1. Introduction
CuO-Fe
2O
3 nanocomposite receives considerable attention because it is applied as a catalyst, gas sensor, anode for batteries, and anti-corrosive hydrophobic coating [
1,
2,
3]. This composite combines the potential characteristics of CuO components and Fe
2O
3. Copper oxide (CuO), which is one of the p-type semiconductors, has excellent optical, electrical, physical, and magnetic properties [
4]. Meanwhile, Fe
2O
3 is one of the n-type semiconductors which has the most stable phase among the oxides of iron, corrosion resistance, high efficiency, non-toxic nature, inexpensive, and environmentally friendly [
5,
6]. The combination of CuO and Fe
2O
3 into a nanocomposite causes the physical properties of CuO-Fe
2O
3 to be better than CuO and Fe
2O
3 alone [
7,
8].
In the past decade, various methods have been proposed to produce CuO-Fe
2O
3 nanocomposite in various sizes and shapes. They include electrochemical reactions, phase separation, spin-coating, sol-gel, particle-filling coprecipitation, and milling. Among these processes, the milling and spin-coating methods are easy and inexpensive [
9]. However, there has also been no report on the preparation of CuO-Fe
2O
3 composites using natural materials. The use of natural materials increases the added value of the functional materials and also has the advantage of inheriting the actual chemical composition and structure of the raw materials.
From previous studies, it was reported that CuO-Fe
2O
3 composite has high photocatalytic activity. The photocatalytic activity obtained in degrading optimum methylene blue with CuO-Fe
2O
3 was 95.9% [
1] and 83% using Fe
2O
3, CuO, and CuFe
2O
4 phases [
10]. Conversely, the hydrophobic properties decrease with increasing photocatalytic activity. This can be altered by introducing the right dopant. This study reports on the synthesis of PS/CuO-Fe
2O
3 nanocomposite using a simple and inexpensive method. The effect of calcination temperature on the structural and hydrophobic properties of PS/CuO-Fe
2O
3 composites was investigated. The spin-coating method was used to coat PS/CuO-Fe
2O
3 nanocomposites on the surface of the glass preparation.
2. Materials and Methods
2.1. Materials
The materials used in this study were copper rock (92.7% Cu element concentration) and iron powder (87.5% Fe concentration) obtained from community mining in West Sumatra. Other materials include distilled water, alcohol, hydrochloric acid (HCL) 36%, polyethylene glycol (PEG) 400 (PT.Brataco, Bandung, Indonesia), polystyrene, and tetrahydrofuran (Merck Indonesia, Jakarta, Indonesia). These materials were commercially available. Glass slides of 1 cm × 1 cm size were used as a substrate for spin coating.
2.2. Tenorite (CuO) Preparation
The copper rock was initially washed before use, crushed using a mortar, and homogenized with a 150 mesh sieve. The copper powder obtained was milled using High Energy Milling Ellipse 3D Motion (HEM-E3D) method for 20 h. It was then added to 50 mL of 2 M HCl, stirred and heated at a temperature of 90 °C for 1 h. The copper powder obtained was cooled for 1 h, then added with 60 mL of distilled water followed by filtering using Whatman paper grade 41. The gel was dried and crushed using a mortar until smoothness was achieved. Subsequently, it was washed with pure water to remove HCl. The powder obtained was calcined at 650 °C for 3 h at atmospheric pressure to obtain the tenorite (CuO) phase [
11]. Furthermore, the heating powder was pulverized using HEM with milling time variations of 0, 10, 20, 30, and 40 h to obtain nanotenorites.
2.3. Hematite (Fe2O3) Preparation
The iron grain was initially washed using water and crushed using steel mortar until it passed through a 200 mesh sieve. The sand obtained was mashed using a ball mill. Milling is carried out with a ball to powder ratio of 10:1 using a tungsten carbide bottle and a tungsten carbide ball with a diameter of 10 mm. The WC (tungsten carbide) vial has an inner diameter of 54.5 mm, an outer diameter of 66.3 mm and a height of 65.4 mm with a capacity of 125 mL. A grinding speed of 150 rpm was kept constant throughout the experiment and the milling balls were 20 g for each milling time. The type of milling ball used is small carbon steel, as many as 40 pieces, weighing 0.2 g per piece; 4 medium-sized milling balls weighing 0.5 g per piece. Meanwhile, there are 2 large milling balls weighing 3.55 g per piece. The powder obtained was withdrawn with a magnet, separating it from other minerals. Subsequently, the withdrawn powder was heated at 900 °C to obtain the hematite phase (Fe2O3). Furthermore, hematite was mashed using HEM with milling time variations of 2, 5, and 10 h to obtain nanometer-sized particles.
2.4. Preparation of PS/CuO-Fe2O3 Nanocomposite
PS/CuO-Fe2O3 nanocomposite was prepared as follows: 15 mL of tetrahydrofuran (THF), 0.1 g of PEG and 2 g of polystyrene were put into a 100 mL beaker. The nanohematite powder (Fe2O3) and nanotenorite (CuO) were added to it with variations of Cu molar ratio to Fe 1:3, 2:2, and 3:1. The mixture was stirred using a magnetic stirrer at a speed of 500 rpm for 60 min, so that all the components were evenly mixed to form a PS/CuO-Fe2O3 nanocomposite gel.
2.5. Hydrophobic Thin Layer Preparation
PS/CuO-Fe2O3 nanocomposite coating using spin-coating method was conducted with a glass substrate that has been washed with alcohol. The entire surface of the substrate was then covered. Furthermore, the substrate was rotated at a spin coating rate of 1000 rpm for 60 s. Samples were heated for 1 h with variations in calcination temperature of 30 °C, 100 °C, 150 °C, 200 °C, and 250 °C for each composition of 1:3, 2:2, and 3:1.
2.6. Characterization
The X-ray powder (XRD) diffraction pattern was recorded with XRD type X’Pert PRO PANalytical PW30/40. The crystallite size was calculated using the Scherrer equation;
D = 0.9
λ/
β cos
θ,
D is the crystal size,
λ is the wavelength of radiation (
λ = 0.154184 nm for CuKα),
θ is the Bragg angle,
B is FWHM (full width at half maximum) of the selected peak, and
K is the material constant, commonly
K ≈ 0.9 [
12]. SEM type INSPECT-S50 was used to obtain the surface structure and particle size. All measurements were made at room temperature. Water contact angles with the composite coating were taken using a NIKON D5200 camera with the sessile drop method with volume 0.01 mL, a micro syringe at 25 °C, and image-J software (V 1.5, National Institutes of Health, Bethesda, MD 20814, USA).
2.7. Photocatalytic Activity
The PS/CuO-Fe
2O
3 nanocomposite gel was added to 10 mg/L of methyl orange (MO) and dissolved in 1000 mL of distilled water to show photocatalyst activity. Furthermore, it was dried in the sun with a time variation of 2, 4, and 6 h. The degradation percentage was calculated from the value of absorbance before and after irradiation [
13] using a GENESYS 10S Uv-Vis spectrometer.
4. Discussion
Based on the synthesis of PS/CuO-Fe
2O
3 nanocomposites, the diffraction pattern showed the basic ingredients of composites. They include hematite, tenorite, and the presence of copper ferrite phase was also identified. The presence of the copper ferrite phase in the PS/CuO-Fe
2O
3 nanocomposite was also discovered in other studies [
12,
13]. The formation of this phase was due to the presence of iron oxide and Cu oxide CuFe
2O
4 with the general formula AB
2O
4, where A and B are metal ions with valency of +2 and +3 respectively. Copper Ferrite (CuFe
2O
4) has an inverse spinel structure with 8 Cu
2+ ions and 16 Fe
3+ ions in one unit cell, with a composite particle size of 92 nm. This is greater than the particle size of CuO-Fe
2O
3 composites discovered in other studies, which ranged from 27 to 49 nm [
1]. The large particle size obtained is due to the synthesis carried out using natural materials which contain the actual chemical composition of the raw material and suboptimal sample purity.
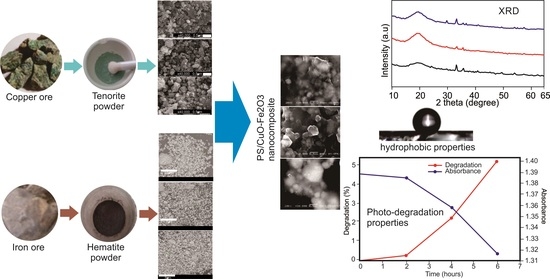
The hydrophobic test showed that there was an effect of calcination temperature and composition towards the water contact angle on the surface of PS/CuO-Fe2O3 nanocomposites. The higher the calcination temperature, the more the contact angle increases to its optimum limit and further decreases as heating continues. The optimum contact angle was detected in the composition of CuO-Fe2O3 at 3:1 and calcination temperature of 200 °C.
The rough surface morphology increased the hydrophobicity of the material. This was explained when water dropped on a rough surface with a nanometer scale, trapping air and preventing water from getting into the rough surface. Therefore, the surface of particles which interacted with water became smaller. This was also in accordance with the Cassie and Baxter model which states that the smaller the surface area of the particles that interacts with water, the greater the contact angle formed [
6,
17].
The highest value of contact angle obtained by PS/CuO-Fe
2O
3 nanocomposites at 125.46° was contradictory considering that the composite was a p and n-type semiconductor material with high photocatalyst activity [
1]. Materials with high photocatalytic activity are more hydrophilic than hydrophobic, based on their properties. The high contact angle obtained was related to the filler (polystyrene) used in the synthesis of PS/CuO-Fe
2O
3 nanocomposites. The fillers used were originally polarized, but in order to produce waterproof nanocomposite properties, they were coated with polystyrene which is a matrix with hydrophobic properties [
17,
18]. Therefore, the coating was able to modify the filler that was originally polarized into a non-polar nanocomposite. The change in angle obtained is related to either polar or non-polar surface of the substrate. On a polar surface, wetness increases (the contact angle reduces). Conversely, on the non-polar surface, wetness reduces (the contact angle increases) [
19]. This low wetness was also due to the cohesive force between water molecules being greater than the adhesive force between water and the surface of the nanocomposite layer. Consequently, water droplets were formed on the nanocomposite layer surface. The hydrophobic surface resisted wetness, because the cohesive force was greater than the adhesive force. Therefore, the substrate in this study was initially not hydrophobic (polar), but by increasing calcination temperature to the optimum, the surface properties became hydrophobic (non-polar). Based on the photocatalyst test on PS/CuO-Fe
2O
3 nanocomposite, it was discovered that the photocatalyst activity was very small at 0.24% (
Figure 9).
The photocatalyst mechanism can be described by the following Equations:
CuO-Fe2O3, when subjected to a photon energy, causes an electron jump from the valence band to the conduction band. This jump of electrons causes holes that can interact to form radicals. The resulting conduction band electrons (e−) react with oxygen molecules to form superoxide radicals . Likewise, the holes react with H2O to form OH radicals. The resulting OH radicals cut the bonds from methyl orange (C14H14N3NaO3S) to (NaO3C6SH6N and C8H10N2O2). OH radicals break the bonds (NaO3C6SH6N and C8H10N2O2) to become aliphatic acid. OH radicals break aliphatic acid bonds into CO2 and H2O.
Based on FTIR characterization, it is known that the chemical composition of the surface of the test material consists of vibrations of O-H, Fe-O, Cu-O, and C-H. The presence of C-H groups (hydrocarbon compounds) sourced from polystyrene acts as a hydrophobic agent. The presence of hydrocarbons on the surface of this test material indicates that the surface is nonpolar. When water is dropped on a polar surface, the water cannot bind to the surface, as a result, the water molecules only bond with each other to form a sphere.
The combination of the polystyrene matrix with CuO and Fe
2O
3 was able to modify the polar properties of the fillers (CuO and Fe
2O
3) into nonpolar nanocomposites. The chemical content of this sample can be seen from the results of FTIR characterization. The functional group analysis of the Ps/CuO-Fe
2O
3 nanocomposite samples was carried out to determine the absorption of the wave numbers of the Ps/CuO-Fe
2O
3 nanocomposite functional groups. FTIR is used as a characterization tool to analyze functional groups. The area of the wave number used in functional group analysis using FTIR is in the range of 600–4000 cm
−1. The results of characterization using FTIR on the Ps/CuO-Fe
2O
3 nano composite sample showed that the peak absorption of the Ps/CuO-Fe
2O
3 composite was in the area of wave numbers 3687.07 cm
−1, 3026.79 cm
−1, 2921.81 cm
−1, 2162.27 cm
−1, 1987.89 cm
−1, 1800.34 cm
−1, 1451.07 cm
−1, 979.79 cm
−1, 901.55 cm
−1, 756.15 cm
−1, 689.90 cm
−1, wave number 3687.07 cm
−1 absorption peak for O-H vibration, wave number 3026.79 cm
−1 which is the FTIR spectrum for C-H, and wave number 2921.81 cm
−1 absorption peak for CH
2 vibrations [
20], wave number 689.90 cm
−1 which represents the FTIR spectrum for the Fe-O group [
18], and wave number 979.79 cm
−1 which represents the FTIR spectrum for CuO.
FTIR characterization illustrates that there are chemical elements of polystyrene compounds, THF, CuO, and Fe2O3 in nanocomposite samples, so that the presence of polystyrene compounds causes the non-polar nature of the sample.
If water is dropped on a surface that contains non-polar components, the polar water cannot unite with the surface, because the attractive force between water molecules is stronger than the taric force between water molecules and molecules on the surface, consequently the hydrogen bonding force between water molecules will happen compared to the London force.
The hydrogen bonding force between water molecules in the form of positive hydrogen atoms will attract the oxygen atoms of other water molecules. Hydrogen bonds cause water molecules to tend to unite (cohesion). The nature of cohesion causes water to have the ability to resist strain (water molecular bonds are not easily broken). The strong cohesion between water molecules at the boundary between water and air seems to form a strong enough “skin” called surface tension; therefore, water will make a curve on the surface of Ps/CuO-Fe2O3 nanocomposite.