for SARS-CoV-2 detection. RSC Adv. 2021, 11, 3816–3826. [Google Scholar] [CrossRef]
- de Souza, F.A.L.; Amorim, R.G.; Prasongkit, J.; Scopel, W.L.; Scheicher, R.H.; Rocha, A.R. Topological line defects in graphene for applications in gas sensing. Carbon N. Y. 2018, 129, 803–808. [Google Scholar] [CrossRef] [Green Version]
- Rocha, A.R.; García-suárez, V.M.; Bailey, S.W.; Lambert, C.J.; Ferrer, J.; Sanvito, S. Towards molecular spintronics. Nat. Mater. 2005, 4, 335–339. [Google Scholar] [CrossRef]
- Li, J.H.; Wu, J.; Yu, Y.X. DFT exploration of sensor performances of two-dimensional WO3 to ten small gases in terms of work function and band gap changes and I-V responses. Appl. Surf. Sci. 2021, 546, 149104. [Google Scholar] [CrossRef]
- Prasongkit, J.; de Freitas Martins, E.; de Souza, F.A.L.; Scopel, W.L.; Amorim, R.G.; Amornkitbamrung, V.; Rocha, A.R.; Scheicher, R.H. Topological Line Defects Around Graphene Nanopores for DNA Sequencing. J. Phys. Chem. C 2018, 122, 7094–7099. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Choi, Y.K.; Kim, H.J.; Scheicher, R.H.; Cho, J.H. Physisorption of DNA nucleobases on h-BN and graphene: VdW-corrected DFT calculations. J. Phys. Chem. C 2013, 117, 13435–13441. [Google Scholar] [CrossRef] [Green Version]
- Civitarese, T.; Zollo, G. Triggering amino acid detection by atomistic resolved tunneling current signals in graphene nanoribbon devices for peptide sequencing. ACS Appl. Nano Mater. 2021, 4, 363–371. [Google Scholar] [CrossRef]
- Feliciano, G.T.; Sanz-Navarro, C.; Coutinho-Neto, M.D.; Ordejón, P.; Scheicher, R.H.; Rocha, A.R. Capacitive DNA detection driven by electronic charge fluctuations in a graphene nanopore. Phys. Rev. Appl. 2015, 3, 1–7. [Google Scholar] [CrossRef]







| 1D | 2D | |||||
|---|---|---|---|---|---|---|
| LDM | Single-Walled Carbon Nanotubes | Silicon Nanowire | Graphene | Transition Metal Dichalcogenides | Black Phosphorus | MXenes |
 |  |  |  |  |  | |
| Bandgap (eV) | 0.6−1.8 [73] | 1.1 [73] | 0 [73] | 0−3.4 [73] | 1.59 [73] | 0−1.82 [74,75] |
| Bandgap Tunability (eV) | −0.3 [73] | 3 [73] | 0.25 [73] | ±1 [73] | 0.1 [73] | 1.25−1.80 [75] |
| Carrier Mobility (cm2 V−1 s−1) | 105 [73] | 400 [73] | 105 [73] | 1−102 [73] | 103 [73] | 7.41 × 104 (Ti2CO2) [76] |
| Conductivity (S/cm) | 102−106 [77] | 2.95 × 10−2 [78] | 6300 [66] | 10−4 (MoS2) [77] | N/A | 2410 (Ti3C2) [77] |
| ION/IOFF ratio | 104 [79] | 106 [80] | 107 [81] | 1 × 108 (MoS2) [82] | 105 [83] | N/A |
| Surface Area (m2 g−1) | 600 [77] | 323.47 (Porous) [84] | 2630 [85] | 8.6 (MoS2) [77] | 2630 [86] | 93.6 (Ti3C2) [77] |
| Active Sites | sp2 orbital of carbon (large π system), defects [66] | Silanol (Si-OH) groups on the surface of the native oxide layer [66] | sp2 orbital of carbon (large π system), defects [66] | p orbital of chalcogenide atoms, defects [66] | pZ orbital of P atom [66] | O, OH, and F on the surface [66] |
| Resulting interactions | π−π interaction, Van der Waals, charge transfer, covalent bond [66] | Covalent bond [66] | π−π interaction, Van der Waals, charge transfer, covalent bond [66] | Charge transfer [66] | Charge transfer [66] | van der Waals, H-bond, charge transfer [66] |
| Functionalization strategies commonly used to immobilize antibodies on the surface | Non-covalent functionalization by π−π interaction via PBASE; Covalent functionalization by promoting carboxyl groups on the surface followed by EDC-NHS crosslinker [87] | Covalent attachment of antibodies to the NW surface through silane derivatives, i.e., APTES [88] | Non-covalent functionalization by π−π interaction via PBASE; Covalent functionalization by promotion carboxyl groups on the surface followed by EDC-NHS crosslinker [87] | Physical adsorption [89]; Disulfide linkages between the MoS2 nanosheets and the chemically reduced antibodies with exposed sulfhydryl (−SH) groups [90] | Covalent functionalization of the protective oxide layer with silane derivatives, i.e., APTES [68] | Soy phospholipid, PEGylation, photo-grafting, photopolymerization, APTES [91] |
| Surface properties | High chemical stability, hydrophobic [56] | Low chemical stability [92] | High chemical stability, hydrophobic [25] | Hydrophobic [93] | Poor chemical stability [25] | Hydrophilic [94] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Freitas Martins, E.; Pinotti, L.F.; de Carvalho Castro Silva, C.; Rocha, A.R. Addressing the Theoretical and Experimental Aspects of Low-Dimensional-Materials-Based FET Immunosensors: A Review. Chemosensors 2021, 9, 162. https://doi.org/10.3390/chemosensors9070162
de Freitas Martins E, Pinotti LF, de Carvalho Castro Silva C, Rocha AR. Addressing the Theoretical and Experimental Aspects of Low-Dimensional-Materials-Based FET Immunosensors: A Review. Chemosensors. 2021; 9(7):162. https://doi.org/10.3390/chemosensors9070162
Chicago/Turabian Stylede Freitas Martins, Ernane, Luis Francisco Pinotti, Cecilia de Carvalho Castro Silva, and Alexandre Reily Rocha. 2021. "Addressing the Theoretical and Experimental Aspects of Low-Dimensional-Materials-Based FET Immunosensors: A Review" Chemosensors 9, no. 7: 162. https://doi.org/10.3390/chemosensors9070162